Key Points
Question
Is there an association of the extent and timing of cardiac implantable electronic device infection with all-cause mortality?
Findings
In this cohort study of 19 559 patients undergoing cardiac implantable electronic device procedures, 177 developed infection. Individuals with early localized infections were not at higher risk of all-cause mortality, those with early systemic infections and delayed localized infections were at greater risk of mortality, and those with delayed systemic infections were at greatest risk of mortality.
Meaning
Findings suggest that early detection and treatment of cardiac implantable electronic device–related infections may be important in reducing associated mortality.
Abstract
Importance
Cardiac implantable electronic device (CIED) infection is a potentially devastating complication with an estimated 12-month mortality of 15% to 30%. The association of the extent (localized or systemic) and timing of infection with all-cause mortality has not been established.
Objective
To evaluate the association of the extent and timing of CIED infection with all-cause mortality.
Design, Setting, and Participants
This prospective observational cohort study was conducted between December 1, 2012, and September 30, 2016, in 28 centers across Canada and the Netherlands. The study included 19 559 patients undergoing CIED procedures, 177 of whom developed an infection. Data were analyzed from April 5, 2021, to January 14, 2023.
Exposures
Prospectively identified CIED infections.
Main Outcomes and Measures
Time-dependent analysis of the timing (early [≤3 months] or delayed [3-12 months]) and extent (localized or systemic) of infection was performed to determine the risk of all-cause mortality associated with CIED infections.
Results
Of 19 559 patients undergoing CIED procedures, 177 developed a CIED infection. The mean (SD) age was 68.7 (12.7) years, and 132 patients were male (74.6%). The cumulative incidence of infection was 0.6%, 0.7%, and 0.9% within 3, 6, and 12 months, respectively. Infection rates were highest in the first 3 months (0.21% per month), reducing significantly thereafter. Compared with patients who did not develop CIED infection, those with early localized infections were not at higher risk for all-cause mortality (no deaths at 30 days [0 of 74 patients]: adjusted hazard ratio [aHR], 0.64 [95% CI, 0.20-1.98]; P = .43). However, patients with early systemic and delayed localized infections had an approximately 3-fold increase in mortality (8.9% 30-day mortality [4 of 45 patients]: aHR, 2.88 [95% CI, 1.48-5.61]; P = .002; 8.8% 30-day mortality [3 of 34 patients]: aHR, 3.57 [95% CI, 1.33-9.57]; P = .01), increasing to a 9.3-fold risk of death for those with delayed systemic infections (21.7% 30-day mortality [5 of 23 patients]: aHR, 9.30 [95% CI, 3.82-22.65]; P < .001).
Conclusions and Relevance
Findings suggest that CIED infections are most common within 3 months after the procedure. Early systemic infections and delayed localized infections are associated with increased mortality, with the highest risk for patients with delayed systemic infections. Early detection and treatment of CIED infections may be important in reducing mortality associated with this complication.
This cohort study evaluates the association of the extent and timing of cardiac implantable electronic device infection with all-cause mortality.
Introduction
Cardiac implantable electronic device (CIED) infection is a potentially devastating complication of CIED procedures. The estimated incidence of CIED infections ranges between 1% and 2% for all procedures and varies with procedure type.1,2,3,4,5 Retrospective data indicate that the incidence of CIED infections is highest within the first 12 months of device implant, with a gradual reduction over time.6 To date, there is little prospective information regarding the timing of CIED infections within the first 12 months after CIED procedures.
Patient mortality associated with CIED infections is also substantial. In-hospital mortality ranges between 5% and 10%,7,8 whereas 12-month all-cause mortality ranges between 15% and 30%.9,10 Although the extent of infection (ie, localized vs systemic) is associated with all-cause mortality for patients with CIED infections,11 the association between infection timing and mortality has yet to be elucidated, to our knowledge.
In this cohort study of the Prevention of Arrhythmia Device Infection Trial (PADIT),3,12,13 we analyzed the timing of CIED infections within the initial 12 months of a CIED procedure and the association of the timing and extent of infection with all-cause mortality.
Methods
The study design of PADIT has been previously described.12 In brief, this was a prospective cluster crossover randomized trial involving 28 sites across Canada and the Netherlands, evaluating the differences between incremental antimicrobial treatment and conventional treatment in the prevention of CIED infections. Overall, 19 559 patients from 28 centers who were undergoing CIED procedures were recruited for this cohort study between December 1, 2012, and September 30, 2016. Each center recruited for a 24-month period, followed by 12 months of prospective follow-up. In general, prospective follow-up occurred at 2 to 4 weeks, 3 months, 6 months, 9 months, and 12 months after the device procedure. From this cohort, 1717 patients (8.8%) died during follow-up, and 177 patients (0.9%) developed a CIED infection. Treatment of patients who developed CIED infections was directed by the implant cardiologist, supported by infectious disease physicians at the respective centers. This treatment included a course of both intravenous and oral antibiotics directed at isolated pathogens, or empirically when no pathogen was identified, along with consideration of device extraction, depending on clinical status.
