Abstract
Rationale and Objective:
Hyperglycemia exacerbates the progression of chronic kidney disease (CKD), but most glucose-lowering therapies do not address morbidities associated with CKD. Sodium-glucose cotransporter-2 (SGLT2) inhibitors offer potential benefits to patients with diabetes and CKD, but their effectiveness may be diminished with impaired kidney function. We aimed to evaluate the safety and effectiveness of bexagliflozin, a novel SGLT2 inhibitor, in patients with type 2 diabetes and CKD.
Study Design:
Phase 3, double-blind, placebo-controlled, multi-center, multi-national, randomized trial
Setting and Participants:
54 sites across 4 countries. Patients with CKD stage 3a or 3b, type 2 diabetes mellitus, and a hemoglobin A1c between 7.0% −10.5% and eGFR 30–59 ml/min/1.73 m2 who were taking oral hypoglycemic agents for 8 weeks.
Interventions:
Bexagliflozin, 20 mg daily versus placebo, for 24 weeks.
Outcomes:
Primary outcome was the change in percent hemoglobin A1c from baseline to week 24 weeks. Secondary endpoints included changes in body weight, systolic blood pressure, albuminuria and hemoglobin A1c stratified by CKD stage.
Results:
312 patients across 54 sites were analyzed. Bexagliflozin lowered hemoglobin A1c by 0.37% [95% CI 0.20, 0.54]; p <0.001 compared to placebo. Patients with CKD stage 3a (eGFR 45 to < 60) and 3b (eGFR 30 to < 45) experienced reductions in hemoglobin A1c of 0.31% (p = 0.007) and 0.43% (p = 0.002), respectively. Bexagliflozin lowered body weight (1.61 kg, p < 0.001), systolic blood pressure (3.8 mmHg, p = 0.02), fasting plasma glucose (0.76 mmol/L, p = 0.003) and albuminuria (geometric mean ratio reduction of 20.1%, p = 0.03). The frequency of adverse events was not detectably different across treatment groups.
Limitations:
Not designed to evaluate impact of treatment on long-term kidney disease and cardiovascular outcomes.
Conclusions:
Bexagliflozin reduces hemoglobin A1c in patients with diabetes and stage 3a/3b CKD and appears to be well tolerated. Additional observed benefits included reduction in body weight, systolic blood pressure, and albuminuria.
Funding:
Trial was sponsored by Theracos Sub, LLC.
Trial Registration:
Keywords: Type 2 diabetes, SGLT2 inhibitor, chronic kidney disease, renal impairment, bexagliflozin
Plain Language Summary:
Hyperglycemia accelerates the progression of chronic kidney disease (CKD). Few oral glucose-lowering therapies are effective for patients with diabetes and CKD, especially in stage 3b CKD. This study reports results from a phase 3, randomized, controlled trial comparing bexagliflozin, a novel sodium-glucose cotransporter-2 inhibitor, to placebo, in patients with stage 3a and 3b CKD. Patients treated with bexagliflozin had significantly lower hemoglobin A1c levels, body weight, systolic blood pressure, and albuminuria. Adverse events were similar between treatment groups. Bexagliflozin may be a safe and effective agent for the treatment of hyperglycemia in patients with stage 3a and 3b CKD.
Introduction
Type 2 diabetes mellitus is one of the leading causes of morbidity and mortality worldwide.1–3 Chronic kidney disease (CKD) develops in over 1/3 of diabetic patients.4,5 Tight glycemic control and agents that block the renin-angiotensin-aldosterone axis can delay progression of macrovascular and microvascular complications of diabetes.6,7 As kidney function and estimated glomerular filtration rate (eGFR) worsen, oral anti-diabetic treatment options become increasingly limited.8–11 Thus, novel oral therapies that can be used safely and effectively in patients with diabetes and CKD are needed.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a class of oral antidiabetic medications that reduce hyperglycemia by decreasing renal proximal tubular reabsorption of glucose, thereby inducing glucosuria.12 Agents in this class lower hemoglobin A1c and decrease body weight and blood pressure; some members of the class have been shown to improve cardiovascular and kidney outcomes.13–16 However, relatively few studies have been reported for the CKD population,17,18 and SGLT2 inhibitors have generally not been effective in reducing hemoglobin A1c in patients with stage 3 CKD.19,20 To date, no SGLT2 inhibitor has been approved for the management of type 2 diabetes in patients with stage 3b CKD.
Bexagliflozin is an SGLT2 inhibitor with high potency and high selectivity for the SGLT2 transporter. It elicits a prominent and predictable glucosuria in experimental models.21 In previous clinical trials, bexagliflozin has demonstrated hemoglobin A1c lowering comparable to other members of the SGLT2 inhibitor class in subjects with type 2 diabetes who have eGFR > 60 mL min−1 per 1.73 m2 (ClinicalTrials.gov NCT02715258, NCT02769481, NCT02956044). The current study was intended to determine if the glucose-lowering benefit of bexagliflozin could be extended safely into populations of diabetic patients with more moderately impaired kidney function, with particular emphasis on those with eGFR less than 45 mL min−1 per 1.73 m2.
Methods
Study Design
Data were collected in a phase 3, multi-center, multi-national, randomized, double-blind, placebo-controlled, parallel group trial intended to evaluate the safety and effectiveness of bexagliflozin in patients with type 2 diabetes mellitus, KDIGO (kidney disease improving global outcomes) CKD stage 3a/3b,22 and inadequate glycemic control (with hemoglobin A1c ≥ 7.0%). The study was conducted between September 2016 and January 2018 at 54 investigational sites in the United States, Spain, France and Japan. Patients who met the inclusion and exclusion criteria were enrolled in a 1-week, placebo run-in period. After run-in, eligible patients were randomized in a 1:1 ratio to receive bexagliflozin (20 mg) or placebo for 24 weeks in an outpatient setting, with a final study visit at week 26. Randomization was stratified by screening hemoglobin A1c level (7.0% to 8.5% or 8.6% to 10.5%), insulin-treated or non-insulin treated status, and stage 3a CKD (eGFR 45 to < 60 mL min−1 per 1.73 m2) or stage 3b CKD (eGFR 30 to < 45 mL min−1 per 1.73 m2) classification. Randomization and study drug allocation were performed via a central interactive web response system. Investigators, patients and the sponsor team remained blinded to allocation groups for the duration of the trial. Allocation codes were maintained by a designated statistician not involved in study operations.
This study was sponsored by Theracos Sub, LLC and coordinated by the Translational Medicine Group at Massachusetts General Hospital (Boston, MA). The study protocol and statistical analysis plan were developed by the MGH Translational Medicine Group. The trial was registered with ClinicalTrials.gov (NCT02836873) and is part of an ongoing phase 3 development program involving multiple clinical trials. Prior to study initiation the study protocol, informed consent documents and all subject recruitment information were approved by a central institutional review board (IRB) for investigational sites in the US and by ethics committees for investigational sites in other countries. The study conduct adhered to the principles set forth in the Declaration of Helsinki. All patients provided written informed consent. A Data and Safety Monitoring Board reviewed unblinded aggregate data periodically.
The required sample size for the study was calculated using a two-group t-test assuming two-sided significance of 0.05, placebo-corrected mean reduction of hemoglobin A1c of 0.4% in the treatment arm and a standard deviation of 1%. Under these assumptions, a sample size of 133 patients per arm was estimated to yield 90% power to detect a treatment difference between the bexagliflozin and placebo arms. This sample size determination was based on the assumptions at a specific time point of week 24, without consideration of correlated repeated measurements. The goal was to recruit 300 participants, comprising a minimum of 135 subjects in each CKD stage, to account for a potential loss of approximately 12% of subjects due to early withdrawal.
