Abstract
Pyronaridine, tilorone and quinacrine are cationic molecules that have in vitro activity against Ebola, SARS-CoV-2 and other viruses. All three molecules have also demonstrated in vivo activity against Ebola in mice, while pyronaridine showed in vivo efficacy against SARS-CoV-2 in mice. We have recently tested these molecules and other antivirals against human organic cation transporters (OCTs) and apical multidrug and toxin extruders (MATEs). Quinacrine was found to be an inhibitor of OCT2, while tilorone and pyronaridine were less potent, and these displayed variability depending on the substrate used. To assess whether any of these three molecules have other potential interactions with additional transporters, we have now screened them at 10 μM against various human efflux and uptake transporters including P-gp, OATP1B3, OAT1, OAT3, MRP1, MRP2, MRP3, BCRP, as well as confirmational testing against OCT1, OCT2, MATE1 and MATE2K. Interestingly, in this study tilorone appears to be a more potent inhibitor of OCT1 and OCT2 than pyronaridine or quinacrine. However, both pyronaridine and quinacrine appear to be more potent inhibitors of MATE1 and MATE2K. None of the three compounds inhibited MRP1, MRP2, MRP3, OAT1, OAT3, P-gp or OATP1B3. Similarly, we previously showed that tilorone and pyronaridine do not inhibit OATP1B1 and have confirmed that quinacrine behaves similarly. In total, these observations suggest that the three compounds only appear to interact with OCTs and MATEs to differing extents, suggesting they may be involved in fewer clinically relevant drug-transporter interactions involving pharmaceutical substrates of the other major transporters tested.
Introduction
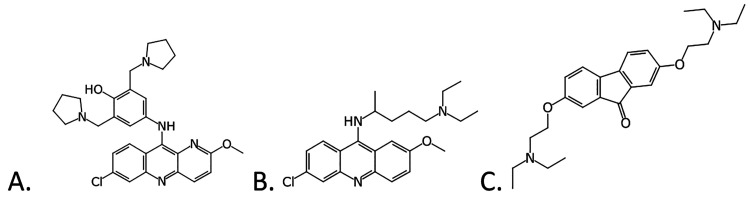
The search for broad spectrum antivirals may benefit from alternative approaches to high-throughput screening. A recent computational approach was taken with a published high-throughput screen of 868 molecules tested in a viral pseudotype entry assay and an Ebola virus (EBOV) replication assay.1,2 This Bayesian model enabled virtual screening of 2320 compounds and identified three active compounds: tilorone, quinacrine and pyronaridine3 (Figure 1). Recombinant, infectious EBOV encoding GFP was then used for testing the efficacy of compounds using HeLa cells and all had nanomolar EC50 values.3 These three molecules were later found to be active in the mouse model of EBOV infection and provided substantial insights into their mechanism of action.4−10 These three molecules were further assessed against 3 EBOV strains and 2 Marburg virus isolates.8 All block the entry stage of infection in a pseudotype assay for EBOV.8 The pyronaridine mouse EBOV efficacy study also provided preliminary insights into how pyronaridine may possess antiviral activity, as cytokine and chemokine panels suggested immunomodulatory actions during an infection.5 Orally administered pyronaridine was tested in guinea pigs alongside tilorone and favipiravir, where pyronaridine and favipiravir resulted in statistically significant 40% and 45% increase in survival rates versus control, respectively,8 a statistically significant increased survival compared to the control. Pursuit of the potential target of these computationally identified drugs pointed us to docking compounds in the EBOV glycoprotein structure and experimental validation. These docking predictions were then validated using microscale thermophoresis to calculate dissociation constants (Kd). The Kd values for pyronaridine (7.34 μM), tilorone (0.73 μM) and quinacrine (7.55 μM)10 were lower than for the positive control toremifene (24.83 μM), which is very similar to the literature value of 16 μM run under similar conditions.13 In summary, the accumulated in vitro and in vivo data gathered for tilorone, quinacrine and pyronaridine suggest that they share a common target or mechanism for the inhibition of EBOV,5−9,14 (i.e., these three molecules block viral entry and bind to the EBOV glycoprotein, although there may be other potential targets involved).
Figure 1.
Chemical structures of compounds tested in this study. (A) Pyronaridine tetraphosphate (pyronaridine), (B) quinacrine, (C) tilorone.
