Abstract
Surfactants are a group of amphiphilic molecules (i.e., having both hydrophobic and hydrophilic domains) that are a vital part of nearly every contemporary industrial process such as in agriculture, medicine, personal care, food, and petroleum. In general surfactants can be derived from (i) petroleum-based sources or (ii) microbial/plant origins. Petroleum-based surfactants are obvious results from petroleum products, which lead to petroleum pollution and thus pose severe problems to the environment leading to various ecological damages. Thus, newer techniques have been suggested for deriving surfactant molecules and maintaining environmental sustainability. Biosurfactants are surfactants of microbial or plant origins and offer much added advantages such as high biodegradability, lesser toxicity, ease of raw material availability, and easy applicability. Thus, they are also termed “green surfactants”. In this regard, this review focused on the advantages of biosurfactants over the synthetic surfactants produced from petroleum-based products along with their potential applications in different industries. We also provided their market aspects and future directions that can be considered with selections of biosurfactants. This would open up new avenues for surfactant research by overcoming the existing bottlenecks in this field.
1. Introduction
The term “surfactant” comes from “surface active agents”, which are molecules that adsorb on the water–surface interface and reduce water’s surface tension to enhance the cleaning of surfaces.1,2 They are also known as amphiphiles because they have polar heads, also known as hydrophilic heads, that have an attraction for polar solvents, and nonpolar tails, also known as hydrophobic tails.2,3 The molecular structures of these molecules help reduce the cohesive forces between water molecules, resulting in the lowering of surface tension.1−5 They possess other qualities that allow them to be used in applications other than lowering surface tension6,7 such as emulsifiers,8−11 foaming agents,12−16 corrosion inhibitors,17−21 and antistatic agents.22−25 Surfactants have been used in practically every industry because of their physicochemical characteristics.25 These include paints,26 inks,27,28 coatings,29−31 adhesives, paper and pulp, petroleum and oil, plastics, resins, textiles and fibers, detergents, agricultural, food, cosmetics, pharmaceutical, and various industrial applications.32 Originally, surfactants were only created from renewable resources such as plant oils or animal fat. The majority of surfactants in use today are either only partly or slowly biodegradable, which results in environmental damage and toxicological problems.33,34 For example, eutrophication is one of the direct effects on the environment due to the use of synthetic surfactants that include phosphates.35−38 Cleaning products and detergents with phosphate surfactants are the principal sources of phosphate in aquatic systems.39 As a result, there is a high demand for biodegradable products developed through green chemistry to prevent environmental pollution (the elimination of the use or generation of hazardous substances in the design, manufacture, and application of chemical products worldwide). The growing legal and societal pressures for these substances to be biodegradable and produced in a sustainable manner have fueled research into new degradable surfactants of synthetic or biological origin.40 Also, diminishing petrochemical stocks and environmental degradation have created a drive toward the identification of novel renewable bioresources for efficient surfactant production. Next-generation renewable surfactant inventions must be produced effectively, come from reliable and sustainable feedstocks, and have physicochemical characteristics that are on par with or better than those of petrochemical surfactants.41,42 All of these requirements must be met while achieving a low manufacturing cost. The green surfactants currently in use come from two different sources: biosurfactants, which are produced by bacteria as they increase the availability of hydrocarbons, and oleo surfactants, which are sourced from feedstocks such oils and fats of plant and animal origins.43,44 Oleochemical-based surfactants are more biocompatible and easily biodegradable than petroleum-based ones.45 Fatty acids, fatty alcohols, fatty amines, and glycerol are the main oleochemical feedstocks. The development of modern biotechnology has made it feasible to generate many forms of biosurfactants from microbes and vegetable oils that are biodegradable, have low toxicity, and behave similarly to synthetic surfactants.46
There are many negative environmental consequences of using synthetic surfactants, including their high levels of toxicity and poor biodegradability. These materials have a negative impact on wastewater treatment as well as aquatic microbial populations, fish and other aquatic life, and plant photochemical energy conversion efficiency.46 With over 15 million tons of surfactants used worldwide each year and an estimated 60% of them ending up in the aquatic environment, it is urgently necessary to find substitutes that have fewer environmental impacts.47−49 The origins and natural uses of biosurfactants are discussed, along with their benefits over synthetic alternatives, such as their low toxicity and biodegradability. This review describes the current methods of surfactant production, the future trends, cleaner and sustainable production methods, and an extensive comparison of performance parameters between green and petroleum-based surfactants.
2. Classification of Surfactants
For better clarity and ease of understanding, surfactants can be classified on the basis of their physicochemical properties, their sources, and/or the hydrophilic and hydrophobic moieties present in the molecule.
2.1. Classification of Surfactants According to the Charges of Their Headgroups
2.1.1. Anionic Surfactants
Anionic surfactants are surfactants that have anionic functionalities at their heads. These anionic groups include phosphate, sulfate, sulfonate, and carboxylate. They tend to give negatively charged surfactant ions when dissolved in water.50−52 Such surfactants are finding applicability in shampoos, laundering, dishwashing formulations, etc.50−52 One class of common soap surfactants is sodium stearates (comprise >50% of global usage).53 Some examples of anionic surfactants are dioctyl sodium sulfosuccinate (DOSS; used as wetting agent in coatings, toothpaste, etc.), linear alkylbenzene sulfonates (LASs; used in laundry detergents, dishwasher detergents, etc.), and sodium lauryl ether sulfate (SLES; used in shampoos, bath products, etc.). Subtypes include (i) soaps (CnH2n+1COO–X), (ii) LASs (CnH2n+1SO3–X), (iii) alkyl ether sulfates (AESs; CnH2+1–(OCH2CH2)n–OSO3X), and (iv) alcoholic sulfates (R–O–SO3X). It has been seen that anionic surfactants exhibit relatively nontoxic characteristics.54
2.1.2. Cationic Surfactants
Cationic surfactants (CSs) have positively charged heads and usually find application as good emulsifiers. These surfactants have also been reported for numbers of bactericidal and topical antiseptic properties in various previous studies.55 Thereby, they are greatly used in the manufacturing of bathroom and hand sanitizers. Cationic surfactants are often used as fabric softeners, hydrophobic agents, hydrotropes, etc.55 The cationic head functionality of a CS tends to disrupt bacterial cell membranes, and therefore CSs are used as antibacterial agents.56,57 However, the use of CSs should be within specific limits given by regulatory agencies. Examples of CSs are methylbenzethonium and benzalkonium QA (quaternary ammonium). These CSs include quaternary ammonium compounds [QACs; R1R2R3R4N+X], esters of QACs [RCO–O–CH2CH2–N(CH3)2], and derivatives of pyridines and imidazolines [NC5H5]+·R1—C=N—(CH2)2—N—R2+].
2.1.3. Zwitterionic Surfactants
Zwitterionic surfactants are also known as amphoteric surfactants and contain both cationic and anionic functionalities in the same molecule.58−60 The anionic part can be varied, wherein the cationic functionality has primary, secondary, or tertiary amines or QACs. The behavior of the cationic or anionic nature of such a surfactant is usually prone to pH changes. Groups of such surfactants include carboxylic acid/quaternary ammonium (e.g., lauryl-N,N-(dimethylammonio)butyrate), sulfuric acid/quaternary ammonium (e.g., surfactin), phosphoric acid/quaternary ammonium (e.g., miltefosine), phospholipids, and miscellaneous (e.g., lauryldimethylamine N-oxide).
2.1.4. Nonionic Surfactants
Nonionic surfactants are surfactants which do not undergo ionization in aqueous solutions. As they do not undergo ionization in aqueous solution, they tend to retain higher stability and are less affected by or prone to strong electrolytes.61−64 They have been known for their excellent compatibility with other surfactants and have solubilities in both aqueous and organic solvents.64 The inclusion of a polyethylene glycol chain, obtained through polycondensation of ethylene oxide, makes a high proportion of these nonionic surfactants hydrophilic. As the strength of hydrogen bonds weakens with rising temperature, so do the water solubilities of nonionic surfactants. Nonionic surfactants are likely the most often employed in drug delivery applications.63 Polyol esters, polyoxyethylene esters, poloxamers, and Pluronics can all be used as nonionic surfactants. As previously stated, nonionic surfactants have a unique feature known as a cloud point.64 The cloud point is the temperature at which the nonionic surfactant begins to phase separate from the cleaning solution.64 The cleaning solution becomes cloudy as a result of this. As a result, this cloud point is thought to be the optimal temperature for detergency. Polyethylene glycol is a significant component of polyoxyethylene esters (PEGs).64 Nonionic surfactants that are often used are ethers of fatty alcohols. Nonionic surfactants aid in lowering the hardness sensitivity of the surfactant system. Alkylphenolethoxylates [RO(CH2CH2O)nH (R = alkylphenol group)], alcohol ethoxylates [CnH2+1(OCH2CH2)NOH], and nonylphenols are noncharged hydrophilic parts of nonionic surfactants.
2.2. Classification of Surfactants Based on Solubility
Another way to classify surfactants is based on their solubility profiles.65,66 For example, surfactants soluble in water would be of “hydrophilic” nature, while those soluble in lipids can be termed “hydrophobic” (“lipophilic”) surfactants. In this instance, ionic surfactants are hydrophilic in nature, while nonionic surfactants can either be hydrophilic or hydrophobic. Such characteristics of nonionic surfactants are based on the balance between the hydrophilic group and the lipophilic group. This is usually quantified on the basis of the hydrophilic–lipophilic balance (HLB) scale. Other indicators are the cloud point for nonionic surfactants (the temperature at which the mixture starts to phase separate and two phases appear, thus becoming cloudy) and the Krafft point for ionic surfactants (the Krafft point is the minimum temperature at which a surfactant can form micelles).67
2.3. Sugar-Based Surfactants
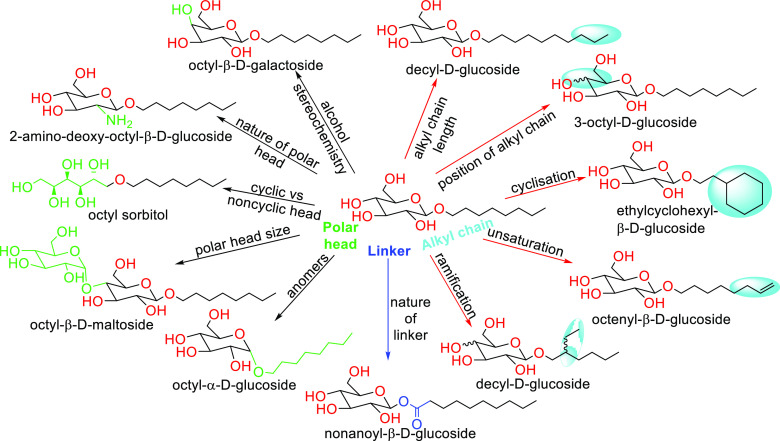
Representing 95% of the world’s biomass volume generated, carbohydrates are the most abundant organic compounds worldwide.68 Although their industrial-scale production has been relatively new, sugar-based surfactants have been gaining increasing attention due to their advantages with respect to their performance, consumer health, and environmental compatibility as compared to existing surfactants.69 Sugar-based surfactants, such as sorbitan esters, sucrose esters, alkyl polyglycosides, and fatty acid glucamides, are gaining popularity due to improvements in performance, consumer health, and environmental compatibility over some traditional formulations. The very interesting review published by Gaudin et al.70 describes the impact of the chemical structure on amphiphilic properties of sugar-based surfactants. Sugar-based surfactants can also be classified based on their stereochemistry. Figure 1 illustrates possible systematic modifications of sugar-based surfactants centered on octyl-β-d-glucoside.
Figure 1.
Possible systematic modifications of sugar-based surfactants centered on octyl-β-d-glucoside. Adapted with permission from ref (70). Copyright 2019 Elsevier.
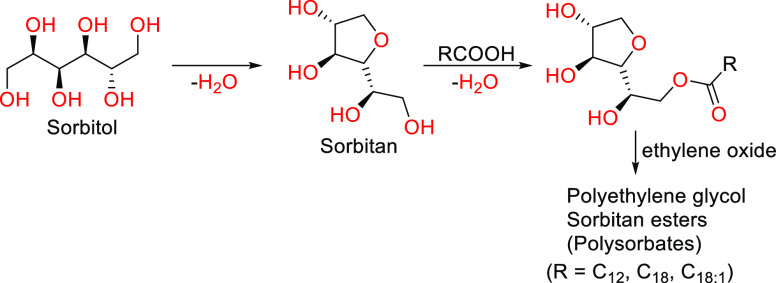
2.3.1. Sorbitan Esters
Sorbitan esters have been around since 1938, when the industrial processes to synthesize them were established. There are two processes to manufacture them, one of which occurs in two steps. The process begins with the conversion of sorbitol into 1,4-sorbitan by dehydration, followed by esterification with fatty acids of technical grade (Scheme 1).68 These two steps can occur independently or simultaneously, with both processes being used industrially to produce sorbitan esters.68,69 Sorbitan esters are known commercially as “Span” (e.g., Span 80 is a biodegradable surfactant based on a natural fatty acid (oleic acid) and sugar alcohol sorbitol). These esters are relatively hydrophobic; hence they tend to find applications in the formation and formulation of water-in-oil emulsions. To make these molecules more hydrophilic, it is common to derivatize sorbitan esters by reacting them with ethylene oxide to form sorbitan ester ethoxylates. Commercially called “Tween”, polyethoxylated sorbitan monoesters are surfactants ideal for creating oil-in-water emulsions due to their hydrophilicity.68,69
Scheme 1. Synthesis of Sorbitan Esters by Intramolecular Dehydration of Sorbitol in the Presence of Acid.
RCOOH: fatty acids. Adapted with permission from ref (68). Copyright 1999 John Wiley and Sons.
Sorbitan esters mainly find use as emulsifiers in pharmaceuticals, foods,3,71 cosmetic products,72−74 emulsion polymerization,75 explosives, and other specific applications. In contrast to traditional nonionic surfactants, sucrose fatty acid esters’ hydrophilic–lipophilic balance (HLB) may be altered by switching from one to eight fatty acid residues attached to a sucrose ring. It is quite intriguing to look at how the number of linked fatty acid residue affects the phase behavior and self-organized structures in a water/sucrose fatty acid ester system. In comparison to ionic surfactants, nonionic surfactants have the advantage of allowing anything to alter molecular structures, particularly the hydrophilic moiety, to produce surfactants with a wide range of HLB. For nonionic surfactants of the polyoxyethylene type, the HLB is altered by varying the polymerization level of the polyoxyethylene group. Due to this benefit, in water/polyoxyethylene-type surfactant systems, a wide range of surfactant aggregates, both with positive and negative curvatures, are seen in a phase diagram as a function of the surfactant’s HLB number. The global sorbitan esters market is slated to top USD 756.4 million in 2021. Sales of sorbitan esters are expected to grow by 5.6% compound annual growth rate (CAGR) between 2021 and 2031.76
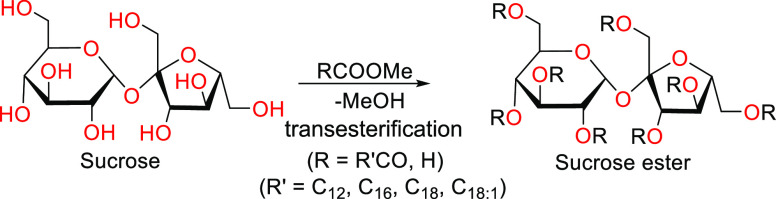
2.3.2. Sucrose Esters
Sucrose is an organic compound abundantly produced and is available at high purity at low cost. Sucrose esters are primarily produced by combining the primary hydroxyl group of a nonreducing sugar moiety and a methyl ester of a fatty acid.77 This is achieved using a transesterification reaction between sucrose and the fatty acid ester, followed by further purification to get the desired product (Scheme 2).2 A large range of amphiphilic products can be obtained by controlling the extent of esterification as well as the choice of fatty acid used as the hydrophilic moiety. The most hydrophilic products are those with a high content of monoester, while the least hydrophilic products are those with a higher ratio of multiple esterification products. This range of hydrophilicity, along with relatively low dermatological impact, provides this class of surfactants with a versatility that allows it to have a wide range of applications in personal care products, cosmetic care applications, food emulsifiers, and certain specialty detergent products.2,48 The sucrose esters market size was valued at USD 72 million in 2022 and is projected to reach USD 111.6 million by 2030, growing at a CAGR of 5.20% from 2023 to 2030.2
Scheme 2. Synthesis of Sucrose Esters by Base Catalyzed Transesterification with Fatty Acid Methyl Esters (R′COOMe).
Adapted with permission from ref (68). Copyright 1999 John Wiley and Sons.
2.3.3. Glucose-Derived Surfactants
Under the umbrella of glucose-derived surfactants, there are two groups of compounds which have been focused upon: alkyl polyglycosides (APGs) and alkyl glucamides (or fatty acid glucamides).
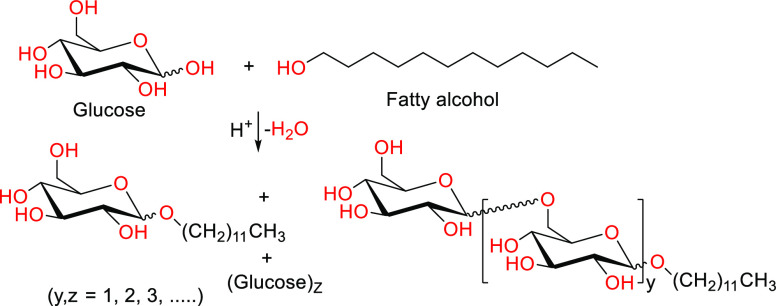
There is currently an extremely strong interest in the synthesis of APGs, which is done by a direct reaction between glucose and a fatty alcohol, with the alcohol being in large excess to minimize and reduce sugar oligomerization and promoting the desired product (Scheme 3).42 An alternate method is transacetalization between a short-chain alkyl glycoside and a long-chain fatty alcohol.42 The mixture of alkyl oligoglycosides, alkyl monoglycosides, and alkyl polyglycosides that results from both synthesis methods depends on the proportion of glucose/fatty alcohol used as reactants. The product’s surfactant properties are determined by the length of the carbon chain and the average number of glucose units polymerized—the degree of polymerization.68 The primary attractiveness of APG is due to their environmental compatibility as they have high biodegradability and low aquatic toxicity. They also possess desirable dermatological properties as they are mild to the skin and eye, which have made this class of surfactants attractive to personal care products.48 C12/C14 APGs have applications as liquid dishwashing agents, personal care products, and detergents, while C8/C10 APGs are used as agrochemicals, hard surface cleaners, and industrial and institutional cleaners.
Scheme 3. Reaction of Glucose and Fatty Alcohol to Form Alkyl Polyglycosides (APGs) Carrying Units of Glucose.
Adapted with permission from ref (68). Copyright 1999 John Wiley and Sons.
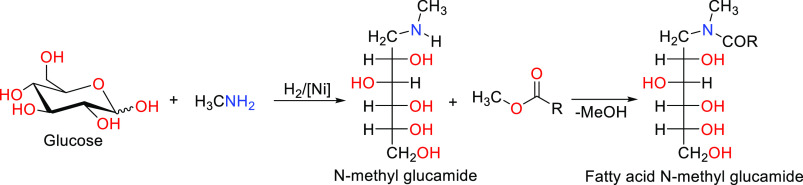
Fatty acid glucamides, or N-methylglucamides (NMGAs),48 are an additional class of glucose-derived surfactants produced industrially. The synthesis procedure involves reacting glucose with methylamine under reductive conditions to form N-methylglucamine, which is further converted with a fatty acid methyl ester to the respective fatty acid amide (Scheme 4).11 In comparison to APGs, NMGAs have only one carbohydrate molecule attached to the chain. However, their physicochemical properties are comparable to those of APGs.82 Currently, NMGAs with C12/C14 and C16/C18 alkyl chains are exclusively used by The Procter & Gamble Company (P&G) in liquid dishwashing agents and powdered and liquid detergents.
Scheme 4. Formation of Fatty Acid N-Methylglucamide with Two-Step Process.
Adapted with permission from ref (68). Copyright 1999 John Wiley and Sons.
2.3.4. Alkyl Polyglycoside (APG) Derivatives
APGs have been employed as raw materials for the synthesis of specialty surfactants intending to slightly adjust the properties of APGs to the desired impact due to the simplicity of procurement and availability.78 Despite the fact that a wide variety of items can be produced using straightforward techniques, only a small number of goods—methyl glucoside esters and a number of specialized esters—have successfully entered the market.78
By esterification of methyl glucoside with methyl esters of oleic or stearic acid, one can enhance the lipophilicity of the molecule. Compared to APGs with the same chain length, methyl glucoside esters are sparingly soluble in water and yet show excellent emulsification properties. Methyl glucoside esters have found applications as emollients, emulsifying and moisturizing agents, and thickeners in cosmetic products and formulations. The hydrocarbon chain length and degree of substitution can be altered to obtain specific water-in-oil emulsification activity. The series of specialty esters, namely citrates, tartrates, and sulfosuccinates, have applications in personal care products.68,78
2.4. Classification of Surfactants Based on Feedstocks
2.4.1. Surfactants Manufactured Using Synthetic or Petrochemical Feedstocks
Oil, gas, and chemical processing all offer synthetic or petrochemical feedstocks for surfactant manufacturing. The resultant molecules, synthetic alcohols, can be further processed or reacted to form a variety of surfactant molecules (including those of alkylation, ethoxylation, or sulfation). Due to their synthetic nature, the molecular structures of these compounds may be adjusted during manufacturing to achieve specialized physical and performance properties of surfactant molecules. They are also chemically versatile, which allows them to be compatible with a wide range of other chemicals and substances. These include petroleum-based surfactants.79 A recent review by Ng et al.79 summarizes recent advances of biosurfactants for waste and pollution bioremediation compared with petroleum-based surfactants.
