Abstract
Background
Cabotegravir delivered as a long-acting intramuscular injection has shown superior efficacy to oral tenofovir-emtricitabine as pre-exposure prophylaxis (PrEP) for HIV. Cabotegravir pharmacokinetics (PK), like those of other long-acting depot preparations, exhibit variability between individuals and between injection occasions.
Aim
To describe the population pharmacokinetics of long-acting cabotegravir (CAB-LA).
Methods
Using available PK measurements from 133 participants in the HIV Prevention Trials Network (HPTN) 077 trial, we analyzed CAB-LA PK data using nonlinear mixed-effects modeling to develop a population PK model.
Results
A two-compartment model with first order absorption best described the CAB-LA PK. The analysis identified between-occasional variability (BOV, i.e., differences in PK within one individual from one injection to the next) as a significant covariate affecting the absorption rate, with an estimated contribution of BOV to PK variability on the absorption rate (Ka) of 38.5%. Sex and body weight were identified as significant covariates influencing the absorption rate and apparent clearance of CAB-LA after intramuscular injection at various doses and frequencies. Male participants had 67% higher Ka than female participants. Serially adding to the model body weight on clearance, Sex on Ka, and BOV on Ka led to a decrease in the objective function value (OFV) of 24.4, 36, and 321.4, respectively.
Conclusion
The public availability of this model will facilitate and enable a wide variety of future clinically relevant simulations to inform the optimal use of CAB-LA.
Keywords: Antiretrovirals, Pharmacometrics, HIV/AIDS, Infectious Diseases
Introduction
The significant preventive efficacy of oral HIV pre-exposure prophylaxis (PrEP) has been undermined by the reality of incomplete adherence to daily oral medications, in both the real world and clinical trials context.1–3 The advent of novel, less-frequent dosing approaches for biomedical prevention offers the possibility of improved adherence and therefore improved protection against HIV.4 Cabotegravir, a compound closely chemically related to dolutegravir, is an integrase strand transfer inhibitor (INSTI) with potent activity against HIV in vitro and in vivo and characteristics that support its long-acting delivery as an extended-release injectable suspension.5,6
Cabotegravir pharmacokinetics (PK) differ according to formulation. When orally administered, it exhibits linear PK with multiple dose administration, with immediate absorption and low inter-individual variability in PK parameters, including rate of absorption (ka) and rate of clearance.7 When delivered as a long-acting injectable suspension, cabotegravir (CAB-LA) exhibits “flip-flop” PK that is defined by its absorption rate rather than its elimination, with a high degree of inter-individual variability.8,9
Dosing frequency of injectable CAB-LA has evolved as human data has accrued. The ÉCLAIR trial of CAB-LA among HIV-negative men administered a dose of 800 mg IM every 12 weeks, which had been predicted from modelling of human data following oral CAB (CAB n=288) and CAB LA (n=93) administration, including 9 healthy participants who received 2 quarterly 800mg doses.10 However, the concentration target (set at 4x protein-adjusted [PA]-IC90, or 0.664μg/mL), as determined to be relevant for protection in NHP SHIV challenge studies, was achieved in only one-third of ÉCLAIR participants.11 Modeling indicated that both (1) reducing the dose and dose frequency to 600 mg IM every 8 weeks and (2) adding a “loading dose” by giving an earlier second dose 4-weeks after the first dose (before starting every 8 weeks dosing thereafter) would be more likely to achieve target plasma concentrations in most people. The Phase 2a HIV Prevention Trials Network (HPTN) 077 study, which characterized the safety and PK of CAB-LA in 199 healthy adults, was already in progress using a dose of 800 mg CAB-LA IM q 12 weeks for 3 injections total (Cohort I), when the ÉCLAIR modeling data became available. Consequently, the HPTN 077 clinical trial was amended to include a sequentially enrolled second cohort using 600mg CAB-LA IM q 8weeks for 5 injections total, including the initial two injections separated by 4 weeks (Cohort 2).12,13
“Tail-phase” PK after the terminal intramuscular (IM) injection was examined in 177 of the HPTN 077 participants, and a sex-based divergence in PK was observed (sex at birth, hereafter referred to as sex). The median time between the last IM injection and cabotegravir concentration declining to non-quantifiable concentrations (< 0.025 μg/mL) was significantly longer in women, 67.3 weeks (IQR 29.1–89.6; range 17.7–225.5), compared to 43.7 weeks (interquartile range (IQR) 31.1–66.6; range 20.4–152.5) for men (p=0.0003).14 Accordingly, the apparent cabotegravir terminal half-life (t1/2app) was 1.33-fold longer (95% CI 1.06–1.68; p=0 014) in women than in men. The t1/2app was also longer for participants with greater than or equal to median body-mass index (BMI) than those below median BMI (1 31-fold higher, 95% CI 1.06–1.63; p=0 015). These two factors, however, only explained 10% of the elimination-phase PK variability of CAB-LA; other significant contributors to its PK variability are as yet undefined. There may be a difference in CAB-LA PK for any given dose depending on whether depot location is subcutaneous or intramuscular; in a companion tissue PK study to HPTN 077, when location of injection depot was visualized with magnetic resonance imaging (MRI), recipients with subcutaneous depot locations had lower peak concentrations, longer terminal half-life, and higher area under the concentration curve (AUC) than individuals whose depot location was intramuscular.15
Large double-blind, double-dummy randomized controlled phase 3 efficacy trials followed, which compared the HIV preventive efficacy of CAB-LA to that of oral tenofovir disoproxil fumarate-emtricitabine (F-TDF), and found superiority in HIV preventive efficacy of CAB-LA in both cisgender women (HPTN 084) and cisgender men and transgender women who have sex with men (HPTN 083).16,17 Importantly, F-TDF has been established to be highly effective as PrEP in the setting of excellent adherence. PK-PD relationships underlying the finding of superior preventive efficacy of injectable CAB over oral F-TDF remain incompletely understood, partially due to the high rate of efficacy of both strategies. Four incident cases of HIV occurred in participants during the blinded phase of the HPTN 083 study, despite mostly on time injections and cabotegravir concentrations above the 4x PA-IC90 target at 95% of visits; prior to detection of viral infection, one of those cases fell below 4x PA-IC90 once, two cases fell below 8x PA-IC90 once, and one never fell below 8x PA-IC90.18 The timing of these concentration dips occurred between the first and second injection in three out of the four cases. In HPTN 084, there was one incident case of HIV that occurred during the injection phase of the study; several of that participant’s injections were administered late, and drug concentrations were below target concentrations at the first HIV positive visit. It would be advantageous to understand, based on modelling of the pharmacokinetic data from HPTN 077, what alternative dosing strategies could result in safely achieving the maximal proportion of individuals above whatever target protective concentration is eventually established from PK-PD analysis of HPTN 083 and HPTN 084 (tentatively, >8x PA-IC90). Alternative strategies could include changes in dosages or intervals based on individual characteristics (e.g., sex or weight/BMI), reconsideration of an oral lead-in or oral dosing overlap (just as likely to be complicated by the adherence challenges of oral dosing as when oral dosing precedes injection), or more frequent early IM dosing as a load. Strategies accounting for the impact of occasional delayed injections or the occasional anomalous instance of an injection with lower than expected exposures (as observed in several of the HPTN 083/084 breakthrough cases of HIV) could help maintain protective concentrations. Finally, simulations to support a precision medicine-based individualized therapeutic drug monitoring approach could potentially play a role one day in optimizing CAB-LA dosing for HIV prevention.