Patient Population
CKD stage and eGFR were calculated using the 4-variable Modification of Diet in Renal Disease (MDRD) equation.23 To be eligible, a prospective participant was required to have been a diabetic man or non-pregnant woman of age ≥ 20 years, either treatment naïve or currently managed by a regimen of approved hypoglycemic agents that had not changed in the preceding 8 weeks; have a baseline eGFR between 30 and 59 mL min−1 per 1.73 m2; and have a body mass index (BMI) ≤ 45 kg m-2. Disqualifying conditions included a diagnosis of type I diabetes mellitus, the presence of a hemoglobinopathy that could interfere with A1c measurement, or a history of > 1 episode of symptomatic hypoglycemia per week; patients were also excluded if they had a history of cancer not in remission for > 3 years, a myocardial infarction, stroke, or hospitalization for unstable angina/heart failure within 3 months of screening, use of an SGLT2 inhibitor within 3 months of screening, or had prior kidney replacement therapy (dialysis or transplant).
All participants were instructed to continue their existing anti-diabetic regimens for the duration for the study. Investigators were directed to prescribe additional medication for hyperglycemia at any time if deemed medically necessary. If a patient had an episode of 3 consecutive days of fasting serum glucose ≥ 270 mg dL−1 from week 1 to week 6, ≥ 240 mg dL−1 from week 7 to week 12, or ≥ 200 mg dL−1 from week 12 to week 24 or had fasting serum glucose ≥ 250 mg dL−1 associated with severe clinical signs or symptoms of hyperglycemia, the prescription of additional medication, dictated by the medical judgement of the site principal investigator, was permitted.
Outcomes
The primary endpoint was the change in percent hemoglobin A1c from baseline to week 24. Additional endpoints included the change from baseline to week 24 of fasting plasma glucose, body weight, blood pressure and urine albumin to creatinine ratio, as well as the change in percent hemoglobin A1c within the subgroups defined by stage 3a or stage 3b CKD at baseline. The proportion of patients that achieved a hemoglobin A1c < 7%, or of patients with baseline BMI ≥25 kg m−2 that achieved ≥5% reduction of body weight was also documented.
Safety
Safety monitoring included assessments of vital signs, physical examinations, electrocardiograms, urinalyses, and blood specimens. In addition, histories of adverse events and changes in concomitant medication use were recorded. Samples for laboratory testing were processed at regional central facilities. Adverse events of special interest were prospectively defined based on available safety data and regulatory information. Patients were provided with glucometers to measure and upload serum glucose values and were instructed to keep a daily glucose dairy for review during study visits. Suspected acute kidney injury (AKI) was addressed via a standard monitoring protocol using creatinine-based KDIGO definitions (Supplementary Figure 1).24 Urinary tract infection was defined as a positive urinalysis for leukocyte esterase and/or nitrites plus symptoms and/or a culture of 105 colony forming units of one bacterial species. All suspected major adverse cardiac events (MACE) were forwarded to a cardiovascular endpoint committee for blinded adjudication based on prospectively defined event definitions.
Statistical Analysis
Effectiveness analyses were performed in an intention-to-treat (ITT) manner. The primary outcome of change in percent hemoglobin A1c from baseline to week 24 was analyzed using a mixed-effect model repeated measures (MMRM) analysis of covariance (ANCOVA) to generate an estimate of treatment difference at 24 weeks. The model included region, insulin-treated status, baseline eGFR, treatment, visit, treatment-by-visit interaction, and the baseline hemoglobin A1c value as fixed effect covariates. An unstructured covariance matrix was assumed. Data from Weeks 6, 12, and 24 were used in the model. A last observation carried forward method was used to impute hemoglobin A1c values after rescue medication. Secondary continuous outcomes were analyzed using all available data at each week. Similar to the primary outcome, MMRM analysis (with additional baseline value of the outcome as a fixed effect covariate) was utilized. Proportion of hemoglobin A1c<7% was analyzed using mixed-effects logistic regression for repeated measures assuming an unstructured covariance matrix, using the same covariates as the primary outcome. Urine albumin to creatinine ratio (UACR) data were log transformed prior to analysis. Change in log transformed UACR from baseline to week 24 was analyzed by ANCOVA model. The fixed effect included region, baseline hemoglobin A1c value, insulin-treated status, baseline eGFR, and treatment group. The log transformed baseline UACR values were used as covariate as well. The adjusted geometric mean ratio of relative change from baseline in UACR and the 95 % CI by treatment group were calculated as the antilog of the LS mean and 95 % CI of log transformed values, converted to percentages. The effect of group assignment on early withdrawal was assessed by Kaplan Meier analysis.
All patients who took at least one dose of double-blind study medication were included in the safety analysis. An interim analysis was not performed. All analyses were performed using SAS version 9.3 (Cary, NC).
Results
Demographics
A total of 312 patients (157 in the bexagliflozin arm and 155 in the placebo arm) were included in the final analysis. (Figure 1). At baseline, 166 participants had CKD stage 3a and 146 had CKD stage 3b. Normoalbuminuria (< 30 mg g−1 UACR) was observed in 116/312 (37%) patients, microalbuminuria (30–299 mg g−1 UACR) in 118/312 (38%) and macroalbuminuria (≥ 300 mg g−1 UACR) in 78/312 (25%). The mean duration of diabetes as assessed by medical history was nearly 16 years, and the mean (SD) baseline hemoglobin A1c was 7.98% (0.798). Approximately 56% of patients were prescribed insulin as part of their regimen for glycemic control. The mean (SD) age was 69.6 (8.32) years, 116/312 (37%) were women and 171/312 (55%) self-described as white or Caucasian. The mean (SD) baseline BMI was 30.2 (5.87) kg m−2 and the mean SBP (SD) was 137 (14.5) mm Hg. Baseline characteristics, including variables used to stratify randomization (Table 1), and early withdrawal rates (Supplementary Figure 2) were similar between treatment groups. Frequently used concomitant baseline medications are presented in Table 2.
