Abstract
Recent studies have revealed a high diversity of pleurostomatid ciliates in brackish habitats. Here, a novel species, Pseudolitonotus spirelis gen. et sp. n., isolated from a mangrove wetland of southern China, was investigated based on living observation, protargol staining, and molecular analyses. The new genus Pseudolitonotus gen. n. is characterized by the last left somatic kinety (LKn) being shortened and none of the right somatic kineties extending to the anterior end of the cell, thus distinguishing it from all known pleurostomatid genera. The type species, Pseudolitonotus spirelis sp. n., is characterized by the possession of two macronuclear nodules, 11–15 right and 7–9 left kineties, a single contractile vacuole subterminally located, extrusomes evenly spaced along the entire ventral margin and some forming an “apical group”, two types of cortical granules, and the bottom of the oral slit invariably being twisted. Litonotus gracilis (Pan et al. Eur J Protistol 51:494–506, 2015) is believed to be another member of this new genus as its LKn and right somatic kineties are all shortened. Hence, a new combination, Pseudolitonotus gracilis (Pan et al., 2015) comb. n., is suggested and its diagnosis is improved. Molecular phylogenetic analyses based on SSU rDNA sequence data reveal that Pseudolitonotus gen. n. is monophyletic and groups with Apolitonotus (Pan et al. J Eukaryot Microbiol 67:252–262, 2020) of the family Protolitonotidae (Wu et al. Zool Scr 46:245–253, 2017). However, the familial assignment of this new genus is uncertain based on current data.
Keywords: Biodiversity, Litonotus gracilis, Molecular phylogeny, New combination, New genus, New species
Introduction
Ciliated protozoa (ciliates) are widely distributed in a range of habitats (Bai et al. 2020; Liu et al. 2021; Lynn 2008; Wu et al. 2021; Zhang et al. 2021). This includes marine ecosystems where they play key roles in ecological processes, such as the remineralization of nutrients, connecting the classic food chain and the microbial loop (Azam et al. 1983). Most of our knowledge of the diversity, systematics, and ecology of ciliates derives from studies of temperate ecosystems. Far less is known about ciliates from tropical and sub-tropical ecosystems. For instance, mangrove ciliates are relatively poorly studied (Hu et al. 2019). Thus, to understand mangrove ecosystems, we need to improve our appreciation of their ciliate biodiversity. Here, we take a step toward that end by examining one order that occurs in mangrove swamps and similar benthic habitats.
Ciliates of the order Pleurostomatida Schewiakoff, 1896 are characterized by their laterally flattened body, slit-like cytostome, densely ciliated right side, and sparsely ciliated left side with bristle-like cilia (Lynn 2008). To date, about 300 nominal species of pleurostomatids have been reported from marine, freshwater, and terrestrial habitats worldwide (Buddenbrock 1920; Carey 1992; Dragesco 1954, 1960, 1965; Kahl 1931; Song and Wilbert 1989; Song et al. 2009; Stokes 1893; Wu et al. 2021a, b). They are assigned to 13 genera and four families, mainly based on the ciliary patterns of both somatic and perioral kineties and features of their extrusomes (Pan et al. 2020; Vďačný et al. 2015; Wu et al. 2017). Since the beginning of the twenty-first century, about 35 new species, three new genera and two new families have been reported from marine and brackish water habitats, including intertidal zones, mangrove wetlands, mariculture ponds, and estuaries (Chen et al. 2011; Hu et al. 2019; Lin et al. 2004, 2005a, b, 2007a, b, 2008; Pan et al. 2010, 2013, 2014, 2015, 2020; Song et al. 2009; Wu et al. 2013, 2014, 2015a, b, 2017, 2021a, b). Both single gene- and multiple gene-based phylogenetic analyses support the monophyly of the order Pleurostomatida (Pan et al. 2020; Vďačný et al. 2011, 2015, 2021a; Wu et al. 2017, 2021a, b; Zhang et al. 2012), which is consistent with morphological studies (Corliss 1979). However, there are several genera for which molecular data are lacking (Heminotus, Opisthodon, Amphileptiscus and Apoamphileptus) or for which only one SSU rDNA sequence is available (e.g., Apolitonotus, Siroloxophyllum and Pseudoamphileptus) in the GenBank database. Therefore, expanded sampling is needed to further explore the diversity of pleurostomatids using a range of methods (Warren et al. 2017).
In this study, a pleurostomatid was isolated from a mangrove wetland in the city of Zhanjiang, Guangdong Province, southern China (Fig. 1). After morphological and molecular studies and comparison with known species, this isolate could not be assigned to any known genus. Therefore, a new genus and new species, Pseudolitonotus spirelis gen. et sp. n., is suggested.
Fig. 1.
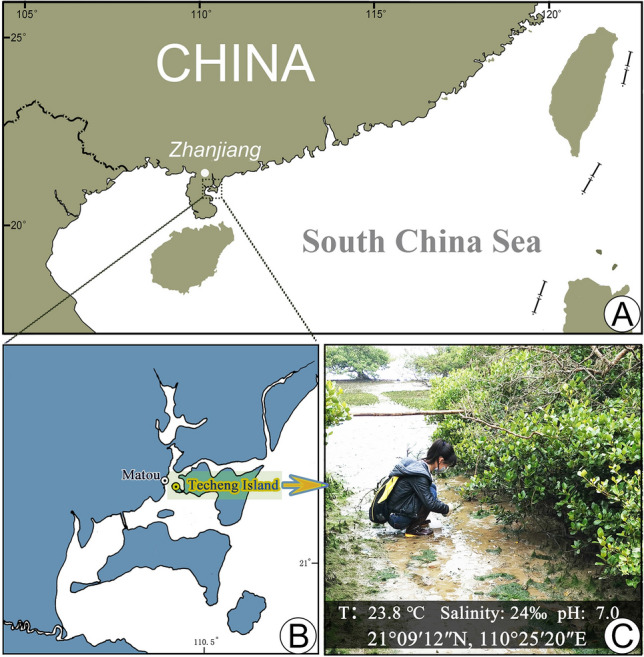
The sampling site. A Map of southern China with sampling area marked by the square. B Map of Techeng Island and surrounding area, with a yellow circle indicating the location of the sampling site. C A view of the sampling site
ZooBank registration
This work: urn:lsid:zoobank.org:pub:09138052-FC78-4BAF-96B4-1BC70E5B924F
Pseudolitonotus gen. n.: urn:lsid:zoobank.org:act:E3D805BE-AD0B-4FE1-BA9D-1E5615C3DDB1
Pseudolitonotus spirelis sp. n.: urn:lsid:zoobank.org:act:F1259A08-5894–4688-92B2-18EEFE6A8477
Results
Taxonomy
Class: Litostomatea Small and Lynn, 1981
Subclass: Haptoria Corliss, 1974
Order: Pleurostomatida Schewiakoff, 1896
Family: incertae familiae
Genus: Pseudolitonotus gen. n.
