Abstract
Purpose
To investigate the evolution of COVID-19 patient characteristics and multiorgan injury across the pandemic.
Methods
This retrospective cohort study consisted of 40,387 individuals tested positive for SARS-CoV-2 in the Montefiore Health System in Bronx, NY, between March 2020 and February 2022, of which 11,306 were hospitalized. Creatinine, troponin, and alanine aminotransferase were used to define acute kidney injury (AKI), acute cardiac injury (ACI) and acute liver injury, respectively. Demographics, comorbidities, emergency department visits, hospitalization, intensive care utilization, and mortality were analyzed across the pandemic.
Results
COVID-19 positive cases, emergency department visits, hospitalization and mortality rate showed four distinct waves with a large first wave in April 2020, two small (Alpha and Delta) waves, and a large Omicron wave in December 2021. Omicron was more infectious but less lethal (p = 0.05). Among hospitalized COVID-19 patients, age decreased (p = 0.014), female percentage increased (p = 0.023), Hispanic (p = 0.028) and non-Hispanic Black (p = 0.05) percentages decreased, and patients with pre-existing diabetes (p = 0.002) and hypertension (p = 0.04) decreased across the pandemic. More than half (53.1%) of hospitalized patients had major organ injury. Patients with AKI, ACI and its combinations were older, more likely males, had more comorbidities, and consisted more of non-Hispanic Black and Hispanic patients (p = 0.005). Patients with AKI and its combinations had 4-9 times higher adjusted risk of mortality than those without.
Conclusions
There were shifts in demographics toward younger age and proportionally more females with COVID-19 across the pandemic. While the overall trend showed improved clinical outcomes, a substantial number of COVID-19 patients developed multi-organ injuries over time. These findings could bring awareness to at-risk patients for long-term organ injuries and help to better inform public policy and outreach initiatives.
Keywords: Omicron, SARS-CoV-2, Acute cardiac injury, Acute kidney injury
1. Introduction
Two years into the Coronavirus disease 2019 (COVID-19) pandemic [[1], [2], [3]], close to half a billion individuals have been infected and over 6 million have died from COVID-19 disease world-wide (http://www.coronvirus.jhu.edu/, March 22, 2022). In the United States, SARS-CoV-2 have infected over 80 million individuals and killed close to a million. COVID-19 disease imposes a heavy health and socioeconomic burden [4] and exacerbates health and healthcare disparities [[5], [6], [7]].
The COVID-19 pandemic has evolved considerably with multiple waves of infection driven by new variants. The compositions of age, sex, race/ethnicity, comorbidities, multiorgan injury, hospitalization, critical care utilization, and mortality rate have changed substantially across the pandemic [8,9]. In addition, there have been remarkable progress made in effective treatments and vaccines, as well as in public health measures (i.e., wearing masks, social distancing, etc.) [10] to improve outcomes and curb the spread of infection. A few studies have suggested that COVID-19 disproportionally affects racial and ethnic minority groups [5,11]. Living conditions, household density, occupational exposure, and access to quality care, among others may contribute to racial disparities in increased susceptibility to SARS-CoV-2 infection and worse outcomes [[12], [13], [14], [15]]. Many of the studies to date involved patients early in the COVID-19 pandemic. Studies that evaluated COVID-19 patient characteristics, risk factors, incidences of multiple organ injuries and clinical outcomes across the pandemic to date are sparse [[16], [17], [18]]. Improved understanding of COVID-19 patient characteristics across the pandemic could help to better manage future COVID-19 disease, inform public policy and outreach initiatives.
The purpose of this work was to investigate the characteristics of COVID-19 patients with multiorgan injury and clinical outcomes across the COVID-19 pandemic in a large academic health system in the Bronx, New York.
2. Methods
2.1. Data source
This retrospective cohort study was approved by the Einstein-Montefiore Institutional Review Board with a waiver for informed consent (#2021–13658). The data comes from the Montefiore Health System, one of the largest academic healthcare systems in New York City with multiple hospitals and clinics located in the Bronx, the lower Hudson Valley, and Westchester County serving a large, low-income, and racially and ethnically diverse population that was hit hard by COVID-19 early in the pandemic [9,19]. Health data were searched and extracted as described previously [9,[20], [21], [22], [23], [24], [25]] via the Observational Medical Outcomes Partnership (OMOP) Common Data Model (CDM) version 6. OMOP CDM represents healthcare data from diverse sources, which are stored in standard vocabulary concepts [26], allowing for the systematic analysis of disparate observational databases, including data from the electronic medical record system, administrative claims, and disease classifications systems (e.g., ICD-10, SNOWMED, LOINC, etc.). ATLAS, a web-based tool developed by the Observational Health Data Sciences and Informatics (OHDSI) community that enables navigation of patient-level, observational data in the CDM format, was used to search vocabulary concepts and facilitate cohort building. Data were subsequently exported and queried as SQLite database files using the DB Browser for SQLite (version 3.12.0). To ensure data accuracy, our team performed extensive cross validation of all major variables extracted by manual chart reviews on subsets of patients [9,[20], [21], [22], [23], [24], [25]].
From March 11, 2020, to February 20, 2022, there were 40,387 individuals who tested positive for SARS-CoV-2 by polymerase chain reaction, of which 11,306 were hospitalized and included in this study. Demographics, comorbidities, and primary outcomes (unadjusted mortality and covariate-adjusted mortality odds ratios) were tabulated. Demographic data included age, sex, ethnicity, and race. Chronic comorbidities included obesity, diabetes, congestive heart failure, chronic kidney disease (CKD), coronary artery disease (CAD), hypertension, chronic obstructive pulmonary disease (COPD) and asthma. Clinical laboratory tests included creatinine (Cr), Troponin-T (TNT), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) that were used to define organ injuries (see below for definitions of organ injuries). Other data analyzed include emergency department (ED) visits, hospitalization, ICU admission, anticoagulant use, and steroid use.
2.2. Definitions of organ injuries
AKI was defined using KDIGO criteria either as a 0.3 mg/dl increase in serum creatinine within 48 h or a 1.5× increase in serum creatinine within a 7-day iterative window [[27], [28], [29], [30]]. Approximately 57% of patients had preexisting creatinine baseline values prior to hospitalization with COVID-19. For patients who did not have creatinine baseline values, the baseline creatinine was defined as the lowest creatinine value during hospitalization [31,32]. ACI was defined as a serum troponin T (TNT) level above the 99th percentile upper reference limit (>0.014 ng/mL) any time during hospitalization [30,[33], [34], [35], [36]]. ALI was defined as ALT >1 ULN [>40 U/L] and AST>1 ULN [>35 U/L] were considered to have liver injury [[37], [38], [39], [40], [41]]. These definitions were applied to any time during hospitalization. In this work, the term “no injury (NI)” is used to refer to individuals with COVID-19 who did not experience AKI, ACI and/or ALI during hospitalization. The average and standard deviation values of creatinine, troponin, alanine aminotransferase and aspartate aminotransferase used to triage cases were 1.49 ± 1.60 mg/dl, 0.38 ± 2.79 ng/ml, 61.4 ± 238.9 U/L, 71.1 ± 398.2 U/L.
