Abstract
Background
Vaccination reduces mortality from infectious disease, which is the leading cause of death in children under 5 and bears a particularly high burden in low- and middle-income countries. The Global Vaccine Action Plan (2011–2020) has set a target of 90% vaccine coverage for all vaccines included in national immunization programs by 2020. The objectives of this study were to estimate vaccine coverage among children in Madagascar, Cambodia, and Senegal and to identify the risk factors associated with incomplete vaccination.
Methods
Using data from a community-based prospective cohort that included all newborn of some areas from 2012 to 2018 in these 3 countries, vaccine coverage was estimated for BCG, hepatitis B, oral polio, pentavalent (targeting diphtheria, tetanus, pertussis, hepatitis B, and Haemophilus influenzae type b), and measles vaccines. Risk factor analysis was performed with logistic regression models to identify correlates of incomplete vaccination.
Results
A total of 3606 children were followed up, and vaccine coverage was below the 90% threshold for most vaccines in all countries. Coverage was higher for vaccines recommended at birth and at 6 weeks, while a decrease in coverage for subsequent doses was observed for vaccines requiring several doses (23–47 points). Low birth weight (<2500 g) was an important risk factor for nonvaccination for vaccines recommended at birth in all 3 countries (adjusted odds ratio [95% confidence interval] ranging from 1.93 [1.11–3.38] to 4.28 [1.85–9.37]).
Conclusions
Vaccine coverage for common childhood vaccines was lower than World Health Organization recommendations, and multidisciplinary approaches may help to improve vaccine coverage and timeliness.
Keywords: Cambodia, Madagascar, risk factors, Senegal, vaccine coverage
Among the 3606 children followed up from a community-based cohort in Cambodia, Madagascar, and Senegal, vaccine coverage was below World Health Organization recommendations. Low birth weight was an important risk factor for nonvaccination for vaccines recommended at birth.
Introduction
Despite the 3 million deaths prevented each year by childhood vaccination [1], the burden of vaccine-preventable diseases is still high in low- and middle-income countries (LMICs) [2]. A recent measles outbreak in Madagascar, with >100 000 reported cases and nearly 1000 deaths, highlights ongoing vulnerability to infectious diseases for which effective and widely available vaccines exist [3]. The Global Vaccine Action Plan (2011–2020) has defined vaccine coverage (VC) targets to be reached by 2020 [4]: vaccines included in national programs should reach 90% VC nationally, and 80% VC in every district.
The main sources of VC data in LMICs are national reports, Demographic and Health Surveys (DHSs), and Multiple Indicator Cluster Surveys (MICSs). National reports are typically based on data collected from healthcare facilities, thus missing children whose parents do not seek care. DHSs and MICSs are based on cluster surveys designed to be representative of the national population, but the sampling might not be exhaustive for each selected cluster. All of these data sources have some limitations [5], justifying different approaches to estimate VC, in relation to 2020 targets.
It is also necessary to identify the determinants associated with noncompletion of vaccine schedules to inform and optimize interventions. Several studies, mostly based on DHS, have examined these determinants [6–9] and found common factors associated with nonvaccination, for example, low household wealth, low parental education, lower maternal age at delivery, or distance from health facility. However, they did not investigate risk factors on a vaccine-by-vaccine basis, and some factors may be specific to the vaccine delivered at birth.
The goal of this study was to estimate VC of vaccines included in national immunization programs among children in 3 LMICs (Cambodia, Madagascar, and Senegal), and to identify risk factors associated with incomplete immunization.
METHODS
Study Design
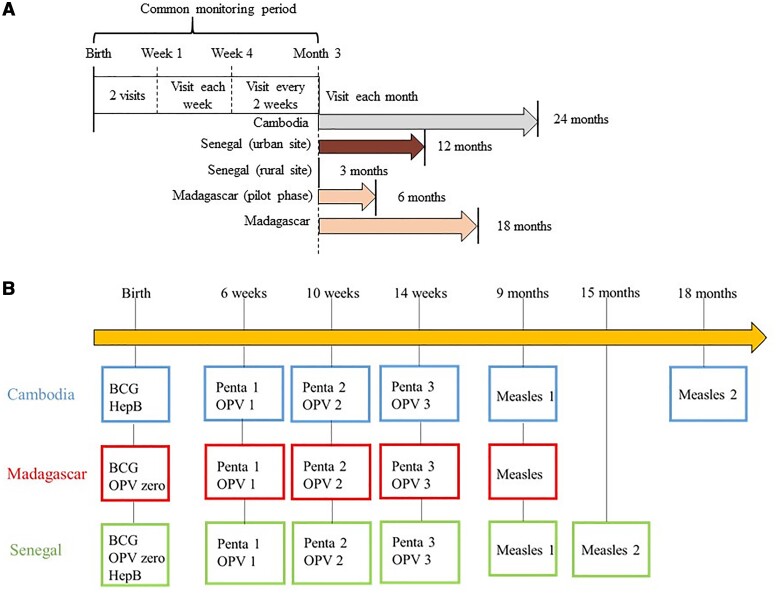
This study is based on data from the BIRDY (Bacterial Infections and Antibiotic-Resistant Diseases Among Young Children in Low-Income Countries) cohort [10]. Details of the study design have been described elsewhere [11]. In brief, this cohort took place in Madagascar (2012–2018), Cambodia (2014–2018), and Senegal (2014–2018), across both an urban site and a rural site in each country. All children were included at birth and followed up during home visits up to the maximum duration of follow-up. For financial and logistical reasons, duration of follow-up was country specific: in Madagascar, 6 months during the pilot phase (2012–2014) and 18 months thereafter (2014–2018); in Cambodia, 24 months; and, in Senegal, 3 months in the rural site and 1 year in the urban site (Figure 1A). At each home visit, the birth parent was asked if the child had received a vaccine since the last visit. Then, the type of vaccine and the date of immunization were checked in the child’s vaccination card.
Figure 1.
Study design of the Bacterial Infections and Antibiotic-Resistant Diseases Among Young Children in Low-Income Countries (BIRDY) cohort and national immunization programs in Cambodia, Madagascar, and Senegal. A, Schedule of home visits of the BIRDY cohort by country and site. B, Vaccines recommended in the national immunization programs of Cambodia, Madagascar, and Senegal and included in the analysis. (Other vaccines are recommended in these countries, but not covered in this study.) A dose of hepatitis B vaccine is recommended at birth, within the first 24 hours, in Cambodia and Senegal but not in Madagascar. One dose of oral polio vaccine is recommended at birth, within the first 14 days, in Senegal and Madagascar only. A second dose of measles vaccine is recommended at 15 months in Senegal and 18 months in Cambodia, whereas in Madagascar, only 1 dose is recommended. Abbreviations: HepB, hepatitis B vaccine; OPV zero, oral polio vaccine at birth; OPV 1, oral polio vaccine recommended at 6 weeks; OPV 2, oral polio vaccine recommended at 10 weeks; OPV 3, oral polio vaccine recommended at 14 weeks; Penta, pentavalent vaccine (diphtheria, tetanus, pertussis, Haemophilus influenzae type b, hepatitis B).