2.4.2. Surfactants Manufactured Using Renewable Feedstocks
These surfactants are also known as “natural based” (also known as “biobased” or “oleo”) surfactants, as they have origins in natural sources such as microbes, plants, and marine.80 To manufacture fatty alcohols, plant oils need to be chemically processed (including esterification, hydrogenation, and distillation). Despite differing plant origins, these alcohols are comparable to their synthetic equivalents and hence go through the same type of subsequent chemical processing steps to produce the end surfactant. Natural feedstocks may be seen as more environmentally sustainable; nevertheless, other factors must be acknowledged when assessing sustainability, and all aspects of the surfactant life cycle must be examined.
2.4.2.1. Microbial Surfactants
Microbial surfactants (Figure 2), or second-generation biosurfactants, are surfactants which are derived from microbial origins, harnessing the biosynthetic machinery available in the biotic community to synthesize the desired molecule.80−84 Surfactants under this category are also termed “green surfactants” or “biosurfactants”. In contrast to synthetically produced surfactants, which are typically categorized according to the characteristics of the polar groups they contain, biosurfactants are typically categorized according to the chemical compositions of their molecules and the microbes from which they originated.80−84
Figure 2.
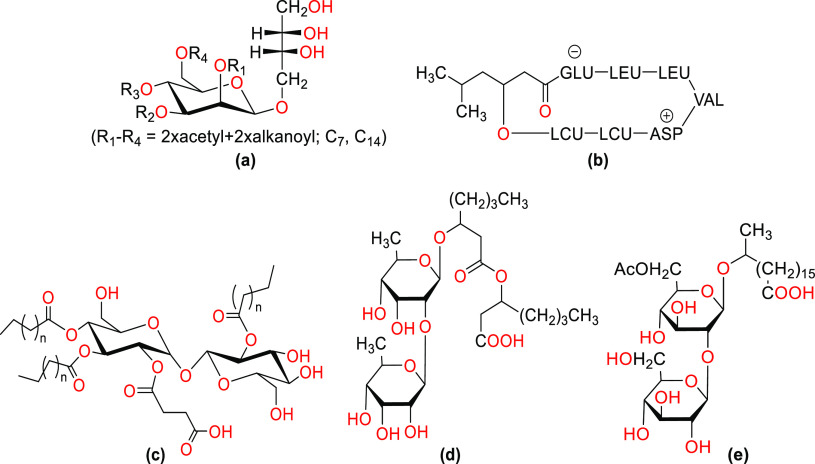
Types of microbial surfactants: (a) mannosylerythritol lipid; (b) surfactin; (c) trehalose lipid; (d) sophorolipid; (e) rhamnolipid.
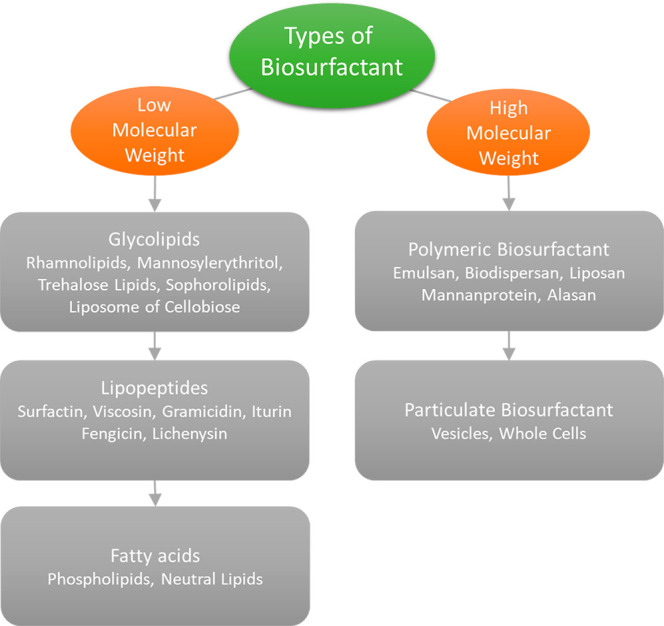
With an average mass ranging from 500 to 1500 Da, biosurfactants are widely divided into low molecular mass and high molecular mass biosurfactants.83 While larger molecular mass biosurfactants are better at stabilizing emulsions, smaller molecular mass biosurfactants are better at lowering surface or interfacial tension. While lipopeptides, glycolipids, and phospholipids have low molecular weights and are commonly referred to as biosurfactants, lipoproteins and lipopolysaccharide have high molecular weights and are usually referred to as bioemulsifiers.80−84
Glycolipids are the class of low molecular weight molecules that have been the subject of the greatest research, with the most commonly known being the sophorolipids (sophorose lipids, SPLs), rhamnolipids (rhamnose lipids, RMLs), trehalose lipids, and mannosylerythritol lipids (MELs).85−87 Glycolipids have been reported to have wide ranges of applications in pharmaceutical formulations.88−90
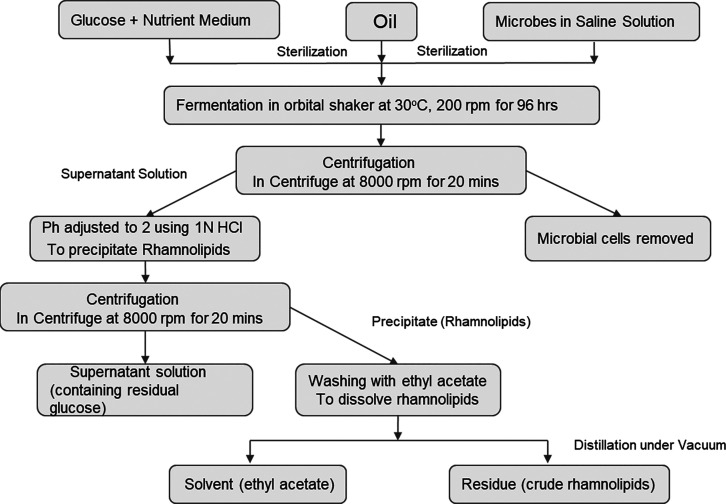
Rhamnolipids are glycolipids that contain rhamnose (a pentose monosaccharide, 6-deoxy-l-mannose) linked to a fatty acid tail ranging from 8 to 16 carbon carbons in length, although generally β-hydroxydecanoic acid (C10) is there.91 These biosurfactants are well-known globally, and depending on the number of rhamnoses present in the molecule groups, their architectures can be split into two groups: monorhamnolipids and dirhamnolipids (one rhamnose group).91 The main method of producing rhamnolipids uses the pathogenic Gram-negative bacterium, Pseudomonas aeruginosa. Rhamnolipids are frequently utilized in environmental processes including the bioremediation of water and soils that have been contaminated by metals, petroleum, or other xenobiotic substances. Apart from bioremediation rhamnolipids are used in food processing, protein folding, microbial fuel cells, and the creation of nanoparticles.92−100
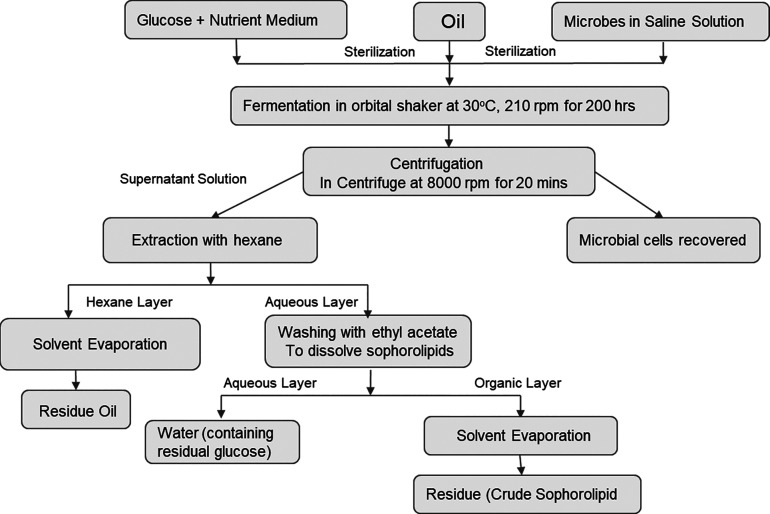
Sophorolipids are another type of glycolipidic biosurfactants that have sophorose (a hydrophilic disaccharide consisting of two glucose residues connected by a β-1,2 glycosidic linkage) connected by a glycosidic linkage to a C16–C18 hydroxylated fatty acid which may or may not be acetylated.101 Sophorolipids can be either acidic (containing a carboxyl group) or lactonic (containing a cyclic ester). Yeasts are used for sophorolipid production, with strains such as Torulopsis bombicola and Starmerella bombicola being mainly used.102 These molecules often have applications in beauty and personal care products, household cleaning products, and biopesticides.101−104
Mannosylerythritol lipids are among the biosurfactants with the most exciting potential, as they are widely manufactured from vegetable oils with the help of Pseudozyma antarctica.105 These biosurfactants are characterized by mannose and erythritol linked to a fatty acid and are further classified based on the length of the hydrophobic fatty acid, degree of saturation, and degree of acetylation (mono-, di-, or triacetylated). They are primarily used in the formulation of beauty and personal care products.105−109
Trehalose lipids are made by species of the genera Mycobacterium, Rhodococcus, Corynebacterium, and Nocardia and consist of trehalose disaccharides coupled with a fatty acid, primarily mycolic acid.110,111 The size, structure, degree of unsaturation, and amount of carbon atoms in the trehalolipids produced by various organisms differ.111
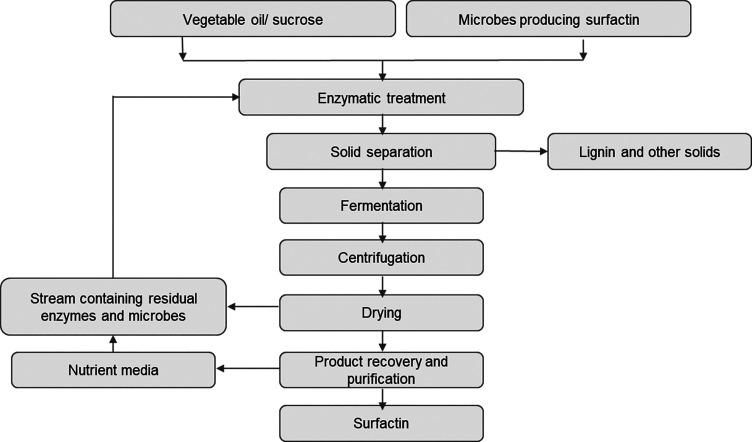
The most well-known and well-known biosurfactant in the lipopeptide family is surfactin, which is also one of the strongest biosurfactants ever discovered. This cyclic lipopeptide is made by the species Bacillus subtilis and contains seven long-chain hydrophobic amino acids linked to a fatty acid chain by a lactone bond. Due to its antibacterial, antimycoplasmal, antiviral, and antitumoral characteristics, surfactin offers a wide range of potential biological applications. In the food business, it can also work well as a stabilizer, emulsifier, and surface modifier.112−114 In 2021, the microbial biosurfactants market was around USD 16.1 million. It is estimated to increase at 3.9% CAGR through 2032.115
2.4.2.2. Amino Acid/Peptide Based Surfactants
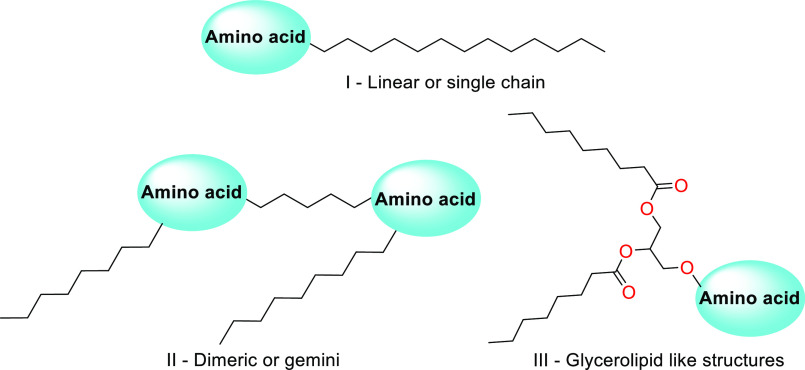
Amino acids as raw materials for surfactant preparations have gained immense importance since the last century.116,117 Amino acid/peptide based surfactants mostly have applications in the area of life sciences and biomedicine as drug carriers, antiviral agents, mediators in DNA, or DNA transfection and gene delivery agents in gene therapy.122,123 Previously, they were used for medicinal and cosmeceutical applications; however, they have been thoroughly investigated nowadays for many of their surfactant applications. The chemical structure, length, and number of fatty acid chains would be varied for nonpolar long-chain compounds (hydrophobic moiety) coupled with polar amino acids/peptides (hydrophilic moiety) resulting in molecules with higher surfactant properties. Considering this, vast varieties of amino acid/peptide structures are available for building amino acid/peptide based surfactants; one can regulate the surfactant properties of end molecules.116,117 Moreover, such variety is also applicable due to the structural diversity among chemical moieties, biological properties, and physicochemical properties. The amino acids and long aliphatic chains can be joined to synthesize three major structures of amino acid based surfactants: (1) linear or single chain, I; (2) dimeric or gemini, II; and (3) glycerolipid-like structures, III116,117 (Figure 3). Linear structures (I) typically made up of an amino acid with at least one hydrophobic tail. Gemini or dimeric (amphipathic) structures (II) consist of two polar heads (two amino acids) and two hydrophobic tails every molecule. Glycerolipid-like structures (III) can be viewed as monoglyceride, diglyceride, and phospholipid analogues. They are made up of one polar head and one or two hydrophobic moieties that are joined together by a glycerol skeleton. Natural α-amino acids are coupled by extensive aliphatic chains connected to the α-COOH, α-amino, or side chains to form a linear, or single-chain, amino acid surfactant. Hence, the fatty acids or alkyl halides are capable of reacting with the amino groups to yield the respective N-acyl or N-alkyl derivatives. On the other hand, N-alkyl amides and esters can be created by condensing the carboxyl group of with alkyl amines or aliphatic alcohols.118−121
Figure 3.
Types of amino acid based surfactant: (I) linear or single chain; (II) dimeric or gemini; (III) glycerolipid-like/glycolipid-like.
Gemini surfactants, which have two hydrophilic and two hydrophobic groups in each molecule connected by a spacer chain, are an example of a particular kind of amphipathic chemicals.122 These molecules, which have good surface activity, can also be thought of as single-chain conventional surfactant dimers.122,123 Gemini surfactants made of cysteine have been created to boost their effectiveness while lowering their environmental impact. Gemini surfactants have had large numbers of biomedical and pharmaceutical applications reported very recently.124−127
Glycerol-amino acids, a subclass of lipoamino acids that are similar to mono- and diacylglycerides and phospholipids, are amino acid glyceride conjugates. They consist of one or two aliphatic chains joined by ester bonds to the glycerol backbone, together with an amino acid serving as the polar head, and have biological applications, too.128−132
As per previously published reports, this class of surfactants can be synthesized via (1) chemical methods, (2) enzymatic methods, or, usually, (3) a combination of both methodologies.132 Thus, saturated single-chain, double-chain, gemini, and amino acid glycerolipid conjugate surfactants formed from various ionic character amino acids have all been found to be highly biodegradable, with low toxicity, ecotoxicity, and irritant effects.
The global amino acid based surfactants market size was estimated at USD 528.58 million in 2021 and is projected to reach USD 1163.05 million by 2028, exhibiting a CAGR of 11.92% during the forecast period.133
2.4.2.3. Glycerol-Based Surfactants
Globally, the production of emulsifiers is estimated to be of the order of 300 000 t.134 Esters formed from glycerol and fatty acids are referred to as glycerides, more commonly known as acylglycerols. Glycerol has three hydroxyl functional groups, all of which can be esterified with one, two, or three fatty acids to form specific monoglycerides, diglycerides, and triglycerides.134−136 Fats and vegetable oils contain triglycerides which can be broken down into monoglycerides and diglycerides (also called partial glycerides) due to the activity of natural enzymes.
Pure partial glycerides are a type of nonionic surfactant which show no charge. Pure monoglycerides and diglycerides have been proven to be efficient and effective surfactants and hence have multiple applications.134,136 Glycerol monostearate (GMS) is a monoglyceride that has been used as a food additive as an emulsifier, thickening agent, anticaking agent, and preservative; as an emulsifier for oils and waxes; and as a control release agent and solidifier for pharmaceutical agents. Magnesium stearate (MG) and its derivatives make up 75% of the food emulsifiers used in the world.136
Partial glycerides exist majorly in three crystalline forms: α, β, and β′. The α crystalline forms are the most functional and can be converted into the β form, which are the most stable and moderately functional.135
Glycerol-based surfactants can be synthesized through either direct esterification with glycerol and fatty acids or transesterification with glycerol and natural fats/oils or fatty acid methyl esters. Chemical syntheses of mono- and diglycerides are done by glycerolysis of fats and oils under high temperatures with inorganic catalysts.137 The global polyglycerol esters market was valued at USD 9.8 billion in 2021 and is expected to reach USD 15.24 billion by 2029, registering a CAGR of 5.60% during the forecast period of 2022–2029.137
Thus, herein this review shall be focusing on two major categories of surfactants: (I) petroleum-based surfactants or “petro-based surfactants” and (II) surfactants derived from biological origins or “biosurfactants” or “green surfactants”.
3. Petroleum-Based Surfactants (Petro-Based Surfactants): Their Origins, Advantages, and Limitations
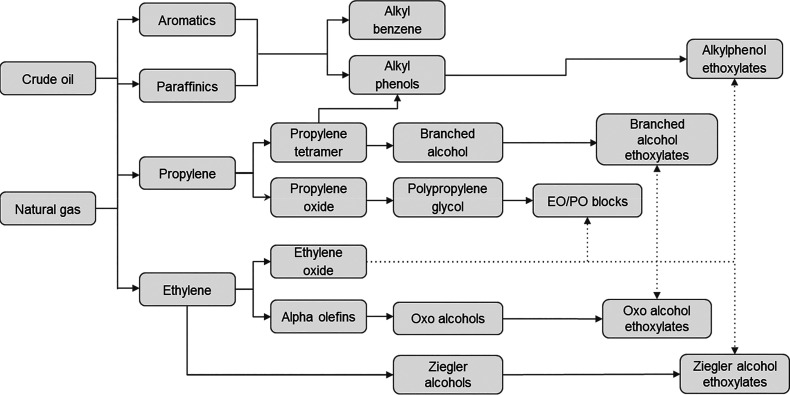
Oil, gas, and chemical processing are used to create synthetic or petrochemical feedstocks (as shown in Figure 4). Through the advancement of petrochemical processing, particularly petroleum cracking, which yields unsaturated, short-chain hydrocarbons, it was possible to obtain hydrophobic structures for surfactant molecules through polymerization of these alkenes, such as ethylene or propylene, which gives way to surfactants with C9–C18 carbon chains.138 The derived chemicals can be further processed or reacted to create a variety of other surfactant molecules, including through alkylation, ethoxylation, and sulfation (Schemes 5–8 illustrate the chemical pathways that lead to the creation of petroleum-based surfactants). Because they are made of synthetic materials, manufacturers may manipulate the molecular structures to develop products with precisely defined physical and performance properties. They are also chemically adaptable, making a diverse variety of other chemicals and substances compatible with them. They can thus be combined with various elements, such as other surfactants, to create a completed formulation whose qualities are adapted to the needs of certain applications. Table 1 highlights sources, properties, and application of some well-known petrochemical surfactants. Due to their high toxicity, low biodegradability, and dependence on petroleum and its derivatives for their synthesis, synthetic surfactants have come under fire, particularly from environmental advocacy groups. These synthetic compounds typically contain lengthy carbon chains, branching, or aromatic groups, which impede biodegradation and cause a host of environmental issues.
Figure 4.
Flowchart representing formation of petro-based surfactants from nonrenewable sources.
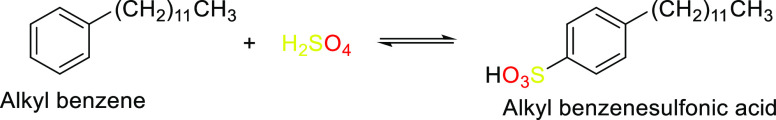
Scheme 5. Reaction Forming Alkylbenzene Sulfonic Acid from Alkylbenzene.
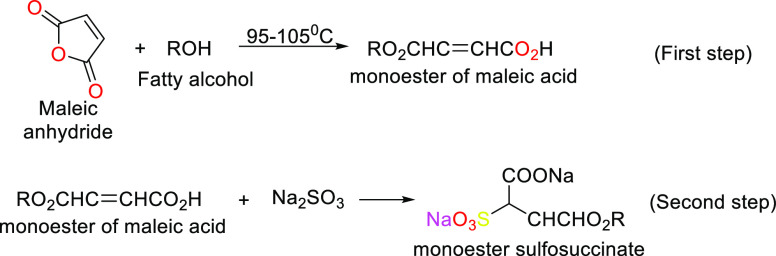
Scheme 8. Reaction Forming Monoester Sulfosuccinate.