Figure 1:
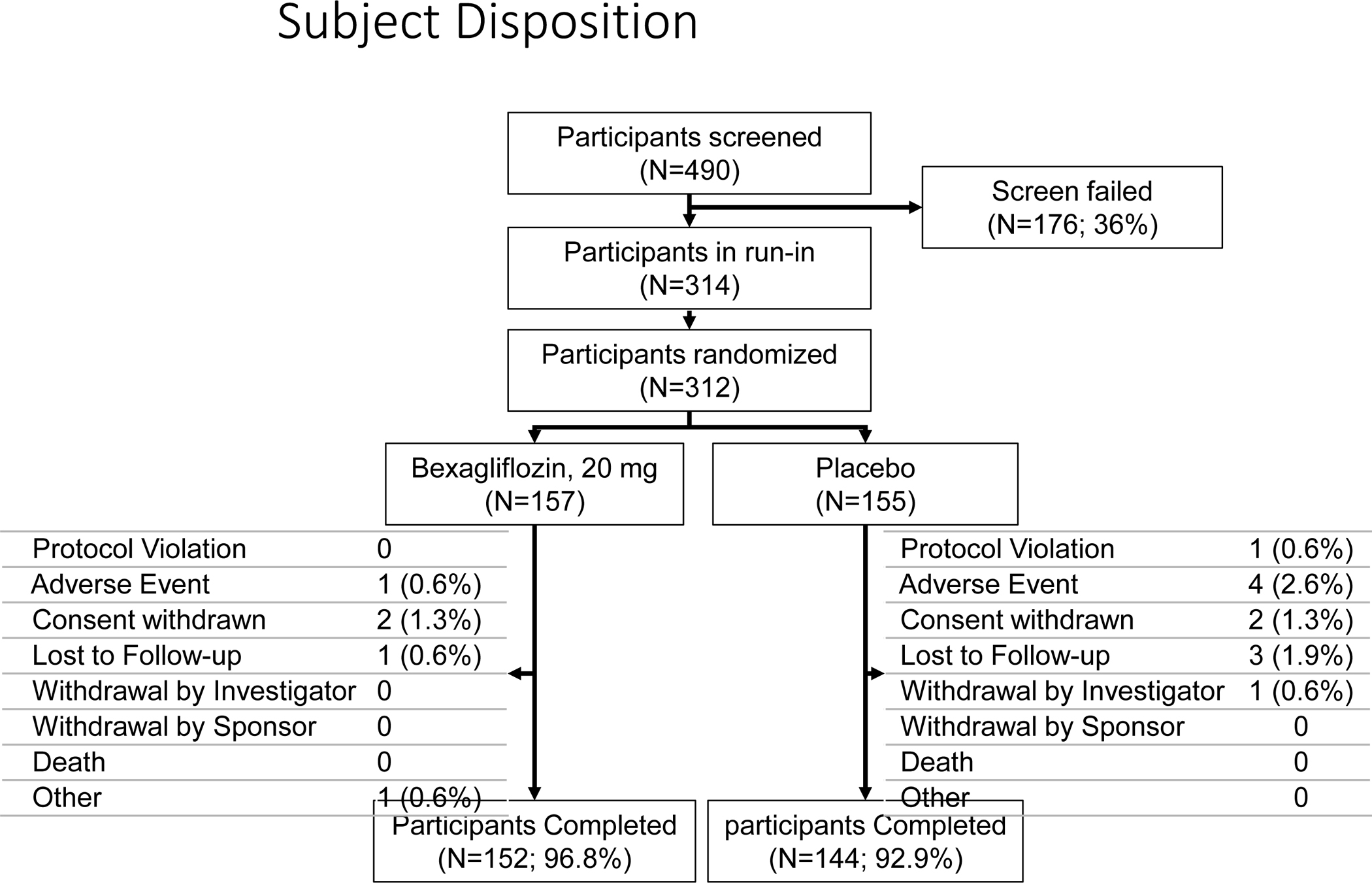
CONSORT flow chart of patient disposition.
Table 1:
Baseline Characteristics by Randomization Status.
| Bexagliflozin, 20 mg (N=157) |
Placebo (N=155) |
Total (N=312) |
|
|---|---|---|---|
|
| |||
| Gender, n (%) | |||
| Woman | 65 (41.4) | 51 (32.9) | 116 (37.2) |
| Man | 92 (58.6) | 104 (67.1) | 196 (62.8) |
|
| |||
| Age (years) Mean (SD) | 69.3 (8.36) | 69.9 (8.29) | 69.6 (8.32) |
|
| |||
| Race, n (%) | |||
| White | 83 (52.9) | 88 (56.8) | 171 (54.8) |
| Black or African-American | 9 (5.7) | 6 (3.9) | 15 (4.8) |
| Asian | 61 (38.9) | 59 (38.1) | 120 (38.5) |
| Other | 4 (2.5) | 2 (1.3) | 6 (1.9) |
|
| |||
| Country of Investigational Site | |||
| France | 12 (7.6) | 16 (10.3) | 28 (9.0) |
| Spain | 34 (21.7) | 31 (20.0) | 65 (20.8) |
| USA | 53 (33.8) | 50 (32.3) | 103 (33.0) |
| Japan | 58 (36.9) | 58 (37.4) | 116 (37.2) |
|
| |||
| BMI, Mean (SD) | 30.29 (5.988) | 30.10 (5.774) | 30.20 (5.874) |
|
| |||
| Body Weight (kg), Mean (SD) | 82.90 (20.509) | 82.59 (21.196) | 82.75 (20.820) |
|
| |||
| SBP (mm Hg), Mean (SD) | 135.9 (14.25) | 137.6 (14.75) | 136.8 (14.50) |
|
| |||
| Hemoglobin A1c (%), Mean (SD) | 8.01 (0.786) | 7.95 (0.812) | 7.98 (0.798) |
|
| |||
| Hemoglobin A1c Group, n (%) | |||
| 7.0 to 8.5% | 124 (79.0) | 123 (79.4) | 247 (79.2) |
| 8.6 to 10.5% | 33 (21.0) | 32 (20.6) | 65 (20.8) |
|
| |||
| Fasting Plasma Glucose (mmol L−1), Mean (SD) | 8.61 (2.525) | 8.63 (2.246) | 8.62 (2.387) |
|
| |||
| Subjects in eGFR Sub-group at Baseline, n (%)1 | |||
| Stage 3a CKD: eGFR 45 to <60 | 86 (54.8) | 80 (51.6) | 166 (53.2) |
| Stage 3b CKD: eGFR 30 to <45 | 71 (45.2) | 75 (48.4) | 146 (46.8) |
|
| |||
| eGFR (mL min−1 per 1.73 m2), Mean (SD) | 45.44 (8.565) | 44.78 (8.085) | 45.11(8.323) |
|
| |||
| eGFR (mL min−1 per 1.73 m2), Mean (SD) Sub-group | |||
| eGFR 45 to <60: Stage 3a CKD | 51.76 (5.307) | 51.27 (4.404) | 51.52 (4.884) |
| eGFR 30 to <45: Stage 3b CKD | 37.79 (4.572) | 37.87 (4.629) | 37.83 (4.586) |
|
| |||
| UACR Group, n (%) | |||
| Normoalbuminuria (UACR<30 mg g−1) | 61 (38.9) | 55 (35.5) | 116 (37.2) |
| Microalbuminuria (≥ 30 and <300 mg g−1) | 65 (41.4) | 53 (34.2) | 118 (37.8) |
| Macroalbuminuria (≥ 300 mg g−1) | 31 (19.7) | 47 (30.3) | 78 (25.0) |
|
| |||
| Duration of Diabetes, Mean (SD) (years) | 15.54 (9.198) | 16.28 (8.977) | 15.91 (9.082) |
|
| |||
| Anti-diabetic Treatment, n (%) | |||
| Insulin | 86 (54.8) | 88 (56.8) | 174 (55.8) |
| Alpha Glucosidase Inhibitors | 8 (5.1) | 2 (1.3) | 10 (3.2) |
| Biguanides | 57 (36.3) | 73 (47.1) | 130 (41.7) |
| Dipeptidyl Peptidase 4 Inhibitors | 71 (45.2) | 59 (38.1) | 130 (41.7) |
| Glucagon-like Peptide-1 Receptor Agonists | 16 (10.2) | 11 (7.1) | 27 (8.7) |
| Meglitinides | 11 (7.0) | 12 (7.7) | 23 (7.4) |
| Sulfonylureas | 35 (22.3) | 34 (21.9) | 69 (22.1) |
| Thiazolidinediones | 13 (8.3) | 10 (6.5) | 23 (7.4) |
| Combination Oral Agents | 6 (3.8) | 6 (3.9) | 12 (3.8) |
Abbreviations: BMI = body mass index; CKD = chronic kidney disease; SBP = systolic blood pressure; SD = standard deviation; UACR = Urine Albumin-to-Creatinine Ratio. eGFR = estimated glomerular filtration rate.
