Abstract
Hematodinium is a type of parasitic dinoflagellate that infects marine crustaceans globally. The parasite lives mainly in the hemolymph or hemocoels of affected hosts, and results in mortalities due to malfunction or loss of functions of major organs. In recent years, the parasite had developed into an emerging epidemic pathogen not only affecting wild populations of economically valuable marine crustaceans in western countries but also the sustainable yield of aquaculture of major crabs in China. The epidemics of the parasitic diseases expanded recently in the coastal waters of China, and caused frequent outbreaks in aquaculture of major crab species, especially Portunus trituberculatus and Scylla paramamosain. In addition, the pathogen infected two species of co-cultured shrimps and multiple cohabitating wild crabs, implying it is a significant threat to the sustainable culture of commercially valuable marine crustaceans. In particular, the polyculture system that is widely used along the coast of China may facilitate the spread and transmission of the pathogen. Thus, to provide a better understanding of the biological and ecological characteristics of the parasitic dinoflagellate and highlight important directions for future research, we have reviewed the current knowledge on the taxonomy, life cycle, pathogenesis, transmission and epidemiology of Hematodinium spp. Moreover, ecological countermeasures have been proposed for the prevention and control of the emerging infectious disease.
Keywords: Parasitic dinoflagellate, Diversity, Life cycle, Pathogenesis, Outbreak mechanism, Ecological control
Introduction
Hematodium is a parasitic dinoflagellate that infects marine crustaceans (Chatton and Poisson 1931). The parasitic dinoflagellate shares similar biological characteristics to typical planktonic dinoflagellates, and include the dinokaryon structure, unique mitosis and the presence of athecate dinospores (Ris and Kublai 1974). However, unlike planktonic dinoflagellates, the parasitic Hematodinium lives mainly in the hemolymph or hemocoels of affected organs and tissues (e.g., hepatopancreas, heart, gills, and muscle) (Stentiford and Shields 2005; Wheeler et al. 2007). The presence of the parasitic dinoflagellate leads to mortality in the affected hosts particularly in the late stages of infections when massive proliferations of the parasitic cells caused malfunction or loss of function to the major organs (e.g., hepatopancreas and heart) of the affected crustacean hosts (Field and Appleton 1995; Taylor et al. 1996; Wheeler et al. 2007). Hematodinium can infect a wide range of phylogenetically-related crustacean hosts. To date, Hematodinium or Hematodinium-like infections had been reported in over 40 species of marine crabs, shrimps, lobsters and amphipods (Li and Xu 2014; Shields 1994; Small et al. 2012) (Table 1).
Table 1.
List of decapod hosts reported as infected with Hematodinium parasites around the world
| Host types | Host species | Parasite species | Locations |
|---|---|---|---|
| Crab | Callinectes sapidus | Hematodinium perezi | Maryland and Virginia, USA |
| Callinectes similes | Hematodinium sp. | Georgia, USA | |
| Cancer borealis | Hematodinium sp. | New York Bight, USA | |
| Cancer irroratus | Hematodinium sp. | New York Bight, USA | |
| Cancer pagurus | Hematodinium sp. |
English Channel, UK South coast, Ireland Clyde, Scotland |
|
| Carcinus maenas |
Hematodinium perezi Hematodinium perezi Hematodinium sp. |
Arcachon and Penpoull, France English Channel, UK Clyde, Scotland |
|
| Chionoecetes angulatus | Hematodinium sp. | British Columbia, Canada | |
| Chionoecetes bairdi | Hematodinium sp. | Alaska, USA | |
| Chionoecetes opilio | Hematodinium sp. |
Alaska, USA Newfoundland, Canada British Columbia, Canada |
|
| Chionoecetes tanneri | Hematodinium sp. | Vancouver Island, Canada | |
| Eurypanopeus depressus | Hematodinium sp. | Virginia, USA | |
| Hyas araneus | Hematodinium sp. | West coast, Greenland | |
| Hyas coarctatus | Hematodinium sp. | Bering Sea | |
| Helice tientsinensis | Hematodinium perezi | Shandong Province, China | |
| Libinia emerginata | Hematodinium sp. | Georgia, USA | |
| Liocarcinus depurator |
Hematodinium perezi Hematodinium sp. Hematodinium sp. |
English Channel, France Clyde, Scotland Kattegat, Denmark |
|
| Lithodes couesi | Hematodinium sp. | Vancouver Island, Canada | |
| Menippe mercenaria | Hematodinium sp. | Georgia, USA | |
| Necora puber | Hematodinium sp. |
South Brittany, France Weymouth, UK Clyde Sea, Scotland |
|
| Neopanope sayi | Hematodinium sp. |
Maryland, USA North Carolina, USA |
|
| Ovalipes ocellatus | Hematodinium sp. | New York Bight, USA | |
| Pagurus bernhardus | Hematodinium sp. |
Clyde, Scotland Kattegat, Denmark |
|
| Pagurus prideaux | Hematodinium sp. | Clyde, Scotland | |
| Panopeus pollicaris | Hematodinium sp. | Virginia, USA | |
| Paralithodes camtschaticus | Hematodinium sp. | Sea of Okhotsk, Russia | |
| Paralithodes platypus | Hematodinium sp. | Sea of Okhotsk, Russia | |
| Portumnus latipes | Hematodinium perezi | Wimereux, France | |
| Portunus armatus |
Hematodinium australis Hematodinium australis |
Moreton Bay, Australia Shark Bay, Australia |
|
| Portunus pelagicus | Hematodinium australis | Moreton Bay, Australia | |
| Portunus trituberculatus | Hematodinium perezi |
Zhejiang Province, China Shandong Province, China Hebei Province, China |
|
| Scylla Serrata |
Hematodinium sp. Hematodinium sp. Hematodinium sp. |
Moreton Bay, Australia Zhejiang Province, China Guangdong Province, China |
|
| Trapezia areolata | Hematodinium sp. | Great Barrier Reef, Australia | |
| Trapezia coeruleab | Hematodinium sp. | Great Barrier Reef, Australia | |
| Munida rugosa | Hematodinium sp. | Clyde, Scotland | |
| Lobster | Nephrops norvegicus | Hematodinium sp. |
Clyde and West coast, Scotland Skagerrak, Sweden Kattegat, Denmark |
| Exopalaemon carinicauda | Hematodinium sp. | Zhejiang Province, China | |
| Shrimp | Penaeus monodon | Hematodinium sp. | Shandong Province, China |
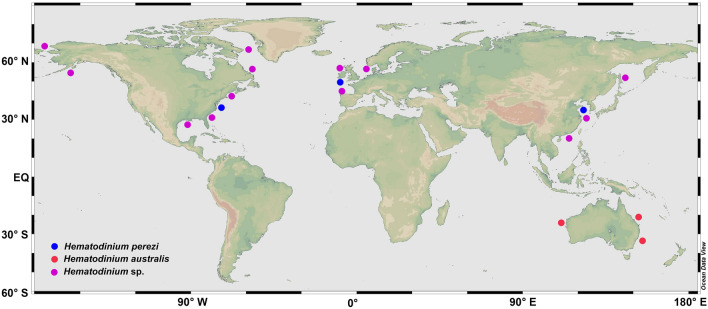
The parasitic dinoflagellate caused epidemics in a variety of commercially important crustaceans globally, and resulted in significant economic losses to local fisheries and mariculture (Li and Xu 2014; Small 2012; Stentiford and Shields 2005) (Fig. 1). In the coastal waters of the USA, the parasite infected various species of cohabitated wild crabs (e.g., Libinia dubia, Pagurus pollicaris and Eurypanopeus depressus) in addition to its major host Callinectes sapidus (Pagenkopp et al. 2012). Since 2004, Hematodinium spp. infections had been identified as the causative agents of “milky blood disease” in the cultured Chinese swimming crabs, Portunus trituberculatus (Li et al. 2013; Xu et al. 2007a) and the “Yellow water disease” in cultured mud crabs, Scylla paramamosain (formerly known as Scylla serrata) (Li et al. 2008; Xu et al. 2007b) along the Chinese coast. In Shandong Province, China, the prevalence of Hematodinium sp. infections ranged from 15 to 88% in cultured P. trituberculatus during outbreaks of disease with accumulative mortalities of more than 97% in laboratory trials (Li et al. 2013; Li et al. unpublished data; Wang et al. 2017a). More recently, the parasite has developed into an emerging cause of epidemics affecting the wild populations of economic valuable marine crustaceans in western countries and also the sustainable yield of farmed major crabs in China (Li et al. 2013; Morado et al. 2012; Shields 2012; Small 2012; Wang et al. 2017a, b).