We and others15−17 have recently shown that these compounds also possess in vitro activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Tilorone and pyronaridine are in clinical trials, the latter in combination with artesunate. Using A549-ACE2 cells which support SARS-CoV2 growth to about 107 PFU/mL, pyronaridine showed SARS-CoV-2 inhibition demonstrating IC50 = 0.23 μM and a good selectivity index, and binding to SARS-CoV-2 spike receptor-binding domain (RBD) (Kd = 0.62 μM).15 We have recently expanded on our earlier in vitro characterization of pyronaridine against SARS-CoV-215 by testing against the papain-like protease (PLpro) that is essential for maturation of viral polyproteins, dysregulation of host inflammation, and antiviral immune responses in SARS-CoV-2, ultimately demonstrating in vivo efficacy against infection in a 3 day mouse model.19
The many membrane transporters that belong to the solute carrier and ATP-binding cassette superfamilies work to control the uptake, efflux and homeostasis of many nutrients that are physiologically relevant as well as xenobiotics that we are constantly exposed to. However, these transporters can also influence the pharmacokinetics of many drugs and, in addition, may be used as potential targets for prodrug approaches. These transporters are quite promiscuous in their selectivity, which increases the probability for potential interactions. Current guidance from the U.S. FDA recommends screening nine drug transporters: OAT1, OAT3, OCT2, OATP1B1, OATP1B3, P-glycoprotein (P-gp), BCRP, MATE1, and MATE2K for the evaluation of small molecule drug-drug interactions (DDIs).20,21 Drug transporters play a major role in determining absorption, distribution, metabolism, and distribution (ADME) and are key determinants of DDIs and adverse drug reactions (ADRs), some of which can be predicted early from targeted preclinical studies. Recent guidance recommendations have suggested various additional transporters with emerging clinical relevance for assessment.21,22 As the three molecules we have focused on are potential clinical candidates, we have considered their potential for interacting with transporters including organic cation transporters (the basolateral organic cation transporters, (OCT)1 and OCT2; and the apical multidrug and toxin extruders, (MATE)1 and MATE2-K) and have used computational and in vitro approaches to evaluate this activity.23 All three molecules previously showed substrate dependent inhibition for OCT2 (low μM to tens of μM using radiolabeled atenolol, metformin or MPP) and were modest inhibitors of MATE1, while quinacrine showed substrate dependent inhibition of MATE2K. We have also recently tested tilorone and pyronaridine against OATP1B124 at 20 μM and neither showed inhibition at this concentration. Tilorone, quinacrine and pyronaridine were also found in most cases to have IC50 values >1 mM for ENT1 and ENT2 inhibition.25
These initial results prompted us to evaluate additional transporters, including P-gp, OATP1B3, OAT1, OAT3, MRP1, MRP2, MRP3, and BCRP, for inhibition by these antivirals as well as testing the effect of quinacrine against OATP1B1 and confirming the inhibitory specificity of all three compounds against OCT1, OCT2, MATE1 and MATE2K.
Experimental Section
Compounds
Tilorone hydrochloride and quinacrine dihydrochloride dihydrate were purchased from Cayman Chemical Company (Ann Arbor, MI), and pyronaridine tetraphosphate was purchased from BOC Sciences (Shirley, NY).
Transporter Studies
Compounds were evaluated by Eurofins Discovery Services for transporter inhibition following the same basic protocol: Cells expressing a human recombinant transporter of interest were seeded into 96-well plates at 30,000 cells/well for 2 or 3 days prior to testing. Test compounds were prepared in assay buffer (HBSS-HEPES, pH 7.4) so that the final testing concentration was 10 μM (20 μM for OATP1B1 tilorone and pyronaridine, previously24) and 1% DMSO. Compounds are added to the cells and preincubated for 15 min at 37 °C. Substrate was then added to the plate and incubated for 20 min at 37 °C. Cells were then washed with cold assay buffer and read for fluorescence (fluorescent substrates noted in Table 1).
Table 1. Transporter Inhibition Assay Details.
| Transporter | Cell Line | Substrate | Background Determination Style | Reference Inhibitor |
|---|---|---|---|---|
| P-gp | MDR1-MDCKII | Calcein AM (2 μM) | 1 | Verapamil |
| BCRP | BCRP-CHO | Hoechst 33342 (5 μM) | 1 | Ko 143 |
| MRP1 | MRP1-HEK | Calcein AM (6 μM) | 1 | MK571 |
| MRP2 | MRP2-HEK | CDCF (20 μM) | 1 | MK571 |
| MRP3 | MRP3-HEK | CDCF (20 μM) | 1 | MK571 |
| OATP1B1 | OATP1B1-CHO | Fluorescein methotrexate (5 μM) | 2 | Rifampicin |
| OATP1B3 | OATP1B3-CHO | Fluorescein methotrexate (5 μM) | 2 | Rifampicin |
| OAT1 | OAT1-CHO | 6-carboxyfluorescein (10 μM) | 2 | Probenecid |
| OAT3 | OAT3-CHO | 6-carboxyfluorescein (10 μM) | 2 | Probenecid |
| OCT1 | OCT1-CHO | ASP+ (1 μM) | 2 | Verapamil |
| OCT2 | OCT2-CHO | ASP+ (5 μM) | 2 | Verapamil |
| MATE1 | MATE1-HEK | DAPI (1 μM) | 2 | Verapamil |
| MATE2K | MATE2K-HEK | DAPI (2 μM) | 2 | Verapamil |
Percent of control was calculated by subtracting the mean reading of the assay in the presence of the control inhibitor from the reading in the presence of the compound, divided by the difference between the vehicle control and the background, multiplied by 100%:
where “compound” refers to the fluorescence reading when the test compound is present, and “T1” refers to the mean reading when the test compound is absent. “Background” is determined one of two ways: (1) the mean reading at the highest effective concentration of the reference inhibitor or (2) the reading when both test compound and substrate are absent.