While the manufacturer of CAB-LA (ViiV Healthcare) has developed and presented a population PK model for the drug based on a large and rich dataset of available PK data across many clinical studies, parameters such as between subject variability or between occasion variability are not available, and uncertainty in parameter estimates is not completely characterized.19,20 Simulations based on that model have been performed, for example, in the HIV treatment context, where CAB LA is dosed per FDA label instructions every 4 weeks or every 8 weeks, along with long-acting rilpivirine (RPV LA). Those simulations have supported resuming CAB LA and RPV LA without a loading dose for treatment delays of less than 1 month, and reloading with a 1.5x higher than usual dose for treatment interruptions greater than 1 month. Simulations in the setting of PrEP and every 2 month dosing overall have also supported reloading (with usual dose once a month for two injections followed by every 2 months thereafter) in the setting of unplanned dose interruptions of > 8 weeks. 21,22
As the HPTN 077 study offers rich multi-dose pharmacokinetic data in both men and women given varying dosing approaches (600 mg q8 weeks and 800 mg q 12 weeks) and includes PK tail data, it is an important data source to inform a population pharmacokinetic model of CAB-LA, which can potentially answer key questions about the use of CAB-LA as PrEP. Therefore, we aimed to develop a population PK model that adequately describes the HPTN 077 data specifically, from which future clinically important simulations can be performed to model the adequacy of alternative dosing strategies in various subgroups, support future clinical trial design, and overall contribute to the optimization of CAB-LA as PrEP.
Methods
Clinical Trial
Methods and findings of the parent HPTN 077 study have been previously published.13,14 As the present work was a secondary analysis of deidentified data, it was deemed Not Human Subjects Research, thus ethics committee and IRB approval requirements did not apply. Out of a total of 199 participants in the parent HPTN 077 trial, 177 received at least one injection, of whom 151 had PK data available. Of those 151, one individual did not have an initial plasma concentration before the first injection, and 17 individuals only had PK measurements after oral formulation; these 18 were removed from the NONMEM dataset, giving a total of 133 individuals included in this analysis. Participants first received 30 mg cabotegravir by mouth daily for an oral lead-in period of 28 days; participants without safety concerns during oral lead-in and at least 75% adherence by pill count continued on to receive injections. Participants in Cohort 1 received injections of CAB LA 800 mg IM every 12 weeks for 3 injection cycles, administered as two split 400 mg (2 mL) IM injections in the gluteal muscle. Participants in Cohort 2 received two injections of CAB LA 600 mg (3 mL) IM separated by 4 weeks, followed by 600 mg every 8 weeks for 3 additional injections thereafter.
Model development
We analyzed CAB-LA PK data from HPTN 077 using nonlinear mixed-effects modeling with NONMEM® (7.3.0) to develop a population pharmacokinetic model. The ADVAN13 subroutine and first-order conditional estimation method with interaction were used. We used RStudio (version 1.4) for dataset preparation, data visualization, and diagnostic plot generation. A likelihood-based approach (Method 3) was used to handle measurements below the lower limit of quantitation at 0.025 μg/mL.23 The model-building process was guided by changes in the NONMEM objective function value and diagnostic plots. An alpha threshold of 0.05 was set for the model improvement threshold (corresponding to a change in NONMEM objective function value (OFV) of 3.84 units for 1 degree of freedom).
Structural model
One-compartment and two-compartment models were explored for CAB-LA. The parameters used to describe the pharmacokinetics of cabotegravir include apparent clearance (CL/F), apparent central volume (Vc/F), apparent peripheral volume (Vp/F), apparent intercompartmental clearance (Q/F), and first-order absorption rate (Ka). Since all the patients received a 4-week oral lead-in, the concentration measurement for each individual before the first injection dose was used as the initial condition of the differential equations.
Random effect model
Inter-individual variability (IIV) with a log-normal distribution was supported for all the PK parameters:
Where the represents the individual value of the parameter , the represents the typical value of the parameter P, and the denotes the IIV, which is assumed to have a normal distribution with mean equal to 0 and variance equal to .
Proportional, additive and a combined additive and proportional error models were tested to describe the residual unexplained variability:
Where the represent the observed concentration of subject at time , the represent the predicted concentration, and represent the proportional and additive error. They were assumed to be normally distributed, with mean = 0 and variances of and
For the parameters Ka and CL, the between occasion variability (BOV) was evaluated as an additional level of random effect:
Covariate model
Once the base model was established, covariates including body weight, BMI, sex, and age were first explored by visualization. We tested the potential covariate relationship of body weight, BMI, sex, race/ethnicity, and age on CL, Q, V2 and V3, and bodyweight, BMI, sex, race/ethnicity, and age on Ka. We used both empirical Bayes estimates and a post-hoc ANOVA as well as directly incorporating these covariate effects into the model. Potential covariate relationships identified by the exploratory visualization were then studied using stepwise forward selection and backward elimination. For the forward selection, a decrease of the OFV more than 3.84 was considered significant (p<0.05). For the backward elimination, an increase of OFV more than 6.63 was considered significant (p<0.01). The continuous covariates were modeled using this equation:
Where the denotes the individual typical value of parameter, denotes the individual covariate, and denotes the population median of the covariate. The categorical covariate, sex, was modeled using this equation:
To determine relevant predictors of BOV, the distribution of standard deviations for the etas was visualized by the following covariates: sex, race, and BMI (obese versus non-obese, using a cut-off of 30 kg/m2). These were compared using a Welch 2-sample t-test with shared variance (for BMI and sex) and one-way ANOVA (for race.) 12 individuals who received only one injection were excluded from this analysis. A unique BOV distribution was also tested for the sex covariate affecting Ka.