Fig. 1.
The global distribution and diversity of the parasitic dinoflagellate Hematodinium. Note: blue dots representing the type species—Hematodinium perezi, red dots representing Hematodinium australis, pink dots representing the other unclassified Hematodinium species
The epidemics caused by Hematodinium parasite were comparable to the catastrophic viral diseases in shrimp and gaffkemia infection in the American lobster in terms of their impact to fisheries and host populations (Morado et al. 2012; Rowley et al. 2015; Stentiford and Shields 2005). More alarmingly, Hematodinium sp. infections were identified also in the shrimps, Exopalaemon carinicauda and Penaeus monodon cultured with Portunus trituberculatus in polyculture ponds in the coastal areas of China (Wang et al. 2017a; Xu et al. 2010). This suggests a potential threat to the sustainable culture of penaeid shrimps in China. Compared to the considerable attention given to viral and bacterial pathogens, studies on parasitic Hematodinium are insufficient to enable proposing a practical strategy for preventing transmission. The limited understanding on the complete life cycle of the parasitic dinoflagellate (Appleton and Vickerman 1998; Li et al. 2011a) reflects the lack of a complete understanding of transmission among different hosts. The waterborne route via dinospores has been considered to be the main route of disease transmission in the natural environment (Li et al. 2011b; Stentiford and Shields 2005) although the actual mechanism still needs to be verified. In addition, polyculture ponds and rotation culture systems are widely deployed for large scale commercial culture of major crustaceans in China (Wang et al. 2004), which may facilitate the spread and transmission of the parasite. Thus, comprehensive studies on outbreaks as well as the biological and environmental driving mechanisms of epidemics are sorely needed.
The current knowledge on the taxonomy, life cycle, pathogenesis, transmission, epidemiology of Hematodinium spp. has been reviewed to provide a better understanding on the biological and ecological characteristics of the parasite and to highlight important directions for future research. Moreover, ecological countermeasures for prevention and control of the disease have been proposed.
Taxonomy and phylogeny of Hematodinium spp.
As indicated in the systematical database of Algaebase, the genus Hematodinium belongs in the family Syndiniceae, order Syndinida (Syndiniales to botanists), Class Dinophyceae (Guiry and Guiry 2020). Due to the limited understanding on their life cycle and lack of distinct characteristics, only two species of Hematodinium have been formally described, the type species—H. perezi and a second species—H. australis. H. perezi was first reported from the portunid crabs, Carcinus maenas and Liocarcinus depurator in the coastal waters of France (Chatton and Poisson 1931). Conversely, H. australis was identified in the sand crab, Portunus pelagicus, from Moreton Bay, Australia (Hudson and Shields 1994). H. australis was discriminated from H. perezi based on the size of the trophonts, the presence of rounded plasmodia, and its distribution. These data were supported by subsequent molecular studies (Hudson and Adlard 1996). Most Hematodinium or Hematodinium-like organisms identified in other hosts have not been officially described. Additionally, the latest phylogenetic analysis on the acquired 18S rDNA sequences of Hematodinium spp. indicated fine genetic differences in the type species H. perezi. Three genotypes of H. perezi were further discriminated based on phylogenetic analyses of the ITS regions: genotype I in L. depurator from the English Channel, genotype II in P. trituberculatus and S. paramamosain from China, and genotype III in C. sapidus from North America (Small et al. 2012). With the accumulation of more molecular evidence, the taxonomy, diversity and biogeography of the parasitic dinoflagellates in the genus Hematodinium will surely be revealed.
Life cycles of Hematodinium spp.
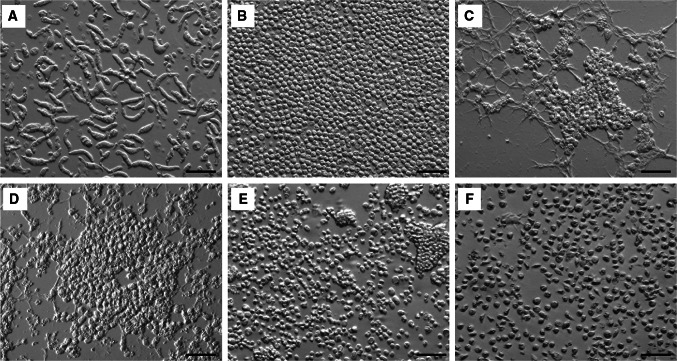
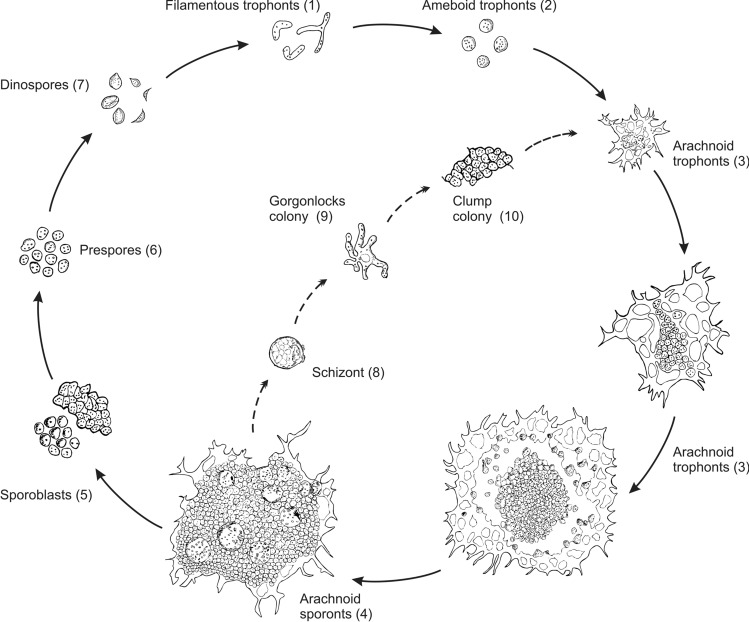
Due to the difficulty of establishing continuous in vitro cultures, only the life cycles of two Hematodinium isolates have been comprehensively studied (Appleton and Vickerman 1998; Li et al. 2011a). In 1998, Appleton and Vickerman successfully isolated Hematodinium sp. from the Norway lobster, Nephrops norvegicus, set up continuous cultures in the laboratory, and characterized the complete in vitro life cycle. In brief, dinospores develop into filamentous trophonts, which further differentiate into a ‘gorgonlocks’ filamentous assemblage, the gorgonlocks assemblage then develops into a ‘clump’ colony. This develops into more filamentous trophonts, or a structurally complicated arachnoid trophont leading to growth and sporogony to produce sporoblasts, which further develop into the macro-dinospores or micro-dinospores. In 2011, Li et al. established a continuous culture of the Hematodinium sp. infecting the American blue crab, Callinectes sapidus (Fig. 2), and reported the first in vitro life cycle (Fig. 3). The Hematodinium sp. from C. sapidus shared most of the similar life stages as an isolate from N. norvegicus, but underwent a distinct pathway after formation of arachnoid trophonts (Li et al. 2011a). In particular, arachnoid trophonts were able to release “schizonts” in the late stage of development, which could develop directly into ‘gorgonlocks’.
Fig. 2.