The transporter inhibition assay details are summarized in Table 1, and additional details are provided as follows: BCRP inhibition studies were performed as described previously26 with the following modifications: CHO cells expressing human recombinant BCRP (BCRP-CHO) were used, the reference inhibitor was Ko 143, test compounds were preincubated with cells at 37 °C for 15 min, and incubation time after addition of substrate was 20 min.
P-gp inhibition studies were performed as described previously27 with the following modifications: Calcein AM final concentration was 2 μM, verapamil was used as the reference inhibitor, test compounds were preincubated with cells at 37 °C for 15 min, and incubation time after addition of substrate was 20 min.
OCT1 inhibition studies were performed as described previously28 with the following modifications: CHO cells expressing human recombinant OCT1 (OCT1-CHO) were used, the fluorescent substrate ASP+ was used as at a concentration of 1 μM, the reference inhibitor was verapamil, test compounds were preincubated with cells at 37 °C for 15 min, and incubation time after addition of substrate was 20 min.
OCT2 inhibition studies were performed as described previously29 with the following modifications: CHO cells expressing human recombinant OCT2 (OCT2-CHO) were used, the reference inhibitor was verapamil, test compounds were preincubated with cells at 37 °C for 15 min, and incubation time after addition of substrate was 20 min.
MATE1 and MATE2K inhibition studies were performed as described previously30 with the following modifications: HEK cells stably expressing human recombinant MATE1 or MATE2K (MATE1-HEK, MATE2K-HEK) were used, and the substrate DAPI was used at 1 μM and 2 μM more MATE1 and MATE2K, respectively. The reference inhibitor was verapamil, test compounds were preincubated with cells at 37 °C for 15 min, and incubation time after addition of substrate was 20 min.
MRP1 inhibition studies were performed as described previously31 with the following modifications: HEK cells expressing human recombinant MRP1 (MRP1-HEK) were used, Calcein AM was used at a concentration of 6 μM, test compounds were preincubated with cells at 37 °C for 15 min, and incubation time after addition of substrate was 20 min.
MRP2 inhibition studies were performed as described previously32 with the following modifications: whole HEK cells expressing human recombinant MRP2 (MRP2-HEK) were used, the fluorescent substrate 5(6)-carboxy-2′,7′-dichlorofluorescein (CDCF) was used as at a concentration of 20 μM, test compounds were preincubated with cells at 37 °C for 15 min, and incubation time after addition of substrate was 20 min.
MRP3 inhibition studies were performed as described previously33 with the following modifications: HEK cells expressing human recombinant MRP3 (MRP3-HEK) were used, CDCF was used as at a concentration of 20 μM, test compounds were preincubated with cells at 37 °C for 15 min, and incubation time after addition of substrate was 20 min.
OAT1 inhibition studies were performed as described previously34 with the following modifications: the substrate 6-carboxyfluorescein was used at a final concentration of 10 μM, test compounds were preincubated with cells at 37 °C for 15 min, and incubation time after addition of substrate was 20 min.
OAT3 inhibition studies were performed as described previously35 with the following modifications: CHO cells expressing human recombinant OAT3 (OAT3-CHO) were used, test compounds were preincubated with cells at 37 °C for 15 min, and incubation time after addition of substrate was 20 min.
OATP1B1 and OATP1B3: inhibition studies were performed as described previously36 with the following modification: test compounds were preincubated with cells at 37 °C for 15 min.
Results and Discussion
Tilorone, pyronaridine and quinacrine (Figure 1) represent promising antiviral8,15−17 molecules as they have excellent in vitro ADME properties (e.g., good metabolic stability, Caco-2, human plasma protein binding 52–95.1%).5,6,14 Pyronaridine is the major component of the EU-approved antimalarial Pyramax, (which is a combination antimalarial therapy with artesunate) and has a long half-life.5,15 Additionally, our previous study described tilorone as a selective inhibitor of acetylcholinesterase included in a Eurofins SafetyScreen44 panel, which demonstrated that tilorone was inactive against the other 43 pharmacologically relevant targets in that screen.39 To date, assessment of the interactions of these three molecules with human drug transporters has been limited.23,24 Tilorone, pyronaridine and quinacrine were therefore tested at a single concentration of 10 μM for their potential to inhibit transport for 12 uptake and efflux transporters (Figure 2). We also tested quinacrine for activity against OATP1B1, to supplement our previous data on tilorone and pyronaridine.24
Figure 2.
