Abstract
Technological and medical advances over the past few decades epitomize human capabilities. However, the increased life expectancies and concomitant land-use changes have significantly contributed to the release of ∼830 gigatons of CO2 into the atmosphere over the last three decades, an amount comparable to the prior two and a half centuries of CO2 emissions. The United Nations has adopted a pledge to achieve “net zero”, i.e., yearly removing as much CO2 from the atmosphere as the amount emitted due to human activities, by the year 2050. Attaining this goal will require a concerted effort by scientists, policy makers, and industries all around the globe. The development of novel materials on industrial scales to selectively remove CO2 from mixtures of gases makes it possible to mitigate CO2 emissions using a multipronged approach. Broadly, the CO2 present in the atmosphere can be captured using materials and processes for biological, chemical, and geological technologies that can sequester CO2 while also reducing our dependence on fossil-fuel reserves. In this review, we used the curated literature available in the CAS Content Collection to present a systematic analysis of the various approaches taken by scientists and industrialists to restore carbon balance in the environment. Our analysis highlights the latest trends alongside the associated challenges.
Introduction
Carbon dioxide (CO2) is a critical component for plant life and thus animal and human life. Combustion of carbon-containing fuels to CO2 allows humans to live almost anywhere on Earth and provides power for industrial production. However, as shown in Figure 1, rapid growth of atmospheric CO2 concentrations has undesired consequences, including global warming, where the past 40 years have seen temperatures rise at a rate (0.18 °C/decade) that is more than twice that (0.08 °C/decade) in the 100 years prior.1 CO2 emissions from naturally pre-existing and anthropogenic activities outweigh uptake and sequestration pathways, resulting in a cumulative buildup of atmospheric CO2 levels. Thus, global warming, atmospheric CO2 levels, and world population are interconnected.2−4
Figure 1.
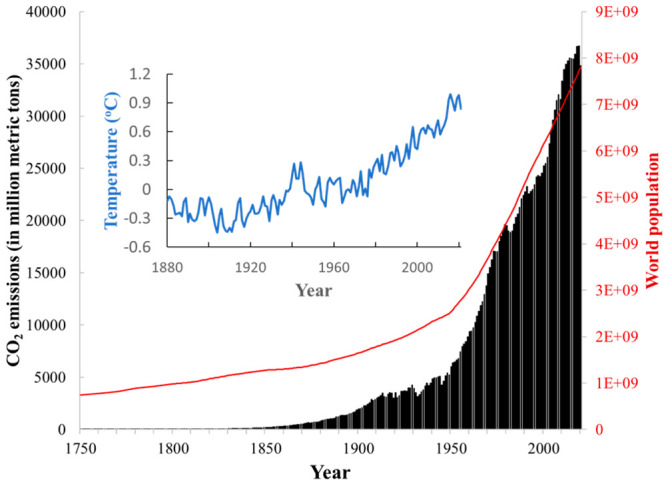
World population growth (red line) and annual CO2 emissions (black bars) from fossil-fuel use and industrial production over the years 1750–2020;5,6 the annual deviation relative to 1910–2000 average global temperature over the years 1880–2020 (blue graph).9
Today, our global population is almost 8 billion and atmospheric CO2 levels are ∼417 ppm (ppm).5 Land use changes and fossil-fuels usage account for ∼40 billion metric tons/year CO2 emissions globally as of 2021.6,7 In comparison, the world population was ∼2.5 billion in 1950 and the global CO2 emissions were estimated at ∼11.5 billion metric tons/year. According to the predictions by an Intergovernmental Panel for Climate Change (IPCC), global warming is likely to reach 1.5 °C between 2030 and 2052 (relative to preindustrial levels between 1850 and 1900). Due to the far-reaching ecological consequences the international community adopted significant CO2-emissions reduction targets (“race to zero” by 2050) in 2015 at the 21st Conference of Parties on Climate Change.8
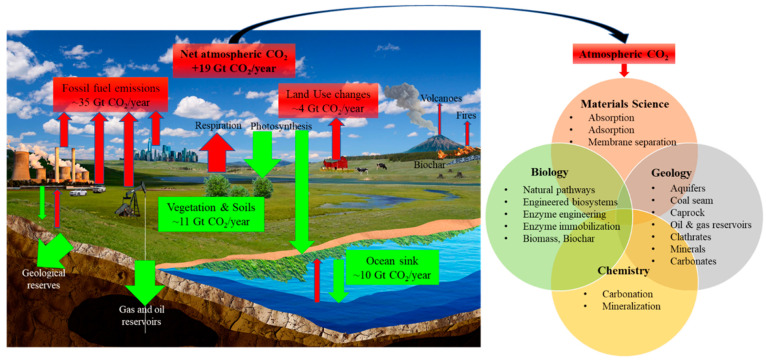
Given these societal challenges and because CO2 is nontoxic, nonflammable, plentiful, and a renewable carbon source, it has been utilized as a sustainable feedstock as part of multipronged approaches to decarbonize the atmosphere via capture and biological, chemical, and geological sequestration or storage. Carbon or CO2 Capture and Storage (CCS) technologies are focused on reducing the amounts of CO2 released into the atmosphere by separating it from other gases, compressing, transporting and finally storing the captured CO2 far away from the atmosphere, avoiding any leakage back into the ecosystem.10,11 The high costs associated with these technologies have limited large-scale annual capture and storage capacity to only about 0.1% of global CO2 emissions,12 but this number is predicted to go up to 19% by 2050.13 CO2 in its purified or impure form (i.e., present in gas mixtures) can be directly utilized as is (enhanced oil recovery, food and beverage industries) or as a raw material for conversion into other substances via geological (carbonates/soda ash, rock and saline formations, minerals, coalbeds, aquifers, etc.), biological (carbohydrates, lipids, proteins, secondary metabolites, etc.), and chemical (carbon monoxide, alkanes, alcohols, alkenes, acids, etc.) processes via carbon or CO2 Capture and Utilization (CCU) technologies.14Figure 2 shows the carbon cycle and human contributions to CO2 emissions and potentially useful technologies for capture and sequestration of CO2.
Figure 2.
Global processes contributing to atmospheric CO2 emissions (red arrows) and sequestration (green arrows), and global efforts to mitigate net emissions through technologies classified into different disciplines of science. Global average CO2 fluxes for the decade 2011–2020 are shown for processes that significantly impact the global carbon cycle via CO2 release (red boxes) or capture (green boxes), as numbers of gigatons (Gt) CO2 per year.6
In this paper, we present a systematic analysis of the latest trends in CO2 capture and sequestration research utilizing data from the CAS Content Collection of journal and patent publications spanning the years 2001–2021. We focus on technologies capable of reducing global atmospheric CO2 levels. The current global CO2 emissions are at about 40 gigatons/year; therefore, a technology should have the potential to sequester gigaton quantities of CO2 each year.15 We first provide an overview of publication trends by time, country/region, and keywords. Then, the technologies, methods, materials, and chemical substances involved in CO2 separation, capture, sequestration and use are discussed and their publication trends analyzed to provide an overview of current developments in CO2 mitigation strategies.
Research Trends in CO2 Capture and Sequestration
The CAS Content Collection is the largest human-curated collection of published scientific knowledge, In this work, about 18500 CO2 capture and sequestration-related documents containing relevant terms in title, abstract, or CAS-indexed areas were found with publication years between 2001 and 2021 (see the Supporting Information for method descriptions of search strategy and terminologies). We chose to use a sample of available documents rather than a comprehensive set of documents in order to ensure that trends in CCS research are accurately represented.
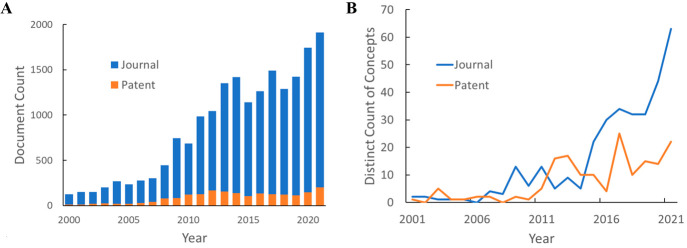
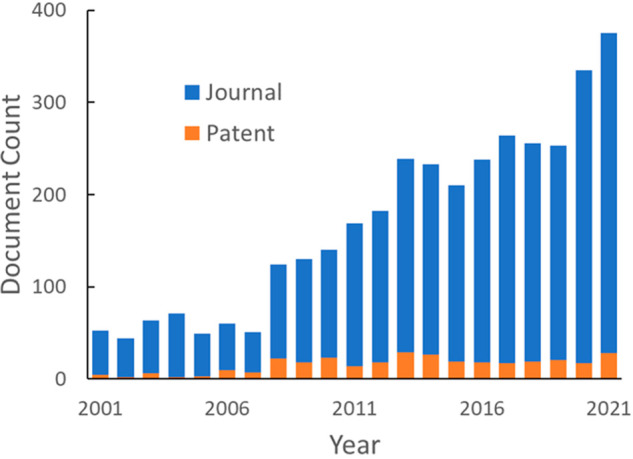
Figure 3 shows the annual publication volume related to CO2 capture and sequestration. Overall publication numbers increased rapidly in the early 2000s, slowed down after mid-2010s, and recently experienced rapid growth. The initial steady increase can be attributed to the urgency of reducing atmospheric CO2 levels triggered by global efforts. However, the absence of strong support in carbon capture and storage projects evident in small investment and economic incentives given to the CCS process compared to other technologies may have been the cause of stabilizing publication numbers afterward. Alternatively, we see an interdependence between oil prices and climate policies; low oil prices are likely to make the expenses of CO2 capture technologies difficult to tolerate, even with the use of captured CO2 in enhanced oil recovery to offset capture costs. It can also be seen from Figure 3A that most of the documents are journal publications. Patent publications only account for ∼10% in this document pool, whereas on average, patents account for a third of the total documents in the CAS Content Collection within similar time frame, suggesting a relatively small commercial interest in this research topic.16 However, the distinct count of concepts being introduced to this field showed a steep increase in the last 10 years, especially in journal publications (Figure 3B), suggesting new ideas or methods being tested.
Figure 3.
Overall publication trend of documents on CO2 capture and sequestration-related research: document count (A); count of distinct concepts in publications (B).
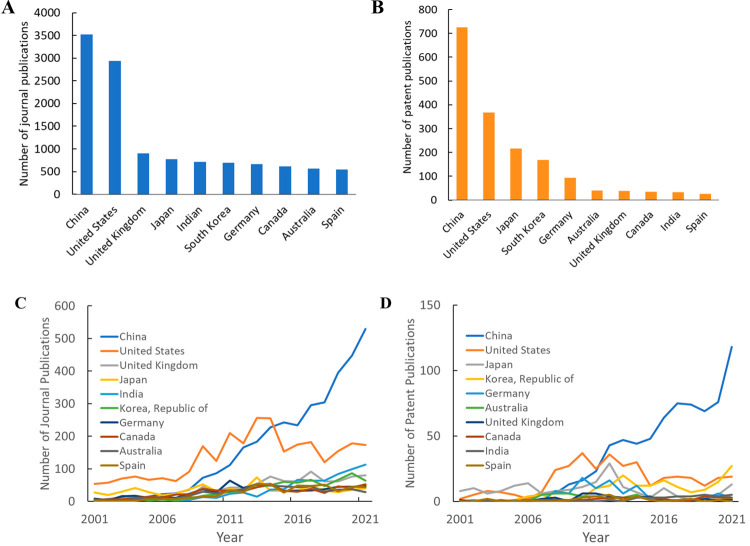
We also grouped the publications according to the country/region of the first author affiliated organization (journal publications) or the country/region of the patent assignee (patent publications). Figure 4 shows that the same ten countries/regions are responsible for the largest numbers of both journal and patent publications. Publication trends over the past 20 years show that China has steady growth in both journal and patent publications, while India shows similar growth in journal publications; these increases may be driven by the increasing CO2 emissions in both China and India (Figure 5). In CCS literature, the United States shows a small peak in the years of 2013 and 2014 and constant publication numbers since 2015. It is clear that researchers from China are driving publication trends in CCS, as removing articles from China-based authors showed publication numbers remaining roughly constant from 2015 to 2019 before increasing in 2020 and 2021 (Figure 6).
Figure 4.
Top 10 countries in the total numbers of publications related to CO2 capture and sequestration from 2001 to 2021: (A) total number of journal publications for each country; (B) total number of patent publications for each country; (C) journal publication trends of the top 10 countries; (D) patent publication trends of the top 10 countries.
Figure 5.
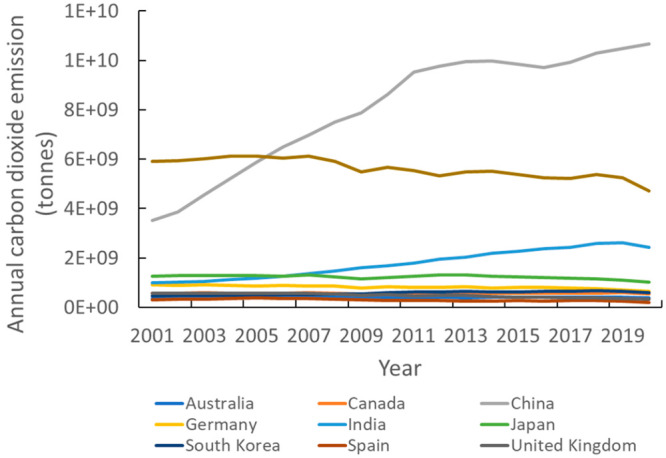
Annual CO2 emission levels of those countries with top publication numbers.
Figure 6.
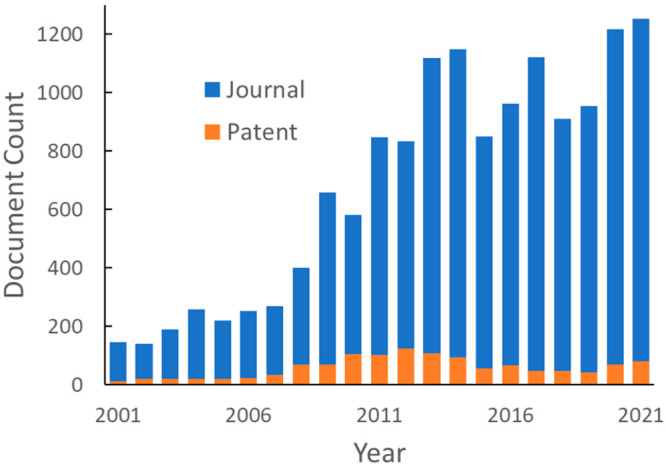
Global document publication trends of journals and patents when documents published by the organizations from China were removed.
Carbon Capture Processes
In this section we focus on the methods and materials used for CO2 capture and separation from other gases in industrial settings. Annual volumes of related publications from 2000 to 2021 are shown in Figure 7, where the pattern is similar to that for total publications related to CO2 sequestration and utilization (Figure 3A). Publication numbers were low prior to 2007, increased sharply afterward, peaked in the early 2010s, and stabilized afterward. The publication trends for CO2 separation and capture (Figure 7) are more abrupt than the overall publication trends shown in Figure 3A but likely originate from similar causes. CO2 capture and separation technologies are closer to implementation than other CO2 sequestration research and are more sensitive to economic incentives and oil prices, consistent with the observed publication trends. This section will begin with an introduction to carbon capture systems (industrial setting for flue gas handling), followed by reviews of capture methods (physical/chemical processes and materials used).
Figure 7.
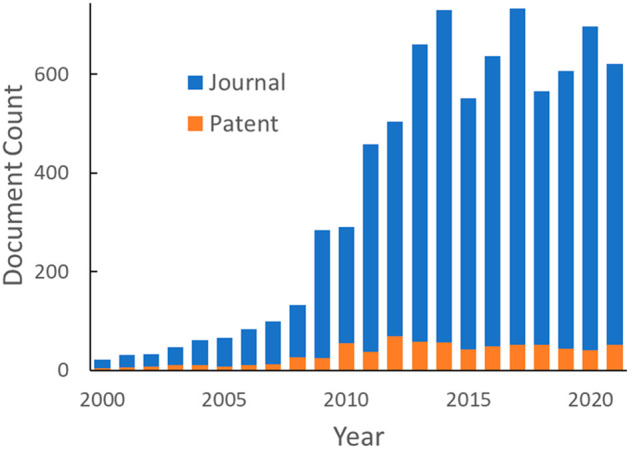
Publication trend on CO2 capture and separation, 2000–2021.