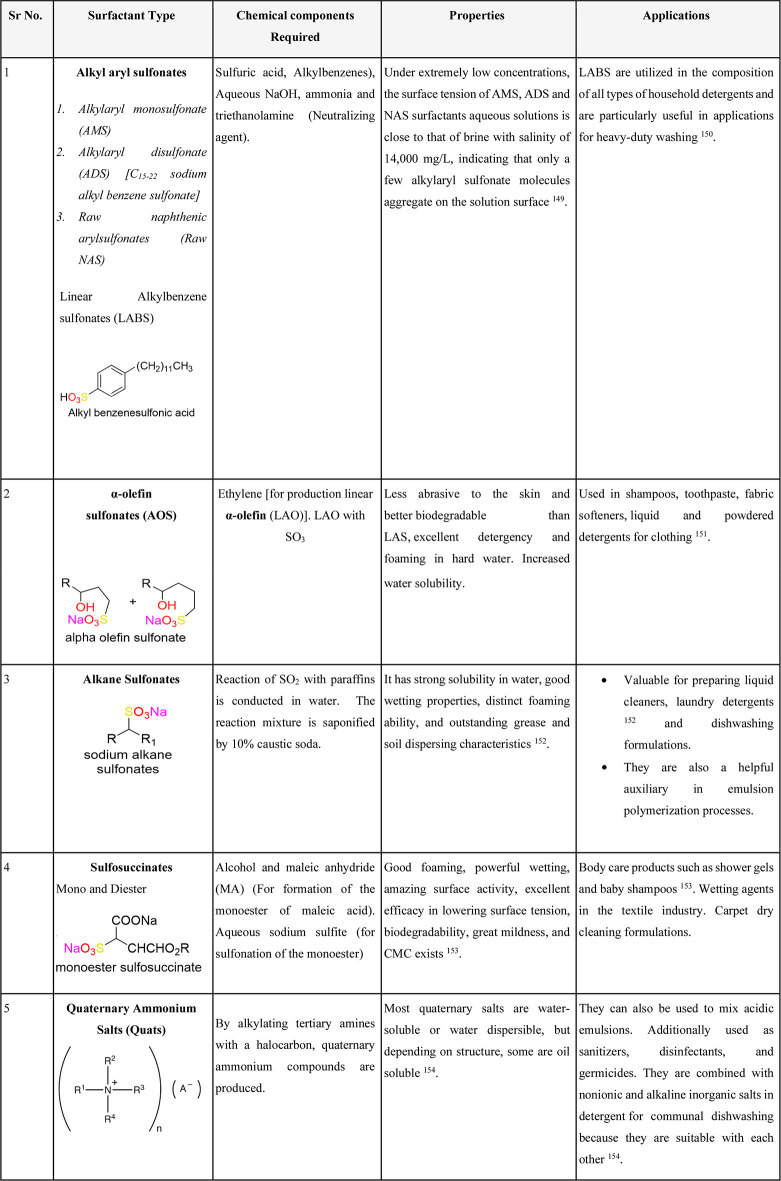
Table 1. Properties, Advantages, and Sources of Some Well-Known Petroleum-Based Surfactants149−154.
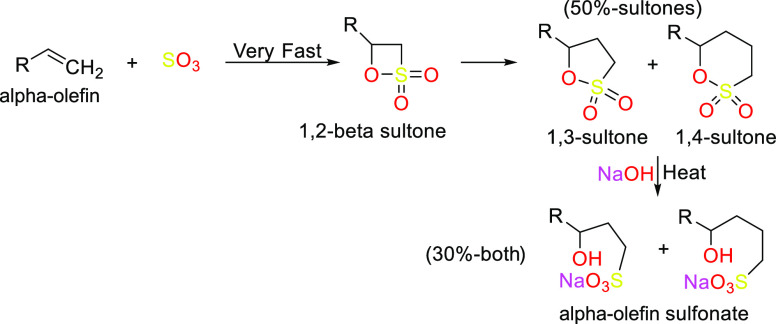
Scheme 6. Reaction Forming α-Olefin Sulfonates.
Scheme 7. Reaction Forming Alkane Sulfonates.
3.1. Advantages
Petro-based surfactants have been in use for decades, and gradually, with increasing demand, their production has increased enormously with a huge extent of advancement in technology also. Despite extensive research in the field of biosurfactants, they are still struggling with the research and development stage of production. The actual part of the study to infer applications of these surfactants is neglected, due to which petroleum-based surfactants are major promising molecules to serve the demands of humankind. With continuous advancements in research, scientists have come up with many biodegradable petroleum-derived surfactants such as LASs and paraffin sulfonates, which are used worldwide in various industries giving satisfactory results. This type of surfactant is economical and has many upper edges compared with other classes of surfactants.139
The low shelf life and lack of supply of biosurfactants due to low production yields are major concerns for their compatibility in the surfactant industry.140
Some specific advantages of petroleum-derived surfactants are given in sections 3.1.1–3.1.4.
3.1.1. Linear Alkylbenzene Sulfonates (LASs)
LASs have an advantage because of their low price ($2,400–2,800/MT) and their superior performance due to a low vapor pressure ((3–5) × 10–13 Pa), with a critical micelle concentration (CMC) of 0.1 mg/L. They form clear solutions in water at concentrations up to 250 mg/L. Linear alkylbenzene sulfonic acid is among the most widely used synthetic surfactants by volume.141 This chemical is biodegradable and environmentally friendly.
It has a low salt content and a good water solubility.142
It is completely compatible with hard water.142
It is resistant to hydrolysis in hot acid or alkali.
It is fully ionized; as a result, low pH has no effect on solubility.142
3.1.2. Paraffin Sulfonates, Secondary n-Alkanesulfonates (SASs)
These are used largely in light-duty liquid home detergents that are liquid (LDLs).143
The polymerization of vinyl polymers uses an SAS as an emulsifier.47
It serves as an antistatic agent in a variety of polymers, including polyvinyl chloride (PVC) and polystyrene.47
The water solubility is desirable.47
Its aqueous liquids have low viscosities.47
SAS shows good compatibility with skin.
At low temperature it exhibits biodegradability.47
3.1.3. α-Olefin sulfonates (AOSs)144
AOSs are used in variety of household, personal care soaps, and detergents.
They are stable over a wide range of pHs.
They are cold water and hard water compatible.
They are good foaming agents and are mild on skin.
They are sulfate free.
3.1.4. Sulfosuccinate Esters
Monoesters are used in cosmetics and, when combined with other anionic surfactants, help to lessen the irritation that anionic surfactants can cause to the skin and eyes.145,146
Amide monoesters are among the anionic surfactants that are the least irritating to the eyes because they may be manufactured electrolyte-free and are entirely soluble in organic solvents.145,146
3.2. Limitations of Petroleum-Based Surfactants
Petroleum-based surfactants that have been chemically created degrade slowly when exposed to microbes. As a result, they could bioaccumulate or produce byproducts that are harmful to the environment. Early in the 1960s, persistent foams began to blanket numerous bodies of water, such as rivers and lakes that received wastewater from big towns, which led to an ecological imbalance since the thick layer of foam inhibited photosynthesis and oxygen dissolution.42 This was caused by inefficient alkylbenzene sulfonates (ABSs). As a result, governments of developed countries passed environmental laws to limit the use of ABSs in detergents around 1965. Because of this, less expensive surfactants like linear alkylbenzene sulfonates were created. However, surfactants continue to be a concern of sewage treatment facilities, as well as of freshwater resources like lakes and rivers, in developing countries.79
Another problem is the phosphate content in surfactants which is responsible for eutrophication. Eutrophication results in the abrupt and explosive growth of algae and other plankton which consumes the oxygen dissolved in water, suffocating fish and other aquatic plant and animal life. This indirectly harms humans as many desirable aquatic species are at a loss or extinct. Decrease in species diversity, increase in plant and animal biomass, increase in turbidity, increase in the rate of sedimentation, and shortening of the lifespan of the lake are adverse effects owing to eutrophication.79
Also, petro-based surfactants will soon start facing problems of raw material shortages due to the rapid depletion and exhaustion of petroleum and fossil fuels in the near future. Hence industries and researchers worldwide have shifted their attention to biobased surfactants to tackle this issue. Biosurfactants are also fully biodegradable and hence are a better alternative to petroleum-based surfactants.
Surfactant specific limitations are given in sections 3.2.1–3.2.3.
3.2.1. Linear Alkylbenzene Sulfonates (LASs)
With the exception of alcohol, sodium alkylbenzene sulfonate (LAS) is not soluble in organic solvents. The crucial quality for elimination in the environment is that LAS readily, quickly, and fully degrades in aerobic environments.147 However, under anaerobic conditions, LAS only experiences primary biodegradation. There has not been any proof that LAS completely biodegrades in anaerobic environments. LAS might irritate the skin.
3.2.2. Sulfosuccinate Esters
Acidic and hot alkaline solutions hydrolyze these surfactants. Dialkyl esters cause skin irritation (monoesters do not).148
3.2.3. POE Alkylphenols, Alkylphenol “Ethoxylates” (APEs), RC6H4(OC2H4)nOH
The rates of biodegradation for APEs are slower than those for other nonionic surfactants such as linear alcohol ethoxylates, even though they are fully biodegradable in aerobic environments.140 Compared to the parent APE, the intermediates of aerobic biodegradation are more hazardous to fish and other aquatic species.140 Although no evidence of APE endocrine disrupting activity in actual environmental systems has been established, there are reports that APEs may exhibit it in model systems in laboratory testing.
4. Biosurfactants: Green Generation of Specialty Chemicals (Also Termed “Green Surfactants”)
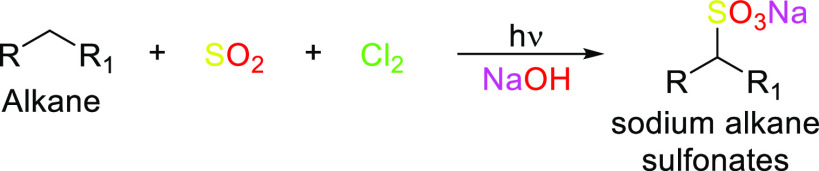
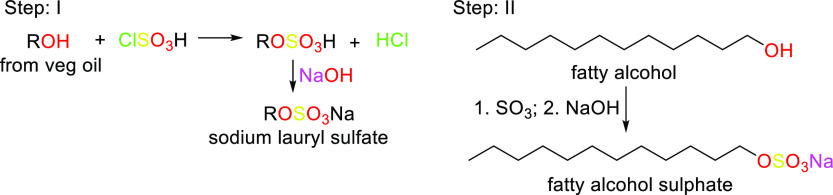
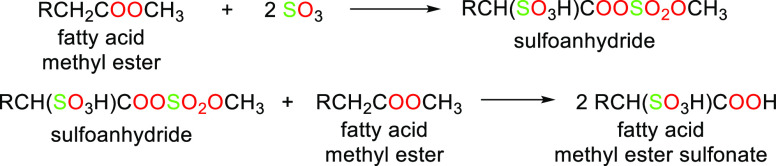
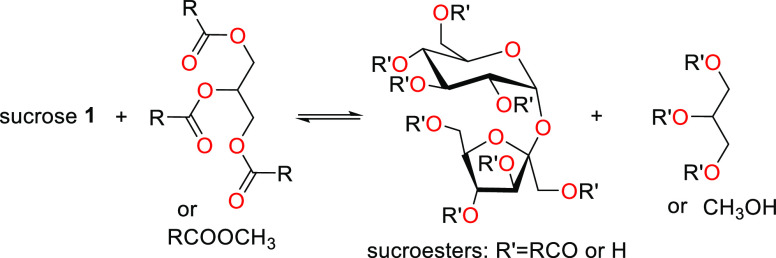
The shift from using petrochemicals to using renewable materials as starting raw materials for surfactant synthesis has been promoted by the concept of “going green” due to environmental concerns as well as legislation and government restrictions over toxic detergents in products, resulting in what we now refer to as “green surfactants” as suitable substitutes.54 This new category of products is more biocompatible, and biodegradable, and meets the rising consumer demand for products which are “greener”, that is, milder, more effective, and less environmentally impactful. The term “green surfactants” refers to biobased amphiphilic molecules that are either obtained naturally or can be synthesized from renewable raw materials.54 They are also sometimes referred to as “biosurfactants”. First-generation biosurfactants are those directly extracted from animal-based or plant-based raw materials or directly synthesized chemically. Multiple raw materials can be used and chemically modified to yield green surfactants or biosurfactants.54 In particular, triglycerides, carbohydrate sources, and certain organic acids have been extremely useful as starting materials in green surfactant synthesis. Examples of surfactants produced through chemical synthesis are saponins, fatty alcohol sulfates (as shown in Scheme 9), fatty acid methyl ester sulfonates (as shown in Schemes 10 and 11), sugar esters, alkyl polyglucosides, and alkanolamines. Table 2 lists the sources, properties, and uses of a few well-known oleochemical-based surfactants. Second-generation biosurfactants refer to those that make use of the biosynthetic machinery of organisms of the biotic community (microbes, yeasts, plants, etc.) through biological processes (biocatalysis or fermentation) to produce the surfactants, from which the desired product is extracted and purified (Figures 5–8 highlight downstream processes of some popular microbial surfactants). Primarily examples of these biosurfactants are microbial surfactants such as glycolipids, specifically rhamnolipids and sophorolipids, and lipopeptides, specifically surfactin, produced in this way. The sources, properties, and uses of a few well-known oleochemical-based surfactants are highlighted in Table 3.
Scheme 9. Different Routes to Form Fatty Alcohol Sulfates.
Scheme 10. Reaction to Form Fatty Acid Methylesters.
Scheme 11. Reaction to Form Sucroesters.
Table 2. Properties, Advantages, and Sources of Some Well-Known Microbial Based Surfactants.
| no. | name | microbe | carbon source | properties | applications | ref |
|---|---|---|---|---|---|---|
| 1 | rhamnolipids | Pseudomonas aeruginosa, Pseudomonas cepacia, Pseudomonas spp. | olive oil, canola oil, soybean oil refinery waste | molecular weights in the range 500–650 Da; CMC of 15 mg/L; reduce surface tension to 29 mN/m; good foaming and wetting abilities; antibacterial and antiviral properties | enhanced oil recovery; cleanup of oil spills in aquatic media; proposed as concrete additives; in cosmetics as promoters | (140) |
| 2 | sophorose lipids (sophorolipids) | Candida sphaerica, Starmerella bombicola, Cutaneotrichosporonmucoides | canola oil, safflower oil, animal fat | molecular weights 600–706 Da (depending on type of sophorolipid); CMC 82 mg/L; surface tension 37 mN/m; good wetting ability; lactonic form inhibits growth of certain microbes | ethoxylated sophorolipids used as skin moisturizers; humectants in cosmetics; sophorolipid esters used in lipstick and hair products; acidic forms of sophorose lipids used as agents for healing, desquamation, and macrophage activation; release of bitumen from tar sands; improvement of oral hygiene by getting rid of bacterial biofilms or preventing growth of further bacterial cultures in the mouth | (140) |
| 3 | surfactin | Bacillus subtilis, Bacillus nealsonii | sucrose, vegetable oil | reduces surface tension from 72 to 27 mN/m low CMC | in food industry as surface concentration (CMC) modifier, emulsifier, and stabilizer; incorporated in detergents to remove tough stains like coffee and vegetable oils; also exhibit antifungal, antibacterial, and antimycoplasmal activities | (168) |
| 4 | mannosylerythritol | Pseudozyma antarctica, Ustilago nuda | soybean oil, sunflower oil | reduces surface tension to 28–40 mN/m; CMCs of (2–5) × 10–4 M | moisturizing property similar to those of ceramides; widely used in skin care industry as an antiaging and moisturizing agent having cytoprotective and antioxidant qualities; also exhibit antibacterial activity against Gram-positive bacteria | (107) |
Figure 5.
Classification of biosurfactants based on molecular weight.
Figure 8.
Manufacturing process for surfactin.
Table 3. Properties, Advantages, and Sources of Some Well-Known Oleo-Based Surfactants.
| no. | name | source | properties | applications | ref |
|---|---|---|---|---|---|
| 1 | fatty alcohol sulfates | tallow, palm kernels, coconut oil | demonstrate strong emulsifying and dispersing abilities in hard water | used in polymerization process as an emulsifying agent and personal care and hygiene industry in products such as toothpastes, cosmetics, and synthetic soaps | (169, 170) |
| 2 | fatty acid methyl esters (FAMEs) | waste palm oil | exhibit high emulsifying and dispersing properties in hard water; moderate level of foaming | low interfacial tension for oil-in-water system and environmentally safe nature, so find application in detergents containing no phosphates | (171, 172) |
| 3 | sucroesters | beet or cane sucrose, FAME from vegetable oil | very low CMC values and higher esters provide more lipophilic surfactants that can help stabilize “water-in-oil” emulsions | widely used in personal care and food industries | (173) |
| 4 | alkyl polyglycosides | fatty alcohols (palm kernel or coconut oil (C12–C14 carbon chain)) and rapeseed or palm oil (C16–18 carbon chain) and glucose from wheat, corn starch, or potatoes | lessen the irritating effects of surfactant mixtures and have good compatibility with the skin, eyes, and mucous membranes | widely used in cosmetic creams due to their effective emulsifying of water and oil | (173) |
| 5 | fatty acid glucamides | glucose, derived from starches | low potential for irritation due to polyol structure and synergistic effects with other types of surfactants; due to affinity for calcium ions and limited solubility, glucamides have drawbacks and must always be mixed with sequestering agents to prevent precipitation | glucamides with C16/18 and C12/14 alkyl chains are used in liquid dishwashing agents and powdered and liquid | (173) |
| 6 | sorbitan esters | sorbitol (glucose derivative) | HLB values of various sorbitan esters (such as laurates, oleates, or stearates) are typically in the range 1–8 | used as emulsifiers and solubilizers in food, medicinal, and cosmetic products | |
| 7 | fatty amine ethoxylates | fatty amides from coconut oil or palm kernel, or tallow; ethoxylated fatty amines from coco, stearyl, oleyl, tallow, and lauryl amines (2–50 mol of EO/mol of amine hydrophobe) | average molecular weight between 174 and 798 g/mol; length and level of ethoxylation of fatty alcohol chain affect physical and chemical characteristics of fatty amine ethoxylate | used as lubricant agricultural adjuvants, antistatic agent, detergents ingredient, and acid thickener | (174, 175) |
| 8 | gemini surfactants | palm oil fractions (cheap and abundant source) | outperform traditional surfactants thanks to their outstanding surface activity characteristics; exhibit solubilizing, wetting, foaming, antibacterial, and lime soap dispersion capabilities at incredibly low CMC levels | recently discovered that gemini surfactants are very intriguing as possible vehicles for transportation of bioactive compounds utilized in gene therapy | (121−124) |
Figure 6.
Manufacturing process for rhamnolipids.
Figure 7.
Manufacturing process for sophorolipids.
The structural characteristics of the product green surfactants serve as the foundation for both the classification of these substances and the determination of their physicochemical properties. As with any surfactant, they contain a hydrophobic moiety (saturated, unsaturated, hydroxylated, or branched) and a hydrophilic moiety (ester, carboxylate, hydroxyl group, phosphate, peptide, amino acid, or carbohydrate). As previously mentioned, such products are considered extremely important in the current scenario, due to their ecological soundness and low (or no) toxicity and high biodegradability. Such green surfactants are also considered vastly versatile compounds due to their applications in the petroleum, chemical, pharmaceutical, food, metals, textiles, cosmetics, and agriculture industries.54
4.1. Production/Synthesis and Extraction Methods of Green Surfactants or Biosurfactants
In this section, we outline a few of the methods or routes available for the production of green surfactants or biosurfactants including their biosyntheses.
4.1.1. Biosynthesis
The amphiphilic structure in biosurfactants consists of a long-chain fatty acid and the hydrophilic motif (which includes amino acid, carboxylic acid, phosphate, etc.).155,156 The synthesis of their hydrophobic and hydrophilic components is carried out by two primary metabolic pathways, namely hydrocarbon and carbohydrate. The pathways for synthesis of these two types of precursors are diversified and rely on distinct sets of enzymes. Because the first enzymes involved in the synthesis of these precursors are often regulatory enzymes, there are some commonalities in their synthesis and regulation. There are detailed descriptions of synthetic pathways for these the major hydrophobic and hydrophilic motifs available in the literature. However, a comprehensive summary by Hommel and Ratledge155 may be handy to read. Per Syldatk and Wagner,157 there are four possibilities which can exist for synthesis of linkages and involved hydrophilic (HPL) and hydrophobic (HB) moieties of biosurfactants:158
-
(1)
HPL and HB moieties synthesized de novo by two independent pathways
-
(2)
HPL synthesized de novo and HB induced by substrate
-
(3)
HB synthesized de novo and HPL substrate dependent
-
(4)
both HB and HPL substrate dependent
The chemical synthesis for a biosurfactant depends on the molecule and desired surfactant. Due to the vastness, there is no overall generalization that can be made as each mechanism and route will be different as suggested above.