Owing to clinical equipoise of the initial study question and the cluster crossover study design, all centers' ethics boards approved the study with a waiver of consent for treatment. Ten centers required written patient consent for additional data collection, which was obtained at follow-up. This observational cohort study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Data Collection
The primary outcome of PADIT was the occurrence of CIED infections requiring hospitalization within the first 12 months of the procedure. Adjudication of end points was performed by 2 infectious disease experts (P.G. and Y.L.) who were blinded to the treatment received, with all discrepancies resolved by the adjudication committee. The extent of infection was determined as being localized (skin, subcutaneous, or pocket infection), systemic (bacteremia or endocarditis), or both. Endocarditis was diagnosed if the patient met definite modified Duke criteria.14 For the present study, patients who were deemed to have concomitant localized and systemic infection were categorized as having systemic infection. The timing of infection was determined as either early or delayed. Early infection was considered present if it occurred 3 months or earlier after the procedure, whereas delayed infection was categorized as occurring between 3 and 12 months after the procedure. The microbiology of pathogens causing CIED infections in PADIT has been previously reported.15
Statistical Analysis
Cumulative incidence rates of overall, localized, and systemic infections were estimated with the cumulative incidence function method rather than with the typically used Kaplan-Meier method because the latter may overestimate the risk of infection in the presence of competing risks (such as death).16 Infection rates (per 100 person-months) were calculated for monthly intervals within the first 3 months after the CIED procedure and for 3 monthly intervals within the first 12 months after the CIED procedure, with additional stratification according to treatment group, procedure type, and PADIT risk score factors (procedure number, age, decreased kidney function, immunocompromised, and type of procedure). In comparing patients with early vs delayed infection and those with localized vs systemic CIED infection, continuous data were reported as mean (SD) or median (IQR) values as appropriate. The 2-sample t test compared normally distributed variables, whereas the Wilcoxon rank sum test compared nonnormally distributed variables. Categorical data were presented as frequency and percentage and were compared with the χ2 test or the Fisher exact test. We evaluated the association between CIED infection and all-cause mortality by treating infection status as a time-dependent covariate. Initially, all patients were considered as having no infection. If CIED infection occurred, patients were reclassified as having infection, and that status remained during follow-up. The time-dependent covariate took the most recent infection status at all-cause mortality or the end of the study. We constructed 2 time-dependent Cox proportional hazards models with or without accounting for the timing (early or delayed) and extent (localized or systemic) of infection. Patients enrolled in PADIT who did not experience infection (n = 19 382) served as the control group. Both models were adjusted for sex, heart failure, type 1 or type 2 diabetes, duration of procedure, randomized antibiotic treatment, and PADIT risk score factors.13 A 2-sided P < .05 was considered statistically significant. Considering the exploratory nature of the study, no adjustment was made for multiple testing. All statistical analyses were conducted with SAS, version 9.4 (SAS Institute). Data were analyzed from April 5, 2021, to January 14, 2023.
Results
Of the 19 559 patients, 177 developed a CIED infection requiring hospitalization. The mean (SD) age was 68.7 (12.7) years, 132 patients were male (74.6%), and 45 were female (25.4%). Data regarding race and ethnicity were not collected owing to the lack of reported associations between race and ethnicity and CIED infection outcomes. There were 109 cases (62%) of localized infection and 68 cases (38%) of systemic infection. Of these patients, 22 had systemic infection only, whereas there were 46 patients with concomitant systemic and localized infection. In addition, 119 infections (67%) occurred within the first 3 months (early), and 58 (33%) occurred between 3 and 12 months (delayed).
Incidence of CIED Infections Within the First 12 Months
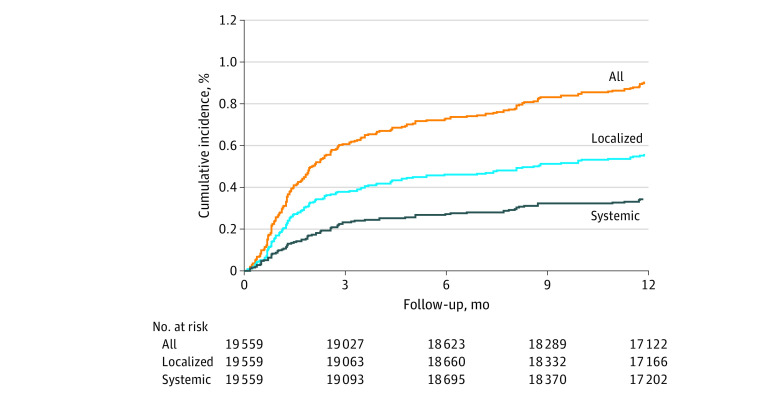
The cumulative incidence of CIED infections is shown in Figure 1. The incidence of infection was 0.6%, 0.7%, and 0.9% within 3, 6, and 12 months, respectively, after a CIED procedure.