Morphological observation of the in vitro life stages of Hematodinium isolated from the American blue crab, Callinectes sapidus. a Filamentous trophonts; b Ameboid trophonts; c Arachnoid trophonts; d Arachnoid sporonts; e Sporoblasts; f Prespores and dinospores. Note: Hematodinium cells were cultured using the methodology described in Li et al. (2011a). Scale bar = 50 μm
Fig. 3.
Schematic diagram of the developmental cycle of Hematodinium perezi ex. Callinectes sapidus. (1) Filamentous trophonts represent the initial stage of infection in new crab hosts, which were usually observed in the hemolymph of diseased crabs with light infections. (2) Ameboid trophonts were also observed in circulating hemolymph of diseased crabs, usually present in moderately infected hosts or coexisting with filamentous trophonts in lightly infected hosts. (3) Arachnoid trophonts were the main proliferating stages of the parasites, dwelling in internal organs (e.g., hepatopancreas and heart). (4) Arachnoid sporonts were the late development stage of arachnoid syncytia that were ready to release sporoblasts and/or pansporoblasts. (5) Sporoblasts, single cells or cellular clumps were released from arachnoid sporonts when fully developed. These cells were observed in hemolymph of diseased crab with chronic and heavy infections. (6) Prespores were the transition stage between sporoblasts and dinospores, and may occasionally be observed in hemolymph of diseased crab with chronic and heavy infections. (7) Motile dinospores, microspores or macrospores represent the final stages of the development life cycle. Dinospores were observed in aquaria holding diseased crab under sporulation, but were rarely observed in the hemolymph of diseased crabs. (8) Pansporoblasts besides normal sporoblasts were also developed from arachnoid sporonts, which may represent an alternate pathway for proliferation of parasites in crab hosts (dashed arrows). (9) Gorgonlocks colonies derived from pansporoblasts that underwent segmentation, and eventually developed into clump colonies. (10) Clump colonies morphologically look like aggregated sporoblasts and can also be observed in circulating hemolymph of heavily infected crabs. Clump colonies will develop into arachnoid trophonts when subcultured in vitro (from Li et al. 2011a)
Partial life cycles were known for Hematodinium sp. infecting the snow crab, Chionoecetes bairdi (Eaton et al. 1991), in which the ovoid plasmodial trophont produced vegetative cells (= amoeboid trophonts) that developed into prespores and dinospores (Meyers et al. 1987, 1990). Subsequently, in vitro cultures of Hematodinium from P. trituberculatus were established successfully in laboratory (Wang et al. 2017a). This isolate underwent similar developmental processes as Hematodinium sp. from C. sapidus. However, the formation and development of “schizonts” had not been observed in the batches of laboratory cultures. As indicated, there are overt differences among the life histories of parasitic dinoflagellate Hematodinium and its genetic relatives, i.e., planktonic dinoflagellate, despite that the life cycle of the type species H. perezi is still unknown. To date, there are few studies on the complete life cycle of Hematodinium spp. It is still unclear whether there are alternate or reservoir hosts that participate in the life cycles. Besides, the biological roles of macro- and microdinospores remain obscure. Nevertheless, the zygotic or resting stages that planktonic dinoflagellates experience occur regularly in the natural environment. Thus, it is of great importance to conduct comprehensive studies on the complete life cycle of Hematodinium spp. to enable a better understanding of the biological characteristics as well as the transmission and spreading mechanism of this parasite.
The transmission mechanism of Hematodinium spp.
Cannibalism, sexual transmission and waterborne transmission are the general routes of transmission for viruses and pathogenic bacteria. Therefore, previous studies on the transmission and spreading mechanisms of the parasitic dinoflagellate Hematodinium were focused on these pathways. Meyers et al. (1996) reported the presence of Hematodinium parasites in the seminal duct of infected Chionoecetes bairdi, and speculated that sexual transmission may be one of the routes of distribution and spreading. However, no further research or evidence has been reported to verify this hypothesis. Like other marine protozoan pathogens, cannibalism was generally assumed to be route of transmission for the parasitic dinoflagellate Hematodinium. However, there was not any consistent conclusion regarding this route of transmission in previous studies. For example, Hudson and Shields (1994) failed to transmit H. australis to Portunus pelagicus through ingestion of infected crab tissues. On the contrary, Sheppard et al. (2003) reported successful transmission of Hematodinium disease to juvenile American blue crabs, C. sapidus, through ingestion of infected tissues. Subsequently, Li et al. (2011b) conducted a series of feeding experiments with young and adult blue crabs, and concluded that cannibalism was not an effective route of transmission. Meanwhile, waterborne transmission, via dinospores, had been speculated to be the effective route of transmission for the parasite (Shields 1994; Stentiford and Shields 2005).
To date, increasing evidence has been accumulated to support the effectiveness of waterborne transmission. Firstly, dinospores could directly develop into filamentous trophont in vitro cultures, which were normally identified as the early stage of infection in affected hosts (Appleton and Vickerman 1998). Secondly, sporulation of heavily infected hosts had been observed in laboratories, and involved the release of a large number of dinospores into water column (106 ~ 108 cells/ml) (Appleton and Vickerman 1998; Li et al. 2010; Shields and Squyars 2000). Additionally, both the macro- and microdinospores of Hematodinium sp. are able to produce infections when inoculated into C. bairdi (Eaton et al. 1991). Finally, Shields et al. (2017) and Huchin-Mian et al. (2017) concluded that H. perezi could be transmitted naturally to juvenile American blue crabs by “sentinel” deployments in endemic areas of the parasitic diseases. However, it is unknown whether the dinospores constitute the actual infectious stage, act as gametes for further development, or are just an intermediate stage proceeding to resting cysts. Besides, it is not clear how dinospores invade susceptible hosts, despite that Hematodinium spp. had been thought to parasitize potential hosts during molting or physical damage/injuries (Eaton et al. 1991; Meyers et al. 1990; Shields et al. 2007; Stentiford et al. 2001a). The answers to these questions are the keys to reveal the transmission and spreading mechanism of Hematodinium spp.
The pathogenesis of Hematodinium spp.