Table 2:
Frequently Used Concomitant Medications
| Bexagliflozin 20 mg (N=157), n (%) |
Placebo (N=155), n (%) |
Total (N=312), n (%) |
|
|---|---|---|---|
|
| |||
| Any Concomitant Medication | 157 (100) | 154 (99.4) | 311 (99.7) |
| Drugs Used in Diabetes | 149 (94.9) | 149 (96.1) | 298 (95.5) |
| Insulin | 89 (56.7) | 91 (58.7) | 180 (57.7) |
| Non-Insulin | 125 (79.6) | 126 (81.3) | 251 (80.4) |
| Alpha Glucosidase Inhibitors | 8 (5.1) | 2 (1.3) | 10 (3.2) |
| Biguanides | 57 (36.3) | 73 (47.1) | 130 (41.7) |
| Dipeptidyl Peptidase 4 Inhibitors | 71 (45.2) | 61 (39.4) | 132 (42.3) |
| Glucagon-like Peptide-1 Receptor Agonists | 17 (10.8) | 13 (8.4) | 30 (9.6) |
| Meglitinides | 12 (7.6) | 12 (7.7) | 24 (7.7) |
| Sulfonylureas | 35 (22.3) | 37 (23.9) | 73 (23.1) |
| Thiazolidinediones | 13 (8.3) | 10 (6.5) | 23 (7.4) |
| Combination Oral Agents | 6 (3.8) | 6 (3.9) | 12 (3.8) |
| Lipid Modifying Agents | 133 (84.7) | 120 (77.4) | 253 (81.1) |
| Agents Acting on the Renin-Angiotensin System | 113 (72.0) | 117 (75.5) | 230 (73.7) |
| Antithrombotic Agents | 93 (59.2) | 102 (65.8) | 195 (62.5) |
| Drugs for Gastric Acid Related Disorders | 66 (42.0) | 75 (48.4) | 141 (45.2) |
| Diuretics | 65 (41.4) | 66 (42.6) | 131 (42.0) |
| Calcium Channel Blockers | 58 (36.9) | 67 (43.2) | 125 (40.1) |
| Beta Blocking Agents | 58 (36.9) | 59 (38.1) | 117 (37.5) |
| Analgesics | 55 (35.0) | 48 (31.0) | 103 (33.0) |
| Antibacterials For Systemic Use | 39 (24.8) | 36 (23.2) | 75 (24.0) |
| Antigout Agents | 34 (21.7) | 31 (20.0) | 65 (20.8) |
| Antiinflammatory And Antirheumatic Agents | 34 (21.7) | 25 (16.1) | 59 (18.9) |
| Antianemic Agents | 26 (16.6) | 24 (15.5) | 50 (16.0) |
| Urologicals | 23 (14.6) | 24 (15.5) | 47 (15.1) |
| Psycholeptics | 20 (12.7) | 23 (14.8) | 43 (13.8) |
Concomitant medication is any medication that the participant had been taking prior to enrollment and was expected to continue for some portion of the trial, as well as any medication other than the investigational product that was taken during the course of the trial. Medications are coded using WHO-DD version March 2016. Anatomical Therapeutic Chemical (ATC) classes and preferred terms are presented.
Change in Hemoglobin A1c from Baseline to Week 24
Subjects treated with bexagliflozin had a mean reduction in hemoglobin A1c of 0.61% whereas those treated with placebo had a mean reduction of 0.24%, yielding a placebo-corrected reduction of 0.37% [95% CI 0.20, 0.54]; p <0.001 (Figure 2). Rescue medication was provided to 26 patients, (9 in the bexagliflozin arm and 17 in the placebo arm). Including observed values after the administration of rescue medication, subjects treated with bexagliflozin had a mean reduction in hemoglobin A1c of 0.59% whereas those treated with placebo had a mean reduction of 0.31%, a placebo-corrected reduction in hemoglobin A1c of 0.28% [95% CI 0.10, 0.46]; p = 0.003.
Figure 2:
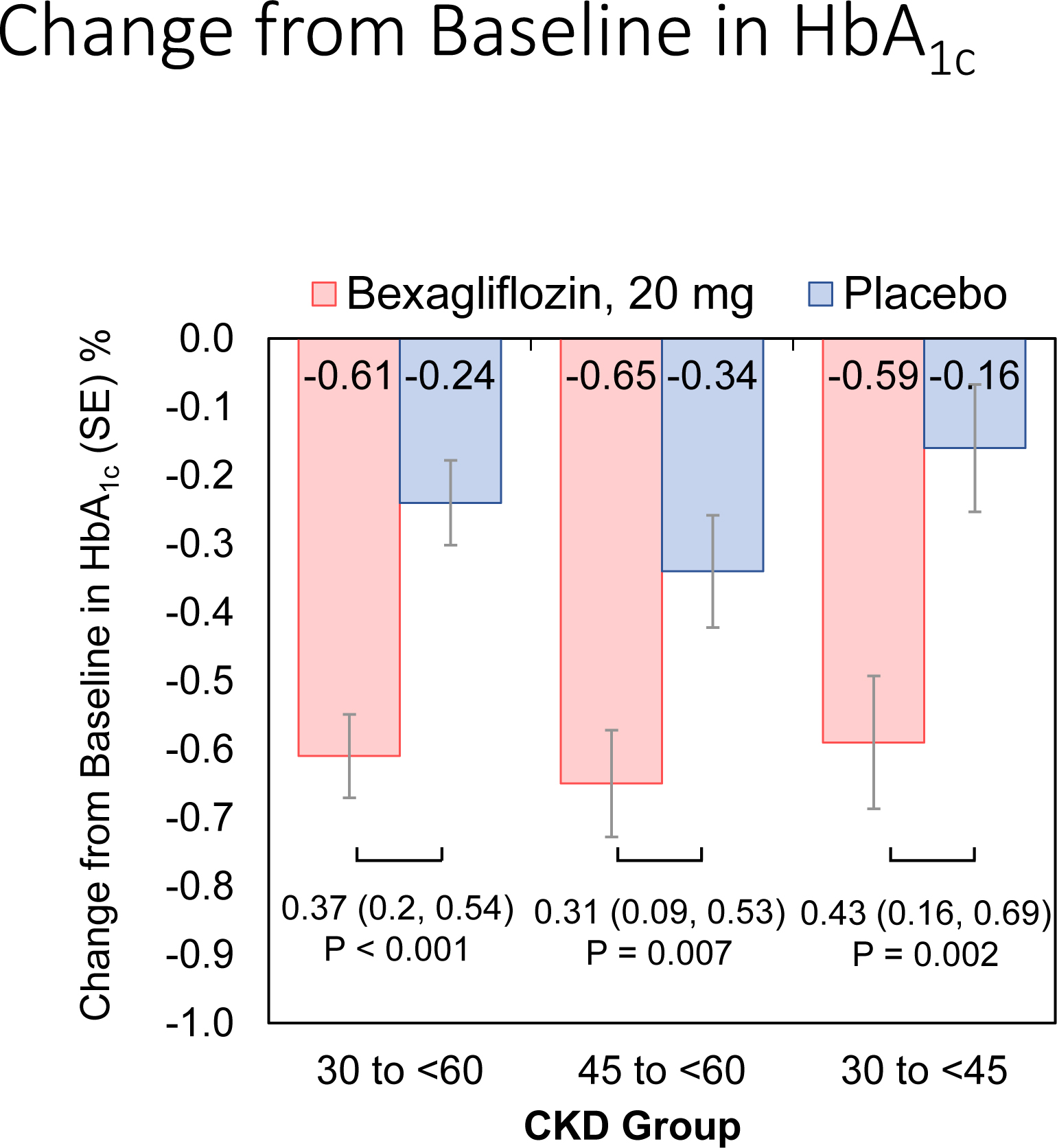
Estimated mean change from baseline in hemoglobin A1c by treatment and kidney function group at week 24
Key: HbA1c (hemoglobin A1c), SE (standard error), CKD (chronic kidney disease)
The analysis used a mixed-effects repeated measures model that included region, insulin-treated status, baseline eGFR, treatment, visit, treatment-by-visit interaction, and the baseline hemoglobin A1c value as a fixed effect covariate. An unstructured covariance matrix was assumed. Data from Weeks 6, 12, and 24 were used in the model. The last post-baseline observation before rescue medication was carried forward.