Figure 1. Infection Rate Over Time Estimated by the Cumulative Incidence Function Method.
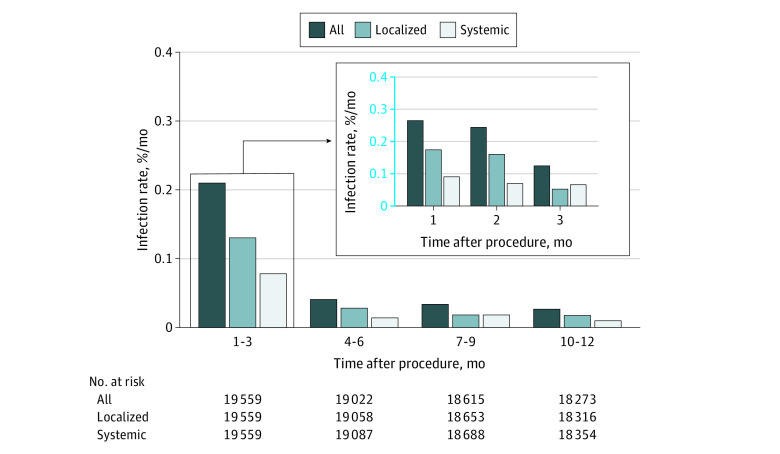
The overall CIED infection rates, including localized and systemic infections, are presented in Figure 2. As expected, indexed infection rates were highest in the first 3 months (0.21% per month, resulting in 67% of all infections [119 of 177 patients]) and reduced significantly by months 10 to 12 (0.026% per month, representing 8% of all infections [14 of 177 patients]). This trend of highest infection rates within the initial 3 months with a precipitous decrease thereafter was consistent for both localized and systemic infections.
Figure 2. Monthly Infection Rate According to Extent of Infection.
Monthly CIED infection rates, stratified according to treatment group, procedure type, and PADIT risk score, are shown in the eFigure in Supplement 1. There was a consistent trend showing greatest infection risk within the initial 3 months after CIED procedure, followed by a decrease, regardless of treatment group (conventional or incremental antibiotics), procedure type (new implant or reintervention), or PADIT risk score. Although patients undergoing reinterventions (compared with requiring new implants) and with higher PADIT risk scores had greater infection rates, all subgroups demonstrated this trend.
Timing and Extent of CIED Infections
In comparing patients with early vs delayed CIED infection, there were no significant differences with respect to baseline demographic characteristics, including age, sex, medical history, procedure type, and procedure duration (Table 1). In comparing patients with localized vs systemic CIED infection, a significantly higher proportion of revision procedures was present for those with localized infections vs systemic infection (47 of 109 patients [43.1%] vs 16 of 68 patients [23.5%]; P = .008), whereas underlying diabetes (27 of 68 patients [39.7%] vs 28 of 109 patients [25.7%]; P = .049) and new pacemaker implants (11 of 68 patients [16.2%] vs 5 of 109 patients [4.6%]; P = .009) were more common among patients with systemic infections than localized infections (Table 1).
Table 1. Baseline Characteristics for Early (≤3 Months) vs Delayed (3-12 Months) Infection and Localized vs Systemic Infection.
| Characteristic | All, No. (%) (n = 177) | Infection, No. (%) | P value | Infection, No. (%) | P value | ||
|---|---|---|---|---|---|---|---|
| Early (n = 119) | Delayed (n = 58) | Localized (n = 109) | Systemic (n = 68) | ||||
| Demographic characteristic and history | |||||||
| Age, mean (SD), y | 68.7 (12.7) | 68.2 (12.2) | 69.6 (13.6) | .51 | 67.7 (13.1) | 70.3 (11.8) | .18 |
| Female | 45 (25.4) | 31 (26.1) | 14 (24.1) | .78 | 25 (22.9) | 20 (29.4) | .34 |
| Male | 132 (74.6) | 88 (73.9) | 44 (75.9) | 84 (77.1) | 48 (70.6) | ||
| Type 1 or type 2 diabetes | 55 (31.1) | 33 (27.7) | 22 (37.9) | .17 | 28 (25.7) | 27 (39.7) | .049 |
| Heart failure | 94 (53.1) | 66 (55.5) | 28 (48.3) | .37 | 55 (50.5) | 39 (57.4) | .37 |
| Chronic kidney disease | 41 (23.2) | 27 (22.7) | 14 (24.1) | .83 | 22 (20.2) | 19 (27.9) | .23 |
| Penicillin allergy | 28 (15.8) | 20 (16.8) | 8 (13.8) | .61 | 17 (15.6) | 11 (16.2) | .92 |
| Immunocompromised | 7 (4.0) | 5 (4.2) | 2 (3.4) | >.99 | 4 (3.7) | 3 (4.4) | >.99 |
| Type of procedure | |||||||
| New | |||||||
| PM | 16 (9.0) | 12 (10.1) | 4 (6.9) | .49 | 5 (4.6) | 11 (16.2) | .009 |
| ICD | 18 (10.2) | 15 (12.6) | 3 (5.2) | .12 | 10 (9.2) | 8 (11.8) | .58 |
| Generator replacementa | |||||||
| PM | 24 (13.6) | 15 (12.6) | 9 (15.5) | .60 | 17 (15.6) | 7 (10.3) | .32 |
| ICD | 22 (12.4) | 17 (14.3) | 5 (8.6) | .28 | 17 (15.6) | 5 (7.4) | .11 |