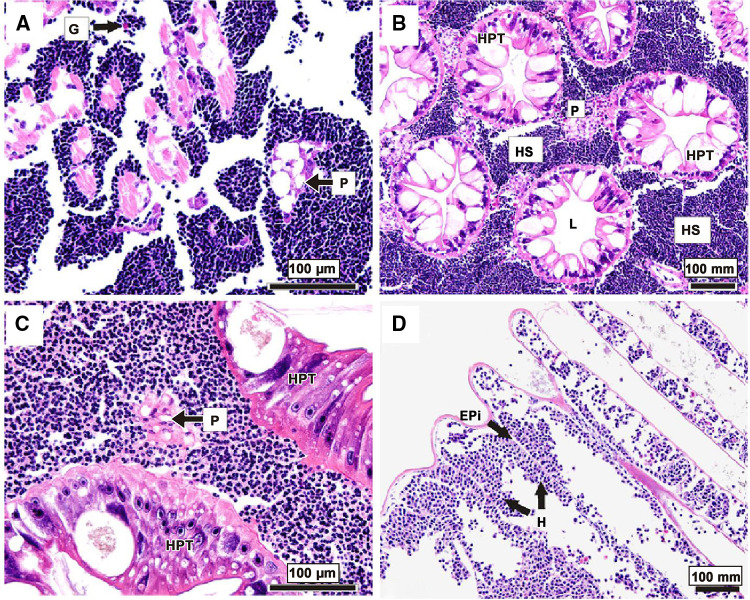
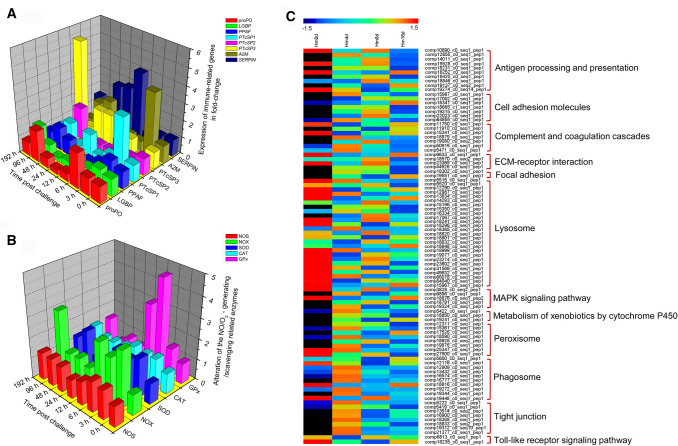
Hematodinium infection resulted in a series of physiological and pathological changes in the hemolymph and major tissues (e.g., hepatopancreas, gill, heart and muscle) of affected crustacean hosts (Huang et al. 2019; Stentiford and Shields 2005; Wang et al. 2015; Wheeler et al. 2007). Hematodinium infections could not only induce decreases in the number of circulating hemocytes (Li et al. 2015a) but also lead to detrimental biochemical changes in the hemolymph of the crustacean hosts (Shields et al. 2003; Patrzia and Giorgio 2018). Hematodinium infections were able to lead to elevation in the rate of oxygen consumption, suppression of the oxygen carrying capacity of hemolymph, and subsequent respiratory dysfunction in affected hosts (Shields et al. 2003; Taylor et al. 1996). Moreover, the activities of biochemical enzymes involved in the metabolic processes of carbohydrates, lipids and amino acids were significantly impacted by the parasitic infection (Shields et al. 2003). During the late stage of infection, the rapid proliferation of Hematodinium parasites led to absorption and consumption of large amounts of nutrients from the crustacean hosts, and to significantly reduced levels of glycogen in the hemolymph and hepatopancreas (Shields et al. 2003; Stentiford et al. 2001c). Along with the progression of the Hematodinium infections, overt pathological alterations (e.g., cellular damage and necrosis) were observed in hosts’ major organs/tissues, including the hepatopancreas, heart, gills and muscles (Huang et al. 2019; Wang et al. 2015; Wheeler et al. 2007) (Fig. 4). The severe respiratory/metabolic dysfunction, nutrient deletion as well as systemic tissue damages resulted in the eventual mortality of Hematodinium-infected hosts (Stentiford and Shields 2005). In addition, Hematodinium parasites could induce multifaceted immunological responses in affected crustacean hosts (Fig. 5). The parasite caused alterations in the expression of multiple immune-related genes and exhibited immunosuppressive effects on its hosts’ proPO system and Toll-like signaling pathway (Li et al. 2015a, b, c). The parasitic infection imbalanced the hosts’ cellular redox homeostasis via dysregulation of hosts’ cellular ROS/RNS-generating/scavenging system (Li et al. 2016), and were able to manipulate hosts’ miRNAs (Li et al. 2018). Hematodinium parasites resulted in suppressive and adverse effects on crustacean hosts’ immunity by inhibiting the critical immune responses mediated by the key pattern recognition receptors (e.g., C-lectin, SR class B, Toll), regulating the complement and coagulation pathway, and disturbing the cellular redox homeostasis as well as the cell adhesion molecules and extracellular matrix (Li et al. 2019). Meanwhile, the latest progress on the host-parasite interactions between the parasite’s phylogenetic relatives and their hosts suggest new directions to further explore the interplay mechanism between Hematodinium sp. and its crustacean hosts. For example, some specific life cycle stages of apicomplexan parasites (e.g., Plasmodium merozoites, Schistosoma larvae) possessed immune evasion mechanisms by mimicking the naturally-expressed host cellular surface proteins or glycans (Belachew 2018; Wright and Rayner 2014; Yoshino et al. 2013). Besides, apicomplexan parasites could hijack host immune defense functions to their benefit by subverting their host’s miRNAs (Hakimi and Cannella 2011). Similarly, the Hematodinium parasite may also possess immune evading ability to outsmart the host immune defenses at a certain life cycle stage (Rowley et al. 2015). Thus, further studies may be needed to emphasize the stage-specific immune responses of affected hosts against the parasitic infections and to reveal the possible evading mechanisms of Hematodinium.
Fig. 4.
Histopathological changes in the tissues of snow crab, Chionoecetes opilio, heavily infected by the parasitic dinoflagellate Hematodinium. Note: the tissue samples were stained with hematoxylin and eosin staining. Massive numbers of parasites can be observed in the hemal spaces in the tissue of heart (a), hepatopancreas (b, c) and gills (d). G granulocytes, P fixed phagocytes, HPT hepatopancreatic tubule, HS hemal sinus, H Hematodinium parasites, Epi epithelium (from Wheeler et al. 2007)
Fig. 5.
Multifaceted immune responses of Chinese swimming crabs, Portunus trituberculatus, to Hematodinium parasites. a The transcriptional immuno-suppressive effects of Hematodinium parasites on crustacean host’s proPO system [data originally from Li et al. (2015a; b)]; b significant imbalance of the crustacean host’s redox homeostasis via dysregulation of the NO/O2.−generating and antioxidant systems after Hematodinium challenge (data originally from Li et al. 2016); c numerous differentially expressed immune-related proteins and the significantly enriched immune pathways were found in crustacean hosts after Hematodinium challenge by iTRAQ proteomics. The immune system of the crustacean hosts were seriously suppressed and damaged by the Hematodinium parasites via inhibition of pattern recognition receptors (C-lectin, SR class B, and Toll)-mediated immune pathways, regulation of the complement and coagulation pathway, dysregulation of important cell adhesion molecules and extracellular matrix, and imbalance of the cellular redox homeostasis at translational levels [data originally from (Li et al. 2019)]
Outbreaks of Hematodinium diseases in wild populations of marine crustaceans
The seasonality of Hematodinium infections is an overt epidemiological feature of the parasite–host model in wild populations. Moreover, most infections of Hematodinium exhibit strong seasonal peaks in prevalence, but the patterns are not the same for each host system. Long-term monitoring of Nephrops norvegicus in the Clyde, Scotland indicated higher prevalence of Hematodinium sp. infections in winter and spring (Field et al. 1992, 1998; Stentiford et al. 2001a, b), In the Chionoecetes bairdi fishery of southeastern Alaska, the prevalence of Hematodinium sp. infections increased through spring, peaked in summer, then declined into fall and winter as infected hosts died (Eaton et al. 1991; Love et al. 1993; Meyers et al. 1990). In the American blue crab, Callinectes sapidus, the prevalence of H. perezi exhibited a bimodal pattern of prevalence, with a strong peak in the autumn and a following moderate peak in the following spring (Messick and Shields 2000; Sheppard et al. 2003). In general, the prevalence of Hematodinium spp. infections in boreal host species peaks in summer (C. bairdi) or autumn (C. opilio), whereas in temperate species the peaks occur mainly in the autumn (C. sapidus and possibly N. puber) or late winter and spring (N. norvegicus, Carcinus maenas, Carcinus pagurus) (Davies et al. 2019; Huchin-Mian et al. 2018; Stentiford and Shields 2005).
Besides the seasonal effect of temperature, several studies suggested its effect on development of Hematodinium spp. infections in affected hosts. In the in vitro cultures of Hematodinium from N. norvegicus, the development of its life cycle could proceed normally within the temperature range of 8–15 °C (Appleton and Vickerman 1998). In C. sapidus, the intensity of H. perezi infections increased in waters with temperature > 15 °C, and decreased when the temperature was below 16 °C (Messick et al. 1999). The in vitro cultures of Hematodinium from C. sapidus indicated also slow or retardant development of the parasite life stages when experimental temperatures were below 15 °C (Li et al. unpublished data). Salinity is a key factor limiting the spread of H. perezi in C. sapidus populations. Thus, outbreaks of Hematodinium disease were reported mainly to occur in high salinity waters, with rare cases of infections in waters below 18 (salinity) (Messick and Shields 2000; Messick and Sinderman 1992; Newman and Johnson 1975). Similarly, the prevalence of Hematodinium infections in N. norvegicus was correlated positively with salinity (Briggs and McAliskey 2002). Further laboratory studies indicated that the dinospores lost viability promptly in low salinity water (< 15), thereby lacking the ability to cause new infections even with the presence of susceptible hosts (Li et al. unpublished data).
