Abstract
Chromoblastomycosis (CBM) is a difficult-to-treat, chronic fungal infection of the skin and subcutaneous tissue. The evidence base for treatment is scarce, with no standardized therapeutic approach. Chronicity of CBM infection is postulated to be due in part to a failure of host cell–mediated immunity to generate a proinflammatory response sufficient for fungal clearance. We present a case of a chronic chromoblastomycosis lesion of the hand present for nearly 4 decades, previously refractory to itraconazole monotherapy, that was successfully treated with a combination of posaconazole and adjunctive immunotherapy with topical imiquimod, a Toll-like receptor 7 agonist. Serial biopsies and images demonstrate the clinical and histopathological improvement of the lesion. Randomized trials of antifungal therapy with adjunctive imiquimod are warranted to determine whether a combination of antifungal and host-directed therapy improves outcomes for this neglected tropical mycosis.
Keywords: chromoblastomycosis, endemic mycosis, Fonsecaea pedrosoi, imiquimod, neglected fungal disease
Chromoblastomycosis (CBM) is a difficult-to-treat, chronic skin and subcutaneous fungal infection, recognized as a neglected tropical disease (NTD) by the World Health Organisation. Infection occurs following traumatic inoculation of melanized fungi into subcutaneous tissues, most often Fonsecaea spp. or Cladophialophora spp., which reside in soil and plant matter in (sub)tropical climates [1]. CBM largely affects outdoor, agricultural, and forestry workers; exposed areas of the skin such as the hands, feet, limbs, and face represent the most common sites of infection. Chronic lesions develop over months and years, and, while infection usually remains localized, the disfiguring lesions can significantly limit limb function and impact ability to work and psychosocial well-being. Complications include secondary bacterial infections, lymphoedema, and, rarely, transformation to squamous cell carcinoma [1, 2]. The true global burden of CBM is unknown due to a lack of epidemiological data; however, cases have been reported across Central and Southern America, Africa, the Caribbean, Asia, and Australia [3]. Depending on the site and extent of lesions, antifungal therapy and surgical excision are the mainstay of treatment, with adjunctive physical therapies occasionally used (cryotherapy, thermotherapy, light-based therapy) [1]. The evidence base for treatment is scarce, with no standardized therapeutic approach. Chronicity of CBM infection is postulated to be due in part to a failure of host cell–mediated immunity to generate a proinflammatory response sufficient for fungal clearance [1]. We present a case of a chronic chromoblastomycosis lesion of the hand present for nearly 4 decades, previously refractory to itraconazole monotherapy, that was successfully treated with a combination of posaconazole and adjunctive immunotherapy with topical imiquimod.
CASE
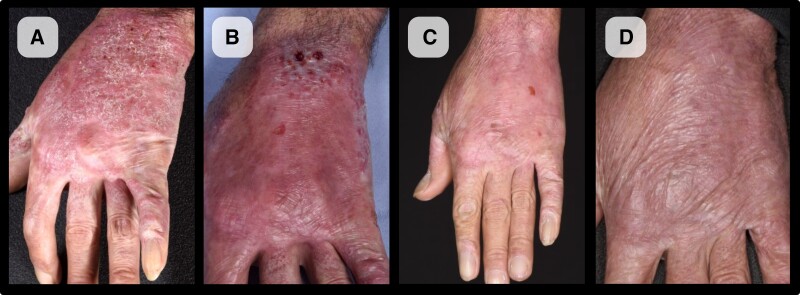
A 61-year-old forestry worker from Brazil, with no past medical history, presented with a 37-year history of a chronic lesion of the left hand. It developed at the site of a wood-cutting injury on the left little finger; over a period of 4 months following the injury, the lesion progressively spread across the dorsum of the hand, associated with pruritus and dysesthesia. Functionality of the hand was limited, with impaired finger flexion and poor grip due to sclerotic contractures. Examination revealed an erythematous, sclerotic, and verrucous plaque extending across the entire dorsal aspect of the left hand (Figure 1A).
Figure 1.
Chromoblastomycosis evolution.