Vaccines schedules were defined as per the Expanded Programme on Immunization, which varied slightly across countries (Figure 1B). Vaccines considered for VC estimation were as follows:
At birth: hepatitis B vaccine <24 hours from birth, oral polio vaccine (OPV) <14 days from birth, and BCG vaccine <14 days from birth.
After birth: pentavalent vaccine (targeting diphtheria, tetanus, whooping cough, hepatitis B, and Haemophilus influenzae type b), OPV (3 doses for Cambodia and Madagascar and 2 doses for Senegal, due to the different duration of follow-up in each country), and measles vaccine (1 dose). As a catch-up is recommended during the first year of life for BCG, we also considered BCG VC at 12 months.
Some vaccines included in the national program were introduced during the study, such as rotavirus and pneumococcal vaccines, and some were country specific (yellow fever and Japanese encephalitis vaccines in Senegal and Cambodia, respectively). Data on these vaccines were thus not collected in this study. VC was assessed for each dose of each vaccine at each site for as long as follow-up at that site allowed. Details of the study populations assessed, combining the local vaccine schedule and length of follow-up at each site, are presented in Supplementary Table 1. To calculate VC for each dose, the proportion of children vaccinated was calculated with its 95% confidence interval (CI). For each estimate, the numerator was taken as the number of children vaccinated with that particular dose given a corresponding minimum duration of follow-up (defined in Supplementary Table 1), and the denominator as the total number of children still followed up for that duration.
Statistical Analysis
Comparisons were made between countries and sites using the χ2 test (or Fisher exact test) for qualitative variables and the Student's t-test (or nonparametric Mann-Whitney test) for quantitative variables. The tests were 2-tailed, and the significance threshold was set at .05. The median was used to categorize the quantitative variables. Low birth weight (LBW) was defined as <2500 g. Due to different distributions across countries, low education was defined as none or primary in Cambodia and Madagascar and none in Senegal.
Vaccines considered as outcomes for risk factors of incomplete vaccination were as follows:
At birth: absence of hepatitis B vaccine <24 hours, absence of OPV <14 days, and absence of BCG vaccine <14 days. Hepatitis B vaccine was not considered for Senegal as this vaccine was only recommended in 2016, thus limiting sample size.
After birth: <2 doses of pentavalent vaccine and <2 doses of OPV among children followed up for at least 4 months. The OPV dose at birth was not considered here in the calculation of doses for Madagascar and Senegal. Only OPV doses at 6 and 10 weeks were considered. In the rural site in Senegal, vaccines recommended after birth were not considered due to insufficient follow-up.
Logistic regression models were used to identify factors associated with incomplete immunization, with separate models considered for each country.
Explanatory variables included in regression models were sociodemographic characteristics, pregnancy history, and child characteristics. We considered LBW as a proxy of prematurity. Indeed, in a perspective of an intervention, the measurement of birth weight is more accessible whereas the estimation of the gestational age is very challenging in LMIC settings. After univariate analysis across all variables, a manual backward stepwise procedure was used to determine which variables to include in multivariate analysis, using an inclusion threshold P = .2 and a significance threshold P = .05. The sex of the child and the site were forced in all multivariate analysis. Some variables were correlated (birth parent age and parity; birth parent education and occupation) and to avoid multi-collinearity, the variable with the strongest association with the outcome was kept in the model.
Birth weight was missing for some children, so 2 sensitivity analyses were performed. First, all children without birth weight were assumed to have LBW; second, these children were assumed to have normal birth weight.
All analyses were carried out in R software (version 3.6.1).
Patient Consent Statement
The study was approved by the ethics committees of Madagascar (036-MSANP/CE and 068-MSANP/CE), Senegal (SEN 14–20), and Cambodia (108 NEHCR) and the Institutional Review Board of Institut Pasteur, France (IRB/2016/08/03). All parents or guardians of the participants gave written informed consent.
RESULTS
Study Population
Overall, 3693 infants were born in the time frame of the BIRDY cohort across Cambodia, Madagascar, and Senegal. Of these, 3606 were followed up at least once during the first week of life (see Supplementary Figure 1 for details).
Birth parent came more often from rural areas in Madagascar (60.9%) and Cambodia (54.7%), and more often from the urban site in Senegal (59.7%) (P < .001; Table 1). Respectively, 73.5%, 54.1%, and 24.3% of birth parents in Senegal, Cambodia, and Madagascar did not go to school or only attended primary school (P < .001). In Cambodia, 3.9% of the newborns had LBW. This proportion was 10.1% in Madagascar and 7.7% in Senegal (P < .001).
Table 1.