Carbon Capture Systems
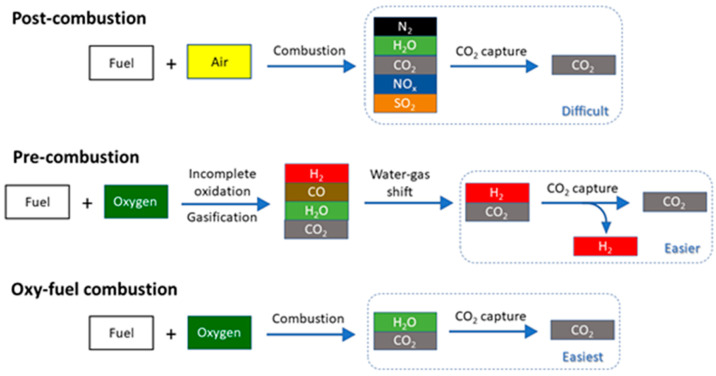
The removal of CO2 from power plant flue gases, the single largest source of human CO2 emissions, has been a major focus of carbon dioxide capture research.17−19 The three most widely studied techniques are postcombustion capture, precombustion capture, and oxy-fuel combustion capture.
Postcombustion Capture
Most thermal power plants generate electricity by burning fuel (most often pulverized coal or natural gas) in air to release its thermal energy. The heat boils water into steam which propels a turbine to generate electricity; the combustion generates CO2 and water vapor, which, along with nitrogen from the air, are major components of the flue gas. Postcombustion capture, the most popular method, removes CO2 from this gas mixture. It can be straightforwardly retrofitted to existing power plants and is the only commercialized carbon capture technique. The major disadvantage of postcombustion CO2 capture is that flue gas, diluted with large amounts of nitrogen carried over from air, has low pressure and low CO2 concentration, making the separation difficult and energy intensive.
Precombustion Capture
Precombustion capture is carried out in power plants where fossil fuels are utilized differently. A limited amount of pure oxygen is supplied, with or without steam, to partially oxidize the fuel, producing synthesis gas (syngas) comprised primarily of carbon monoxide, hydrogen, and some carbon dioxide. This hot gas mixture contains thermal and chemical energy, which are converted to electricity through a steam turbine and a gas turbine, respectively. The whole process, termed the integrated gasification combined cycle (IGCC), is more energy efficient than combustion and has simpler emission control.20 If CO2 capture is desired, the cooled syngas, instead of going straight to the gas turbine, is subjected to a water-gas-shift reaction to convert carbon monoxide into CO2. The CO2/H2 mixture then goes through a separation unit to remove CO2 and produce high-purity hydrogen. The CO2/H2 mixture has a simple composition, is at high pressure, and contains CO2 in high concentration, making CO2 separation much easier and less energy intensive than postcombustion capture. However, retrofitting power plants for precombustion carbon capture is much more difficult than for postcombustion, and the production of pure oxygen for partial oxidation is energy intensive.
Oxy-Fuel Combustion Capture
As the term implies, in oxy-fuel combustion the fuel is combusted in pure oxygen instead of air. The flue gas generated thus comprises predominantly CO2 and water vapor, which are easily separated by condensation of water. The biggest challenge facing oxy-fuel combustion is the high energy and cost required to produce pure oxygen, which is used in much larger quantities than in IGCC. The simplified schemes for the three CO2 capture techniques are shown in Figure 8.
Figure 8.
Simplified schematics of CO2 capture processes.
Chemical Looping Combustion Capture
In this emerging CO2 capture technology, treatment of a fuel with a metal oxide partially reduces the latter while yielding a waste stream containing only CO2 and H2O.21 The reduced metal oxide is then oxidized with air to regenerate metal oxide, which is returned to the fuel stream to complete the cycle. Chemical looping may be viewed as a variant of oxy-fuel combustion in which a metal oxide to metal transition acts as an oxygen carrier, obviating the expensive process for generating pure oxygen. However, the chemical looping combustion of solid and liquid fuels is much more complicated than the combustion of gaseous fuels. In addition, the fluidized bed reactors needed for combustion are complex, while the processes and apparatus needed to move solids between the combustion and reoxidation chambers are complicated and not easy to optimize, making the technology expensive to use. The above four CO2 capture processes are compared in Table 1.
Table 1. Comparison of CO2 Capture Processes.
| processes | advantages | disadvantages | retrofit difficulty |
|---|---|---|---|
| postcombustion | more mature technology, least expensive | low-pressure stream with low CO2 concentration undermines separation efficiency, CO2/N2 separation difficult | low |
| precombustion | high-pressure stream with high CO2 concentration, CO2/H2 separation easier | only works for gasification or reforming plants, no industrial application yet, pure oxygen expensive | moderate |
| oxy-fuel | facile CO2/H2O separation | pure oxygen production very costly | high |
| chemical looping | facile CO2/H2O separation | technology in early stage; more complicated process and equipment | high |
Direct Air Capture
An alternative to CO2 capture from industrial plants is to capture it directly from the environment.22 Direct air capture (DAC) can be carried out by absorption or adsorption and has the potential to achieve negative emissions if clean energy is used in the process without generating extra CO2.23 DAC plants are small and can be placed where needed, such as near carbon storage, use, or emission sites.24,25 DAC projects have recently been strongly supported by substantial funding, and 19 DAC plants have been established worldwide.26,27 Unfortunately, because of the very low CO2 concentration in the atmosphere (412 ppm), the theoretical energy required to capture one ton of CO2 from air is several times that of capturing it from power plant emissions,28 and the current cost of capture is higher than the price of CO2; thus, DAC is not profitable today.
The numbers of publications related to the different processes for CO2 capture are shown in Figure 9, where postcombustion capture has a significantly higher publication volume than all other processes. Publication volumes for the three primary techniques all increased starting in the late 2000s but peaked in the early or mid 2020s, displaying a generally decreasing trend afterward. Chemical looping and direct air capture, the newest emerging technologies, have low but increasing publication volumes, consistent with their lack of technical maturity.
Figure 9.
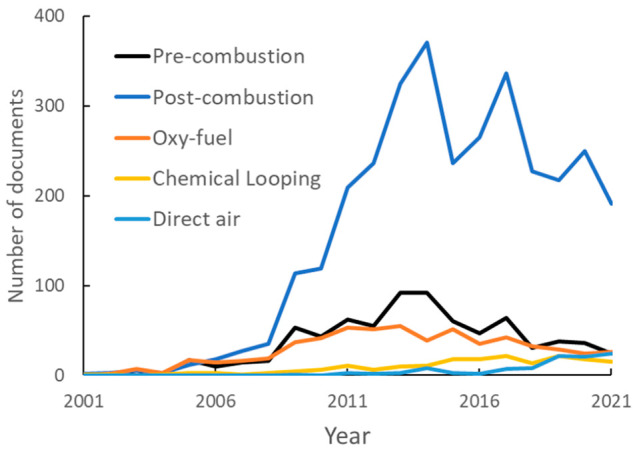
Publication volumes related to various CO2 capture processes, 2001–2021.
Methods for Capturing CO2 from Flue Gas
The most studied methods for separating CO2 from gas mixtures include: (1) absorption into solvents, (2) adsorption into porous solid adsorbents, and (3) filtration using membranes.
Absorption
Absorption of CO2 may be carried out chemically or physically. In chemical absorption, an alkali absorbent solution is brought into contact with the gas mixture to neutralize CO2 and form carbamate or bicarbonate salts.29,30 The resulting solution is then transferred to a regenerator (reboiler) to release the CO2 and recover the solvent. Monoethanolamine (MEA) is the most widely used absorbent and is the only one currently used in commercial applications.31 Other widely studied amines include diethanolamine (DEA),32 methyldiethanolamine (MDEA),33 piperazine,34 and 2-amino-2-methyl-1-propanol (AMP).35 Amine-based absorption is effective even for low-pressure streams with low CO2 concentrations, making it particularly suitable for postcombustion capture. Their drawbacks include limited thermal and oxidative stability,36 the high thermal energy required for solvent regeneration,37 solvent evaporation, and the corrosiveness of the absorbents.38
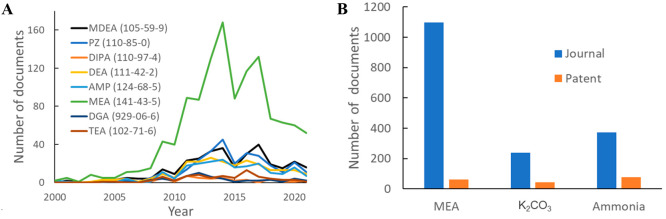
The numbers of studies related to CO2 capture using the most popular amines are shown in Figure 10. MEA has clearly been the most studied absorbent over time, while the publication volumes for most amines peaked in the mid-2010s and then decreased.
Figure 10.
(A) Annual publication volumes related to different amine absorbents. Abbreviations: TEA, triethanolamine; MDEA, methyldiethanolamine; PZ, piperazine; DIPA, diisopropanolamine; DEA, diethanolamine; AMP, aminomethylpropanol; MEA, monoethanolamine; DGA, diglycolamine. (B) Total publication volumes for MEA, K2CO3, and ammonia.
Electrochemical solvent regeneration, where CO2 is stripped from the stream by electrochemically generated copper ions, has been reported.39,40 The processes were found to be more energy efficient than thermal regeneration and can be carried out at normal temperatures, thus minimizing solvent loss and thermal degradation.
Ammonia is more stable, less expensive, and less corrosive than other amines.41 However, ammonia boils at −33 °C and has a high vapor pressure, leading to rapid loss of absorbent and deterioration of absorption capacity. Ammonia loss can be mitigated by absorption of CO2 at low temperature,42 but the absorption efficiency is compromised and the energy requirements for cooling decrease energy efficiency. Washing with water or acids also improves ammonia retention43,44 but generates large amounts of wastewater or chemical waste.
Potassium carbonate acts as an absorber by reacting with CO2 to form potassium bicarbonate.45 It shares most of ammonia’s advantages while being nonvolatile, enabling CO2 absorption at much higher temperatures.46 Its major disadvantage is a low absorption rate owing to poor CO2 mass transfer. Amine and amino acid promoters that form intermediates with CO2 to facilitate the generation of bicarbonate ions have been investigated.47 Grimekis et al. demonstrated that adding piperazine and MEA improved absorption rates and CO2 solubility at the same time, while MDEA and glycine significantly impacted CO2 solubility.47,200 Li et al. reported enhancements in both absorption and desorption by using glycine or lysine as promoters.48 The total journal and patent publication volumes for MEA, potassium carbonate, and ammonia are shown in Figure 10B. Both K2CO3 and ammonia have much lower journal publication numbers than MEA; interestingly, there are more patents on ammonia than on MEA.
Besides being studied as additives for other absorbents, amino acid salts have also attracted attention as CO2 absorbents themselves. They share the benefits of amines and carbonates and have high absorption capacity along with low toxicity and vapor pressure.49 Their major disadvantage is the easy formation of precipitates upon CO2 absorption due to their ionic nature, which complicates heat and mass transfer.50
For direct air capture (DAC), due to the low CO2 concentration in the air, the leading absorbents used are strong base solutions such as KOH or NaOH.51 The use of strong base absorbents means large amounts of energy are needed to separate CO2 from the absorbent, which exacerbates the high cost of DAC. Mahmoudkhani et al. achieved significant reduction in absorbent regeneration energy and temperature by using sodium trititanate in place of calcium hydroxide for causticization.52 More recently, Shu et al. reported an electrochemical process using an electrochemical cell having a pH gradient, allowing for reduction in energy consumption as well as simultaneous desorption and regeneration.53
Computer-aided molecular design has been conducted to identify new structures or commercially available substances that have not been explored as CO2 absorbents. Salazar et al. studied 50 amines that were prescreened using solubility and boiling point data, selecting three that showed much lower theoretical reboiler duty than MEA for postcombustion capture.54 Papadopoulos et al. modeled the solubility and partial pressure of CO2 and identified both new as well as commercially available alternative amines that outperformed MEA in overall absorption/desorption cycles.55
Physical absorption relies on physical dissolution of CO2 as the driving force.56 The method is effective only at high pressure and lower temperature and thus is much more suitable for precombustion capture. However, physical absorption using noncorrosive solvents is much less demanding on equipment than chemical absorption and has been practiced commercially. In addition, regeneration of physical solvents is much less energy intensive since the dissolved CO2 can be easily released through depressurization or moderate heating. Commonly studied good CO2 solvents include methanol,57 Selexol (polyethylene glycol dimethyl ether),58N-methyl-2-pyrrolidone (NMP),59 and propylene carbonate.60 The advantages and limitations of these solvents are summarized in Borhani’s review.61 All four solvents have similar CO2 solubilities at 25 °C. In general, solvents with higher molecular weights such as Selexol have lower vapor pressures, leading to less solvent loss, but suffer from high viscosities which impair mass transfer. Methanol, despite its low cost, has a very high vapor pressure, necessitating refrigeration and water washing to minimize solvent loss. Propylene carbonate’s vapor pressure is higher than Selexol’s but is still low enough to not require water washing. Because it dissolves hydrogen poorly, propylene carbonate is selective for CO2 in precombustion capture. Chen et al. reported optimized propylene carbonate solutions containing 2-methylimidazole and ethylene glycol, which demonstrated significantly improved CO2 solubility and selectivity over hydrogen, methane, and nitrogen.60
The publication numbers related to different physical absorbents are shown in Figure 11, where methanol dominates the other three absorbents in both journal and patent publication volumes. The much lower document numbers compared to those in Figure 10B indicates physical absorption’s lower popularity in comparison to chemical absorption.
Figure 11.
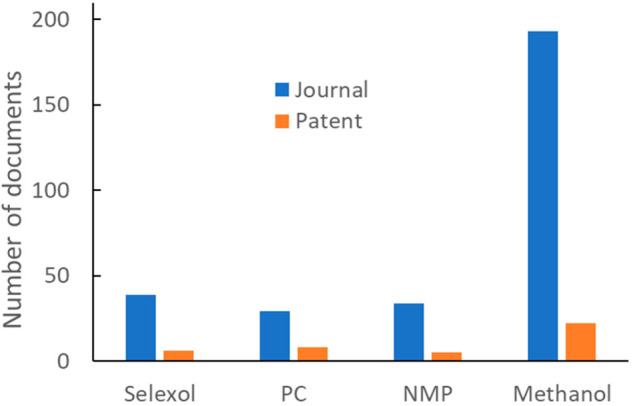
Publication volumes for physical absorbents.
Ionic liquids (ILs) are salts with low melting points, enabling them to stay in a liquid state within wide temperature ranges during normal applications. Typical examples are imidazolium salts such as 1-butyl-3-methylimidazolium tetrafluoroborate ([BmIm][BF4]).62 They have recently drawn strong interest as alternative CO2 solvents thanks to their very low vapor pressure, lower flammability, good thermal stability, and structural tunability.63 However, physical properties such as high viscosity combined with the high cost of ILs has limited research into and commercial application of ILs.64
Adsorption
In adsorption, porous solid adsorbents with large surface areas bind CO2. Adsorption methods are compatible with precombustion, postcombustion, and direct air capture. The solid materials used are more stable, less toxic, and easier to handle compared to liquid absorbents. The most studied adsorbents include carbon (activated carbon, biochar, charcoal, etc.), zeolites, and metal–organic frameworks (MOFs), whose advantages and limitations are summarized in Table S1.
Recent research on adsorbents is focused on improving CO2 uptake and adsorption kinetics and enhancing dimensional stability and reusability, as well as overcoming moisture sensitivity (for zeolites and MOFs).65−68 Optimizing adsorbents for CO2 uptake and selectivity does not necessarily guarantee their applicability in real applications. The design of temperature-swing (favorable for postcombustion capture) or pressure-swing (favorable for precombustion capture) adsorption–desorption cycles, as well as different reactor configurations, all pose specific performance and stability requirements on adsorbents and can induce uncertainties in the practical success of an adsorbent that performed well in the lab.69 More sophisticated modeling to efficiently screen the numerous possible structures (particularly for MOFs) and to predict their performance under complex processing conditions is needed to increase the industrial application potential of CO2 adsorption.
Voskian et al. reported an electrochemical device for CO2 adsorption, utilizing a polyanthraquinone–carbon nanotube composite electrode, where CO2 is captured via reductive addition to the quinones and released by discharging.70,71 The electro-swing adsorption–desorption process is more energy efficient than temperature-swing and pressure-swing cycles, and the compact electrochemical cells are easy to fabricate and scale up.