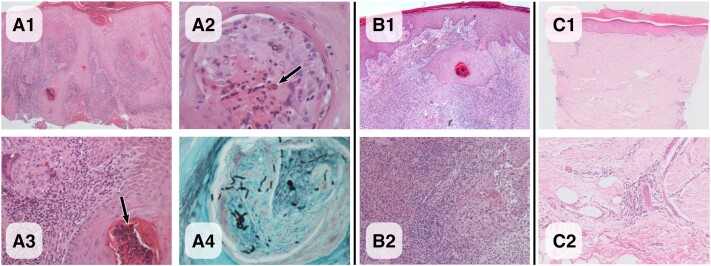
Histology (Figure 2A1–A4) revealed several pigmented muriform (sclerotic) fungal cells or “copper pennies” within dermal suppurative granulomas, pathognomonic for chromoblastomycosis. There were also several organisms within hair follicles undergoing apparent transepidermal elimination. Tissue scarring was evident, with hyperkeratosis and pseudoepilthelimatous hyperplasia and an inflammatory infiltrate containing lymphocytes, eosinophils, and plasma cells. Although rounded pigmented yeast forms were readily visualized on the hematoxylin and eosin (H&E) sections, hyphal forms were only appreciated on the PAS and Grocott stains (Figure 2A4). Fungal culture was performed on Saboroud dextrose agar for 5 days and was negative; however, agents of CBM such as Fonseacaea spp. can take up to 2 weeks to grow. In light of histology findings, a panfungal (ITS region-directed) PCR was performed, which was negative; however, this was done on formalin-fixed rather than fresh tissue, which likely reduced the sensitivity of the assay.
Figure 2.
Chromoblastomycosis histology.
Over 5 years before presentation, he had been treated in Brazil with 18 months of itraconazole, adjunctive cryotherapy, and various topical antifungal creams (unknown identity), but clinical response was poor and the lesion persisted. At our institution, he was initiated on 300-mg oral posaconazole tablets daily and had a therapeutic trough level (1.03 mg/mL) when checked following 3 months of treatment. There were no adverse effects with prolonged therapy. Adjunctive daily treatment with topical 5% imiquimod cream was applied to the lesion for the first 12 months of antifungal therapy.
Figures 1 and 2 illustrate the evolution of the clinical appearance of the lesion with treatment, alongside corresponding histology of serial biopsies performed at baseline, 5 months, and 12 months to assess treatment response.
On review at 6 weeks post-treatment, plaques had reduced but his skin was erythematous; this inflammatory reaction persisted at 5 months, with the corresponding biopsy demonstrating a more pronounced inflammatory infiltrate, as would be expected as a result of imiquimod treatment (Figures 1B and 2B1–B2), which acts by potentiating the inflammatory response. This settled, and by 12 months there was significant improvement and healing of the lesion, with reduced erythema and thickness of the plaque and improved finger flexion (Figure 1C). Biopsy at 12 months (Figure 2C1–2) demonstrated minimal inflammation, no fungal cells were visualized on H&E and Grocott stains, and fungal cultures remained negative.
At 1 year post–completion of treatment, there was no clinical evidence of relapse, and hand function (finger flexion and grip) was much improved. Given the risk of squamous cell carcinoma due to chronic inflammation and the pseudoepithelial hyperplasia visualized on biopsy, the patient remains under clinical review.
DISCUSSION
As an NTD, the antifungal drug of choice for CBM should be affordable and well tolerated, with good oral bioavailability and subcutaneous tissue penetration to allow for prolonged treatment in outpatient settings. The mold-active triazoles (itraconazole, posaconazole, voriconazole, isavuconazole) and terbinafine have good in vitro activity against Fonsecaea spp. and Cladophialophora spp. [4]. Itraconazole is often used as first-line antifungal therapy based on wide availability and evidence from a small number of observational cohorts, many of which were performed 2 decades ago when itraconazole and terbinafine were the most readily available oral antifungals with activity against fungi causing CBM [1, 5–9]. However, cure rates with this regimen are unsatisfactory (∼20%–70%), and relapses are common [5–9]. We do not know whether therapeutic drug monitoring (TDM) was performed during this patient's itraconazole treatment in Brazil; it may be that prior treatment failure was contributed to by poor oral bioavailability and hence inadequate tissue levels of itraconazole. TDM is important to ensure that therapeutic drug levels are achieved but in many settings is unavailable. The evidence base for the use of posaconazole in the treatment of CBM is extremely limited [10–12]; thus, it is considered a second-line treatment option when other therapy has failed [13]. We chose posaconazole because of its predictable oral bioavailability, tolerability, and adequate penetration into the human dermis [14]. However, in many resource-limited CBM-endemic areas, access to second-line options is limited by their expense; this may improve as generic versions become available. Advocacy for access to a wider range of antifungals and for antifungal TDM is an important step to facilitate management of CBM (and other fungal infections) in low- and middle-income settings.