4.1.2. Fermentation
Biosurfactants can be produced by a variety of microorganisms such as bacteria, fungi, and yeasts and are diverse. Their natures, chemical compositions, and amounts, depend on the microorganisms producing them.158,159 These organisms have the ability to use potentially noxious substrates as they have been isolated from contaminated soil, effluents, or wastewater sources. Hence, the possible substrates are vast and can broadly be described as agro-industrial waste, industrial wastes of plant and animal origin, and other industrial wastes. Agro-industrial waste refers to vegetable oils and oil wastes, oil mill waste effluents (OMWE), and starchy waste materials such as potato processing effluents. Industrial wastes of plant and animal origins refer to waste such as dairy industry whey, animal fat, molasses, and soy molasses. Other industrial wastes refer to soap stock.158,159
Since such a process depends on microorganisms, culture variables including agitation, pH, the concentration of metal ions, temperature, dilution rate, and aeration, and the kinds of carbon and nitrogen sources affect the type, quality, and quantity of biosurfactant production. By altering these conditions, the same microorganism can produce a different biosurfactant such as by changing the substrate used. By closely studying them, one can optimize the physicochemical properties of the desired biosurfactant, too.158,159
4.1.3. Enzymatic Methods
Enzymes can be isolated, immobilized, and used for producing base biosurfactants as well as used for processing or post-treatment to produce other biosurfactants with modified properties. By using enzymes, one can often find an alternate route for the synthesis of biobased surfactants instead of using fermentation.85
The most well-known example of this is using immobilized lipases to produce sugar esters. Solvent-free esterification of a simple APG by using fatty acid and Candida antarctica lipase has been carried out under multiple conditions (molten fatty acid or in various solvents) with different yields obtained.160 As such, other lipases or enzymes can be used to synthesize a whole range of biosurfactants, especially amino acid based esters and amines.161
4.1.4. Ultrasonication
Ultrasound refers to sounds inaudible to the human ear and is subdivided into power ultrasound (20–100 kHz), high-frequency ultrasound (20 kHz–2 MHz), and diagnostic ultrasound (above 1 MHz). Practically, ultrasound is used in two ranges: low-intensity (high-frequency 100 kHz–1 MHz, low power of less than 1 W/cm2) and high-intensity (low-frequency 2–100 kHz, high power of 10–1000 W/cm2). Depending on the intensity, the physical technology can have different biological impacts from beneficial to destructive.162
The primary effects are due to the phenomenon of cavitation, which causes chemical and physical changes. It has been shown that ultrasonication increases cell permeability and, in turn, promotes or releases cellular metabolites and/or cells themselves.162 Ultrasound can be employed in different stages, via probes or bath ultrasound systems. A theory is that low-intensity ultrasound promotes mass transfer through the boundary layer, cellular membrane, and even the cytosol, by reducing the boundary layer thickness. Additionally, mass transfer induced by ultrasonication can alter the active sites of enzymes and therefore alter the enzymatic activity.163 On the contrary, high-intensity ultrasound can cause cellular membrane disruption and can damage vital macromolecules or even induce lysis. Hence, the utilization of mild ultrasound can stimulate and control microorganism activity along with other processes such as fermentation to increase efficiency and productivity.162
4.1.5. Hydrodynamic Cavitation (HC)
The fundamental idea behind hydrodynamic cavitation is that when a liquid passes through a constriction or small opening, such as an orifice plate, venturi, or throttling valve, the pressure of the liquid increases at the expense of local pressure, and the pressure around the vena contracta falls below the threshold pressure, creating cavities. Due to the persistent pressure reduction, the eventual collapse of these cavities releases a tremendous amount of energy and can result in high-pressure shock waves and free radicals with temperatures up to 10 000 K.164
The extraction of integral molecules from natural products is effectively done on an industrial scale by the use of efficient cell disruption techniques such as cavitation. Even though ultrasound-based cavitation has wider applications, HC is a viable option due to its efficiency and scale-up application. Depending on the tools being used and the type of cell being disrupted, the mechanism of cell disruption varies. When compared to the ultrasound-assisted approach, the lipid extraction percentage from wet microalgae using venturi type HC is substantially greater.164,165
4.1.6. Microwave-Assisted Extraction (MAE)
The extraction method known as microwave-assisted extraction (MAE) couples microwaves with conventional solvent extraction. By applying microwaves for heating in the extraction process, one can expedite the kinetics of extraction and make it more efficient.166
Microwaves are nonionizing electromagnetic radiation with frequencies in the range 0.3–300 GHz. The field simultaneously provides heating by two mechanisms: dipole rotation and ionic conduction. By doing so, one can cause changes in the cell structure. The process’s acceleration and higher yield may be the outcome of two transport phenomena, mass and heat transfer, which both work in the same direction, both from the inside to the outside.167 One of the major benefits that modern MAE offers is its solvent-free applications in extracting natural products in an environmentally friendly green way.
In MAE, extraction occurs by exposing the desired compounds and cells to the solvent through cell rupture. The free water molecules present in the cell matrix are heated, which results in the localized heating and expansion of the cell itself. As a result, the desired metabolites find it easier to flow out of the cells.166
Multiple parameters and factors may affect the MAE extraction yield such as (i) extraction time and cycle, (ii) the solvent system and solvent to feed ratio, (iii) contact surface area and water content, (iv) microwave power and extraction temperature, and (v) stirring. By optimizing the conditions based on these parameters through detailed study and trial and error, one can achieve the best possible yield for MAE.167
4.2. Advantages of Green Surfactants or Biosurfactants
Green surfactants or biosurfactants have lower toxicities.32,42
They have increased effectiveness at extreme temperatures or pH values.
They have higher biodegradability; that is, compared to chemical surfactants, biosurfactants are environmentally beneficial substances that break down quickly into simpler metabolites. Different bioremediation and biosorption technologies have successfully utilized biosurfactants made from marine microorganisms.32,42
They improve hydrocarbon degradation: Biosurfactants, as opposed to chemical surfactants, offer special qualities like low toxicity and high biodegradability, selectivity, and surface activity. They can also create more stable emulsions. These characteristics make them appealing for improved hydrocarbon recovery methods since they are maintained even at high temperatures and a variety of pH and salinity ranges.32,42
Biosurfactants have been discovered to be more effective and efficient than chemical surfactants in terms of their surface and interfacial activities. Furthermore, the CMC values of biosurfactants are significantly lower than those of chemical surfactants.32,42
4.3. Limitations of Green Surfactants or Biosurfactants
Large-scale production of biosurfactants is complex and difficult.
Some biosurfactants may be as toxic as synthetic biosurfactants.
Biosurfactants may compete with the hydrocarbon as a preferred substrate.
Biosurfactant production is not economically viable.
Many research groups are focusing on finding ways to produce biosurfactants at lower costs by employing readily accessible and renewable bioresources as their primary raw materials.
The biotechnological methods required in the synthesis of biosurfactants are somewhat costly, and surfactant purification is quite difficult. All biotechnology product costs are heavily influenced by downstream processing. Different biosurfactants have been isolated, purified, and categorized using a wide variety of analytical techniques. There has been a significant amount of research on upstream biosurfactant generation to improve productivity and yield, but there has not been much done on downstream purification.176
4.4. Properties of Biosurfactants
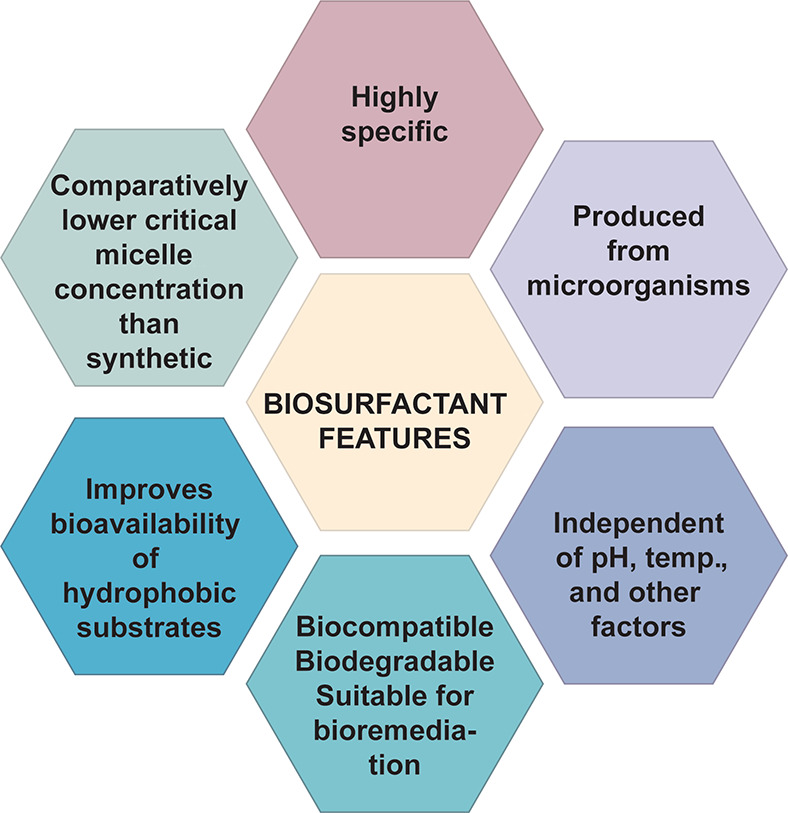
The properties of biosurfactants are summarized in Figure 9.177
Figure 9.
General properties of biosurfactants.
4.4.1. Interfacial Tension
Biosurfactants are more effective than synthetic surfactants by a factor of 10–40. A study conducted on biosurfactant-mediated oil recovery by McInerney et al. showed that Bacillus mojavensis strain JF-2 bacteria, compared to average values of 28–29 mN/m, reduced interfacial tension by almost 2 orders of magnitude.178 With or without 2,3-butanediol (cosurfactant) present, raising the salinity enhanced the interfacial tension. The interfacial tension measured at the lowest point was 0.1 mN/m.178
4.4.2. pH and Temperature
The pH and temperature of the environment have little impact on the surface activity of many biosurfactants. Lichenysin, a biosurfactant produced from Bacillus licheniformis JF-2, is unaffected with a pH range of 4.5–9.0 and temperatures as high as 50 °C. Similarly, a lipopeptide produced by B. subtilis LB5a maintained its surface activity for 6 months at a temperature of 121 °C and in a high salt environment. The biosurfactant is stable at pH 4–10 and high temperatures up to 120 °C, and a NaCl content of up to 10% (w/v) maintains emulsification action.179
4.4.3. Biodegradability and Low Toxicity
The best instrument for bioremediation without having any negative effects on the environment is biosurfactants. They are far safer than synthetic surfactants and more environmentally friendly. Environmental bioremediation, enhanced oil recovery, pharmaceuticals, and food processing have all seen a rise in the use of biosurfactants due to their special qualities, such as increased biodegradability and lower toxicity. A comparative study on biosurfactants done by Muthusamy et al. between a synthetic surfactant, Marlon A-350, and a biosurfactant produced by P. aeruginosa showed that the biosurfactant was nontoxic. In contrast, the synthetic surfactant was highly harmful in all assays and its characteristics.180
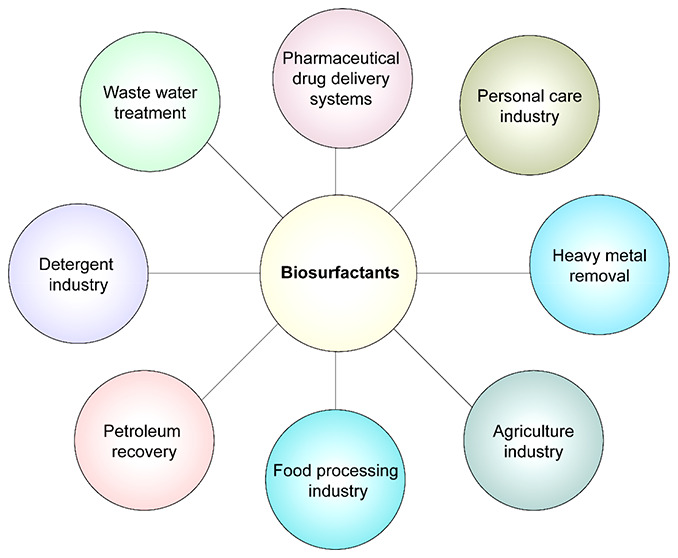
4.5. Applications of Biosurfactants
4.5.1. Applications in Cosmetics
In comparison to synthetic surfactants, the newly created biosurfactants are biocompatible, nontoxic, biodegradable, and milder on the skin and exhibit greater interfacial activity. Sophorolipids, mannosylerythritol, and rhamnolipids lipids have been claimed to have the most uses in the cosmetic industry. Chemically altered sophorolipid produced by Torulopsis bombicola has been observed to contain a natural moisturizing component. Also, because of their antimicrobial qualities, sophorolipids play an essential role in the treatment of body odors, dandruff, and acne. Rhamnolipids find application in toothpaste, nail care products, and deodorants and serve a significant role as an antiwrinkle agent. Mannosylerythritol lipids are utilized in antiaging skincare treatments.177
4.5.2. Applications of Biosurfactants in Contaminated Soils
Heavy metals are contributing to serious environmental issues. The most common heavy metals found in contaminated soils are lead (Pb), mercury (Hg), arsenic (As), cadmium (Cd), chromium (Cr), zinc (Zn), copper (Cu), and nickel (Ni), which can cause a variety of health problems in humans, animals, and plants. Biosurfactants derived from plants and microorganisms have demonstrated superior performance in removing heavy metals from contaminated soil.181 There are three major steps involved in the extraction of heavy metals from soil via washing with a biosurfactant solution. The sorption of biosurfactant molecules at the interfaces between sludge (wet soil) and metal in aqueous solution separates heavy metals adsorbed on the surface of soil particles. The metal will then be absorbed by biosurfactants and electrostatically trapped within the micelle. Ultimately, the biosurfactant can be retrieved with the use of the membrane separation method.181−186
4.5.3. Biosurfactants as Antimicrobial Agents
The rise in antibiotic resistance is driving research into novel antimicrobial strategies.186−188 Antibiotic adjuvants or enhancers are compounds that have little or no antimicrobial activity but improve antibiotic action or prevent resistance when used in conjunction with antibiotics. Because of their membrane-destabilizing properties, biosurfactants are being considered as alternative antimicrobial agents or as adjuvants for traditional antibiotics in the situation of increasing drug resistance in pathogenic bacteria and the necessity for new lines of therapy.189−192 A recent review by De Giani et al.190 summarizes perspectives of biosurfactants with antimicrobial activity.
4.5.4. Biosurfactants in Agriculture
To fulfill the growing demands of the human population and to accomplish sustainable agriculture, green surfactants are currently necessary. Biosurfactants produced by bacteria, yeasts, and fungi are some possibilities for green surfactants. In agriculture, biosurfactants are employed to get rid of plant diseases and boost nutrient bioavailability for helpful plant bacteria.177 The quality of agricultural soil may be significantly increased with biosurfactant remediation. An estimated 0.2 million tons of surfactants are used annually in the formulation of crop protection and pesticides. The method of hydrophilization utilizing biosurfactants results in good wettability, suppression of pesticide toxicants, and even dispersion of fertilizers in the soil. The proliferation of Rhizobacteria in the rhizosphere is accelerated in several ways, aiding in the stimulation of plant growth. These biosurfactants enhance the soil’s quality and stimulate plant–beneficial microbial interactions. These biosurfactants have greater benefits than the synthetic surfactants currently employed in pesticides since they are environmentally safe, are inexpensive, and help the soil’s beneficial bacteria grow.177
4.5.5. Biosurfactants in Bionanotechnology
Presently, the next generation of green chemistry or bioengineering nanocatalyst sources is thought to be composed of biosurfactants generated from microbes and nanoparticles.193 Environmental remediation holds great promise for the production of nanoparticles by biosurfactant-mediated synthesis. The nanoparticles produced with biosurfactant must, however, be commercially feasible. Biosurfactant microbes may stabilize and reduce nanoparticle formation. Nanoparticles like gold, silver, and titanium are produced by microorganisms.193
4.5.6. Biosurfactants in Cancer Therapeutics
Biosurfactants have a crucial ability to regulate mammalian cell functionalities and, thus, would also be used in cancer therapeutics. These molecules have been reported for maintaining varieties of functionalities such as cell immune responses and differentiations, signal transduction, etc. For example, glycolipids were found to growth arrest and apoptosis of melanoma B16 cells of mice. The potential applications of such biosurfactants as antitumor agents have been detailed in Table 4. Increase in reactive oxygen species (ROS) generation, suppressing Bcl-2 expression, promoting cytochrome c (Cyto-c) release, activation of caspase pathways, inhibiting 12-O-tetradecanoylphorbol-13-acetate (TPA) induced migration, colony formation, and invasion through MMP-9 regulation, apoptosis, DNA damage, etc. can be key pathways adopted by biosurfactants causing anticancer effects.194
Table 4. Antitumor Activities Reported for Some Biosurfactants.
| biosurfactant | description | activity | ref |
|---|---|---|---|
| monoolein | cervical cancer, leukemia cancer | growth inhibition | (195) |
| serratamolide | B-chronic lymphocytic leukemia | apoptosis induction | (196) |
| viscosin | metastatic prostate cancer | migration inhibition | (197) |
| mannosylerythritol lipids (MELs) | myelogenous leukemia | growth inhibition, differentiation | (198) |
| sophorolipids | promyelocytic leukemia | interaction with plasma membrane | (199) |
| succinoyl trehalose lipids (STLs) | promyelocytic leukemia | growth inhibition, differentiation | (200) |
| surfactin or surfactin-like biosurfactants | myelogenous leukemia | growth inhibition | (200, 201) |
| lipopeptides of Bacillus | cancer cells | killing and suppression of invasion | (202) |
| surfactin | suppress proliferation of LoVo cells | inducing proapoptotic activity and arresting the cell cycle | (203) |
| human breast carcinoma cell line | causing reactive oxygen species (ROS) generation | (204) | |
| iturin | alveolar adenocarcinoma A549; colon adenocarcinoma HCT-15 | growth inhibition | (205, 206) |
| fengycin | lung malignancy cell 95D | growth inhibition | (207) |
| Bacillus subtilis fmbJ-derived fengycin | colon cancer cell line HT29 | inhibiting development and progression | (208) |
5. Remediation of Surfactants
Better management of surfactant usage and disposal has become a necessity of the hour, at both the industrial and domestic levels. Strict guidelines should be followed for properly remediating surfactants before disposal. Oxidation-based approaches, photocatalytic degradation, foam fractionation, electrochemical degradation, and microbial biodegradation are among the techniques used to treat surfactants.209 In recent years, biosurfactants have attracted prospective interest for use in the environmental remediation of organic and inorganic contaminants, particularly in the removal of heavy metals from soil and water, cosmetics, and pharmaceutical products, as well as in enhanced oil recovery.209−212 Biosurfactants have also applications as microbial-enhanced oil recovery (MEOR).210
6. Comparison with Established Products with Respect to Performance and Cost
Green surfactants, i.e., biosurfactants, are known to have properties like self-assembly, reduction of surface and interfacial tension, emulsification, and adsorption which make them applicable in various applications. Also, their low toxicity makes biosurfactants potentially more useful and attractive than traditionally used surfactants.213
6.1. Critical Micelle Concentration (CMC)
Biosurfactants generally have CMC values ranging from 1 to 200 mg/L, which are on the lower side as compared to petroleum- and oleo-based surfactants. One previous study showed the comparison of CMCs of biosurfactants derived from B. subtilis EG1 with traditional surfactants. Table 5 indicates the CMCs of given biosurfactants along with HLB values.
Table 5. Critical Micelle Concentrations and Hydrophilic–Lipophilic Balances of the Different Commercial Surfactants Assayed in a Previous Study213.
| surfactant | CMC (g/L) | HLB |
|---|---|---|
| Glucopon 215 | 0.241 | 13 |
| Glucopon 600 | 0.028 | 11.2 |
| Glucopon 650 | 0.073 | 11.9 |
| Findet 10/15 | 0.152 | 9.6 |
| Findet 1214N/23 | 0.021 | 14.4 |
| Findet 9Q/21.5NF | 0.034 | 12.8 |
| LAS | 1.018 | – |
The findings showed that B. subtilis EG1 producing biosurfactant is significantly more effective than synthetic surfactants at reducing surface tension.213
Additionally, biosurfactants have the ability to significantly lower interfacial and surface tension. In comparison to synthetic surfactants, they are even quite effective under adverse situations like high temperatures, acidic pH levels, and salinity.214,215
6.2. Emulsification
In applications that require low surfactant concentrations, biosurfactants may be appealing due to their low CMC values and high exhibited emulsifying abilities.213 The stabilities of the resulting water-in-oil emulsions varied between the two surfactants, according to a comparison between rhamnolipid biosurfactant and an amphiphilic quaternary ammonium salt (containing 75 wt % diacetyl dimethylammonium chloride in water–isopropanol solvent). Rhamnolipid was 83% less efficient than the quaternary ammonium salt at 0.01 wt %, and at 1.5 wt %, its emulsion stability was half that of the quaternary ammonium salt (3 min compared to 130 min).216
At greater concentrations, the emulsion stabilities of the two surfactants vary dramatically. Rhamnolipids stop the binding and aggregation of hydrate crystallites at concentrations of 0.05% or higher. In comparison to SDS, rhamnolipid has also demonstrated greater kerosene emulsification effectiveness in the pH range 6–9.