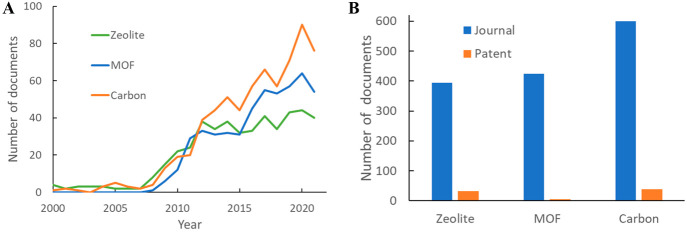
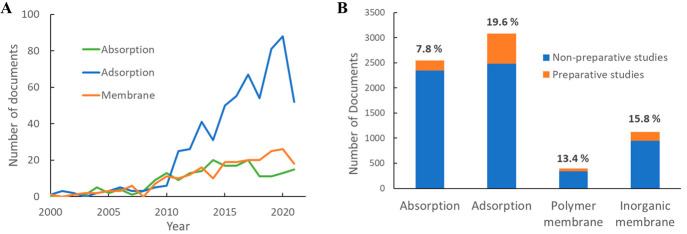
From the trends of publications adopting various adsorbents (Figure 12A), it can be seen that, while publications on zeolites increased little after 2012, those related to carbon had steady growth since 2007. Figure 12B shows the total publication volumes for the adsorbents, where the extremely small patent number for MOFs is worth noting, albeit consistent with the fact that related research is more focused on lab studies of new MOF structures.
Figure 12.
(A) Annual CO2 adsorption publication volumes using zeolites, MOFs, and carbonaceous materials. (B) Total relevant publication volumes.
Membranes
CO2 capture by membrane filtration is still an emerging technology, mainly due to low gas permeabilities and consequent poor separation efficiencies.72 Membrane-based processes offer lower material costs and operational simplicity and flexibility. Applications in precombustion and postcombustion have both been widely studied.73,74 For precombustion capture (CO2/H2 separation), H2 passes through the membrane to the other side (permeate side), leaving CO2 at the feed side, whereas in postcombustion capture (CO2/N2 separation), separation is achieved by CO2 preferentially passing through the membrane. Separation mechanisms are different depending on the membrane material and the gas stream, including (1) size sieving, where the membrane’s pore size is large enough to allow only the smaller gas molecule to pass through, (2) surface diffusion, where the surfaces of the membrane and pores are occupied by one gas through preferential adsorption and become inaccessible to the other gas, which therefore tends to stay at the feed side while the more adsorbable gas moves to the other side, and (3) solution diffusion, where the more soluble gas preferentially dissolves into and then diffuses through the membrane.75 While the first two mechanisms work for porous membranes, the third occurs during separation using dense membranes. The most studied membranes can be classified into dense inorganic membranes, porous inorganic membranes, and polymer membranes, as summarized in Table S2.
Most studied polymer membranes have been nonporous (dense) membranes. However, emerging materials such as conjugated microporous polymers,76 polymers of intrinsic microporosity,77 and thermally rearranged polymers,78 where pores with controlled architectures are introduced to organic polymers to improve CO2 permeability and CO2/N2 selectivity, have recently intrigued researchers. Polymer membrane matrices with inorganic fillers, combining the permeability and thermal stability of inorganic materials with mechanical strength and processability of polymers, have also shown promise.79 Husna et al. prepared surface-modified UiO-66-NH2 by grafting an anhydride-terminated polyimide onto the MOF. The modified filler had improved compatibility with microporous polyimide matrices, and the blended membranes demonstrated improved resistance to thermal aging and plasticization, with CO2 permeability and CO2/N2 selectivity surpassing the Robeson 2008 upper bound.80
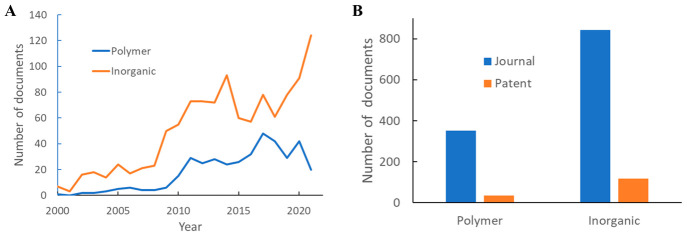
The publication trends of CO2 capture using polymer membranes and inorganic membranes, as well as their overall publication volumes, are individually shown in Figure 13. Here, inorganic membranes surpass polymer membranes in both journal and patent volumes, with remarkable growth in the most recent years. It should be noted that the actual relative prevalence of inorganic membrane studies is likely even higher—in our analysis method, documents containing polymer substances are deemed polymer-membrane-related, yet one cannot rule out the possibilities of inorganic membranes studied in these documents. The journal and patent publication volumes of some of the most studied polymer and inorganic membranes are shown in Figure 14.
Figure 13.
(A) Annual publication numbers on polymer and inorganic membranes for CO2 capture. (B) Total related publication volumes.
Figure 14.
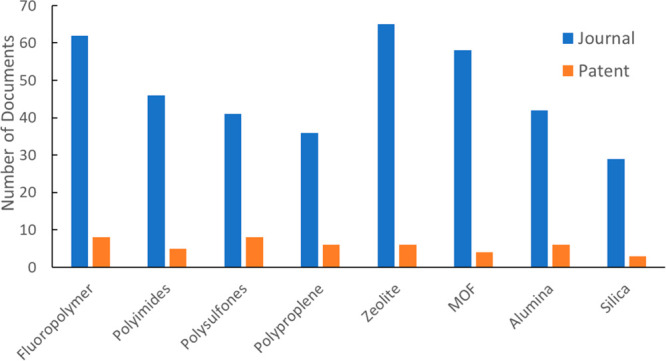
Publication volumes on representative polymer and inorganic membranes for CO2 capture.
Comparisons of Methods and Concept Map Analysis
The three carbon capture methods (absorption, adsorption, membrane) are compared in Table 2.
Table 2. Comparison of CO2 Capture Methods.
| methods | most suitable process | advantages | disadvantages | technical maturity |
|---|---|---|---|---|
| absorption | postcombustion | more mature technology, lower cost, simple operation | corrosive solvent used, high solvent loss, high energy required for solvent regeneration | moderate |
| adsorption | precombustion | continuous operation, environmentally friendly | low CO2 selectivity, difficult to manage solid/gas contact to maximize adsorption capacity, too many potential candidates, actual performance of adsorbents difficult to predict | low |
| membranes | postcombustion, precombustion | simple and flexible system, environmentally friendly, no regeneration needed | low CO2 permeability, energy intensive, membrane material easily compromised | very low |
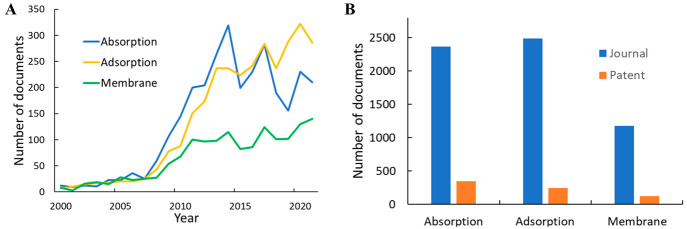
The numbers of publications related to CO2 capture using absorption, adsorption, and membranes from 2001 to 2021 are shown in Figure 15. Here, absorption-related studies grew substantially up to 2014 and then decreased, whereas adsorption and membranes kept growing, albeit at slower paces after 2010. Absorption has been studied in the patent literature more frequently than adsorption. This observation is consistent with absorption capture being relatively more mature and closer to industrial applications. Membrane separation, on the other hand, has much lower numbers for all publication types compared to the other two techniques, with patent publication volume several times lower than that of absorption, consistent with it being an emerging technology.
Figure 15.
Publication volumes related to different CO2 capture methods: (A) publication trends, 2001–2021; (B) total publication volumes, 2001–2021.
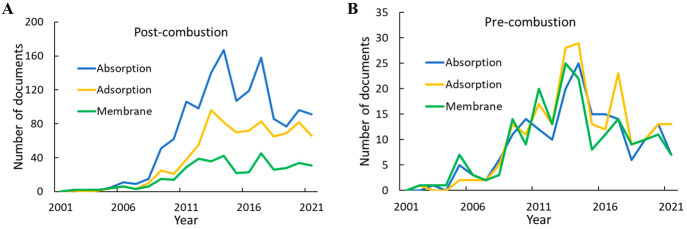
To get insights into the prevalence of various CO2 capture methods (absorption, adsorption, membranes) studied in different processes (postcombustion and precombustion), the numbers of publications involving co-occurrences of the corresponding terms are shown in Figure 16, which suggest that absorption has been studied the most for postcombustion capture; for precombustion, on the other hand, the three methods have almost equal shares of publications. This is to be expected, given that precombustion produces streams with high pressure and high CO2 concentration that are relatively easy for all separation methods, whereas the dilute CO2 in postcombustion’s CO2/N2 stream favors absorption capture.
Figure 16.
Publication volumes related to different CO2 capture methods, 2001–2021, used in (A) postcombustion capture and (B) precombustion capture.
The occurrence frequency of chemical preparation of substances in publications can be one indicator of a certain field’s technical maturity. The publication trends and total publication volumes for studies involving the synthesis of at least one substance are shown in Figure 17. The numbers of preparative studies involving adsorption capture had the fastest rate of increase (Figure 17A) as well as the highest total publication volume (Figure 17B, orange portion of bars) compared to those related to absorption and membranes. 19.6% of all adsorption-related publications concern synthesis, also the highest of all capture methods, with the ratio being only 7.8% for absorption studies. Membrane separation, commonly considered an emerging technology, features a lower percentage of preparative studies than adsorption; researchers have likely focused more on modification of existing materials and membrane fabrication and characterization.
Figure 17.
Publication volumes for CO2 capture publications involving chemical synthesis: (A) annual publication trends; (B) total publication numbers and percentages of preparative studies.
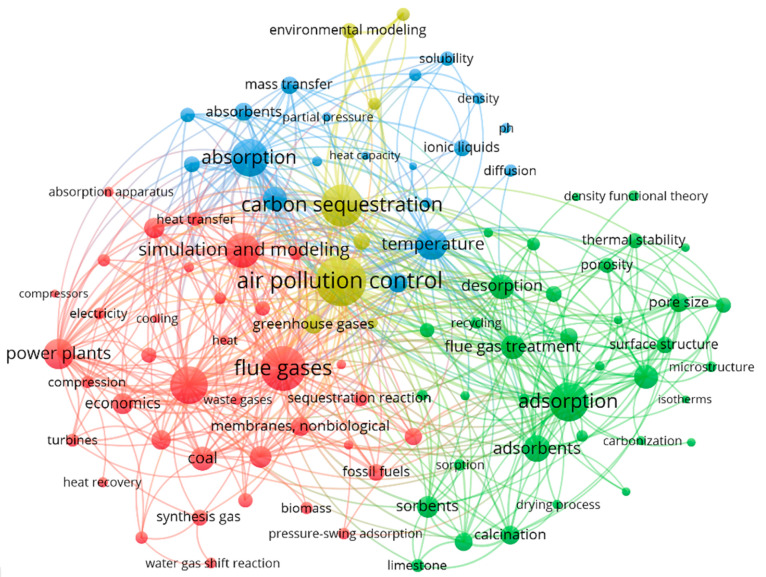
To further shed light on the status of development in CO2 capture and sequestration, we have also analyzed the prevalence and connections between different concepts occurring in related publications. The results are shown in Figure 18, where the size of a node reflects the number of times the corresponding concept occurred in the literature, lines between every two nodes denote co-occurrences in the same publication, and distances between nodes indicate the frequencies at which the concepts co-occurred. One interesting observation of the graph is that the concept “simulation and modeling” is nearer to “absorption” than “adsorption”, the latter instead being adjacent to common adsorbent characterizations such as “pore size” and “surface structure”. The relative material and operation simplicity for absorption capture likely explains the prevalence of its modeling studies.
Figure 18.
Prevalence and co-occurrence of concepts related to CO2 capture.
To help understand recent advancements in real applications of carbon capture, some examples of existing or planned operations of various capture methods are listed in Table 3. As the information shows, carbon capture from flue gas using amine solvents has strong commercial prospects, but direct air capture is also receiving attention, despite being considered the most expensive and energy intensive.
Table 3. Industrial Operations and Projects Using Various Carbon Capture Methods.
| technologies | applications |
|---|---|
| direct air capture | Climeworks, currently capturing 4000 tons annually, raised $634 million;81 Carbon Clean raised $150 million;82 Carbon Engineering and 1PointFive plan to capture 1 million tons annually by 203583 |
| amine-based capture from power plants | Acorn CCS project, partnership among Shell, Harbour Energy, and Pale Blue Dot Energy, is planned to open in mid-2020s and store 5–10 million tons per year84 |
| DOE-funded project outside Bakersfield, California, will capture CO2 from a gas-fired power plant, using a solvent system developed by Fluor85 | |
| Shell’s Cansolv technology for postcombustion capture will be fitted to the gas-emitting stacks of the VPI Immingham power station in the UK, to capture up to 95% of the CO2 in the flue gas; the system was also installed in a Canadian power plant to capture 1 million tonnes annually86 | |
| ExxonMobil Low Carbon Solutions will develop 20 CCS projects with an initial investment of $3 billion87 | |
| solid sorbents | CCS firm Svante raised $75 million to develop nanoporous MOF sorbent to capture CO2 from flue gas and from the air87 |
CO2 Sequestration Methods
Once CO2 is captured, it can be sequestrated and stored by chemical or geological processes. CO2 can also be sequestered biologically, where carbon capture and sequestration are accomplished in one step by living organisms. Recent research progresses and publication trends in these methods will be discussed.
Biological CO2 Sequestration
Natural biological CO2 fixation via plant photosynthesis accounts for the largest CO2 influx (440 gigatons/year) from the earth’s atmosphere, of which 2–3% remains locked in the land for decades.8,15 In addition, about 50% of this amount is fixed by marine primary producers.88 Biological CO2 fixation reactions are highly selective and often require little resources, spurring interest in developing biomimetic and biobased technologies for CO2 capture and sequestration.89 The past decade has witnessed a rapid increase in the number of related journal publications (Figure 19), while recently, viable and cost-effective negative-emissions technologies have been developed, collectively referred to as Bioenergy with Carbon Capture and Storage (BECCS), for utilizing biomass (derived from biologically fixed CO2) as the energy source to capture and permanently store CO2.90−93 According to a report published in 2019,94 five facilities were utilizing this technology to capture ∼1.5 million tons of CO2 per year. However, a recent study predicts that BECCS has a global potential to sequester up to 5.2 gigatons of CO2.94 The accelerated publication activity related to BECCS over the last 6 years reflects these recent developments (Figure 20).
Figure 19.
Publication trends for biological CO2-sequestration research between the years 2000 and 2021.
Figure 20.
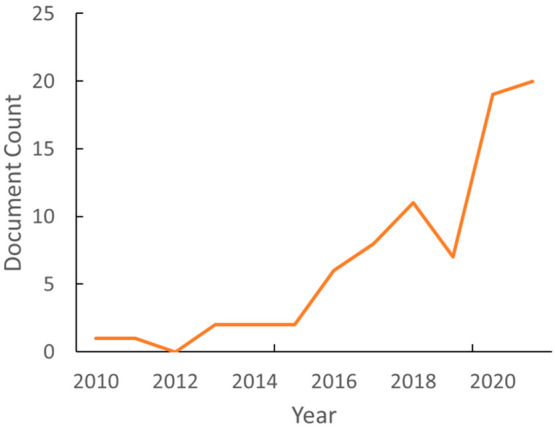
Trends of publications exploring the potential of BECCS as a large-scale negative-emissions technology.
Biological-System Level CO2 Sequestration Studies
Primary producers are known to utilize six pathways for CO2 fixation, which represent billions of years of evolution and optimization for survival and reproduction of the host organisms (Table 4).95,96 Because of their importance, prior research efforts focused on these biological systems have been useful starting points to capture and sequester atmospheric CO2.12,15,97−100 Among the six CO2 fixation pathways, the reductive pentose phosphate pathway or the Calvin–Benson–Bassham (CBB) cycle is the prevalent mechanism used by all plants and algae, and most autotrophic bacteria. It is also the most economically relevant pathway.101 The other five pathways are only present in a small number of bacteria or archaea but nevertheless provide clues regarding the unique environments in which the host organisms thrive.102 Recent efforts include engineering natural CO2 fixation pathways in non-native organisms103−109 and engineering synthetic CO2 fixation pathways into organisms.110−114 An emerging new concept combines microbial or algal cell factories with electrochemistry to directly convert CO2.115 Each approach represents a promising line of investigation potentially leading to the capture of gigaton quantities of CO2 from the atmosphere using natural biological hosts.