We further performed analyses evaluating effects of both race/ethnicity and BMI on Ka and clearance for the cabotegravir model. We used both empirical Bayes estimates and a post-hoc ANOVA as well as directly incorporating these covariate effects into the model.
Results
Concentration-time plots by sex are shown, for visualization, for the two cohorts in Figure 1. There were 259 BLQ concentration measurements in total, all of them in the washout phase; all of the BLQ measurements were kept in the model. Demographics by cohort, summarized in Table 1, are notable for a study population that was 67% female and 41% Black. The model building process is summarized in Table 2. A two-compartment model with first order absorption best described the CAB-LA PK. A schema of the final structural model is shown in Figure 2. The PK of CAB-LA was best characterized by the following differential equations:
Figure 1. Visualization of the concentration: time curves for CAB-LA, by sex at birth.
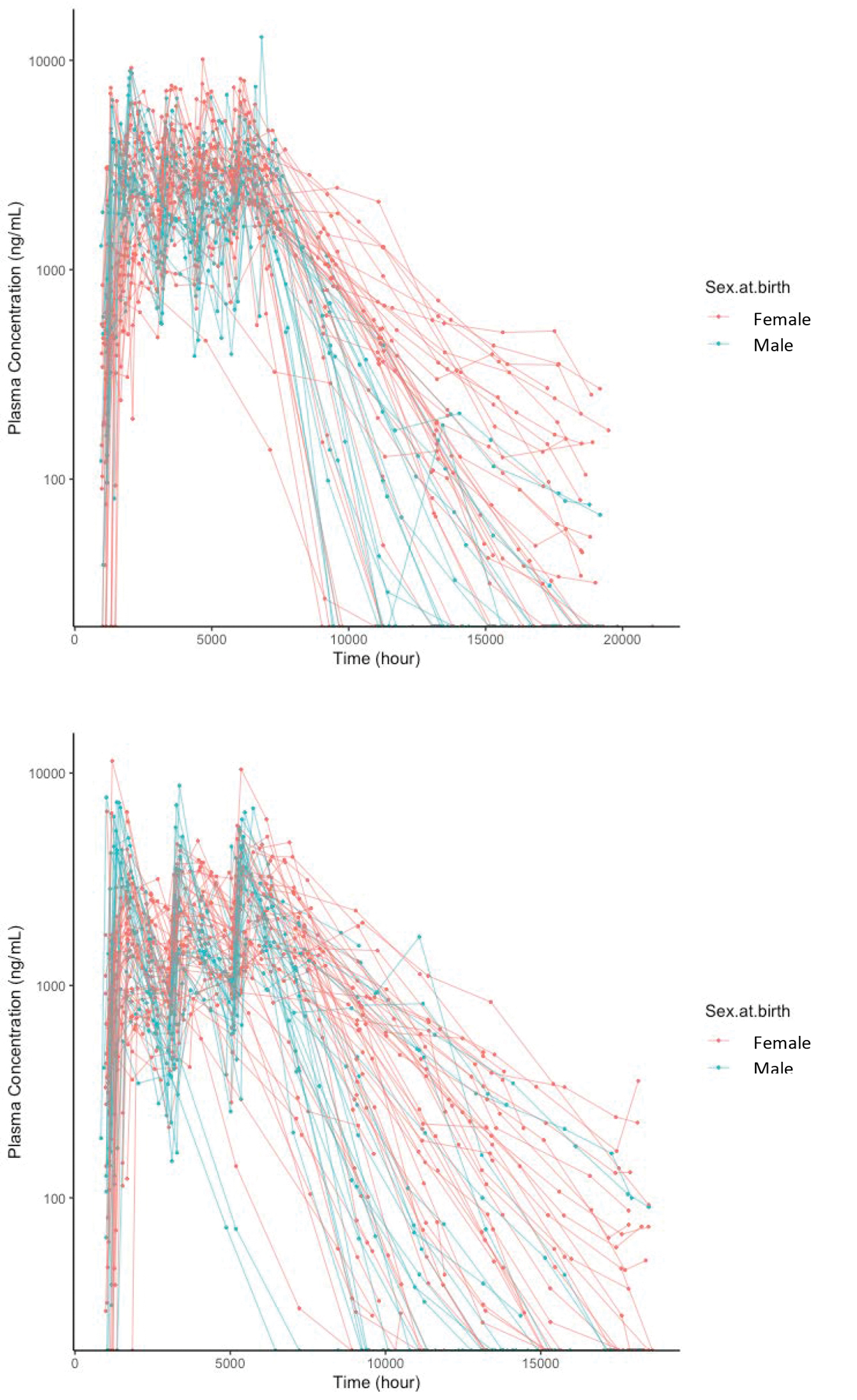
Table 1.
Demographics of HPTN 077 Participants
| Characteristic | Cohort 1 (n=82) | Cohort 2 (n=69) | Total Cohort (n=151) | Data used for modeling (n=133) |
|---|---|---|---|---|
|
| ||||
| Cabotegravir dosing1 | 800 mg IM q 12 wk | 600 mg IM q 8 wk | ||
|
| ||||
| Age (yrs) at Enrollment, median (IQR) | 29.5 (24,41) | 30 (23,36) | 30 (24,39) | 29 (24,38) |
| BMI (kg/m2) at Entry, median (IQR) | 27.4 (24.1,32.6) | 25.7 (22.0,32.0) | 26.8 (23.2,32.3) | 26.6 (23.0,32.8) |
| Weight (kg), median (IQR) | 78.0 (67.6,94.3) | 72.0 (60.2,86.2) | 74.7 (62.15,91.85) | 74.7 (61.0,91.4) |
| Sex at birth2, n (%) | ||||
| Female | 54 (66%) | 46 (67%) | 100 (66%) | 89 (67%) |
| Male | 28 (34%) | 23 (33%) | 51 (34%) | 44 (33%) |
| Race, n (%) | ||||
| Non-Hispanic white | 29 (35%) | 13 (19%) | 42 (28%) | 36 (27%) |
| Non-Hispanic black | 31 (38%) | 33 (48%) | 64 (42%) | 55 (41%) |
| Latino | 19 (23%) | 17 (25%) | 36 (24%) | 35 (26%) |
| Non-Hispanic Asian | 0 (0%) | 3 (4%) | 3 (2%) | 2 (2%) |
| Non-Hispanic mixed/other | 3 (4%) | 3 (4%) | 6 (4%) | 5 (4%) |
Cohort 1 received 3 total injections with no load; Cohort 2 received 5 total injections—the first 2 separated by 4 weeks as an initial load, and the last 3 separated by 8 weeks.
There were 6 transgender men (TGM) and 1 transgender woman (TGW) in the HPTN 077 study, who were categorized according to sex at birth; that is, TGW with the individuals born male, and TGM with the individuals born female.