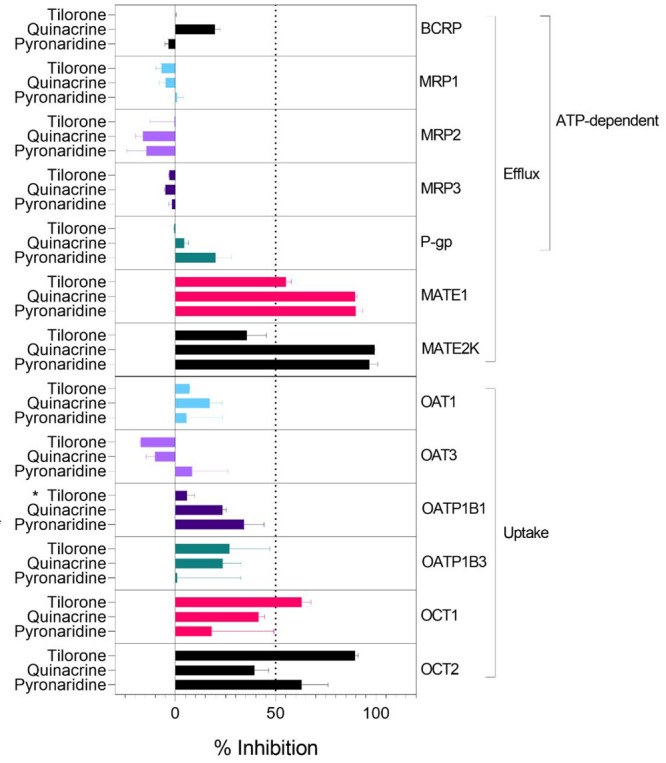
Inhibition of pharmaceutically relevant transporters described in the FDA guidance20 by the antivirals tilorone, quinacrine and pyronaridine. Compounds were tested at 10 μM for the ability to inhibit the transport of fluorescent substrates in cell-based assays. *Indicates these compounds were tested at 20 μM, and these results were previously published.24
Both pyronaridine and quinacrine appear to be more potent inhibitors of MATE1 and MATE2K than tilorone using DAPI as the probe substrate. Previously we showed that quinacrine was a slightly more potent inhibitor of MATE1 and OCT2 substrates.23 Tilorone and pyronaridine were more potent inhibitors of atenolol as a substrate for OCT1, whereas quinacrine was a more potent inhibitor of OCT1 when using MPP as the substrate.23 These differences may suggest subtle inhibitor-substrate dependencies.
None of these three compounds inhibited MRP1, MRP2, MRP3, OAT1, OAT3, P-gp or OATP1B3 to an appreciable extent. We had also previously shown OATP1B124 was not inhibited by tilorone or pyronaridine at 20 μM, and here we show that it is also not inhibited by quinacrine at 10 μM. We did not generate IC50 data for these transporters, as there was no potent inhibition with these molecules and the MATE and OCT1 study previously suggested μM inhibition at concentrations likely in excess of plasma concentrations of these compounds (100s nM inhibitors of viruses as described).
The results for P-gp are particularly surprising, as it has been previously reported that pyronaridine was a modulator (inhibitor) of P-gp in tumor cell lines as well as in mice with xenografts, enhancing the antitumor activity of doxorubicin,40 though the authors noted that this effect was possibly the result of pyronaridine binding intracellular doxorubicin, instead of direct interference of P-gp function by pyronaridine. Additionally, although it was shown that P-gp was overexpressed in these cell lines and xenografts, this study did not quantify or investigate the influence of any other efflux transporters present in these cells on the reversal of multidrug resistance or as possible targets of pyronaridine. Pyronaridine has been described to induce apoptosis via mitochondrial depolarization, caspase 3 activation, inhibition of cell cycle progression and by directly intercalating with cellular DNA at very high concentrations in one study.41 Another study by the same group showed that pyronaridine inhibits topoisomerase II in a dose-dependent manner, slows tumor growth and extends survival in mice xenografted with breast cancer cells.42 We have recently demonstrated that pyronaridine shows cytotoxicity in neuroblastoma (SH-SY5Y IC50 1.70 μM; SK-N-AS IC50 3.45 μM) and fibrosarcoma (HT-1080 IC50 = 4.23 μM) cell lines, while quinacrine was less potent (SH-SY5Y IC50 = 8.57 μM; SK-N-AS IC50 NA; HT-1080 IC50 = 29.1 μM).43
There is evidence to suggest that several viruses, including Marburgvirus, EBOV, and SARS-CoV-2,44−47 may infiltrate the immune-privileged site that harbors sperm production. Therefore, there is considerable interest in identifying compounds that cross the blood-testis barrier (BTB), as this compartment can act as a viral reservoir.48 The equilibrative nucleoside transporters (ENTs) are present at the BTB, where they can facilitate antiviral drug disposition to eliminate a sanctuary site for viruses detectable in semen.49,50 Predicting interactions with these transporters can be used to aid the development of novel compounds that can cross the BTB using the ENT1-ENT2 transepithelial transport pathway.25,51,52 These include novel antivirals and chemotherapeutics that are substrates for these widely expressed transporters. Most recently, we applied computational and experimental approaches to investigate interactions of the antiviral drugs remdesivir, tilorone, pyronaridine, quinacrine, hydroxychloroquine, molnupiravir and its active metabolite EIDD-1931 with ENT1 and ENT2.25 Remdesivir was the most potent inhibitor of ENT-mediated [3H] uridine uptake (ENT1 IC50 = 38.3 μM; ENT2 IC50 = 75.1 μM), while pyronaridine, tilorone and quinacrine had IC50 values >1 mM in most cases.25 It is therefore less likely these molecules may interfere with ENTs at their likely therapeutic concentrations. In recent work, the immunohistochemical staining of human testicular tissues showed OCTs had some staining on the basal membrane of Sertoli cells at the BTB, but OCTNs were much more pronounced.53 Others have also described the functional expression of OCTs and OCTNs in the BTB.54,55.23 As all three of these molecules had previously described substrate-dependent inhibition for hOCT2 (low μM to tens of μM), this would imply that if molecules were also substrates of OCTN1, they may be able to cross the BTB and have efficacy in the male genital tract. Therefore, the assessment of OCTN transport in future may shed light on whether these transporters could facilitate quinacrine, tilorone and pyronaridine crossing the BTB.