2.3. Data analysis
For all COVID-19 patients, monthly incidences were plotted in absolute counts and percentages for: i) COVID-19 positive cases; ii) COVID-19-related ED visits, hospitalization, and ICU admission; and iii) mortality. Monthly incidences of hospitalized and non-hospitalized COVID-19 patients separated by pediatric (≤21 years old) and adults were also plotted.
For hospitalized patients, monthly incidences were plotted in absolute counts and percentages for: i) any injury (AKI, ACI and/or ALI), individual organ injuries, and mortality; ii) anticoagulant and steroid uses; and iii) demographics and comorbidities.
Patients were grouped and tabulated into NI, AKI, ALI, ACI, AKI + ALI, ACI + AKI, ACI + ALI, ACI + AKI + ALI (mutually exclusive). Demographics, comorbidities, mortality rates, and mortality odds ratios adjusted for age and sex were tabulated for each injury group. Whether certain race and ethnicity were overrepresented in each organ injury group were analyzed by comparing with the percentage of each race and ethnicity to relative percent of each injury group. We also quantified the percent of patients with AKI, ACI and ALI (not mutually exclusive).
2.4. Statistical analysis
Age, expressed as median (interquartile range, IQR), was compared between groups using the Kruskall-Wallis test. Group differences in categorical variables (race, ethnicity, comorbidities) were tested using χ2 tests. In addition to unadjusted mortality rate (%), logistic regression with covariate-adjusted odds ratios (aOR) and 95% confidence intervals (CI) for mortality were estimated for each organ injury group with NI as the reference group. Trends of some demographic and comorbidity variables across the pandemic were evaluated using linear regression. Two-sided p-values <0.05 were considered statistically significant.
3. Results
3.1. COVID-19 positive cases, ED visits, hospitalization, ICU admission, and mortality
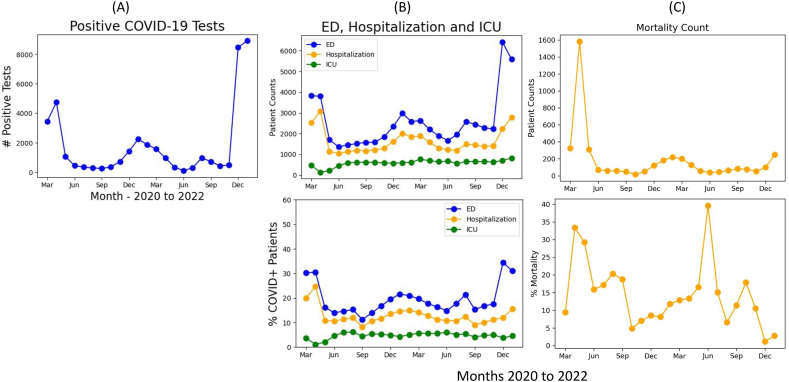
Between March 11, 2020, to February 20, 2022, there were 40,387 individuals who tested positive for SARS-CoV-2. There were 4 distinct waves. The first wave peaked in April 2020, a second wave peaked in December 2020 (Alpha variant), a third small wave peaked in August 2021 (Delta variant), and a disproportionally large fourth wave peaked in January 2022 (Omicron variant) (Fig. 1A). Note that partial month data for February 2022 were not plotted. COVID-19 positive cases largely returned to baseline in late Feb 2022 in our catchment area.
Fig. 1.
(A) Monthly counts of COVID-19 positivity cases, (B) monthly count and percentage relative to COVID-19 positivity cases for ED visits, hospitalization, intensive care unit (ICU), and (C) monthly count and percentage relative to COVID-19 positivity cases for mortality of all COVID-19 patients.
Trends in absolute and relative ED visits and hospitalization showed similar patterns across the pandemic (Fig. 1B). Although ED visits were high during Omicron, there was relatively low hospitalization. ICU admissions were relatively constant. The absolute mortality count was high during the first wave, dropped substantially after, rose during the Alpha wave, did not rise substantially during the Delta wave, and rose again during the Omicron wave (Fig. 1C). In percentage, mortality rate was lowest in the Omicron wave.
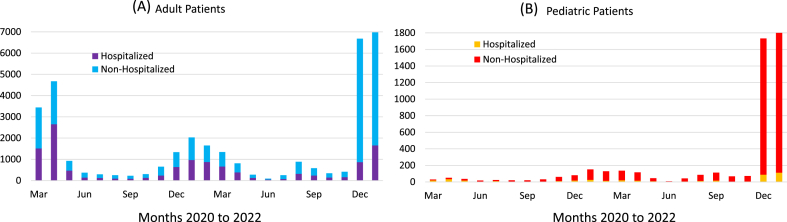
Fig. 2 shows the monthly incidences of non-hospitalized and hospitalized patients for adult and pediatric COVID-19 patients. Pediatric (≤21 years old) cases were highest in the Omicron wave. Hospitalization trends for pediatric patients were relatively low compared to adults. There was a significant increase in pediatric hospitalization during Omicron.
Fig. 2.
Monthly counts of hospitalized and non-hospitalized patients for (A) adult and (B) pediatric (≤21 years old) COVID-19 positive patients.
3.2. Incidence of organ injuries of hospitalized COVID-19 patients
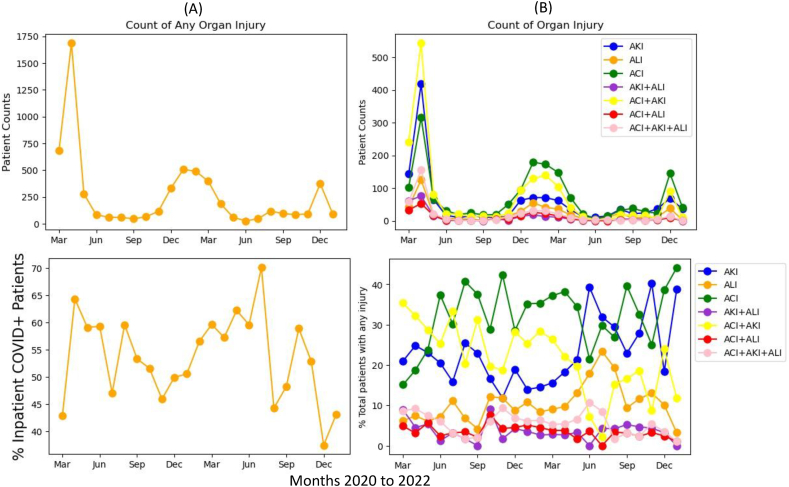
Between March 11, 2020, and February 20, 2022, there were 11,306 hospitalized COVID-19 patients. Fig. 3A depicts the incidence of organ injury in hospitalized patients, which followed a similar trend as COVID-19 positive patients. In percentages of hospitalized COVID-19 patients, the incidence of organ injuries ranged from 45% to 70% with large fluctuations and relatively low incidence in the Omicron wave.