Table 4. CO2 Fixation Pathways Used by Biological Organisms.
| pathway | host organisms |
|---|---|
| Calvin–Benson–Bassham | plants, algae, bacteria |
| Wood–Ljungdahl (W-L) | bacteria, archaea |
| reductive tricarboxylic acid (rTCA) | bacteria |
| 3-hydroxypropionate | bacteria |
| 3-hydroxypropionate 4-hydroxybutyrate | archaea |
| dicarboxylate 4-hydroxybutyrate cycle | archaea |
The most important challenge in employing photosynthetic organisms for CO2 capture lies in scaling up the process. Because this involves the use of photobioreactors, open ponds, or raceway ponds, scaling is hindered by large surface area requirements, light requirements, low productivity, and contamination possibilities.116 Closed systems have been proposed to overcome some of these limitations.117
Recently, chemoautotrophic organisms including Ralstonia eutropha have been exploited for industrial applications due to the CBB pathway that works with other pathways to sequester CO2 into bioplastics.118,119 Furthermore, growth of this organism at scale is simple and can be genetically engineered.120−128
In our curated list of publications, a significant number of the biology-related publications (∼1600 out of ∼3900) had identifiable terms in the abstract that could be linked to photosynthetic organisms that use the CBB pathway for CO2 fixation. 70% of these documents were published in the past decade, suggestive of the recent focus on photosynthetic organisms as the biological chassis of choice for CO2-remediation studies (Figure 21).
Figure 21.
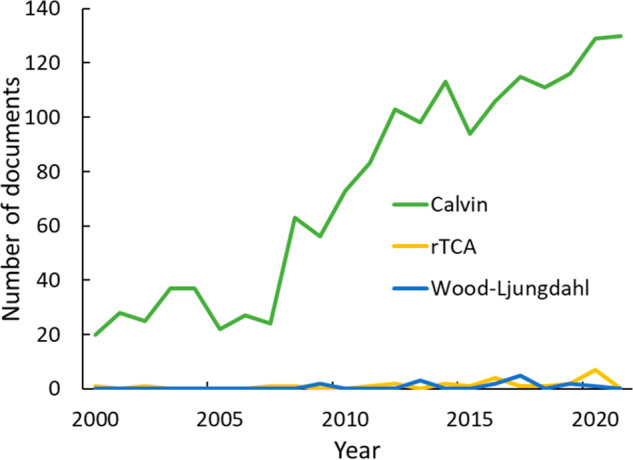
Publication numbers with keywords in the abstract of studies with host organisms containing photosynthetic CBB cycle, the rTCA cycle, or the W-L pathway between the years 2000 and 2021.
Bacteria and algae have rapidly become popular natural biosystems of choice for CO2-sequestration studies due to their extremely versatile metabolic capabilities, shorter lifecycles, natural abundance, simpler growth requirements, and bioremediation potential and recent advances in genetic manipulation capabilities (Figure 22). Analysis of a subset of our curated publication data set comprising 1343 journal articles and 103 patents published between 2017 and 2021 indicated that it is becoming increasingly attractive to utilize bacteria (mostly cyanobacteria) and algae as cellular factories to sequester CO2 because they can deliver a sustainable and renewable platform to produce biofuels and high-value products, wastewater and flue-gas remediation, and biomitigation of unwanted nutrients.129−137 In one study, the authors report the use of a novel metagenomic approach to analyze the microbial communities in a cold subsurface high-CO2 aquifer2 fixation. Metabolic analyses at the organism level provided insights into the biochemical cycles that support subsurface life under the extreme condition of CO2 saturation, which predominantly involved the use of CBB and WL pathways in tandem.135
Figure 22.
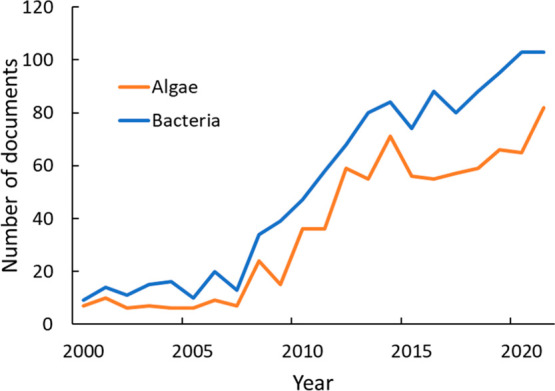
Publication number retrievals from our selected and curated data set for the use of algae or bacteria as the biological system for CO2-sequestration studies.
Agricultural and forestry-related activities contribute significantly to global CO2 emissions. Due to their longer life cycles and less amenability to genetic modification, CO2 sequestration studies have not focused on the production of bioproducts using CO2 fixation in plants. Instead, CO2 sequestration studies utilizing plants have focused on using plant biomass, especially from energy crops, as sustainable and renewable feedstocks for fermentation or biochar production.91,138−141 The overall publication trends for biological CO2 sequestration research indicate a growing interest in using both natural and engineered biosystems and enzymes as modules to capture CO2 in innovative ways and convert it into useful bioproducts, while reducing our dependence on fossil fuel. It is notable that the past few years have seen a number of these applications also integrate flue gas and bioremediation into CO2-sequestration strategies.
Chemical Methods for CO2 Sequestration
Chemical methods for CO2 sequestration are methods that convert carbon dioxide by chemical means into other materials such as mineral carbonates or concrete which sequester carbon for significant periods of time. CO2 may also be converted into reduced forms that can be used either as fuels or in the manufacture of organic compounds or fuels which sequester carbon dioxide for a shorter span.
Concrete
4.4 billion tons of concrete is manufactured worldwide,142 which generates 7–8% of total human CO2 emissions.143 Concrete is made from water, cement, and rocks or sand (aggregate). Cement is prepared from limestone (calcium carbonate, CaCO3), silica (SiO2), iron ore, furnace slag, and clay or slate.144 Heating the mixture at high temperature (1800 °C) drives off carbon dioxide to generate calcium oxide and calcium silicates. 60% of CO2 emissions in concrete manufacture comes from the decarbonation of limestone, and the remaining 40% comes from the energy needed to make the cement.145 The calcium salts in powdered cement react with water at the time of use to form a paste containing calcium hydroxides and silicates, which adhere to the aggregate and bind it into a single mass.144 Over time, the calcium hydroxides in concrete absorb carbon dioxide from the atmosphere, forming more stable calcium carbonates which strengthen the concrete in the weeks after installation and over the service life of the structure. Between 10 and 30% of CO2 emitted during cement manufacture is reabsorbed during its service life.145 Carbon dioxide can also be added during concrete pouring to incorporate more CO2 and to increase concrete strength. When concrete structures are demolished, the concrete can be broken into aggregates which can be recycled into new concrete, reducing concrete’s energy consumption. Concrete wastes also absorb carbon dioxide when left exposed to air, but only 1% of concrete wastes are left exposed long enough to absorb significant amounts of CO2.145
Reduction of CO2 emissions can be obtained by improving the efficiency of heating or using renewable energy sources for cement production, by capturing CO2 liberated in cement manufacture, by carbonating concrete during installation, and by allowing concrete wastes to remain exposed to air during demolition. In addition, the recycling of concrete to form aggregate may reduce the amount of cement needed for new construction.
While concrete with no net CO2 emissions is possible using these advances, it requires most of the concrete service lifetime to reach carbon neutrality and requires additional exposure of concrete wastes to air to absorb CO2.145 Carbonation (addition of additional CO2 to concrete while setting) is unlikely to be used unless it increases concrete strength.146,147 The use of concrete containing recycled aggregates may require modified processes to install and may require more expensive reinforcing materials or equipment,148 although it could reduce CO2 emissions by up to 50% over new concrete manufacture. Reducing the carbon footprint of concrete to zero, however, is likely to require replacement of concrete with other less carbon-intensive materials.
Mineral Carbonation
Mineral carbonation is the sequestration of carbon dioxide by forming stable metal carbonate salts such as calcium and magnesium carbonates.149 Sequestration can be performed either below ground (in situ) or above ground (ex situ) using excavated minerals or metal salts. Natural minerals containing calcium or magnesium oxides or silicates such as wollastonite, olivine, and serpentine will absorb carbon dioxide to form carbonates, as will ammonia or other bases. Cheap wastes like steel slag, mining wastes such as asbestos and nickel tailings, red mud from alumina manufacturing, waste ash from sources such as incinerators, and alkaline paper mill waste can also absorb CO2 but may require careful handling to prevent environmental contamination.
Mineral carbonation can be performed directly (by treatment of the dry or slurried minerals with carbon dioxide) or indirectly (by conversion of the minerals to metal oxides or hydroxides followed by carbonation). Direct carbonation in the solid phase is limited by mass transport and is generally slow unless high-surface-area absorbents are used. Carbonation of minerals in aqueous solution is fast, but the dissolution of minerals in water is slow. The solubility of minerals in water is improved with acids, with hydrochloric or acetic acid being the most common acids used,149 but both acids are corrosive and difficult or impossible to recover, increasing the costs of their use further.
Mineral carbonation in some cases yields valuable materials. Precipitated calcium carbonate (PCC), for example, has been sold for $320/ton, while ultrapure calcium carbonate obtained from carbonation can yield revenue of >$9000/ton.149,150 The use of mineral-carbonation-derived carbonates, however, would only sequester a small fraction of human CO2 emissions.
Ex situ mineral carbonation is likely to be an economical way to sequester carbon dioxide if waste products (such as concrete wastes or ash) are used as sources for metal carbonates. Most indirect methods result in uneconomical carbonation. If temporary sequestration is desired, the processes can be made profitable by selling the carbonates (particularly pure and ultrapure CaCO3), but the market for carbonates is much smaller than the scale that would be needed to capture a significant fraction of human carbon dioxide emissions. In situ methods are likely to be permanent methods for CO2 sequestration, requiring minimal monitoring, and are economical, but sequestrated carbon dioxide is difficult or impossible to reintegrate into the carbon cycle.
Technologies for geological and carbonate-forming methods of CO2 mitigation are likely more mature than those of other chemical methods, and their costs and benefits are better known. Of the keywords searched, the largest number of documents discussed carbonation and mineralization (Figure 23). References to carbonation are high but stabilize after 2011, while publications involving concrete for CO2 sequestration follow a different pattern. The number of articles on concrete is significantly smaller (though some concrete articles may be included in documents discussing carbonation). The lower level of interest in CaCO3 than in other products of CO2 reduction may be evidence that in situ mineralization has attracted more interest than ex situ mineralization.
Figure 23.
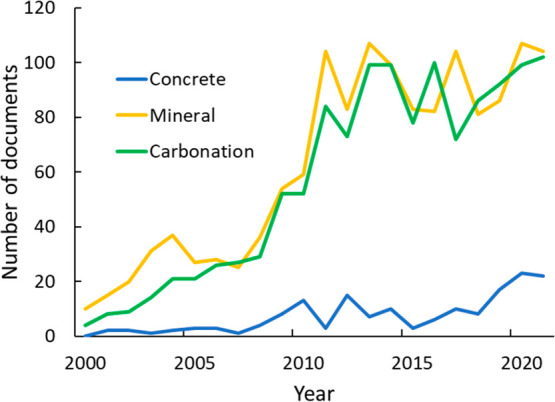
CO2 sequestration publications discussing carbonation, concrete, and mineralization during the period 2001–2021.
The advantages and disadvantages of concrete carbonation, as well as examples of their applications, are listed in Table 5.
Table 5. Comparisons and Application Examples of Concrete Carbonation and Mineralization.
| method | advantages | disadvantages | examples of companies using the method |
|---|---|---|---|
| concrete carbonation | forms stronger concrete than uncarbonated concrete, may accelerate CO2 uptake over standard concrete installation, currently in limited but non-negligible use | costs money (equipment, CO2 capture or purchase), concrete curing still requires significant time to take up CO2 | Solidia, Carbon Cure, Carbon Built |
| mineralization | produces directly saleable products from captured CO2, currently in use | requires reagents in addition to captured CO2, time frame of CO2 sequestration not clear (CO2 may be released by intentional or unintentional acidification), market for mineral products limited relative to need for CO2 sequestration | Carbon Free |
Geological Sequestration of Carbon Dioxide
Carbon geosequestration relates to the process of injecting captured carbon dioxide in deep porous geologic formations for long-term storage. Captured CO2 is compressed to elevated pressures, converted into a supercritical fluid, and then transported mostly by pipelines to the injection site.152 Any method used for geological storage of CO2 should be able to store it for a minimum of 1000 years with a leakage rate of less than 0.1% per year.
Most estimates suggest that sufficient capacity exists to store many thousands of gigatons (Gt) of CO2 with only a small risk of surface leakage in the following 10000 years. However, the level of uncertainty of these estimates depends on the formation (type and heterogeneity), the physical and chemical processes accompanying CO2 storage, the method being used to determine the storage capacity, and the amount of available data.153,154 Several assessments of regional storage capacity were conducted in Europe, China, Japan, Canada, and the United States, yet making direct comparisons of their results poses a problem due to their different underlying assumptions. A method to better assess the CO2 storage capacity worldwide using globally available data sets was developed at MIT as part of a larger project to use Integrated Assessment Models (IAMs).155 Their Economic Prediction and Policy Analysis (EPPA) model estimated between 8000 and 55000 gigatons of accessible geologic storage capacity for carbon dioxide using current storage technology and that storage capacity is not a limiting factor for carbon dioxide sequestration technology in most regions even if stringent emissions reductions are required.
The multiple requirements for site selection and successful long-term CO2 storage include (1) large capacity for storage of the site, (2) high porosity and permeability in the reservoir, (3) sealing caprock, (4) no fault planes near the site of injection and low seismicity, (5) deeper than 800 m (about 2600 ft) so CO2 remains supercritical, (6) wellbore construction must withstand long-term storage without compromising caprock sealing capacity,156 (7) easily accessible and monitored site, and (8) subhydrostatic pore pressure.157 Other considerations include distance from CO2 sources, population density and local public acceptance, reliability of the storage operation, legal accessibility, and the deployment model used.158
Therefore, site options for geological sequestration of CO2 include saline aquifers (porous reservoirs that contain saltwater),159 unmineable coal sites, shales and underground depleted oil and gas reservoirs,160−162 declining oil and gas fields,163,164 deep ocean waters, ocean floor or sediments,165−167 and basalts or reactive rock formations.168
CO2 sequestration via solid gas hydrates (clathrates), including storage in deep oceanic basins, sediments under the sea floor, permafrost regions, methane hydrate reservoirs, and depleted oil and gas fields partially saturated with water has received increased attention in the past years due to its potential storage capacity in the hundreds of thousands of Gt.167,169
Deep saline aquifers are one of the best candidates for CO2 storage because they are widespread, have large storage capacity and ideal geologic properties, cannot be used for human consumption or agriculture, and are isolated from the environment. However, this process involves complicated reactions among CO2, brine solution, and rock formations, which could potentially affect the integrity and storage efficiency of the well over the long term.153,170 The efficiency of trapping mechanisms and the movement of CO2 through the rock are strongly influenced by the CO2–brine–rock wettability, the pressure and temperature, salinity, and dissolved ions.171 These trapping processes take place at different rates and over many years, even thousands of years.
Several million tons of CO2 were injected in saline formations at several successful sites without issue: the Sleipner and Snohvit projects in the North Sea,172 the Quest project in Canada using the Basal Cambrian Sands,173 and the Mt. Simon sandstone in Illinois. However, CO2 injection was stopped at one site in In Salah, Algeria, due to caprock fracture. These projects indicate that CO2 storage can be safely accomplished if site selection, injection and postinjection operations, and monitoring of the formation are rigorously evaluated and implemented.
Besides these storage sites, CO2 has been used extensively in the past 40 years in enhanced oil recovery (EOR) operations.174 Typically, oil recovery increases by 10–15% with EOR due to the solubility of CO2 in oil. Up to two-thirds of the injected CO2 returns with the extracted oil and is usually reinjected into the reservoir to minimize operating costs and trap more CO2 in the oil reservoir. The major drawback of CO2 storage using EOR is that today’s processes use naturally occurring CO2 (i.e., CO2 that was previously underground) due to its lower costs compared to CO2 from anthropogenic sources. Also, EOR projects are driven by the economics of oil production and not by CO2 storage and do not take advantage of the full potential of the oil field to store additional CO2 once no additional oil can be extracted.
Similar to EOR, injection of CO2 in tight gas sands, shales, and coal seams is used to recover gas by displacement in a process called enhanced gas recovery (EGR).175,176 CO2 storage in coalbeds is quite different from storage in oil and gas fields or saline formations because the trapping mechanism is by adsorption as opposed to storage in rock pore space. Here, CO2 is preferentially adsorbed onto the coal micropore surface, displacing the existing methane.177
The use of former fossil-fuel reservoirs for geosequestration of CO2 is attractive for many reasons. Rock reservoirs have sufficient porosity and permeability to promote massive CO2 volume injections, while oil and gas fields have a geological barrier preventing upward migration and leakage of CO2 into shallower formations (proven by having stored hydrocarbons for thousands to millions of years without appreciable leakage). Meanwhile, the existing infrastructure and industrial setup required for fluid injection can be utilized while the reservoirs have already been geologically characterized, tested, and monitored. Moreover, revenues from the produced gas/oil can be used to help offset the current high costs of CO2 sequestration. However, sequestering CO2 while extracting oil or gas is probably not the best way to mitigate the environmental impact of CO2 emissions.