Outbreaks of Hematodinium diseases in the cultured stocks of marine crustaceans
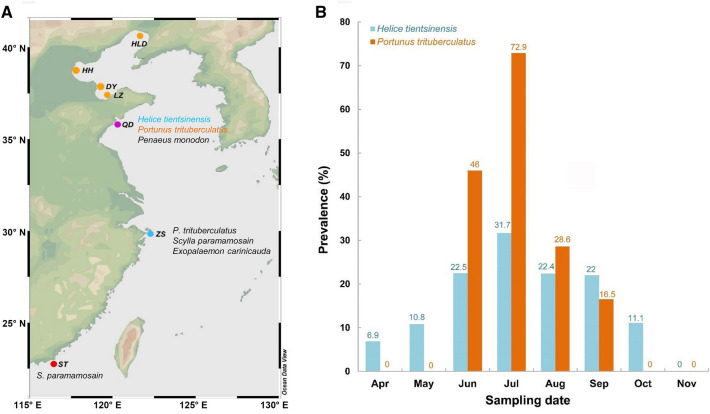
In China, the swimming crab P. trituberculatus and mud crab S. paramamosain sustain important mariculture industries in China with annual yields of 125,000 tons and 148,000 tons in 2016, respectively (Fisheries Bureau of Agriculture Ministry of China 2017). Since 2004, the epidemics caused by the parasitic dinoflagellate Hematodinium had been subsequently identified and reported to occur in aquaculture facilities in the coastal waters of Zhejiang, Guangdong, Shandong and Hebei provinces (Li et al. 2008, 2013; Xu et al. 2007a, b; Xu et al. 2010; Wang et al. 2017a, c) (Fig. 6a), which caused substantial economic losses to local producers. Previous studies have focused mainly on pathogen detection, host identification, the host immune response and the life cycle of representative strains. Limited knowledge had been accumulated to reveal the ecological aspects of the parasitic disease. Epidemics were more frequently reported in those aged polyculture ponds with limited water exchange. The peaks of Hematodinium infections were reported usually between July and September when water temperatures were high, or during rain and the typhoon season when dramatic environmental changes occurred (Li et al. 2013; Wang et al. 2017a; Xu et al. 2007a, b) (Fig. 6b). Yet, which environmental factors are closely associated with the outbreaks of the epidemics and their driving mechanisms still need to be comprehensively studied.
Fig. 6.
a Occurrence of Hematodinium infections in marine crustacean sampled along the coasts of China. HLD Huludao, Liaoning province, HH Huanghua, Hebei province, DY Dongying, Shandong province, LZ Laizhou, Shandong province, QD Qingdao, Shandong province, ZS Zhoushan, Zhejiang province, ST Shantou, Guangdong province. Colored dots indicated the locations of Hematodinium-positive samples (from Wang et al. 2017a; Li et al. unpublished data). b Prevalences of Hematodinium infection in cultured Chinese swimming crabs, Portunus trituberculatus (collected in 2014), and wild mudflat crabs, Helice tientsinensis (collected in 2018), from the polyculture facilities located in Qingdao, Shandong province. The sample sizes and prevalences of Hematodinium infection were shown above the columns in the bar chart [data originally from Wang et al. (2017a) and Huang et al. (2019)]
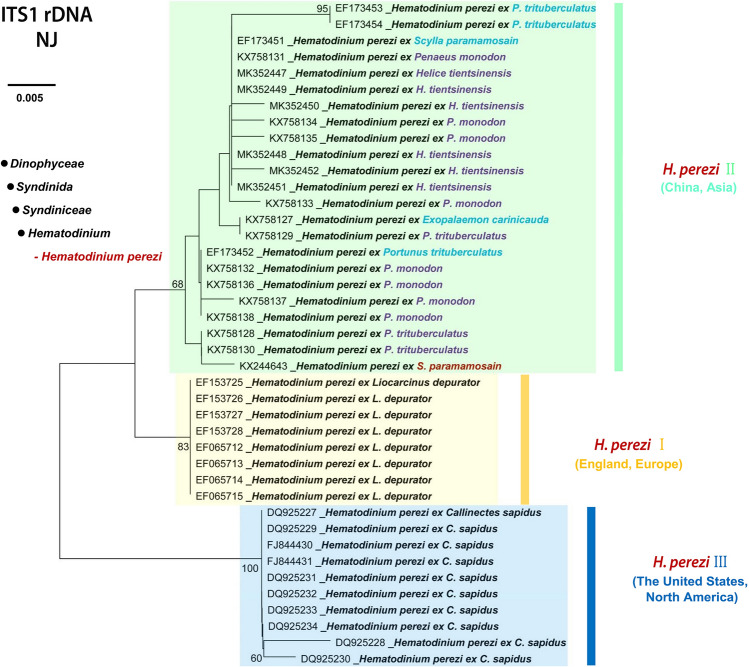
Crustaceans do not possess an adaptive immune system, and mostly rely on innate immunity to defend against invading pathogens (Cerenius et al. 2008; Li and Xiang 2013). There are not any commercial vaccines control particular pathogens; chemotherapy may be achieved by use of broad-spectrum antibiotics (Ringø et al. 2012; Wang et al. 2017b). Certainly, the unregulated usage of antibiotics or pesticides in aquaculture could result in serious negative impacts to the environment and to food safety issues (Rico et al. 2012; Sapkota et al. 2008). To date, there has not been any research to prevent and control Hematodinium diseases even though the parasites have been identified and reported in wild crabs since 1930s. Moreover, there have been frequently occurring epidemics in multiple commercially valuable marine crustaceans (Stentiford and Shields 2005). Indeed, Hematodinium, may be easily maintained and transmitted within the same species or among different species of susceptible hosts in the polyculture pond system, which is widely deployed for aquaculture of marine crabs in China. Furthermore, molecular evidence confirmed the homology of the Hematodinium spp. infecting the major cultured crabs and the two species of co-cultured shrimps (Huang et al. 2019) (Fig. 7). This implies the single origin of the pathogen even though multiple ITS haplotypes had been detected in populations of H. perezi II along the coasts of China (Xiao et al. 2016) (Fig. 8).
Fig. 7.
Phylogenetic analysis of Hematodinium spp. identified in cultured marine crustaceans along the coasts of China. The phylogenetic tree was based on genetic distance analysis of the first internal transcribed spacer (ITS1) of rRNA, and constructed with the Maximum Likelihood method in the MEGA7 software. The ITS1 sequences of Hematodinium spp. derived from Portunus trituberculatus, Scylla paramamosain (formerly known as Scylla Serrata), Helice tientsinensis, Penaeus monodon, Exopalaemon carinicauda formed one sister monophyletic clade (genotype II) to the clade (genotype I) containing the sequences of H. perezi identified in Liocarcinus depurator from northern Europe, and to the clade (genotype III) containing the sequences of H. perezi identified in Callinectes sapidus from North America. Bootstrap values (expressed as percentages of 1000 replications) greater than 50% were shown at branching points. Scale bar, 0.005 substitutions per nucleotide position [data originally from Huang et al. (2019)]
Fig. 8.