Diagnosis
Pleurostomatid in which the last left somatic kinety (LKn) is shortened and none of the right somatic kineties extend to the anterior end of the body.
Type species
Pseudolitonotus spirelis sp. n.
Etymology
The genus name is a composite of the Greek prefix “Pseudo” (not genuine; sham) and the generic name Litonotus, referring to its similarity to the well-known genus Litonotus Wrzesniowski, 1870 in terms of its body shape and ciliary pattern. Masculine gender.
Species assignable
Pseudolitonotus spirelis sp. n. and Pseudolitonotus gracilis (Pan et al., 2015) comb. n.
Remarks
This genus is distinguished from all known pleurostomatid genera by the shortened LKn and none of the right somatic kineties extending to the cell apex.
Pseudolitonotus spirelis sp. n. (Figs. 2, 3; Table 1)
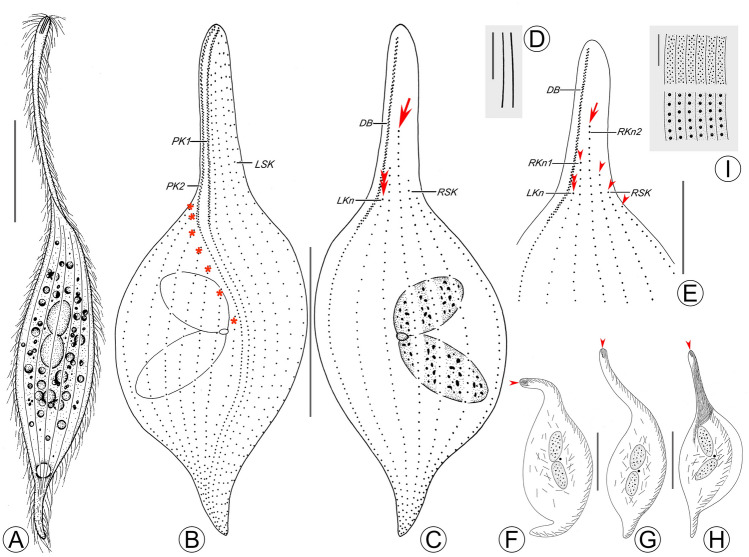
Fig. 2.
Pseudolitonotus spirelis gen. et sp. n. in vivo (A, D, I) and stained with protargol (B, C, E–H). A Right lateral view of a representative cell. B, C Ciliary patterns of right (B) and left (C) side of the holotype specimen, arrow indicates that the longest right somatic kinety does not extend to cell apex, asterisks mark the right somatic kineties in the leftmost region that are shortened along the perioral kineties, double arrowheads mark the shortening of the last left somatic kinety. D Extrusomes. E Ciliary pattern of anterior region of left side, arrowheads mark the right somatic kineties that do not extend to the cell apex, arrow shows the longest somatic kinety, double arrowheads point to the shortening of the last left somatic kinety. F–H Distribution of extrusomes, arrowheads show the “apical group”. I Cortical granules. DB dorsal brush, LKn last left somatic kinety, LSK left somatic kinety, PK1 perioral kinety 1, PK2 perioral kinety 2, RKn1 last right somatic kinety, RKn2 penultimate right somatic kinety, RSK right somatic kinety. Scale bars: 50 μm in (A–C, F–H); 10 μm in (D, E, I)
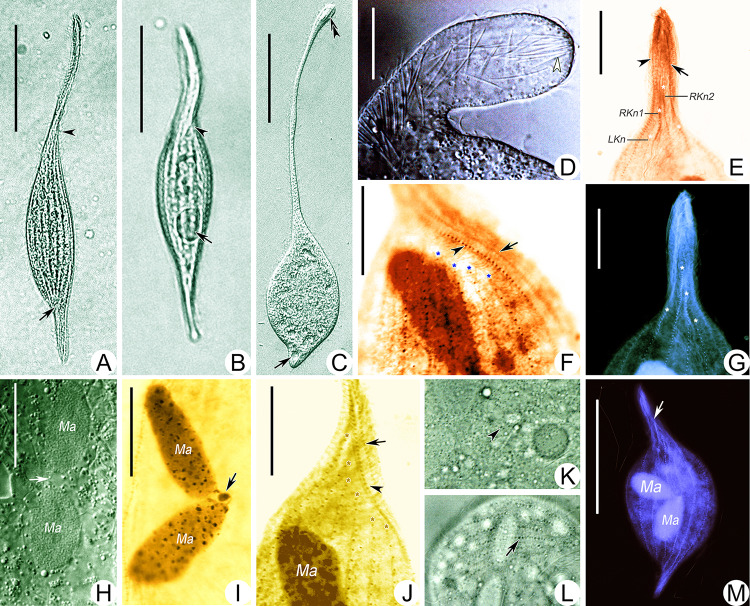
Fig. 3.
Photomicrographs of Pseudolitonotus spirelis gen. et sp. n. in vivo (A–D, H, K, L) and stained with protargol (E–G*, I, J, M*). A Right lateral view of a representative cell, arrowhead marks the twisted portion at the bottom of the oral area. B, C Contracted (B) and extended (C) individuals, arrowheads point to the twisted portion at the bottom of the oral area, arrows indicate the contractile vacuole, double arrowhead marks the “apical group” of extrusomes. D Anterior part of cell, arrowhead shows the “apical group” of extrusomes. E Left view of anterior region of cell of the holotype specimen, to show perioral kinety 2 (arrow), the dorsal brush kinety (arrowhead) and the shortened somatic kineties (asterisks). G Left view of anterior region of cell, asterisks mark the shortened somatic kineties. H, I Nuclear apparatus, arrow shows the micronucleus. K, L To show the smaller (arrowhead) and larger (arrow) cortical granules. M Ciliary pattern, arrow points to the longest somatic kinety (RKn2). LKn last left somatic kinety, RKn1 last right somatic kinety, RKn2 penultimate right somatic kinety. Scale bars: 100 μm in (A–C, M); 20 μm in (D–J). *Images G and M were false-colored to reveal structures (Adobe Photoshop CS6)
Table 1.
