Abstract
As a group, the halophilic archaea (class Halobacteria) are the most salt-requiring and salt-resistant microorganisms within the domain Archaea. Halophilic archaea flourish in thalassohaline and athalassohaline environments and require over 100–150 g/L NaCl for growth and structural stability. Natural hypersaline environments vary in salt concentration, chemical composition and pH, and occur in climates ranging from tropical to polar and even under-sea. Accordingly, their resident haloarchaeal species vary enormously, as do their individual population compositions and community structures. These diverse halophilic archaeal strains are precious resources for theoretical and applied research but assessing their taxonomic and metabolic novelty and diversity in natural environments has been technically difficult up until recently. Environmental DNA-based high-throughput sequencing technology has now matured sufficiently to allow inexpensive recovery of massive amounts of sequence data, revealing the distribution and community composition of halophilic archaea in different hypersaline environments. While cultivation of haloarchaea is slow and tedious, and only recovers a fraction of the natural diversity, it is the conventional means of describing new species, and provides strains for detailed study. As of the end of May 2020, the class Halobacteria contains 71 genera and 275 species, 49.8% of which were first isolated from the marine salt environment and 50.2% from the inland salt environment, indicating that both thalassohaline and athalassohaline environments contain diverse halophilic archaea. However, there remain taxa that have not yet been isolated in pure culture, such as the nanohaloarchaea, which are widespread in the salt environment and may be one of the hot spots in the field of halophilic archaea research in the future. In this review, we focus on the cultivation strategies that have been used to isolate extremely halophilic archaea and point out some of the pitfalls and challenges.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42995-020-00087-3.
Keywords: Halobacteria, Growth, Culture, Cultivation, Large-scale isolation, Medium formulation
Introduction
Haloarchaea (class Halobacteria; phylum Euryarchaeota) are members of the domain Archaea and inhabit high salt environments such as brines, salt lakes and salty soils, where they often grow to such high cell densities that their red pigments give the water a pink–red coloration (Oren 2014a). They are widely distributed around the globe, are halophilic, generally requiring salt concentrations of at least 15% (2.5 mol/L) for optimum growth, with many species able to flourish in saturating salt conditions (~ 35%, 6 mol/L) where few other organisms can survive (Oren et al. 2009). While haloarchaea are often the dominant microorganisms in these extreme environments, other archaea may also be present either in the water column, such as nanohaloarchaea, or in the underlying muds, such as halophilic (anaerobic) methanogenic archaea, for example members of the Methanosarcinales and Methanomicrobiales (Andrei et al. 2012; Abdallah et al. 2018). Several halophilic bacterial species can also compete well at the highest salinities, such as Salinibacter (Antón et al. 2013; Oren 2013). The nanohaloarchaea (Candidatus Nanohaloarchaeota), so named because of their small cell size (< 0.8 µm), diminutive genomes (< 1.2 Mb) and halophilic lifestyle (Narasingarao et al. 2012; Vavourakis et al. 2016), have yet to be cultured in isolation and their classification has undergone considerable change (Aouad et al. 2018). Originally placed within the phylum Euryarchaeota (Narasingarao et al. 2012) they were later reclassified in the candidate phylum Nanohaloarchaeota within the ‘DPANN’ superphylum (Rinke et al. 2013). Some members of Candidatus Nanohaloarchaeota in hypersaline environments may be free-living, however, two recent cultivation studies could only maintain them as co-cultures with haloarchaeal partners (Hamm et al. 2019; La Cono et al. 2020). As dominant microorganisms inhabiting hypersaline environments, haloarchaea are major drivers of various biogeochemical cycles in these extreme ecosystems (Farias et al. 2017; Oren 2015). With poorly studied metabolisms and high salt concentrations both inside and outside their cells, haloarchaea have long been considered potential sources for the discovery of bioactive compounds and novel, extremophilic enzymes (Litchfield 2011; Waditee-Sirisattha et al. 2016). It is to be anticipated that large-scale isolation and cultivation of genetically diverse strains of haloarchaea will lead to the discovery of new products and processes of biotechnological importance (Akolkar et al. 2010; Amoozegar et al. 2017). The aim of this review is to briefly explore the cultivation of the halophilic archaea of the class Halobacteria isolated from thalassohaline and athalassohaline environments.
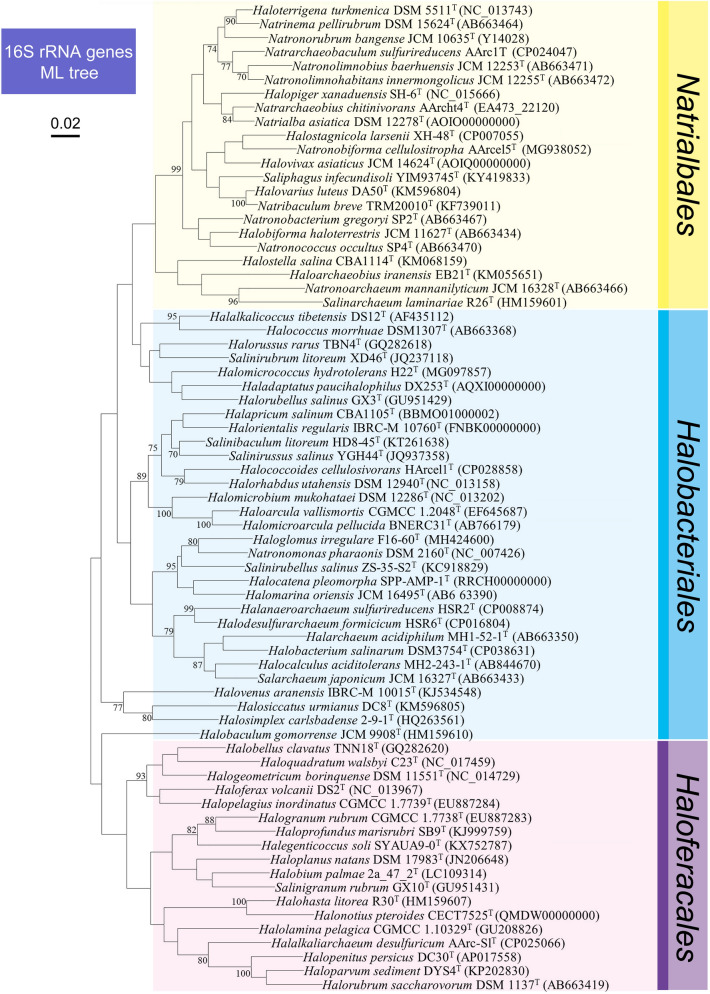
The diversity of the class Halobacteria
The class Halobacteria is currently divided into three orders: Halobacteriales, Haloferacales and Natrialbales, based on whole-genome phylogenetic analyses, conserved signature proteins and conserved signature insertions/deletions (Gupta et al. 2015). More recently, two additional order-level taxa have been detected but remain to be formally described (Aouad et al. 2018). The order Halobacteriales contains three families: Halobacteriaceae, Haloarculaceae and Halococcaceae; the order Haloferacales contains two families, Halorubraceae and Haloferacaceae; and the order Natrialbales contains a single family, Natrialbaceae (Gupta et al. 2016). As of the end of June 2020, class Halobacteria contained 71 genera (Fig. 1), 51 of which were recently reviewed by Amoozegar et al. (2017), and the remaining 20 genera are listed and described in Supplementary Table S1. Among the current 275 species of class Halobacteria, 49.8% were first described from type strains isolated from marine salt environments (thalassohaline), such as coastal solar salterns, while 50.2% were described from type strains isolated from inland salt environments, many of which are athalassohaline and some, such as highly alkaline soda lakes (Soliman and Trüper 1982; Vavourakis et al. 2016), exceedingly so. While most species are aerobic heterotrophs, Halorhabdus tiamatea SARL4BT was the first haloarchaeon isolated from an anoxic, deep-sea brine, an unusual athalassohaline environment created by the evaporation, and subsequent exposure and flooding, of ancient seas (Antunes et al. 2008). This strain grew best under strictly anaerobic conditions. It could also grow under microaerophilic conditions but aerobic growth was poor, revealing a high degree of adaptation to this type of extreme biotope. More recently, genera of strictly anaerobic acetotrophic (Sorokin et al. 2016) and lithoheterotrophic (Sorokin et al. 2017) haloarchaea have been described, Halanaeroarchaeum and Halodesulfurarchaeum, respectively. Low-temperature hypersaline environments also harbor haloarchaea. Halorubrum lacusprofundi was isolated from Deep Lake, a hypersaline, Antarctic lake dominated by haloarchaea (Demaere et al. 2013) and with an average yearly temperature of around – 15 °C (Bowman et al. 2000). While the optimum growth temperature of Hrr. lacusprofundi is in the mesophile range, it shows considerable adaptation to low (mostly subzero) temperatures (Franzmann et al. 1988; Tschitschko et al. 2016). These examples demonstrate that both thalassohaline and athalassohaline environments contain abundant and diverse halophilic archaea.