In summary, after evaluating transporter interactions as recommended in the FDA guidance,20 our results indicate that any human efflux and uptake transporter interactions with pyronaridine, tilorone and quinacrine are likely limited to OCT1 and MATE1 (and even then these may be above the likely circulating concentrations of the drugs). Therefore, their concomitant use with other drugs that are substrates or inhibitors for these transporters may require further detailed evaluation. The future testing of analogs of these molecules will also be valuable to further understand the structure activity relationships for OCT1 and MATE1 selectivity.
Glossary
Abbreviations Used
- CDCF
5(6)-carboxy-2′,7′-dichlorofluorescein
- BTB
blood-testis barrier
- DDI
drug-drug interaction
- EBOV
Ebola virus
- PLpro
papain-like protease
- RBD
receptor-binding domain
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
This work was supported by National Institutes of Health National Institute of General Medical Sciences grants: 1R41GM131433 to S.E. and S.H.W. and R44GM122196 to S.E.
The authors declare the following competing financial interest(s): S.E. is founder and owner of Collaborations Pharmaceuticals, Inc. P.A.V., T.R.L. and A.C.P. are employees. The company has patents filed for the antiviral and other activities of these compounds. All other authors have no conflicts of interest.
References
- Madrid P. B.; Chopra S.; Manger I. D.; Gilfillan L.; Keepers T. R.; Shurtleff A. C.; Green C. E.; Iyer L. V.; Dilks H. H.; Davey R. A.; Kolokoltsov A. A.; Carrion R. Jr; Patterson J. L.; Bavari S.; Panchal R. G.; Warren T. K.; Wells J. B.; Moos W. H.; Burke R. L.; Tanga M. J. A systematic screen of FDA-approved drugs for inhibitors of biological threat agents. PLoS One 2013, 8 (4), e60579 10.1371/journal.pone.0060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid P. B.; Panchal R. G.; Warren T. K.; Shurtleff A. C.; Endsley A. N.; Green C. E.; Kolokoltsov A. A.; Davey R. A.; Manger I. D.; Gilfillan L.; Bavari S.; Tanga M. J. Evaluation of Ebola Virus Inhibitors for Drug Repurposing. ACS Inf Dis 2015, 1, 317–326. 10.1021/acsinfecdis.5b00030. [DOI] [PubMed] [Google Scholar]
- Ekins S.; Freundlich J.; Clark A.; Anantpadma M.; Davey R.; Madrid P. Machine learning models identify molecules active against Ebola virus in vitro. F1000Res. 2015, 4, 1091. 10.12688/f1000research.7217.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft S. L.; Duparc S.; Arbe-Barnes S. J.; Craft J. C.; Shin C. S.; Fleckenstein L.; Borghini-Fuhrer I.; Rim H. J. Review of pyronaridine anti-malarial properties and product characteristics. Malar J. 2012, 11, 270. 10.1186/1475-2875-11-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane T. R.; Massey C.; Comer J. E.; Anantpadma M.; Freundlich J. S.; Davey R. A.; Madrid P. B.; Ekins S. Repurposing The Antimalarial Pyronaridine Tetraphosphate To Protect Against Ebola Virus Infection. PLoS Negl Trop Dis 2019, 13, e0007890 10.1371/journal.pntd.0007890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane T. R.; Comer J. E.; Freiberg A. N.; Madrid P. B.; Ekins S. Repurposing Quinacrine Against Ebola Virus Infection In vivo. Antimicrob. Agents Chemother. 2019, 63, e01142 10.1128/AAC.01142-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantpadma M.; Lane T.; Zorn K. M.; Lingerfelt M. A.; Clark A. M.; Freundlich J. S.; Davey R. A.; Madrid P.; Ekins S. Ebola Virus Bayesian Machine Learning Models Enable New In Vitro Leads. ACS Omega 2019, 4, 2353–2361. 10.1021/acsomega.8b02948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane T. R.; Massey C.; Comer J. E.; Freiberg A. N.; Zhou H.; Dyall J.; Holbrook M. R.; Anantpadma M.; Davey R. A.; Madrid P. B.; Ekins S. Pyronaridine Tetraphosphate Efficacy Against Ebola Virus Infection in Guinea Pig. Antiviral Res. 2020, 181, 104863. 10.1016/j.antiviral.2020.104863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane T. R.; Dyall J.; Mercer L.; Goodin C.; Foil D. H.; Zhou H.; Postnikova E.; Liang J. Y.; Holbrook M. R.; Madrid P. B.; Ekins S. Repurposing Pyramax®, quinacrine and tilorone as treatments for Ebola virus disease. Antiviral Research 2020, 182, 104908. 