| CRT | 21 (11.9) | 13 (10.9) | 8 (13.8) | .58 | 10 (9.2) | 11 (16.2) | .16 |
| Revision (pocket or lead) | 63 (35.6) | 42 (35.3) | 21 (36.2) | .91 | 47 (43.1) | 16 (23.5) | .008 |
| New | |||||||
| CRT-P | 3 (1.7) | 2 (1.7) | 1 (1.7) | >.99 | 1 (0.9) | 2 (2.9) | .56 |
| CRT-D | 20 (11.3) | 11 (9.2) | 9 (15.5) | .22 | 9 (8.3) | 11 (16.2) | .11 |
| Duration of procedure, h | |||||||
| <1 | 98 (55.4) | 63 (52.9) | 35 (60.3) | .46 | 63 (57.8) | 35 (51.5) | .56 |
| 1-1.5 | 45 (25.4) | 31 (26.1) | 14 (24.1) | 25 (22.9) | 20 (29.4) | ||
| >1.5-2 | 16 (9.0) | 12 (10.1) | 4 (6.9) | 11 (10.1) | 5 (7.4) | ||
| >2 | 17 (9.6) | 13 (10.9) | 4 (6.9) | 10 (9.2) | 7 (10.3) | ||
| Other procedure on pocket, No. | 49 (27.7) | 28 (23.5) | 21 (36.2) | .08 | 35 (32.1) | 14 (20.6) | .10 |
| 1 | 27 (15.3) | 17 (14.3) | 10 (17.2) | 21 (19.3) | 6 (8.8) | ||
| 2 | 17 (9.6) | 9 (7.6) | 8 (13.8) | 11 (10.1) | 6 (8.8) | ||
| >2 | 5 (2.8) | 2 (1.7) | 3 (5.2) | 3 (2.8) | 2 (2.9) | ||
| Incremental antibiotics | 78 (44.1) | 53 (44.5) | 25 (43.1) | .86 | 50 (45.9) | 28 (41.2) | .54 |
Abbreviations: CRT, cardiac resynchronization therapy; CRT-D, CRT with defibrillator; CRT-P, CRT with pacemaker; ICD, implantable cardioverter defibrillator; PM, pacemaker.
Replacement indicates generator change (when battery reaches elective replacement indicator or end of life), and revision indicates interventions for device pocket (eg, evacuation of significant hematoma) or lead (eg, lead upgrade procedures, reimplant of dislodged leads).
Baseline characteristics stratified into groups with early localized, early systemic, delayed localized, and delayed systemic infections are shown in eTable 1 in Supplement 1. There were no significant differences in baseline characteristics, procedure duration, or number of procedures, although the proportion of new pacemaker implants was higher for patients with systemic infections (7 of 45 patients [15.6%] with early systemic infections, 4 of 23 patients [17.4%] with delayed systemic infections, 5 of 74 patients [6.8%] with early localized infections, and 0% with delayed localized infections; P = .02).
Microbiology of CIED Infections
Data regarding the microbiology of CIED infections are shown in eTable 2 in Supplement 1. Systemic infections were more likely to be monomicrobial, whereas localized infections were more likely to occur without an identifiable pathogen. In addition, early systemic infections were more likely to be due to Staphylococcus aureus. No significant differences were detected regarding the sensitivity of the isolated pathogens to cephazolin or vancomycin between the groups.
Association of Timing and Extent of Infection With Outcomes
Outcome data associated with the timing and extent of infection revealed significant differences in 30-day mortality rates between groups (eTables 3 and 4 in Supplement 1). Patients with delayed systemic infections had the highest 30-day mortality rate, at 21.7% (95% CI, 7.5%-43.7%; 5 of 23 patients). Patients with delayed localized (8.8% [95% CI, 1.9%-23.7%; 3 of 34 patients]) and early systemic infections (8.9% [95% CI, 2.5%-21.2%; 4 of 45 patients]) had similar 30-day mortality rates. There were no deaths (0% [95% CI, 0%-4.9%; 0 of 74 patients]) within 30 days among patients with early localized infections. Regarding treatment received, 157 of 177 patients (88.7%) with CIED infection underwent a surgical intervention, a system explant, or both, with no significant differences observed between the groups. For the 20 patients for whom a surgical intervention was not attempted, 30-day mortality was 20% (4 of 20 patients) compared with 5.1% 30-day mortality (8 of 157 patients) for those for whom an intervention was attempted (P = .03).