Fig. 1.
Maximum-Likelihood phylogenetic tree based on 16S rRNA gene sequences, showing the relationships among current members within the class Halobacteria. Bar represents expected substitutions per nucleotide position. Gene accession numbers are provided in the brackets
Cultivation of halophilic archaea from thalassohaline environments
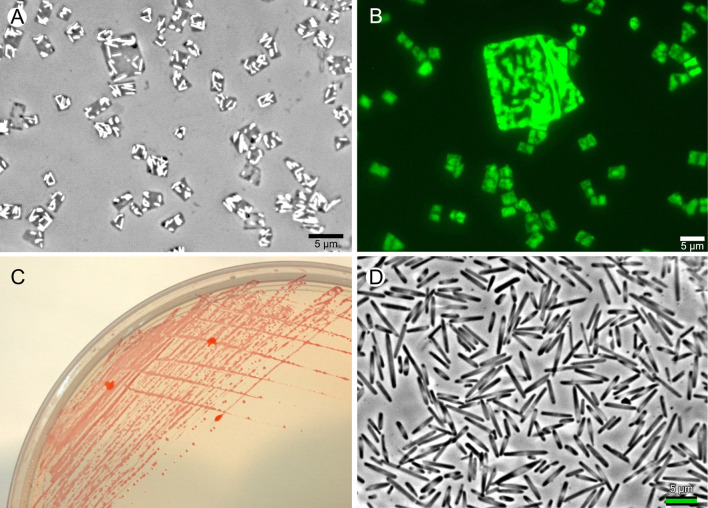
Thalassohaline environments are derived from marine origin and have salt ratios similar to those of marine waters (Ventosa et al. 2015). The pH of thalassohaline environments is near neutral to slightly alkaline. Marine solar salterns and coastal hypersaline ponds or lagoons are two common kinds of thalassohaline environments that have been extensively studied (Clementino et al. 2008; Fernández et al. 2014; Oren 2002), resulting in the isolation and detection of numerous and diverse species of haloarchaea. Cell shapes vary widely, and include rods, cocci, flattened and pleomorphic forms, with individual species often displaying two or more, distinct cell shapes in cell populations (Oren 2014b). One extraordinary example is the square haloarchaea of Walsby, which was first described in 1980 in a brine sample taken from a hypersaline pool (sabkha) in a coastal plain at sea level just south of Nabq, on the Sinai Peninsula (Walsby 1980). These cells grow as extremely thin, flat squares, and can reach high cell densities in natural waters. Their isolation in pure culture in the laboratory was first reported only in 2004 (Bolhuis et al. 2004; Burns et al. 2004a), leading to the taxonomic description of Haloquadratum walsbyi gen. nov., sp. nov. 3 years later (Burns et al. 2007). Two different cultivation methods were used to isolate strains of Haloquadratum walsbyi. Strain C23T (Australia) was recovered using an extinction–dilution culturing technique while strain HBSQ001 (Spain) was obtained by serial enrichment over a 2-year period. Both strains preferred low-nutrient media containing pyruvate (Burns et al. 2007). The use of low-nutrient media with greatly reduced levels of complex organic nutrients (e.g, yeast extract, tryptone or peptone) has been shown to be important in recovering many significant species of environmental bacteria, such as the SAR11 clade member Candidatus Pelagibacter ubique (Rappé et al. 2002). The cell morphology of Haloquadratum is shown in Fig. 2a, b, along with an example of its growth on solid medium (Fig. 2c). This organism requires patience and skill to isolate and grow in the laboratory and very few new strains have been reported since 2007. In 2007, the Haloquadratum strain Bajool9 (JCM 15065; Fig. 2b) was isolated using the same extinction-dilution method described earlier by Burns et al. (2004a). Briefly, a natural water sample was diluted in DBCM2 medium to achieve a cell concentration of about 100 cells/ml, and this was dispensed into the first row (4 wells) of five plastic tissue-culture grade cluster trays (6 × 4 format, a total of 24 wells, 3 ml capacity/well). Five additional 5-fold dilutions were then dispensed into the remaining five rows of the five trays, making a total of 20 replicates for each dilution. Trays were incubated for four weeks at 37 °C at which time growth was visible by eye as pink-colored turbidity. Samples were taken from wells of the highest dilution displaying turbidity and screened by PCR to identify those wells with Haloquadratum. From these, a second round of extinction dilution cultivation was performed to achieve a pure culture.
Fig. 2.
Examples of halophilic archaea that are not easily cultivated. A Haloquadratum strain Bajool9 (JCM 15065), phase contrast microscopy, showing prominent gas vesicles; B Haloquadratum strain Bajool9 (JCM 15065), fluorescent microscopy after staining with acridine orange. A partly-folded giant cell is seen in the middle of the field; C Haloquadratum walsbyi C23T growing on agarose solidified DBCM2 medium, showing small colonies after six weeks incubation; D Antarctic isolate Halohasta litchfieldiae growing as a biofilm on a glass cover slip. Scale bars of micrographs represent 5 µm.