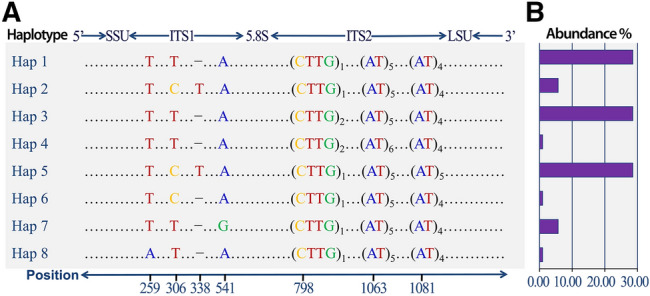
Nucleotide variations (a) and abundance (b) of the 8 ITS haplotypes detected in the populations of Hematodinium perezi (genotype II) along the coasts of China. The sequence analysis revealed only 8 ITS haplotypes with combinations of the dimorphic alleles at the seven polymorphic loci. The three most common haplotypes (Hap 1, Hap 3, Hap 5) accounted for 85.71% of the total number of sequences. Hap 2 and Hap 7 occurred 6 times, with a frequency of 5.71% respectively, and the rest of the three haplotypes (Hap 4, Hap 6 and Hap 8) only occurred one time in the entire population (from Xiao et al. 2016)
Besides those co-cultured shrimps, Hematodinium infections have been also identified in multiple wild crabs cohabiting with the major cultured species, especially in the mudflat crab Helice tientsinensis, which is one of the dominant wild species living in the waterways connecting to polyculture ponds or cohabiting with cultured crabs in the polyculture ponds of Shandong province (Huang et al. 2019). There were constant Hematodinium infections in mudflat crabs from April to November, with a prevalence of 5.8–31.7% (Fig. 6b). Juvenile P. trituberculatus were usually seeded and cultured in the polyculture ponds from early May until the end of October. Thus, it is possible that the mudflat crabs harboring Hematodinium infections may be an important source of the pathogen for cultured P. trituberculatus in the polyculture ponds. So, to isolate or quarantine the cultured P. trituberculatus from Hematodinium infected wild crabs may serve as potential countermeasure to control further spread of the pathogen. In addition, latest sentinel studies revealed successful transmission of the Hematodinium disease to juvenile blue crabs, C. sapidus, through cohabitating with naturally infected crabs in epidemic waters (Huchin-Mian et al. 2017; Shields et al. 2017). This implies the potential role of dinospores during transmission of the parasitic disease as well as a likely strategy to control transmission of the pathogen in aquaculture. Thus, with the accumulated knowledge about the life cycle, pathogenesis, transmission and outbreak mechanism of the parasitic dinoflagellate Hematodinium, the practical measures for prevention and control of the pathogen in polyculture systems will be achievable in the future.
Future directions
Since 1930s, the parasitic dinoflagellate Hematodinium had been identified as an infectious pathogen causing epidemic diseases in wild populations of various marine crabs. The result is significant economic loss to the fisheries of several commercially valuable marine crustaceans globally. The epidemics of parasitic diseases of farmed major crab species have increased in China in recent years resulting in substantial losses, and impacting on the sustainable development of mariculture. Previous studies have focused mainly on pathogen detection, host identification, epidemiology, disease ecology, and life cycles of representative strains. Clearly, there are obvious knowledge gaps compared to other marine pathogens that significantly impact marine crustaceans. Firstly, we foresee that the taxonomy, phylogenetic relations and biogeographical characteristics of Hematodinium spp. globally will be key objectives for future studies. Such data will reveal the origin and global dispersion of the disease. The host-parasite interaction between the parasite and its crustacean hosts will be the second key direction, i.e., how does the parasite manipulate its crustacean hosts, and thereby survives and proliferates internally. Thirdly, the spread and transmission mechanism of the parasite among different susceptible hosts will be another key direction for future studies. Answers to these questions will lead to proposals for practical disease prevention and control strategies for use in aquaculture of marine crustaceans.
Acknowledgements
This research was financially supported by the NSFC-Shandong Joint program (Grant No. U1906214) and the general program (Grant No. 41676102) of National Natural Science Foundations of China.
Author contributions
CL wrote the original draft, ML and QH revised the manuscript. All authors reviewed and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Animal and human rights statement
This article does not contain human participants or animals.
Footnotes
Edited by Xin Yu.
References
- Appleton PL, Vickerman K. In vitro cultivation and developmental cycle in culture of a parasitic dinoflagellate (Hematodinium sp.) associated with mortality of the Norway lobster (Nephrops norvegicus) in British waters. Parasitology. 1998;116:115–130. doi: 10.1017/S0031182097002096. [DOI] [PubMed] [Google Scholar]
- Belachew EB. Immune response and evasion mechanisms of Plasmodium falciparum parasites. J Immunol Res. 2018;2018:6529681. doi: 10.1155/2018/6529681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs RP, McAliskey M. The prevalence of Hematodinium in Nephrops norvegicus from the western Irish Sea. J Mar Biol Assoc UK. 2002;82:427–433. doi: 10.1017/S0025315402005684. [DOI] [Google Scholar]
- Cerenius L, Lee BL, Söderhäll K. The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol. 2008;29:263–271. doi: 10.1016/j.it.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Chatton E, Poisson R. Sur l’existence, dans le sang des Crabes, de Péridiniens parasites: Hematodinium perezi n. g., n. sp. (Syndinidae) C R Seances Soc Biol. 1931;105:553–557. [Google Scholar]
- Davies CE, Batista FM, Malkin SH, Thomas JE, Bryan CC, Crocombe P, Coates CJ, Rowley AF. Spatial and temporal disease dynamics of the parasite Hematodinium sp. in shore crabs Carcinus maenas. Parasite Vector. 2019;12:472. doi: 10.1186/s13071-019-3727-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton WD, Love DC, Botelho C, Meyers TR, Imamura K, Koeneman T. Preliminary results on the seasonality and life cycle of the parasitic dinoflagellate causing bitter crab disease in Alaskan tanner crabs (Chionoecetes bairdi) J Invertebr Pathol. 1991;57:426–434. doi: 10.1016/0022-2011(91)90147-I. [DOI] [PubMed] [Google Scholar]
- Field RH, Appleton PL. A Hematodinium-like dinoflagellate infection of the Norway lobster Nephrops norvegicus: observations on pathology and progression of infection. Dis Aquat Organ. 1995;22:115–128. doi: 10.3354/dao022115. [DOI] [Google Scholar]
- Field RH, Chapman CJ, Taylor AC, Neil DM, Vickerman K. Infection of the Norway lobster Nephrops norvegicus by a Hematodinium-like species of dinoflagellate on the west coast of Scotland. Dis Aquat Organ. 1992;13:1–15. doi: 10.3354/dao013001. [DOI] [Google Scholar]
- Field RH, Hills JM, Atkinson RJA, Magill S, Shanks AM. Distribution and seasonal prevalence of Hematodinium sp. infection of the Norway lobster (Nephrops norvegicus) around the west coast of Scotland. ICES J Mar Sci. 1998;55:846–858. doi: 10.1006/jmsc.1998.0357. [DOI] [Google Scholar]
- Fisheries Bureau of Agriculture Ministry of China . China fishery statistical yearbook. Beijing: China Agriculture Press; 2017. [Google Scholar]
- Guiry MD, Guiry GM (2020) Algae base. World-wide electronic publication, National University of Ireland, Galway https://www.algaebase.org. Accessed 21 July 2020