Selected terms “aquifer”, “saline”, “brine”, “geological”, “shale”, “seam”, “caprock”, “underground storage”, “deep sea storage”, “seismic”, and “clathrate” related to geological storage of CO2 were used to search the CAS Content Collection for articles published between 2000 and 2021. According to the extracted data, publications in this field increased gradually up to 2013, while showing a decline in publications afterward (Figure 24). Individual search terms showed similar trends except for “shale” and “clathrate”, whih displayed an upward movement in the past 4 years, albeit in fewer numbers (data not shown). Research on CO2 storage in the form of clathrates is still in development, with limited field data available, but remains promising due to its potential for high volumes of CO2 to be sequestered. While the “geological” term was used most frequently in publications, as expected, the “aquifer”, “saline”, and “brine” terms returned more publications than the rest of the search terms, reflecting more interest in this specific storage site (Figure 25). This trend is replicated in a network diagram showing the top 1000 co-occurring concepts within documents with the term “aquifer” appearing as the top geological term in comparison with the rest of the geological search terms that we used (data not shown). In the same network diagram, the simulation and modeling concept indicated a strong affiliation with geological processes, as expected, since numerical programs are used for assessment of the storage capacity of the formation as well as for estimation of geological CO2 storage security for leakage risks.
Figure 24.
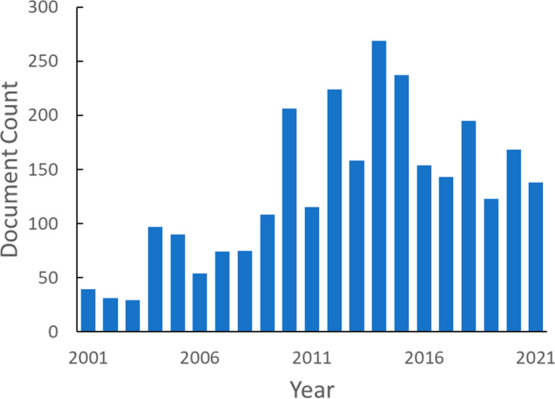
Publications related to geological storage of CO2 from 2001 to 2021.
Figure 25.
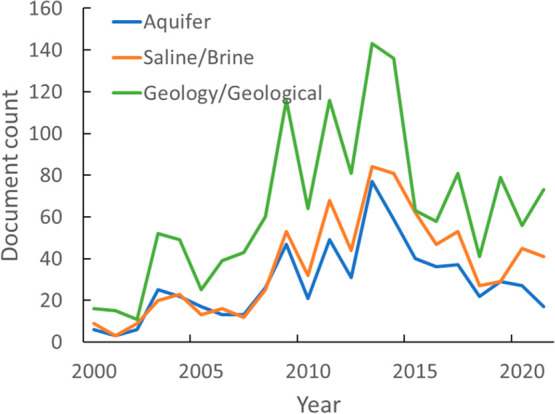
Global publication trends for journal publications that contain “aquifer”, “saline”/“brine”, and “geology”/“geological” search terms.
Main Issues and Potential Leakage Pathways
Once injected in a well, CO2 plumes will rise via buoyant forces, due to lower density than its surroundings. CO2 then spreads laterally upon encountering caprock until it finds a gap. Fault planes or fracture networks near the injection zone increase the possibility of gaps, which would be potentially dangerous to life in the surrounding area. CO2 can potentially migrate into shallow groundwater aquifers and compromise water quality by releasing trace metals such as Sr, Zn, Co, and Ba and organic compounds and/or change the water’s pH.178,179
Deep coal seams that are not economically viable sources for coal mining are generally used for sequestration of CO2. Despite the many advantages of these sites, the injected CO2 may chemically and physically alter the coal matrix and induce its swelling and mobilization of polycyclic aromatic hydrocarbons (PAHs) in the coal seam.180 This mobilization of PAHs may cause environmental issues, as PAHs are harmful even at relatively low concentrations.
Induced seismicity is also a cause for concern, but it is not expected to be a significant problem at geological CO2 storage sites if good engineering practices are followed.181 The measures generally taken to alleviate such effects consist of fluid pressure management. For example, the injection of CO2 is often conducted simultaneously with the coextraction of formation brine in saline aquifers or extraction of oil/gas at EOR/EGR sites, which can control the amplitude of the overpressure in the reservoir and along faults.182
CO2 pipelines pose a risk to local population and the environment, as the presence of water and other impurities within CO2 may lead to operational problems related to corrosion, gas hydrate, and ice formation and thus accidental release of CO2. The exact levels of impurities will vary depending on the source and capture process. Therefore, apart from dehydration, gas treatment is required. The level of impurities that can be tolerated will depend on the storage method (or end use) and the transportation method. Other challenges to CO2 transport through pipelines consist of pipeline design and maintaining the CO2 in a supercritical phase.152
Regulation of CO2 Injection and Environmental Monitoring
Estimation and quantitative predictions of geological CO2 storage security suggest geological storage is a secure, resilient, and feasible option for reducing global climate change even when applying worst-case values for each scenario.183 CO2 becomes safer and more secure the longer it stays in the ground due to a range of physical processes, with mineralization being the ultimate goal as trapping of CO2 becomes permanent.
Best practices include monitoring of the injection process and deploying surface and subsurface sensing technologies to allow for risk assessment and mitigation of potential release of CO2 from wellbores, faults, and other migration pathways, including CO2 leakage from pressurized pipelines during transport.153,184 Monitoring allows leak detection with enough warning to minimize the amount lost, and to quantify the leak size. Simulations are also used in predicting the pressure buildup in the formation, fluid flow, and geomechanical and geochemical processes at the injection site. Research focused on improving the fundamental understanding and modeling of various aspects of geological storage and monitoring of CO2 has been carried out over the past decade.185
The Safe Drinking Water Act (SDWA) requires the EPA to regulate underground injection activities to prevent contamination of underground sources of drinking water (USDW). EPA has issued regulations for six classes of underground injection wells. Class II wells are used to inject fluids related to oil and gas production, including injection of CO2 for EOR. Class VI wells are used to inject CO2 for geological storage.186,187 To protect potable water, EPA requires that carbon storage project owners applying for permits define an Area of Review (AoR) in which all risks to underground sources of drinking water and the leakage potential of legacy wells located within the AoR be identified. The AoR is an estimate of the project footprint and is used to develop monitoring plans to ensure protection of USDWs.188 Either the area of review is assigned a fixed radius (depending on the well type) or it is defined using computational modeling as the edge of the pressure front, whichever is larger. A suggested possibility to reduce the uncertainty of long-term storage of CO2 and to decrease the impact of wells on the migrating CO2 plume is to inject CO2 below the maximum penetration of most wells.
Conclusions
The past two decades have seen dramatic growth in research and application of CO2 capture methods and subsequent chemical, biological, and geological sequestration. Absorption using amine solutions is the most mature CO2 capture method and the only one in large-scale applications, whereas persistent research interest in absorption and membrane filtration is evident despite challenges in industrial applications. Postcombustion has drawn by far the most research interest owing to its lower cost and relative ease to retrofit existing plants, but it only favors absorption capture methods. Precombustion methods, on the other hand, can accommodate any of the capture methods because of the easier separation of CO2 from their gas streams and their flexibilities. Although overall publication volumes related to CO2 capture largely stopped growing since the mid-2010s, the trends are not universal for all specific fields, and continuous publication growth can still be observed for some methods and materials.
Carbon Capture and Storage technologies are attractive to industries such as fossil-fuel extraction and cement, steel, and fertilizer production, as they can continue to function, and CCS receives greater attention because of the ability to allow business as usual. However, CCS is seen as controversial by some environmental groups, as this technology seems to perpetuate fossil-fuel exploration and risks delaying decarbonization efforts.
The use of biomass via BECCS to capture carbon is likely a rapidly deployable and effective method to sequester CO2 at low cost without major alterations in land use. Enzymes, particularly RubisCO and carbonic anhydrase, provide an intermediate strategy for CO2 capture and an alternative to physical and chemical capture methods. Of the chemical methods, mineral carbonation (likely ex situ) may provide the most expedient method to capture CO2 emissions, while concrete carbonation may be useful if it improves concrete strength and reduces overall concrete use.
Injection of large quantities of CO2 into underground reservoirs where it can be securely and permanently stored can be successfully achieved with economic incentives to accelerate field-scale applications of CO2 sequestration. Significant advances in site characterization, monitoring, and leak assessment and management have occurred in the past 10 years. Legal and regulatory protocols have also been put into place in the US. Over time, the leakage risk decreases while the permanence of the storage increases, but the effectiveness of a site to securely store CO2 at a geological time scale is very difficult to define. Moreover, uncertainties persist over the liabilities of parties after the site is closed. Nevertheless, scaling up and worldwide deployment and coordination of these technologies and strategies should be the focus for upcoming years. Since high-purity CO2 streams are required for storage, future research will also have to address ways to reduce the cost of CO2 capture and sequestration processes to be cost-competitive with other carbon-free options.
Acknowledgments
We sincerely appreciate Laura Czuba for project coordination and Peter Jap and Cristina Tomeo for insightful discussions. We are also grateful to Manuel Guzman, Gilles Georges, Michael Dennis, Dawn George, Cynthia Casebolt, and Hong Xie for leadership and support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c05070.
Advantages and disadvantages of common CO2 adsorbents, comparison of different types of membranes for CO2 separation, and methods for obtaining data from the CAS Content Collection (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Lindsey R. D. L.Climate Change: Global Temperature. https://www.climate.gov/news-features/understanding-climate/climate-change-global-temperature (accessed 3/14/2022).
- Bongaarts J.; O’Neill B. C. Global warming policy: Is population left out in the cold?. Science 2018, 361 (6403), 650–652. 10.1126/science.aat8680. [DOI] [PubMed] [Google Scholar]
- O’Neill B. C.; Dalton M.; Fuchs R.; Jiang L.; Pachauri S.; Zigova K. Global demographic trends and future carbon emissions. Proc. Natl. Acad. Sci. U. S. A. 2010, 107 (41), 17521–17526. 10.1073/pnas.1004581107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill B. C.; Liddle B.; Jiang L.; Smith K. R.; Pachauri S.; Dalton M.; Fuchs R. Demographic change and carbon dioxide emissions. Lancet 2012, 380 (9837), 157–164. 10.1016/S0140-6736(12)60958-1. [DOI] [PubMed] [Google Scholar]
- World Population Prospects 2019. https://population.un.org/wpp/ (accessed 3/14/2022).
- Friedlingstein P.; Jones M. W.; O’Sullivan M.; Andrew R. M.; Bakker D. C. E.; Hauck J.; Le Quéré C.; Peters G. P.; Peters W.; Pongratz J.; Sitch S.; Canadell J. G.; Ciais P.; Jackson R. B.; Alin S. R.; Anthoni P.; Bates N. R.; Becker M.; Bellouin N.; Bopp L.; Chau T. T. T.; Chevallier F.; Chini L. P.; Cronin M.; Currie K. I.; Decharme B.; Djeutchouang L.; Dou X.; Evans W.; Feely R. A.; Feng L.; Gasser T.; Gilfillan D.; Gkritzalis T.; Grassi G.; Gregor L.; Gruber N.; Gürses D.; Harris I.; Houghton R. A.; Hurtt G. C.; Iida Y.; Ilyina T.; Luijkx I. T.; Jain A. K.; Jones S. D.; Kato E.; Kennedy D.; Klein Goldewijk K.; Knauer J.; Korsbakken J. I.; Körtzinger A.; Landschützer P.; Lauvset S. K.; Lefèvre N.; Lienert S.; Liu J.; Marland G.; McGuire P. C.; Melton J. R.; Munro D. R.; Nabel J. E. M. S.; Nakaoka S.-I.; Niwa Y.; Ono T.; Pierrot D.; Poulter B.; Rehder G.; Resplandy L.; Robertson E.; Rödenbeck C.; Rosan T. M.; Schwinger J.; Schwingshackl C.; Séférian R.; Sutton A. J.; Sweeney C.; Tanhua T.; Tans P. P.; Tian H.; Tilbrook B.; Tubiello F.; van der Werf G.; Vuichard N.; Wada C.; Wanninkhof R.; Watson A.; Willis D.; Wiltshire A. J.; Yuan W.; Yue C.; Yue X.; Zaehle S.; Zeng J., Global Carbon Budget 2021. Earth Syst. Sci. Data Discuss. 2011, in review. [Google Scholar]
- The Life-or-Death Race to Improve Carbon Capture. https://cen.acs.org/environment/greenhouse-gases/capture-flue-gas-co2-emissions/99/i26 (accessed 3/14/2022).
- Hepburn C.; Adlen E.; Beddington J.; Carter E. A.; Fuss S.; Mac Dowell N.; Minx J. C.; Smith P.; Williams C. K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575 (7781), 87–97. 10.1038/s41586-019-1681-6. [DOI] [PubMed] [Google Scholar]
- Global Time Series. https://www.ncdc.noaa.gov/cag/global/time-series/globe/land_ocean/ann/1/1880-2022 (accessed 3/14/2022).
- Yaashikaa P. R.; Senthil Kumar P.; Varjani S. J.; Saravanan A. A review on photochemical, biochemical and electrochemical transformation of CO2 into value-added products. Journal of CO2 Utilization 2019, 33, 131–147. 10.1016/j.jcou.2019.05.017. [DOI] [Google Scholar]
- Leung D. Y. C.; Caramanna G.; Maroto-Valer M. M. An overview of current status of carbon dioxide capture and storage technologies. Renewable and Sustainable Energy Reviews 2014, 39, 426–443. 10.1016/j.rser.2014.07.093. [DOI] [Google Scholar]
- Kamkeng A. D. N.; Wang M.; Hu J.; Du W.; Qian F. Transformation technologies for CO2 utilisation: Current status, challenges and future prospects. Chemical Engineering Journal 2021, 409, 128138. 10.1016/j.cej.2020.128138. [DOI] [Google Scholar]
- Scott V.; Gilfillan S.; Markusson N.; Chalmers H.; Haszeldine R. S. Last chance for carbon capture and storage. Nature Climate Change 2013, 3 (2), 105–111. 10.1038/nclimate1695. [DOI] [Google Scholar]
- Zheng Y.; Zhang W.; Li Y.; Chen J.; Yu B.; Wang J.; Zhang L.; Zhang J. Energy related CO2 conversion and utilization: Advanced materials/nanomaterials, reaction mechanisms and technologies. Nano Energy 2017, 40, 512–539. 10.1016/j.nanoen.2017.08.049. [DOI] [Google Scholar]
- Majumdar A.; Deutch J. Research Opportunities for CO2 Utilization and Negative Emissions at the Gigatonne Scale. Joule 2018, 2 (5), 805–809. 10.1016/j.joule.2018.04.018. [DOI] [Google Scholar]
- Baum Z. J.; Bird R. E.; Yu X.; Ma J. Lithium-Ion Battery Recycling–Overview of Techniques and Trends. ACS Energy Letters 2022, 7 (2), 712–719. 10.1021/acsenergylett.1c02602. [DOI] [Google Scholar]
- Global Greenhouse Gas Emissions Data. https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data (accessed 3/14/2022).
- Salazar Duarte G.; Schürer B.; Voss C.; Bathen D. Adsorptive Separation of CO2 from Flue Gas by Temperature Swing Adsorption Processes. ChemBioEng. Reviews 2017, 4 (5), 277–288. 10.1002/cben.201600029. [DOI] [Google Scholar]
- Lively R. P.; Chance R. R.; Kelley B. T.; Deckman H. W.; Drese J. H.; Jones C. W.; Koros W. J. Hollow Fiber Adsorbents for CO2 Removal from Flue Gas. Ind. Eng. Chem. Res. 2009, 48 (15), 7314–7324. 10.1021/ie9005244. [DOI] [Google Scholar]
- Moioli S.; Giuffrida A.; Gamba S.; Romano M. C.; Pellegrini L.; Lozza G. Pre-combustion CO2 capture by MDEA process in IGCC based on air-blown gasification. Energy Procedia 2014, 63, 2045–2053. 10.1016/j.egypro.2014.11.220. [DOI] [Google Scholar]
- Lyngfelt A. Chemical Looping Combustion: Status and Development Challenges. Energy Fuels 2020, 34 (8), 9077–9093. 10.1021/acs.energyfuels.0c01454. [DOI] [Google Scholar]
- McQueen N.; Gomes K. V.; McCormick C.; Blumanthal K.; Pisciotta M.; Wilcox J. A review of direct air capture (DAC): scaling up commercial technologies and innovating for the future. Progress in Energy 2021, 3 (3), 032001. 10.1088/2516-1083/abf1ce. [DOI] [Google Scholar]
- Terlouw T.; Treyer K.; Bauer C.; Mazzotti M. Life Cycle Assessment of Direct Air Carbon Capture and Storage with Low-Carbon Energy Sources. Environ. Sci. Technol. 2021, 55 (16), 11397–11411. 10.1021/acs.est.1c03263. [DOI] [PubMed] [Google Scholar]
- Patel H. A.; Byun J.; Yavuz C. T. Carbon Dioxide Capture Adsorbents: Chemistry and Methods. ChemSusChem 2017, 10 (7), 1303–1317. 10.1002/cssc.201601545. [DOI] [PubMed] [Google Scholar]
- Choi S.; Drese J. H.; Jones C. W. Adsorbent Materials for Carbon Dioxide Capture from Large Anthropogenic Point Sources. ChemSusChem 2009, 2 (9), 796–854. 10.1002/cssc.200900036. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Energy Announces an Additional $6 Million in Funding for Four Direct Air Capture Projects. https://www.energy.gov/fecm/articles/us-department-energy-announces-additional-6-million-funding-four-direct-air-capture (accessed 3/14/2022).