Inspired by the cultivation and isolation of Haloquadratum walsbyi, a defined medium (NOM) with pyruvate as carbon source was used to recover diverse halophilic archaea from marine solar salterns and from salted Laminaria (a brown alga) (Cui et al. 2010). Since then, cultivation studies using this medium, as well as those used by other laboratories, have resulted in the isolation and description of 14 new genera: Halobellus, Halogranum, Halohasta, Halolamina, Halopelagius, Halorientalis, Halorubellus, Halorussus, Salinarchaeum, Salinibaculum, Salinigranum, Salinirubellus, Salinirubrum, and Salinirussus were described (Amoozegar et al. 2017; Cui et al. 2017; Han and Cui 2020; Hou et al. 2018). Like Halorubrum lacusprofundi, Halohasta litchfieldiae (strain tADL) was isolated from the Antarctic hypersaline waters of Deep Lake, but it was not readily cultivable on standard media, and isolation used the liquid end-point dilution method with DBCM2 medium (Mou et al. 2012). The only change to the method described above for Hqr. walsbyi Bajool9 was that the incubation temperature was lowered to 30 °C, to accommodate organisms living in a cold environment. The cell morphology of Halohasta litchfieldiae is shown in Fig. 2, where it has formed a thin biofilm on glass. Recently, a serial dilution method was used to isolate Halocatena pleomorpha SPP-AMP-1T from a man-made saltpan in India used for commercial salt production. The medium used was DSMZ 1184 supplemented with ampicillin (Verma et al. 2020). In addition to salinity, pH plays an important role in shaping halophilic archaeal community composition in specific hypersaline environments (Walsh et al. 2005). Halarchaeum members are moderately acidophilic, can grow at pH 4.0–7.2 (optimum, pH 4.5–5.5), and have been recovered from solar salt made from seawater in Australia, Indonesia, Japan, and the Philippines (Minegishi et al. 2010, 2013; Shimane et al. 2015; Yamauchi et al. 2013a, b). To date, there appear to be no reports of the cultivation of obligately alkaliphilic haloarchaea from marine solar salterns or lagoons. Almost without exception, the currently described obligately alkaliphilic haloarchaea have been isolated from athalassohaline environments (usually hypersaline soda lakes). Isolation and cultivation of haloarchaea from ancient, underground halite deposits has been reported by several independent groups (Gramain et al. 2011; Mormile et al. 2003; Stan-Lotter et al. 1999). For example, Halosimplex carlsbadense 2–9-1T was isolated from salt crystals taken from the 250-million-year-old Salado formation in southeastern New Mexico (Vreeland et al. 2002) and grew only on defined media supplemented with either a combination of acetate and glycerol, glycerol and pyruvate, or pyruvate alone.
Cultivation of halophilic archaea from athalassohaline environments
Athalassohaline environments have ionic compositions that differ significantly from that of sea water (Oren 2002). Most examples are based on inland salt lakes where the chemical composition has been largely formed or heavily influenced by leaching from the underlying and surrounding minerals. For example, the Dead Sea is a hypersaline terminal lake with high levels of Mg2+ (about 1.9 mol/L), severely limiting the growth of microorganisms (Oren and Gurevich 1995). The pH of these hypersaline environments can vary widely, with the Dead Sea being around 6.0 while those of alkaline soda lakes in Africa, China, India, and elsewhere reaching up to 11 or higher (Oren 2002; Sorokin et al. 2015; Vavourakis et al. 2016). Using traditional cultivation media, diverse halophilic archaea have been isolated from the Dead Sea, including Haloarcula marismortui (Oren et al. 1990), Halorubrum sodomense (Oren 1983), Halobaculum gomorrense (Oren et al. 1995), Haloferax volcanii (Mullakhanbhai and Larsen 1975) and Haloplanus natans (Bardavid et al. 2007). The haloalkaliphilic archaea make up a distinct physiological group within the class Halobacteria in that they are confined to hypersaline soda lakes and are obligately alkaliphilic (Mwatha and Grant 1993). Early described examples include Natronobacterium gen. nov. and Natronococcus gen. nov. (Tindall et al. 1984). Species of Natronobacterium were isolated on peptone-containing media adjusted to pH 9.5 while species belonging to Natronococcus were isolated using an enrichment method based on lake water supplemented with yeast extract (1%, w/v) and KNO3 (0.1% w/v). In 1999, the genus Natronorubrum was established to accommodate two novel species, Natronorubrum bangense and Natronorubrum tibetense, isolated from the Bange soda lake in Tibet, China, using a complex medium containing the following ingredients (g/L): casamino acids 7.5, yeast extract 10, trisodium citrate 3.0, MgSO4·7H2O 0.3, KCl 2.0, NaCl 200, Na2CO3 8.0, and trace amounts of Fe2+ and Mn2+ (Xu et al. 1999). For convenience, haloalkaliphiles isolated from sediments or near-bottom brines (e.g., Natronolimnobius and Natrarchaeobius) have been included in the next section along with soil haloarchaea.
Saline soils also carry haloarchaea and are athalassohaline due to the mixture of salts with various mineral components of the soil. The variety of soil compositions and structures provide enormous spatial heterogeneity for diverse halophilic archaea. Natribaculum breve and Natribaculum longum were isolated from saline soil of the Lop Nur region in Xinjiang, northwest China using modified S-G medium (pH 7.5) (Liu et al. 2015). Over the same period, Halovarius luteus, a close relative of Natribaculum (showing 98.44–99.08% nucleotide sequence identity of their 16S rRNA genes), was recovered on a standard peptone/YE plate (MGM; 23% salts) from a brine sample of Urmia lake, a hypersaline lake in north-west Iran (Dyall-Smith 2009; Mehrshad et al. 2015). While this is an inland lake, the predominance of Na+ and Cl− ions makes it close to thalassohaline in character. Using the same cultivation and isolation procedure, Halosiccatus urmianus was isolated from the same environment (Mehrshad et al. 2016). Haloparvum sedimenti was isolated from rock salt from the Jiangcheng Salt Mine, Yunnan province, China (Chen et al. 2016). Recovery of this species used conventional plating on MGM (18% salts) agar. Halanaeroarchaeum sulfurireducens was the first obligately anaerobic sulfur-respiring haloarchaeon and was isolated from mixed anaerobic sediments of the hypersaline chloride-sulfate lakes of Kulunda Steppe, Russia (Sorokin et al. 2016). It was recovered using anaerobic enrichment with acetate and elemental sulfur at 4 mol/L NaCl. Enrichment strategies (anaerobic or aerobic) were also applied to the isolation of the following halophilic archaea: Halalkaliarchaeum desulfuricum, which was isolated from anaerobic sediment sampled at the hypersaline alkaline Searles Lake in California (USA) (Sorokin et al. 2019b); Halococcoides cellulosivorans, recovered from the surface brines and sediments of hypersaline athalassic lakes in the Kulunda Steppe (Altai region, Russia) using amorphous cellulose as the growth substrate (Sorokin et al. 2019c); Halodesulfurarchaeum formicicum, isolated from mixed anaerobic sediments of hypersaline chloride−sulfate lakes in Kulunda Steppe (Altai, Russia) (Sorokin et al. 2017); Natrarchaeobaculum sulfurireducens, isolated from various soda lakes in Central Asia, Africa and USA (Sorokin et al. 2020); Natrarchaeobius chitinivorans and Natrarchaeobius haloalkaliphilus, both enriched and isolated from hypersaline alkaline lakes with chitin as their growth substrate (Sorokin et al. 2019a), and Natronobiforma cellulositropha enriched and isolated in pure culture from surface brines and sediments of hypersaline alkaline lakes in various geographical locations with various forms of insoluble cellulose as growth substrate (Sorokin et al. 2018). Halegenticoccus soli was isolated from a shore soil sample of Ebi lake in the Xinjiang region of China. The authors used a modified Gause medium containing starch as carbon source (Liu et al. 2019). Haloalkaliphiles that can grow by dissimilatory sulfur reduction (Natronolimnobius and Halalkaliarchaeum) have recently been reported (Sorokin et al. 2019b), as has the proposed transfer of species within the genus Natronolimnobius to two new genera, Natronolimnohabitans and Natrarchaeobaculum (Sorokin et al. 2020). Supplementary Table S2 shows the compound composition of each medium for culturing Halobacteria.