Morphological characteristics of Pseudolitonotus spirelis gen. et sp. n. based on protargol impregnated specimens (all measurements in μm)
| Character | H | Min | Max | Mean | SD | n |
|---|---|---|---|---|---|---|
| Body length | 185 | 115 | 280 | 190.5 | 43.83 | 19 |
| Body width | 55 | 40 | 75 | 52.5 | 10.55 | 19 |
| Number of RKa | 13 | 11 | 15 | 12.2 | 1.31 | 19 |
| Number of LKb | 9 | 7 | 9 | 8.7 | 0.58 | 19 |
| Number of Ma | 2 | 2 | 2 | 2 | 0 | 19 |
| Length of Ma | 30 | 15 | 40 | 30.0 | 7.30 | 19 |
| Width of Ma | 10 | 8 | 20 | 12.4 | 3.50 | 19 |
| Number of DB | 61 | 46 | 78 | 59 | 8.53 | 19 |
| Distance to RKn2c | 35 | 25 | 60 | 36.5 | 9.26 | 19 |
| Length of Ex | 15 | 12 | 16 | 14.5 | 1.07 | 19 |
| Length of Na | 40 | 30 | 75 | 48.4 | 12.25 | 19 |
DB dorsal brush, Ex extrusomes, H holotype, LK left kineties, Ma macronuclear nodules, Max maximum, Mean arithmetic mean, Min minimum, n sample size, Na nematodesmata, RK right kineties, RKn2 penultimate right kinety, SD standard deviation
aPerioral kinety 2 included
bPerioral kinety 1 and dorsal brush kinety included
cDistance from anterior end to the penultimate right kinety
Diagnosis
Pseudolitonotus about 160–350 µm in vivo; two macronuclear nodules; one micronucleus; 11–15 right and 7–9 left kineties; single contractile vacuole located subterminally; extrusomes bar-shaped, evenly spaced along entire ventral margin and some clustered together to form an “apical group” at anterior end of cell; bottom of oral slit invariably twisted; two types of cortical granules.
Type locality and ecological features
A mangrove wetland on Techeng Island in the city of Zhanjiang, Guangdong Province, China (21°09′12′′ N, 110°25′20′′ E). Water temperature 23.8 °C, salinity 24‰, pH 7.0.
Deposition of slides
A protargol slide with the holotype specimen circled in ink (registration no. WL2012040602-01A), and a second protargol slide with several paratype specimens (registration no. WL2012040602-01B), are deposited in the Laboratory of Protozoology, Ocean University of China (OUC), China.
Etymology
The Latin adjective spirel·is, -is, -e ([m, f, n]; twist) refers to the bottom of “neck” (oral slit) invariably having a twist.
Morphology and ciliary pattern
Body size about 160–350 µm × 30–45 µm in vivo. Body shape Litonotus-like, i.e., slender lanceolate, contractile, with sharply pointed posterior end and a long conspicuous neck-like region that is about half cell-length when fully extended (Figs. 2A, 3A, B). Bottom of oral area twisted to right side in all specimens (n > 20), conspicuously twisted when cell contracts (Figs. 2A, 3A, B), and can be detected in protargol-stained specimens (Fig. 3F, J). Two ovoidal macronuclear nodules, each about 20–25 µm × 10–15 µm in vivo, located in mid-body region (Figs. 2F–H, 3H, I). Single ovoidal micronucleus, located between macronuclear nodules, about 2–3 µm across (Fig. 3H, I). One contractile vacuole, about 15–20 µm in diameter, subterminally located (Figs. 2A, 3A–C). Extrusomes bar-shaped, about 15 µm long and 0.2 µm wide in vivo, densely and evenly spaced along entire ventral margin, some clustered together to form a conspicuous “apical group” at anterior end of cell (Fig. 2A, F–H) that can be detected with DIC microscopy (Fig. 3C, D). Pellicle thin with inconspicuous longitudinal furrows on right side within which ciliary rows are located (Fig. 3A). Two kinds of cortical granules: type 1 dot-like, ca. 0.2 µm across, grayish, irregularly scattered between ciliary rows (Figs. 2I, 3K); type 2 globular, ca. 1–1.5 µm across, grayish, regularly arranged in a single line between adjacent ciliary rows (Figs. 2I, 3L). Right side densely ciliated with cilia ca. 8 µm long; left side sparsely ciliated. Cytoplasm colorless to pale yellow, often with numerous refringent globules ca. 2 µm across and several food vacuoles 3–5 µm across that renders main part of body opaque (Fig. 3A, B). Locomotion by swimming or by gliding on substrate.
Ciliary pattern as shown in Figs. 2B, C, E, 3E–G, J, M. Excluding perioral kinety 2 (PK2), ten to 14 right somatic kineties (Figs. 2B, C, 3F, J) none of which extend to cell apex; penultimate right somatic kinety (RKn2) is the longest of them (Figs. 2C, E, 3E, G, M); leftmost region of RKn2 shortened along PK2; rightmost region of RKn2 shortened along dorsal brush kinety (DB) (Figs. 2B, C, E, 3E–G, J). Left side with 7–9 ciliated kineties including perioral kinety 1 (PK1) and dorsal brush kinety (DB) which extends to about 40% of cell length and is composed of narrowly spaced dikinetids (Fig. 2B, C, E); last left somatic kinety (LKn) does not extend to cell apex and is shorter than last right somatic kinety (RKn1) (Figs. 2C, E, 3E, G).
Two perioral kineties along oral slit. Perioral kinety 1 left of oral slit, comprises dikinetids in anterior 40% and extends posteriorly as a row of monokinetids (Figs. 2B, 3F, J). Perioral kinety 2 right of oral slit, comprises regularly spaced dikinetids in anterior 40% and monokinetids in posterior 60% (Figs. 2B, 3F, J). Perioral kineties invariably twisted towards right side (Fig. 3F, J). Nematodesmata well-developed, all originating from kinetosomes of perioral kinety and extending into cytoplasm (Fig. 2H).
SSU rDNA sequence and phylogenetic analyses
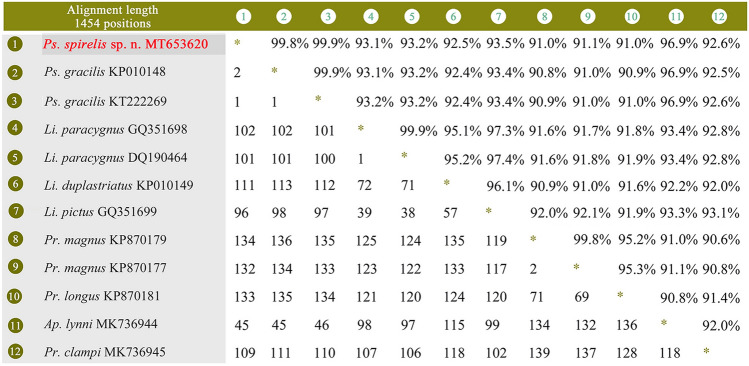
The SSU rDNA sequence of Pseudolitonotus spirelis sp. n. has been deposited in GenBank with accession number, length, and GC content as follows: MT653620, 1492 bp, 43.16%. The sequence identities of the SSU rDNA between the new species and its morphologically similar and closely related species were 91.0–99.9%, i.e., 1 to 134 nucleotide site differences (Figs. 4, 5).