- Direct Air Capture: More Efforts Needed; 2021, https://www.iea.org/reports/direct-air-capture (accessed 3/9/2022).
- Novel carbon capture and utilisation technologies. https://sapea.info/topic/carbon-capture/ (accessed 3/14/2022).
- Kortunov P. V.; Siskin M.; Baugh L. S.; Calabro D. C. In Situ Nuclear Magnetic Resonance Mechanistic Studies of Carbon Dioxide Reactions with Liquid Amines in Aqueous Systems: New Insights on Carbon Capture Reaction Pathways. Energy Fuels 2015, 29 (9), 5919–5939. 10.1021/acs.energyfuels.5b00850. [DOI] [Google Scholar]
- Said R. B.; Kolle J. M.; Essalah K.; Tangour B.; Sayari A. A Unified Approach to CO2-Amine Reaction Mechanisms. ACS Omega 2020, 5 (40), 26125–26133. 10.1021/acsomega.0c03727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya P. D.; Kenig E. Y. Absorption of CO2 into aqueous blends of alkanolamines prepared from renewable resources. Chem. Eng. Sci. 2007, 62 (24), 7344–7350. 10.1016/j.ces.2007.08.015. [DOI] [Google Scholar]
- MacInnes J. M.; Ayash A. A.; Dowson G. R. M. CO2 absorption using diethanolamine-water solutions in a rotating spiral contactor. Chemical Engineering Journal 2017, 307, 1084–1091. 10.1016/j.cej.2016.08.123. [DOI] [Google Scholar]
- Zheng Y.; El Ahmar E.; Simond M.; Ballerat-Busserolles K.; Zhang P. CO2 Heat of Absorption in Aqueous Solutions of MDEA and MDEA/Piperazine. Journal of Chemical & Engineering Data 2020, 65 (8), 3784–3793. 10.1021/acs.jced.9b01163. [DOI] [Google Scholar]
- Norouzbahari S.; Shahhosseini S.; Ghaemi A. Chemical absorption of CO2 into an aqueous piperazine (PZ) solution: development and validation of a rigorous dynamic rate-based model. RSC Adv. 2016, 6 (46), 40017–40032. 10.1039/C5RA27869D. [DOI] [Google Scholar]
- Svensson H.; Hulteberg C.; Karlsson H. T. Precipitation of AMP Carbamate in CO2 Absorption Process. Energy Procedia 2014, 63, 750–757. 10.1016/j.egypro.2014.11.083. [DOI] [Google Scholar]
- Shoukat U.; Knuutila H. K. Effect of Various Parameters on the Thermal Stability and Corrosion of CO2-Loaded Tertiary Amine Blends. Energies 2020, 13 (10), 2626. 10.3390/en13102626. [DOI] [Google Scholar]
- Kemper J.; Ewert G.; Grünewald M. Absorption and regeneration performance of novel reactive amine solvents for post-combustion CO2 capture. Energy Procedia 2011, 4, 232–239. 10.1016/j.egypro.2011.01.046. [DOI] [Google Scholar]
- Ooi Z. L.; Tan P. Y.; Tan L. S.; Yeap S. P. Amine-based solvent for CO2 absorption and its impact on carbon steel corrosion: A perspective review. Chinese Journal of Chemical Engineering 2020, 28 (5), 1357–1367. 10.1016/j.cjche.2020.02.029. [DOI] [Google Scholar]
- Stern M. C.; Hatton T. A. Bench-scale demonstration of CO2 capture with electrochemically-mediated amine regeneration. RSC Adv. 2014, 4 (12), 5906. 10.1039/c3ra46774k. [DOI] [Google Scholar]
- Rahimi M.; Diederichsen K. M.; Ozbek N.; Wang M.; Choi W.; Hatton T. A. An Electrochemically Mediated Amine Regeneration Process with a Mixed Absorbent for Postcombustion CO2 Capture. Environ. Sci. Technol. 2020, 54 (14), 8999–9007. 10.1021/acs.est.0c02595. [DOI] [PubMed] [Google Scholar]
- Al-Hamed K. H. M.; Dincer I. A comparative review of potential ammonia-based carbon capture systems. Journal of Environmental Management 2021, 287, 112357. 10.1016/j.jenvman.2021.112357. [DOI] [PubMed] [Google Scholar]
- Mathias P. M.; Reddy S.; O’Connell J. P. Quantitative evaluation of the chilled-ammonia process for CO2 capture using thermodynamic analysis and process simulation. International Journal of Greenhouse Gas Control 2010, 4 (2), 174–179. 10.1016/j.ijggc.2009.09.016. [DOI] [Google Scholar]
- Lillia S.; Bonalumi D.; Fosbøl P. L.; Thomsen K.; Jayaweera I.; Valenti G. Thermodynamic and kinetic properties of NH3-K2CO3-CO2-H2O system for carbon capture applications. International Journal of Greenhouse Gas Control 2019, 85, 121–131. 10.1016/j.ijggc.2019.03.019. [DOI] [Google Scholar]
- Wang F.; Zhao J.; Miao H.; Zhao J.; Zhang H.; Yuan J.; Yan J. Current status and challenges of the ammonia escape inhibition technologies in ammonia-based CO2 capture process. Applied Energy 2018, 230, 734–749. 10.1016/j.apenergy.2018.08.116. [DOI] [Google Scholar]
- Borhani T. N. G.; Azarpour A.; Akbari V.; Wan Alwi S. R.; Manan Z. A. CO2 capture with potassium carbonate solutions: A state-of-the-art review. International Journal of Greenhouse Gas Control 2015, 41, 142–162. 10.1016/j.ijggc.2015.06.026. [DOI] [Google Scholar]
- Feng Q.; Sun B.; Wang L.; Shao L. Enhancement of CO2 Absorption into K2CO3 Solution by Cyclohexane in a High-Shear Reactor. Energy Fuels 2019, 33 (7), 6628–6633. 10.1021/acs.energyfuels.9b01199. [DOI] [Google Scholar]
- Hu G.; Smith K.; Wu Y.; Kentish S.; Stevens G. Recent Progress on the Performance of Different Rate Promoters in Potassium Carbonate Solvents for CO2 Capture. Energy Procedia 2017, 114, 2279–2286. 10.1016/j.egypro.2017.03.1374. [DOI] [Google Scholar]
- Grimekis D.; Giannoulidis S.; Manou K.; Panopoulos K. D.; Karellas S. Experimental investigation of CO2 solubility and its absorption rate into promoted aqueous potassium carbonate solutions at elevated temperatures. Int. J. Greenhouse Gas Control 2019, 81, 83–92. 10.1016/j.ijggc.2018.12.008. [DOI] [Google Scholar]
- Li Y.; Wang L. a.; Tan Z.; Zhang Z.; Hu X. Experimental studies on carbon dioxide absorption using potassium carbonate solutions with amino acid salts. Sep. Purif. Technol. 2019, 219, 47–54. 10.1016/j.seppur.2019.03.010. [DOI] [Google Scholar]
- Hu G.; Smith K. H.; Wu Y.; Mumford K. A.; Kentish S. E.; Stevens G. W. Carbon dioxide capture by solvent absorption using amino acids: A review. Chinese Journal of Chemical Engineering 2018, 26 (11), 2229–2237. 10.1016/j.cjche.2018.08.003. [DOI] [Google Scholar]
- Sanchez-Fernandez E.; Heffernan K.; van der Ham L.; Linders M. J. G.; Brilman D. W. F.; Goetheer E. L. V.; Vlugt T. J. H. Analysis of Process Configurations for CO2 Capture by Precipitating Amino Acid Solvents. Ind. Eng. Chem. Res. 2014, 53 (6), 2348–2361. 10.1021/ie402323r. [DOI] [Google Scholar]
- Keith D. W.; Holmes G.; St. Angelo D.; Heidel K. A Process for Capturing CO2 from the Atmosphere. Joule 2018, 2 (8), 1573–1594. 10.1016/j.joule.2018.05.006. [DOI] [Google Scholar]
- Mahmoudkhani M.; Keith D. W. Low-energy sodium hydroxide recovery for CO2 capture from atmospheric air—Thermodynamic analysis. International Journal of Greenhouse Gas Control 2009, 3 (4), 376–384. 10.1016/j.ijggc.2009.02.003. [DOI] [Google Scholar]
- Shu Q.; Legrand L.; Kuntke P.; Tedesco M.; Hamelers H. V. M. Electrochemical Regeneration of Spent Alkaline Absorbent from Direct Air Capture. Environ. Sci. Technol. 2020, 54 (14), 8990–8998. 10.1021/acs.est.0c01977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar J.; Diwekar U.; Joback K.; Berger A. H.; Bhown A. S. Solvent Selection for Post-Combustion CO2 Capture. Energy Procedia 2013, 37, 257–264. 10.1016/j.egypro.2013.05.110. [DOI] [Google Scholar]
- Papadopoulos A. I.; Badr S.; Chremos A.; Forte E.; Zarogiannis T.; Seferlis P.; Papadokonstantakis S.; Galindo A.; Jackson G.; Adjiman C. S. Computer-aided molecular design and selection of CO2 capture solvents based on thermodynamics, reactivity and sustainability. Molecular Systems Design & Engineering 2016, 1 (3), 313–334. 10.1039/C6ME00049E. [DOI] [Google Scholar]
- Selexol. https://netl.doe.gov/research/coal/energy-systems/gasification/gasifipedia/selexol (accessed 3/14/2022).
- Décultot M.; Ledoux A.; Fournier-Salaün M.-C.; Estel L. Solubility of CO2 in methanol, ethanol, 1,2-propanediol and glycerol from 283.15 K to 373.15 K and up to 6.0 MPa. J. Chem. Thermodyn. 2019, 138, 67–77. 10.1016/j.jct.2019.05.003. [DOI] [Google Scholar]
- Porter R. T. J.; Fairweather M.; Kolster C.; Mac Dowell N.; Shah N.; Woolley R. M. Cost and performance of some carbon capture technology options for producing different quality CO 2 product streams. International Journal of Greenhouse Gas Control 2017, 57, 185–195. 10.1016/j.ijggc.2016.11.020. [DOI] [Google Scholar]
- Bohloul M. R.; Vatani A.; Peyghambarzadeh S. M. Experimental and theoretical study of CO2 solubility in N-methyl-2-pyrrolidone (NMP). Fluid Phase Equilib. 2014, 365, 106–111. 10.1016/j.fluid.2013.12.019. [DOI] [Google Scholar]
- Chen W.; Chen M.; Yang M.; Zou E.; Li H.; Jia C.; Sun C.; Ma Q.; Chen G.; Qin H. A new approach to the upgrading of the traditional propylene carbonate washing process with significantly higher CO2 absorption capacity and selectivity. Applied Energy 2019, 240, 265–275. 10.1016/j.apenergy.2019.01.236. [DOI] [Google Scholar]
- N.Borhani T.; Wang M. Role of solvents in CO2 capture processes: The review of selection and design methods. Renewable and Sustainable Energy Reviews 2019, 114, 109299. 10.1016/j.rser.2019.109299. [DOI] [Google Scholar]
- Daryayehsalameh B.; Nabavi M.; Vaferi B. Modeling of CO2 capture ability of [Bmim][BF4] ionic liquid using connectionist smart paradigms. Environmental Technology & Innovation 2021, 22, 101484. 10.1016/j.eti.2021.101484. [DOI] [Google Scholar]
- Aghaie M.; Rezaei N.; Zendehboudi S. A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renewable and Sustainable Energy Reviews 2018, 96, 502–525. 10.1016/j.rser.2018.07.004. [DOI] [Google Scholar]
- Cuéllar-Franca R. M.; García-Gutiérrez P.; Hallett J. P.; Mac Dowell N. A life cycle approach to solvent design: challenges and opportunities for ionic liquids - application to CO2 capture. Reaction Chemistry & Engineering 2021, 6 (2), 258–278. 10.1039/D0RE00409J. [DOI] [Google Scholar]
- Acevedo S.; Giraldo L.; Moreno-Piraján J. C. Adsorption of CO2 on Activated Carbons Prepared by Chemical Activation with Cupric Nitrate. ACS Omega 2020, 5 (18), 10423–10432. 10.1021/acsomega.0c00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo L.; Vargas D. P.; Moreno-Piraján J. C. Study of CO2 Adsorption on Chemically Modified Activated Carbon With Nitric Acid and Ammonium Aqueous. Frontiers in Chemistry 2020, 8, 1. 10.3389/fchem.2020.543452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C.; Yu S.; Ma K.; Liang B.; Tang S.; Liu C.; Yue H. Amine-functionalized mesoporous monolithic adsorbents for post-combustion carbon dioxide capture. Chemical Engineering Journal 2021, 413, 127675. 10.1016/j.cej.2020.127675. [DOI] [Google Scholar]
- Philip F. A.; Henni A. Enhancement of post-combustion CO2 capture capacity by incorporation of task-specific ionic liquid into ZIF-8. Microporous Mesoporous Mater. 2022, 330, 111580. 10.1016/j.micromeso.2021.111580. [DOI] [Google Scholar]
- Park J.; Rubiera Landa H. O.; Kawajiri Y.; Realff M. J.; Lively R. P.; Sholl D. S. How Well Do Approximate Models of Adsorption-Based CO2 Capture Processes Predict Results of Detailed Process Models?. Ind. Eng. Chem. Res. 2020, 59 (15), 7097–7108. 10.1021/acs.iecr.9b05363. [DOI] [Google Scholar]
- Stauffer N. W.A New Approach to Carbon Capture. https://news.mit.edu/2020/new-approach-to-carbon-capture-0709 (accessed 3/24/2022).
- Voskian S.; Hatton T. A. Faradaic Electro-Swing Reactive Adsorption for CO2 Capture. Energy Environ. Sci. 2019, 12, 3530. 10.1039/C9EE02412C. [DOI] [Google Scholar]
- Tong Z.; Sekizkardes A. Recent Developments in High-Performance Membranes for CO2 Separation. Membranes 2021, 11 (2), 156. 10.3390/membranes11020156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano L.; Gubis J.; Bierman G.; Kapteijn F. Conceptual design of membrane-based pre-combustion CO2 capture process: Role of permeance and selectivity on performance and costs. J. Membr. Sci. 2019, 575, 229–241. 10.1016/j.memsci.2018.12.063. [DOI] [Google Scholar]
- Xu J.; Wang Z.; Qiao Z.; Wu H.; Dong S.; Zhao S.; Wang J. Post-combustion CO2 capture with membrane process: Practical membrane performance and appropriate pressure. J. Membr. Sci. 2019, 581, 195–213. 10.1016/j.memsci.2019.03.052. [DOI] [Google Scholar]
- Ji G.; Zhao M., Membrane Separation Technology in Carbon Capture. In Recent Advances in Carbon Capture and Storage; InTech: 2017. [Google Scholar]
- Lee J.-S. M.; Cooper A. I. Advances in Conjugated Microporous Polymers. Chem. Rev. 2020, 120 (4), 2171–2214. 10.1021/acs.chemrev.9b00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi Shamsabadi A.; Rezakazemi M.; Seidi F.; Riazi H.; Aminabhavi T.; Soroush M. Next generation polymers of intrinsic microporosity with tunable moieties for ultrahigh permeation and precise molecular CO2 separation. Prog. Energy Combust. Sci. 2021, 84, 100903. 10.1016/j.pecs.2021.100903. [DOI] [Google Scholar]
- Luo S.; Zhang Q.; Zhu L.; Lin H.; Kazanowska B. A.; Doherty C. M.; Hill A. J.; Gao P.; Guo R. Highly Selective and Permeable Microporous Polymer Membranes for Hydrogen Purification and CO2 Removal from Natural Gas. Chem. Mater. 2018, 30 (15), 5322–5332. 10.1021/acs.chemmater.8b02102. [DOI] [Google Scholar]
- Ahmad M. Z.; Navarro M.; Lhotka M.; Zornoza B.; Téllez C.; de Vos W. M.; Benes N. E.; Konnertz N. M.; Visser T.; Semino R.; Maurin G.; Fila V.; Coronas J. Enhanced gas separation performance of 6FDA-DAM based mixed matrix membranes by incorporating MOF UiO-66 and its derivatives. J. Membr. Sci. 2018, 558, 64–77. 10.1016/j.memsci.2018.04.040. [DOI] [Google Scholar]
- Husna A.; Hossain I.; Choi O.; Lee S. M.; Kim T. H. Efficient CO2 Separation Using a PIM-PI-Functionalized UiO-66 MOF Incorporated Mixed Matrix Membrane in a PIM-PI-1 Polymer. Macromol. Mater. Eng. 2021, 306 (10), 2100298. 10.1002/mame.202100298. [DOI] [Google Scholar]
- Bettenhausen C.Cash Rushes in for CO2 Removal. https://cen.acs.org/business/investment/Cash-rushes-CO2-removal/100/i14 (accessed 12/28/2022).