Media, problems and solutions regarding cultivation of halophilic archaea
In the early cultivation studies, enriched media containing casamino acids, yeast extract or peptone as carbon, nitrogen or energy sources were used for both neutrophilic (Sehgal and Gibbons 1960) and alkali-halophilic haloarchaea (Tindall et al. 1980). Later, an acidic enrichment medium was devised to enrich the moderately acidophilic members of the genus Halarchaeum (Minegishi et al. 2010). In addition to using these nutrient-rich media to isolate halophilic archaea from diverse hypersaline environments, low-nutrient media have been used to cultivate species unable to grow on the traditional enriched media (Vreeland et al. 2002). Compared with the nutrient-rich media, some halophilic archaea seem to grow better on low-nutrient media (Burns et al. 2004a, 2007). The formation of colonies of eutrophic halophilic archaea can be slowed down, while that of colonies of halophilic archaea that prefer low concentration and simple nutrition may be stimulated.
In addition to providing the correct nutrient concentration and the inclusion of utilizable substrates, the agar used for growth on solid media must be of high quality (e.g., agarose, Difco Bacto Agar) or washed to remove any residual growth inhibitors, as haloarchaea are particularly sensitive to contaminants (Dyall-Smith 2009). Most haloarchaea are sensitive to detergents, so laboratory glassware should be rinsed with distilled water after washing. The contamination of a commercial peptone preparation with bile acids has also been reported to lyse cultures of haloarchaea and should be avoided (Kamekura et al. 1988).
For cultivation studies of environmental samples, a variety of media and conditions should be used. Incubation time is an important factor, with some species able to form colonies on plates within a week while others can take at least eight weeks (Burns et al. 2004b). Haloquadratum walsbyi does not grow well at all on solid media (Fig. 2) and is best grown in liquid media (Burns et al. 2007).
Neutral-halophilic (pH7) and alkali-halophilic (pH9) media can be simultaneously prepared to cultivate neutral and alkaliphilic halophilic archaea that may exist in the same environmental sample (Tao et al. 2020). After one month or even half a year of long-term cultivation, the groups that prefer nutrient-rich and those like low-concentration or simple nutrition can have the opportunity to grow and form colonies.
Future perspectives
The past 10 years have witnessed a rapid expansion in cultivated members of the class Halobacteria, with 43 new genera added over that time, demonstrating the great diversity of this group. Environmental DNA-based high-throughput sequencing can now rapidly and comprehensively describe the diversity of halophilic archaea present in different hypersaline environments, and this has revealed a plethora of taxa yet to be cultivated (He et al. 2019). Strategies to culture these strains will need to be developed, including the formulation of novel media and testing a range of conditions. Some halophilic archaea prefer low-nutrient media containing acetate, pyruvate or similar compounds for growth, and the formulation of optimum cultivation media and conditions will maximize the recovery of diverse halophilic archaea from different hypersaline environments. The use of high-quality medium components such as washed or purified agar, detergent-free culture vessels, long incubation times and reduced nutrient concentration should all be considered when devising strategies for cultivation of haloarchaea. The recent successes with cultivating Candidatus Nanohaloarchaeota as co-cultures with Halobacteria opens up an exciting new hot spot in the field of halophilic archaea research. It is anticipated that improvements in cultivation technology (also termed ‘culturomics’ (Durán-Viseras et al. 2020; Greub 2012), such as the use of robotics and high-throughput microchambers (Nichols et al. 2010) will greatly increase the ability to cultivate diverse halophilic archaea from different hypersaline environments, so that many thousands of isolates can be recovered and analyzed simultaneously (Berdy et al. 2017).
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 31770005, 32070003) and the National Science and Technology Fundamental Resources Investigation Program of China (No. 2017FY100302, 2019FY100700).
Author contributions
HLC conceived the idea to this work and wrote the manuscript, and MDS improved the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and human rights statement
The article does not contain any studies related to human participants or animals.
Footnotes
SPECIAL TOPIC: Cultivation of uncultured microorganisms.
Edited by Chengchao Chen.
Contributor Information
Heng-Lin Cui, Email: cuihenglin@ujs.edu.cn.