10.1016/j.antiviral.2020.104908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane T. R.; Ekins S. Towards the Target: Tilorone, Quinacrine and Pyronaridine Bind to Ebola Virus Glycoprotein. ACS Med. Chem. Lett. 2020, 11, 1653–1658. 10.1021/acsmedchemlett.0c00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.; Ren J.; Harlos K.; Jones D. M.; Zeltina A.; Bowden T. A.; Padilla-Parra S.; Fry E. E.; Stuart D. I. Toremifene interacts with and destabilizes the Ebola virus glycoprotein. Nature 2016, 535 (7610), 169–172. 10.1038/nature18615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekins S.; Lingerfelt M. A.; Comer J. E.; Freiberg A. N.; Mirsalis J. C.; O’Loughlin K.; Harutyunyan A.; McFarlane C.; Green C. E.; Madrid P. B. Efficacy of Tilorone Dihydrochloride against Ebola Virus Infection. Antimicrob. Agents Chemother. 2018, 62 (2), e01711 10.1128/AAC.01711-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl A. C.; Fritch E. J.; Lane T. R.; Tse L. V.; Yount B. L.; Sacramento C. Q.; Fintelman-Rodrigues N.; Tavella T. A.; Maranhao Costa F. T.; Weston S.; Logue J.; Frieman M.; Premkumar L.; Pearce K. H.; Hurst B. L.; Andrade C. H.; Levi J. A.; Johnson N. J.; Kisthardt S. C.; Scholle F.; Souza T. M. L.; Moorman N. J.; Baric R. S.; Madrid P. B.; Ekins S. Repurposing the Ebola and Marburg Virus Inhibitors Tilorone, Quinacrine, and Pyronaridine: In Vitro Activity against SARS-CoV-2 and Potential Mechanisms. ACS Omega 2021, 6 (11), 7454–7468. 10.1021/acsomega.0c05996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S.; Ko M.; Lee J.; Choi I.; Byun S. Y.; Park S.; Shum D.; Kim S. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob. Agents Chemother. 2020, 64, e00819 10.1128/AAC.00819-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae J.-Y.; Lee G. E.; Park H.; Cho J.; Kim Y.-E.; Lee J.-Y.; Ju C.; Kim W.-K.; Kim J. I.; Park M.-S.. Pyronaridine and artesunate are potential antiviral drugs against COVID-19 and influenza. bioRxiv, ver. 1, 2020. https://www.biorxiv.org/content/10.1101/2020.07.28.225102v1 (accessed March 22 2023).
- Puhl A. C.; Gomes G. F.; Damasceno S.; Godoy A. S.; Noske G. D.; Nakamura A. M.; Gawriljuk V. O.; Fernandes R. S.; Monakhova N.; Riabova O.; Lane T. R.; Makarov V.; Veras F. P.; Batah S. S.; Fabro A. T.; Oliva G.; Cunha F. Q.; Alves-Filho J. C.; Cunha T. M.; Ekins S. Pyronaridine Protects against SARS-CoV-2 Infection in Mouse. ACS Infect Dis 2022, 8 (6), 1147–1160. 10.1021/acsinfecdis.2c00091. [DOI] [PubMed] [Google Scholar]
- FDA . Clinical Drug Interaction Studies — Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions Guidance for Industry, 2020.
- Giacomini K. M.; Huang S. M.; Tweedie D. J.; Benet L. Z.; Brouwer K. L.; Chu X.; Dahlin A.; Evers R.; Fischer V.; Hillgren K. M.; Hoffmaster K. A.; Ishikawa T.; Keppler D.; Kim R. B.; Lee C. A.; Niemi M.; Polli J. W.; Sugiyama Y.; Swaan P. W.; Ware J. A.; Wright S. H.; Yee S. W.; Zamek-Gliszczynski M. J.; Zhang L.. Membrane transporters in drug development. Nat. Rev. Drug Discov 2010, 9 ( (3), ), 215–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamek-Gliszczynski M. J.; Taub M. E.; Chothe P. P.; Chu X.; Giacomini K. M.; Kim R. B.; Ray A. S.; Stocker S. L.; Unadkat J. D.; Wittwer M. B.; Xia C.; Yee S. W.; Zhang L.; Zhang Y. International Transporter, C. Transporters in Drug Development: 2018 ITC Recommendations for Transporters of Emerging Clinical Importance. Clin Pharmacol Ther 2018, 104 (5), 890–899. 10.1002/cpt.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez Guerrero L. J.; Zhang X.; Zorn K. M.; Ekins S.; Wright S. H. Cationic Compounds with SARS-CoV-2 Antiviral Activity and their Interaction with OCT/MATE Secretory Transporters. J. Pharmacol Exp Ther 2021, 379, 96–107. 10.1124/jpet.121.000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane T. R.; Urbina F.; Zhang X.; Fye M.; Gerlach J.; Wright S. H.; Ekins S. Machine Learning Models Identify New Inhibitors for Human OATP1B1. Mol. Pharmaceutics 2022, 19 (11), 4320–4332. 