Characteristics of Included Children and Their Birth Parent by Country and Site, 2012–2018
| Characteristic | Cambodia | Madagascar | Senegal | P Valueb | |||
|---|---|---|---|---|---|---|---|
| Birth Parenta | (n = 786) | (n = 2055) | (n = 725) | ||||
| Rural (n = 430) |
Urban (n = 356) |
Rural (n = 1251) |
Urban (n = 804) |
Rural (n = 292) |
Urban (n = 433) |
||
| Education | <.001 | ||||||
| None | 20 (4.7) | 34 (9.6) | 25 (2.0) | 8 (1.0) | 87 (29.8) | 171 (39.5) | |
| Primary | 216 (50.2) | 155 (43.5) | 287 (22.9) | 179 (22.3) | 110 (37.7) | 165 (38.1) | |
| Secondary or university | 194 (45.1) | 167 (46.9) | 939 (75.1) | 617 (76.7) | 95 (32.5) | 97 (22.4) | |
| Occupation | <.001 | ||||||
| Unemployed or student | 102 (23.7) | 171 (48.0) | 949 (75.9) | 468 (58.2) | 238 (81.5) | 317 (73.2) | |
| Employed | 328 (76.3) | 185 (52.0) | 302 (24.1) | 336 (41.8) | 54 (18.5) | 116 (26.8) | |
| No. of previous live-born children | <.001 | ||||||
| 0 | 202 (47.0) | 130 (36.5) | 419 (33.5) | 345 (42.9) | 75 (25.7) | 117 (27.0) | |
| ≥1 | 228 (53.0) | 226 (63.5) | 832 (66.5) | 459 (57.1) | 217 (74.3) | 316 (73.0) | |
| No. of prenatal visits | <.001 | ||||||
| <4 | 91 (21.2) | 153 (43.0) | 553 (44.2) | 418 (52.0) | 230 (78.8) | 285 (65.8) | |
| ≥4 | 301 (70.0) | 202 (56.7) | 698 (55.8) | 386 (48.0) | 55 (18.8) | 146 (33.7) | |
| Missing data | 38 (8.8) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 7 (2.4) | 2 (0.5) | |
| Age at delivery, y, mean (SD) | 27.1 (5.0) | 27.0 (5.9) | 25.8 (6.4) | 25.4 (6.6) | 27.7 (6.8) | 27.8 (6.3) | <.001 |
| Place of delivery | <.001 | ||||||
| Healthcare structure | 420 (97.7) | 355 (99.7) | 643 (51.4) | 599 (74.5) | 286 (97.9) | 421 (98.8) | |
| Home | 10 (2.3) | 1 (0.3) | 601 (48.0) | 202 (25.1) | 6 (2.1) | 5 (1.2) | |
| Missing data | 0 (0.0) | 0 (0.0) | 7 (0.6) | 3 (0.4) | 0 (0.0) | 7 (1.6) | |
| Cesarean delivery | <.001 | ||||||
| No | 383 (89.1) | 304 (85.4) | 1182 (94.5) | 686 (85.3) | 280 (95.9) | 416 (96.1) | |
| Yes | 43 (10.0) | 52 (14.6) | 69 (5.5) | 118 (14.7) | 8 (2.7) | 11 (2.5) | |
| Missing data | 4 (0.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (1.4) | 6 (1.4) | |
| Neonatesc | (n = 789) | (n = 2078) | (n = 739) | ||||
| Rural (n = 432) |
Urban (n = 357) |
Rural (n = 1264) |
Urban (n = 814) |
Rural (n = 297) |
Urban (n = 442) |
||
| Weight at delivery, g, mean (SD) | 3075 (429.7) | 3149 (438.4) | 3007 (448.1) | 2929 (476.2) | 3092 (534.0) | 3022 (423.4) | <.001 |
| Weight <2500 g at delivery | <.001 | ||||||
| No | 409 (94.7) | 343 (96.1) | 1140 (90.2) | 691 (84.9) | 256 (86.2) | 324 (73.3) | |
| Yes | 18 (4.2) | 13 (3.6) | 97 (7.7) | 112 (13.8) | 30 (10.1) | 27 (6.1) | |
| Missing data | 5 (1.1) | 1 (0.3) | 27 (2.1) | 11 (1.3) | 11 (3.7) | 91 (20.6) | |
| Pretermd | <.001 | ||||||
| No | 407 (94.2) | 349 (97.8) | 1096 (86.7) | 650 (79.9) | 265 (89.2) | 387 (87.6) | |
| Yes | 23 (5.3) | 8 (2.2) | 158 (12.5) | 159 (19.5) | 8 (2.7) | 22 (5.0) | |
| Missing data | 2 (0.5) | 0 (0.0) | 10 (0.8) | 5 (0.6) | 24 (8.1) | 33 (7.5) | |
| Sex | .4 | ||||||
| Male | 205 (47.5) | 170 (47.6) | 622 (49.2) | 431 (52.9) | 137 (46.1) | 230 (52.0) | |
| Female | 223 (51.6) | 187 (52.4) | 642 (50.8) | 383 (47.1) | 159 (53.6) | 207 (46.9) | |
| Missing data | 4 (0.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 5 (1.1) | |
Data are presented as No. (%) unless otherwise indicated.
Abbreviation: SD, standard deviation.
Total = 3566 birth parent, including 38 who had twin pregnancies and 1 who had a triplet pregnancy.
Comparison between countries.
Total = 3606 children.
Birth before 37 weeks‘ gestation.
Vaccine Coverage
Vaccines Recommended at Birth
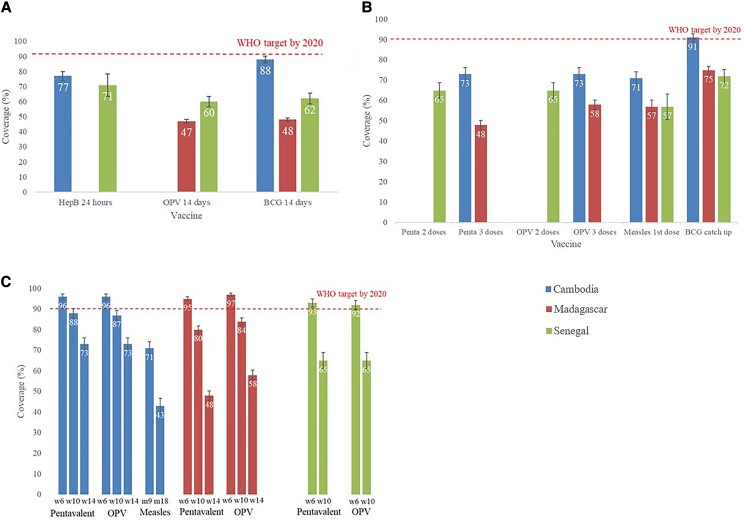
A complete list of VC estimates is provided in Supplementary Table 2. The hepatitis B vaccine was administered at birth in 77% (95% CI, 74%–80%) of children in Cambodia and 71% (95% CI, 63%–78%) in Senegal (Figure 2A). For OPV, 47% (95% CI, 45%–49%) of children in Madagascar and 60% (95% CI, 57%–64%) in Senegal received a dose at birth. The BCG vaccine was administered in the first 14 days to 88% (95% CI, 86%–91%) of children in Cambodia, 48% (95% CI, 46%–50%) in Madagascar, and 62% (95% CI, 58%–65%) in Senegal.
Figure 2.