6.3. Toxicity
In general, biosurfactants have low toxicity. There is numerous research that has investigated the toxicities of biosurfactants in aquatic life, plants, and human cell lines. According to a study, using biosurfactants made from Candida lipolytica at concentrations 0.5–2 times the CMC had no impact on plant root length or seed germination.81 In a recent study, the toxicities of natural and synthetic biosurfactants were compared. In aquatic habitats, both a naturally occurring monorhamnolipid and a synthetic monorhamnolipid had EC50 levels that were “somewhat hazardous” according to the EPA. Additionally, a human cell line’s cytotoxicity and biodegradability (measured by the xCELLigence assay) were dependent on the stereochemistry of the synthetic rhamnolipid.217
6.4. Cost Analysis
Synthetic surfactants are substantially less expensive than biosurfactants when comparing pricing (see Table 6).217
Table 6. Market Prices of Some Popular Surfactants.
| type of surfactant | name of sufactant | cost/kg222,223 |
|---|---|---|
| petro-based | α-olefin sulfonates | $1.00–$2.00 |
| alkyl ether sulfates | $0.8–$0.9 | |
| linear alkylbenzene | $1.4–$1.7 | |
| alkyl phenol ethoxylates | $2.19 | |
| oleochemical | sorbitan monooleates (SPAN) | $1.30–$1.50 |
| polysorbate 80 (TWEEN) | $1.60–$2.60 | |
| microbial surfactants | rhamnolipids | $25.00–$40.00 |
| sophorolipids | $30.00/L |
The prices of synthetic surfactants are significantly lower than those of biosurfactants. The high cost of production is mainly due to the fermentation and product purification steps. Rhamnolipids still cannot be recovered and purified on an industrial scale using any downstream technique that is both cost-effective and compelling. For the manufacturing of biosurfactants, downstream processing is responsible for 70–80% of total production costs. A significant barrier to the commercialization of biosurfactants is the economics of manufacturing.218
7. Screening Biosurfactant Efficiencies
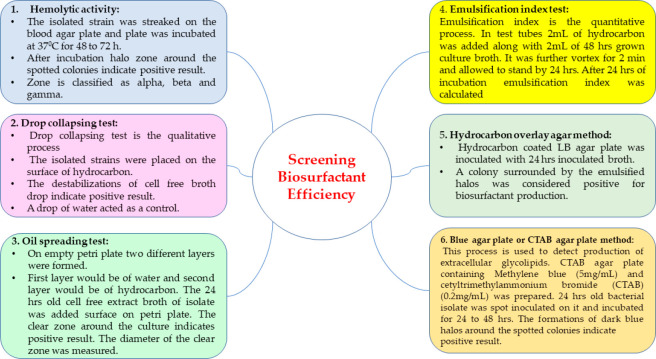
Biosurfactants can be screened with the use of methods such as the hemolysis test, oil spreading test, drop collapse method, emulsification index, hydrocarbon overlay agar method, and blue agar plate method (Figure 10).219
Figure 10.
Methods used to test biosurfactant efficiencies.
8. Market and Future Prospects
During the forecast period of 2021–2028, the global surfactants market is anticipated to expand at a CAGR of 4.9%, rising from $41.22 billion in 2021 to $57.81 billion in 2028. Surfactants are produced by the industry at a rate of approximately 17 million metric tons yearly, some of which come into direct contact with customers and most of which are eventually released as effluent. In light of this volume, solving green issues is a crucial subject for a sector that is dealing with expanding regulation and customer awareness.219 In 2020, the market for green surfactants was estimated to be worth close to USD 2.54 billion. The global green surfactants market is projected to expand at a CAGR of 5.7% from 2022 to 2027, reaching a value of $3.56 billion by 2026. The sector is expanding because of the growing demand for green surfactants made from waste biomass and agricultural raw materials.220 The booming personal care sector is contributing to the continuous rise of the market for green surfactants. This industry is expanding as a result of the increased attention being paid to health, beauty, and personal hygiene, which in turn is assisting the market for green surfactants.221Table 7 displays a list of companies that produce novel green surfactants or use them in their products advancing sustainability in the process. Figure 11 demonstrates a typical comparison between synthetic surfactants and biosurfactants.177
Table 7. Current Marketed Products Containing Green Surfactants.
| no. | company | product/surfactant | description | ref |
|---|---|---|---|---|
| 1 | Dow | EcoSense SL-60 HA surfactant | This is a special emulsifier and glycolipid surfactant for rinse-off and leave-on applications that is 100% bioderived. It is a biosurfactant and natural emulsifier made from sugar that has a high concentration of acid sophorolipid. | (223) |
| 2 | Dow | EcoSense 3000 surfactant; personal care grade | It is a nonionic surfactant made from plants that is easily biodegradable and creates good to excellent stable foam for use in applications. It is comprised of 65% formulation water, and the remaining 35% solid content is considered derived naturally. | (224) |
| 3 | BASF | BioToLife | The component, which is based on sophorolipid, prevents the spread of several dangerous microbes. When used for skin, scalp, or oral hygiene, this promotes the development of a balanced microecosystem. | (225) |
| 4 | BASF | BASF APG | Under the brand name Plantacare, BASF sells its APG range made entirely of plants. The all-purpose sugar surfactants have a great ecological and toxicological profile, a history of being mild, and other useful properties. | (226) |
| 5 | Evonik | REWOFERM RL 100 | It has both primary and secondary surfactant functions. It has the ability to ease difficult formulations because of its special characteristics in terms of effectiveness, formulability, and endurance. | (227) |
| 6 | Clariant | Velsan SC | It has reduced glucose from wheat or corn and the fatty acid from palm or coconut oil: sorbitan caprylate. It is entirely natural. It functions as a preservative booster, giving the option to employ fewer preservatives while yet maintaining the safety of cosmetics. It also functions as an emulsifier or emollient at the same time. | (228) |
| 7 | Croda | Arlasilk EFA; INCI linoleamidopropyl PG-dimonium chloride phosphate | Arlasilk EFA is a sunflower oil derived fatty acid carrier which not only acts as surfactant but also helps in countering irritation and also provide mildness. Due to its good compatibility with a variety of surfactants, it is increasingly used in rinse-off and leave-on skin care products. | (229) |
| 8 | Croda | OleoCraft HP-33; INCI polyamide-4 | The ingredient is derived from vegetable and is a rheology modifier for high polarity liquids and water used in cosmetics and personal care products. It helps in forming a stable emulsion of oil in gelled water using polar organic liquids such as ethanol, butylene, and glycol without the help of surfactants. | (230) |
| 9 | Unilever | Unilever’s Quix dishwashing liquid | The product uses a rhamnolipid, a type of biosurfactant which is mild on skin, 100% biobased, and biodegradable. Collaborating with Evonik, Unilever was able to deliver a sustainable and cleaner product with better performance. They believe in scaling up the innovation and exploring new technologies offering renewable and greener products. | (231) |
| 10 | Solvay | Mirasoft SL L60 and Mirasoft SL A60 | Solvay introduced Mirasoft, a high-perfomance glycolipid biosurfactant developed from sugar and rapeseed oil, to suit broad applications in beauty care like shampoos, creams, face wash, and shower gels. These surfactants provide the same performance as synthetics and are biodegradable. This approach represents a dramatic shift in favor of sustainability and makes sustainable beauty concepts a reality. | (232) |
| 11 | AGAE Technologies, USA | rhamnolipid biosurfactants | The products can be used in pharmaceuticals, cosmetics, personal care products, bioremediation (in situ and ex situ), and enhanced oil recovery (EOR). | (233) |
| 12 | Fraunhofer IGB, Germany | glycolipid and cellobiose lipid biosurfactants | These are used in cleaning products, dishwashing liquids, and pharmaceutical products (bioactive properties). | (234) |
Figure 11.
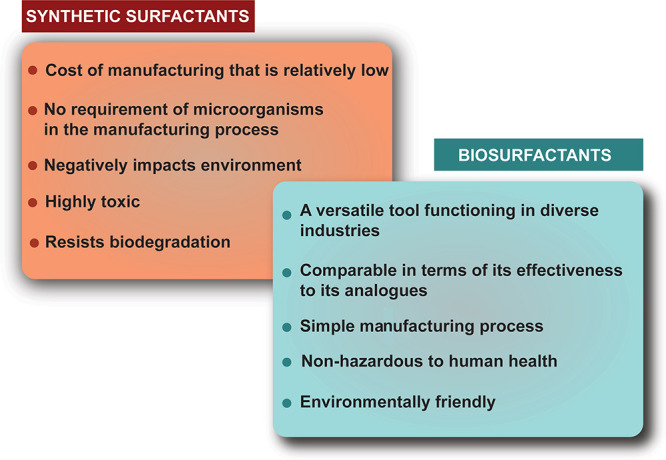
Comparison between synthetic surfactants and biosurfactants.
This has led to a rise in the cultivation of natural oils, the origin of which, particularly tropical oils, is a major source of concern. Even though manufacturers have joined groups like the Roundtable on Sustainable Palm Oil (RSPO), there is still much disagreement over the true cradle-to-gate effects of using land for renewable chemical feedstocks. It is interesting to note that Clariant introduced its GlucoPure Sense surfactant in 2017, which utilizes European sunflower oil as opposed to oils from tropical regions. As an alternative to cocamidopropyl betaines generated from coconut oil, BASF has also developed amphoteric betaine surfactants for use in formulations for hair care.222
Green surfactants provide a significant and expanding contribution to the business, albeit the extent of that contribution will depend on how people define what is natural, biobased, and sustainable. Additionally, even though different customer product and personal care companies have shown interest in 100% biobased surfactants, the market has not yet evaluated whether consumers are prepared to pay premium pricing for them. A further factor driving the global market for green surfactants is the strict government laws on the use of chemicals that may have a harmful impact on the environment. These rules are encouraging manufacturers to employ more eco-friendly and sustainable goods. As businesses try to get away from surfactants made from petroleum sources, green surfactants are becoming more and more desirable.
9. Industrial Challenges of Biobased Surfactants
Many of the synthetic procedures required or involved in biosurfactants’ production utilizes harsh conditions.235 It is interesting to note that several processes are still using hazardous solvents, toxic acid catalysts, or toxic base catalysts. This definitely has environmental issues regarding toxicities. Thus, one important challenge in synthesizing or purifying biosurfactants is to have proper reaction optimizations for solvents or catalysts. Recent literature also demonstrates the usage of various enzymes in reaction optimizations, which definitely aids in sustainability.236 Enzymes’ primary disadvantages are their considerably greater costs as compared to chemical catalysts and their slower reaction rates. The requirement for sustainability (reduced operating energy, less waste, and safer operating conditions) is essential, nevertheless, as energy prices are predicted to increase. One other challenge includes the higher pricing of biobased surfactants and biosurfactants; with this hurdle it is indeed difficult to meet the expectations of price-sensitive Asian customers. Further, the higher complexities and lower efficiencies of microbial surfactants also disappoint in their industrial production. For example, the average price of sophorolipids is USD 34/kg as compared to sodium dodecyl sulfate and amino acid surfactants that are priced at USD 1–4/kg.237 But with technical advancements like integrated separation, sophorolipid surfactants may be produced at a lower operating cost of USD 2530/ton, making them comparable in cost to other specialty surfactants.237 Increased biosurfactant sustainability without noticeably greater performance is not well received since typical customers will not be prepared to pay a premium for items made from biomaterials.
Therefore, improving biosurfactant manufacturing at a reduced cost is crucial for achieving an economically viable method and guaranteeing future market stability.237 Recently, our group has also optimized various procedures pertaining to a few biosurfactants with the ease of freely available raw materials.238−243 The dependence of the demand for biosurfactants on the volatility and economic meltdown of downstream end-user industries is another issue. The performance of the overall macroeconomic environment is known to have an impact on industries that are relevant for biosurfactant applications, including oil and gas, improved oil recovery, the food sector, construction, textiles, paints, pharmaceuticals, and detergents. The coronavirus disease (COVID-19) pandemic also affects end-user industrial demand and raises concerns about the sustainability of the raw material supply. The sustainability of raw materials is a significant issue since they account for up to 50% of the cost of producing glycolipids and 10–30% of the price of other biosurfactant products. Of the cost of production, 60% goes in purification; however, this may be reduced when biosurfactants in their raw forms are used.
Additionally, a number of operational elements offer crucial controls to reduce the expenses associated with the manufacturing of biosurfactants. The fermentation and purification can be improved with batch cycles’ optimization, which also aids in shortening the time between two batches during production. The most crucial element in the manufacturing economics of the manufacture of biosurfactants at industrial sizes is productivity. The most effective batch-sequencing campaign reduces the frequency of starting and shutdown to reduce production downtime and boost productivity. Last, but not least, the development of biosurfactant products will be hampered by costly and time-consuming legal restrictions. Manufacturers of biosurfactants pay a significant cost of compliance in addition to the price of product development.
10. Summary
The traditional usage of petroleum-based surfactants is a result of their inexpensive production costs, long shelf lives, widespread availability, and improved performance at lower temperatures. They also offer formula flexibility owing to their branching, odd, and even hydrocarbon chains. The necessity to discover a renewable alternative that is safe for the environment arises from their nonrenewability and environmental damage. The increasing interest of industries in developing environmentally safe products has led to biotechnological advances involving the synthesis of green surfactants. Biosurfactants and oleo surfactants are examples of green surfactants gaining popularity and have been looked at as a breakthrough for the replacement of synthetic surfactants. There has been a rise in the production of these surfactants over a decade, and they are likely to touch the mark of 2.6 billion in 2023.52 However, the high cost of production of these surfactants poses a problem for its scale-up.
The main obstacles to the manufacture of biosurfactants include low productivity, extensive downstream processing, and a lack of adequate understanding of the bioreactor systems.
Although these problems are a cause of concern, there has been a substantial increase in the variety of manufacturing processes of these green surfactants. Also, chemical giants like Dow, BASF, Croda, Evonik, and Clariant are investing heavily in the research and development of these products and have proved so by launching them in the market. We believe that in the coming decades there will be a complete phasing out of petroleum-based surfactants, giving rise to newer, greener, and sustainable surfactants.
Acknowledgments
The authors of this article are thankful to the Institute of Chemical Technology, Mumbai, India. H.K.A.Y. would like to thank the Deanship of Graduate Studies and Research, Ajman University, UAE, for their support in providing assistance in article processing charges for this review.
Glossary
Abbreviations
- APG
alkyl polyglycoside
- LAS
linear alkylbenzene sulfonate
- SAS
secondary n-alkanesulfonate
- AOS
α-olefin sulfonate
- APE
alkylphenol “ethoxylate”
- HC
hydrodynamic cavitation
- MAE
microwave-assisted extraction
- CMC
critical micelle concentration
- DOSS
dioctyl sodium sulfosuccinate
- LABSA
linear alkylbenzene sulfonic acid
- SLES
sodium lauryl ether sulfate
- AES
alkyl ether sulfate
- CS
cationic surfactant
- QAC
quaternary ammonium compound
- PEG
polyoxyethylene ester
- HLB
hydrophilic–lipophilic balance
- CAGR
compound annual growth rate
- USD
U.S. dollar
- APG
alkyl polyglycoside
- NMGA
N-methylglucamide
- P&G
The Procter & Gamble Company
- SPL
sophorose lipid
- RML
rhamnose lipid
- MEL
mannosylerythritol lipid
- DNA
deoxyribonucleic acid
- GMS
glycerol monostearate
- PVC
polyvinyl chloride
- AMS
alkylaryl monosulfonate
- ADS
alkylaryl disulfonate
- LAO
linear α-olefin
- MA
maleic anhydride
- HPL
hydrophilic
- HB
hydrophobic
- OMWE
oil mill waste effluents
- FAMEs
fatty acid methyl esters
- NaCl
sodium chloride
- MMP-9
metalloproteinase-9
- LoVo
cell line isolated in 1971 from the large intestine of a white, 56-year-old, male with grade IV Dukes C colorectal cancer
- HCT-15
HCT-15 cells were isolated from the large intestine of a male with Dukes C colorectal cancer and can be used for cancer and toxicology research
- MEOR
microbial-enhanced oil recovery
- SDS
sodium dodecyl sulfate
- COVID-19
coronavirus disease
Author Contributions
○ V.S.N., C.C., H.K.A.Y., and S.N.M.: These authors made equal contributions.
The authors declare no competing financial interest.