Table 2.
Model building steps3
| Model | BSV | OFV | Change in OFV4 | |
|---|---|---|---|---|
|
| ||||
| 1 | 1 compartment, First-order absorption, Combined RUV | Ka, CL, V2 | 25420 | |
| 2 | 2 compartment, First-order absorption, Combined RUV | Ka, CL, V2, V3, Q | 25396.6 | −23.3 |
| 3 | 2 compartment, First-order absorption, Combined RUV, WT on CL (fixed=0.75) | Ka, CL, V2, V3, Q | 25372.2 | −24.435 |
| 4 | 2 compartment, First-order absorption, Combined RUV, WT on CL (fixed=0.75), SEX on Ka | Ka, CL, V2, V3, Q | 25336.17 | −36.029 |
| 5 | 2 compartment, First-order absorption, Combined RUV, WT on CL (fixed=0.75), SEX on Ka, BOV on Ka | Ka, CL, V2, V3, Q | 25014.74 | −321.428 |
RUV= residual unexplained variability; BSV= between subject variability; OFV= objective function value; WT= weight; BOV= between occasion variability
Compared with immediately previous model
Figure 2. Schema of pharmacokinetic model for long-acting injectable cabotegravir.
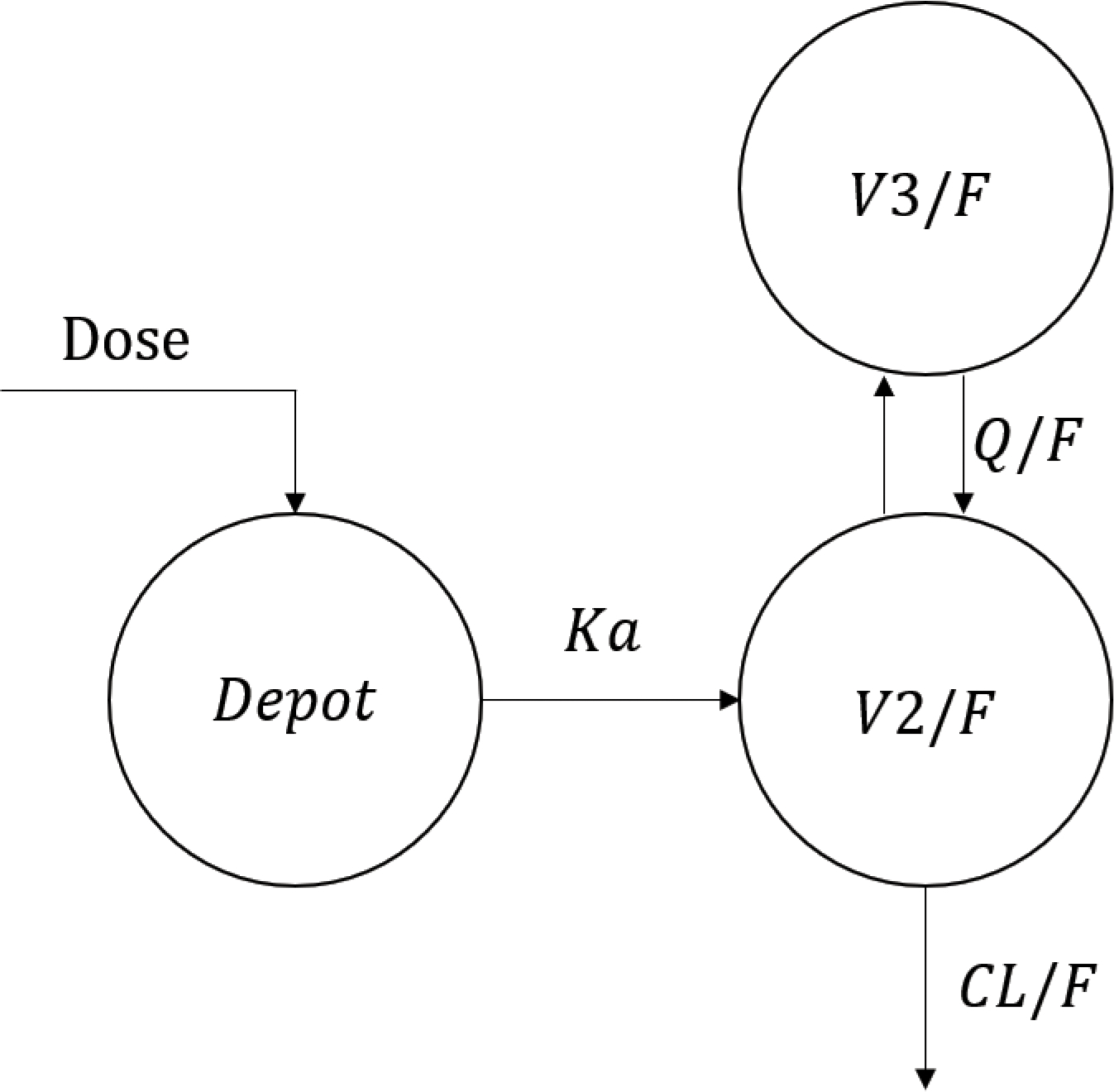
Here, represents the amount of drug in the depot, represents the concentration in the central compartment, and represents the concentration in the peripheral compartment.
In the final model, the between-participant variability was supported for all the parameters, and the between-occasional variability was supported for Ka when evaluated as an additional level of random effect. Covariate relationships of sex at birth on Ka and body weight on CL were identified (Figure 4). A unique BOV distribution for Ka by sex, BMI (Obese/others) and race was not significantly different across these covariates. The first-order absorption rate constant of female sex was estimated to be 0.0003 h−1, which is 40% less than male sex (0.0005 h−1). The relationship between body weight and clearance was described by the classic allometric relationship, with the exponent fixed to 0.75. Estimated values of PK parameters are summarized in Table 3. According to the diagnostic plots (shown in Figure 3), the PK profile of CAB-LA was well characterized.
Figure 4. A. Covariate relationships between body weight and apparent clearance (CL/F); B. Covariate relationship between sex and first-order absorption rate.