Vaccination coverage among children in Cambodia, Madagascar, and Senegal, 2012–2018. A, Vaccination coverage of BCG, oral polio, and hepatitis B vaccines at birth among children in Cambodia, Madagascar, and Senegal, 2012–2018. B, Vaccination coverage of catch-up BCG, pentavalent (diphtheria, tetanus, pertussis, Haemophilus influenzae type b, hepatitis B) (3 or 2 doses), oral polio (3 or 2 doses), and measles vaccines among children in Cambodia, Madagascar, and Senegal, 2012–2018. C, Vaccination coverage of different recommended doses of pentavalent, oral polio, and measles vaccines in Cambodia, Madagascar, and Senegal, 2012–2018. Abbreviations: BCG 14 days, BCG vaccine within the first 14 days; BCG catch-up, BCG vaccine within the first year; HepB 24 hours, hepatitis B vaccine within 24 hours after birth; Measles m9, measles vaccine recommended at 9 months; OPV 14 days, oral polio vaccine within the first 14 days from birth; OPV w6, oral polio vaccine recommended at 6 weeks; OPV 2 doses, oral polio vaccine 2 doses recommended at 6 and 10 weeks; OPV 3 doses, oral polio vaccine 3 doses recommended at 6, 10, and 14 weeks; Pentavalent, pentavalent vaccine (diphtheria, tetanus, pertussis, Haemophilus influenzae type b, hepatitis B); Penta 2 doses, pentavalent vaccine 2 doses recommended at 6 and 10 weeks; Penta 3 doses, pentavalent vaccine 3 doses recommended at 6, 10, and 14 weeks; Pentavalent w6, pentavalent vaccine recommended at 6 weeks; WHO, World Health Organization.
Vaccines Recommended After Birth
The catch-up dose of BCG vaccine at 1 year had the highest VC among vaccines recommended after birth: 91% (95% CI, 89%–93%) in Cambodia, 75% (95% CI, 73%–77%) in Madagascar, and 72% (95% CI, 69%–75%) in Senegal (Figure 2B). VC for all other such vaccines was <80% (Figure 2B). For the pentavalent vaccine, VC was 73% (95% CI, 70%–76%) in Cambodia and 48% (95% CI, 46%–50%) in Madagascar for doses at 6, 10, and 14 weeks, and 65% (95% CI, 61%–69%) in Senegal for doses at 6 and 10 weeks. For OPV, 73% (95% CI, 70%–76%) of children in Cambodia and 58% (95% CI, 56%–60%) in Madagascar received all doses of oral vaccine recommended at 6, 10, and 14 weeks, while 65% (95% CI, 61%–69%) of children in Senegal received doses at 6 and 10 weeks. For measles vaccine, 71% (95% CI, 68%–75%) of children in Cambodia, 57% (95% CI, 54%–60%) in Madagascar, and 57% (95% CI, 50%–63%) in Senegal had received the first dose.
Coverage Evolution of Pentavalent, Oral Polio, and Measles Vaccine Doses
For vaccine schedules with multiple doses, a decrease in VC was observed with later dose number for all vaccines (Figure 2C). In Cambodia, VC for pentavalent vaccine decreased from 96% (95% CI, 94%–97%) for the first dose to 73% (95% CI, 70%–76%) for the third dose. Similar declines were observed for OPV, while measles VC dropped from 71% (95% CI, 67%–74%) for the first dose to 43% (95% CI, 39%–47%) for the second dose. In Madagascar, VC for the pentavalent vaccine decreased from 95% (95% CI, 94%–96%) for the first dose to 48% (95% CI, 46%–50%) for the third dose and dropped from 97% (95% CI, 96%–98%) to 58% (95% CI, 56%–60%) for OPV. In Senegal, pentavalent VC decreased from 93% (95% CI, 91%–95%) for the first dose to 65% (95% CI, 61%–69%) for the second dose, and for OPV fell from 92% (95% CI, 89%–94%) to 65% (95% CI, 61%–69%).
Risk Factors for Incomplete Vaccination
Vaccines Recommended at Birth
Low birth weight was associated with lack of BCG vaccination in Cambodia (adjusted odds ratio [aOR], 4.28 [95% CI, 1.85–9.37]), Madagascar (aOR, 2.40 [95% CI, 1.75–3.32]), and Senegal (aOR, 1.93 [95% CI, 1.11–3.38]) (Table 2). Results were similar in Cambodia for hepatitis B vaccine (aOR, 3.48 [95% CI, 1.66–7.31]; Supplementary Table 3), as well as for OPV at birth in Madagascar (aOR, 2.38 [95% CI, 1.73–3.30]; Supplementary Table 4) and Senegal (aOR, 2.52 [95% CI, 1.44–4.45]; Supplementary Table 4). Sensitivity analyses accounting for missing birth weight data yielded similar results, regardless of the scenario considered (Supplementary Tables 5 and 6).
Table 2.
Risk Factors Associated With Incomplete Vaccination for BCG Vaccine Within the First 14 Days From Birth in Cambodia, Madagascar, and Senegal
| Risk Factor | Cambodia (n = 789) | Madagascar (n = 2078) | Senegal (n = 739) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||||
| OR (95% CI) | P Value | aOR (95% CI) | P Value | OR (95% CI) | P Value | aOR (95% CI) | P Value | OR (95% CI) | P Value | aOR (95% CI) | P Value | |
| Sex | ||||||||||||
| Male (Ref) | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Female | 1.14 (.73–1.78) | .57 | 1.10 (.70–1.74) | .67 | 1.06 (.89–1.26) | .52 | 1.04 (.87–1.25) | .66 | 0.95 (.70–1.28) | .73 | 0.93 (.67–1.29) | .66 |
| Site | ||||||||||||
| Urban (Ref) | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Rural | 0.51 (.33–.80) | .003 | 0.46 (.29–.72) | .001 | 1.16 (.98–1.39) | .09 | 1.03 (.85–1.25) | .78 | 0.84 (.62–1.14) | .26 | 0.76 (.54–1.06) | .11 |
| Education | ||||||||||||
| Low education (Ref) | 1 | … | 1 | 1 | 1 | … | ||||||
| High education | 0.94 (.60–1.45) | .77 | … | 0.47 (.38–.58) | <.001 | 0.54 (.44–.68) | <.001 | 0.78 (.55–1.09) | .15 | … | ||
| No. of previous live-born children | ||||||||||||
| 0 (Ref) | 1 | … | 1 | … | 1 | … | ||||||
| ≥1 | 0.63 (.41–.98) | .04 | … | 1.37 (1.15–1.64) | .001 | … | 0.97 (.70–1.36) | .87 | … | |||
| No. of prenatal visits | ||||||||||||
| <4 (Ref) | 1 | … | 1 | 1 | 1 | 1 | ||||||
| ≥4 | 0.63 (.40–.99) | .04 | … | 0.51 (.43–.61) | <.001 | 0.63 (.52–.76) | <.001 | 0.65 (.46–.92) | .02 | 0.61 (.41–.88) | .01 | |
| Place of delivery | ||||||||||||
| Healthcare structure (Ref) | … | … | 1 | 1 | … | … | ||||||
| Home | … | … | 2.54 (2.12–3.05) | <.001 | 2.28 (1.87–2.78) | <.001 | … | … | ||||
| Cesarean delivery | ||||||||||||
| No (Ref) | 1 | … | 1 | … | 1 | … | ||||||
| Yes | 0.91 (.43–1.75) | .79 | … | 0.69 (.51–.92) | .01 | … | 3.15 (1.35–7.92) | .01 | … | |||
| Weight <2500 g at delivery | ||||||||||||
| No (Ref) | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Yes | 4.06 (1.77–8.73) | <.001 | 4.28 (1.85–9.37) | <.001 | 2.25 (1.66–3.08) | <.001 | 2.40 (1.75–3.32) | <.001 | 1.77 (1.02–3.06) | .04 | 1.93 (1.11–3.38) | .02 |
Sex of child and site were forced in all multivariate analyses.