- Bettenhausen C.Linde, BP Team up on Carbon Capture as Carbon Clean Raises Funds. https://cen.acs.org/environment/greenhouse-gases/Linde-BP-team-carbon-capture/100/i18 (accessed 12/28/2022).
- Bettenhausen C. Carbon Engineering, Oxy Join for Air Capture. C&EN Global Enterp. 2022, 100, 13. 10.1021/cen-10021-buscon9. [DOI] [Google Scholar]
- Bettenhausen C.Carbon-Capture Projects Proliferate. 10.1021/cen-09927-buscon2 (accessed 12/28/2022). [DOI]
- Bomgardner M. M. A Carbon-Capture Project Stalls; Another Advances. C&EN Global Enterp. 2020, 98, 11. 10.1021/cen-09831-buscon2. [DOI] [Google Scholar]
- Carbon Capture and Storage. https://www.shell.com/energy-and-innovation/carbon-capture-and-storage.html#iframe=L3dlYmFwcHMvQ0NTX0dsb2JlLw (accessed 12/28/2022).
- Bettenhausen C. ExxonMobil Creates CO2 Capture Business. C&EN Global Enterp. 2021, 99, 12. 10.1021/cen-09905-buscon10. [DOI] [Google Scholar]
- Pierella Karlusich J. J.; Bowler C.; Biswas H. Carbon Dioxide Concentration Mechanisms in Natural Populations of Marine Diatoms: Insights From Tara Oceans. Frontiers in Plant Science 2021, 12, 1. 10.3389/fpls.2021.657821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleku G. A.; Roberts G. W.; Titchiner G. R.; Leys D. Synthetic Enzyme-Catalyzed CO2 Fixation Reactions. ChemSusChem 2021, 14 (8), 1781–1804. 10.1002/cssc.202100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obersteiner M.; Azar C.; Kauppi P.; Möllersten K.; Moreira J.; Nilsson S.; Read P.; Riahi K.; Schlamadinger B.; Yamagata Y.; Yan J.; van Ypersele J. P. Managing Climate Risk. Science 2001, 294 (5543), 786–787. 10.1126/science.294.5543.786b. [DOI] [PubMed] [Google Scholar]
- Fajardy M.; Mac Dowell N. Can BECCS deliver sustainable and resource efficient negative emissions?. Energy Environ. Sci. 2017, 10 (6), 1389–1426. 10.1039/C7EE00465F. [DOI] [Google Scholar]
- Baik E.; Sanchez D. L.; Turner P. A.; Mach K. J.; Field C. B.; Benson S. M. Geospatial analysis of near-term potential for carbon-negative bioenergy in the United States. Proc. Natl. Acad. Sci. U. S. A. 2018, 115 (13), 3290–3295. 10.1073/pnas.1720338115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday C.; Hatton T. A. Net-Negative Emissions through Molten Sorbents and Bioenergy with Carbon Capture and Storage. Ind. Eng. Chem. Res. 2020, 59 (52), 22582–22596. 10.1021/acs.iecr.0c04512. [DOI] [Google Scholar]
- Consoli C.Bioenergy and Carbon Capture and Storage Elsevier: 2019. [Google Scholar]
- Appel A. M.; Bercaw J. E.; Bocarsly A. B.; Dobbek H.; DuBois D. L.; Dupuis M.; Ferry J. G.; Fujita E.; Hille R.; Kenis P. J. A.; Kerfeld C. A.; Morris R. H.; Peden C. H. F.; Portis A. R.; Ragsdale S. W.; Rauchfuss T. B.; Reek J. N. H.; Seefeldt L. C.; Thauer R. K.; Waldrop G. L. Frontiers, Opportunities, and Challenges in Biochemical and Chemical Catalysis of CO2 Fixation. Chem. Rev. 2013, 113 (8), 6621–6658. 10.1021/cr300463y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fast A. G.; Papoutsakis E. T. Stoichiometric and energetic analyses of non-photosynthetic CO2-fixation pathways to support synthetic biology strategies for production of fuels and chemicals. Current Opinion in Chemical Engineering 2012, 1 (4), 380–395. 10.1016/j.coche.2012.07.005. [DOI] [Google Scholar]
- Velvizhi G.; Balakumar K.; Dharanidharan S. Sequestering of CO2 to Value-Added Products through Various Biological Processes. Next Generation Biomanufacturing Technologies 2019, 1329, 261–284. 10.1021/bk-2019-1329.ch012. [DOI] [Google Scholar]
- Nisar A.; Khan S.; Hameed M.; Nisar A.; Ahmad H.; Mehmood S. A. Bio-conversion of CO2 into biofuels and other value-added chemicals via metabolic engineering. Microbiological Research 2021, 251, 126813. 10.1016/j.micres.2021.126813. [DOI] [PubMed] [Google Scholar]
- Gonzales J. N.; Matson M. M.; Atsumi S. Nonphotosynthetic Biological CO2 Reduction. Biochemistry 2019, 58 (11), 1470–1477. 10.1021/acs.biochem.8b00937. [DOI] [PubMed] [Google Scholar]
- Mistry A. N.; Ganta U.; Chakrabarty J.; Dutta S. A review on biological systems for CO2 sequestration: Organisms and their pathways. Environmental Progress & Sustainable Energy 2019, 38 (1), 127–136. 10.1002/ep.12946. [DOI] [Google Scholar]
- Ducat D. C.; Silver P. A. Improving carbon fixation pathways. Curr. Opin. Chem. Biol. 2012, 16 (3–4), 337–344. 10.1016/j.cbpa.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Even A.; Noor E.; Milo R. A survey of carbon fixation pathways through a quantitative lens. Journal of Experimental Botany 2012, 63 (6), 2325–2342. 10.1093/jxb/err417. [DOI] [PubMed] [Google Scholar]
- Antonovsky N.; Gleizer S.; Milo R. Engineering carbon fixation in E. coli: from heterologous RuBisCO expression to the Calvin-Benson-Bassham cycle. Curr. Opin. Biotechnol. 2017, 47, 83–91. 10.1016/j.copbio.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Mattozzi M. d.; Ziesack M.; Voges M. J.; Silver P. A.; Way J. C. Expression of the sub-pathways of the Chloroflexus aurantiacus 3-hydroxypropionate carbon fixation bicycle in E. coli: Toward horizontal transfer of autotrophic growth. Metabolic Engineering 2013, 16, 130–139. 10.1016/j.ymben.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Guadalupe-Medina V.; Wisselink H. W.; Luttik M. A. H.; de Hulster E.; Daran J.-M.; Pronk J. T.; van Maris A. J. A. Carbon dioxide fixation by Calvin-Cycle enzymes improves ethanol yield in yeast. Biotechnology for Biofuels 2013, 6 (1), 125. 10.1186/1754-6834-6-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P.-F.; Zhang G.-C.; Walker B.; Seo S.-O.; Kwak S.; Liu J.-J.; Kim H.; Ort D. R.; Wang S.-G.; Jin Y.-S. Recycling Carbon Dioxide during Xylose Fermentation by Engineered Saccharomyces cerevisiae. ACS Synth. Biol. 2017, 6 (2), 276–283. 10.1021/acssynbio.6b00167. [DOI] [PubMed] [Google Scholar]
- Gassler T.; Sauer M.; Gasser B.; Egermeier M.; Troyer C.; Causon T.; Hann S.; Mattanovich D.; Steiger M. G. The industrial yeast Pichia pastoris is converted from a heterotroph into an autotroph capable of growth on CO2. Nat. Biotechnol. 2020, 38 (2), 210–216. 10.1038/s41587-019-0363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleizer S.; Ben-Nissan R.; Bar-On Y. M.; Antonovsky N.; Noor E.; Zohar Y.; Jona G.; Krieger E.; Shamshoum M.; Bar-Even A.; Milo R. Conversion of Escherichia coli to Generate All Biomass Carbon from CO2. Cell 2019, 179 (6), 1255–1263. e12 10.1016/j.cell.2019.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B.; Zhao Y.; Yang J. Recent Advances in Developing Artificial Autotrophic Microorganism for Reinforcing CO2 Fixation. Frontiers in Microbiology 2020, 11, 1. 10.3389/fmicb.2020.592631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durall C.; Lindberg P.; Yu J.; Lindblad P. Increased ethylene production by overexpressing phosphoenolpyruvate carboxylase in the cyanobacterium Synechocystis PCC 6803. Biotechnology for Biofuels 2020, 13 (1), 16. 10.1186/s13068-020-1653-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe H.; Joo J. C.; Cho D. H.; Kim M. H.; Lee S. H.; Jung K. D.; Kim Y. H. Efficient CO2-Reducing Activity of NAD-Dependent Formate Dehydrogenase from Thiobacillus sp. KNK65MA for Formate Production from CO2 Gas. PLoS One 2014, 9 (7), e103111. 10.1371/journal.pone.0103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwander T.; Schada von Borzyskowski L.; Burgener S.; Cortina N. S.; Erb T. J. A synthetic pathway for the fixation of carbon dioxide in vitro. Science 2016, 354 (6314), 900–904. 10.1126/science.aah5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H.; Li X.; Duchoud F.; Chuang D. S.; Liao J. C. Augmenting the Calvin-Benson-Bassham cycle by a synthetic malyl-CoA-glycerate carbon fixation pathway. Nat. Commun. 2018, 9 (1), 1. 10.1038/s41467-018-04417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yishai O.; Bouzon M.; Döring V.; Bar-Even A. In Vivo Assimilation of One-Carbon via a Synthetic Reductive Glycine Pathway in Escherichia coli. ACS Synth. Biol. 2018, 7 (9), 2023–2028. 10.1021/acssynbio.8b00131. [DOI] [PubMed] [Google Scholar]
- Kondaveeti S.; Abu-Reesh I. M.; Mohanakrishna G.; Bulut M.; Pant D. Advanced Routes of Biological and Bio-electrocatalytic Carbon Dioxide (CO2) Mitigation Toward Carbon Neutrality. Frontiers in Energy Research 2020, 8, 1. 10.3389/fenrg.2020.00094. [DOI] [Google Scholar]
- de Mooij T.; de Vries G.; Latsos C.; Wijffels R. H.; Janssen M. Impact of light color on photobioreactor productivity. Algal Research 2016, 15, 32–42. 10.1016/j.algal.2016.01.015. [DOI] [Google Scholar]
- Li K.; An X.; Park K. H.; Khraisheh M.; Tang J. A critical review of CO2 photoconversion: Catalysts and reactors. Catal. Today 2014, 224, 3–12. 10.1016/j.cattod.2013.12.006. [DOI] [Google Scholar]
- Thakur I. S.; Kumar M.; Varjani S. J.; Wu Y.; Gnansounou E.; Ravindran S. Sequestration and utilization of carbon dioxide by chemical and biological methods for biofuels and biomaterials by chemoautotrophs: Opportunities and challenges. Bioresour. Technol. 2018, 256, 478–490. 10.1016/j.biortech.2018.02.039. [DOI] [PubMed] [Google Scholar]
- Burkart M. D.; Hazari N.; Tway C. L.; Zeitler E. L. Opportunities and Challenges for Catalysis in Carbon Dioxide Utilization. ACS Catal. 2019, 9 (9), 7937–7956. 10.1021/acscatal.9b02113. [DOI] [Google Scholar]
- Li Z.; Xin X.; Xiong B.; Zhao D.; Zhang X.; Bi C. Engineering the Calvin-Benson-Bassham cycle and hydrogen utilization pathway of Ralstonia eutropha for improved autotrophic growth and polyhydroxybutyrate production. Microbial Cell Factories 2020, 19 (1), 228. 10.1186/s12934-020-01494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satagopan S.; Tabita F. R. RubisCO selection using the vigorously aerobic and metabolically versatile bacterium Ralstonia eutropha. FEBS Journal 2016, 283 (15), 2869–2880. 10.1111/febs.13774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panich J.; Fong B.; Singer S. W. Metabolic Engineering of Cupriavidus necator H16 for Sustainable Biofuels from CO2. Trends Biotechnol. 2021, 39 (4), 412–424. 10.1016/j.tibtech.2021.01.001. [DOI] [PubMed] [Google Scholar]
- Villanueva M. Engineering Ralstonia Eutropha to Convert CO2/Waste Stream into Useful Chemicals Using a Synthetic Biology Approach. Uses 2016, 1. 10.15445/02012015.34. [DOI] [Google Scholar]
- Bi C.; Su P.; Müller J.; Yeh Y.-C.; Chhabra S. R.; Beller H. R.; Singer S. W.; Hillson N. J. Development of a broad-host synthetic biology toolbox for ralstonia eutropha and its application to engineering hydrocarbon biofuel production. Microbial Cell Factories 2013, 12 (1), 107. 10.1186/1475-2859-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Opgenorth P. H.; Wernick D. G.; Rogers S.; Wu T.-Y.; Higashide W.; Malati P.; Huo Y.-X.; Cho K. M.; Liao J. C. Integrated Electromicrobial Conversion of CO2 to Higher Alcohols. Science 2012, 335 (6076), 1596–1596. 10.1126/science.1217643. [DOI] [PubMed] [Google Scholar]
- Müller J.; MacEachran D.; Burd H.; Sathitsuksanoh N.; Bi C.; Yeh Y.-C.; Lee T. S.; Hillson N. J.; Chhabra S. R.; Singer S. W.; Beller H. R. Engineering of Ralstonia eutropha H16 for Autotrophic and Heterotrophic Production of Methyl Ketones. Appl. Environ. Microbiol. 2013, 79 (14), 4433–4439. 10.1128/AEM.00973-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. S.; Oh Y. H.; Jang Y.-A.; Kang K. H.; David Y.; Yu J. H.; Song B. K.; Choi J.-i.; Chang Y. K.; Joo J. C.; Park S. J. Recombinant Ralstonia eutropha engineered to utilize xylose and its use for the production of poly(3-hydroxybutyrate) from sunflower stalk hydrolysate solution. Microbial Cell Factories 2016, 15 (1), 95. 10.1186/s12934-016-0495-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmann A.; Fricke W. F.; Reinecke F.; Kusian B.; Liesegang H.; Cramm R.; Eitinger T.; Ewering C.; Pötter M.; Schwartz E.; Strittmatter A.; Voß I.; Gottschalk G.; Steinbüchel A.; Friedrich B.; Bowien B. Genome sequence of the Bioplastic-producing “Knallgas” bacterium Ralstonia eutropha H16. Nat. Biotechnol. 2006, 24 (10), 1257–1262. 10.1038/nbt1244. [DOI] [PubMed] [Google Scholar]
- Zhou W.; Wang J.; Chen P.; Ji C.; Kang Q.; Lu B.; Li K.; Liu J.; Ruan R. Bio-mitigation of carbon dioxide using microalgal systems: Advances and perspectives. Renewable and Sustainable Energy Reviews 2017, 76, 1163–1175. 10.1016/j.rser.2017.03.065. [DOI] [Google Scholar]
- Raheem A.; Prinsen P.; Vuppaladadiyam A. K.; Zhao M.; Luque R. A review on sustainable microalgae based biofuel and bioenergy production: Recent developments. Journal of Cleaner Production 2018, 181, 42–59. 10.1016/j.jclepro.2018.01.125. [DOI] [Google Scholar]
- Cheng J.; Zhu Y.; Zhang Z.; Yang W. Modification and improvement of microalgae strains for strengthening CO2 fixation from coal-fired flue gas in power plants. Bioresour. Technol. 2019, 291, 121850. 10.1016/j.biortech.2019.121850. [DOI] [PubMed] [Google Scholar]
- Chai W. S.; Tan W. G.; Halimatul Munawaroh H. S.; Gupta V. K.; Ho S.-H.; Show P. L. Multifaceted roles of microalgae in the application of wastewater biotreatment: A review. Environ. Pollut. 2021, 269, 116236. 10.1016/j.envpol.2020.116236. [DOI] [PubMed] [Google Scholar]
- Kumar M.; Sundaram S.; Gnansounou E.; Larroche C.; Thakur I. S. Carbon dioxide capture, storage and production of biofuel and biomaterials by bacteria: A review. Bioresour. Technol. 2018, 247, 1059–1068. 10.1016/j.biortech.2017.09.050. [DOI] [PubMed] [Google Scholar]
- Duarte J. H.; de Morais E. G.; Radmann E. M.; Costa J. A. V. Biological CO2 mitigation from coal power plant by Chlorella fusca and Spirulina sp. Bioresour. Technol. 2017, 234, 472–475. 10.1016/j.biortech.2017.03.066. [DOI] [PubMed] [Google Scholar]
- Yunus I. S.; Wichmann J.; Wördenweber R.; Lauersen K. J.; Kruse O.; Jones P. R. Synthetic metabolic pathways for photobiological conversion of CO2 into hydrocarbon fuel. Metabolic Engineering 2018, 49, 201–211. 10.1016/j.ymben.2018.08.008. [DOI] [PubMed] [Google Scholar]
- Huang C. C. W.; Huang S.; Su T.; Yu J.; Luo S.; Li G.; Wu D.; Zheng S.; Tsuge K.. Recombinant Microorganism for Carbon Fixation Reaction and Method for Reducing Carbon Dioxide in the Environment. Taiwan Pat. TWI641686B, 2018.