Mike L. Dyall-Smith, Email: mike.dyallsmith@gmail.com
References
- Abdallah MB, Karray F, Kallel N, Armougom F, Mhiri N, Quéméneur M, Cayol J-L, Erauso G, Sayadi S. Abundance and diversity of prokaryotes in ephemeral hypersaline lake Chott El Jerid using Illumina Miseq sequencing, DGGE and qPCR assays. Extremophiles. 2018;22:811–823. doi: 10.1007/s00792-018-1040-9. [DOI] [PubMed] [Google Scholar]
- Akolkar AV, Durai D, Desai AJ. Halobacterium sp. SP1(1) as a starter culture for accelerating fish sauce fermentation. J Appl Microbiol. 2010;109:44–53. doi: 10.1111/j.1365-2672.2009.04626.x. [DOI] [PubMed] [Google Scholar]
- Amoozegar MA, Siroosi M, Atashgahi S, Smidt H, Ventosa A. Systematics of haloarchaea and biotechnological potential of their hydrolytic enzymes. Microbiology. 2017;163:623–645. doi: 10.1099/mic.0.000463. [DOI] [PubMed] [Google Scholar]
- Andrei A-Ş, Banciu H, Oren A. Living with salt: metabolic and phylogenetic diversity of archaea inhabiting saline ecosystems. FEMS Microbiol Lett. 2012;330:1–9. doi: 10.1111/j.1574-6968.2012.02526.x. [DOI] [PubMed] [Google Scholar]
- Antón J, Lucio M, Peña A, Cifuentes A, Brito-Echeverría J, Moritz F, Tziotis D, López C, Urdiain M, Schmitt-Kopplin P, Rosselló-Móra R. High metabolomic microdiversity within co-occurring isolates of the extremely halophilic bacterium Salinibacter ruber. PLoS ONE. 2013;8:e64701. doi: 10.1371/journal.pone.0064701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes A, Taborda M, Huber R, Moissl C, Nobre MF, da Costa MS. Halorhabdus tiamatea sp. nov., a non-pigmented, extremely halophilic archaeon from a deep-sea, hypersaline anoxic basin of the Red Sea, and emended description of the genus Halorhabdus. Int J Syst Evol Microbiol. 2008;58:215–220. doi: 10.1099/ijs.0.65316-0. [DOI] [PubMed] [Google Scholar]
- Aouad M, Taib N, Oudart A, Lecocq M, Gouy M, Brochier-Armanet C. Extreme halophilic archaea derive from two distinct methanogen Class II lineages. Mol Phylogenet Evol. 2018;127:46–54. doi: 10.1016/j.ympev.2018.04.011. [DOI] [PubMed] [Google Scholar]
- Bardavid RE, Mana L, Oren A. Haloplanus natans gen. nov., sp. nov., an extremely halophilic, gas-vacuolate archaeon isolated from Dead Sea-Red Sea water mixtures in experimental outdoor ponds. Int J Syst Evol Microbiol. 2007;57:780–783. doi: 10.1099/ijs.0.64648-0. [DOI] [PubMed] [Google Scholar]
- Berdy B, Spoering AL, Ling LL, Epstein SS. In situ cultivation of previously uncultivable microorganisms using the ichip. Nat Protoc. 2017;12:2232–2242. doi: 10.1038/nprot.2017.074. [DOI] [PubMed] [Google Scholar]
- Bolhuis H, te Poele EM, Rodríguez-Valera F. Isolation and cultivation of Walsby’s square archaeon. Environ Microbiol. 2004;6:1287–1291. doi: 10.1111/j.1462-2920.2004.00692.x. [DOI] [PubMed] [Google Scholar]
- Bowman JP, McCammon SA, Rea SM, McMeekin TA. The microbial composition of three limnologically disparate hypersaline Antarctic lakes. FEMS Microbiol Lett. 2000;183:81–88. doi: 10.1111/j.1574-6968.2000.tb08937.x. [DOI] [PubMed] [Google Scholar]
- Burns DG, Camakaris HM, Janssen PH, Dyall-Smith ML. Cultivation of Walsby’s square haloarchaeon. FEMS Microbiol Lett. 2004;238:469–473. doi: 10.1016/j.femsle.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Burns DG, Camakaris HM, Janssen PH, Dyall-Smith ML. Combined use of cultivation-dependent and cultivation-independent methods indicates that members of most haloarchaeal groups in an Australian crystallizer pond are cultivable. Appl Environ Microbiol. 2004;70:5258–5265. doi: 10.1128/AEM.70.9.5258-5265.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns DG, Janssen PH, Itoh T, Kamekura M, Li Z, Jensen G, Rodríguez-Valera F, Bolhuis H, Dyall-Smith ML. Haloquadratum walsbyi gen. nov., sp. nov., the square haloarchaeon of Walsby, isolated from saltern crystallizers in Australia and Spain. Int J Syst Evol Microbiol. 2007;57:387–392. doi: 10.1099/ijs.0.64690-0. [DOI] [PubMed] [Google Scholar]
- Chen S, Liu H-C, Zhou J, Xiang H. Haloparvum sedimenti gen. nov., sp. nov., a member of the family Haloferacaceae. Int J Syst Evol Microbiol. 2016;66:2327–2334. doi: 10.1099/ijsem.0.001033. [DOI] [PubMed] [Google Scholar]
- Clementino MM, Vieira RP, Cardoso AM, Nascimento APA, Silveira CB, Riva TC, Gonzalez ASM, Paranhos R, Albano RM, Ventosa A, Martins OB. Prokaryotic diversity in one of the largest hypersaline coastal lagoons in the world. Extremophiles. 2008;12:595–604. doi: 10.1007/s00792-008-0162-x. [DOI] [PubMed] [Google Scholar]
- Cui H-L, Sun F-F, Gao X, Dong Y, Xu X-W, Zhou Y-G, Liu H-C, Oren A, Zhou P-J. Haladaptatus litoreus sp. nov., an extremely halophilic archaeon from a marine solar saltern, and emended description of the genus Haladaptatus. Int J Syst Evol Microbiol. 2010;60:1085–1089. doi: 10.1099/ijs.0.015933-0. [DOI] [PubMed] [Google Scholar]
- Cui HL, Lü ZZ, Li Y, Zhou Y. Salinirussus salinus gen. nov., sp. nov., isolated from a marine solar saltern. Int J Syst Evol Microbiol. 2017;67:3622–3626. doi: 10.1099/ijsem.0.002182. [DOI] [PubMed] [Google Scholar]
- Demaere MZ, Williams TJ, Allen MA, Brown MV, Gibson JAE, Rich J, Lauro FM, Dyall-Smith M, Davenport KW, Woyke T, Kyrpides NC, Tringe SG, Cavicchioli R. High level of intergenera gene exchange shapes the evolution of haloarchaea in an isolated Antarctic lake. Proc Natl Acad Sci USA. 2013;110:16939–16944. doi: 10.1073/pnas.1307090110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durán-Viseras A, Andrei A-S, Vera-Gargallo B, Ghai R, Sánchez-Porro C, Ventosa A. Culturomics-based genomics sheds light on the ecology of the new haloarchaeal genus Halosegnis. Environ Microbiol. 2020 doi: 10.1111/1462-2920.15082. [DOI] [PubMed] [Google Scholar]
- Dyall-Smith ML (2009) The halohandbook: Protocols for haloarchaeal genetics. http://www.haloarchaea.com/resources/halohandbook/. Accessed on Aug 11 2020