Fig. 3.
Monthly counts and percentages for (A) any organ injury and (B) individual injury groups of hospitalized COVID-19 patients. AKI: acute kidney injury, ALI: acute liver injury, ACI: acute cardiac injury.
Fig. 3B shows the absolute and relative compositions of individual organ injuries. In absolute counts, incidences followed similar patterns as COVID-19 positive case counts, with ACI counts being the highest in all 4 waves, followed by ACI + AKI. AKI counts were high during the first wave but dropped in subsequent waves. In relative counts, AKI + ACI incidence was highest during the first wave but dropped in subsequent waves (p < 0.05), AKI and ACI rose and became more dominant towards a later phase of the pandemic. The incidence of all other organ injuries remained low and relatively constant across the pandemic.
3.3. Anticoagulant and steroid treatment of hospitalized COVID-19 patients
Supplemental Figure 1 shows the percentages of hospitalized COVID-19 patients treated with anticoagulant and steroid drugs. On average, 48 ± 13% of patients received anticoagulants, which peaked during the first wave, again during the Delta and Omicron waves, but did not peak during the Alpha wave. Steroid usage was relatively constant across the pandemic averaging 26 ± 8% of hospitalized COVID-19 patients.
3.4. Demographics and comorbidities across the pandemic in hospitalized patients
Supplemental Figure 2A shows trends for age, female sex, Black/African American patients, and Hispanic patients among those hospitalized for COVID-19 across the pandemic. The average age decreased over time (slope = −0.96, R2 = 0.25, p = 0.014). The % of female was low early in the pandemic but increased slowly across the pandemic (slope = 0.78, R2 = 0.22, p = 0.023). The % of Hispanic patients was low early in the pandemic, rose substantially around Jun 2020, and decreased steady thereafter (slope = −0.63, R2 = 0.63, p = 0.0001, fitting data from Jun 2020 forward). The % of Black/African American patients dropped after the initial wave early on (p < 0.05) but rose slightly and plateaued.
Supplemental Figure 2B shows the compositions of comorbidities of hospitalized COVID-19 patients across the pandemic. Incidence of hypertension decreased steadily across the pandemic from 45% to 30% (slope = −0.45, R2 = 0.19, p = 0.04) and incidence of diabetes also decreased steadily across the pandemic from 38% to 28% (slope = −0.71, R2 = 0.36, p = 0.002). The other comorbidities (obesity, CKD, CAD, HF and COPD), which were comparatively less prevalent, remained relatively constant across the pandemic.
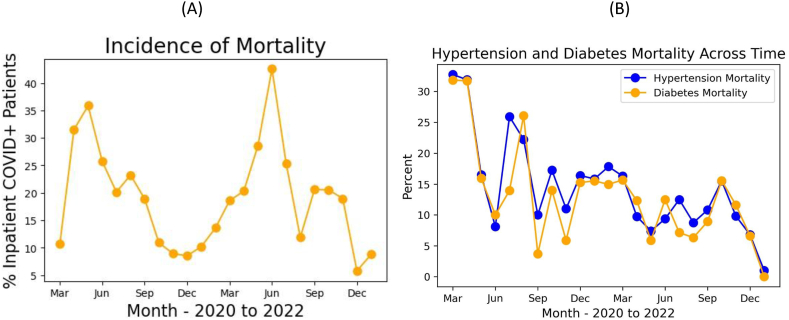
The mortality rate of hospitalized COVID-19 patients was high during the first wave in April and May 2020 (∼35%), dropped to ∼10% thereafter, did not peak during the Alpha wave, but peaked during the Delta wave (Fig. 4A). Mortality rate of hospitalized patients was low during the Omicron wave. Note that the overall mortality rates of hospitalized-only patients were higher than those of all COVID-19 positive patients (Fig. 1C). Mortality rate of hospitalized COVID-19 patients with pre-existing diabetes and hypertension markedly decreased across the pandemic (p < 0.05) (Fig. 4B).
Fig. 4.
Monthly mortality in percentage of (A) all hospitalized COVID-19 patients, and (B) those with hypertension and diabetes.
3.5. Demographics and comorbidities of hospitalized COVID-19 patients
Table 1 summarizes the incidence, demographics, comorbidities, mortality and adjusted mortality odds ratios for different organ injury groups of hospitalized COVID-19 patients. The incidence of NI, AKI, ALI, ACI, AKI + ALI, ACI + AKI, ACI + ALI, ACI + AKI + ALI, were, respectively, 46.9%, 11.2%, 4.6%, 14.7%, 2.3%, 14.6%, 2%, and 3.6% (mutually exclusive). There were 31.7% who had AKI, 34.9% had ACI and 12.5% had ALI (not mutually exclusive).
Table 1.
Demographics and comorbidities of NI, AKI, ALI ACI, AKI + LI, ALI + ACI, ACI + AKI and ACI + AKI + ALI groups. Group comparison of categorical variables in frequencies and percentages used chi-squared test or Fisher exact tests. Group comparison of continuous variables in means and SEMs (standard error of means) used the Mann-Whitney U test. Abbreviation: CKD, chronic kidney disease. All values are in n (%) unless otherwise specified. Note that all variables shown of all injury groups were significant compared to those of the NI group. *, p < 0.01. **, p < 0.001. ***, p < 0.0001.