- Sefton B. C. W.Novel Microbial Biomass Based Feed Products. [Google Scholar]
- Intergovernmental Panel on Climate C., Agriculture, Forestry and Other Land Use (AFOLU). Climate Change 2014 Mitigation of Climate Change, 2014, https://www.ipcc.ch/report/ar5/wg3/ (accessed 3/9/2022).
- Rashidi N. A.; Yusup S. Biochar as potential precursors for activated carbon production: parametric analysis and multi-response optimization. Environmental Science and Pollution Research 2020, 27 (22), 27480–27490. 10.1007/s11356-019-07448-1. [DOI] [PubMed] [Google Scholar]
- Tan X.-f.; Liu S.-b.; Liu Y.-g.; Gu Y.-l.; Zeng G.-m.; Hu X.-j.; Wang X.; Liu S.-h.; Jiang L.-h. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour. Technol. 2017, 227, 359–372. 10.1016/j.biortech.2016.12.083. [DOI] [PubMed] [Google Scholar]
- Rampolla B.Carbon Blocks for Atmospheric Carbon Sequestration. https://contest.techbriefs.com/2017/entries/sustainable-technologies/8461 (accessed 3/14/2022).
- Hilburg J.Concrete production produces eight percent of the world’s carbon dioxide emissions. https://www.archpaper.com/2019/01/concrete-production-eight-percent-co2-emissions/ (accessed 3/17/2022).
- Malsang I.Concrete: the World’s 3rd Largest CO2 Emitter. https://phys.org/news/2021-10-concrete-world-3rd-largest-co2.html (accessed 3/14/2022).
- How Cement is Made. https://www.cement.org/cement-concrete/how-cement-is-made (accessed 3/14/2022).
- Tanzer S. E.; Blok K.; Ramírez A. Curing time: a temporally explicit life cycle CO2 accounting of mineralization, bioenergy, and CCS in the concrete sector. Faraday Discuss. 2021, 230, 271–291. 10.1039/D0FD00139B. [DOI] [PubMed] [Google Scholar]
- Pu Y.; Li L.; Wang Q.; Shi X.; Luan C.; Zhang G.; Fu L.; El-Fatah Abomohra A. Accelerated carbonation technology for enhanced treatment of recycled concrete aggregates: A state-of-the-art review. Construction and Building Materials 2021, 282, 122671. 10.1016/j.conbuildmat.2021.122671. [DOI] [Google Scholar]
- Tam V. W. Y.; Butera A.; Le K. N. Mechanical properties of CO2 concrete utilising practical carbonation variables. Journal of Cleaner Production 2021, 294, 126307. 10.1016/j.jclepro.2021.126307. [DOI] [Google Scholar]
- Reis D. C.; Quattrone M.; Souza J. F. T.; Punhagui K. R. G.; Pacca S. A.; John V. M. Potential CO2 reduction and uptake due to industrialization and efficient cement use in Brazil by 2050. Journal of Industrial Ecology 2021, 25 (2), 344–358. 10.1111/jiec.13130. [DOI] [Google Scholar]
- Yadav S.; Mehra A. A review on ex situ mineral carbonation. Environmental Science and Pollution Research 2021, 28 (10), 12202–12231. 10.1007/s11356-020-12049-4. [DOI] [PubMed] [Google Scholar]
- Calcium Carbonate Price Trend and Forecast. https://www.chemanalyst.com/Pricing-data/calcium-carbonate-1158 (accessed 3/14/2022).
- Onyebuchi V. E.; Kolios A.; Hanak D. P.; Biliyok C.; Manovic V. A systematic review of key challenges of CO2 transport via pipelines. Renewable and Sustainable Energy Reviews 2018, 81, 2563–2583. 10.1016/j.rser.2017.06.064. [DOI] [Google Scholar]
- Ajayi T.; Gomes J. S.; Bera A. A review of CO2 storage in geological formations emphasizing modeling, monitoring and capacity estimation approaches. Petroleum Science 2019, 16 (5), 1028–1063. 10.1007/s12182-019-0340-8. [DOI] [Google Scholar]
- Brennan S. T. B. R. C.; Merrill M. D.; Freeman P. A.; Ruppert L. F.. A Probabilistic Assessment Methodology for the Evaluation of Geologic Carbon Dioxide Storage; 2010, https://pubs.usgs.gov/of/2010/1127/ (accessed 3/9/2022). [Google Scholar]
- Kearns J.; Teletzke G.; Palmer J.; Thomann H.; Kheshgi H.; Chen Y.-H. H.; Paltsev S.; Herzog H. Developing a Consistent Database for Regional Geologic CO2 Storage Capacity Worldwide. Energy Procedia 2017, 114, 4697–4709. 10.1016/j.egypro.2017.03.1603. [DOI] [Google Scholar]
- Jahanbakhsh A.; Liu Q.; Hadi Mosleh M.; Agrawal H.; Farooqui N. M.; Buckman J.; Recasens M.; Maroto-Valer M.; Korre A.; Durucan S. An Investigation into CO2-Brine-Cement-Reservoir Rock Interactions for Wellbore Integrity in CO2 Geological Storage. Energies 2021, 14 (16), 5033. 10.3390/en14165033. [DOI] [Google Scholar]
- Bachu S. Screening and selection criteria, and characterisation techniques for the geological sequestration of carbon dioxide (CO2). Developments and Innovation in Carbon Dioxide (CO2) Capture and Storage Technology 2010, 27–56. 10.1533/9781845699581.1.27. [DOI] [Google Scholar]
- Esposito R. A.; Monroe L. S.; Friedman J. S. Deployment Models for Commercialized Carbon Capture and Storage. Environ. Sci. Technol. 2011, 45 (1), 139–146. 10.1021/es101441a. [DOI] [PubMed] [Google Scholar]
- Bachu S. Review of CO2 storage efficiency in deep saline aquifers. International Journal of Greenhouse Gas Control 2015, 40, 188–202. 10.1016/j.ijggc.2015.01.007. [DOI] [Google Scholar]
- Sharma S.; Agrawal V.; McGrath S.; Hakala J. A.; Lopano C.; Goodman A. Geochemical controls on CO2 interactions with deep subsurface shales: implications for geologic carbon sequestration. Environmental Science: Processes & Impacts 2021, 23 (9), 1278–1300. 10.1039/D1EM00109D. [DOI] [PubMed] [Google Scholar]
- Paluszny A.; Graham C. C.; Daniels K. A.; Tsaparli V.; Xenias D.; Salimzadeh S.; Whitmarsh L.; Harrington J. F.; Zimmerman R. W. Caprock integrity and public perception studies of carbon storage in depleted hydrocarbon reservoirs. International Journal of Greenhouse Gas Control 2020, 98, 103057. 10.1016/j.ijggc.2020.103057. [DOI] [Google Scholar]
- Agartan E.; Gaddipati M.; Yip Y.; Savage B.; Ozgen C. CO2 storage in depleted oil and gas fields in the Gulf of Mexico. International Journal of Greenhouse Gas Control 2018, 72, 38–48. 10.1016/j.ijggc.2018.02.022. [DOI] [Google Scholar]
- Dai Z.; Middleton R.; Viswanathan H.; Fessenden-Rahn J.; Bauman J.; Pawar R.; Lee S.-Y.; McPherson B. An Integrated Framework for Optimizing CO2Sequestration and Enhanced Oil Recovery. Environmental Science & Technology Letters 2014, 1 (1), 49–54. 10.1021/ez4001033. [DOI] [Google Scholar]
- Wei N.; Jiao Z.; Ellett K.; Ku A. Y.; Liu S.; Middleton R.; Li X. Decarbonizing the Coal-Fired Power Sector in China via Carbon Capture, Geological Utilization, and Storage Technology. Environ. Sci. Technol. 2021, 55, 13154. 10.1021/acs.est.1c01144. [DOI] [PubMed] [Google Scholar]
- Qanbari F.; Pooladi-Darvish M.; Hamed Tabatabaie S.; Gerami S. Storage of CO2 as hydrate beneath the ocean floor. Energy Procedia 2011, 4, 3997–4004. 10.1016/j.egypro.2011.02.340. [DOI] [Google Scholar]
- Rehman A. N.; Bavoh C. B.; Pendyala R.; Lal B. Research Advances, Maturation, and Challenges of Hydrate-Based CO2 Sequestration in Porous Media. ACS Sustainable Chem. Eng. 2021, 9 (45), 15075–15108. 10.1021/acssuschemeng.1c05423. [DOI] [Google Scholar]
- Zheng J.; Chong Z. R.; Qureshi M. F.; Linga P. Carbon Dioxide Sequestration via Gas Hydrates: A Potential Pathway toward Decarbonization. Energy Fuels 2020, 34 (9), 10529–10546. 10.1021/acs.energyfuels.0c02309. [DOI] [Google Scholar]
- Gislason S. R.; Wolff-Boenisch D.; Stefansson A.; Oelkers E. H.; Gunnlaugsson E.; Sigurdardottir H.; Sigfusson B.; Broecker W. S.; Matter J. M.; Stute M. Mineral sequestration of carbon dioxide in basalt: A pre-injection overview of the CarbFix project. International Journal of Greenhouse Gas Control 2010, 4 (3), 537–545. 10.1016/j.ijggc.2009.11.013. [DOI] [Google Scholar]
- Sahu C.; Sircar A.; Sangwai J. S.; Kumar R. Effect of Methylamine, Amylamine, and Decylamine on the Formation and Dissociation Kinetics of CO2 Hydrate Relevant for Carbon Dioxide Sequestration. Ind. Eng. Chem. Res. 2022, 61 (7), 2672–2684. 10.1021/acs.iecr.1c04074. [DOI] [Google Scholar]
- Jun Y.-S.; Giammar D. E.; Werth C. J. Impacts of Geochemical Reactions on Geologic Carbon Sequestration. Environ. Sci. Technol. 2013, 47 (1), 3–8. 10.1021/es3027133. [DOI] [PubMed] [Google Scholar]
- Iglauer S. CO2-Water-Rock Wettability: Variability, Influencing Factors, and Implications for CO2 Geostorage. Acc. Chem. Res. 2017, 50 (5), 1134–1142. 10.1021/acs.accounts.6b00602. [DOI] [PubMed] [Google Scholar]
- Ringrose P. S. The CCS hub in Norway: some insights from 22 years of saline aquifer storage. Energy Procedia 2018, 146, 166–172. 10.1016/j.egypro.2018.07.021. [DOI] [Google Scholar]
- Duong C.; Bower C.; Hume K.; Rock L.; Tessarolo S. Quest carbon capture and storage offset project: Findings and learnings from 1st reporting period. International Journal of Greenhouse Gas Control 2019, 89, 65–75. 10.1016/j.ijggc.2019.06.001. [DOI] [Google Scholar]
- Du F.; Nojabaei B. A Review of Gas Injection in Shale Reservoirs: Enhanced Oil/Gas Recovery Approaches and Greenhouse Gas Control. Energies 2019, 12 (12), 2355. 10.3390/en12122355. [DOI] [Google Scholar]
- Huo P.; Zhang D.; Yang Z.; Li W.; Zhang J.; Jia S. CO 2 geological sequestration: Displacement behavior of shale gas methane by carbon dioxide injection. International Journal of Greenhouse Gas Control 2017, 66, 48–59. 10.1016/j.ijggc.2017.09.001. [DOI] [Google Scholar]
- Hamza A.; Hussein I. A.; Al-Marri M. J.; Mahmoud M.; Shawabkeh R.; Aparicio S. CO2 enhanced gas recovery and sequestration in depleted gas reservoirs: A review. J. Pet. Sci. Eng. 2021, 196, 107685. 10.1016/j.petrol.2020.107685. [DOI] [Google Scholar]
- Mukherjee M.; Misra S. A review of experimental research on Enhanced Coal Bed Methane (ECBM) recovery via CO2 sequestration. Earth-Science Reviews 2018, 179, 392–410. 10.1016/j.earscirev.2018.02.018. [DOI] [Google Scholar]
- Zheng L.; Nico P.; Spycher N.; Domen J.; Credoz A. Potential impacts of CO2 leakage on groundwater quality of overlying aquifer at geological carbon sequestration sites: A review and a proposed assessment procedure. Greenhouse Gases: Science and Technology 2021, 11 (5), 1134–1166. 10.1002/ghg.2104. [DOI] [Google Scholar]
- Varadharajan C.; Tinnacher R. M.; Trautz R. C.; Zheng L.; Dafflon B.; Wu Y.; Reagan M. T.; Birkholzer J. T.; Carey J. W. A Review of Studies Examining the Potential for Groundwater Contamination From CO2 Sequestration. Geological Carbon Storage 2018, 305–326. 10.1002/9781119118657.ch15. [DOI] [Google Scholar]
- Perera M. S. A. Influences of CO2 Injection into Deep Coal Seams: A Review. Energy Fuels 2017, 31 (10), 10324–10334. 10.1021/acs.energyfuels.7b01740. [DOI] [Google Scholar]
- Mignan A.; Broccardo M.; Wang Z. Comprehensive Survey of Seismic Hazard at Geothermal Sites by a Meta-Analysis of the Underground Feedback Activation Parameter afb. Energies 2021, 14 (23), 7998. 10.3390/en14237998. [DOI] [Google Scholar]
- Kroll K. A.; Buscheck T. A.; White J. A.; Richards-Dinger K. B. Testing the efficacy of active pressure management as a tool to mitigate induced seismicity. International Journal of Greenhouse Gas Control 2020, 94, 102894. 10.1016/j.ijggc.2019.102894. [DOI] [Google Scholar]
- Alcalde J.; Flude S.; Wilkinson M.; Johnson G.; Edlmann K.; Bond C. E.; Scott V.; Gilfillan S. M. V.; Ogaya X.; Haszeldine R. S. Estimating geological CO2 storage security to deliver on climate mitigation. Nat. Commun. 2018, 9 (1), 1. 10.1038/s41467-018-04423-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi P.; Bikkina P.; Wang Q. Consequence analysis of accidental release of supercritical carbon dioxide from high pressure pipelines. International Journal of Greenhouse Gas Control 2016, 55, 166–176. 10.1016/j.ijggc.2016.10.010. [DOI] [Google Scholar]
- Rodosta T.; Ackiewicz M. U.S. DOE/NETL Core R&D Program for Carbon Storage Technology Development. Energy Procedia 2014, 63, 6368–6378. 10.1016/j.egypro.2014.11.672. [DOI] [Google Scholar]
- Federal Requirements Under the Underground Injection Control (UIC) Program for Carbon Dioxide (CO2) Geologic Sequestration (GS) Wells Final Rule. https://www.epa.gov/uic/federal-requirements-under-underground-injection-control-uic-program-carbon-dioxide-co2-geologic (accessed 3/14/2022).
- Class VI - Wells used for Geologic Sequestration of Carbon Dioxide. https://www.epa.gov/uic/class-vi-wells-used-geologic-sequestration-carbon-dioxide (accessed 3/14/2022).
- Nicot J.-P.; Oldenburg C. M.; Bryant S. L.; Hovorka S. D. Pressure perturbations from geologic carbon sequestration: Area-of-review boundaries and borehole leakage driving forces. Energy Procedia 2009, 1 (1), 47–54. 10.1016/j.egypro.2009.01.009. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.