- Farias ME, Rasuk MC, Gallagher KL, Contreras M, Kurth D, Fernandez AB, Poiré D, Novoa F, Visscher PT. Prokaryotic diversity and biogeochemical characteristics of benthic microbial ecosystems at La Brava, a hypersaline lake at Salar de Atacama, Chile. PLoS ONE. 2017;12:e0186867. doi: 10.1371/journal.pone.0186867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández AB, Vera-Gargallo B, Sánchez-Porro C, Ghai R, Papke RT, Rodriguez-Valera F, Ventosa A. Comparison of prokaryotic community structure from Mediterranean and Atlantic saltern concentrator ponds by a metagenomic approach. Front Microbiol. 2014;5:196. doi: 10.3389/fmicb.2014.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzmann PD, Stackebrandt E, Sanderson K, Volkman JK, Cameron DE, Stevenson PL, Mcmeekin TA, Burton HR. Halobacterium lacusprofundi sp. nov., a halophilic bacterium isolated from Deep Lake, Antarctica. Syst Appl Microbiol. 1988;11:20–27. [Google Scholar]
- Gramain A, Díaz GC, Demergasso C, Lowenstein TK, McGenity TJ. Archaeal diversity along a subterranean salt core from the Salar Grande (Chile) Environ Microbiol. 2011;13:2105–2121. doi: 10.1111/j.1462-2920.2011.02435.x. [DOI] [PubMed] [Google Scholar]
- Greub G. Culturomics: a new approach to study the human microbiome. Clin Microbiol Infect. 2012;18:1157–1159. doi: 10.1111/1469-0691.12032. [DOI] [PubMed] [Google Scholar]
- Gupta RS, Naushad S, Baker S. Phylogenomic analyses and molecular signatures for the class Halobacteria and its two major clades: a proposal for division of the class Halobacteria into an emended order Halobacteriales and two new orders, Haloferacales ord. nov. and Natrialbales ord. nov., containing the novel families Haloferacaceae fam. nov. and Natrialbaceae fam. nov. Int J Syst Evol Microbiol. 2015;65:1050–1069. doi: 10.1099/ijs.0.070136-0. [DOI] [PubMed] [Google Scholar]
- Gupta RS, Naushad S, Fabros R, Adeolu M. A phylogenomic reappraisal of family-level divisions within the class Halobacteria: proposal to divide the order Halobacteriales into the families Halobacteriaceae, Haloarculaceae fam. nov., and Halococcaceae fam. nov., and the order Haloferacales into the families, Haloferacaceae and Halorubraceae fam nov. Antonie Van Leeuwenhoek. 2016;109:565–587. doi: 10.1007/s10482-016-0660-2. [DOI] [PubMed] [Google Scholar]
- Hamm JN, Erdmann S, Eloe-Fadrosh EA, Angeloni A, Zhong L, Brownlee C, Williams TJ, Barton K, Carswell S, Smith MA, Brazendale S, Hancock AM, Allen MA, Raftery MJ, Cavicchioli R. Unexpected host dependency of Antarctic Nanohaloarchaeota. Proc Natl Acad Sci USA. 2019;116:14661–14670. doi: 10.1073/pnas.1905179116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Cui H-L. Salinibaculum litoreum gen. nov., sp. nov., isolated from salted brown alga Laminaria. Int J Syst Evol Microbiol. 2020;70:2879–2887. doi: 10.1099/ijsem.0.004114. [DOI] [PubMed] [Google Scholar]
- He S, Tan J, Hu W, Mo C. Diversity of archaea and its correlation with environmental factors in the Ebinur Lake wetland. Curr Microbiol. 2019;76:1417–1424. doi: 10.1007/s00284-019-01768-8. [DOI] [PubMed] [Google Scholar]
- Hou J, Zhao YJ, Zhu L, Cui HL. Salinirubellus salinus gen. nov., sp. nov., isolated from a marine solar saltern. Int J Syst Evol Microbiol. 2018;68:1874–1878. doi: 10.1099/ijsem.0.002757. [DOI] [PubMed] [Google Scholar]
- Kamekura M, Oesterhelt D, Wallace R, Anderson P, Kushner D. Lysis of halobacteria in bacto-peptone by bile acids. Appl Environ Microbiol. 1988;54:990–995. doi: 10.1128/aem.54.4.990-995.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Cono V, Messina E, Rohde M, Arcadi E, Ciordia S, Crisafi F, Denaro R, Ferrer M, Giuliano L, Golyshin PN, Golyshina OV, Hallsworth JE, Spada GL, Mena MC, Merkel AY, Shevchenko MA, Smedile F, Sorokin DY, Toshchakov SV, Yakimov MM. Symbiosis between nanohaloarchaeon and haloarchaeon is based on utilization of different polysaccharides. Proc Natl Acad Sci USA. 2020;117:20223–20234. doi: 10.1073/pnas.2007232117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litchfield CD. Potential for industrial products from the halophilic archaea. J Ind Microbiol Biotechnol. 2011;38:1635–1647. doi: 10.1007/s10295-011-1021-9. [DOI] [PubMed] [Google Scholar]
- Liu Q, Ren M, Zhang LL. Natribaculum breve gen. nov., sp. nov. and Natribaculum longum sp. nov., halophilic archaea isolated from saline soil. Int J Syst Evol Microbiol. 2015;65:604–608. doi: 10.1099/ijs.0.060541-0. [DOI] [PubMed] [Google Scholar]
- Liu B, Rao MPN, Yin X-Q, Li X, Salam N, Zhang Y, Alkhalifah DHM, Hozzein WN, Li W-J. Description of Halegenticoccus soli gen. nov., sp. nov., a halophilic archaeon isolated from a soil sample of Ebi lake. Extremophiles. 2019;23:521–528. doi: 10.1007/s00792-019-01104-9. [DOI] [PubMed] [Google Scholar]
- Mehrshad M, Amoozegar MA, Makhdoumi A, Rasooli M, Asadi B, Schumann P, Ventosa A. Halovarius luteus gen. nov., sp. nov., an extremely halophilic archaeon from a salt lake. Int J Syst Evol Microbiol. 2015;65:2420–2425. doi: 10.1099/ijs.0.000279. [DOI] [PubMed] [Google Scholar]
- Mehrshad M, Amoozegar MA, Makhdoumi A, Fazeli SAS, Farahani H, Asadi B, Schumann P, Ventosa A. Halosiccatus urmianus gen. nov., sp. nov., a haloarchaeon from a salt lake. Int J Syst Evol Microbiol. 2016;66:725–730. doi: 10.1099/ijsem.0.000781. [DOI] [PubMed] [Google Scholar]
- Minegishi H, Echigo A, Nagaoka S, Kamekura M, Usami R. Halarchaeum acidiphilum gen. nov., sp. nov., a moderately acidophilic haloarchaeon isolated from commercial solar salt. Int J Syst Evol Microbiol. 2010;60:2513–2516. doi: 10.1099/ijs.0.013722-0. [DOI] [PubMed] [Google Scholar]
- Minegishi H, Yamauchi Y, Echigo A, Shimane Y, Kamekura M, Itoh T, Ohkuma M, Usami R. Halarchaeum nitratireducens sp. nov., a moderately acidophilic haloarchaeon isolated from commercial sea salt. Int J Syst Evol Microbiol. 2013;63:4202–4206. doi: 10.1099/ijs.0.054668-0. [DOI] [PubMed] [Google Scholar]