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio; Ref, reference.
BCG uptake was higher among children whose birth parents attended ≥4 visits during pregnancy in Madagascar (aOR, 0.63 [95% CI, .52–.76]) and Senegal (aOR, 0.61 [95% CI, .41–.88]). Similar results were found for OPV at birth in Madagascar (aOR, 0.61 [95% CI, .51–.74]) and Senegal (aOR, 0.60 [95% CI, .41–.87]) (Supplementary Table 4).
In Madagascar, children whose birth parents delivered at home were more at risk of not being vaccinated with BCG than those whose birth parents delivered in healthcare facilities (aOR, 2.28 [95% CI, 1.87–2.78]). We found similar results for OPV at birth in Madagascar (aOR, 2.12 [95% CI, 1.74–2.58]; Supplementary Table 4).
Vaccines Recommended After Birth
Similar risk factors for incomplete vaccination were identified for vaccines recommended after birth. For pentavalent vaccine in Cambodia and Madagascar, respectively, children whose birth parents were more educated (aORs, 0.61 [95% CI, .38–.97] and 0.66 [95% CI, .51–.86]) and who attended ≥4 visits during pregnancy (aORs, 0.39 [95% CI, .25–.63] and 0.66 [95% CI, .52–.84]) were more likely to be completely vaccinated (Table 3). We found similar results for OPV in Cambodia and Madagascar (aORs, 0.56 [95% CI, .35–.89] and 0.66 [95% CI, .50–.87], respectively, for children with highly educated birth parents; and aORs, 0.38 [95% CI, .24–.60] and 0.64 [95% CI, .49–.83], respectively, for children whose birth parents attended ≥4 prenatal visits; Supplementary Table 7).
Table 3.
Risk Factors Associated With Incomplete Immunization (<2 Doses) of Pentavalent Vaccine in Cambodia, Madagascar, and Senegal
| Risk Factor | Cambodia (n = 761a) | Madagascar (n = 1733a) | Senegal (n = 261a) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||||
| OR (95% CI) | P Value | aOR (95% CI) | P Value | OR (95% CI) | P Value | aOR (95% CI) | P Value | OR (95% CI) | P Value | aOR (95% CI) | P Value | |
| Sex | ||||||||||||
| Male (Ref) | 1 | 1 | 1 | 1 | 1 | … | ||||||
| Female | 0.83 (.54–1.28) | .40 | 0.85 (.54–1.34) | .49 | 1.23 (.97–1.56) | .09 | 1.25 (.98–1.59) | .07 | 0.71 (.37–1.33) | .29 | 0.79 (.38–1.63) | .53 |
| Site | ||||||||||||
| Urban (Ref) | 1 | 1 | 1 | 1 | … | … | ||||||
| Rural | 0.71 (.46–1.09) | .11 | 0.85 (.53–1.36) | .50 | 0.82 (.65–1.05) | .11 | 0.84 (.66–1.07) | .16 | … | … | ||
| Education | ||||||||||||
| Low education (Ref) | 1 | 1 | 1 | 1 | 1 | … | ||||||
| High education | 0.60 (.38–.94) | .03 | 0.61 (.38–.97) | .04 | 0.63 (.48–.81) | <.001 | 0.66 (.51–.86) | .002 | 1.07 (.47–2.26) | .86 | … | |
| No. of previous live-born children | ||||||||||||
| 0 (Ref) | 1 | … | 1 | … | 1 | … | ||||||
| ≥1 | 1.52 (.97–2.41) | .07 | … | 1.39 (1.08–1.79) | .01 | … | 1.53 (.72–3.56) | .29 | … | |||
| No. of prenatal visits | ||||||||||||
| <4 (Ref) | 1 | 1 | 1 | 1 | 1 | … | ||||||
| ≥4 | 0.37 (.24–.59) | <.001 | 0.39 (.25–.63) | <.001 | 0.62 (.49–.78) | <.001 | 0.66 (.52–.84) | .001 | 1.38 (.73–2.64) | .32 | … | |
| Cesarean delivery | ||||||||||||
| No (Ref) | 1 | … | 1 | … | 1 | … | ||||||
| Yes | 0.93 (.45–1.75) | .84 | … | 0.91 (.60–1.36) | .66 | … | 0.74 (.04–4.45) | .78 | … | |||
| Weight <2500 g at delivery | ||||||||||||
| No (Ref) | 1 | … | 1 | … | 1 | … | ||||||
| Yes | 1.04 (.30–2.73) | .95 | … | 1.29 (.87–1.87) | .19 | … | 1.25 (.27–4.25) | .74 | … | |||
Sex of child and site were forced in all multivariate analyses (only sex of child in Senegal).
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio; Ref, reference.
Children followed up to at least 4 months (in Senegal, only children from urban site).
We did not find any significant factor associated with incomplete pentavalent vaccination in Senegal in our analysis (Table 3), nor for OPV (Supplementary Table 7).
DISCUSSION
In a prospective community-based cohort with longitudinal follow-up conducted across 3 LMICs, we showed that VC is below the levels recommended by the World Health Organization (WHO), when considering vaccine timeliness in respect of vaccine schedules. For vaccines requiring multiple doses, we also showed that VC declined significantly for later doses across all vaccines and countries studied. We further identified that LBW was a major factor associated with the absence of vaccination at birth.