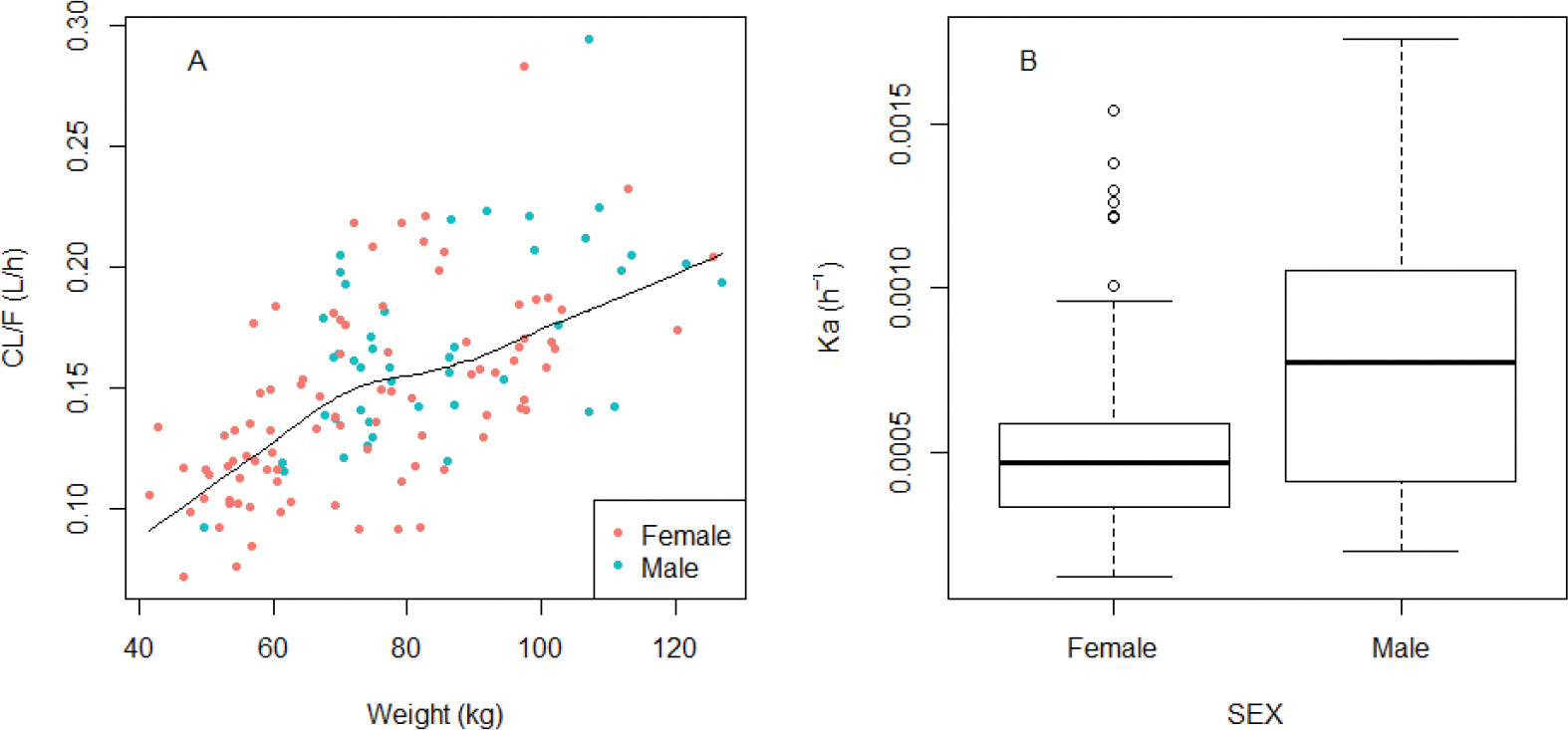
Table 3.
Final estimates of long-acting injectable Cabotegravir pharmacokinetic parameters, between subject variability, and residual variability2
| Parameter | Estimate | RSE% | Shrinkage% |
|---|---|---|---|
|
| |||
| Ka(Female)(1/h) | 0.0003 | 8 | |
| Ka(Male)(1/h) | 0.0005 | 6 | |
| CL(L/h) | 0.148 | 2 | |
| V2(L) | 7.84 | 3 | |
| V3(L) | 6.99 | 6 | |
| Q(L/h) | 0.225 | 10 | |
| BOV on Ka | 38.5% | 4 | |
| BSV on Ka | 82.8% | 11 | 32 |
| BSV on CL | 21.6% | 8 | 9 |
| BSV on V2 | 154.6% | 15 | 43 |
| BSV on V3 | 231.1% | 20 | 49 |
| BSV on Q | 69.6% | 86 | 85 |
| σ1(prop) | 26.3% | 2 | |
| σ2(add (ng/mL)) | 6.8 | 4 | |
Ka= absorption rate constant; CL= clearance; V2= volume of distribution of the Central compartment; V3= volume of distribution of the Peripheral compartment; Q=intercompartmental clearance; BOV= between-occasion variability; BSV= between-subject variability; σ1=proportional residual error; σ2= additive residual error; RSE= relative standard error. All PK parameter estimates are /F. All estimates for variability are reported as %CV.
Figure 3. Goodness-of-fit plots for the final model.