| Among hospitalized COVID-19 patients (N = 11306), the number of patients with organ injuries | ||||||||
|---|---|---|---|---|---|---|---|---|
| NI | AKI | ALI | ACI | AKI + ALI | ACI + AKI | ACI + ALI | ACI + AKI + ALI | |
| Incidence | 5303 (46.9%) | 1268 (11.2%) | 523 (4.6%) | 1661 (14.7%) | 259 (2.3%) | 1652 (14.6%) | 228 (2.0%) | 412 (3.6%) |
| Demographics | ||||||||
| Age, median (IQR) | 59 (43, 75) | 65 (54, 76)*** | 57 (45, 69) | 73 (64.5, 82.5)*** | 63 (52, 74) | 74 (65, 83)*** | 66 (58, 74)*** | 70 (61, 79)*** |
| Females | 2960 (55.8%) | 630 (49.7%)** | 240 (45.9%)*** | 749 (45.1%)*** | 103 (39.8%)*** | 738 (44.7%)*** | 86 (37.7%)*** | 143 (34.7%)*** |
| Black, not Hispanic (3670, 32.4%) | 1573 (29.7%) | 448 (35.3%)** | 128 (24.5%)* | 596 (35.9%)*** | 74 (28.6%) | 616 (37.3%)*** | 83 (36.4%)* | 152 (12.1%)* |
| White, not Hispanic (1324, 11.7%) | 653 (12.3%) | 144 (11.4%) | 60 (11.5%) | 173 (10.4%) | 37 (14.3%) | 190 (11.5%) | 17 (7.5%) | 50 (14.8%) |
| other (1681, 14.9%) | 841 (15.9%) | 179 (14.1%)* | 78 (14.9%) | 222 (13.4%)* | 43 (16.6%) | 227 (13.7%)** | 30 (13.2%) | 61 (36.2%) |
| Hispanic (4631, 41.0%) | 2236 (42.2%) | 497 (39.2%) | 257 (49.1%)* | 670 (40.3%) | 105 (40.5%) | 619 (37.5%)** | 98 (43.0%) | 149 (36.2%)* |
| Comorbidities | ||||||||
| Diabetes | 1434 (27.0%) | 467 (36.8%)*** | 164 (31.4%)* | 702 (42.3%)*** | 83 (32.0%) | 701 (42.4%)*** | 121 (53.1%)*** | 176 (42.7%)*** |
| Congestive Heart Failure | 327 (6.2%) | 129 (10.2%)*** | 31 (5.9%) | 394 (23.7%)*** | 15 (5.8%) | 406 (24.6%)*** | 55 (24.1%)*** | 82 (19.9%)*** |
| CKD | 497 (9.4%) | 195 (15.4%)*** | 38 (7.3%) | 542 (32.6%)*** | 34 (13.1%) | 464 (28.1%)*** | 87 (38.2%)*** | 111 (26.9%)*** |
| Hypertension | 1806 (34.1%) | 520 (41.0%)*** | 182 (34.8%) | 1011 (60.9%)*** | 93 (35.9%) | 884 (53.5%)*** | 153 (67.1%)*** | 215 (52.2%)*** |
| Coronary artery disease | 67 (1.3%) | 21 (1.7%) | 4 (0.8%) | 93 (5.6%)*** | 4 (1.5%) | 75 (4.5%)*** | 13 (5.7%)*** | 17 (4.1%)** |
| COPD/Asthma | 738 (13.9%) | 160 (12.6%) | 72 (13.8%) | 258 (15.5%) | 27 (10.4%) | 232 (14.0%) | 32 (14.0%) | 44 (10.7%) |
| Obesity | 853 (16.1%) | 215 (17.0%) | 93 (17.8%) | 264 (15.9%) | 46 (17.8%) | 255 (15.4%) | 30 (13.2%) | 70 (17.0%) |
| Mortality | ||||||||
| Unadjusted mortality rates | 350 (6.6%) | 307 (24.2%)*** | 34 (6.5%) | 341 (20.5%)*** | 60 (23.2%)*** | 716 (43.3%)*** | 54 (23.7%)*** | 193 (46.8%)*** |
| Adjusted mortality odds ratios[95% CI] | 1 | 4.03 [3.37, 4.82] | 0.96 [0.65,1.36] | 2.06 [1.73, 2.46] | 4.12 [3.00, 5.58] | 6.92 [5.92, 8.11] | 3.43 [2.38, 4.87] | 9.25 [7.21, 11.86] |
Hospitalized COVID-19 patients in every organ injury group were older than those in the NI group (p < 0.0001), with ACI + AKI, ACI and ACI + AKI + ALI group being the oldest (74, 73 and 70 years old, respectively). All organ injury groups had fewer females (p < 0.0001).
By comparing the percentages of race and ethnicity versus the percentages of each injury group, we found non-Hispanic Black patients were overrepresented in most organ injury groups (AKI, ACI, ACI + AKI and ACI + ALI) as compared to the NI group (p < 0.05). Conversely, Black patients were underrepresented in the ALI group (p < 0.05) and ACI + AKI + ALI groups (p < 0.05). In contrast, non-Hispanic White patients were not overrepresented in any of the organ injury groups as compared to the NI group (p > 0.05).
All organ injury groups, except ALI and ALI + AKI, had significantly more diabetes, congestive heart failure, CKD, hypertension, and coronary artery disease compared to the NI group among hospitalized COVID-19 patients (p < 0.05). COPD/asthma and obesity (except COPD/asthma in the ACI group (p < 0.05)) were not significantly different between injury groups and NI group (p > 0.05).
3.6. In-hospital mortality of hospitalized COVID-19 patients
The unadjusted mortality of hospitalized COVID-19 patients with NI was 6.6%. The unadjusted mortality of hospitalized COVID-19 patients with AKI, ALI, ACI, AKI + ALI, ACI + AKI, ACI + ALI, ACI + AKI + ALI, were 24.2%, 6.5%, 20.5%, 23.2%, 43.3%, 23.7%, and 46.8%, respectively, with the corresponding mortality odds ratios (adjusted for significant comorbidities and demographics) to be 4.03, 0.96, 2.06, 4.12, 6.92, 3.43, and 9.25 relative to the NI group (Table 1).
4. Discussion
This study investigated the COVID-19 patient characteristics and multiorgan injury across the pandemic. The major findings are: i) COVID-19 positive cases showed four distinct waves with the Omicron wave being more infectious but less lethal, and infecting more children compared to the original strain; ii) age, percent male, percent Hispanic and non-Hispanic Black patients, and prevalence of pre-existing diabetes and hypertension decreased across the pandemic; iii) more than half of hospitalized COVID-19 patients had major organ injuries, and those with organ injuries were older, more likely males, had more comorbidities, and were overrepresented for non-Hispanic Black and Hispanic patients; and iv) COVID-19 patients with AKI and its combinations had 4 to 9 times higher covariate-adjusted risk of mortality than COVID-19 patients without organ injuries.
4.1. Trends across the pandemic
Different coronavirus variants, seasonality, vaccination rate, increased awareness, and health policies, among others, contributed to the magnitudes of different waves [10]. A significant number of patients who visited the ED were hospitalized. ICU admission was relatively constant across the pandemic, likely reflecting the maximum ICU capacity assigned for COVID-19 patients [42,43]. The majority of death occurred during the first wave. There was overall improvement in clinical outcomes in terms of ED visits, hospitalization, and mortality rate after the first wave, interrupted by each subsequent surge of infection [44,45]. The Omicron variant was highly infectious, but mortality rate was relatively low consistent with a previous report [46,47].
Prior to the Omicron wave, pediatric COVID-19 cases were very low. Omicron variant infected comparatively more pediatric patients, consistent with prior reports [48]. Possible explanations are that schools returned to in-person instruction during the Omicron wave, kids were vaccinated or received boosters later than adults, or kids were more susceptible to the Omicron variant [49], although other explanations are possible. Nonetheless, pediatric hospitalization rates during Omicron were low compared to adults.
During the first wave of COVID-19, the highest incidence of organ injuries was a combination of ACI and AKI, suggestive of the negative impacts of COVID-19 disease on heart and kidney, consistent with prior findings [[1], [2], [3],[23], [24], [25],29]. The relative incidence of ACI + AKI decreased whereas the relative AKI and ACI incidences increased with time.