In Cambodia, our estimates of VC were concordant with the latest Cambodia DHS and WHO estimates for hepatitis B vaccine at birth, BCG vaccine at birth and with 1-year catch-up, and OPV and pentavalent vaccine doses at 6 and 10 weeks [12, 13]. In Madagascar, coverage estimates were consistent with the latest MICS and WHO estimates for BCG, OPV, and pentavalent vaccine doses recommended at 6 and 10 weeks [14, 15]. In Senegal, hepatitis B vaccine dose coverage at birth was similar to WHO estimates [16]. In contrast, coverage of OPV at birth in Madagascar was 10 points lower [14]. BCG VC in Senegal was 20–25 points lower than previous estimates [8, 16]. Finally, coverage of the pentavalent and OPV doses recommended at 14 weeks was 10–20 points lower in Cambodia [12, 13] and 20–40 points lower in Madagascar [15], respectively, than DHS and WHO estimates. Several factors can be hypothesized to explain these discrepancies. First, the limited duration of follow-up of the cohort did not allow us to capture possible catch-up vaccinations (pilot study in Madagascar: 6 months and rural site in Senegal: 3 months). Indeed, all children could potentially benefit from catch-up vaccination after leaving the study. Data from the DHSs and MICSs were collected using a cross-sectional design among children up to 4 years old, thus precluding assessment of whether vaccination timing respects recommended vaccine schedules, but accounting for potential catch-up vaccinations. This difference in methodology could explain why the coverage rates found in our study were lower than those found in the DHSs. However, it is important to underline that our study design allowed us to have information that the DHSs do not capture on the timing of vaccination, suggesting a delay in some vaccine uptake. Second, VC estimates from our study were derived from 2 sites in each country, and it is possible that the areas in our study have lower VC than national-level estimates from other work.
A striking finding from our study is that VC declined significantly for later doses compared to earlier ones, across all vaccines and countries studied. Our results are consistent with the literature. In Cambodia and Madagascar, a drop in pentavalent vaccine coverage between doses recommended at 6 and 14 weeks has been observed previously [12, 17]. One hypothesis is that this drop may be due to lack of interaction with the healthcare system and thus lack of opportunity to be vaccinated. Child’s contact with health structures depends on the child health events, access to healthcare, and cultural and socioeconomic factors. Moreover, even if a child seeks care for a health event, his/her parents might not bring his/her vaccination card, thus preventing the healthcare provider from checking the vaccination status. This suggests that a possible intervention to create opportunity of vaccination and increase immunization coverage might be to structure the child’s follow-up with compulsory consultations around the ages when vaccines are recommended.
Another interesting finding is that certain vaccines recommended on the same schedule had different coverage within a given country. This difference may be explained by the organization of healthcare structures. For example, in Senegal, coverage at birth of hepatitis B vaccine exceeded BCG vaccine and OPV. This may be explained by the fact that BCG vaccine and OPV are administered by the routine vaccination service during weekdays only, whereas the hepatitis B vaccine is available in maternity wards and can be given on any day. In Madagascar, coverage of the pentavalent vaccine was lower than that of OPV. This may be explained by possible mass vaccinations targeting polio vaccine, in which all children in a given age group, regardless of disease or vaccination history, are vaccinated [18]. Modifiable logistical or care organization issues such as stock-outs in distribution channels or possibly freezer breakdowns would need to be explored to better understand insufficient and asymmetric immunization coverage.
We found that LBW was associated with risk of nonvaccination for all vaccines recommended at birth in Cambodia, Madagascar, and Senegal. This result is consistent with a study conducted in Ghana, which found that LBW was a risk factor for neonatal BCG undervaccination (aOR, 1.64–2.42) [19]. Prematurity is one of the leading causes of LBW [20], and although it is recommended that preterm infants be vaccinated on the same schedules as full-term infants [21, 22], vaccinations in this at-risk population are often delayed [23, 24]. Children born prematurely are at increased risk of vaccine-preventable diseases [25], due in part to their immature immune system. Barriers to immunization of LBW infants should be explored among both parents and healthcare professionals in order to provide tailored educational programs targeting this vulnerable population.
In our study, low birth parent education was associated with incomplete child vaccination for pentavalent vaccine and OPV in Cambodia and Madagascar, but also for hepatitis B vaccine at birth in Cambodia and BCG vaccine in Madagascar. This risk factor has been found previously in Madagascar for BCG, pentavalent vaccine, and OPV [6], and in Ethiopia for all vaccinations recommended in that country [26]. Higher education is often associated with a better awareness on preventive care such as childhood vaccination services in LMICs [27]. Our finding reinforces the need to use approaches and tools adapted to less educated populations to improve awareness of vaccines and their effectiveness in protecting children.
Having fewer prenatal visits was associated with incomplete child vaccination schedules for pentavalent vaccine and OPV in Cambodia and Madagascar, and BCG vaccine in Madagascar and Senegal. This risk factor had also been found in previous literature in Cambodia for BCG and pentavalent vaccines [28] and in Senegal for all vaccinations recommended in that country [7]. This limited prenatal care may reflect a lower accessibility or even affinity of the birth parent for the healthcare system, which may explain lower adherence to vaccination.
Home delivery was associated with incomplete child vaccination schedules for vaccines at birth in Madagascar and may also reflect this lower accessibility or affinity for engagement with healthcare systems. This risk factor has also been found in studies conducted in Ghana [29] for all vaccinations recommended in these countries. Indeed, in our study, coverage of vaccines administered at birth was lower in Madagascar than in Cambodia and Senegal, which may be explained by its higher rate of home delivery. Vaccines may be less available for deliveries that takes place at home, and it may therefore be important to sensitize traditional birth attendants to the importance of promoting immunization in countries where home deliveries are still common.
Our study has some limitations. It is possible that children who did not complete the full follow-up might have had different vaccination coverage, either better or worse, than those who did. In addition, children included up in the context of a longitudinal cohort study may have benefited from more available vaccination than children from the general population. Therefore, real vaccination coverage may be even lower than found here. Also, children followed in this study came from 2 sites, 1 urban and 1 rural, in each of the countries, and not the whole country, which may limit the generalization of the results at the national level. Moreover, it is possible that an insufficient sample size limited our analysis of risk factors, particularly in Senegal, where significantly fewer children were enrolled relative to the other 2 countries in the study. Finally, as mentioned in the Methods, some of the vaccines included in the national programs were either introduced during the study or were country specific, and thus were not included in our analysis.
The main strength of this study is its community-based approach, which makes it possible to follow-up children who do not go to the health centers either at the time of their birth (home birth) or later during their follow-up. In addition, we were able to compare and show differences in vaccination coverage of vaccines recommended with the same schedule. Finally, as our study was a prospective cohort, we were able to collect missing information on the next visit when families did not have child’s vaccination card with them, which would not have been possible with cross-sectional studies.
CONCLUSIONS
We showed that vaccine coverage for common childhood vaccines was lower than recommendations from WHO, when considering vaccine timeliness in respect of vaccine schedules, in Cambodia, Madagascar, and Senegal. This result suggests that too many children are still not vaccinated in time. LBW was a strong risk factor for nonvaccination for vaccines recommended at birth in all 3 countries. Multidisciplinary approaches targeting birth parents and healthcare providers across urban and rural sites may help to improve vaccine coverage and timeliness.