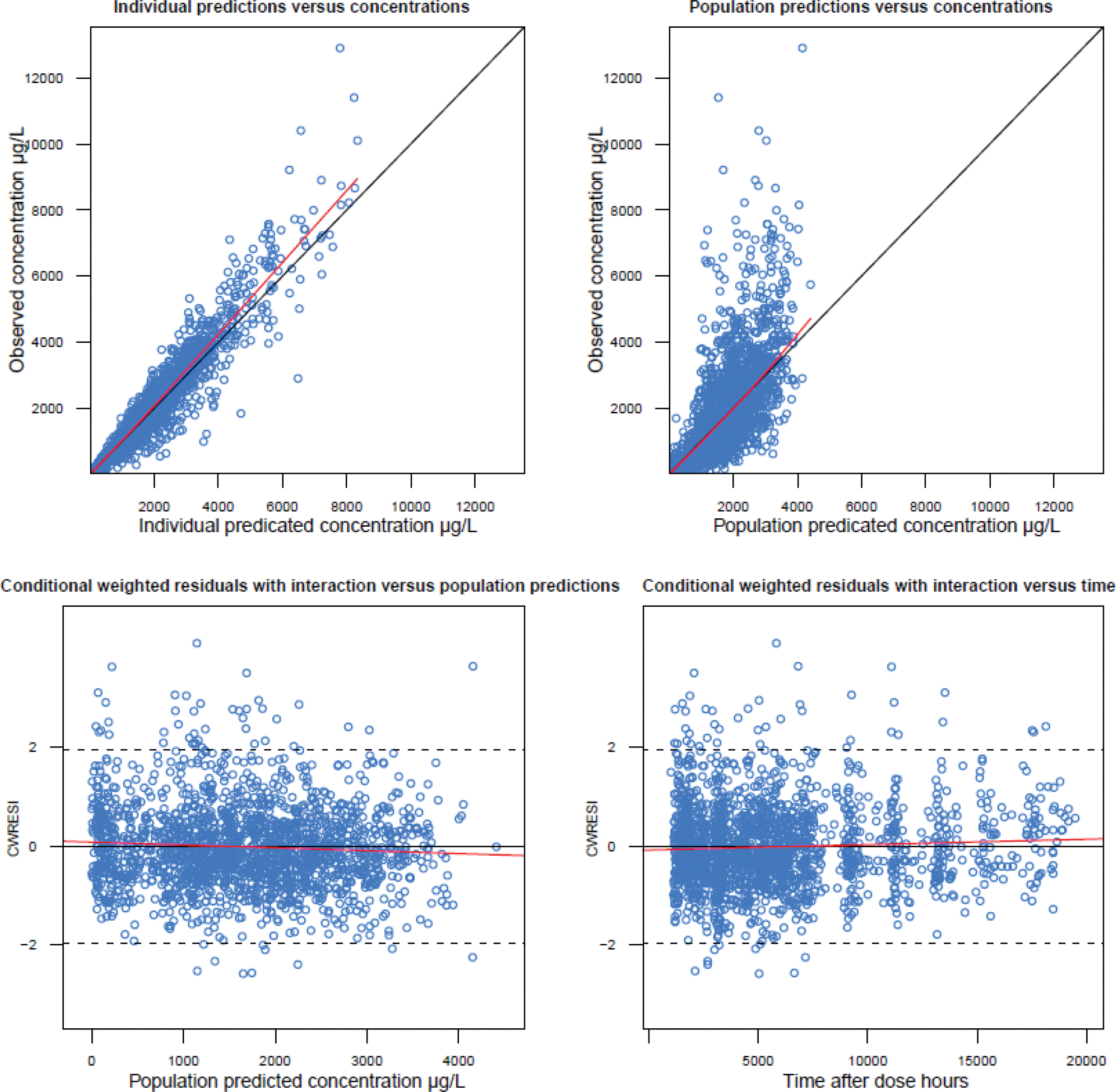
Regarding covariate effects, no significant effects of the covariate effects of BMI and race/ethnicity on Cl or Ka were found. In fact, the incorporation of these covariates into the model actually worsened the model fit (manifested by a positive change in minus-2 log likelihood values).
Simulations
Using our model, we performed simulations to determine the expected range of cabotegravir Ctrough in selected circumstances. As the range of BMI in our study population was 16.5 to 50 kg/m2, we simulated the CAB plasma PK profiles after q 8-week CAB-LA dosing of 10,000 males and 10,000 females at the extremes of BMI—at 16.5 kg/m2 and 50 kg/m2. (Table 4). Simulated cabotegravir Ctrough for the lowest extreme of BMI was higher than for the highest extreme of BMI (2.707 and 1.102 ug/mL for male participants and 3.275 and 1.368 ug/mL for female participants, respectively). As Body Mass Index was found to be less predictive in our model than body weight, we back-extrapolated body weight from these BMI’s based on the average heights for males and females in the U.S. based on the 2015–2016 NHANES database.24 Because ethnicity/race was not found to have a significant impact on Ka, simulations by ethnicity were not performed.
Table 4.
Simulated steady-state Cabotegravir Trough (Ctrough)1 for extremes of BMI:
| CAB Ctrough (ug/mL, Median [90% prediction interval]) for BMI 16.5 kg/m2 (Body weight 43.0kg F; 50.6kg M) | CAB Ctrough (ug/mL, Median [90% prediction interval]) for BMI 50 kg/m2 (Body weight 130.6kg F; 153.9kg M) | |
|---|---|---|
| Male | 2.707 [0.931, 5.648] ug/mL | 1.102 [0.339, 2.355] ug/mL |
| Female | 3.275 [1.291, 6.676] ug/mL | 1.368 [0.527, 2.828] ug/mL |
For reference: CAB PA-IC90: 0.166 μg/mL; 4*PA-IC90: 0.664 μg/mL; 8*PA-IC90: 1.33 μg/mL
In addition, in order to better understand determine the expected range of Ctroughs after the first cabotegravir injection and following multiple injections, accounting for BOV, and to understand whether intra-individual variability related to injection becomes less clinically relevant after multiple injections, we simulated CAB plasma PK profiles for 1,000 males and 1,000 females of average body weight according to 2015–2016 NHANES database.24 We generated cabotegravir Ctrough concentrations after CAB-LA administration both after first injection and at steady state. (Table 5) The simulated median Ctrough after first injection was lower than the median trough at steady state (0.99 and 1.75 ug/mL respectively for men, and 0.76 and 2.04 ug/mL respectively for women), consistent with a described phenomenon of accumulation.25
Table 5.
Simulated Cabotegravir Trough (Ctrough)1 After First Injection and at Steady State
| Ctrough after first injection median [90% prediction interval] (ug/mL) | Ctrough at steady state median [90% prediction interval] (ug/mL) | |
|---|---|---|
| Male | 0.99 [0.25,2.68] | 1.75 [0.62,2.68] |
| Female | 0.76 [0.17,2.57] | 2.04 [0.91,4.11] |
For reference: CAB PA-IC90: 0.166 μg/mL; 4*PA-IC90: 0.664 μg/mL; 8*PA-IC90: 1.33 μg/mL
Discussion
To better understand the absorption and drug disposition of CAB-LA, we developed a population pharmacokinetic model based on data from HIV negative research participants in the HPTN 077 trial. We found that a two-compartment model with first-order absorption provided the best fit for the data, which is consistent with a previous study.19 We tested several covariates (including weight, BMI, race/ethnicity, sex, and age) for their influence on PK parameters. In the final model, we identified weight as a significant covariate affecting the apparent clearance, and sex as a significant influence on Ka. According to the final estimate, the first-order absorption rate constant in women was 40% lower than in men (Figure 4). As has been previously described, CAB-LA exhibits the flip-flop kinetics typical of long-acting injectables, where the apparent terminal half-life is governed by the absorption rate constant, Ka.26 In a previous single-dose study, there was a non-statistically significant trend of higher CAB-LA plasma exposures in female compared to male participants after subcutaneous administration and higher plasma exposures in male participants after a split IM injection.8 Sex-based differences in absorption rate may reflect different skin-to-muscle depth or different fat content and distribution within tissues at the site of injection depot. In the previous PK analysis of this CAB-LA data from HPTN 077, BMI above the median, in addition to female sex, was identified as a covariate significantly associated with lower Ka.14 However, in our analysis, this covariate relationship was not detected, as BMI did not add statistically to the characterization of the Ka once sex was incorporated. Because sex had a stronger effect, it was included first in the modelling sequence.