Participants with stage 3a CKD that were treated with bexagliflozin had a mean reduction in hemoglobin A1c of 0.65% whereas those treated with placebo had a mean reduction of 0.34%, a placebo-corrected reduction of 0.31% [95% CI 0.09, 0.53]; p = 0.007. Participants in stage 3b CKD that were treated with bexagliflozin had a mean reduction in hemoglobin A1c of 0.59% whereas those treated with placebo had a mean reduction of 0.16%, a placebo-corrected reduction of 0.43% [95% CI 0.16, 0.69]; p = 0.002 (Figure 2). The magnitudes of the reduction in hemoglobin A1c in the bexagliflozin arm were similar between the stage 3a and stage 3b populations, but the placebo effect was more pronounced in the stage 3a population, leading to an apparent increase in treatment effect in those with more severe kidney disease.
Other Endpoints at 24 Weeks
Change in hemoglobin A1c over time is reported in Figure 3A. At 24 weeks, bexagliflozin increased the proportion of patients who achieved hemoglobin A1c < 7% (34% vs. 22%; p = 0.007). The treatment effect was apparent as early as 6 weeks (p = 0.001) and the trend continued for the entire course of 24 weeks with an odds ratio of 2.26 favoring bexagliflozin treatment (Figure 3B). Bexagliflozin