References
- Ricardo F.; Ruiz-Puentes P.; Reyes L. H.; Cruz J. C.; Alvarez O.; Pradilla D. Estimation and prediction of the air-water interfacial tension in conventional and peptide surface-active agents by random Forest regression. Chem. Eng. Sci. 2023, 265, 118208. 10.1016/j.ces.2022.118208. [DOI] [Google Scholar]
- De S.; Malik S.; Ghosh A.; Saha R.; Saha B. A review on natural surfactants. RSC Adv. 2015, 5 (81), 65757–65767. 10.1039/C5RA11101C. [DOI] [Google Scholar]
- Kralova I.; Sjöblom J. Surfactants Used in Food Industry: A Review. J. Dispersion Sci. Technol. 2009, 30 (9), 1363–1383. 10.1080/01932690902735561. [DOI] [Google Scholar]
- Dias M. A. M.; Nitschke M. Bacterial-derived surfactants: an update on general aspects and forthcoming applications. Braz. J. Microbiol. 2023, 54, 103–123. 10.1007/s42770-023-00905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtil E.; Oztop M. H. Mechanism of adsorption for design of role-specific polymeric surfactants. Chem. Pap. 2023, 10.1007/s11696-022-02636-9. [DOI] [Google Scholar]
- Margaritis A.; Zajic J. E.; Gerson D. F. Production and surface-active properties of microbial surfactants. Biotechnol. Bioeng. 1979, 21 (7), 1151–1162. 10.1002/bit.260210706. [DOI] [Google Scholar]
- Czajka A.; Hazell G.; Eastoe J. Surfactants at the Design Limit. Langmuir 2015, 31 (30), 8205–8217. 10.1021/acs.langmuir.5b00336. [DOI] [PubMed] [Google Scholar]
- Tcholakova S.; Denkov N. D.; Lips A. Comparison of solid particles, globular proteins and surfactants as emulsifiers. Phys. Chem. Chem. Phys. 2008, 10 (12), 1608–1627. 10.1039/b715933c. [DOI] [PubMed] [Google Scholar]
- Ribeiro H. M.; Morais J. A.; Eccleston G. M. Structure and rheology of semisolid o/w creams containing cetyl alcohol/non-ionic surfactant mixed emulsifier and different polymers. Int. J. Cosmet. Sci. 2004, 26 (2), 47–59. 10.1111/j.0412-5463.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- Yan N.; Ni P.; Zhang M. Preparation and properties of polyurea microcapsules with non-ionic surfactant as emulsifier. J. Microencapsulation 1993, 10 (3), 375–383. 10.3109/02652049309031527. [DOI] [PubMed] [Google Scholar]
- Rodríguez-López L.; Rincón-Fontán M.; Vecino X.; Cruz J. M.; Moldes A. B. Biological Surfactants vs. Polysorbates: Comparison of Their Emulsifier and Surfactant Properties. Tenside Surfactants Deterg. 2018, 55 (4), 273–280. 10.3139/113.110574. [DOI] [Google Scholar]
- Wang H.; Guo W.; Zheng C.; Wang D.; Zhan H. Effect of Temperature on Foaming Ability and Foam Stability of Typical Surfactants Used for Foaming Agent. J. Surfactants Deterg. 2017, 20 (3), 615–622. 10.1007/s11743-017-1953-9. [DOI] [Google Scholar]
- Bureiko A.; Trybala A.; Kovalchuk N.; Starov V. Current applications of foams formed from mixed surfactant-polymer solutions. Adv. Colloid Interface Sci. 2015, 222, 670–677. 10.1016/j.cis.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Montufar E. B.; Traykova T.; Planell J. A.; Ginebra M.-P. Comparison of a low molecular weight and a macromolecular surfactant as foaming agents for injectable self setting hydroxyapatite foams: Polysorbate 80 versus gelatine. Mater. Sci. Eng., C 2011, 31 (7), 1498–1504. 10.1016/j.msec.2011.06.008. [DOI] [Google Scholar]
- Thompson J. E.A Practical Guide to Contemporary Pharmacy Practice; Williams & Wilkins: 1998. [Google Scholar]
- Panda A.; Kumar A.; Mishra S.; Mohapatra S. S. Soapnut: A replacement of synthetic surfactant for cosmetic and biomedical applications. Sustain. Chem. Pharm. 2020, 17, 100297. 10.1016/j.scp.2020.100297. [DOI] [Google Scholar]
- Zhu Y.; Free M. L.; Woollam R.; Durnie W. A review of surfactants as corrosion inhibitors and associated modeling. Prog. Mater. Sci. 2017, 90, 159–223. 10.1016/j.pmatsci.2017.07.006. [DOI] [Google Scholar]
- Shalabi K.; Helmy A. M.; El-Askalany A. H.; Shahba M. M. New pyridinium bromide mono-cationic surfactant as corrosion inhibitor for carbon steel during chemical cleaning: Experimental and theoretical studies. J. Mol. Liq. 2019, 293, 111480. 10.1016/j.molliq.2019.111480. [DOI] [Google Scholar]
- Osman M. M.; El-Ghazawy R. A.; Al-Sabagh A. M. Corrosion inhibitor of some surfactants derived from maleic-oleic acid adduct on mild steel in 1 M H2SO4. Mater. Chem. Phys. 2003, 80 (1), 55–62. 10.1016/S0254-0584(01)00588-0. [DOI] [Google Scholar]
- Sliem M. H.; Afifi M.; Bahgat Radwan A.; Fayyad E. M.; Shibl M. F.; Heakal F. E.-T.; Abdullah A. M. AEO7 Surfactant as an Eco-Friendly Corrosion Inhibitor for Carbon Steel in HCl solution. Sci. Rep. 2019, 9 (1), 2319. 10.1038/s41598-018-37254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyab M. A. Application of nonionic surfactant as a corrosion inhibitor for zinc in alkaline battery solution. J. Power Sources 2015, 292, 66–71. 10.1016/j.jpowsour.2015.05.040. [DOI] [Google Scholar]
- Zheng A.; Xu X.; Xiao H.; Li N.; Guan Y.; Li S. Antistatic modification of polypropylene by incorporating Tween/modified Tween. Appl. Surf. Sci. 2012, 258 (22), 8861–8866. 10.1016/j.apsusc.2012.05.105. [DOI] [Google Scholar]
- Griffith R. M. The effect of surfactants on the terminal velocity of drops and bubbles. Chem. Eng. Sci. 1962, 17 (12), 1057–1070. 10.1016/0009-2509(62)80084-0. [DOI] [Google Scholar]
- Clint J. H.Surfactants: applications in plastics. In Plastics Additives: An A-Z Reference; Pritchard G., Ed.; Springer Netherlands: 1998; pp 604–612. [Google Scholar]
- Joshi T. A short history and preamble of surfactants. Int. J. Appl. Chem. 2017, 13, 283–292. [Google Scholar]
- Hellgren A.-C.; Weissenborn P.; Holmberg K. Surfactants in water-borne paints. Prog. Org. Coat. 1999, 35 (1), 79–87. 10.1016/S0300-9440(99)00013-2. [DOI] [Google Scholar]
- Deng Y.; Zheng X.; Bai Y.; Wang Q.; Zhao J.; Huang J. Surfactant-controlled ink drying enables high-speed deposition of perovskite films for efficient photovoltaic modules. Nat. Energy 2018, 3 (7), 560–566. 10.1038/s41560-018-0153-9. [DOI] [Google Scholar]
- Zhmud B.; Tiberg F.. Surfactants in ink-jet inks. In Surfactants in Polymers, Coatings, Inks and Adhesives; Blackwell: 2020; pp 211–244. [Google Scholar]
- Swarup S.; Schoff C. K. A survey of surfactants in coatings technology. Prog. Org. Coat. 1993, 23 (1), 1–22. 10.1016/0033-0655(93)80002-R. [DOI] [Google Scholar]
- Trier X.; Granby K.; Christensen J. H. Polyfluorinated surfactants (PFS) in paper and board coatings for food packaging. Environ. Sci. Pollut. Res. 2011, 18 (7), 1108–1120. 10.1007/s11356-010-0439-3. [DOI] [PubMed] [Google Scholar]
- Wokosin K. A.; Schell E. L.; Faust J. A. Emerging investigator series: surfactants, films, and coatings on atmospheric aerosol particles: a review. Environ. Sci. Atmos. 2022, 2 (5), 775–828. 10.1039/D2EA00003B. [DOI] [Google Scholar]
- Tato J. V.; Seijas J. A.; Vázquez-Tato M. P.; Meijide F.; de Frutos S.; Jover A.; Fraga F.; Soto V. H. Introduction to Biosurfactants. Biosurfactants for a Sustainable Future 2021, 1–42. 10.1002/9781119671022.ch1. [DOI] [Google Scholar]
- Rebello S.; Asok A. K.; Mundayoor S.; Jisha M. S.. Surfactants: Chemistry, Toxicity and Remediation. In Pollutant Diseases, Remediation and Recycling; Lichtfouse E., Schwarzbauer J., Robert D., Eds.; Springer International Publishing: 2013; pp 277–320. [Google Scholar]
- Henkel M.; Müller M. M.; Kügler J. H.; Lovaglio R. B.; Contiero J.; Syldatk C.; Hausmann R. Rhamnolipids as biosurfactants from renewable resources: Concepts for next-generation rhamnolipid production. Process Biochem. 2012, 47 (8), 1207–1219. 10.1016/j.procbio.2012.04.018. [DOI] [Google Scholar]
- Ostroumov S. A. Inhibitory analysis of top-down control: new keys to studying eutrophication, algal blooms, and water self-purification. Hydrobiologia 2002, 469 (1), 117–129. 10.1023/A:1015559123646. [DOI] [Google Scholar]
- Ostroumov S. A. The Synecological Approach to the Problem of Eutrophication. Dokl. Biol. Sci. 2001, 381 (1), 559–562. 10.1023/A:1013378505630. [DOI] [PubMed] [Google Scholar]
- Khan M. N.; Mohammad F.. Eutrophication: Challenges and Solutions. In Eutrophication: Causes, Consequences and Control; Ansari A. A., Gill S. S., Eds.; Springer Netherlands: 2014; Vol. 2, pp 1–15. [Google Scholar]
- Phukan M. M.; Sangma S. R.; Kalita D.; Pankaj P. P.; Das P. P.; Bora P.; Saha J.; Manoj K.; Hazarika N.; Kataki R.. Chapter 18 - Next-generational biosurfactant and their practical application in the food industry. In Applications of Next Generation Biosurfactants in the Food Sector; Inamuddin, Adetunji C. O., Eds.; Academic Press: 2023; pp 361–389. [Google Scholar]
- Dereszewska A. K.; Cytawa S.; Tomczak-Wandzel R.; Medrzycka K. The Effect of Anionic Surfactant Concentration on Activated Sludge Condition and Phosphate Release in Biological Treatment Plant. Polym. J. Environ. Stud. 2015, 24 (1), 83–91. 10.15244/pjoes/28640. [DOI] [Google Scholar]
- Tripathy D. B.; Mishra A.; Clark J.; Farmer T. Synthesis, chemistry, physicochemical properties and industrial applications of amino acid surfactants: A review. C. R. Chim. 2018, 21 (2), 112–130. 10.1016/j.crci.2017.11.005. [DOI] [Google Scholar]
- Tripathi L.; Irorere V. U.; Marchant R.; Banat I. M. Marine derived biosurfactants: a vast potential future resource. Biotechnol. Lett. 2018, 40 (11), 1441–1457. 10.1007/s10529-018-2602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P.; Trybala A.; Starov V.; Pinfield V. J. Effect of synthetic surfactants on the environment and the potential for substitution by biosurfactants. Adv. Colloid Interface Sci. 2021, 288, 102340. 10.1016/j.cis.2020.102340. [DOI] [PubMed] [Google Scholar]
- Madeddu S.; Ueckerdt F.; Pehl M.; Peterseim J.; Lord M.; Kumar K. A.; Krüger C.; Luderer G. The CO2 reduction potential for the European industry via direct electrification of heat supply (power-to-heat). Environmental Research Letters 2020, 15 (12), 124004. 10.1088/1748-9326/abbd02. [DOI] [Google Scholar]
- Santos D. K. F.; Rufino R. D.; Luna J. M.; Santos V. A.; Sarubbo L. A. Biosurfactants: Multifunctional Biomolecules of the 21st Century. Int. J. Mol. Sci. 2016, 17 (3), 401. 10.3390/ijms17030401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadani A.; Kafle A.; Ogura T.; Akamatsu M.; Sakai K.; Sakai H.; Abe M. Current perspective of sustainable surfactants based on renewable building blocks. Curr. Opin. Colloid Interface Sci. 2020, 45, 124–135. 10.1016/j.cocis.2020.01.002. [DOI] [Google Scholar]
- Sarubbo L. A.; Silva M. d. G. C.; Durval I. J. B.; Bezerra K. G. O.; Ribeiro B. G.; Silva I. A.; Twigg M. S.; Banat I. M. Biosurfactants: Production, properties, applications, trends, and general perspectives. Biochem. Eng. J. 2022, 181, 108377. 10.1016/j.bej.2022.108377. [DOI] [Google Scholar]
- Rosen M. J.; Kunjappu J. T.. Surfactants and Interfacial Phenomena; John Wiley & Sons: 2012. [Google Scholar]
- Ortiz M. S.; Alvarado J. G.; Zambrano F.; Marquez R. Surfactants produced from carbohydrate derivatives: A review of the biobased building blocks used in their synthesis. J. Surfactants Deterg. 2022, 25 (2), 147–183. 10.1002/jsde.12581. [DOI] [Google Scholar]
- Cserháti T.; Forgács E.; Oros G. Biological activity and environmental impact of anionic surfactants. Environ. Int. 2002, 28 (5), 337–348. 10.1016/S0160-4120(02)00032-6. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Liu X.; Zhang M.; Wang T.; Tang H.; Jia Y. The effect of pH/PAC on the coagulation of anionic surfactant wastewater generated in the cosmetic production. J. Environ. Chem. Eng. 2023, 11 (2), 109312. 10.1016/j.jece.2023.109312. [DOI] [Google Scholar]
- Bazel Y. R.; Antal I. P.; Lavra V. M.; Kormosh Z. A. Methods for the determination of anionic surfactants. J. Anal. Chem. 2014, 69 (3), 211–236. 10.1134/S1061934814010043. [DOI] [Google Scholar]
- Bartnik F. G.Interaction of anionic surfactants with proteins, enzymes, and membranes. In Anionic Surfactants: Biochemistry, Toxicology, Dermatology; Gloxhuber C., Klunstler K., Eds.; Surface Science Series 43; Marcel Dekker: 1992; p 1. [Google Scholar]
- Lin B.; McCormick A. V.; Davis H. T.; Strey R. Solubility of sodium soaps in aqueous salt solutions. J. Colloid Interface Sci. 2005, 291 (2), 543–549. 10.1016/j.jcis.2005.05.036. [DOI] [PubMed] [Google Scholar]
- Rebello S.; Asok A. K.; Mundayoor S.; Jisha M. S. Surfactants: toxicity, remediation and green surfactants. Environ. Chem. Lett. 2014, 12 (2), 275–287. 10.1007/s10311-014-0466-2. [DOI] [Google Scholar]
- Zakharova L. Y.; Pashirova T. N.; Doktorovova S.; Fernandes A. R.; Sanchez-Lopez E.; Silva A. M.; Souto S. B.; Souto E. B. Cationic Surfactants: Self-Assembly, Structure-Activity Correlation and Their Biological Applications. Int. J. Mol. Sci. 2019, 20 (22), 5534. 10.3390/ijms20225534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mynacik D.; Devinsky F.; Lacko I. Influence of counterions on antimicrobial activity of quaternary ammonium salts. Eur. J. Pharm. Sci. 1996, 4 (4), S191. 10.1016/S0928-0987(97)86582-7. [DOI] [Google Scholar]
- Chen A.; Karanastasis A.; Casey K. R.; Necelis M.; Carone B. R.; Caputo G. A.; Palermo E. F. Cationic Molecular Umbrellas as Antibacterial Agents with Remarkable Cell-Type Selectivity. ACS Appl. Mater. Interfaces 2020, 12 (19), 21270–21282. 10.1021/acsami.9b19076. [DOI] [PubMed] [Google Scholar]
- Zhou M.; Li S.; Zhang Z.; Wang C.; Luo G.; Zhao J. Progress in the Synthesis of Zwitterionic Gemini Surfactants. J. Surfactants Deterg. 2017, 20 (6), 1243–1254. 10.1007/s11743-017-2014-0. [DOI] [Google Scholar]
- FernLey G. W. Zwitterionic surfactants: Structure and performance. J. Am. Oil Chem. Soc. 1978, 55 (1), 98–103. 10.1007/BF02673397. [DOI] [Google Scholar]
- Gerola A. P.; Costa P. F. A.; Quina F. H.; Fiedler H. D.; Nome F. Zwitterionic surfactants in ion binding and catalysis. Curr. Opin. Colloid Interface Sci. 2017, 32, 39–47. 10.1016/j.cocis.2017.10.002. [DOI] [Google Scholar]
- Jiao J. Polyoxyethylated nonionic surfactants and their applications in topical ocular drug delivery. Adv. Drug Delivery Rev. 2008, 60 (15), 1663–1673. 10.1016/j.addr.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Cserháti T. Alkyl Ethoxylated and Alkylphenol Ethoxylated Nonionic Surfactants: Interaction with Bioactive Compounds and Biological Effects. Environ. Health Perspect. 1995, 103 (4), 358–364. 10.1289/ehp.103-1519097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G. P.; Rajeshwarrao P. Nonionic surfactant vesicular systems for effective drug delivery—an overview. Acta Pharm. Sin. B 2011, 1 (4), 208–219. 10.1016/j.apsb.2011.09.002. [DOI] [Google Scholar]
- Lindman B.; Medronho B.; Karlström G. Clouding of nonionic surfactants. Curr. Opin. Colloid Interface Sci. 2016, 22, 23–29. 10.1016/j.cocis.2016.01.005. [DOI] [Google Scholar]
- Bou-Chacra N.; Melo K. J. C.; Morales I. A. C.; Stippler E. S.; Kesisoglou F.; Yazdanian M.; Löbenberg R. Evolution of Choice of Solubility and Dissolution Media After Two Decades of Biopharmaceutical Classification System. AAPS Journal 2017, 19 (4), 989–1001. 10.1208/s12248-017-0085-5. [DOI] [PubMed] [Google Scholar]
- de Guertechin L. O.Classification of surfactants. In Handbook of Cosmetic Science and Technology; Barel A. O., Maibach H. I., Eds.; CRC Press: 2001; pp 431–450. [Google Scholar]
- Nishikido N.; Kobayashi H.; Tanaka M. Pressure effect on the Krafft points of ionic surfactants. J. Phys. Chem. 1982, 86 (16), 3170–3172. 10.1021/j100213a021. [DOI] [Google Scholar]
- Hill K.; Rhode O. Sugar-based surfactants for consumer products and technical applications. Lipid/Fett 1999, 101 (1), 25–33. . [DOI] [Google Scholar]
- Allen D. K.; Tao B. Y. Carbohydrate-alkyl ester derivatives as biosurfactants. J. Surfactants Deterg. 1999, 2 (3), 383–390. 10.1007/s11743-999-0093-4. [DOI] [Google Scholar]
- Gaudin T.; Lu H.; Fayet G.; Berthauld-Drelich A.; Rotureau P.; Pourceau G.; Wadouachi A.; Van Hecke E.; Nesterenko A.; Pezron I. Impact of the chemical structure on amphiphilic properties of sugar-based surfactants: A literature overview. Adv. Colloid Interface Sci. 2019, 270, 87–100. 10.1016/j.cis.2019.06.003. [DOI] [PubMed] [Google Scholar]
- González-Peña M. A.; Ortega-Regules A. E.; Anaya de Parrodi C.; Lozada-Ramírez J. D. Chemistry, Occurrence, Properties, Applications, and Encapsulation of Carotenoids—A Review. Plants 2023, 12 (2), 313. 10.3390/plants12020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Final Report on the Safety Assessment of Sorbitan Caprylate, Sorbitan Cocoate, Sorbitan Diisostearate, Sorbitan Dioleate, Sorbitan Distearate, Sorbitan Isostearate, Sorbitan Olivate, Sorbitan Sesquiisostearate, Sorbitan Sesquistearate, and Sorbitan Triisostearate. Int. J. Toxicol. 2002, 21, 93–112. 10.1080/10915810290096414. [DOI] [PubMed] [Google Scholar]
- Moldes A. B.; Rodríguez-López L.; Rincón-Fontán M.; López-Prieto A.; Vecino X.; Cruz J. M. Synthetic and Bio-Derived Surfactants Versus Microbial Biosurfactants in the Cosmetic Industry: An Overview. Int. J. Mol. Sci. 2021, 22 (5), 2371. 10.3390/ijms22052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Final Report on the Safety Assessment of PEG-6, -8, and -20 Sorbitan Beeswax. Int. J. Toxicol. 2001, 20, 27–38. 10.1080/10915810152902565. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Dubé M. A.. Green Emulsion Polymerization Technology. In Polymer Reaction Engineering of Dispersed Systems; Pauer W., Ed.; Springer International Publishing, 2018; Vol. I, pp 65–100. [Google Scholar]
- Sorbitan Esters Market Outlook (2023–2033). FMI, March 2023. https://www.futuremarketinsights.com/reports/sorbitan-esters-market (accessed 2022-10-19).