Fig. 4.
The sequence identities (upper right) and numbers of nucleotide differences (lower left) of SSU rDNA sequences between Pseudolitonotus spirelis gen. et sp. n. and morphologically similar and/or closely related species. The new species is in bold font
Fig. 5.
Unmatched sites from SSU rDNA sequence alignment of Pseudolitonotus spirelis gen. et sp. n. with morphologically similar and/or closely related species sequences included in the phylogenetic analyses. Numbers indicate the unmatched site positions. Missing sites are indicated by dashes (-) and matched sites are marked with dots (.)
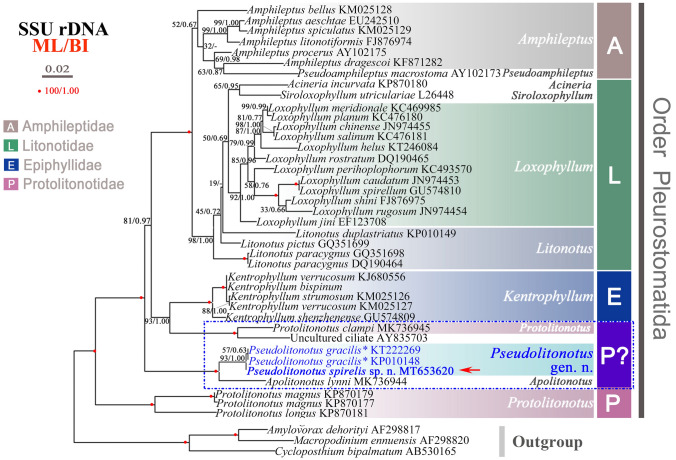
The topologies of trees constructed using each of the two algorithms were almost identical, so only the maximum likelihood (ML) tree is shown (Fig. 6). In both analyses, the order Pleurostomatida is monophyletic. The family Protolitonotidae is divided into three clades. The first clade comprises three sequences of two Protolitonotus species (Pr. magnus and Pr. longus). The second clade, which contains Apolitonotus lynni, is a sister group to the genus Pseudolitonotus gen. n., which is represented by Ps. spirelis sp. n. and two populations of Ps. gracilis comb. n. The third clade, which consists of Protolitonotus clampi and an unidentified ciliate, is sister group to the genus Kentrophyllum with moderate to full support (ML/BI, 93/1.00). The other two families, Amphileptidae and Litonotidae, are sister groups and each is monophyletic.
Fig. 6.
Maximum likelihood (ML) tree inferred from 42 SSU rDNA sequences of pleurostomatid and trichostomatid (outgroup) ciliates, revealing the phylogenetic position of Pseudolitonotus spirelis gen. et sp. n. (arrow). Bootstrap values of ML analysis and the posterior probabilities of Bayesian inference analysis (BI) are given at nodes. Dashes indicate incongruity between BI and ML trees. All branches are drawn to scale. GenBank accession numbers are given after names of species. Scale bar corresponds to two substitutions per 100 nucleotide positions. *Designated as Litonotus gracilis in Pan et al. (2015)
Discussion
Comments on Pseudolitonotus gen. n.
Ciliary patterns of both the somatic and the perioral kineties are important characters for the classification of pleurostomatids (Foissner 1984; Foissner et al. 1995; Lynn 2008). Among the known genera of the order Pleurostomatida, the last left somatic kinety (LKn) extends to the anterior end of the cell whereas in Pseudolitonotus gen. n. the LKn is obviously shortened. Consequently, this new genus can be separated from other pleurostomatid genera.
The ciliary pattern on the right side of the cell is one of the most important characters for the identification of pleurostomatids at family and/or genus level (Lynn 2008; Vďačný et al. 2015; Wu et al. 2017). Hitherto, the order Pleurostomatida was divided into four families based mainly on the ciliary pattern of the right side, i.e., Amphileptidae (single-suture), Epiphyllidae (double-suture), Protolitonotidae (semi-suture), and Litonotidae (no suture) (Vďačný et al. 2015; Wu et al. 2017). The right somatic kineties of Pseudolitonotus gen. n. do not extend to the anterior end of cell but instead are progressively shortened from the middle to both sides, thus distinguishing it from all known pleurostomatid genera. Pseudolitonotus gen. n. cannot to be assigned to any of the four pleurostomatid families based on their current diagnostic characters (Vďačný et al. 2015; Wu et al. 2017). However, we are hesitant to suggest that the shortening of the right somatic kineties and/or the LKn is a diagnostic character at family level. Therefore, we regard Pseudolitonotus gen. n. as incertae familiae at this time.
Revision of Litonotus gracilis Pan et al., 2015
Litonotus gracilis was originally described by Pan et al. (2015) who also sequenced its SSU rRNA gene. In their phylogenetic analyses, two populations of Litonotus gracilis clustered with Kentrophyllum rather than with its congeners or other litonotids (Pan et al. 2015). Although Pan et al. (2015) noted that some right somatic kineties (RSKs) of L. gracilis are shortened along the oral slit in the typical Litonotus pattern, they overlooked that none of the RSKs extend to the apical region of the cell (see Fig. 2G in Pan et al. 2015). We re-examined the type specimens of Litonotus gracilis and discovered that all of the RSKs are shortened, either along the dorsal margin or along the perioral kineties (Fig. 7B–D). Furthermore, Pan et al. (2015) did not recognize that the LKn, does not extend to the cell apex (Fig. 7A, E, F). The patterns of both the RSKs and LKn in L. gracilis are diagnostic characters of Pseudolitonotus gen. n. In addition, the two populations of Litonotus gracilis group with Pseudolitonotus spirelis sp. n. in the SSU rDNA tree to form a well-supported clade (ML/BI, 93/1.00) (Fig. 6). Hence, we suggest that Litonotus gracilis Pan et al., 2015 should be assigned to Pseudolitonotus gen. n. as a new combination, i.e., Pseudolitonotus gracilis (Pan et al., 2015) comb. n. (original combination: Litonotus gracilis Pan et al., 2015), and its diagnosis and ciliary pattern are improved based on original and current observations of the holotype and paratype specimens (Fig. 7).
Fig. 7.