Supplementary Material
Contributor Information
Florian Verrier, Université Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay, Anti-infective Evasion and Pharmacoepidemiology Team, Centre de recherche en épidémiologie et santé des populations, Inserm, Montigny-le-Bretonneux, France; Epidemiology and Modelling of Antibiotic Evasion, Université Paris-Cité, Institut Pasteur, Paris, France.
Agathe de Lauzanne, Epidemiology and Public Health Unit, Institut Pasteur du Cambodge, Phnom Penh, Cambodia.
Jean-Baptiste Niokhhor Diouf, Centre Hospitalier Roi Baudouin Guédiawaye, Dakar, Senegal.
Andrianirina Zafitsara Zo, Pediatric Ward, Centre Hospitalier de Soavinandriana, Antananarivo, Madagascar.
Lison Ramblière, Université Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay, Anti-infective Evasion and Pharmacoepidemiology Team, Centre de recherche en épidémiologie et santé des populations, Inserm, Montigny-le-Bretonneux, France; Epidemiology and Modelling of Antibiotic Evasion, Université Paris-Cité, Institut Pasteur, Paris, France.
Perlinot Herindrainy, Unité d’épidémiologie et de recherche clinique, Institut Pasteur de Madagascar, Antananarivo, Madagascar.
Fatoumata Diene Sarr, Unité d’épidémiologie des maladies infectieuses, Institut Pasteur de Dakar, Dakar, Senegal.
Touch Sok, Ministry of Health, Phnom Penh, Cambodia.
Muriel Vray, Unité d’épidémiologie des maladies infectieuses, Institut Pasteur de Dakar, Dakar, Senegal.
Jean-Marc Collard, Unité de bactériologie expérimentale, Institut Pasteur de Madagascar, Antananarivo, Madagascar.
Laurence Borand, Center for Tuberculosis Research, Division of Infectious Diseases, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Epidemiology and Public Health Unit, Institut Pasteur du Cambodge, Phnom Penh, Cambodia.
Elsa Kermorvant-Duchemin, Assistance Publique–Hôpitaux de Paris, Department of Neonatology, Hôpital Universitaire Necker-Enfants Malades and Université de Paris, Paris, France.
Elisabeth Delarocque-Astagneau, Université Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay, Anti-infective Evasion and Pharmacoepidemiology Team, Centre de recherche en épidémiologie et santé des populations, Inserm, Montigny-le-Bretonneux, France; Assistance Publique–Hôpitaux de Paris, Groupe hospitalier universitaire Paris-Saclay Université, Epidemiology and Public Health, Raymond Poincaré Hospital, Garches, France.
Didier Guillemot, Université Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay, Anti-infective Evasion and Pharmacoepidemiology Team, Centre de recherche en épidémiologie et santé des populations, Inserm, Montigny-le-Bretonneux, France; Epidemiology and Modelling of Antibiotic Evasion, Université Paris-Cité, Institut Pasteur, Paris, France; Assistance Publique–Hôpitaux de Paris, Public Health, Medical Information, Clinical Research, Paris -Saclay, Le Kremlin-Bicêtre, France.
Bich-Tram Huynh, Université Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay, Anti-infective Evasion and Pharmacoepidemiology Team, Centre de recherche en épidémiologie et santé des populations, Inserm, Montigny-le-Bretonneux, France; Epidemiology and Modelling of Antibiotic Evasion, Université Paris-Cité, Institut Pasteur, Paris, France.
for the Bacterial Infections and Antibiotic-Resistant Diseases Among Young Children in Low-Income Countries (BIRDY) Study Group:
Aina Nirina Randriamamonjiarison, Tanjona Antsa Volahasina, Fanjalalaina Rasoanaivo, Feno Manitra Jacob Rakotoarimanana, Tanjona Bodonirina Raheliarivao, Frédérique Randrianirina, Thida Chon, Sophie Goyet, Alexandra Kerleguer, Véronique Ngo, Siyin Lach, Pring Long, Arnaud Tarantola, Marguerite Diatta, Joseph Faye, Abdoulaye Seck, Michael Padget, Armiya Youssouf Abdou, and Benoit Garin
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
BIRDY Study Group. Members from the Institut Pasteur in Madagascar include Aina Nirina Randriamamonjiarison, Tanjona Antsa Volahasina, Fanjalalaina Rasoanaivo, Feno Manitra Jacob Rakotoarimanana, Tanjona Bodonirina Raheliarivao, and Frédérique Randrianirina. Members from the Institut Pasteur in Cambodia include Thida Chon, Sophie Goyet, Alexandra Kerleguer, Véronique Ngo, Siyin Lach, Pring Long, and Arnaud Tarantola. Members from the Institut Pasteur in Senegal include Marguerite Diatta, Joseph Faye, and Abdoulaye Seck. Members from the Institut Pasteur in Paris include Michael Padget, Armiya Youssouf Abdou, and Benoit Garin.
Author contributions. F. V. conducted formal data analysis, interpreted the data, drafted the initial manuscript, and created the figures. B.-T. H. conceptualized and designed the study, coordinated data collection, supervised data analysis, and contributed to critical revision for important intellectual content. L. R. contributed to the analysis of the data and critically reviewed the manuscript for important intellectual content. A. L., J.-B. N. D., A. Z. Z., P. H., D. S., T. S., M. V., J.-M. C., and L. B., implemented and supervised the course of the study, collected data, and critically reviewed the manuscript for important intellectual content. E. K.-D., E. D.-A., and D. G. conceptualized and designed the study, designed the data collection instruments, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Acknowledgments. We are grateful to all birth parents and their newborns, physicians, laboratory staff, field investigators, community workers, and collaborators for their participation in this project; David R. M. Smith for his proofreading in English; and the Department of International Affairs and the Centre de Recherche Translationnelle–Coordination Clinique of Institut Pasteur for their support in the management of the study.
Data availability . Data cannot be shared publicly because data privacy is subject to French, Cambodian, Senegalese, and Malagasy regulations. Data are available from the Ethics Committee (contact via irb@pasteur.fr) for researchers who meet the criteria for access to confidential data.
Disclaimer. The funders had no role in study design, data collection and analysis, preparation of the manuscript, or decision to submit the manuscript for publication.
Financial support . This work was supported by the Department of International Cooperation of the Principality of Monaco (https://en.gouv.mc/Government-Institutions/The-Government/Ministry-of-Foreign-Affairs-and-Cooperation/Department-of-International-Cooperation), MSD Avenir (http://www.msdavenir.fr/), and Total Foundation (https://www.foundation.total/fr).