treatment over 24 weeks resulted in an average 1.61 kg body weight reduction compared to placebo (95% CI 1.00, 2.22; p < 0.001). The body weight reduction was consistent throughout the 24-week treatment period (Figure 3C). More bexagliflozin-treated patients with baseline BMI ≥ 25 kg m−2 had a ≥ 5% decrease in body weight compared to placebo (19% vs. 9%, p = 0.03). Bexagliflozin lowered SBP by 3.8 [95% CI 0.6, 7.1] mm Hg (p = 0.02; Figure 3D) and decreased fasting plasma glucose by 0.76 [95% CI 0.26, 1.26] mmol/L (p = 0.003; Figure 3E). A decrease in diastolic blood pressure of 3.0 mm Hg was observed in bexagliflozin-treated patients, though this was not meaningfully different from those who received placebo (2.1 mm Hg). At week 24, those treated with bexagliflozin had a relative reduction in geometric mean ratio of UACR of 20.1% [95% CI 2.52%, 34.56%]; p = 0.03 (Figure 3F).
Figure 3:
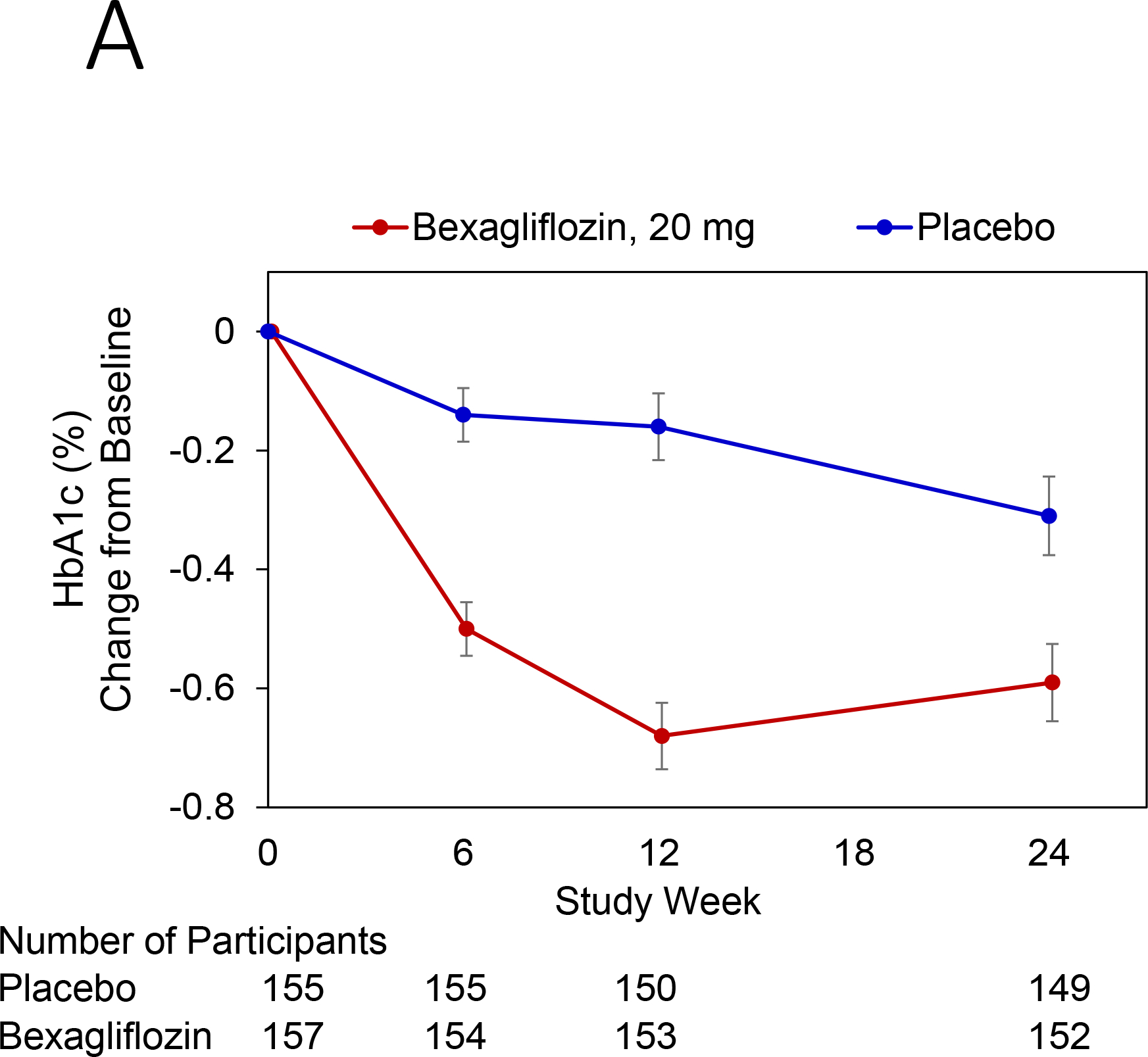
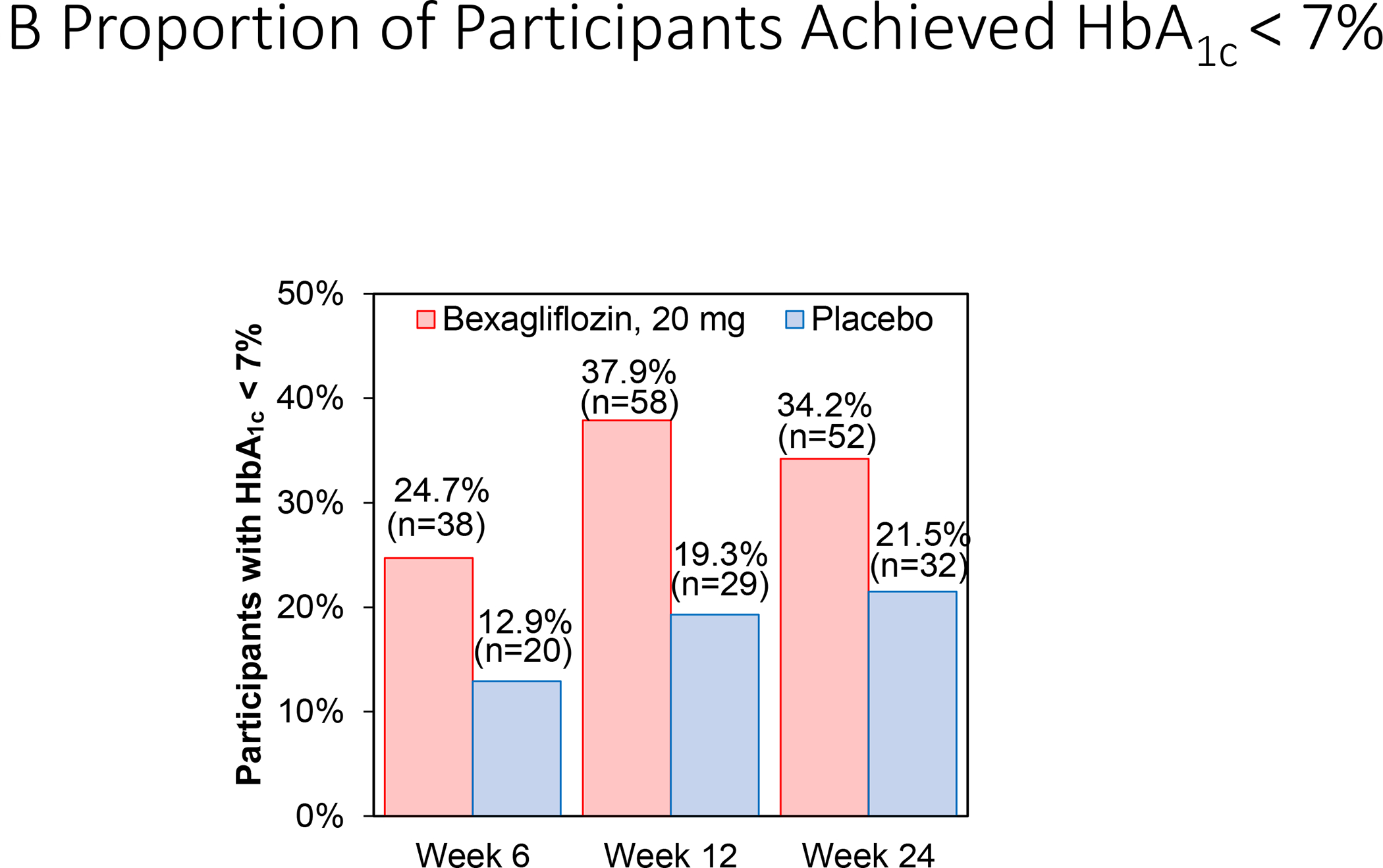
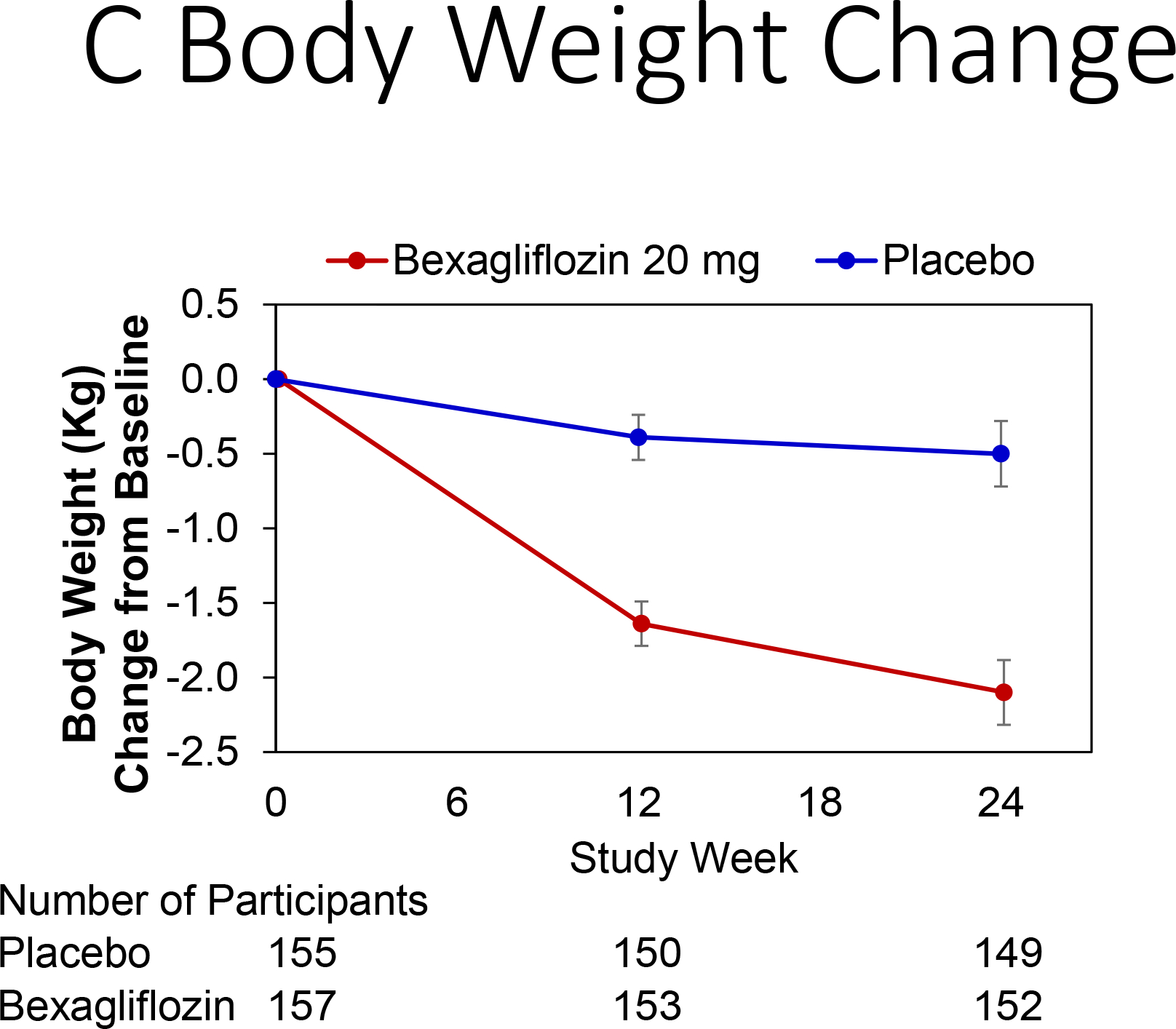
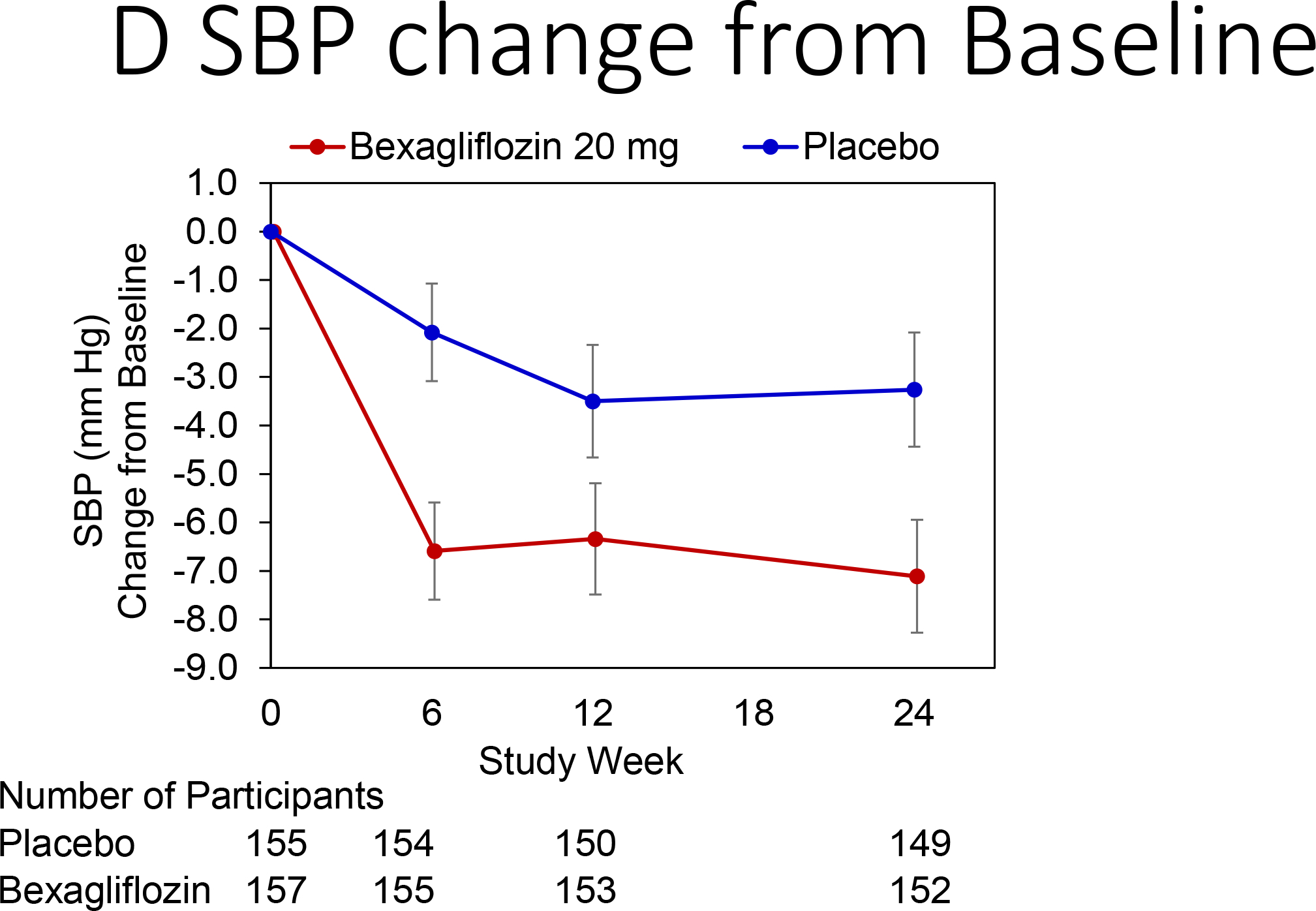
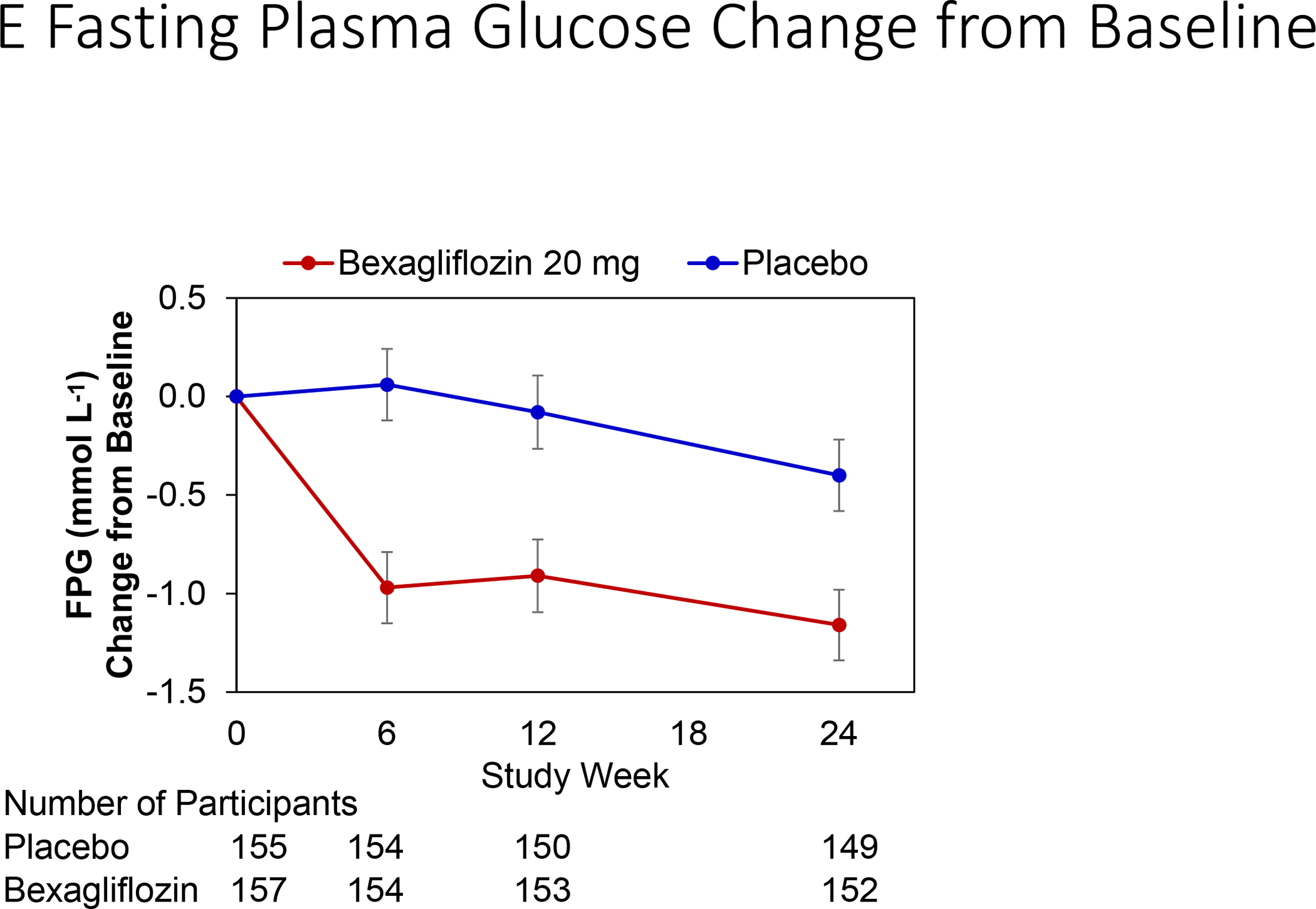
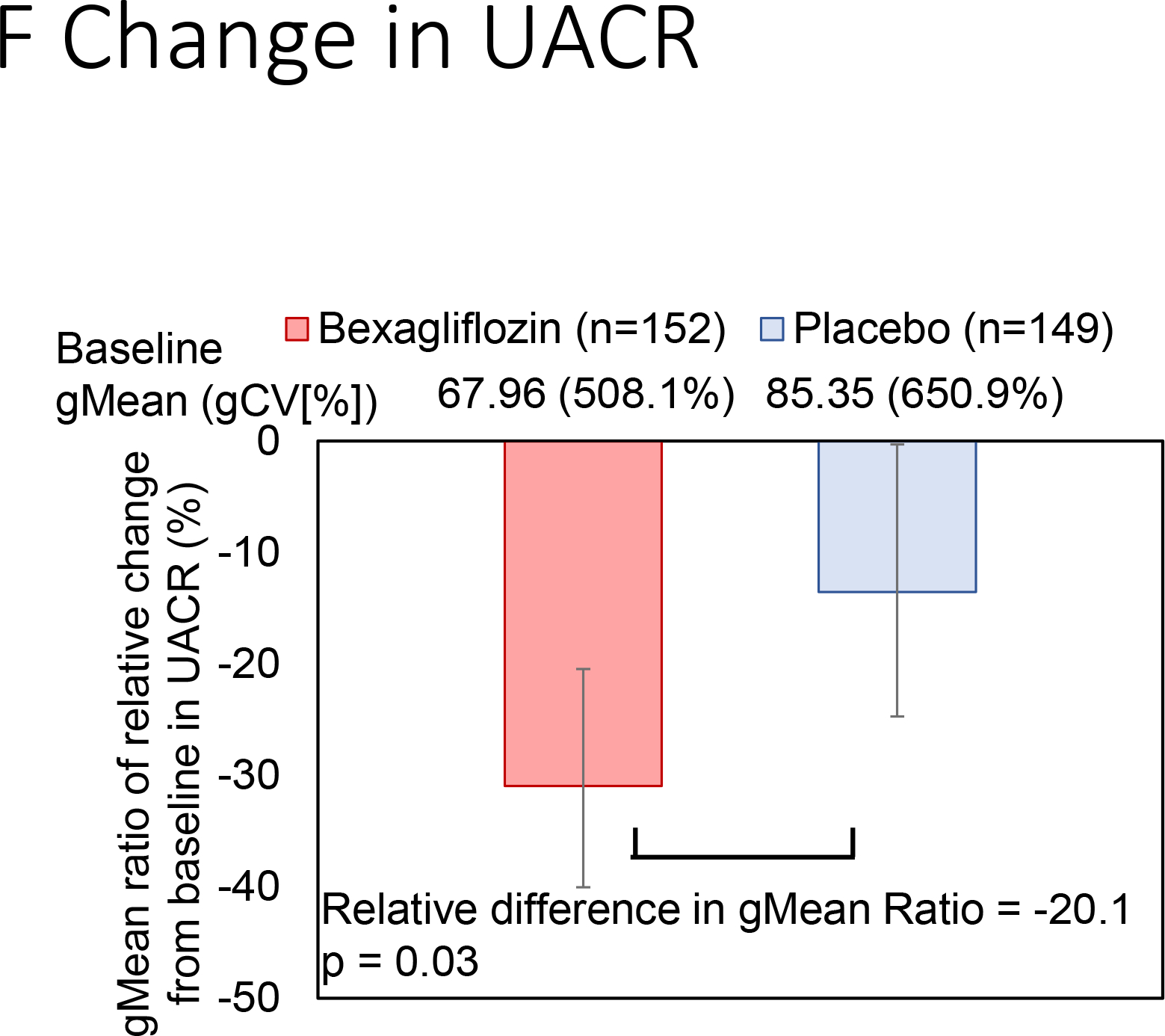