- Mormile MR, Biesen MA, Gutierrez MC, Ventosa A, Pavlovich JB, Onstott TC, Fredrickson JK. Isolation of Halobacterium salinarum retrieved directly from halite brine inclusions. Environ Microbiol. 2003;5:1094–1102. doi: 10.1046/j.1462-2920.2003.00509.x. [DOI] [PubMed] [Google Scholar]
- Mou Y-Z, Qiu X-X, Zhao M-L, Cui H-L, Oh D, Dyall-Smith ML. Halohasta litorea gen. nov. sp. nov., and Halohasta litchfieldiae sp. nov., isolated from the Daliang aquaculture farm, China and from Deep Lake, Antarctica, respectively. Extremophiles. 2012;16:895–901. doi: 10.1007/s00792-012-0485-5. [DOI] [PubMed] [Google Scholar]
- Mullakhanbhai MF, Larsen H. Halobacterium volcanii spec. nov., a Dead Sea halobacterium with a moderate salt requirement. Arch Microbiol. 1975;104:207–214. doi: 10.1007/BF00447326. [DOI] [PubMed] [Google Scholar]
- Mwatha WE, Grant WD. Natronobacterium vacuolata sp. nov., a haloalkaliphilic archaeon isolated from Lake Magadi, Kenya. Int J Syst Bacteriol. 1993;43:401–404. [Google Scholar]
- Narasingarao P, Podell S, Ugalde JA, Brochier-Armanet C, Emerson JB, Brocks JJ, Heidelbert KB, Banfield JF, Allen EE. De novo metagenomic assembly reveals abundant novel major lineage of Archaea in hypersaline microbial communities. ISME J. 2012;6:81–93. doi: 10.1038/ismej.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols D, Cahoon N, Trakhtenberg EM, Pham L, Mehta A, Belanger A, Kanigan T, Lewis K, Epstein SS. Use of ichip for high-throughput in situ cultivation of "uncultivable" microbial species. Appl Environ Microbiol. 2010;76:2445–2450. doi: 10.1128/AEM.01754-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren A. Halobacterium sodomense sp. nov. a Dead Sea halobacterium with an extremely high magnesium requirement. Int J Syst Bacteriol. 1983;33:381–386. [Google Scholar]
- Oren A. Halophilic microorganisms and their environments. Dordrecht: Kluwer Scientific Publishers; 2002. [Google Scholar]
- Oren A. Salinibacter: an extremely halophilic bacterium with archaeal properties. FEMS Microbiol Lett. 2013;342:1–9. doi: 10.1111/1574-6968.12094. [DOI] [PubMed] [Google Scholar]
- Oren A. Taxonomy of halophilic Archaea: current status and future challenges. Extremophiles. 2014;18:825–834. doi: 10.1007/s00792-014-0654-9. [DOI] [PubMed] [Google Scholar]
- Oren A. The Family Halobacteriaceae. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F, editors. The prokaryotes: other major lineages of bacteria and the archaea. Berlin, Heidelberg: Springer; 2014. pp. 41–121. [Google Scholar]
- Oren A. Halophilic microbial communities and their environments. Curr Opin Biotechnol. 2015;33:119–124. doi: 10.1016/j.copbio.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Oren A, Gurevich P. Dynamics of a bloom of halophilic archaea in the Dead Sea. Hydrobiologia. 1995;315:149–158. [Google Scholar]
- Oren A, Ginzburg M, Ginzburg BZ, Hochstein LI, Volcani BE. Haloarcula marismortui (Volcani) sp. nov., nom. rev., an extremely halophilic bacterium from the Dead Sea. Int J Syst Bacteriol. 1990;40:209–210. doi: 10.1099/00207713-40-2-209. [DOI] [PubMed] [Google Scholar]
- Oren A, Gurevich P, Gemmell RT, Teske A. Halobaculum gomorrense gen. nov., sp. nov., a novel extremely halophilic archaeon from the Dead Sea. Int J Syst Bacteriol. 1995;45:747–754. doi: 10.1099/00207713-45-4-747. [DOI] [PubMed] [Google Scholar]
- Oren A, Arahal DR, Ventosa A. Emended descriptions of genera of the family Halobacteriaceae. Int J Syst Evol Microbiol. 2009;59:637–642. doi: 10.1099/ijs.0.008904-0. [DOI] [PubMed] [Google Scholar]
- Rappé MS, Connon SA, Vergin KL, Giovannoni SJ. Cultivation of the ubiquitous SAR11 marine bacterioplankton clade. Nature. 2002;418:630–633. doi: 10.1038/nature00917. [DOI] [PubMed] [Google Scholar]
- Rinke C, Schwientek P, Sczyrba A, Ivanova NN, Anderson IJ, Cheng J-F, Darling A, Malfatti S, Swan BK, Gies EA, Dodsworth JA, Hedlund BP, Tsiamis G, Sievert SM, Liu W-T, Eisen JA, Hallam SJ, Kyrpides NC, Stepanauskas R, Rubin EM, et al. Insights into the phylogeny and coding potential of microbial dark matter. Nature. 2013;499:431–437. doi: 10.1038/nature12352. [DOI] [PubMed] [Google Scholar]
- Sehgal SN, Gibbons NE. Effect of some metal ions on the growth of Halobacterium cutirubrum. Can J Microbiol. 1960;6:165–169. doi: 10.1139/m60-018. [DOI] [PubMed] [Google Scholar]
- Shimane Y, Minegishi H, Echigo A, Kamekura M, Itoh T, Ohkuma M, Tsubouchi T, Usui K, Maruyama T, Usami R, Hatada Y. Halarchaeum grantii sp. nov., a moderately acidophilic haloarchaeon isolated from a commercial salt sample. Int J Syst Evol Microbiol. 2015;65:3830–3835. doi: 10.1099/ijsem.0.000501. [DOI] [PubMed] [Google Scholar]
- Soliman GSH, Trüper HG. Halobacterium pharaonis sp. nov., a new, extremely haloalkaliphilic archaebacterium with low magnesium requirement. Zentralbl Bakteriol Hyg Abt I Orig. 1982;C3:318–329. [Google Scholar]
- Sorokin DY, Toshchakov SV, Kolganova TV, Kublanov IV. Halo(natrono)archaea isolated from hypersaline lakes utilize cellulose and chitin as growth substrates. Front Microbiol. 2015;6:942. doi: 10.3389/fmicb.2015.00942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin DY, Kublanov IV, Yakimov MM, Rijpstra WIC, Sinninghe JS, Damsté JSS. Halanaeroarchaeum sulfurireducens gen. nov., sp. nov., the first obligately anaerobic sulfur-respiring haloarchaeon, isolated from a hypersaline lake. Int J Syst Evol Microbiol. 2016;66:2377–2381. doi: 10.1099/ijsem.0.001041. [DOI] [PubMed] [Google Scholar]