10.1021/acs.molpharmaceut.2c00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. R.; McGrath M. E.; Zorn K. M.; Ekins S.; Wright S. H.; Cherrington N. J. Remdesivir and EIDD-1931 Interact with Human Equilibrative Nucleoside Transporters 1 and 2: Implications for Reaching SARS-CoV-2 Viral Sanctuary Sites. Mol. Pharmacol. 2021, 100, 548. 10.1124/molpharm.121.000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paturi D. K.; Kwatra D.; Ananthula H. K.; Pal D.; Mitra A. K. Identification and functional characterization of breast cancer resistance protein in human bronchial epithelial cells (Calu-3). Int. J. Pharm. 2010, 384 (1–2), 32–8. 10.1016/j.ijpharm.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polli J. W.; Wring S. A.; Humphreys J. E.; Huang L.; Morgan J. B.; Webster L. O.; Serabjit-Singh C. S. Rational Use of in Vitro P-glycoprotein Assays in Drug Discovery. Journal of Pharmacology and Experimental Therapeutics 2001, 299 (2), 620. [PubMed] [Google Scholar]
- Zhang L.; Dresser M. J.; Gray A. T.; Yost S. C.; Terashita S.; Giacomini K. M. Cloning and functional expression of a human liver organic cation transporter. Molecular pharmacology 1997, 51 (6), 913–921. 10.1124/mol.51.6.913. [DOI] [PubMed] [Google Scholar]
- Kido Y.; Matsson P.; Giacomini K. M. Profiling of a prescription drug library for potential renal drug-drug interactions mediated by the organic cation transporter 2. J. Med. Chem. 2011, 54 (13), 4548–58. 10.1021/jm2001629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasujima T.; Ohta K.-y.; Inoue K.; Ishimaru M.; Yuasa H. Evaluation of 4′,6-Diamidino-2-phenylindole as a Fluorescent Probe Substrate for Rapid Assays of the Functionality of Human Multidrug and Toxin Extrusion Proteins. Drug Metab. Dispos. 2010, 38 (4), 715–721. 10.1124/dmd.109.030221. [DOI] [PubMed] [Google Scholar]
- Hamilton K. O.; Topp E.; Makagiansar I.; Siahaan T.; Yazdanian M.; Audus K. L. Multidrug resistance-associated protein-1 functional activity in Calu-3 cells. J. Pharmacol Exp Ther 2001, 298 (3), 1199–205. [PubMed] [Google Scholar]
- Matsson P.; Pedersen J. M.; Norinder U.; Bergström C. A. S.; Artursson P. Identification of novel specific and general inhibitors of the three major human ATP-binding cassette transporters P-gp, BCRP and MRP2 among registered drugs. Pharm. Res. 2009, 26 (8), 1816–1831. 10.1007/s11095-009-9896-0. [DOI] [PubMed] [Google Scholar]
- Weiss J.; Theile D.; Ketabi-Kiyanvash N.; Lindenmaier H.; Haefeli W. E. Inhibition of MRP1/ABCC1, MRP2/ABCC2, and MRP3/ABCC3 by nucleoside, nucleotide, and non-nucleoside reverse transcriptase inhibitors. Drug metabolism and disposition: the biological fate of chemicals 2007, 35 (3), 340–344. 10.1124/dmd.106.012765. [DOI] [PubMed] [Google Scholar]
- Cihlar T.; Ho E. S. Fluorescence-based assay for the interaction of small molecules with the human renal organic anion transporter 1. Anal. Biochem. 2000, 283 (1), 49–55. 10.1006/abio.2000.4633. [DOI] [PubMed] [Google Scholar]
- Rizwan A. N.Dissertation zur Erlangung des Doktorgrades der Mathematisch-Naturwissenschaftlichen Fakultäte, Georg-August-Universität zu Göttingen, 2006. [Google Scholar]
- Gui C.; Obaidat A.; Chaguturu R.; Hagenbuch B. Development of a cell-based high-throughput assay to screen for inhibitors of organic anion transporting polypeptides 1B1 and 1B3. Curr. Chem. Genomics 2010, 4, 1–8. 10.2174/1875397301004010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignaux P. A.; Minerali E.; Lane T. R.; Foil D. H.; Madrid P. B.; Puhl A. C.; Ekins S. The Antiviral Drug Tilorone Is a Potent and Selective Inhibitor of Acetylcholinesterase. Chem. Res. Toxicol. 2021, 34 (5), 1296–1307. 10.1021/acs.chemrestox.0c00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J.; Wang S.; Liu G.; Peng H.; Wang J.; Zhu Z.; Yang C. Pyronaridine, a novel modulator of P-glycoprotein-mediated multidrug resistance in tumor cells in vitro and in vivo. Biochem. Biophys. Res. Commun. 2004, 319 (4), 1124–31. 10.1016/j.bbrc.2004.05.099. [DOI] [PubMed] [Google Scholar]