Time-dependent analysis for the association between CIED infection and all-cause mortality is shown in Table 2. Compared with patients who did not develop CIED infection, those who had infection within 12 months of the procedure had an associated 2.2 times higher risk of all-cause mortality (adjusted hazard ratio [aHR], 2.21; 95% CI, 1.43-3.42; P < .001). When data were stratified by the timing and extent of infection, patients with early localized infections were not statistically significantly different from patients without infection in terms of all-cause mortality risk (aHR, 0.64 [95% CI, 0.20-1.98]; P = .43). However, patients with early systemic infections (aHR, 2.88 [95% CI, 1.48-5.61]; P = .002) and delayed localized infections (aHR, 3.57 [95% CI, 1.33-9.57]; P = .01) were at significantly higher risk for all-cause mortality. Patients who developed a delayed systemic infection were at exceptionally high risk (aHR, 9.30 [95% CI, 3.82-22.65]; P < .001).
Table 2. Adjusted Analysis of Association Between Infection and All-Cause Mortality, With Infection as a Time-Dependent Covariate.
| Model | aHR (95% CI)a | P value |
|---|---|---|
| Time-dependent model 1 | ||
| Any infection | 2.21 (1.43-3.42) | <.001 |
| Time-dependent model 2 | ||
| Early (≤3 mo) | ||
| Systemic infection | 2.88 (1.48-5.61) | .002 |
| Local infection | 0.64 (0.20-1.98) | .43 |
| Delayed (>3 mo) | ||
| Systemic infection | 9.30 (3.82-22.65) | <.001 |
| Local infection | 3.57 (1.33-9.57) | .01 |
Abbreviation: aHR, adjusted hazard ratio.
Adjusted for sex, heart failure, diabetes, duration of procedure, randomized antibiotic treatment, and the components of the PADIT risk score (procedure type, age, chronic kidney disease, immunocompromised, and number of previous procedures).
Discussion
This study prospectively followed up with all 177 patients from the original cohort of 19 559 patients who developed a CIED infection. In our cohort of contemporary cases of CIED infections, the key findings were as follows: (1) infection rates were highest within the initial 3 months after the device procedure, with a gradual decrease during 12 months; (2) patients who experienced an early localized infection did not appear to be at higher risk of all-cause mortality compared with patients with no infection; (3) patients with early systemic infection or those with delayed localized infection were at higher risk of all-cause mortality; and (4) patients with delayed systemic infection were at greatest risk of all-cause mortality.
Incidence of CIED Infections Within the First 12 Months
In our cohort, we found that the greatest infection risk occurred within the first 3 months after the CIED procedure, and this risk was reduced significantly by months 4 to 6. This trend was observed regardless of initial antimicrobial treatment, type of CIED procedure, or PADIT risk score. Previous studies have found an infection rate of approximately 1% to 2% within the first 12 months after the CIED procedure,1,3,4 with an ongoing infection rate of approximately 0.3% per year after the initial 12 months.6 Limited data have also indicated that infection risk is higher within the initial 2 months after a procedure.17 However, these prior studies retrospectively identified CIED infections. Thus, our findings provide prospective corroboration to these previous reports using a large, contemporary cohort of patients who received a CIED.
Timing and Extent of CIED Infections
We did not detect any significant differences in baseline characteristics between patients who had early CIED infections and those with delayed CIED infections. Mechanistically, early CIED infections are mainly due to direct contamination during the procedure.18 In contrast, although delayed CIED infections may also be associated with intraprocedural contamination, they can also be due to bacteremia in the presence of foreign hardware or from seeding with fastidious organisms.19,20 From our data, it appears that underlying patient factors (including age, decreased kidney function, and immunosuppression) and procedural factors (number of prior procedures and procedure type) have similar associations with both early and delayed infections.13
Patients with localized CIED infections were more likely to have undergone a pocket or lead revision procedure. A number of factors could be involved, including direct contamination due to greater pocket manipulation,21 possible traumatic hematoma formation resulting in bacterial colonization,22 or the presence of an avascular device pocket, resulting in suboptimal local antimicrobial treatment.23
Patients with systemic CIED infections were more likely to have underlying diabetes or have undergone a de novo pacemaker implant. Patients with underlying diabetes are at greater risk of both immunosuppression and infections associated with macrovascular complications.24 For example, having a diabetic foot ulcer and a CIED implant may have resulted in more systemic infections in this group, although the infection was not necessarily associated with the presence of the CIED. Less certain are the reasons why patients with systemic infection had a higher proportion of de novo pacemaker implants. One hypothesis is that this population may be more likely to have had a temporary transvenous pacing wire in situ, which is associated with greater infection risk.1 Unfortunately, this level of preprocedural treatment was not captured in the initial trial.