- Szűts A.; Szabó-Révész P. Sucrose esters as natural surfactants in drug delivery systems—A mini-review. Int. J. Pharm. 2012, 433 (1), 1–9. 10.1016/j.ijpharm.2012.04.076. [DOI] [PubMed] [Google Scholar]
- Geetha D.; Tyagi R. Alkyl Poly Glucosides (APGs) Surfactants and Their Properties: A Review. Tenside Surfactants Deterg. 2012, 49 (5), 417–427. 10.3139/113.110212. [DOI] [Google Scholar]
- Ng Y. J.; Lim H. R.; Khoo K. S.; Chew K. W.; Chan D. J. C.; Bilal M.; Munawaroh H. S. H.; Show P. L. Recent advances of biosurfactant for waste and pollution bioremediation: Substitutions of petroleum-based surfactants. Environ. Res. 2022, 212, 113126. 10.1016/j.envres.2022.113126. [DOI] [PubMed] [Google Scholar]
- Kapadia S. G.; Yagnik B. Current trend and potential for microbial biosurfactants. Asian J. Exp. Biol. Sci. 2013, 4 (1), 1–8. [Google Scholar]
- Banat I. M.; Franzetti A.; Gandolfi I.; Bestetti G.; Martinotti M. G.; Fracchia L.; Smyth T. J.; Marchant R. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010, 87 (2), 427–444. 10.1007/s00253-010-2589-0. [DOI] [PubMed] [Google Scholar]
- Marchant R.; Banat I. M. Microbial biosurfactants: challenges and opportunities for future exploitation. Trends Biotechnol. 2012, 30 (11), 558–565. 10.1016/j.tibtech.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Naughton P. J.; Marchant R.; Naughton V.; Banat I. M. Microbial biosurfactants: current trends and applications in agricultural and biomedical industries. J. Appl. Microbiol. 2019, 127 (1), 12–28. 10.1111/jam.14243. [DOI] [PubMed] [Google Scholar]
- Lang S. Biological amphiphiles (microbial biosurfactants). Curr. Opin. Colloid Interface Sci. 2002, 7 (1), 12–20. 10.1016/S1359-0294(02)00007-9. [DOI] [Google Scholar]
- Grüninger J.; Delavault A.; Ochsenreither K. Enzymatic glycolipid surfactant synthesis from renewables. Process Biochem. 2019, 87, 45–54. 10.1016/j.procbio.2019.09.023. [DOI] [Google Scholar]
- Sałek K.; Euston S. R.; Janek T. Phase Behaviour, Functionality, and Physicochemical Characteristics of Glycolipid Surfactants of Microbial Origin. Front. Bioeng. Biotechnol. 2022, 10, 816613. 10.3389/fbioe.2022.816613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto D.; Morita T.; Fukuoka T.; Konishi M.-a.; Imura T. Self-assembling properties of glycolipid biosurfactants and their potential applications. Curr. Opin. Colloid Interface Sci. 2009, 14 (5), 315–328. 10.1016/j.cocis.2009.05.009. [DOI] [Google Scholar]
- Cortés-Sánchez A. d. J.; Hernández-Sánchez H.; Jaramillo-Flores M. E. Biological activity of glycolipids produced by microorganisms: New trends and possible therapeutic alternatives. Microbiol. Res. 2013, 168 (1), 22–32. 10.1016/j.micres.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Pöhnlein M.; Hausmann R.; Lang S.; Syldatk C. Enzymatic synthesis and modification of surface-active glycolipids. Eur. J. Lipid Sci. Technol. 2015, 117 (2), 145–155. 10.1002/ejlt.201400418. [DOI] [Google Scholar]
- Dusane D. H.; Pawar V. S.; Nancharaiah Y. V.; Venugopalan V. P.; Kumar A. R.; Zinjarde S. S. Anti-biofilm potential of a glycolipid surfactant produced by a tropical marine strain of Serratia marcescens. Biofouling 2011, 27 (6), 645–654. 10.1080/08927014.2011.594883. [DOI] [PubMed] [Google Scholar]
- Soberón-Chávez G.; Lépine F.; Déziel E. Production of rhamnolipids by Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2005, 68 (6), 718–725. 10.1007/s00253-005-0150-3. [DOI] [PubMed] [Google Scholar]
- Kumar R.; Das A. J.. Application of Rhamnolipids in Medical Sciences. In Rhamnolipid Biosurfactant: Recent Trends in Production and Application; Springer Singapore: 2018; pp 79–87. [Google Scholar]
- Jiang L.; Shen C.; Long X.; Zhang G.; Meng Q. Rhamnolipids elicit the same cytotoxic sensitivity between cancer cell and normal cell by reducing surface tension of culture medium. Appl. Microbiol. Biotechnol. 2014, 98 (24), 10187–10196. 10.1007/s00253-014-6065-0. [DOI] [PubMed] [Google Scholar]
- Elshikh M.; Funston S.; Chebbi A.; Ahmed S.; Marchant R.; Banat I. M. Rhamnolipids from non-pathogenic Burkholderia thailandensis E264: Physicochemical characterization, antimicrobial and antibiofilm efficacy against oral hygiene related pathogens. New Biotechnology 2017, 36, 26–36. 10.1016/j.nbt.2016.12.009. [DOI] [PubMed] [Google Scholar]
- Yi G.; Son J.; Yoo J.; Park C.; Koo H. Rhamnolipid nanoparticles for in vivo drug delivery and photodynamic therapy. Nanomedicine: Nanotechnology, Biology and Medicine 2019, 19, 12–21. 10.1016/j.nano.2019.03.015. [DOI] [PubMed] [Google Scholar]
- Pratap A.; Wadekar S.; Kale S.; Lali A.; Bhowmick D. N. Non-traditional Oils as Newer Feedstock for Rhamnolipids Production by Pseudomonas aeruginosa (ATCC 10145). J. Am. Oil Chem. Soc. 2011, 88 (12), 1935–1943. 10.1007/s11746-011-1875-z. [DOI] [Google Scholar]
- Oluwaseun A. C.; Phazang P.; Sarin N. B. Significance of rhamnolipids as a biological control agent in the management of crops/plant pathogens. Curr. Trends biomed. Eng. Biosci. 2017, 10 (3), 555788. 10.19080/CTBEB.2017.10.555788. [DOI] [Google Scholar]
- Bayee P.; Amani H.; Najafpour G. D.; Kariminezhad H. Experimental Investigations on Behaviour of Rhamnolipid Biosurfactant as a Green Stabilizer for the Biological Synthesis of Gold Nanoparticles. Int. J. Eng. 2020, 33 (6), 1054–1060. 10.5829/IJE.2020.33.06C.02. [DOI] [Google Scholar]
- Moussa Z.; Chebl M.; Patra D. Interaction of curcumin with 1,2-dioctadecanoyl-sn-glycero-3-phosphocholine liposomes: Intercalation of rhamnolipids enhances membrane fluidity, permeability and stability of drug molecule. Colloids Surf., B 2017, 149, 30–37. 10.1016/j.colsurfb.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Bordas F.; Lafrance P.; Villemur R. Conditions for effective removal of pyrene from an artificially contaminated soil using Pseudomonas aeruginosa 57SJ rhamnolipids. Environ. Pollut. 2005, 138 (1), 69–76. 10.1016/j.envpol.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Intasit R.; Soontorngun N. Enhanced palm oil-derived sophorolipid production from yeast to generate biodegradable plastic precursors. Ind. Crops Prod. 2023, 192, 116091. 10.1016/j.indcrop.2022.116091. [DOI] [Google Scholar]
- Xu R.-Q.; Ma L.; Chen T.; Zhang W.-X.; Chang K.; Wang J.. Sophorolipid inhibits histamine-induced itch by decreasing PLC/IP3R signaling pathway activation and modulating TRPV1 activity. Research Square, January 5, 2023, ver. 1. 10.21203/rs.3.rs-2427024/v1. [DOI] [PMC free article] [PubMed]
- Zhang X.; Wang Y.; Lu J.; Liu M.; Tan W.; Cheng Y.; Tao Y.; Du J.; Wang H. Biosurfactant promoted enzymatic saccharification of alkali-pretreated reed straw. Bioresour. Technol. 2023, 372, 128665. 10.1016/j.biortech.2023.128665. [DOI] [PubMed] [Google Scholar]
- Nascimento M. F.; Keković P.; Ribeiro I. A. C.; Faria N. T.; Ferreira F. C. Novel Organic Solvent Nanofiltration Approaches for Microbial Biosurfactants Downstream Processing. Membranes 2023, 13 (1), 81. 10.3390/membranes13010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matosinhos R. D.; Cesca K.; Carciofi B. A. M.; de Oliveira D.; de Andrade C. J. Mannosylerythritol lipids as green pesticides and plant biostimulants. J. Sci. Food Agric. 2023, 103 (1), 37–47. 10.1002/jsfa.12100. [DOI] [PubMed] [Google Scholar]
- Yang Q.; Shen L.; Yu F.; Zhao M.; Jin M.; Deng S.; Long X. Enhanced fermentation of biosurfactant mannosylerythritol lipids on the pilot scale under efficient foam control with addition of soybean oil. Food Bioprod. Process. 2023, 138, 60–69. 10.1016/j.fbp.2023.01.002. [DOI] [Google Scholar]
- Mawani J. S.; Mali S. N.; Pratap A. P. Formulation and evaluation of antidandruff shampoo using mannosylerythritol lipid (MEL) as a bio-surfactant. Tenside Surfactants Deterg. 2023, 60 (1), 44–53. 10.1515/tsd-2022-2449. [DOI] [Google Scholar]
- de Oliveira Schmidt V. K.; de Vasconscelos G. M. D.; Vicente R.; de Souza Carvalho J.; Della-Flora I. K.; Degang L.; de Oliveira D.; de Andrade C. J. Cassava wastewater valorization for the production of biosurfactants: surfactin, rhamnolipids, and mannosileritritol lipids. World J. Microbiol. Biotechnol. 2023, 39 (2), 65. 10.1007/s11274-022-03510-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalafi H.; Ahmadi S. A comparative study of Mannosylerythritol lipids (MELs) surfactant adsorption upon the Al12N12 and B12N12 nano-cages as potential candidates for detecting MELs. J. Indian Chem. Soc. 2023, 100 (1), 100805. 10.1016/j.jics.2022.100805. [DOI] [Google Scholar]
- Selva Filho A. A. P.; Converti A.; Soares da Silva R. d. C. F.; Sarubbo L. A. Biosurfactants as Multifunctional Remediation Agents of Environmental Pollutants Generated by the Petroleum Industry. Energies 2023, 16 (3), 1209. 10.3390/en16031209. [DOI] [Google Scholar]
- Franzetti A.; Gandolfi I.; Bestetti G.; Smyth T. J. P.; Banat I. M. Production and applications of trehalose lipid biosurfactants. Eur. J. Lipid Sci. Technol. 2010, 112 (6), 617–627. 10.1002/ejlt.200900162. [DOI] [Google Scholar]
- Falardeau J.; Wise C.; Novitsky L.; Avis T. J. Ecological and Mechanistic Insights Into the Direct and Indirect Antimicrobial Properties of Bacillus subtilis Lipopeptides on Plant Pathogens. J. Chem. Ecol. 2013, 39 (7), 869–878. 10.1007/s10886-013-0319-7. [DOI] [PubMed] [Google Scholar]
- Meena K. R.; Kanwar S. S. Lipopeptides as the Antifungal and Antibacterial Agents: Applications in Food Safety and Therapeutics. Biomed Res. Int. 2015, 2015, 473050. 10.1155/2015/473050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaligram N. S.; Singhal R. S. Surfactin-a review on biosynthesis, fermentation, purification and applications. Food Sci. Biotechnol. 2010, 48 (2), 119–134. [Google Scholar]
- Microbial Biosurfactants Market (2022–2032). Fact.MR. https://www.factmr.com/report/microbial-biosurfactants-market (accessed 2023-10-19).
- Morán M. C.; Pinazo A.; Pérez L.; Clapés P.; Angelet M.; García M. T.; Vinardell M. P.; Infante M. R. “Green” amino acid-based surfactants. Green Chem. 2004, 6 (5), 233–240. 10.1039/B400293H. [DOI] [Google Scholar]
- Chandra N.; Tyagi V. K. Synthesis, Properties, and Applications of Amino Acids Based Surfactants: A Review. J. Dispersion Sci. Technol. 2013, 34 (6), 800–808. 10.1080/01932691.2012.695967. [DOI] [Google Scholar]
- Dunuweera S. P.; Rajapakse R. M. S. I.; Rajapakshe R. B. S. D.; Wijekoon S. H. D. P.; Nirodha Thilakarathna M. G. G. S.; Rajapakse R. M. G. Review on Targeted Drug Delivery Carriers Used in Nanobiomedical Applications. Curr. Nanosci. 2019, 15 (4), 382–397. 10.2174/1573413714666181106114247. [DOI] [Google Scholar]
- Gayen K.; Basu K.; Bairagi D.; Castelletto V.; Hamley I. W.; Banerjee A. Amino-Acid-Based Metallo-Hydrogel That Acts Like an Esterase. ACS Appl. Bio Mater. 2018, 1 (5), 1717–1724. 10.1021/acsabm.8b00513. [DOI] [PubMed] [Google Scholar]
- Infante M. R.; Pérez L.; Pinazo A.; Clapés P.; Morán M. C.; Angelet M.; García M. T.; Vinardell M. P. Amino acid-based surfactants. C. R. Chim. 2004, 7 (6), 583–592. 10.1016/j.crci.2004.02.009. [DOI] [Google Scholar]
- Menger F. M.; Keiper J. S. Gemini Surfactants. Angew. Chem., Int. Ed. 2000, 39 (11), 1906–1920. . [DOI] [PubMed] [Google Scholar]
- Menger F. M.; Littau C. A. Gemini-surfactants: synthesis and properties. J. Am. Chem. Soc. 1991, 113 (4), 1451–1452. 10.1021/ja00004a077. [DOI] [Google Scholar]
- Zhou G.; Wang Q.; Li S.; Huang Q.; Liu Z. Effect of a newly synthesized anionic Gemini surfactant composite fracturing system on the wettability of coking coal. Process Saf. Environ. Prot. 2023, 169, 13–23. 10.1016/j.psep.2022.10.084. [DOI] [Google Scholar]
- Lu Z.; Zongjie G.; Qianyu Z.; Xueyan L.; Kexin W.; Baoyan C.; Ran T.; Fang R.; Hui H.; Huali C. Preparation and characterization of a gemini surfactant-based biomimetic complex for gene delivery. Eur. J. Pharm. Biopharm. 2023, 182, 92–102. 10.1016/j.ejpb.2022.12.002. [DOI] [PubMed] [Google Scholar]
- Akram M.; Osama M.; Lal H.; Salim M.; Hashmi M. A.; Din K.-u. Biophysical investigation of the interaction between NSAID ibuprofen and cationic biodegradable Cm-E2O2-Cm gemini surfactants. J. Mol. Liq. 2023, 370, 120972. 10.1016/j.molliq.2022.120972. [DOI] [Google Scholar]
- Ansari F.; Lal H.; Osama M.; Akram M.; Kabir-ud-Din Solution Behavior of Bovine Skin Gelatin in the Presence of Cationic Gemini Surfactants. ChemistrySelect 2023, 8 (3), e202202080 10.1002/slct.202202080. [DOI] [Google Scholar]
- Pavlov R. V.; Gaynanova G. A.; Kuznetsov D. M.; Ivanov Y. A.; Amerkhanova S. K.; Lyubina A. P.; Voloshina A. D.; Zakharova L. Y. A study involving PC-3 cancer cells and novel carbamate gemini surfactants: Is zeta potential the key to control adhesion to cells?. Smart Mater. Struct. 2023, 4, 123–133. 10.1016/j.smaim.2022.09.001. [DOI] [Google Scholar]
- Varka E. M.; Coutouli-Argyropoulou E.; Infante M. R.; Pegiadou S. Synthesis, characterization, and surface properties of phenylalanine-glycerol ether surfactants. J. Surfactants Deterg. 2004, 7 (4), 409–414. 10.1007/s11743-004-0325-7. [DOI] [Google Scholar]
- Lozano N.; Pérez L.; Pons R.; Pinazo A. Diacyl glycerol arginine-based surfactants: biological and physicochemical properties of catanionic formulations. Amino Acids 2011, 40 (2), 721–729. 10.1007/s00726-010-0710-4. [DOI] [PubMed] [Google Scholar]
- Benavides T.; Martınez V.; Mitjans M.; Infante M. a. R.; Moran C.; Clapés P.; Clothier R.; Vinardell M. a. P. Assessment of the potential irritation and photoirritation of novel amino acid-based surfactants by in vitro methods as alternative to the animal tests. Toxicology 2004, 201 (1), 87–93. 10.1016/j.tox.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Infante M. R.; Pérez L.; Morán M. C.; Pons R.; Mitjans M.; Vinardell M. P.; Garcia M. T.; Pinazo A. Biocompatible surfactants from renewable hydrophiles. Eur. J. Lipid Sci. Technol. 2010, 112 (1), 110–121. 10.1002/ejlt.200900110. [DOI] [Google Scholar]
- Adlercreutz P.; Hatti-Kaul R.. Synthesis of surfactants using enzymes. In Surfactants from Renewable Resources; Kjellin M., Johansson I., Eds.; Wiley: 2010; p 143. 10.1002/9780470686607.ch8. [DOI] [Google Scholar]
- Amino Acid based Surfactants Market Trends, Size, Share, Top Players, Opportunities, Revenue, Regional Analysis, Future Growth by 2022–2028. Digital Journal. August 30, 2022. https://www.digitaljournal.com/pr/amino-acid-based-surfactants-market-trends-size-share-top-players-opportunities-revenue-regional-analysis-future-growth-by-2022-2028.
- Johansson I.; Svensson M. Surfactants based on fatty acids and other natural hydrophobes. Curr. Opin. Colloid Interface Sci. 2001, 6 (2), 178–188. 10.1016/S1359-0294(01)00076-0. [DOI] [Google Scholar]
- Sharma K.; Negi S.; Thakur N.; Kishore K. Partial glycerides-an important nonionic surfactant for industrial applications: an overview. J. Biol. Chem. Chron. 2017, 3 (1), 10–19. [Google Scholar]
- Hasenhuettl G. L.Analysis of Food Emulsifiers. In Food Emulsifiers and Their Applications, 2nd ed.; Hasenhuettl G. L., Hartel R. W., Eds.; Springer: 2008; pp 39–62. [Google Scholar]
- Polyglycerol Esters Market Players, Report, Statistics, Trends, Research, Drivers, & Forecast Analysis By 2029. Data Bridge Market Research.https://www.databridgemarketresearch.com/reports/global-polyglycerol-esters-market (accessed 2022-10-19).
- Farias C. B. B.; Almeida F. C. G.; Silva I. A.; Souza T. C.; Meira H. M.; Soares da Silva R. d. C. F.; Luna J. M.; Santos V. A.; Converti A.; Banat I. M.; et al. Production of green surfactants: Market prospects. Electron. J. Biotechnol. 2021, 51, 28–39. 10.1016/j.ejbt.2021.02.002. [DOI] [Google Scholar]
- Piispanen P.Synthesis and Characterization of Surfactants Based on Natural Products. B.Sc. Thesis, Kungl Tekniska Högskolan, Stockholm, 2002. [Google Scholar]
- Ivanković T.; Hrenović J. Surfactants in the environment. Arh. Hig. Rada Toksikol. 2010, 61 (1), 95–109. 10.2478/10004-1254-61-2010-1943. [DOI] [PubMed] [Google Scholar]
- Mungray A. K.; Kumar P. Fate of linear alkylbenzene sulfonates in the environment: A review. Int. Biodeterior. Biodegrad. 2009, 63 (8), 981–987. 10.1016/j.ibiod.2009.03.012. [DOI] [Google Scholar]
- Stamatelatou K.; Pakou C.; Lyberator G.. Occurrence, toxicity, and biodegradation of selected emerging priority pollutants in municipal sewage sludge. In Comprehensive Biotechnology; Elsevier: 2011; Vol. 6, pp 473–484. [Google Scholar]
- Fekarcha L.; Tazerouti A. Surface Activities, Foam Properties, HLB, and Krafft Point of Some n-Alkanesulfonates (C14-C18) with Different Isomeric Distributions. J. Surfactants Deterg. 2012, 15 (4), 419–431. 10.1007/s11743-012-1335-2. [DOI] [Google Scholar]
- Adami I.Production of linear alkylbenzene sulfonate and α-olefin sulfonates. In Handbook of Detergents, Part F: Production; Taylor & Francis: 2009; pp 83–115. [Google Scholar]
- Caryl C. R. Sulfosuccinic Esters. Structure and Wetting Power. Ind. Eng. Chem. Res. 1941, 33 (6), 731–737. 10.1021/ie50378a011. [DOI] [Google Scholar]
- Deepika; Tyagi V. K. Sulfosuccinates as Mild Surfactants. J. Oleo Sci. 2006, 55 (9), 429–439. 10.5650/jos.55.429. [DOI] [Google Scholar]
- Jensen J. Fate and effects of linear alkylbenzene sulphonates (LAS) in the terrestrial environment. Sci. Total Environ. 1999, 226 (2), 93–111. 10.1016/S0048-9697(98)00395-7. [DOI] [PubMed] [Google Scholar]
- Hulzebos E. M.; Maslankiewicz L.; Walker J. D. Verification of literature-derived SARs for skin irritation and corrosion. QSAR & Combinatorial Science 2003, 22 (3), 351–363. 10.1002/qsar.200390025. [DOI] [Google Scholar]
- Luan H.; Gong L.; Yue X.; Nie X.; Chen Q.; Guan D.; Que T.; Liao G.; Su X.; Feng Y. Micellar Aggregation Behavior of Alkylaryl Sulfonate Surfactants for Enhanced Oil Recovery. Molecules 2019, 24 (23), 4325. 10.3390/molecules24234325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penteado J. C. P.; El Seoud O. A.; Carvalho L. R. Linear alkylbenzene sulfonates: chemistry, environmental impact and analysis. Quim. Nova 2006, 29, 1038–1046. 10.1590/S0100-40422006000500025. [DOI] [Google Scholar]
- Wibbertmann A.; Mangelsdorf I.; Gamon K.; Sedlak R. Toxicological properties and risk assessment of the anionic surfactants category: Alkyl sulfates, primary alkane sulfonates, and α-olefin sulfonates. Ecotoxicol. Environ. Saf. 2011, 74 (5), 1089–1106. 10.1016/j.ecoenv.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Bajpai D.; Tyagi V. K. Laundry Detergents: An Overview. J. Oleo Sci. 2007, 56 (7), 327–340. 10.5650/jos.56.327. [DOI] [PubMed] [Google Scholar]
- Adam R. Skin Care of the Diaper Area. Pediatr. Dermatol. 2008, 25 (4), 427–433. 10.1111/j.1525-1470.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- DeLeo P. C.; Huynh C.; Pattanayek M.; Schmid K. C.; Pechacek N. Assessment of ecological hazards and environmental fate of disinfectant quaternary ammonium compounds. Ecotoxicol. Environ. Saf. 2020, 206, 111116. 10.1016/j.ecoenv.2020.111116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel R. K., Ratledge C.. Biosynthetic mechanisms of low molecular weight surfactants and their precursor molecules; Biosurfactants: Production, Properties, Applications; Kosaric N., Ed.; Marcel Dekker: 1993; pp 3–63. [Google Scholar]
- Desai J.; Desai A.. Production of Biosurfactants. In Biosurfactants: Production, Properties, Applications, Kosaric N., Ed.; Marcel Dekker: 1993; pp 65–97. [Google Scholar]
- Syldatk C.; Wagner F.. Production of Biosurfactants. In Biosurfactants and Biotechnology; Kosaric N., Ed.; Marcel Dekker, 1987; pp 89–120. [Google Scholar]
- Desai J. D.; Banat I. M. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 1997, 61 (1), 47–64. 10.1128/mmbr.61.1.47-64.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saharan B.; Sahu R.; Sharma D. A review on biosurfactants: fermentation, applications, current. Genet. Eng. Biotechnol. J. 2011, 29, 1–42. [Google Scholar]
- Neta N. S.; Teixeira J. A.; Rodrigues L. R. Sugar Ester Surfactants: Enzymatic Synthesis and Applications in Food Industry. Crit. Rev. Food Sci. Nutr. 2015, 55 (5), 595–610. 10.1080/10408398.2012.667461. [DOI] [PubMed] [Google Scholar]
- Clapés P.; Rosa Infante M. Amino Acid-based Surfactants: Enzymatic Synthesis, Properties and Potential Applications. Biocatal. Biotransform. 2002, 20 (4), 215–233. 10.1080/10242420290004947. [DOI] [Google Scholar]
- Behzadnia A.; Moosavi-Nasab M.; Ojha S.; Tiwari B. K. Exploitation of Ultrasound Technique for Enhancement of Microbial Metabolites Production. Molecules 2020, 25 (22), 5473. 10.3390/molecules25225473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadnia A.; Moosavi-Nasab M.; Tiwari B. K.; Setoodeh P. Lactobacillus plantarum-derived biosurfactant: Ultrasound-induced production and characterization. Ultrason. Sonochem. 2020, 65, 105037. 10.1016/j.ultsonch.2020.105037. [DOI] [PubMed] [Google Scholar]
- Panda D.; Saharan V. K.; Manickam S. Controlled Hydrodynamic Cavitation: A Review of Recent Advances and Perspectives for Greener Processing. Processes 2020, 8 (2), 220. 10.3390/pr8020220. [DOI] [Google Scholar]
- Rocha e Silva F. C. P.; Rocha e Silva N. M. P.; Luna J. M.; Rufino R. D.; Santos V. A.; Sarubbo L. A. Dissolved air flotation combined to biosurfactants: a clean and efficient alternative to treat industrial oily water. Rev. Environ. Sci. Biotechnol. 2018, 17 (4), 591–602. 10.1007/s11157-018-9477-y. [DOI] [Google Scholar]
- Delazar A.; Nahar L.; Hamedeyazdan S.; Sarker S. D.. Microwave-Assisted Extraction in Natural Products Isolation. In Natural Products Isolation; Sarker S. D., Nahar L., Eds.; Humana Press: 2012; pp 89–115. [DOI] [PubMed] [Google Scholar]
- Veggi P. C.; Martinez J.; Meireles M. A. A.. Fundamentals of Microwave Extraction. In Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice; Chemat F., Cravotto G., Eds.; Springer US: 2013; pp 15–52. [Google Scholar]
- Chen X.; Lu Y.; Shan M.; Zhao H.; Lu Z.; Lu Y. A mini-review: mechanism of antimicrobial action and application of surfactin. World J. Microbiol. Biotechnol. 2022, 38 (8), 143. 10.1007/s11274-022-03323-3. [DOI] [PubMed] [Google Scholar]
- Johansson I.; Somasundaran P.. Handbook for Cleaning/Decontamination of Surfaces; Elsevier: 2007. [Google Scholar]
- Hill K. Industrial development and application of biobased oleochemicals. Pure Appl. Chem. 2007, 79 (11), 1999–2011. 10.1351/pac200779111999. [DOI] [Google Scholar]
- Rabiu A.; Elias S.; Oyekola O.. Oleochemicals from palm oil for the petroleum industry. In Palm Oil; Waisundara V., Ed.; InTechOpen: 2018. 10.5772/intechopen.76771. [DOI] [Google Scholar]
- Jin Y.; Tian S.; Guo J.; Ren X.; Li X.; Gao S. Synthesis, Characterization and Exploratory Application of Anionic Surfactant Fatty Acid Methyl Ester Sulfonate from Waste Cooking Oil. J. Surfactants Deterg. 2016, 19 (3), 467–475. 10.1007/s11743-016-1813-z. [DOI] [Google Scholar]
- Benvegnu T.; Plusquellec D.; Lemiègre L.. Chapter 7 - Surfactants from Renewable Sources: Synthesis and Applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem M. N., Gandini A., Eds.; Elsevier: 2008; pp 153–178. [Google Scholar]
- Hayes D. G.Chapter 11 - Fatty Acids-Based Surfactants and Their Uses. In Fatty Acids; Ahmad M. U., Ed.; AOCS Press: 2017; pp 355–384. [Google Scholar]
- Lang R. F.; Parra-Diaz D.; Jacobs D. Analysis of ethoxylated fatty amines. Comparison of methods for the determination of molecular weight. J. Surfactants Deterg. 1999, 2 (4), 503–513. 10.1007/s11743-999-0099-y. [DOI] [Google Scholar]
- Manga E. B.; Celik P. A.; Cabuk A.; Banat I. M. Biosurfactants: Opportunities for the development of a sustainable future. Curr. Opin. Colloid Interface Sci. 2021, 56, 101514. 10.1016/j.cocis.2021.101514. [DOI] [Google Scholar]
- Gayathiri E.; Prakash P.; Karmegam N.; Varjani S.; Awasthi M. K.; Ravindran B. Biosurfactants: Potential and Eco-Friendly Material for Sustainable Agriculture and Environmental Safety—A Review. Agronomy 2022, 12 (3), 662. 10.3390/agronomy12030662. [DOI] [Google Scholar]
- McInerney M. J.; Maudgalya S.; Knapp R.; Folmsbee M.. Development of Biosurfactant-Mediated Oil Recovery in Model Porous Systems and Computer Simulations of Biosurfactant-Mediated Oil Recovery; University of Oklahoma: 2004.