- Hakimi MA, Cannella D. Apicomplexan parasites and subversion of the host cell microRNA pathway. Trends Parasitol. 2011;27:481–486. doi: 10.1016/j.pt.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Huang Q, Li M, Wang F, Li CW. The parasitic dinoflagellate Hematodinium perezi infecting mudflat crabs, Helice tientsinensis, in polyculture system in China. J Invertebr Pathol. 2019;166:107229. doi: 10.1016/j.jip.2019.107229. [DOI] [PubMed] [Google Scholar]
- Huchin-Mian JP, Small HJ, Shields JD. Patterns in the natural transmission of the parasitic dinoflagellate Hematodinium perezi in American blue crabs, Callinectes sapidus from a highly endemic area. Mar Biol. 2017;164:153. doi: 10.1007/s00227-017-3185-y. [DOI] [Google Scholar]
- Huchin-Mian JP, Small HJ, Shields JD. The influence of temperature and salinity on mortality of recently recruited blue crabs, Callinectes sapidus, naturally infected with Hematodinium perezi (Dinoflagellata) J Invertebr Pathol. 2018;152:8–16. doi: 10.1016/j.jip.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Hudson DA, Adlard RD. Nucleotide sequence determination of the partial SSU rDNA gene and ITS1 region of Hematodinium cf. perezi and Hematodinium-like dinoflagellates. Dis Aquat Organ. 1996;24:55–60. doi: 10.3354/dao024055. [DOI] [Google Scholar]
- Hudson DA, Shields JD. Hematodinium australis n. sp., a parasitic dinoflagellate of the sand crab Portunus pelagicus from Moreton Bay. Australia Dis Aquat Organ. 1994;19:109–119. doi: 10.3354/dao019109. [DOI] [Google Scholar]
- Li FH, Xiang JH. Recent advances in researches on the innate immunity of shrimp in China. Dev Comp Immunol. 2013;39:11–26. doi: 10.1016/j.dci.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Li CW, Xu WJ. Review on parasitic dinoflagellates Hematodinium spp. in major marine crustaceans. Oceanol Limnol Sin. 2014;45:3–12. [Google Scholar]
- Li YY, Xia XA, Wu QY, Liu WH, Lin YS. Infection with Hematodinium sp. in mud crabs Scylla serrata cultured in low salinity water in southern China. Dis Aquat Organ. 2008;82:145–150. doi: 10.3354/dao01988. [DOI] [PubMed] [Google Scholar]
- Li CW, Shields JD, Miller TL, Small HJ, Pagenkopp KM, Reece KS. Detection and quantification of the free-living stage of the parasitic dinoflagellate Hematodinium sp. in laboratory and environmental samples. Harmful Algae. 2010;9:515–521. doi: 10.1016/j.hal.2010.04.001. [DOI] [Google Scholar]
- Li CW, Miller TL, Small HJ, Shields JD. In vitro culture and developmental cycle of the parasitic dinoflagellate Hematodinium sp. from the blue crab Callinectes sapidus. Parasitology. 2011;138:1924–1934. doi: 10.1017/S0031182011001405. [DOI] [PubMed] [Google Scholar]
- Li CW, Wheeler KN, Shields JD. Lack of transmission of Hematodinium sp. in the blue crab Callinectes sapidus through cannibalism. Dis Aquat Organ. 2011;96:249–258. doi: 10.3354/dao02399. [DOI] [PubMed] [Google Scholar]
- Li CW, Song SQ, Liu Y, Chen TT. Hematodinium infections in cultured Chinese swimming crab, Portunus trituberculatus, in northern China. Aquaculture. 2013;396–399:59–65. doi: 10.1016/j.aquaculture.2013.02.022. [DOI] [Google Scholar]
- Li M, Li C, Wang J, Song S. Immune response and gene expression in hemocytes of Portunus trituberculatus inoculated with the parasitic dinoflagellate Hematodinium. Mol Immunol. 2015;65:113–122. doi: 10.1016/j.molimm.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Li M, Li C, Wang J, Song S. Molecular characterization and expression of a novel Toll gene from the swimming crab Portunus trituberculatus. Mol Immunol. 2015;67:388–397. doi: 10.1016/j.molimm.2015.06.028. [DOI] [PubMed] [Google Scholar]
- Li M, Wang J, Song S, Li C. Early transcriptional response to the parasitic dinoflagellate Hematodinium in hepatopancreas of Portunus trituberculatus. J Invertebr Pathol. 2015;130:28–36. doi: 10.1016/j.jip.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Li M, Wang J, Song S, Li C. Molecular characterization of a novel nitric oxide synthase gene from Portunus trituberculatus and the roles of NO/O2−-generating and antioxidant systems in host immune responses to Hematodinium. Fish Shellfish Immunol. 2016;52:263–277. doi: 10.1016/j.fsi.2016.03.042. [DOI] [PubMed] [Google Scholar]
- Li M, Huang Q, Wang J, Li C. Differential expression of microRNAs in Portunus trituberculatus in response to Hematodinium parasites. Fish Shellfish Immunol. 2018;83:134–139. doi: 10.1016/j.fsi.2018.09.011. [DOI] [PubMed] [Google Scholar]
- Li M, Wang J, Huang Q, Li C. Proteomic analysis reveals the immune responses of the hepatopancreas against Hematodinium infection in Portunus trituberculatus. J Proteomics. 2019;197:92–105. doi: 10.1016/j.jprot.2018.11.012. [DOI] [PubMed] [Google Scholar]
- Love DC, Rice SD, Moles DA, Eaton WD. Seasonal prevalence and intensity of Bitter Crab dinoflagellate infection and host mortality in Alaskan Tanner crabs Chionoecetes bairdi from Auke Bay, Alaska, USA. Dis Aquat Organ. 1993;15:1–7. doi: 10.3354/dao015001. [DOI] [Google Scholar]
- Messick GA, Shields JD. Epizootiology of the parasitic dinoflagellate Hematodinium sp. in the American blue crab Callinectes sapidus. Dis Aquat Organ. 2000;43:139–152. doi: 10.3354/dao043139. [DOI] [PubMed] [Google Scholar]
- Messick GA, Sinderman CJ (1992) Synopsis of principal diseases of the blue crab, Callinectes sapidus. NOAA NMFS Tech Memo NMFS-F/NEC-88, Washington, D.C. p 24
- Messick GA, Jordan SJ, Van Heukelem WF. Salinity and temperature effects on Hematodinium sp. in the blue crab Callinectes sapidus. J Shellfish Res. 1999;18:657–662. [Google Scholar]
- Meyers TR, Koeneman TM, Botelho C, Short S. Bitter crab disease: a fatal dinoflagellate infection and marketing problem for Alaskan Tanner crabs Chionoecetes bairdi. Dis Aquat Organ. 1987;3:195–216. doi: 10.3354/dao003195. [DOI] [Google Scholar]
- Meyers TR, Botelho C, Koeneman TM, Short S, Imamura K. Distribution of bitter crab dinoflagellate syndrome in southeast Alaskan tanner crabs, Chionoecetes bairdi. Dis Aquat Organ. 1990;9:37–43. doi: 10.3354/dao009037. [DOI] [Google Scholar]
- Meyers TR, Morado JF, Sparks AK, Bishop GH, Pearson T, Urban D, Jackson D. Distribution of bitter crab syndrome in Tanner crabs (Chionoecetes bairdi, C. opilio) from the Gulf of Alaska and the Bering Sea. Dis Aquat Organ. 1996;26:221–227. doi: 10.3354/dao026221. [DOI] [Google Scholar]
- Morado JF, Siddeek MSM, Mullowney DR, Dawe EG. Protistan parasites as mortality drivers in cold water crab fisheries. J Invertebr Pathol. 2012;110:201–210. doi: 10.1016/j.jip.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Newman MW, Johnson CA. A disease of blue crabs (Callinectes sapidus) caused by a parasitic dinoflagellate, Hematodinium sp. J Parasitol. 1975;63:554–557. doi: 10.2307/3279346. [DOI] [Google Scholar]
- Pagenkopp Lohan KM, Reece KS, Miller TL, Wheeler KN, Small HJ, Shields JD. The role of alternate hosts in the ecology and life history of Hematodinium sp. a parasitic dinoflagellate of the blue crab (Callinectes sapidus) J Parasitol. 2012;98:73–84. doi: 10.1645/GE-2854.1. [DOI] [PubMed] [Google Scholar]
- Patrzia P, Giorgio M. Parasites affect hemocyte functionality in the hemolymph of the invasive Atlantic blue crab Callinectes sapidus from a coastal habitat of the Salento Peninsula (SE Italy) Medit Mar Sci. 2018;19:193–200. doi: 10.12681/mms.13886. [DOI] [Google Scholar]