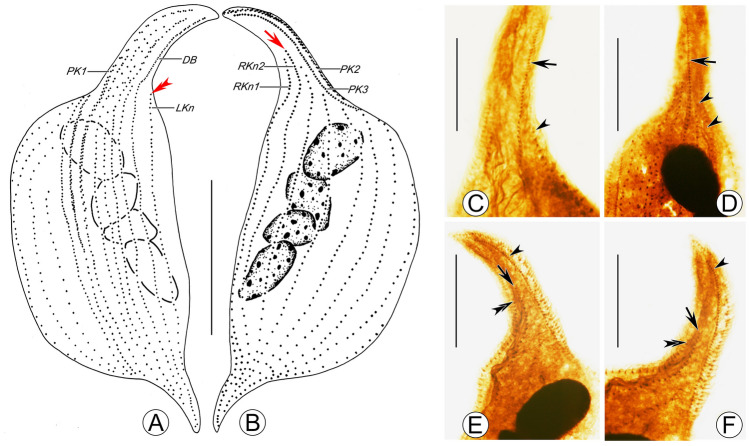
Pseudolitonotus gracilis (Pan et al., 2015) comb. n. stained with protargol (A–F). A B Ciliary patterns of right (B) and left (A) side of the holotype specimen, double arrowhead points to the shortened last left somatic kinety (LKn) and arrow marks the longest right somatic kinety (RKn2) not extending to cell apex. C, D Left view of anterior region of cell, to show the dorsal brush (arrow) and the last left somatic kinety (arrowhead). E, F Right view of anterior region of cell, to show the last right somatic kinety (double arrowhead), the penultimate right somatic kinety (arrow) and perioral kineties (arrowhead). DB dorsal brush, LKn last left somatic kinety, PK1 perioral kinety 1, PK2 perioral kinety 2, PK3 perioral kinety 3, RKn1 last right somatic kinety, RKn2 penultimate right somatic kinety. Scale bars: 100 μm in (A, B); 10 μm in (C–F)
Improved diagnosis of Pseudolitonotus gracilis (Pan et al., 2015) comb. n.
Body about 200–350 µm in vivo, with conspicuous neck that is up to 50% of body length when fully extended; usually four macronuclear nodules; one contractile vacuole subterminally located; bar-shaped extrusomes arranged along oral silt; cortical granules arranged in honeycomb-like pattern; 5–9 left and 12–18 right kineties.
Comments on Pseudolitonotus spirelis sp. n.
In terms of the body size and/or shape, seven pleurostomatid species resemble Pseudolitonotus spirelis sp. n., including: Pseudolitonotus gracilis (Pan et al., 2015) comb. n. (Fig. 7A, B); Protolitonotus clampi Pan et al., 2020 (Fig. 8C, D); Apolitonotus lynni Pan et al., 2020 (Fig. 8A, B); Litonotus duplostriatus (Maupas, 1883) Kahl, 1931 (Fig. 8G, H); L. blattereri Lin et al., 2008 (Fig. 8K, L); L. gongi Lin et al., 2009 (Fig. 8E, F); and L. guae Lin et al., 2009 (Fig. 8I, J) (Table 2). Pseudolitonotus spirelis sp. n. has two traits by which it can be distinguished from similar species: (1) extrusomes clustered together to form an “apical group” at the anterior end of the cell; and (2) the anterior part of the body twisted to the right at the bottom of the oral slit (Table 2). In addition, Pseudolitonotus spirelis sp. n. differs from Ps. gracilis (Pan et al., 2015) comb. n. by having fewer macronuclear nodules (2 vs. 4) and by the distribution patterns of extrusomes (along the entire ventral margin and with an “apical group” vs. along the oral slit only) (Pan et al. 2015).
Fig. 8.
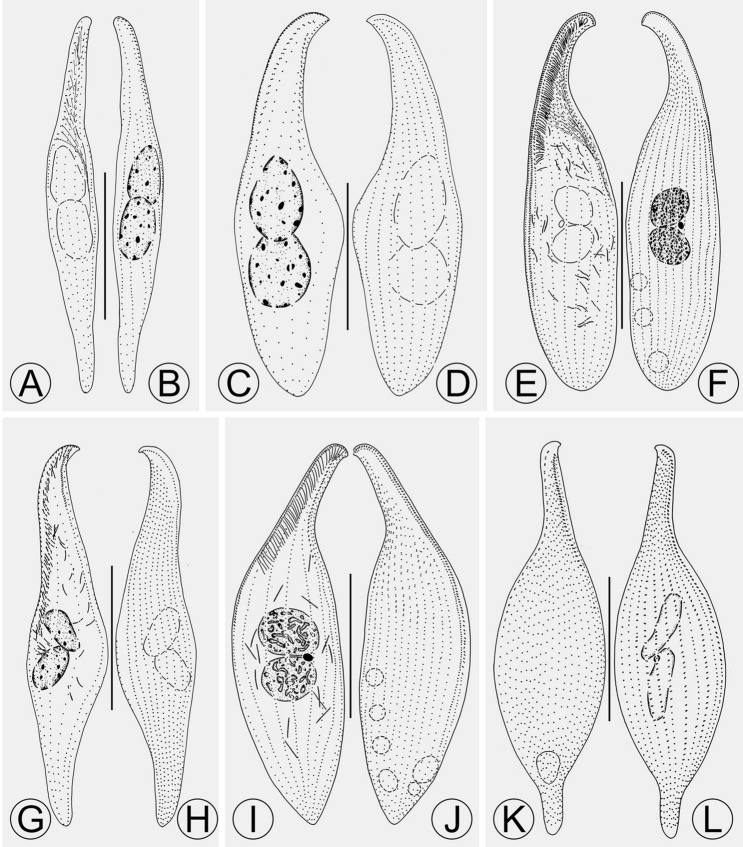
Morphology of species related and/or morphologically similar to Pseudolitonotus spirelis gen. et sp. n. A, B Apolitonotus lynni, from Pan et al. (2020). C, D Protolitonotus clampi, from Pan et al. (2020). E, F Litonotus gongi, from Lin et al. (2009). G, H Litonotus duplostriatus, from Pan et al. (2015). I, J Litonotus guae, from Lin et al. (2009). K, L Litonotus blattereri, from Lin et al. (2008). Scale bars: 50 μm in (A–D); 100 μm in (E–L)
Table 2.