Imiquimod was used as adjunctive treatment based on a handful of published cases reporting success with this agent. The rationale behind use derives from the concept that the chronicity of CBM is at least partly due to a failure of local host cell–mediated immunity [1]. Compared with those with mild disease, patients with severe CBM appear to have a T helper-2-skewed cellular immune response to F. pedrosoi, with high interleukin-10 and low interferon-gamma production [15]. Imiquimod, licensed for the treatment of genital warts, basal cell carcinoma, and actinic keratosis, is a potent TLR7 agonist, which stimulates a Th-1-weighted cellular immune response [16]. A murine F. pedrosoi infection model demonstrated that macrophages recognize the fungus via the pattern recognition receptor (PRR) C-type lectin (CLR), but not Toll-like receptors (TLRs) [17]: Recognition by CLR alone was insufficient to induce an adequate inflammatory response to clear the fungi. However, when co-stimulation of both pattern recognition receptors CLR and TLR was achieved in the murine model using the TLR7 ligand imiquimod, a robust proinflammatory response was induced, which significantly improved fungal clearance. A similar phenomenon likely occurred in our patient, as evidenced by a flare of local inflammation early in treatment. While 2 small case series [18, 19] have demonstrated improvement in the clinical and histopathological appearance of lesions in patients treated with imiquimod (6–19 months) with and without itraconazole, ours is the first case to report clinical cure with the combination of posaconazole and imiquimod, with no evidence of relapse 18 months after cessation of therapy.
This case illustrates the principle of targeting both pathogen and host in the management of a difficult-to-treat, chronic mycosis. Improving access to affordable and effective triazoles and therapeutic drug monitoring in resource-limited settings is a key public health challenge in the treatment of chromoblastomycosis. Delineating the effect of posaconazole, a triazole with good oral bioavailability, from imiquimod was not possible in this case. For lesions refractory to antifungal therapy and/or not suitable for surgical excision, randomized trials of posaconazole therapy, with and without adjunctive imiquimod, are warranted to determine whether a combination of antifungal and host-directed therapy improves outcomes for this neglected tropical mycosis.
Acknowledgments
We would like to thank the patient who gave informed consent to publish their case and accompanying images.
Financial support . None
Ethical statement. Written consent was obtained. The case details the routine clinical care of the patient, and ethics committee approval was not required.
Patient consent. The patient provided written consent for the case and images to be published.
Contributor Information
Clare Logan, Clinical Infection Unit, St George’s University Hospitals NHS Foundation Trust, London, United Kingdom; Institute of Infection & Immunity, St Georges University London, London, United Kingdom.
Manuraj Singh, Department of Cellular Pathology, St George’s University Hospitals NHS Foundation Trust, London, United Kingdom.
Natalya Fox, Department of Dermatology, St George’s University Hospitals NHS Foundation Trust, London, United Kingdom.
Gordon Brown, MRC Centre for Medical Mycology, University of Exeter, Exeter, United Kingdom.
Sreedhar Krishna, Department of Dermatology, St George’s University Hospitals NHS Foundation Trust, London, United Kingdom.
Kristiana Gordon, Department of Dermatology, St George’s University Hospitals NHS Foundation Trust, London, United Kingdom.
Derek Macallan, Clinical Infection Unit, St George’s University Hospitals NHS Foundation Trust, London, United Kingdom; Institute of Infection & Immunity, St Georges University London, London, United Kingdom.
Tihana Bicanic, Clinical Infection Unit, St George’s University Hospitals NHS Foundation Trust, London, United Kingdom; Institute of Infection & Immunity, St Georges University London, London, United Kingdom; MRC Centre for Medical Mycology, University of Exeter, Exeter, United Kingdom.