There were substantial changes in demographics of hospitalized COVID-19 patients across the pandemic. The average age decreased over time, which might be due to decreased susceptibility of older adults who were being vaccinated and received boosters first, and infection that spread among younger individuals more easily due to school re-openings and social gatherings [47,50]. The percent female in the hospitalized cohort increased over time, but the reason is unclear. A few studies have suggested that COVID-19 disproportionally affects minority populations [5,11]. Cultural factors, living conditions, household density, occupational exposure, and access to quality care, amongst others may contribute to potential COVID-19 outcome disparities [[12], [13], [14], [15]]. We found the proportion of Hispanic and non-Hispanic Black patients decreased over time, which could be due to increased awareness of the disease in these populations and the vaccination might have a larger impact on these more susceptible populations early in the pandemic.
There was also a substantial decrease in the composition and mortality rate of hospitalized COVID-19 patients with pre-existing diabetes and hypertension across the pandemic. This could be due to broad availability of COVID-19 vaccines and boosters and improved management of COVID-19 disease, which were differentially more beneficial to these populations.
4.2. Multiorgan injury
Multiorgan injury is a known major complication of COVID-19, with the most reported organ injuries being acute kidney injury (AKI), acute cardiac injury (ACI) and acute liver injury (ALI). A few studies have characterized the risk factors and outcomes of AKI [31,[51], [52], [53], [54], [55], [56]], ACI [[57], [58], [59], [60], [61], [62]] and ALI [38,39], and they have been associated with increased risk of critical illness and mortality in COVID-19 patients. We found more than half of hospitalized COVID-19 patients showed major organ injury. Although previous studies have reported organ injuries in COVID-19 patients [31,38,39,[51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62]], this study is first to compare multiple major organ injuries and their combinations with respect to other clinical variables such as age, sex, race, ethnicity, comorbidities, and mortality across the pandemic in a highly diverse population. ACI, AKI and ACI + AKI had the highest incidence rates, suggesting the heart and kidney are more susceptible to COVID-19 disease. COVID-19 patients with organ injuries were significantly older, more male and had more major comorbidities compared to hospitalized COVID-19 patients without organ injuries. These are known risk factors of severe COVID-19 disease [[1], [2], [3],57,58].
Minority and underserved populations are known to have increased susceptibility to SARS-CoV-2 infection [5,11]. Although health disparity in COVID-19 outcomes in general has been reported previously [24], this is the first study that showed Hispanic and Black patients were more susceptible to multiorgan injuries.
COVID-19 patients with AKI, ACI and their combinations were much more likely to die. Our mortality risk estimates of patients with AKI, ACI and ALI separately are consistent with the literature [31,38,39,[51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62]]. However, there are no studies that compared mortality risk of combined major organ injuries in a large diverse cohort. Hospitalized COVID-19 patients with AKI and its combinations and patients with multiple organ injuries generally had higher risk of mortality than COVID-19 patients without organ injuries. This underscores the importance of identifying patients with AKI and ACI during COVID-19 hospitalization and follow up care. Hospitalized COVID-19 patients with ALI did not have increased mortality risk as compared to those without organ injuries. ALI has been previously reported to be not significantly associated with increased mortality odds ratio, although more severe ALI have been shown to be associated with higher mortality odds ratio [38,39]. Incident rate of ALI is relatively low [38,39].
Mechanisms of organ injuries include direct and indirect effects of SARS-CoV-2 infection. SARS-CoV-2 could directly infect cells within these organs via the ACE2 receptors. Indirect effects could result from hypoxemia, cytokine storm, hyperinflammation, sepsis, ARDS, among others [[1], [2], [3],57,58]. Previous studies have reported that, following COVID-19 hospitalization, ACI develops early on in the first 24 h [36], AKI after 3 days [24,30], and ALI after 7 days [39]. These observations suggest that ACI and AKI may be directly affected by the SARS-CoV-2 virus, whereas ALI might be associated with secondary, treatment effects (such as antiviral, antibacterial or other medications). Previous studies found that most COVID-19 patients with ALI recovered 2.5 months after hospitalization [39], whereas most patients with ACI required longer recovery time [36]. These findings suggest that liver might recover well associated with COVID-19 disease.
4.3. Limitations
Our study has several limitations. During acute COVID-19 management, laboratory tests were limited and did not include all necessary workups to make complete diagnosis, often precluding definitive diagnosis of secondary organ damages or disorders. We made use of readily available laboratory data on most patients. Thus, the classifications of ACI, AKI, and ALI based on TNT, Cr, ALT, respectively, have limitations because some laboratory markers were not specific the organ injury (i.e., NTproBNP is more specific to cardiac injury compared to TNT but NTproBNP are not available for most patients). More vigorous definitions and follow-up diagnosis of organ injuries would be needed. Moreover, changes of these laboratory variables might be more informative. However, many patients did not have pre COVID-19 laboratory data in our health system.
We only assumed that the COVID-19 waves were associated with certain variants and there were likely some mixtures of multiple variants in each wave. Although the Omicron wave returned to baseline in mid-January 2022, some patients were still in the hospitals and their mortality associated with the Omicron wave were not yet known. Our catchment area consisted of a large proportion of Hispanic and Black patients compared to other race and ethnicity groups, thus comparisons whether they were more susceptible to worse outcomes need to be interpreted with caution. We did not investigate the effect of vaccination as vaccination status is challenging to documented. This is a descriptive retrospective study that could not address the underlying cause of organ injuries. As with any retrospective study, there could be unintended patient selection bias and unaccounted confounders.
5. Conclusions
More than half of hospitalized COVID-19 patients had organ injury, and they were older, more likely males, had more comorbidities, and were overrepresented by non-Hispanic Black and Hispanic patients. Patients with AKI, ACI and its combinations were more likely to have worse outcomes. There were improved clinical outcomes, lower incidence of organ injuries, younger age and more female across the pandemic. These findings bring awareness to monitor at-risk patients post COVID-19 to prevent or minimize long-term organ damages and complications. These findings could also help could help to better manage future COVID-19 disease, inform public policy and outreach initiatives.
Author contribution statement
Justin Lu: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Alexandra Buczek; Roman Fleysher; Wouter Hoogenboom: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Benjamin Musheyev; Tim Q Duong: Conceived and designed the experiments, Wrote the paper.