Comparison of Pseudolitonotus spirelis gen. et sp. n. with morphologically similar species
| Species | BLa (μm) | RKb /LKc | n-MAd | pr-APe | pr-Tf | d-Exgc | n & po-CVh | Data source |
|---|---|---|---|---|---|---|---|---|
| Pseudolitonotus spirelis | 260–350 | 11–15/7–9 | 2 | Yes | Yes | Entire ventral | 1, S | Present work |
| Pseudolitonotus gracilis | 200–450 | 12–18/5–9 | 4 | No | Yes | Oral slit | 1, S | Pan et al. (2015) |
| Protolitonotus clampi | 80–130 | 9–11/5 or 6 | 2 | No | No | Oral slit | 1, T | Pan et al. (2020) |
| Apolitonotus lynni | 100–180 | 5–7/4 or 5 | 2 | No | No | Scatter | 1, S | Pan et al. (2020) |
| Litonotus duplostriatus | 90–315 | 11–14/5 or 6 | 2 | No | No | Oral slit | 1, S | Pan et al. (2015) |
| Litonotus blattereri | 100–180 | 15–20/10–14 | 2 | No | No | Entire ventral | 1, T | Lin et al. (2008) |
| Litonotus gongi | 150–300 | 11–17/8–10 | 2 | No | No | Oral slit | 2–4, D | Lin et al. (2009) |
| Litonotus guae | 100–200 | 11–18/6–9 | 2 | No | No | Oral slit | 3–7, D & V | Lin et al. (2009) |
AP apical group, BL body length, CV contractile vacuoles, d distribution, D dorsal, Ex extrusomes, LK left kineties, MA macronuclear nodules, n number, po position, pr presence, RK right kineties, S subterminal, T terminal, V ventral
aBody length in vivo
bPerioral kineties 2 and/or 3 included
cPerioral kinety 1 and dorsal brush kinety included
dNumber of macronuclear nodules
ePresence of apical group
fPresence of twist at the bottom of oral area
gDistribution of extrusomes
hNumber and position of contractile vacuoles
Comments on the phylogeny of Pseudolitonotus gen. n.
In the SSU rDNA tree, Apolitonotus lynni is the sister group to Pseudolitonotus gen. n. with full support (Fig. 6). This close relationship is unexpected considering the differences in their morphology. For example, Pseudolitonotus gen. n. can be clearly separated from Apolitonotus by a combination of: (1) the ciliary pattern on the right side (all right somatic kineties shortened vs. presence of several full-length right somatic kineties); (2) the presence (vs. absence) of extrusomes in the oral region; (3) the number of perioral kineties (2 vs. 3); and (4) the shortened (vs. not shortened) last left somatic kinety (Pan et al. 2020). The morphological characters of Pseudolitonotus gen. n. and Apolitonotus seem insufficient to reveal the phylogeny of either genus. Therefore, greater taxon sampling and more information on the species of Apolitonotus and Pseudolitonotus gen. n., including morphogenetic data and sequences of multiple gene markers, are needed to reveal their evolutionary relationships and to determine the family assignment of Pseudolitonotus gen. n.
Materials and methods
Sample collection and cultivation
Pseudolitonotus spirelis gen. et sp. n. was isolated from a mixture of water and rotting leaves collected on 06 April 2012 from a mangrove wetland (21° 09′ 12′′ N, 110° 25′ 20′′ E) on Techeng Island in the city of Zhanjiang, Guangdong Province, China (Fig. 1), when the water temperature was 23.8 °C, the salinity was 24‰, and the pH was 7.0. Ciliates were cultured at ca. 25 °C in Petri dishes containing about 20 ml of habitat water and two rice grains to facilitate the growth of bacteria as a food source for the ciliates.
Sample observation and identification
Observations of isolated living cells were performed using bright field and differential interference contrast (DIC) microscopy (Nikon Eclipse 80i, Tokyo, Japan) at 100–1,000 × magnifications. Cells were protargol stained following the method of Wilbert (1975). Meristics and morphometrics were obtained from 19 stained specimens at a magnification of 1000 × . Line diagrams of stained specimens were made with the help of a camera lucida at a magnification of 1250 × . Terminology and classification followed that of Wu et al. (2017) and Vďačný et al. (2015).
DNA extraction, PCR amplification and sequencing
Two cells were isolated from the raw cultures, rinsed five times with filtered habitat water (0.22 μm pore size) and then transferred into microfuge tube with ATL buffer. Genomic DNA extraction was performed with a DNeasy Blood & Tissue kit (Qiagen, Shanghai, China) according to the supplier’s instructions. Gene amplification and gene sequencing were carried out following the methods described by Wu et al. (2013).
Phylogenetic analyses
In addition to the new sequence of Pseudolitonotus spirelis, 38 SSU rDNA sequences of other pleurostomatids, including examples of all available genera within the order Pleurostomatida, were acquired from the GenBank database for the phylogenetic analyses (GenBank accession numbers are provided in Fig. 6). Three species of the order Trichostomatia, i.e., Amylovorax dehorityi AF298817, Macropodinium ennuensis AF298820 and Cycloposthium bipalmatum AB530165, were selected as outgroup taxa. Sequences were aligned using Clustal W implemented in Bioedit v. 7.2.6 (Hall 1999) with default parameters and edited and checked by eye to remove primer sequences and highly variable regions. The final alignment was used to construct phylogenetic trees included 1516 characters and 42 taxa.
Maximum likelihood (ML) analysis was conducted using RaxM-HPC2 V. 7.2.8 on XSEDE V. 8.1.11 with parameter settings as given by Stamatakis et al. (2008) via the CIPRES Portal V. 1.15 (http://www.phylo.org). The reliability of internal branches was estimated by bootstrapping with 1000 replicates. Bayesian inference (BI) analysis was conducted with MrBayes 3.1.2 (Ronquist and Huelsenbeck 2003) using the GTR + G + I evolutionary mode indicated by MrModeltest v. 2.0 (Nylander 2004). The chain length of Markov chain Monte Carlo algorithm simulations was run for 106 generations with trees sampled every 100 generations. The first 2500 generations (25%) were discarded as burn-in.
Acknowledgements
This work was supported by the Natural Science Foundation of China (Project Numbers: 42076113, 31761133001, 41576148). Lei Wu was supported by the China Postdoctoral Science Foundation (Project Number: 2018M640796) and the Guangdong Basic and Applied Basic Research Foundation (Project Number: 2020A1515111125). We are grateful to Dr. Weibo Song, Ocean University of China, and Dr. Hongbo Pan, Shanghai Ocean University, for their insightful and constructive comments and suggestions for improving our manuscript.
Author contributions
LW and XL conceived and planned the experiments. LW performed all the experiments and analyzed the phylogeny and wrote the manuscript with support from AW, XL and JL. XL supervised the project. All authors prepared the manuscript and approved the final version.