Comparing our results to those of other studies, the population CL/F estimated in our study (0.148 L/h) was similar to values from a previous study using data from HIV negative adult participants and participants living with HIV-1 (0.175 L/h) as well as the full population PK model for CAB LA (0.151 L/h).19,20 Body weight was found to be a significant predictor of apparent clearance (CL/F). In addition, the central and peripheral volume of distribution (estimated V2/F and V3/F; 7.84 L and 6.99 L, respectively) were slightly higher than previously reported values (7.70 L and 3.14 L).27 These are small VD values, potentially attributable to the fact that cabotegravir is known to be >99% protein bound.
In the final model, the between-occasion variability (BOV) was attributable to differences in absorption rate (Ka) from one injection occasion to the next. The estimated variability from occasion to occasion on the absorption rate (Ka) was 38.5%. This variability is relatively large, but reasonable, for a long-acting injectable formulation, since the absorption of the injectable formulation of cabotegravir is influenced by the depth, vascularity, lymphatics, and fat content of the injection site, as is true of many other long-acting depot formulations, including paloperidone.15,28–30 For comparison, in one population PK study of paliperidone, the BOV on clearance, volume of distribution, and dose fraction in the central compartment were 26% CV, 14% CV, and 0.07 SD, respectively.30 In our examination of whether BOV varied according to characteristics such as sex, race, and BMI, we found no significant associations between any of these characteristics and BOV. Thus, the variability in exposures from one administration to the next was not completely or satisfactorily explained by variable locus of depot according to different fat distribution in women and men. A vulnerability to lower cabotegravir exposures early on in dose administration (i.e., between the first and second injections, as seen in HPTN 083 and 084) could mean various overlap and loading strategies could be productively explored with simulations, to get around the BOV factor with adequate dosing “cushion”. As the HPTN 077 study and many of the clinical trials of CAB-LA did not include many individuals with BMI above 30 kg/m2, simulations and further study to explore and predict CAB LA PK in morbidly obese individuals are an area ripe for future study. Regardless, a better understanding of variables influencing BOV are key to truly personalized dosing, but they have not yet emerged from the data. Currently, long-acting injectable cabotegravir is only recommended for injection in the gluteal region, but there is interest in expanding injection site options, such as anterior thigh self-injection, and it is possible that these absorption kinetics will differ according to body site where injection is administered, due to different fat-to-muscle relationships and other as yet unidentified variables.12
In general, the role of personalized dosing for CAB-LA as PrEP remains unclear, in part because the concentration-response relationship has not been established in this PrEP setting due to rarity of cases of HIV infection during active injection phases of pivotal trials. A component of individualized dosing based on PK measurements could have important implications for efficacy as well as participant-important outcomes like satisfaction and adherence, particularly if it enables a more convenient extended dosing interval in some people, given the considerable variability between individuals and between occasions within the same individual. The publication of this population PK model creates a foundation for asking these important questions, in the populations who will most benefit from this long-acting strategy.
Conclusions
A two-compartment model with first-order absorption best described the pharmacokinetics of CAB-LA. The population pharmacokinetic analysis identified between-occasional variability (i.e., differences in PK within one individual from one injection to the next) as a significant covariate on the absorption rate. Sex and body weight were identified as significant covariates influencing the absorption rate and apparent clearance of CAB-LA after intramuscular injection at various doses and frequencies. The public availability of this model will facilitate and enable a wide variety of future clinically relevant simulations to inform the optimal use of CAB-LA.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
The manufacturer’s previously presented model has characterized the population PK of long-acting cabotegravir (CAB-LA) based on the available PK data across many clinical studies. However, the influence of parameters such as between subject variability or between occasion variability are not available, and uncertainty in parameter estimates is not completely characterized.
WHAT THIS STUDY ADDS
The present study adds to our understanding about the predictors of pharmacokinetic variability with injectable long-acting cabotegravir. In particular, it characterizes for the first time the influence on long-acting cabotegravir pharmacokinetics of the parameter between-occasion variability (BOV)—differences in cabotegravir concentrations within a single individual from one injection to the next.
Funding
The authors gratefully acknowledge the funding support from the HPTN and NIH for the original HPTN 077, study which provided the data for this secondary analysis. EDW receives funding from the NIH NIAID—award number K23 AI165290-01. CWH reports receiving research contract funding and scientific advisory board honorarium and/or travel support from ViiV Healthcare, Gilead, and Merck, and is the founder of PRIÖNDE Biopharma, LLC. ICON Plc provided the license for NONMEM 7 to KLB. All potential conflicts for CWH, EDW and KB managed by Johns Hopkins University.
Footnotes
Conflict of Interest Statement
Dr. Ford is an employee of ViiV/GSK.
This article can be found at: https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/bcp.15477
Data Availability Statement:
De-identified individual participant data that underlie the results reported in this Article (text, tables, figures, and appendices) will be shared upon request. Proposals and data requests should be directed to Sue Li (sli@fredhutch.org). Those requesting de-identified data may be required to sign a data access agreement.