Erin Heninger; Kasra Jabbery; Mahendranath Rangareddy; Devdatta Kanawade; Chandra Nelapat; Selvin Soby; Parsa Mirhaji: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare the following conflict of interests: Wouter S. Hoogenboom, co-author, is employed by Elsevier and functions as an external editor for Heliyon. Wouter S. Hoogenboom had no part in the peer-review process or the editorial decision-making and the work carried out in this manuscript was completed before WSH was employed by Elsevier.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e15277.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., Wang H., Wan J., Wang X., Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Rio C., Collins L.F., Malani P. Long-term health consequences of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.19719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apea V.J., Wan Y.I., Dhairyawan R., Puthucheary Z.A., Pearse R.M., Orkin C.M., Prowle J.R. Ethnicity and outcomes in patients hospitalised with COVID-19 infection in East London: an observational cohort study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-042140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golestaneh L., Neugarten J., Fisher M., Billett H.H., Gil M.R., Johns T., Yunes M., Mokrzycki M.H., Coco M., Norris K.C., Perez H.R., Scott S., Kim R.S., Bellin E. The association of race and COVID-19 mortality. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webb Hooper M., Nápoles A.M., Pérez-Stable E.J. COVID-19 and racial/ethnic disparities. JAMA. 2020;323:2466–2467. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth G.A., Emmons-Bell S., Alger H.M., Bradley S.M., Das S.R., de Lemos J.A., Gakidou E., Elkind M.S.V., Hay S., Hall J.L., Johnson C.O., Morrow D.A., Rodriguez F., Rutan C., Shakil S., Sorensen R., Stevens L., Wang T.Y., Walchok J., Williams J., Murray C. Trends in patient characteristics and COVID-19 in-hospital mortality in the United States during the COVID-19 pandemic. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoogenboom W.S., Pham A., Anand H., Fleysher R., Buczek A., Soby S., Mirhaji P., Yee J., Duong T.Q. Clinical characteristics of the first and second COVID-19 waves in the Bronx, New York: a retrospective cohort study. Lancet Reg Health Am. 2021;3 doi: 10.1016/j.lana.2021.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowling B.J., Aiello A.E. Public health measures to slow community spread of coronavirus disease 2019. J. Infect. Dis. 2020;221:1749–1751. doi: 10.1093/infdis/jiaa123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louis-Jean J., Cenat K., Njoku C.V., Angelo J., Sanon D. Coronavirus (COVID-19) and racial disparities: a perspective analysis. J Racial Ethn Health Disparities. 2020;7:1039–1045. doi: 10.1007/s40615-020-00879-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabarriti R., Brodin N.P., Maron M.I., Guha C., Kalnicki S., Garg M.K., Racine A.D. Association of race and ethnicity with comorbidities and survival among patients with COVID-19 at an urban medical center in New York. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.19795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yehia B.R., Winegar A., Fogel R., Fakih M., Ottenbacher A., Jesser C., Bufalino A., Huang R.H., Cacchione J. Association of race with mortality among patients hospitalized with coronavirus disease 2019 (COVID-19) at 92 US hospitals. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.18039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magesh S., John D., Li W.T., Li Y., Mattingly-App A., Jain S., Chang E.Y., Ongkeko W.M. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic-review and meta-analysis. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.34147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimani M.E., Sarr M., Cuffee Y., Liu C., Webster N.S. Associations of race/ethnicity and food insecurity with COVID-19 infection rates across US counties. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Syrowatka T., Wiadrowska B., Jurek A., Bankowska J., Bojanowska A. [Effect of zineb and maneb on thyroid function] Rocz. Panstw. Zakl. Hig. 1971;22:369–373. [PubMed] [Google Scholar]
- 17.Iuliano A.D., Brunkard J.M., Boehmer T.K., Peterson E., Adjei S., Binder A.M., Cobb S., Graff P., Hidalgo P., Panaggio M.J., Rainey J.J., Rao P., Soetebier K., Wacaster S., Ai C., Gupta V., Molinari N.M., Ritchey M.D. Trends in disease severity and health care utilization during the early omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, december 2020-january 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:146–152. doi: 10.15585/mmwr.mm7104e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adjei S., Hong K., Molinari N.M., Bull-Otterson L., Ajani U.A., Gundlapalli A.V., Harris A.M., Hsu J., Kadri S.S., Starnes J., Yeoman K., Boehmer T.K. Mortality risk among patients hospitalized primarily for COVID-19 during the omicron and delta variant pandemic periods - United States, April 2020-june 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:1182–1189. doi: 10.15585/mmwr.mm7137a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wadhera R.K., Wadhera P., Gaba P., Figueroa J.F., Joynt Maddox K.E., Yeh R.W., Shen C. Variation in COVID-19 hospitalizations and deaths across New York city boroughs. JAMA. 2020;323:2192–2195. doi: 10.1001/jama.2020.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoogenboom W.S., Fleysher R., Soby S., Mirhaji P., Mitchell W.B., Morrone K.A., Manwani D., Duong T.Q. Individuals with sickle cell disease and sickle cell trait demonstrate no increase in mortality or critical illness from COVID-19 - a fifteen hospital observational study in the Bronx, New York. Haematologica. 2021;106:3014–3016. doi: 10.3324/haematol.2021.279222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iosifescu A.L., Hoogenboom W.S., Buczek A.J., Fleysher R., Duong T.Q. New-onset and persistent neurological and psychiatric sequelae of COVID-19 compared to influenza: a retrospective cohort study in a large New York City healthcare network. Int. J. Methods Psychiatr. Res. 2022;31 doi: 10.1002/mpr.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu J.Y., Ho S.L., Buczek A., Fleysher R., Hou W., Chacko K., Duong T.Q. Clinical predictors of recovery of COVID-19 associated-abnormal liver function test 2 months after hospital discharge. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-22741-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu J.Q., Lu J.Y., Wang W., Liu Y., Buczek A., Fleysher R., Hoogenboom W.S., Zhu W., Hou W., Rodriguez C.J., Duong T.Q. Clinical predictors of acute cardiac injury and normalization of troponin after hospital discharge from COVID-19. EBioMedicine. 2022 doi: 10.1016/j.ebiom.2022.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu J.Y., Hou W., Duong T.Q. Longitudinal prediction of hospital-acquired acute kidney injury in COVID-19: a two-center study. Infection. 2022;50:109–119. doi: 10.1007/s15010-021-01646-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu J.Y., Boparai M.S., Shi C., Henninger E.M., Rangareddy M., Veeraraghavan S., Mirhaji P., Fisher M.C., Duong T.Q. Long-term outcomes of COVID-19 survivors with hospital AKI: association with time to recovery from AKI. Nephrol. Dial. Transplant. 2023 doi: 10.1093/ndt/gfad020. [DOI] [PubMed] [Google Scholar]