- Rico A, Satapornvanit K, Haque MM, Min J, Nguyen PT, Telfer TC, van den Brink PJ. Use of chemicals and biological products in Asian aquaculture and their potential environmental risks: a critical review. Rev Aquac. 2012;4:75–93. doi: 10.1111/j.1753-5131.2012.01062.x. [DOI] [Google Scholar]
- Ringø E, Olsen RE, Vecino JLG, Wadsworth S, Song SK. Use of immunostimulants and nucleotides in aquaculture: a review. J Mar Sci Res Dev. 2012;1:104. [Google Scholar]
- Ris H, Kublai DF. An unusual mitotic mechanism in the parasitic protozoan Syndinium sp. J Cell Biol. 1974;60:702–720. doi: 10.1083/jcb.60.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley AF, Smith AL, Davies CE. How does the dinoflagellate parasite Hematodinium outsmart the immune system of its crustacean hosts? PLoS Pathog. 2015;11:e1004724. doi: 10.1371/journal.ppat.1004724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota A, Sapkota AR, Kucharski M, Burke J, McKenzie S, Walker P, Lawrence R. Aquaculture practices and potential human health risks: current knowledge and future priorities. Environ Int. 2008;34:1215–1226. doi: 10.1016/j.envint.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Sheppard M, Walker A, Frischer ME, Lee RF. Histopathology and prevalence of the parasitic dinoflagellate Hematodinium sp, in crabs (Callinectes sapidus, Callinectes similis, Neopanope sayi, Libiniae marginata, Menippemer cenaria) from a Georgia estuary. J Shellfish Res. 2003;22:873–880. [Google Scholar]
- Shields JD. The parasitic dinoflagellates of marine crustaceans. Annu Rev Fish Dis. 1994;4:241–271. doi: 10.1016/0959-8030(94)90031-0. [DOI] [Google Scholar]
- Shields JD. The impact of pathogens on exploited populations of decapod crustaceans. J Invertebr Pathol. 2012;110:211–224. doi: 10.1016/j.jip.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Shields JD, Squyars CM. Mortality and hematology of blue crabs, Callinectes sapidus, experimentally infected with the parasitic dinoflagellate Hematodinium perezi. Fish Bull. 2000;98:139–152. [Google Scholar]
- Shields JD, Scanlon C, Volety A. Aspects of the pathophysiology of blue crabs, Callinectes sapidus, infected with the parasitic dinoflagellate Hematodinium perezi. B Mar Sci. 2003;72:519–535. [Google Scholar]
- Shields JD, Taylor DM, O’Keefe PG, Colbourne E, Hynick E. Epidemiological determinants in outbreaks of bitter crab disease (Hematodinium sp.) in snow crabs, Chionoecetes opilio from Newfoundland. Canada Dis Aquat Organ. 2007;77:61–72. doi: 10.3354/dao01825. [DOI] [PubMed] [Google Scholar]
- Shields JD, Huchin-Mian JP, O'Leary PA, Small HJ. New insight into transmission dynamics of the crustacean pathogen Hematodinium perezi (Dinoflagellata) using a novel sentinel methodology. Mar Ecol Prog Ser. 2017;573:73–84. doi: 10.3354/meps12175. [DOI] [Google Scholar]
- Small HJ. Advances in our understanding of the global diversity and distribution of Hematodinium spp. – Significant pathogens of commercially exploited crustaceans. J Invertebr Pathol. 2012;110:234–246. doi: 10.1016/j.jip.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Small HJ, Shields JD, Reece KS, Bateman K, Stentiford GD. Morphological and molecular characterization of Hematodinium perezi (Dinophyceae: Syndiniales), a dinoflagellate parasite of the harbour crab, Liocarcinus depurator. J Eukaryot Microbiol. 2012;59:54–66. doi: 10.1111/j.1550-7408.2011.00592.x. [DOI] [PubMed] [Google Scholar]
- Stentiford GD, Shields JD. A review of the parasitic dinoflagellates Hematodinium species and Hematodinium-like infections in marine crustaceans. Dis Aquat Organ. 2005;66:47–70. doi: 10.3354/dao066047. [DOI] [PubMed] [Google Scholar]
- Stentiford GD, Chang ES, Chang SA, Neil DM. Carbohydrate dynamics and the crustacean hyperglycemic hormone (CHH): effects of parasitic infection in Norway lobsters (Nephrops norvegicus) Gen Comp Endocrinol. 2001;121:13–22. doi: 10.1006/gcen.2000.7575. [DOI] [PubMed] [Google Scholar]
- Stentiford GD, Neil DM, Atkinson RJA. The relationship of Hematodinium infection prevalence in a Scottish Nephrops norvegicus population to seasonality, moulting and sex. ICES J Mar Sci. 2001;58:814–823. doi: 10.1006/jmsc.2001.1072. [DOI] [Google Scholar]
- Stentiford GD, Neil DM, Coombs GH. Development and application of an immunoassay diagnostic technique for studying Hematodinium infections in Nephrops norvegicus populations. Dis Aquat Organ. 2001;46:223–229. doi: 10.3354/dao046223. [DOI] [PubMed] [Google Scholar]
- Taylor AC, Field RH, Parslow-Williams PJ. The effects of Hematodinium sp.-infection on aspects of the respiratory physiology of the Norway lobster, Nephrops norvegicus (L.) J Exp Mar Bio Ecol. 1996;207:217–228. doi: 10.1016/S0022-0981(96)02649-4. [DOI] [Google Scholar]
- Wang RC, Yang JM, Zheng XD. Current status of polyculture and rotation culture in Chinese mariculture industry. Acta Oceanol Sin. 2004;23:505–512. [Google Scholar]
- Wang J, Li C, Li M, Song S. Morphology and histopathology of parasitic dinoflagellate Hematodinium sp. infecting Portunus trituberculatus. Oceano Limnol Sin. 2015;46:1–10. [Google Scholar]
- Wang JF, Li M, Xiao J, Xu WJ, Li CW. Hematodinium spp. infections in wild and cultured populations of marine crustaceans along the coast of China. Dis Aquat Organ. 2017;124:181–191. doi: 10.3354/dao03119. [DOI] [PubMed] [Google Scholar]
- Wang W, Sun J, Liu C, Xue Z. Application of immunostimulants in aquaculture: current knowledge and future perspectives. Aquac Res. 2017;48:1–23. doi: 10.1111/are.13161. [DOI] [Google Scholar]
- Wang YG, Yang Y, Zhang Z, Li B, Liao MJ, Deng W, Meng FL. Etiological and pathological analyses of massive mortality in cultured crab Portunus trituberculatus along the coasts of Tianjin and Hebei, China. J Fish Sci China. 2017;24:596–605. [Google Scholar]
- Wheeler KN, Shields JD, Taylor DM. Pathology of Hematodinium infections in snow crabs (Chionoecetes opilio) from Newfoundland, Canada. J Invertebr Pathol. 2007;95:93–100. doi: 10.1016/j.jip.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Wright GJ, Rayner JC. Plasmodium falciparum erythrocyte invasion: combining function with immune evasion. PLoS Pathog. 2014;10:e1003943. doi: 10.1371/journal.ppat.1003943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Miao XX, Li CW, Xu WJ, Zhang XL, Wang ZL. Genetic variations of the parasitic dinoflagellate Hematodinium infecting cultured marine crustaceans in China. Protist. 2016;167:597–609. doi: 10.1016/j.protis.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Xu WJ, Sheng XZ, Xu HX, Shi H, Li PF. Dinoflagellates Hematodinium sp. parasitizing the mud crab Scylla serrata. Period Ocean Univ China. 2007;37:916–920. [Google Scholar]
- Xu WJ, Shi H, Xu HX, Small HJ. Preliminary study on the Hematodinium infection in cultured Portunus trituberculatus. Acta Hydrobiol Sin. 2007;31:637–642. [Google Scholar]
- Xu WJ, Xie JJ, Shi H, Li CW. Hematodinium infections in cultured ridgetail white prawns, Exopalaemon carinicauda, in eastern China. Aquaculture. 2010;300:25–31. doi: 10.1016/j.aquaculture.2009.12.024. [DOI] [Google Scholar]
- Yoshino TP, Wu XJ, Gonzale LA, Hooke CH. Circulating Biomphalaria glabrata hemocyte subpopulations possess shared schistosome glycans and receptors capable of binding larval glycoconjugates. Exp Parasitol. 2013;133:28–36. doi: 10.1016/j.exppara.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]