Declarations
Conflict of interest
Author Alan Warren is a member of the Editorial Board for Marine Life Science & Technology. He was not involved in the journal’s review of, or decisions related to, this manuscript.
Animal and human rights statement
We declare that all applicable international, national, and/or institutional guidelines for sampling, care, and experimental use of organisms for the study have been followed and all necessary approvals have been obtained.
References
- Azam F, Fenchel T, Field JG, Gray JS, Meyer-Reil LA, Thingstad F. The ecological role of water-column microbes in the sea. Mar Ecol Prog Ser. 1983;10:257–262. doi: 10.3354/meps010257. [DOI] [Google Scholar]
- Bai Y, Wang R, Song W, Suzuki T, Hu XZ. Redescription of five tintinnine ciliates (Alveolata: Ciliophora: Oligotrichea) from coastal waters of Qingdao, China. Mar Life Sci Technol. 2020;2:209–221. doi: 10.1007/s42995-020-00034-2. [DOI] [Google Scholar]
- Buddenbrock W. Beobachtungen über einige neue oder wenig bekannte marine Infusorien. Arch Protistenk. 1920;41:341–364. [Google Scholar]
- Carey PG. Marine interstitial ciliates: an illustrated key. London: Chapman and Hall; 1992. [Google Scholar]
- Chen RM, Lin XF, Warren A. A new pleurostomatid ciliate, Amphileptus salignus n. sp. (Protozoa, Ciliophora), from mangrove wetlands in southern China. Zootaxa. 2011;3048:62–68. doi: 10.11646/zootaxa.3048.1.4. [DOI] [Google Scholar]
- Corliss JO. The ciliated protozoa: characterization, classification and guide to the literature. 2. New York: Pergamon Press; 1979. [Google Scholar]
- Dragesco J. Diagnoses préliminaries de quelques ciliés nouveaux des sables. Bull Soc Zool Fr. 1954;79:62–70. [Google Scholar]
- Dragesco J. Ciliés mésopsammiques littoraux, systématique, morphologie, écologie. Trav Stat Biol Roscoff. 1960;12:1–356. [Google Scholar]
- Dragesco J. Ciliés mésopsammiques d’Afrique noire. Cah Biol Mar. 1965;6:357–399. [Google Scholar]
- Foissner W. Taxonomie und ökologie einiger Ciliaten (Protozoa, Ciliophora) des Saprobiensystems. I. Genera Litonotus, Amphileptus Opisthodon. Hydrobiologia. 1984;119:193–208. doi: 10.1007/BF00015210. [DOI] [Google Scholar]
- Foissner W, Berger H, Blatterer H, Kohmann F. Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems-Band IV: Gymnostomatea, Loxodes, Suctoria. Informationsberichte Des Bayer. Landesamtes Für Wasserwirtschaft. 1995;1:1–540. [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Hu XZ, Lin XF, Song WB. Ciliate atlas: species found in the South China Sea. Beijing: Science Press; 2019. [Google Scholar]
- Kahl A. Urtiere oder Protozoa I: Wimpertiere oder Ciliata (Infusoria) 2. Holotricha außer den im 1. Teil Behandelten Prostomata Tierwelt Dtl. 1931;21:181–398. [Google Scholar]
- Lin XF, Song WB. Establishment of a new amphileptid genus, Apoamphileptus nov. gen. (Ciliophora, Litostomatea, Pleurostomatida), with description of a new marine species, Apoamphileptus robertsi nov. spec. from Qingdao China. J Eukaryot Microbiol. 2004;51:618–625. doi: 10.1111/j.1550-7408.2004.tb00595.x. [DOI] [PubMed] [Google Scholar]
- Lin XF, Song WB, Warren A. Two new marine pleurostomatid ciliates from China, Amphileptus gui nov. spec. and Amphileptus yuianus nov. spec. (Ciliophora, Pleurostomatida) Eur J Protistol. 2005;41:163–173. doi: 10.1016/j.ejop.2005.01.002. [DOI] [Google Scholar]
- Lin XF, Song WB, Warren A. Taxonomic studies on three marine pleurostomatid ciliates: Kentrophyllum verrucosum (Stokes, 1893) Petz, Song et Wilbert, 1995, Epiphyllum soliforme (Fauré-Frémiet, 1908) gen. n., comb. n. and Amphileptus sikorai sp. n., with the establishment of a new genus Epiphyllum (Ciliophora: Pleurostomatida) Acta Protozool. 2005;44:129–145. [Google Scholar]
- Lin XF, Song WB, Li JQ. Amphileptus aeschtae nov. spec. and Amphileptus eigneri nov. spec. (Ciliophora, Pleurostomatida), two new marine pleurostomatid ciliates from China. Eur J Protistol. 2007;43:77–86. doi: 10.1016/j.ejop.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Lin XF, Song WB, Li JQ. Description of two new marine pleurostomatid ciliates, Loxophyllum choii nov. spec. and L. shini nov. spec. (Ciliophora, Pleurostomatida) from China. Eur J Protistol. 2007;43:131–139. doi: 10.1016/j.ejop.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Lin XF, Li JQ, Gong J, Warren A, Song WB. Taxonomic studies on three marine pleurostomatid ciliates, Litonotus bergeri nov. spec., L. blattereri nov. spec. and L. petzi nov. spec. (Ciliophora, Pleurostomatida) from North China Sea. Eur J Protistol. 2008;44:91–102. doi: 10.1016/j.ejop.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Lin XF, Song WB, Warren A. Pleurostomatids. In: Song WB, Hu XZ, Warren A, editors. Free-living ciliates in the Bohai and Yellow Seas. Beijing: Science Press; 2009. pp. 93–134. [Google Scholar]
- Liu WW, Shin MK, Yi ZZ, Tan YH. Progress in studies on the diversity and distribution of planktonic ciliates (Protista, Ciliophora) in the South China Sea. Mar Life Sci Technol. 2021;3:28–43. doi: 10.1007/s42995-020-00070-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn DH. The ciliated protozoa: characterization, classification, and guide to the literature. 3. Dordrecht: Springer; 2008. [Google Scholar]
- Nylander J. MrModeltest v2. Distributed by the author. Uppsala: Evolutionary Biology Centre, Uppsala University; 2004. [Google Scholar]
- Pan HB, Gao F, Li JQ, Lin XF, Al-Farraj SA, Al-Rasheid KAS. Morphology and phylogeny of two new pleurostomatid ciliates, Epiphyllum shenzhenense n. sp. and Loxophyllum spirellum n. sp. (Protozoa, Ciliophora) from a mangrove wetland, south China. J Eukaryot Microbiol. 2010;57:421–428. doi: 10.1111/j.1550-7408.2010.00492.x. [DOI] [PubMed] [Google Scholar]