- Sorokin DY, Messina E, Smedile F, Roman P, Damsté JSS, Ciordia S, Mena MC, Ferrer M, Golyshin PN, Kublanov IV, Samarov NI, Toshchakov SV, La Cono V, Yakimov MM. Discovery of anaerobic lithoheterotrophic haloarchaea, ubiquitous in hypersaline habitats. ISME J. 2017;11:1245–1260. doi: 10.1038/ismej.2016.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin DY, Khijniak TV, Kostrikina NA, Elcheninov AG, Toshchakov SV, Bale NJ, Damsté JSS, Kublanov IV. Natronobiforma cellulositropha gen. nov., sp. nov., a novel haloalkaliphilic member of the family Natrialbaceae (class Halobacteria) from hypersaline alkaline lakes. Syst Appl Microbiol. 2018;41:355–362. doi: 10.1016/j.syapm.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin DY, Elcheninov AG, Toshchakov SV, Bale NJ, Damsté JSS, Khijniak TV, Kublanov IV. Natrarchaeobius chitinivorans gen. nov., sp. nov., and Natrarchaeobius halalkaliphilus sp. nov., alkaliphilic, chitin-utilizing haloarchaea from hypersaline alkaline lakes. Syst Appl Microbiol. 2019;42:309–318. doi: 10.1016/j.syapm.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin DY, Yakimov M, Messina E, Merkel AY, Bale NJ, Damsté JSS. Natronolimnobius sulfurireducens sp. nov. and Halalkaliarchaeum desulfuricum gen. nov. sp. nov. the first sulfur-respiring alkaliphilic haloarchaea from hypersaline alkaline lakes. Int J Syst Evol Microbiol. 2019;69:2662–2673. doi: 10.1099/ijsem.0.003506. [DOI] [PubMed] [Google Scholar]
- Sorokin DY, Khijniak TV, Elcheninov AG, Toshchakov SV, Kostrikina NA, Bale NJ, Damsté JSS, Kublanov IV. Halococcoides cellulosivorans gen. nov., sp. nov., an extremely halophilic cellulose-utilizing haloarchaeon from hypersaline lakes. Int J Syst Evol Microbiol. 2019;69:1327–1335. doi: 10.1099/ijsem.0.003312. [DOI] [PubMed] [Google Scholar]
- Sorokin DY, Merkel AY, Messina E, Yakimov MM, Itoh T, Mesbah NM, Wiegel J, Oren A. Reclassification of the genus Natronolimnobius: proposal of two new genera, Natronolimnohabitans gen. nov. to accommodate Natronolimnobius innermongolicus and Natrarchaeobaculum gen. nov. to accommodate Natronolimnobius aegyptiacus and Natronolimnobius sulfurireducens. Int J Syst Evol Microbiol. 2020;70:3399–3405. doi: 10.1099/ijsem.0.004186. [DOI] [PubMed] [Google Scholar]
- Stan-Lotter H, McGenity TJ, Legat A, Denner EBM, Glaser K, Stetter KO, Wanner G. Very similar strains of Halococcus salifodinae are found in geographically separated Permo-Triassic salt deposits. Microbiology. 1999;145:3565–3574. doi: 10.1099/00221287-145-12-3565. [DOI] [PubMed] [Google Scholar]
- Tao CQ, Ding Y, Zhao YJ, Cui HL. Natronorubrum halophilum sp. nov. isolated from two inland salt lakes. J Microbiol. 2020;58:105–112. doi: 10.1007/s12275-020-9514-8. [DOI] [PubMed] [Google Scholar]
- Tindall BJ, Mills AA, Grant WD. An alkalophilic red halophilic bacterium with a low magnesium requirement from a Kenyan Soda Lake. Microbiology. 1980;116:257–260. [Google Scholar]
- Tindall BJ, Ross HNM, Grant WD. Natronobacterium gen. nov. and Natronococcus gen. nov., two new genera of haloalkaliphilic archaebacteria. Syst Appl Microbiol. 1984;5:41–57. [Google Scholar]
- Tschitschko B, Williams TJ, Allen MA, Zhong L, Raftery MJ, Cavicchioli R. Ecophysiological distinctions of haloarchaea from a hypersaline antarctic lake as determined by metaproteomics. Appl Environ Microbiol. 2016;82:3165–3173. doi: 10.1128/AEM.00473-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavourakis CD, Ghai R, Rodriguez-Valera F, Sorokin DY, Tringe SG, Hugenholtz P, Muyzer G. Metagenomic insights into the uncultured diversity and physiology of microbes in four hypersaline soda lake brines. Front Microbiol. 2016;7:211. doi: 10.3389/fmicb.2016.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventosa A, de la Haba RR, Sánchez-Porro C, Papke RT. Microbial diversity of hypersaline environments: a metagenomic approach. Curr Opin Microbiol. 2015;25:80–87. doi: 10.1016/j.mib.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Verma A, Pal Y, Kumar P, Krishnamurthi S. Halocatena pleomorpha gen. nov. sp. nov., an extremely halophilic archaeon of family Halobacteriaceae isolated from saltpan soil. Int J Syst Evol Microbiol. 2020;70:3693–3700. doi: 10.1099/ijsem.0.004222. [DOI] [PubMed] [Google Scholar]
- Vreeland RH, Straight S, Krammes J, Dougherty K, Rosenzweig WD, Kamekura M. Halosimplex carlsbadense gen. nov., sp. nov., a unique halophilic archaeon, with three 16S rRNA genes, that grows only in defined medium with glycerol and acetate or pyruvate. Extremophiles. 2002;6:445–452. doi: 10.1007/s00792-002-0278-3. [DOI] [PubMed] [Google Scholar]
- Waditee-Sirisattha R, Kageyama H, Takabe T. Halophilic microorganism resources and their applications in industrial and environmental biotechnology. AIMS Microbiol. 2016;2:42–54. [Google Scholar]
- Walsby AE. A square bacterium. Nature. 1980;283:69–71. [Google Scholar]
- Walsh DA, Papke RT, Doolittle WF. Archaeal diversity along a soil salinity gradient prone to disturbance. Environ Microbiol. 2005;7:1655–1666. doi: 10.1111/j.1462-2920.2005.00864.x. [DOI] [PubMed] [Google Scholar]
- Xu Y, Zhou P, Tian X. Characterization of two novel haloalkaliphilic archaea Natronorubrum bangense gen. nov., sp. nov. and Natronorubrum tibetense gen. nov., sp. nov. Int J Syst Bacteriol. 1999;49:261–266. doi: 10.1099/00207713-49-1-261. [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Minegishi H, Echigo A, Shimane Y, Kamekura M, Itoh T, Ohkuma M, Doukyu N, Inoue A, Usami R. Halarchaeum rubridurum sp. nov., a moderately acidophilic haloarchaeon isolated from commercial sea salt samples. Int J Syst Evol Microbiol. 2013;63:3143–3147. doi: 10.1099/ijs.0.049262-0. [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Minegishi H, Echigo A, Shimane Y, Shimoshige H, Kamekura M, Itoh T, Doukyu N, Inoue A, Usami R. Halarchaeum salinum sp. nov., a moderately acidophilic haloarchaeon isolated from commercial sea salt. Int J Syst Evol Microbiol. 2013;63:1138–1142. doi: 10.1099/ijs.0.044693-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.