- Villanueva P. J.; Martinez A.; Baca S. T.; DeJesus R. E.; Larragoity M.; Contreras L.; Gutierrez D. A.; Varela-Ramirez A.; Aguilera R. J. Pyronaridine exerts potent cytotoxicity on human breast and hematological cancer cells through induction of apoptosis. PLoS One 2018, 13 (11), e0206467 10.1371/journal.pone.0206467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva P. J.; Gutierrez D. A.; Contreras L.; Parra K.; Segura-Cabrera A.; Varela-Ramirez A.; Aguilera R. J. The Antimalarial Drug Pyronaridine Inhibits Topoisomerase II in Breast Cancer Cells and Hinders Tumor Progression In Vivo. Clin Cancer Drugs 2021, 8 (1), 50–56. 10.2174/2212697X08666210219101023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank L.; Puhl A. C.; Havener T. M.; Anderson E.; Foil D. H.; Zorn K. M.; Monakhova N.; Riabova O.; Hickey A. J.; Makarov V.; Ekins S. Multiple approaches to repurposing drugs for neuroblastoma. Bioorg. Med. Chem. 2022, 73, 117043. 10.1016/j.bmc.2022.117043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X.; Blancett C. D.; Koistinen K. A.; Schellhase C. W.; Bearss J. J.; Radoshitzky S. R.; Honnold S. P.; Chance T. B.; Warren T. K.; Froude J. W.; Cashman K. A.; Dye J. M.; Bavari S.; Palacios G.; Kuhn J. H.; Sun M. G. Identification and pathological characterization of persistent asymptomatic Ebola virus infection in rhesus monkeys. Nat. Microbiol 2017, 2, 17113. 10.1038/nmicrobiol.2017.113. [DOI] [PubMed] [Google Scholar]
- Coffin K. M.; Liu J.; Warren T. K.; Blancett C. D.; Kuehl K. A.; Nichols D. K.; Bearss J. J.; Schellhase C. W.; Retterer C. J.; Weidner J. M.; Radoshitzky S. R.; Brannan J. M.; Cardile A. P.; Dye J. M.; Palacios G.; Sun M. G.; Kuhn J. H.; Bavari S.; Zeng X. Persistent Marburg Virus Infection in the Testes of Nonhuman Primate Survivors. Cell Host Microbe 2018, 24 (3), 405–416. 10.1016/j.chom.2018.08.003. [DOI] [PubMed] [Google Scholar]
- Perry D. L.; Huzella L. M.; Bernbaum J. G.; Holbrook M. R.; Jahrling P. B.; Hagen K. R.; Schnell M. J.; Johnson R. F. Ebola Virus Localization in the Macaque Reproductive Tract during Acute Ebola Virus Disease. Am. J. Pathol. 2018, 188 (3), 550–558. 10.1016/j.ajpath.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos R. K.; Camargos V. N.; Azar S. R.; Haines C. A.; Eyzaguirre E. J.; Rossi S. L. SARS-CoV-2 Infects Hamster Testes. Microorganisms 2021, 9 (6), 1318. 10.3390/microorganisms9061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessons from reservoirs. Nat. Med. 2017, 23 ( (8), ), 899. 10.1038/nm.4387. [DOI] [PubMed] [Google Scholar]
- Le Tortorec A.; Dejucq-Rainsford N. HIV infection of the male genital tract - consequences for sexual transmission and reproduction. Int. J. Andrology 2010, 33 (1), e98–e108. 10.1111/j.1365-2605.2009.00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durmus S.; Hendrikx J. J. M. A.; Schinkel A. H. Chapter one- Apical ABC Transporters and Cancer Chemotherapeutic Drug Disposition. Adv. Cancer Res. 2015, 125, 1–41. 10.1016/bs.acr.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Miller S. R.; Lane T. R.; Zorn K. M.; Ekins S.; Wright S. H.; Cherrington N. J. Multiple Computational Approaches for Predicting Drug Interactions with Human Equilibrative Nucleoside Transporter 1. Drug Metab. Dispos. 2021, 49 (7), 479–489. 10.1124/dmd.121.000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. R.; Zhang X.; Hau R. K.; Jilek J. L.; Jennings E. Q.; Galligan J. J.; Foil D. H.; Zorn K. M.; Ekins S.; Wright S. H.; Cherrington N. J. Predicting Drug Interactions with Human Equilibrative Nucleoside Transporters 1 and 2 Using Functional Knockout Cell Lines and Bayesian Modeling. Mol. Pharmacol. 2021, 99 (2), 147–162. 10.1124/molpharm.120.000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hau R. K.; Klein R. R.; Wright S. H.; Cherrington N. J. Localization of Xenobiotic Transporters Expressed at the Human Blood-Testis Barrier. Drug Metab. Dispos. 2022, 50 (6), 770–780. 10.1124/dmd.121.000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T.; Goto A.; Kobayashi D.; Tamai I. Transport of organic cations across the blood-testis barrier. Mol. Pharmaceutics 2007, 4 (4), 600–7. 10.1021/mp070023l. [DOI] [PubMed] [Google Scholar]
- Kobayashi D.; Goto A.; Maeda T.; Nezu J.; Tsuji A.; Tamai I. OCTN2-mediated transport of carnitine in isolated Sertoli cells. Reproduction 2005, 129 (6), 729–36. 10.1530/rep.1.00507. [DOI] [PubMed] [Google Scholar]