References
- 1.Dimitrov DT, BR MA, Donnell D. PrEP adherence patterns strongly impact individual HIV risk and observed efficacy in randomized clinical trials. J Acquir Immune Defic Syndr. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wheeler DP, Fields SD, Beauchamp G, et al. Pre-exposure prophylaxis initiation and adherence among Black men who have sex with men (MSM) in three US cities: results from the HPTN 073 study. J Int AIDS Soc. 2019;22(2):e25223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hebel S, Kahn-Woods E, Malone-Thomas S, et al. Brief Report: Discrepancies Between Self-Reported Adherence and a Biomarker of Adherence in Real-World Settings. J Acquir Immune Defic Syndr. 2020;85(4):454–457. [DOI] [PubMed] [Google Scholar]
- 4.Gulick RM, Flexner C. Long-Acting HIV Drugs for Treatment and Prevention. Annu Rev Med. 2019;70:137–150. [DOI] [PubMed] [Google Scholar]
- 5.Yoshinaga T, Kobayashi M, Seki T, et al. Antiviral Characteristics of GSK1265744, an HIV Integrase Inhibitor Dosed Orally or by Long-Acting Injection. Antimicrobial Agents and Chemotherapy. 2015;59(1):397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowers GD, Culp A, Reese MJ, et al. Disposition and metabolism of cabotegravir: a comparison of biotransformation and excretion between different species and routes of administration in humans. Xenobiotica. 2016;46(2):147–162. [DOI] [PubMed] [Google Scholar]
- 7.Spreen W, Min S, Ford SL, et al. Pharmacokinetics, safety, and monotherapy antiviral activity of GSK1265744, an HIV integrase strand transfer inhibitor. HIV Clin Trials. 2013;14(5):192–203. [DOI] [PubMed] [Google Scholar]
- 8.Spreen W, Ford SL, Chen S, et al. GSK1265744 pharmacokinetics in plasma and tissue after single-dose long-acting injectable administration in healthy subjects. J Acquir Immune Defic Syndr. 2014;67(5):481–486. [DOI] [PubMed] [Google Scholar]
- 9.Spreen W, Williams P, Margolis D, et al. Pharmacokinetics, safety, and tolerability with repeat doses of GSK1265744 and rilpivirine (TMC278) long-acting nanosuspensions in healthy adults. J Acquir Immune Defic Syndr. 2014;67(5):487–492. [DOI] [PubMed] [Google Scholar]
- 10.Ford S, Chiu J, Lovern M, et al. Population PK approach to predict cabotegravir (CAB, GSK1265744) long-acting injectable doses for phase 2b (Ph2b). ICAAC 2014. September 5–9, 2014. Washington, DC. Abstract H-645. ICAAC. Washington, D.C.2014. [Google Scholar]
- 11.Markowitz M, Frank I, Grant RM, et al. Safety and tolerability of long-acting cabotegravir injections in HIV-uninfected men (ECLAIR): a multicentre, double-blind, randomised, placebo-controlled, phase 2a trial. Lancet HIV. 2017;4(8):e331–e340. [DOI] [PubMed] [Google Scholar]
- 12.Landovitz RJ, Li S, Grinsztejn B, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLOS Medicine. 2018;15(11):e1002690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landovitz RJ, Li S, Grinsztejn B, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med. 2018;15(11):e1002690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landovitz RJ, Li S, Eron JJ, et al. Tail-phase safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in HIV-uninfected adults: a secondary analysis of the HPTN 077 trial. Lancet HIV. 2020;7(7):e472–e481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jucker BM, Fuchs EJ, Lee S, et al. Multiparametric magnetic resonance imaging to characterize cabotegravir long-acting formulation depot kinetics in healthy adult volunteers. Br J Clin Pharmacol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landovitz RJ, Donnell D, Clement ME, et al. Cabotegravir for HIV Prevention in Cisgender Men and Transgender Women. N Engl J Med. 2021;385(7):595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delany-Moretlwe S, J. H, Bock P, et al. Long acting injectable cabotegravir is safe and effective in preventing HIV infection in cisgender women: interim results from HPTN 084. HIV R4P (HIV Research for Prevention) 2021. [Google Scholar]
- 18.Marzinke MA, Grinsztejn B, Fogel JM, et al. Characterization of HIV infection in cisgender men and transgender women who have sex with men receiving injectable cabotegravir for HIV prevention: HPTN 083. J Infect Dis. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han K. BM, Patel P, Margolis D, Spreen W, Moore KP, Ford SL 1532. Population Pharmacokinetic (PPK) Modeling and Simulation of Long-Acting (LA) Cabotegravir (CAB) to Inform Strategies Following Dosing Interruptions in HIV-1-Infected Subjects. 2019;6(Suppl 2):S558. Published 2019 Oct 23. doi: 10.1093/ofid/ofz360.1396. Open Forum Infectious Diseases. 2019;2019;6:S558. [DOI] [Google Scholar]
- 20.Han K BM, Lovern M, Paul P, Xiong Y, Spreen W, Moore K, Ford SL. Population pharmacokinetics of cabotegravir in adult healthy subjects and HIV-1 infected patients following administration of oral tablet and long acting intramuscular injection. American Conference on Pharmacometrics. Orlando, FL, USA.2019. [Google Scholar]
- 21.Han K PP, Baker M, Margolis D, Spreen W, Rhinehart A, Landovitz R, Ford S. Cabotegravir (CAB) Long-acting (LA) Phase 3 (Ph3) PrEP Dose Selection Based on Population Pharmacokinetics (PPK) in Healthy and HIV-infected Adults. Research for Prevention. Madrid, Spain: 2018. [Google Scholar]
- 22.Han K BM, Spreen W, Ford S. Cabotegravir Population Pharmacokinetic (PPK) Simulation to Inform Q2M Strategies Following Dosing Interruption. Conference on Retroviruses and Opportunistic Infections (CROI). Virtual2021. [Google Scholar]
- 23.Mould DR, Upton RN. Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst Pharmacol. 2013;2:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fryar CD, Kruszon-Moran D, Gu Q, Ogden CL. Mean Body Weight, Height, Waist Circumference, and Body Mass Index Among Adults: United States, 1999–2000 Through 2015–2016. Natl Health Stat Report. 2018(122):1–16. [PubMed] [Google Scholar]
- 25.Swindells S, Andrade-Villanueva JF, Richmond GJ, et al. Long-Acting Cabotegravir and Rilpivirine for Maintenance of HIV-1 Suppression. N Engl J Med. 2020;382(12):1112–1123. [DOI] [PubMed] [Google Scholar]
- 26.Gibaldi M, Perrier D. Pharmacokinetics / Milo Gibaldi, Donald Perrier. 2nd ed. rev. and expanded. ed. New York: M. Dekker; 1982. [Google Scholar]
- 27.Han K, Patel P, Baker M, Margolis D, Spreen W, Ford S Population Pharmacokinetics of Cabotegravir in Healthy Adult Subjects and HIV-1 Infected Patients Following Administration of Oral Tablet and Long-Acting Intramuscular Injection. 22nd International AIDS Conference. Amsterdam, The Netherlands2018. [Google Scholar]
- 28.Hough D, Lindenmayer JP, Gopal S, et al. Safety and tolerability of deltoid and gluteal injections of paliperidone palmitate in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(6):1022–1031. [DOI] [PubMed] [Google Scholar]
- 29.Samtani MN, Gopal S, Gassmann-Mayer C, Alphs L, Palumbo JM. Dosing and switching strategies for paliperidone palmitate: based on population pharmacokinetic modelling and clinical trial data. CNS Drugs. 2011;25(10):829–845. [DOI] [PubMed] [Google Scholar]
- 30.Samtani MN, Vermeulen A, Stuyckens K. Population pharmacokinetics of intramuscular paliperidone palmitate in patients with schizophrenia: a novel once-monthly, long-acting formulation of an atypical antipsychotic. Clin Pharmacokinet. 2009;48(9):585–600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
De-identified individual participant data that underlie the results reported in this Article (text, tables, figures, and appendices) will be shared upon request. Proposals and data requests should be directed to Sue Li (sli@fredhutch.org). Those requesting de-identified data may be required to sign a data access agreement.