References
- 1. World Health Organization . Immunization coverage.2022. Available at: https://www.who.int/news-room/fact-sheets/detail/immunization-coverage. Accessed 11 November 2020.
- 2. United Nations Children’s Fund . Levels and trends in child mortality 2020. 2020. Available at: https://www.unicef.org/reports/levels-and-trends-child-mortality-report-2020. Accessed 11 November 2020.
- 3. Raherindrasana A, Metcalf CJ, Heraud JM, et al. . Towards better targeting: lessons from a posthoneymoon measles outbreak in Madagascar, 2018–2019. BMJ Glob Health 2020; 5:e003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Global vaccine action plan 2011–2020. 2013. Available at: https://www.who.int/publications-detail-redirect/global-vaccine-action-plan-2011-2020. Accessed 15 May 2021.
- 5. Burton A, Monasch R, Lautenbach B, et al. . WHO and UNICEF estimates of national infant immunization coverage: methods and processes. Bull World Health Organ 2009; 87:535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clouston S, Kidman R, Palermo T. Social inequalities in vaccination uptake among children aged 0–59 months living in Madagascar: an analysis of Demographic and Health Survey data from 2008 to 2009. Vaccine 2014; 32:3533–9. [DOI] [PubMed] [Google Scholar]
- 7. Sarker AR, Akram R, Ali N, Chowdhury ZI, Sultana M. Coverage and determinants of full immunization: vaccination coverage among Senegalese children. Medicina (Kaunas) 2019; 55:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peretti-Watel P, Cortaredona S, Ly EY, et al. . Determinants of childhood immunizations in Senegal: adding previous shots to sociodemographic background. Hum Vaccines Immunother 2020; 16:363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Young B, Sarwar G, Hossain I, Mackenzie G. Risk factors associated with non-vaccination in Gambian children: a population-based cohort study. Trans R Soc Trop Med Hyg 2022; 116:1063–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huynh BT, Padget M, Garin B, Delarocque-Astagneau E, Guillemot D; BIRDY Study Group . Bacterial neonatal sepsis and antibiotic resistance in low-income countries. Lancet 2016; 387:533–4. [DOI] [PubMed] [Google Scholar]
- 11. Huynh BT, Kermorvant-Duchemin E, Chheang R, et al. . Severe bacterial neonatal infections in Madagascar, Senegal, and Cambodia: a multicentric community-based cohort study. PLoS Med 2021; 18:e1003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Institute of Statistics . Cambodia Demographic and Health Survey 2014. 2015. Available at: https://dhsprogram.com/publications/publication-fr312-dhs-final-reports.cfm. Accessed 22 November 2020.
- 13. World Health Organization . Cambodia: WHO and UNICEF estimates of immunization coverage: 2021 revision. Available at: https://cdn.who.int/media/docs/default-source/country-profiles/immunization/2022-country-profiles/immunization_khm_2022.pdf?sfvrsn=12ab08b0_3&download=true. Accessed 22 March 2023.
- 14. United Nations Children’s Fund . MICS 6: Vaccination des enfants.2018. Available at: https://www.unicef.org/madagascar/documents/mics-6-2018-vaccination-des-enfants. Accessed 13 August 2021.
- 15. World Health Organization . Madagascar: WHO and UNICEF estimates of immunization coverage: 2021 revision. Available at: https://cdn.who.int/media/docs/default-source/country-profiles/immunization/2022-country-profiles/immunization_mdg_2022.pdf?sfvrsn=b0979d66_3&download=true. Accessed 22 March 2023.
- 16. World Health Organization . Senegal: WHO and UNICEF estimates of immunization coverage: 2021 revision. Available at: https://cdn.who.int/media/docs/default-source/country-profiles/immunization/2022-country-profiles/immunization_sen_2022.pdf?sfvrsn=5e9d7114_3&download=true. Accessed 22 March 2023.
- 17. Randriatsarafara FM, Ralamboson S, Rahoelison H, Ranjalahy RJ, Ratsimbazafimahefa RH. Respect du calendrier vaccinal selon le programme élargi de vaccination au CSMIU de Moramanga. La Revue Médicale de Madagascar 2014; 4:458–63. [Google Scholar]
- 18. Nimpa MM, Razafiarivao NR, Robinson A, et al. . Efforts towards polio eradication in Madagascar: 1997 to 2017. J Immunol Sci 2021; Special Issue 2:1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O’Leary M, Edmond K, Floyd S, et al. . Neonatal vaccination of low birthweight infants in Ghana. Arch Dis Child 2017; 102:145–51. [DOI] [PubMed] [Google Scholar]
- 20. World Health Organization . Preterm and low birth weight infants. Available at: https://www.who.int/teams/maternal-newborn-child-adolescent-health-and-ageing/newborn-health/preterm-and-low-birth-weight. Accessed 22 March 2023.
- 21. World Health Organization . BCG vaccines: WHO position paper—February 2018. Wkly Epidemiol Rec 2018; 93:73–96.29474026 [Google Scholar]
- 22. World Health Organization . Hepatitis B vaccines: WHO position paper, July 2017—recommendations. Vaccine 2019; 37:223–5. [DOI] [PubMed] [Google Scholar]
- 23. Sisson H, Gardiner E, Watson R. Vaccination timeliness in preterm infants: an integrative review of the literature. J Clin Nurs 2017; 26:4094–104. [DOI] [PubMed] [Google Scholar]
- 24. Mutua MK, Ochako R, Ettarh R, Ravn H, Echoka E, Mwaniki P. Effects of low birth weight on time to BCG vaccination in an urban poor settlement in Nairobi, Kenya: an observational cohort study. BMC Pediatr 2015; 15:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bonhoeffer J, Siegrist CA, Heath PT. Immunisation of premature infants. Arch Dis Child 2006; 91:929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geweniger A, Abbas KM. Childhood vaccination coverage and equity impact in Ethiopia by socioeconomic, geographic, maternal, and child characteristics. Vaccine 2020; 38:3627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Owais A, Hanif B, Siddiqui AR, Agha A, Zaidi AKM. Does improving maternal knowledge of vaccines impact infant immunization rates? A community-based randomized-controlled trial in Karachi, Pakistan. BMC Public Health 2011; 11:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ngy MH, Nakamura K, Ohnishi M, et al. . Improved perinatal health through qualified antenatal care in urban Phnom Penh, Cambodia. Environ Health Prev Med 2007; 12:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moran EB, Wagner AL, Asiedu-Bekoe F, Abdul-Karim A, Schroeder LF, Boulton ML. Socioeconomic characteristics associated with the introduction of new vaccines and full childhood vaccination in Ghana, 2014. Vaccine 2020; 38:2937–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.