Microbiology of CIED Infections
We found that localized CIED infections were more likely not to have an identifiable pathogen, which may have occurred because localized infections can be diagnosed on clinical examination without the requirement for positive blood culture results. Sonification was not routinely performed in our cohort, which has been shown to increase the diagnostic yield in cases of pocket infection.25 In contrast, systemic infections were more likely to be monomicrobial, reflecting the fact that diagnosis is often confirmed only after a positive blood culture result. There were no significant differences in the sensitivity of pathogens to cephazolin or vancomycin between the groups. Although the data are exploratory, this suggests that antibiotic resistance of pathogens was not associated with the observed differences in mortality between the groups.
Association of the Timing and Extent of Infection With Outcomes
We found an interesting association between the timing and extent of CIED infection with respect to all-cause mortality. Patients who had an early localized CIED infection did not have an associated greater mortality compared with patients who did not develop CIED infections. The reasons for this are uncertain. One possibility is that the early detection of localized infections (eg, during routine postimplant assessment) could lead to prompt treatment, thereby mitigating subsequent associations with mortality. All patients in this cohort required hospitalization for their infections, with the majority (157 of 177 patients [88.7%]) requiring reintervention, indicating that infections were serious as opposed to simple superficial wound infections (eg, stitch abscess).
Patients with an early systemic CIED infection had an approximately 3-fold increased risk of all-cause mortality. This finding is consistent with previous studies that have demonstrated that bacteremia is associated with adverse outcomes, such as increased length of stay, greater treatment costs, and higher mortality among patients with CIED infections.11 In addition, we found that patients who have a delayed localized infection are also at an approximately 3-fold increased risk of all-cause mortality. We hypothesize that this novel finding may be explained in part by the presence of underlying patient factors associated with the development of localized infection later than would be expected while predisposing patients to morbidity and mortality. For example, a degree of immunosuppression (not sufficient to register on the PADIT score) may give rise to indolent organisms but also increases susceptibility to worse clinical outcomes. Additionally, it may also be possible that some of these cases were diagnosed only later in their time course, leading to worse outcomes.
Finally, patients who develop delayed systemic CIED infections are at highest risk for all-cause mortality. Here, the confluence of underlying patient factors and bacteremia resulted in a very high 30-day mortality ( 21.7% [5 of 23 patients]).
Although the numbers are small, patients who did not undergo a surgical intervention had an approximately 4-fold increase in 30-day mortality, supporting the usual recommendation to remove all hardware when possible26 and suggesting a possible association between treatment and mortality. In this cohort, most patients who died in the absence of a surgical intervention did so soon after diagnosis or were deemed to be at prohibitive risk for a surgical intervention, which emphasizes the need for surgical exploration and system explantation, when feasible, once CIED infection is diagnosed.
These findings have implications for the evaluation and management of CIED infections. Although early localized infections are most common and require medical attention, they are not associated with increased mortality when adequately treated. Preventive measures should be considered for patients who are at risk for early systemic infections, such as removing unnecessary central lines, ensuring appropriate preprocedural antibiotics, awaiting clearance of blood cultures for those with bacteremia before CIED implant, and judiciously considering the use of antibiotic-eluting envelopes.4,10,27,28,29 Patients should also be taught to monitor for signs of localized infection and systemic infection after implant (including delayed infection), with prompt attendance for medical assessment if infection is present. In addition, education of other physicians, particularly primary care physicians, may be another important step in the early detection and referral of patients with CIED infections. Once CIED infection is established, early surgical exploration and system extraction are indicated. It is hoped that these hypothesis-generating findings will be instructive for future research endeavors in this field.
Strengths and Limitations
We note certain limitations of our study. The 0.9% overall infection rate may be lower than previous clinical data, although it is similar to findings from the Worldwide Randomized Antibiotic Envelope Infection Prevention Trial (WRAP-IT),4 supporting the feasibility of a new target 1% “standard” infection rate. The lack of a statistically significant difference in baseline characteristics is likely associated with the small numbers within each group, which may have also affected our findings in relation to associations with mortality. Furthermore, the follow-up period from the original PADIT was 12 months after the CIED procedure, meaning we were unable to provide longer-term outcome data after CIED infection. In addition, some potentially pertinent baseline characteristics (such as the presence of central venous lines or indwelling catheters) were not available. Nevertheless, this study represents, to our knowledge, the largest prospective cohort of patients with CIED infections. We were unable to determine whether certain factors (such as admission blood test results or clinical parameters) were important in evaluating outcomes, although we were able to adjust for relevant patient factors (including PADIT risk score components). The treatment outcomes in this study were observational, rather than controlled, although we believe that this is representative of contemporary clinical treatment of CIED infections.