- Purwasena I. A.; Astuti D. I.; Syukron M.; Amaniyah M.; Sugai Y. Stability test of biosurfactant produced by Bacillus licheniformis DS1 using experimental design and its application for MEOR. J. Pet. Sci. Eng. 2019, 183, 106383. 10.1016/j.petrol.2019.106383. [DOI] [Google Scholar]
- Muthusamy K.; Gopalakrishnan S.; Ravi T. K.; Sivachidambaram P. Biosurfactants: Properties, commercial production and application. Curr. Sci. 2008, 94 (6), 736–747. [Google Scholar]
- Adamu C. I.; Nganje T. N.; Edet A. Heavy metal contamination and health risk assessment associated with abandoned Barite mines in Cross River State, southeastern Nigeria. Environ. Nanotechnol. Monit. Manag. 2015, 3, 10–21. 10.1016/j.enmm.2014.11.001. [DOI] [Google Scholar]
- Guan R.; Yuan X.; Wu Z.; Wang H.; Jiang L.; Li Y.; Zeng G. Functionality of surfactants in waste-activated sludge treatment: A review. Sci. Total Environ. 2017, 609, 1433–1442. 10.1016/j.scitotenv.2017.07.189. [DOI] [PubMed] [Google Scholar]
- Ibrahim W. M.; Hassan A. F.; Azab Y. A. Biosorption of toxic heavy metals from aqueous solution by Ulva lactuca activated carbon. Egypt. J. Basic Appl. Sci. 2016, 3 (3), 241–249. 10.1016/j.ejbas.2016.07.005. [DOI] [Google Scholar]
- Tang J.; He J.; Liu T.; Xin X. Removal of heavy metals with sequential sludge washing techniques using saponin: optimization conditions, kinetics, removal effectiveness, binding intensity, mobility and mechanism. RSC Adv. 2017, 7 (53), 33385–33401. 10.1039/C7RA04284A. [DOI] [Google Scholar]
- da Rosa C. F. C.; Freire D. M. G.; Ferraz H. C. Biosurfactant microfoam: Application in the removal of pollutants from soil. J. Environ. Chem. Eng. 2015, 3 (1), 89–94. 10.1016/j.jece.2014.12.008. [DOI] [Google Scholar]
- Mali S. N.; Thorat B. R.; Gupta D. R.; Pandey A. Mini-Review of the Importance of Hydrazides and Their Derivatives—Synthesis and Biological Activity. Eng. Proc. 2021, 11 (1), 21. 10.3390/ASEC2021-11157. [DOI] [Google Scholar]
- Desale V. J.; Mali S. N.; Thorat B. R.; Yamgar R. S. Synthesis, admetSAR Predictions, DPPH Radical Scavenging Activity, and Potent Anti-mycobacterial Studies of Hydrazones of Substituted 4-(anilino methyl) benzohydrazides (Part 2). Current Computer - Aided Drug Design 2021, 17 (4), 493–503. 10.2174/1573409916666200615141047. [DOI] [PubMed] [Google Scholar]
- Thorat B. R.; Mali S. N.; Rani D.; Yamgar R. S. Synthesis, In silico and In vitro Analysis of Hydrazones as Potential Antituberculosis Agents. Current Computer - Aided Drug Design 2021, 17 (2), 294–306. 10.2174/1573409916666200302120942. [DOI] [PubMed] [Google Scholar]
- Rodrigues L.; Banat I. M.; Teixeira J.; Oliveira R. Biosurfactants: potential applications in medicine. J. Antimicrob. Chemother. 2006, 57 (4), 609–618. 10.1093/jac/dkl024. [DOI] [PubMed] [Google Scholar]
- De Giani A.; Zampolli J.; Di Gennaro P. Recent Trends on Biosurfactants With Antimicrobial Activity Produced by Bacteria Associated With Human Health: Different Perspectives on Their Properties, Challenges, and Potential Applications. Front. Microbiol. 2021, 12, 655150. 10.3389/fmicb.2021.655150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi C. C.; Santos-Gandelman J. F.; Barros E. M.; Alvarez V. M.; Laport M. S.; Giambiagi-deMarval M. Staphylococcus haemolyticus as a potential producer of biosurfactants with antimicrobial, anti-adhesive and synergistic properties. Lett. Appl. Microbiol. 2016, 63 (3), 215–221. 10.1111/lam.12611. [DOI] [PubMed] [Google Scholar]
- Rout S.; Tambe S.; Deshmukh R. K.; Mali S.; Cruz J.; Srivastav P. P.; Amin P. D.; Gaikwad K. K.; Andrade E. H. d. A.; Oliveira M. S. d. Recent trends in the application of essential oils: The next generation of food preservation and food packaging. Trends Food Sci. Technol. 2022, 129, 421–439. 10.1016/j.tifs.2022.10.012. [DOI] [Google Scholar]
- Jimoh A. A.; Lin J. Biosurfactant: A new frontier for greener technology and environmental sustainability. Ecotoxicol. Environ. Saf. 2019, 184, 109607. 10.1016/j.ecoenv.2019.109607. [DOI] [PubMed] [Google Scholar]
- Rofeal M.; El-Malek F. A. Valorization of Lipopeptides Biosurfactants as Anticancer Agents. Int. J. Pept. Res. Ther. 2021, 27 (1), 447–455. 10.1007/s10989-020-10105-8. [DOI] [Google Scholar]
- Chiewpattanakul P.; Phonnok S.; Durand A.; Marie E.; Thanomsub B. W. Bioproduction and anticancer activity of biosurfactant produced by the dematiaceous fungus Exophiala dermatitidis SK80. J. Microbiol. Biotechnol. 2010, 20 (12), 1664–1671. [PubMed] [Google Scholar]
- Escobar-Díaz E.; López-Martín E. M.; Hernández del Cerro M.; Puig-Kroger A.; Soto-Cerrato V.; Montaner B.; Giralt E.; García-Marco J. A.; Pérez-Tomás R.; Garcia-Pardo A. AT514, a cyclic depsipeptide from Serratia marcescens, induces apoptosis of B-chronic lymphocytic leukemia cells: interference with the Akt/NF-κB survival pathway. Leukemia 2005, 19 (4), 572–579. 10.1038/sj.leu.2403679. [DOI] [PubMed] [Google Scholar]
- Saini H. S.; Barragán-Huerta B. E.; Lebrón-Paler A.; Pemberton J. E.; Vázquez R. R.; Burns A. M.; Marron M. T.; Seliga C. J.; Gunatilaka A. A. L.; Maier R. M. Efficient Purification of the Biosurfactant Viscosin from Pseudomonas libanensis Strain M9–3 and Its Physicochemical and Biological Properties. J. Nat. Prod. 2008, 71 (6), 1011–1015. 10.1021/np800069u. [DOI] [PubMed] [Google Scholar]
- Isoda H.; Nakahara T. Mannosylerythritol lipid induces granulocytic differentiation and inhibits the tyrosine phosphorylation of human myelogenous leukemia cell line K562. Cytotechnology 1997, 25 (1), 191–195. 10.1023/A:1007982909932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda H.; Kitamoto D.; Shinmoto H.; Matsumura M.; Nakahara T. Microbial Extracellular Glycolipid Induction of Differentiation and Inhibition of the Protein Kinase C Activity of Human Promyelocytic Leukemia Cell Line HL60. Int. J. Pept. Res. Ther. 1997, 61 (4), 609–614. 10.1271/bbb.61.609. [DOI] [PubMed] [Google Scholar]
- Sudo T.; Zhao X.; Wakamatsu Y.; Shibahara M.; Nomura N.; Nakahara T.; Suzuki A.; Kobayashi Y.; Jin C.; Murata T.; et al. Induction of the differentiation of human HL-60 promyelocytic leukemia cell line by succinoyl trehalose lipids. Cytotechnology 2000, 33 (1), 259–264. 10.1023/A:1008137817944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X.; Wang A. H.; Jiao R. Z.; Wang C. L.; Mao D. Z.; Yan L.; Zeng B. Surfactin Induces Apoptosis and G2/M Arrest in Human Breast Cancer MCF-7 Cells Through Cell Cycle Factor Regulation. Cell Biochem. Biophys. 2009, 55 (3), 163. 10.1007/s12013-009-9065-4. [DOI] [PubMed] [Google Scholar]
- Wang C. L.; Ng T. B.; Yuan F.; Liu Z. K.; Liu F. Induction of apoptosis in human leukemia K562 cells by cyclic lipopeptide from Bacillus subtilis natto T-2. Peptides 2007, 28 (7), 1344–1350. 10.1016/j.peptides.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Park S. Y.; Kim J.-H.; Lee Y. J.; Lee S. J.; Kim Y. Surfactin suppresses TPA-induced breast cancer cell invasion through the inhibition of MMP-9 expression. Int. J. Oncol. 2013, 42 (1), 287–296. 10.3892/ijo.2012.1695. [DOI] [PubMed] [Google Scholar]
- Cao X.-h.; Wang A.-h.; Wang C.-l.; Mao D.-z.; Lu M.-f.; Cui Y.-q.; Jiao R.-z. Surfactin induces apoptosis in human breast cancer MCF-7 cells through a ROS/JNK-mediated mitochondrial/caspase pathway. Chem. Biol. Interact. 2010, 183 (3), 357–362. 10.1016/j.cbi.2009.11.027. [DOI] [PubMed] [Google Scholar]
- Dey G.; Bharti R.; Dhanarajan G.; Das S.; Dey K. K.; Kumar B.; Sen R.; Mandal M. Marine lipopeptide Iturin A inhibits Akt mediated GSK3β and FoxO3a signaling and triggers apoptosis in breast cancer. Sci. Rep. 2015, 5 (1), 10316. 10.1038/srep10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajare S. N.; Subramanian M.; Gautam S.; Sharma A. Induction of apoptosis in human cancer cells by a Bacillus lipopeptide bacillomycin D. Biochimie 2013, 95 (9), 1722–1731. 10.1016/j.biochi.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Yin H.; Guo C.; Wang Y.; Liu D.; Lv Y.; Lv F.; Lu Z. Fengycin inhibits the growth of the human lung cancer cell line 95D through reactive oxygen species production and mitochondria-dependent apoptosis. Anti-Cancer Drugs 2013, 24 (6), 587–598. 10.1097/CAD.0b013e3283611395. [DOI] [PubMed] [Google Scholar]
- Cheng W.; Feng Y.; Ren J.; Jing D.; Wang C. Anti-tumor role of Bacillus subtilis fmbJ-derived fengycin on human colon cancer HT29 cell line. Neoplasma 2016, 63 (2), 215–222. 10.4149/206_150518N270. [DOI] [PubMed] [Google Scholar]
- Singh P.; Cameotra S. S. Enhancement of metal bioremediation by use of microbial surfactants. Biochem. Biophys. Res. Commun. 2004, 319 (2), 291–297. 10.1016/j.bbrc.2004.04.155. [DOI] [PubMed] [Google Scholar]
- Mohanty S.; Jasmine J.; Mukherji S. Practical Considerations and Challenges Involved in Surfactant Enhanced Bioremediation of Oil. Biomed Res. Int. 2013, 2013, 328608. 10.1155/2013/328608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill P. F.; Dudley R. J.; Churchill S. A. Surfactant-enhanced bioremediation. Waste Manage. (Oxford) 1995, 15 (5), 371–377. 10.1016/0956-053X(95)00038-2. [DOI] [Google Scholar]
- Franzetti A.; Di Gennaro P.; Bestetti G.; Lasagni M.; Pitea D.; Collina E. Selection of surfactants for enhancing diesel hydrocarbons-contaminated media bioremediation. J. Hazard. Mater. 2008, 152 (3), 1309–1316. 10.1016/j.jhazmat.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Vaz D. A.; Gudiña E. J.; Alameda E. J.; Teixeira J. A.; Rodrigues L. R. Performance of a biosurfactant produced by a Bacillus subtilis strain isolated from crude oil samples as compared to commercial chemical surfactants. Colloids Surf., B 2012, 89, 167–174. 10.1016/j.colsurfb.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Hung H.-c.; Shreve G. S. Effect of the Hydrocarbon Phase on Interfacial and Thermodynamic Properties of Two Anionic Glycolipid Biosurfactants in Hydrocarbon/Water Systems. J. Phys. Chem. B 2001, 105 (50), 12596–12600. 10.1021/jp010930k. [DOI] [Google Scholar]
- Nitschke M.; Costa S. G. V. A. O.; Contiero J. Rhamnolipids and PHAs: Recent reports on Pseudomonas-derived molecules of increasing industrial interest. Process Biochem. 2011, 46 (3), 621–630. 10.1016/j.procbio.2010.12.012. [DOI] [Google Scholar]
- Lovaglio R. B.; dos Santos F. J.; Jafelicci M.; Contiero J. Rhamnolipid emulsifying activity and emulsion stability: pH rules. Colloids Surf., B 2011, 85 (2), 301–305. 10.1016/j.colsurfb.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Hogan D. E.; Tian F.; Malm S. W.; Olivares C.; Palos Pacheco R.; Simonich M. T.; Hunjan A. S.; Tanguay R. L.; Klimecki W. T.; Polt R.; et al. Biodegradability and toxicity of monorhamnolipid biosurfactant diastereomers. J. Hazard. Mater. 2019, 364, 600–607. 10.1016/j.jhazmat.2018.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhon Randhawa K. K.; Rahman P. K. S. M. Rhamnolipid biosurfactants—past, present, and future scenario of global market. Front. Microbiol. 2014, 5, 454. 10.3389/fmicb.2014.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almansoory A. F.; Idris M.; Abdullah S. R. S.; Anuar N. Screening for potential biosurfactant producing bacteria from hydrocarbon-degrading isolates. Adv. Environ. Biol. 2014, 639–648. [Google Scholar]
- Green Surfactants Market Size, Share, Price Analysis 2022–2027. Expert Market Research. https://www.expertmarketresearch.com/reports/green-surfactants-market (accessed 2022-08-19).
- Kandasamy R.; Rajasekaran M.; Venkatesan S. K.; Uddin M.. New Trends in the Biomanufacturing of Green Surfactants: Biobased Surfactants and Biosurfactants. In Next Generation Biomanufacturing Technologies; Rathinam N. K., Sani R. K., Eds.; ACS Symposium Series 1329; American Chemical Society, 2019; pp 243–260. 10.1021/bk-2019-1329.ch011. [DOI] [Google Scholar]
- Bland A.Assessing the sustainability and performance of green surfactants. IHS Markit, March 4, 2020. https://ihsmarkit.com/research-analysis/assessing-sustainability-and-performance-of-green-surfactants.html (accessed 2022-08-19).
- EcoSenseTM SL-60 HA Surfactant.
- EcoSenseTM 3000 Surfactant - Personal Care grade.
- BioToLife Postbiotic Rebalance Mouthwash.
- BASF announces 25th anniversary of APG production at Düsseldorf site.
- Entering a new era of surfactants - Evonik Industries.
- Velsan SC preservative booster for cosmetics.
- ArlasilkTM EFA|Personal Care.
- OleoCraftTM HP-33|Personal Care.
- Unilever and Evonik partner to launch green cleaning ingredient.
- Solvay launches new biosurfactants for carbon-neutral and circular beauty care products.
- AGAE|AGAE Technologies.
- Fraunhofer IGB - Wir verbinden Biologie and Technik - Fraunhofer IGB.
- Ismail N. L.; Shahruddin S.; Othman J.. Perspective Chapter: Overview of Bio-Based Surfactant—Recent Development, Industrial Challenge, and Future Outlook. In Surfactants and Detergents: Updates and New Insights; Kumar Dutta A., Ed.; IntechOpen: 2022. 10.5772/intechopen.100542. [DOI] [Google Scholar]
- Rodríguez-López L.; Rincón-Fontán M.; Vecino X.; Cruz J. M.; Moldes A. B. Extraction, separation and characterization of lipopeptides and phospholipids from corn steep water. Sep. Purif. Technol. 2020, 248, 117076. 10.1016/j.seppur.2020.117076. [DOI] [Google Scholar]
- Roelants S.; Van Renterghem L.; Maes K.; Everaert B.; Redant E.; Vanlerberghe B.; Demaeseneire S. L.; Soetaert W.. Microbial biosurfactants: from lab to market. In Microbial Biosurfactants and their Environmental and Industrial Applications; Banat I. M., Thavasi R., Eds.; CRC Press: 2019; pp 340–362. 10.1201/b21950-13. [DOI] [Google Scholar]
- Pal Y.; Mali S. N.; Kale S. B.; Pratap A. P. Improved adsorptive purification and effective separation of acidic and lactonic sophorolipid biosurfactant. J. Indian Chem. Soc. 2022, 99 (11), 100776. 10.1016/j.jics.2022.100776. [DOI] [Google Scholar]
- Pal Y.; Mali S. N.; Pratap A. P. Optimization of the primary purification process of extracting sphorolipid from the fermentation broth to achieve a higher yield and purity. Tenside Surfactants Deterg. 2022, 59 (5), 441–449. 10.1515/tsd-2022-2450. [DOI] [Google Scholar]
- Bhangale A. P.; Wadekar S. D.; Kale S. B.; Mali S. N.; Pratap A. P. Non-traditional oils with water-soluble substrate as cell growth booster for the production of mannosylerythritol lipids by Pseudozyma antarctica (ATCC 32657) with their antimicrobial activity. Tenside Surfactants Deterg. 2022, 59 (2), 122–133. 10.1515/tsd-2021-2366. [DOI] [Google Scholar]
- Sivagiri S. D.; Mali S. N.; Pratap A. P. Improved synthesis of sophorolipid biosurfactants using industrial by-products and their practical application. Tenside Surfactants Deterg. 2022, 59 (1), 17–30. 10.1515/tsd-2021-2365. [DOI] [Google Scholar]
- Pratap A. P.; Mestri R. S.; Mali S. N. Waste derived-green and sustainable production of Sophorolipid. Curr. Opin. Green Sustain. Chem. 2021, 4, 100209. 10.1016/j.crgsc.2021.100209. [DOI] [Google Scholar]
- Singh P.; Mali S.; Sangale N.; Pratap A. P. Synthesis of (2-hydroxyl-3-butoxyl) propyl-succinyl-chitosan (HBP-SCCHS)-an amino sugar anionic surfactant under Microwave Irradiation and its application. Thai J. Pharm. Sci. 2021, 45 (6), 461–469. [Google Scholar]