References
- 1. Queiroz-Telles F, de Hoog S, Santos DWCL, et al. Chromoblastomycosis. Clin Microbiol Rev 2017; 30:233–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol 2018; 93:495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Santos DWCL, de Azevedo CMPES, Vicente VA, et al. The global burden of chromoblastomycosis. PLoS Negl Trop Dis 2021; 15:e0009611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. da Silva Hellwig AH, Heidrich D, Zanette RA, Scroferneker ML. In vitro susceptibility of chromoblastomycosis agents to antifungal drugs: a systematic review. J Glob Antimicrob Resist 2019; 16:108–14. [DOI] [PubMed] [Google Scholar]
- 5. Bonifaz A, Carrasco-Gerard E, Saúl A. Chromoblastomycosis: clinical and mycologic experience of 51 cases. Mycoses 2001; 44:1–7. [DOI] [PubMed] [Google Scholar]
- 6. Queiroz-Telles F, Purim KS, Fillus JN, Bordignon GF, Lameira RP, Van Cutsem JCG. Itraconazole in the treatment of chromoblastomycosis due to Fonsecaea pedrosoi. Int J Dermatol 1992; 31:805–12. [DOI] [PubMed] [Google Scholar]
- 7. Bonifaz A, Martínez-Soto E, Carrasco-Gerard E, Peniche J. Treatment of chromoblastomycosis with itraconazole, cryosurgery, and a combination of both. Int J Dermatol 1997; 36:542–7. [DOI] [PubMed] [Google Scholar]
- 8. Ranawaka RR, Amarasinghe N, Hewage D. Chromoblastomycosis: combined treatment with pulsed itraconazole therapy and liquid nitrogen cryotherapy. Int J Dermatol 2009; 48:397–400. [DOI] [PubMed] [Google Scholar]
- 9. Restrepo A, Gonzalez A, Gomez I, Arango MBC. Treatment of chromoblastomycosis with itraconazole. Ann N Y Acad Sci 1988; 544:504–16. [DOI] [PubMed] [Google Scholar]
- 10. Calvo E, Pastor FJ, Salas V, Mayayo E, Capilla J, Guarro J. Histopathology and antifungal treatment of experimental murine chromoblastomycosis caused by Cladophialophora carrionii. J Antimicrob Chemother 2012; 67:666–70. [DOI] [PubMed] [Google Scholar]
- 11. Negroni R, Tobón A, Bustamante B, Shikanai-Yasuda MA, Patino H, Restrepo A. Posaconazole treatment of refractory eumycetoma and chromoblastomycosis. Rev Inst Med Trop Sao Paulo 2005; 47:339–46. [DOI] [PubMed] [Google Scholar]
- 12. Dupont C, Duong TA, Mallet S, et al. Unusual presentation of chromoblastomycosis due to Cladophialophora carrionii in a renal and pancreas transplant recipient patient successfully treated with posaconazole and surgical excision. Transpl Infect Dis 2010; 12:180–3. [DOI] [PubMed] [Google Scholar]
- 13. Chowdhary A, Meis JF, Guarro J, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis: diseases caused by black fungi. Clin Microbiol Infect 2014; 20:47–75. [DOI] [PubMed] [Google Scholar]
- 14. Felton T, Troke PF, Hope WW. Tissue penetration of antifungal agents. Clin Microbiol Rev 2014; 27:68–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fávero Gimenes VM, De Souza MDG, Ferreira KS, et al. Cytokines and lymphocyte proliferation in patients with different clinical forms of chromoblastomycosis. Microbes Infect 2005; 7:708–13. [DOI] [PubMed] [Google Scholar]
- 16. Schon M, Schon M. The antitumoral mode of action of imiquimod and other imidazoquinolines. Curr Med Chem 2007; 14:681–7. [DOI] [PubMed] [Google Scholar]
- 17. Sousa MG, Reid DM, Schweighoffer E, et al. Restoration of pattern recognition receptor costimulation to treat chromoblastomycosis, a chronic fungal infection of the skin. Cell Host Microbe 2011; 9:436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Sousa MGT, Belda W, Spina R, et al. Topical application of imiquimod as a treatment for chromoblastomycosis. Clin Infect Dis 2014; 58:1734–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Belda W, Criado PR, Passero LFD. Successful treatment of chromoblastomycosis caused by Fonsecaea pedrosoi using imiquimod. J Dermatol 2020; 47:409–12. [DOI] [PubMed] [Google Scholar]