- 26.Hripcsak G., Duke J.D., Shah N.H., Reich C.G., Huser V., Schuemie M.J., Suchard M.A., Park R.W., Wong I.C., Rijnbeek P.R., van der Lei J., Pratt N., Norén G.N., Li Y.C., Stang P.E., Madigan D., Ryan P.B. Observational health data Sciences and Informatics (OHDSI): opportunities for observational researchers. Stud. Health Technol. Inf. 2015;216:574–578. [PMC free article] [PubMed] [Google Scholar]
- 27.Siew E.D., Ikizler T.A., Matheny M.E., Shi Y., Schildcrout J.S., Danciu I., Dwyer J.P., Srichai M., Hung A.M., Smith J.P., Peterson J.F. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin. J. Am. Soc. Nephrol. 2012;7:712–719. doi: 10.2215/CJN.10821011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu J.Y., Wilson J., Hou W., Fleysher R., Herold B.C., Herold K.C., et al. Incidence of new-onset in-hospital and persistent diabetes in COVID-19 patients: comparison with influenza. EBioMedicine. 2023;90:104487. doi: 10.1016/j.ebiom.2023.104487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu J.Y., Babatsikos I., Fisher M.C., Hou W., Duong T.Q. Longitudinal clinical profiles of hospital vs. Community-acquired acute kidney injury in COVID-19. Front. Med. 2021;8 doi: 10.3389/fmed.2021.647023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu J.Y., Buczek A., Fleysher R., Hoogenboom W.S., Hou W., Rodriguez C.J., Fisher M.C., Duong T.Q. Outcomes of hospitalized patients with COVID-19 with acute kidney injury and acute cardiac injury. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.798897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirsch J.S., Ng J.H., Ross D.W., Sharma P., Shah H.H., Barnett R.L., Hazzan A.D., Fishbane S., Jhaveri K.D., Northwell C.-R.C., Northwell Nephrology C.-R.C. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pelayo J., Lo K.B., Bhargav R., Gul F., Peterson E., DeJoy R., Iii, Salacup G.F., Albano J., Gopalakrishnan A., Azmaiparashvili Z., Patarroyo-Aponte G., Rangaswami J. Clinical characteristics and outcomes of community- and hospital-acquired acute kidney injury with COVID-19 in a US inner city hospital system. Cardiorenal Med. 2020;10:223–231. doi: 10.1159/000509182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reichlin T., Hochholzer W., Bassetti S., Steuer S., Stelzig C., Hartwiger S., Biedert S., Schaub N., Buerge C., Potocki M., Noveanu M., Breidthardt T., Twerenbold R., Winkler K., Bingisser R., Mueller C. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N. Engl. J. Med. 2009;361:858–867. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 34.Calvo-Fernandez A., Izquierdo A., Subirana I., Farre N., Vila J., Duran X., Garcia-Guimaraes M., Valdivielso S., Cabero P., Soler C., Garcia-Ribas C., Rodriguez C., Llagostera M., Mojon D., Vicente M., Sole-Gonzalez E., Sanchez-Carpintero A., Tevar C., Marrugat J., Vaquerizo B. Markers of myocardial injury in the prediction of short-term COVID-19 prognosis. Rev. Esp. Cardiol. 2021;74:576–583. doi: 10.1016/j.rec.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A., White H.D., Executive I. Group on behalf of the joint European society of cardiology/American college of cardiology/American heart association/world heart federation task force for the universal definition of myocardial. Fourth universal definition of myocardial infarction (2018) Circulation. 2018;138:e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 36.Lu J.Q., Lu J.Y., Wang W., Liu Y., Buczek A., Fleysher R., Hoogenboom W.S., Zhu W., Hou W., Rodriguez C.J., Duong T.Q. Clinical predictors of acute cardiac injury and normalization of troponin after hospital discharge from COVID-19. EBioMedicine. 2022;76 doi: 10.1016/j.ebiom.2022.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frager S.Z., Szymanski J., Schwartz J.M., Massoumi H.S., Kinkhabwala M., Wolkoff A.W. Hepatic predictors of mortality in severe acute respiratory syndrome coronavirus 2: role of initial aspartate aminotransferase/alanine aminotransferase and preexisting cirrhosis. Hepatology Communications. 2020 doi: 10.1002/hep4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu J.Y., Anand H., Frager S.Z., Hou W., Duong T.Q. Longitudinal progression of clinical variables associated with graded liver injury in COVID-19 patients. Hepatol Int. 2021;15:1018–1026. doi: 10.1007/s12072-021-10228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho S.L., Lu J.Q., Buczek A., Fleysher R., Hou W., Chacko K., Duong T.Q. 2021. Clinical Predictors of Acute Liver Injury Recovery Associated with COVID-19 Two Months after Hospital Discharge. (submitted)) [Google Scholar]
- 40.Bertolini A., van de Peppel I.P., Bodewes F., Moshage H., Fantin A., Farinati F., Fiorotto R., Jonker J.W., Strazzabosco M., Verkade H.J., Peserico G. Abnormal liver function tests in patients with COVID-19: relevance and potential pathogenesis. Hepatology. 2020;72:1864–1872. doi: 10.1002/hep.31480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du M., Yang S., Liu M., Liu J. COVID-19 and liver dysfunction: epidemiology, association and potential mechanisms. Clin Res Hepatol Gastroenterol. 2021;46 doi: 10.1016/j.clinre.2021.101793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fiore M.C., Smith S.S., Adsit R.T., Bolt D.M., Conner K.L., Bernstein S.L., Eng O.D., Lazuk D., Gonzalez A., Jorenby D.E., D'Angelo H., Kirsch J.A., Williams B., Nolan M.B., Hayes-Birchler T., Kent S., Kim H., Piasecki T.M., Slutske W.S., Lubanski S., Yu M., Suk Y., Cai Y., Kashyap N., Mathew J.P., McMahan G., Rolland B., Tindle H.A., Warren G.W., An L.C., Boyd A.D., Brunzell D.H., Carrillo V., Chen L.S., Davis J.M., Dilip D., Ellerbeck E.F., Iturrate E., Jose T., Khanna N., King A., Klass E., Newman M., Shoenbill K.A., Tong E., Tsoh J.Y., Wilson K.M., Theobald W.E., Baker T.B. The first 20 months of the COVID-19 pandemic: mortality, intubation and ICU rates among 104,590 patients hospitalized at 21 United States health systems. PLoS One. 2022;17 doi: 10.1371/journal.pone.0274571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Acosta A.M., Garg S., Pham H., Whitaker M., Anglin O., O'Halloran A., Milucky J., Patel K., Taylor C., Wortham J., Chai S.J., Kirley P.D., Alden N.B., Kawasaki B., Meek J., Yousey-Hindes K., Anderson E.J., Openo K.P., Weigel A., Monroe M.L., Ryan P., Reeg L., Kohrman A., Lynfield R., Bye E., Torres S., Salazar-Sanchez Y., Muse A., Barney G., Bennett N.M., Bushey S., Billing L., Shiltz E., Sutton M., Abdullah N., Talbot H.K., Schaffner W., Ortega J., Price A., Fry A.M., Hall A., Kim L., Havers F.P. Racial and ethnic disparities in rates of COVID-19-associated hospitalization, intensive care unit admission, and in-hospital death in the United States from March 2020 to february 2021. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.30479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tandon P., Leibner E.S., Hackett A., Maguire K., Mashriqi N., Kohli-Seth R. The third wave: comparing seasonal trends in COVID-19 patient data at a large hospital system in New York city. Crit Care Explor. 2022;4 doi: 10.1097/CCE.0000000000000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Egoryan G., Yanez-Bello M.A., Ozcekirdek E.C., Zhang Q., Poudel B., Ozen E., Trelles-Garcia D.P., Chung C.W., Ginsburg B., Friedman H.J., Rodriguez-Nava G. Clinical characteristics and outcomes of the first two waves of the COVID-19 pandemic in a community hospital: a retrospective cohort study. IJID Reg. 2022;3:1–7. doi: 10.1016/j.ijregi.2022.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouzid D., Visseaux B., Kassasseya C., Daoud A., Femy F., Hermand C., Truchot J., Beaune S., Javaud N., Peyrony O., Chauvin A., Ayar P.V., Bourg A., Riou B., Marot S., Bloom B., Cachanado M., Simon T., Freund Y., I.M.E.C.F.C. Group Comparison of patients infected with delta versus omicron COVID-19 variants presenting to paris emergency departments : a retrospective cohort study. Ann. Intern. Med. 2022 doi: 10.7326/M22-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nyberg T., Ferguson N.M., Nash S.G., Webster H.H., Flaxman S., Andrews N., Hinsley W., Bernal J.L., Kall M., Bhatt S., Blomquist P., Zaidi A., Volz E., Aziz N.A., Harman K., Funk S., Abbott S., consortium C.-G.U., Hope R., Charlett A., Chand M., Ghani A.C., Seaman S.R., Dabrera G., De Angelis D., Presanis A.M., Thelwall S. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taytard J., Prevost B., Schnuriger A., Aubertin G., Berdah L., Bitton L., Dupond-Athenor A., Thouvenin G., Nathan N., Corvol H. SARS-CoV-2 B.1.1.529 (omicron) variant causes an unprecedented surge in children hospitalizations and distinct clinical presentation compared to the SARS-CoV-2 B.1.617.2 (delta) variant. Front Pediatr. 2022;10 doi: 10.3389/fped.2022.932170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu F., Ang J.Y. COVID-19 infection in children: diagnosis and management. Curr. Infect. Dis. Rep. 2022;24:51–62. doi: 10.1007/s11908-022-00779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hobbs C.V., Martin L.M., Kim S.S., Kirmse B.M., Haynie L., McGraw S., Byers P., Taylor K.G., Patel M.M., Flannery B., Team C.C.-R. Factors associated with positive SARS-CoV-2 test results in outpatient health facilities and emergency departments among children and adolescents aged <18 Years - Mississippi, september-november 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1925–1929. doi: 10.15585/mmwr.mm6950e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamilton P., Hanumapura P., Castelino L., Henney R., Parker K., Kumar M., Murphy M., Al-Sayed T., Pinnington S., Felton T., Challiner R., Ebah L. Characteristics and outcomes of hospitalised patients with acute kidney injury and COVID-19. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fisher M., Neugarten J., Bellin E., Yunes M., Stahl L., Johns T.S., Abramowitz M.K., Levy R., Kumar N., Mokrzycki M.H., Coco M., Dominguez M., Prudhvi K., Golestaneh L. AKI in hospitalized patients with and without COVID-19: a comparison study. J. Am. Soc. Nephrol. 2020;31:2145–2157. doi: 10.1681/ASN.2020040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ouyang L., Gong Y., Zhu Y., Gong J. Association of acute kidney injury with the severity and mortality of SARS-CoV-2 infection: a meta-analysis. Am. J. Emerg. Med. 2021;43:149–157. doi: 10.1016/j.ajem.2020.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan L., Chaudhary K., Saha A., Chauhan K., Vaid A., Zhao S., Paranjpe I., Somani S., Richter F., Miotto R., Lala A., Kia A., Timsina P., Li L., Freeman R., Chen R., Narula J., Just A.C., Horowitz C., Fayad Z., Cordon-Cardo C., Schadt E., Levin M.A., Reich D.L., Fuster V., Murphy B., He J.C., Charney A.W., Bottinger E.P., Glicksberg B.S., Coca S.G., Nadkarni G.N., Mount Sinai C.I.C., Li L. AKI in hospitalized patients with COVID-19. J. Am. Soc. Nephrol. 2020 doi: 10.1681/ASN.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trabulus S., Karaca C., Balkan, Dincer M.T., Murt A., Ozcan S.G., Karaali R., Mete B., Bakir A., Kuskucu M.A., Altiparmak M.R., Tabak F., Seyahi N. Kidney function on admission predicts in-hospital mortality in COVID-19. PLoS One. 2020;15 doi: 10.1371/journal.pone.0238680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagner J., Garcia-Rodriguez V., Yu A., Dutra B., DuPont A., Cash B., Farooq A. Elevated D-dimer is associated with multiple clinical outcomes in hospitalized covid-19 patients: a retrospective cohort study. SN Compr Clin Med. 2020:1–7. doi: 10.1007/s42399-020-00627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi S., Qin M., Cai Y., Liu T., Shen B., Yang F., Cao S., Liu X., Xiang Y., Zhao Q., Huang H., Yang B., Huang C. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur. Heart J. 2020;41:2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., Gong W., Liu X., Liang J., Zhao Q., Huang H., Yang B., Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lala A., Johnson K.W., Januzzi J.L., Russak A.J., Paranjpe I., Richter F., Zhao S., Somani S., Van Vleck T., Vaid A., Chaudhry F., De Freitas J.K., Fayad Z.A., Pinney S.P., Levin M., Charney A., Bagiella E., Narula J., Glicksberg B.S., Nadkarni G., Mancini D.M., Fuster V., Mount Sinai C.I.C. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J. Am. Coll. Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prasitlumkum N., Chokesuwattanaskul R., Thongprayoon C., Bathini T., Vallabhajosyula S., Cheungpasitporn W. Incidence of myocardial injury in COVID-19-infected patients: a systematic review and meta-analysis. Diseases. 2020;8:40. doi: 10.3390/diseases8040040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., C.-R.C. and the Northwell. Barnaby D.P., Becker L.B., Chelico J.D., Cohen S.L., Cookingham J., Coppa K., Diefenbach M.A., Dominello A.J., Duer-Hefele J., Falzon L., Gitlin J., Hajizadeh N., Harvin T.G., Hirschwerk D.A., Kim E.J., Kozel Z.M., Marrast L.M., Mogavero J.N., Osorio G.A., Qiu M., Zanos T.P. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao Y.H., Zhao L., Yang X.C., Wang P. Cardiovascular complications of SARS-CoV-2 infection (COVID-19): a systematic review and meta-analysis. Rev. Cardiovasc. Med. 2021;22:159–165. doi: 10.31083/j.rcm.2021.01.238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.