Conclusions
CIED infections are most common within the first months after a procedure, decreasing significantly after the initial 3 months. Early localized infections are not associated with an increase in mortality. Early systemic infections and delayed localized infections are associated with an increase in mortality, whereas delayed systemic infections are associated with the greatest risk of mortality. Future studies should consider the potential relevance of infection timing and extent with respect to outcomes. The early detection and treatment of CIED infections is important in reducing mortality associated with this complication.
eFigure. Stratified Analysis of Indexed Infection Rate
eTable 1. Baseline Characteristics According to the Combination of Timing and Extent of Infections
eTable 2. Microbiology of CIED Infections With Patient-Level and Organism-Level Data
eTable 3. Outcome Data According to the Timing and Extent of Infection
eTable 4. Outcome Data According to the Timing and Extent of Infection
Data Sharing Statement
References
- 1.Klug D, Balde M, Pavin D, et al. ; PEOPLE Study Group . Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation. 2007;116(12):1349-1355. doi: 10.1161/CIRCULATIONAHA.106.678664 [DOI] [PubMed] [Google Scholar]
- 2.Krahn AD, Lee DS, Birnie D, et al. ; Ontario ICD Database Investigators . Predictors of short-term complications after implantable cardioverter-defibrillator replacement: results from the Ontario ICD Database. Circ Arrhythm Electrophysiol. 2011;4(2):136-142. doi: 10.1161/CIRCEP.110.959791 [DOI] [PubMed] [Google Scholar]
- 3.Krahn AD, Longtin Y, Philippon F, et al. Prevention of Arrhythmia Device Infection Trial: the PADIT trial. J Am Coll Cardiol. 2018;72(24):3098-3109. doi: 10.1016/j.jacc.2018.09.068 [DOI] [PubMed] [Google Scholar]
- 4.Tarakji KG, Mittal S, Kennergren C, et al. ; WRAP-IT Investigators . Antibacterial envelope to prevent cardiac implantable device infection. N Engl J Med. 2019;380(20):1895-1905. doi: 10.1056/NEJMoa1901111 [DOI] [PubMed] [Google Scholar]
- 5.Han HC, Hawkins NM, Pearman CM, Birnie DH, Krahn AD. Epidemiology of cardiac implantable electronic device infections: incidence and risk factors. Europace. 2021;23(23 suppl 4):iv3-iv10. doi: 10.1093/europace/euab042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansen JB, Jørgensen OD, Møller M, Arnsbo P, Mortensen PT, Nielsen JC. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population-based cohort study of 46299 consecutive patients. Eur Heart J. 2011;32(8):991-998. doi: 10.1093/eurheartj/ehq497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenspon AJ, Patel JD, Lau E, et al. 16-Year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol. 2011;58(10):1001-1006. doi: 10.1016/j.jacc.2011.04.033 [DOI] [PubMed] [Google Scholar]
- 8.Sohail MR, Henrikson CA, Braid-Forbes MJ, Forbes KF, Lerner DJ. Mortality and cost associated with cardiovascular implantable electronic device infections. Arch Intern Med. 2011;171(20):1821-1828. doi: 10.1001/archinternmed.2011.441 [DOI] [PubMed] [Google Scholar]
- 9.Greenspon AJ, Eby EL, Petrilla AA, Sohail MR. Treatment patterns, costs, and mortality among Medicare beneficiaries with CIED infection. Pacing Clin Electrophysiol. 2018;41(5):495-503. doi: 10.1111/pace.13300 [DOI] [PubMed] [Google Scholar]
- 10.Wilkoff BL, Boriani G, Mittal S, et al. ; WRAP-IT Investigators . Impact of cardiac implantable electronic device infection: a clinical and economic analysis of the WRAP-IT trial. Circ Arrhythm Electrophysiol. 2020;13(5):e008280. doi: 10.1161/CIRCEP.119.008280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee DH, Gracely EJ, Aleem SY, Kutalek SP, Vielemeyer O. Differences of mortality rates between pocket and nonpocket cardiovascular implantable electronic device infections. Pacing Clin Electrophysiol. 2015;38(12):1456-1463. doi: 10.1111/pace.12748 [DOI] [PubMed] [Google Scholar]
- 12.Connolly SJ, Philippon F, Longtin Y, et al. Randomized cluster crossover trials for reliable, efficient, comparative effectiveness testing: design of the Prevention of Arrhythmia Device Infection Trial (PADIT). Can J Cardiol. 2013;29(6):652-658. doi: 10.1016/j.cjca.2013.01.020 [DOI] [PubMed] [Google Scholar]
- 13.Birnie DH, Wang J, Alings M, et al. Risk factors for infections involving cardiac implanted electronic devices. J Am Coll Cardiol. 2019;74(23):2845-2854. doi: 10.1016/j.jacc.2019.09.060 [DOI] [PubMed] [Google Scholar]