- Pan HB, Gao F, Lin XF, Warren A, Song WB. Three new Loxophyllum species (Ciliophora: Pleurostomatida) from China with a brief review of the marine and brackish Loxophyllum species. J Eukaryot Microbiol. 2013;60:44–56. doi: 10.1111/jeu.12005. [DOI] [PubMed] [Google Scholar]
- Pan HB, Li LF, Lin XF, Li JQ, Al-Farraj SA, Al-Rasheid KAS. Morphology of three species of Amphileptus (Protozoa, Ciliophora, Pleurostomatida) from the South China Sea, with note on phylogeny of A. dragescoi sp. n. J Eukaryot Microbiol. 2014;61:644–654. doi: 10.1111/jeu.12146. [DOI] [PubMed] [Google Scholar]
- Pan HB, Li LF, Wu L, Miao M, Al-Rasheid KAS, Song WB. Morphology of three Litonotus species (Ciliophora: Pleurostomatida) from China seas, with brief notes on their SSU rDNA-based phylogeny. Eur J Protistol. 2015;51:494–506. doi: 10.1016/j.ejop.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Pan HB, Zhang QQ, Dong JY, Jiang JM. Morphology and phylogeny of two novel pleurostomatids (Ciliophora, Litostomatea), establishing a new genus. J Eukaryot Microbiol. 2020;67:252–262. doi: 10.1111/jeu.12779. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck J. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Song WB, Wilbert N. Taxonomische untersuchungen an aufwuchsciliaten (Protozoa, Ciliophora) im Poppelsdorfer Weiher, Bonn. Lauterbornia. 1989;3:2–221. [Google Scholar]
- Song WB, Warren A, Hu XZ. Free-living ciliates in the Bohai and Yellow Seas. Beijing: Science Press; 2009. [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. A fast bootstrap algorithm for the RAxML web-servers. Syst Biol. 2008;57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- Stokes AC. Notices of some undescribed infusoria from the brackish watershed of the eastern United States. J R Micr Soc. 1893;1:298–302. doi: 10.1111/j.1365-2818.1893.tb05977.x. [DOI] [Google Scholar]
- Vďačný P, Bourland WA, Orsi W, Epstein SS, Foissner W. Phylogeny and classification of the Litostomatea (Protista, Ciliophora), with emphasis on free-living taxa and the 18S rRNA gene. Mol Phylogenet Evol. 2011;59:510–522. doi: 10.1016/j.ympev.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Vďačný P, Rajter Ľ, Shazib SUA, Jang SW, Kim JH, Shin MK. Reconstruction of evolutionary history of pleurostomatid ciliates (Ciliophora, Litostomatea, Haptoria): interplay of morphology and molecules. Acta Protozool. 2015;54:9–29. [Google Scholar]
- Warren A, Patterson DJ, Dunthorn M, Clamp JC, Achilles-Day UEM, Aescht E, Al-Farraj SA, Al-Quraishy S, Al-Rasheid K, Carr M, Day JG, Dellinger M, El-Serehy HA, Fan YB, Gao F, Gao S, Gong J, Gupta R, Hu XZ, Kamra K, et al. Beyond the "Code": A guide to the description and documentation of biodiversity in ciliated protists (Alveolata, Ciliophora) J Eukaryot Microbiol. 2017;64:539–554. doi: 10.1111/jeu.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbert N. Eine verbesserte Technik der Protargolimprägnation für Ciliaten. Mikrokosmos. 1975;64:171–179. [Google Scholar]
- Wu L, Chen RM, Yi ZZ, Li JQ, Warren A, Lin XF. Morphology and phylogeny of three new Loxophyllum species (Ciliophora, Pleurostomatida) from mangrove wetlands of southern China. J Eukaryot Microbiol. 2013;60:267–281. doi: 10.1111/jeu.12032. [DOI] [PubMed] [Google Scholar]
- Wu L, Chen RM, Yi ZZ, Li JQ, Warren A, Lin XF. The morphology of three Loxophyllum species (Ciliophora, Pleurostomatida) from southern China, L. lembum sp. n., L. vesiculosum sp. n. and L. perihoplophorum Buddenbrock, 1920, with notes on the molecular phylogeny of Loxophyllum. J Eukaryot Microbiol. 2014;61:115–125. doi: 10.1111/jeu.12089. [DOI] [PubMed] [Google Scholar]
- Wu L, Yi ZZ, Li JQ, Warren A, Xu HL, Lin XF. Two new brackish ciliates, Amphileptus spiculatus sp. n. and A. bellus sp. n. from mangrove wetlands in southern China, with notes on the molecular phylogeny of the family Amphileptidae (Protozoa, Ciliophora, Pleurostomatida) J Eukaryot Microbiol. 2015;62:662–669. doi: 10.1111/jeu.12225. [DOI] [PubMed] [Google Scholar]
- Wu L, Clamp JC, Yi ZZ, Li JQ, Lin XF. Phylogenetic and taxonomic revision of an enigmatic group of haptorian ciliates, with establishment of the Kentrophyllidae fam. n. (Protozoa, Ciliophora, Litostomatea, Pleurostomatida) PLoS ONE. 2015;10:e0123720. doi: 10.1371/journal.pone.0123720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Jiao XX, Shen Z, Yi ZZ, Li JQ, Warren A, Lin XF. New taxa refresh the phylogeny and classification of pleurostomatid ciliates (Ciliophora, Litostomatea) Zool Scr. 2017;46:245–253. doi: 10.1111/zsc.12193. [DOI] [Google Scholar]
- Wu L, Li JQ, Warren A, Lin XF. Species diversity of the pleurostomatid ciliate genus Amphileptus (Ciliophora, Haptoria), with notes on the taxonomy and molecular phylogeny of three species. Front Mar Sci. 2021;8:642767. doi: 10.3389/fmars.2021.642767. [DOI] [Google Scholar]
- Wu L, Li JQ, Warren A, Lin XF. Morphology and molecular phylogeny of a new brackish pleurostomatid ciliate, Loxophyllum paludosum sp. n. (Ciliophora, Litostomatea, Haptoria), from a mangrove wetland in China. Eur J Protistol. 2021;80:125802. doi: 10.1016/j.ejop.2021.125802. [DOI] [PubMed] [Google Scholar]
- Zhang QQ, Simpson A, Song WB. Insights into the phylogeny of systematically controversial haptorian ciliates (Ciliophora, Litostomatea) based on multigene analyses. Proc Royal Soc B. 2012;279:2625–2635. doi: 10.1098/rspb.2011.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]