- 14.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30(4):633-638. doi: 10.1086/313753 [DOI] [PubMed] [Google Scholar]
- 15.Longtin Y, Gervais P, Birnie DH, et al. Impact of choice of prophylaxis on the microbiology of cardiac implantable electronic device infections: insights from the Prevention of Arrhythmia Device Infection Trial (PADIT). Open Forum Infect Dis. 2021;8(11):ofab513. doi: 10.1093/ofid/ofab513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601-609. doi: 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prutkin JM, Reynolds MR, Bao H, et al. Rates of and factors associated with infection in 200 909 Medicare implantable cardioverter-defibrillator implants: results from the National Cardiovascular Data Registry. Circulation. 2014;130(13):1037-1043. doi: 10.1161/CIRCULATIONAHA.114.009081 [DOI] [PubMed] [Google Scholar]
- 18.de Oliveira JC, Martinelli M, Nishioka SA, et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: results of a large, prospective, randomized, double-blinded, placebo-controlled trial. Circ Arrhythm Electrophysiol. 2009;2(1):29-34. Published correction appears in Circ Arrhythm Electrophysiol. 2009;2(1):e1. doi: 10.1161/CIRCEP.108.795906 [DOI] [PubMed] [Google Scholar]
- 19.Chamis AL, Peterson GE, Cabell CH, et al. Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter-defibrillators. Circulation. 2001;104(9):1029-1033. doi: 10.1161/hc3401.095097 [DOI] [PubMed] [Google Scholar]
- 20.Rohacek M, Weisser M, Kobza R, et al. Bacterial colonization and infection of electrophysiological cardiac devices detected with sonication and swab culture. Circulation. 2010;121(15):1691-1697. doi: 10.1161/CIRCULATIONAHA.109.906461 [DOI] [PubMed] [Google Scholar]
- 21.Poole JE, Gleva MJ, Mela T, et al. ; REPLACE Registry Investigators . Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation. 2010;122(16):1553-1561. doi: 10.1161/CIRCULATIONAHA.110.976076 [DOI] [PubMed] [Google Scholar]
- 22.Essebag V, Verma A, Healey JS, et al. ; BRUISE CONTROL Investigators . Clinically significant pocket hematoma increases long-term risk of device infection: Bruise Control Infection study. J Am Coll Cardiol. 2016;67(11):1300-1308. doi: 10.1016/j.jacc.2016.01.009 [DOI] [PubMed] [Google Scholar]
- 23.Lekkerkerker JC, van Nieuwkoop C, Trines SA, et al. Risk factors and time delay associated with cardiac device infections: Leiden device registry. Heart. 2009;95(9):715-720. doi: 10.1136/hrt.2008.151985 [DOI] [PubMed] [Google Scholar]
- 24.Dryden M, Baguneid M, Eckmann C, et al. Pathophysiology and burden of infection in patients with diabetes mellitus and peripheral vascular disease: focus on skin and soft-tissue infections. Clin Microbiol Infect. 2015;21(suppl 2):S27-S32. doi: 10.1016/j.cmi.2015.03.024 [DOI] [PubMed] [Google Scholar]
- 25.Mason PK, Dimarco JP, Ferguson JD, et al. Sonication of explanted cardiac rhythm management devices for the diagnosis of pocket infections and asymptomatic bacterial colonization. Pacing Clin Electrophysiol. 2011;34(2):143-149. doi: 10.1111/j.1540-8159.2010.02820.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perrin T, Deharo JC. Therapy and outcomes of cardiac implantable electronic devices infections. Europace. 2021;23(23)(suppl 4):iv20-iv27. doi: 10.1093/europace/euab016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mittal S, Wilkoff BL, Kennergren C, et al. The World-wide Randomized Antibiotic Envelope Infection Prevention (WRAP-IT) trial: long-term follow-up. Heart Rhythm. 2020;17(7):1115-1122. doi: 10.1016/j.hrthm.2020.02.011 [DOI] [PubMed] [Google Scholar]
- 28.Blomstrom-Lundqvist C, Ostrowska B. Prevention of cardiac implantable electronic device infections: guidelines and conventional prophylaxis. Europace. 2021;23(suppl 4):iv11-iv19. doi: 10.1093/europace/euab071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boriani G, Vitolo M, Wright DJ, et al. Infections associated with cardiac electronic implantable devices: economic perspectives and impact of the TYRX™ antibacterial envelope. Europace. 2021;23(23)(suppl 4):iv33-iv44. doi: 10.1093/europace/euab126 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Stratified Analysis of Indexed Infection Rate
eTable 1. Baseline Characteristics According to the Combination of Timing and Extent of Infections
eTable 2. Microbiology of CIED Infections With Patient-Level and Organism-Level Data
eTable 3. Outcome Data According to the Timing and Extent of Infection
eTable 4. Outcome Data According to the Timing and Extent of Infection
Data Sharing Statement