Change over time of clinical outcomes by treatment group. Panels: (A) Change in hemoglobin A1c, (B) Proportion of patients who achieved hemoglobin A1c <7%, (C) Change in body weight, (D) Change in systolic blood pressure, (E) Change in fasting plasma glucose, and (F) Change in albuminuria (UACR) at week 24
Key: HbA1c (Hemoglobin A1c), SBP (systolic blood pressure), FPG (fasting plasma glucose), UACR (urinary albumin-creatinine ratio), gMean (geometric mean), gCV (geometric coefficient of variation)
(A), (C), (D), (E): Estimated Mean (± SE) change from baseline in hemoglobin A1c (%), body weight (kg), SBP (mm Hg), and fasting plasma glucose (mmol L−1) for all subjects were summarized. The full model is a mixed-effects repeated measures analysis.
(B) Proportion of subjects with HbA1c < 7% over time was summarized using mixed-effects logistic regression for repeated measures analysis. An unstructured covariance matrix is assumed.
(F) Adjusted geometric mean ratios of relative change from baseline in UACR was calculated as (gmean of week 24 /gmean of baseline) and their 95% CIs calculated as the antilog of the least squares means and 95 % CI of change from baseline in log-transformed, are values minus 1, converted to percentage.
Safety Endpoints
Overall, treatment emergent adverse events were reported for 214 patients (109 [69%] bexagliflozin vs. 105 [68%] placebo). Serious adverse events were reported for 20 patients (11 [7%] bexagliflozin vs. 9 [6%] placebo). An adverse event led to withdrawal from the study for 5 patients (1 [1%] bexagliflozin vs. 4 [3%] placebo) and discontinuation of study drug for 11 patients (4 [3%] bexagliflozin vs. 7 [5%] placebo). No patients died during the study period (Table 3).
Table 3:
Adverse Events by Randomization Status
| Bexagliflozin N=157, n (%) |
Placebo N=155, n (%) |
Total N=312, n (%) |
|
|---|---|---|---|
|
| |||
| Subjects with any TEAEs | 109 (69.4) | 105 (67.7) | 214 (68.6) |
|
| |||
| Total reports of TEAEs | 562 | 533 | 1095 |
|
| |||
| Subjects with any Treatment Related AEs 1 | 60 (38.2) | 42 (27.1) | 102 (32.7) |
|
| |||
| Subjects with any Serious Adverse Event | 11 (7.0) | 9 (5.8) | 20 (6.4) |
|
| |||
| Subjects with any Serious Treatment Related AEs 1 | 1 (0.6) | 0 | 1 (0.3) |
|
| |||
| Subjects with AEs Leading to Dosing Discontinuation 2 | 4 (2.5) | 7 (4.5) | 11 (3.5) |
|
| |||
| Subjects with AEs Leading to Subject Discontinuation 3 | 1 (0.6) | 4 (2.6) | 5 (1.6) |
|
| |||
| Subjects with AE Leading to Death | 0 | 0 | 0 |
|
| |||
| Subjects with any TEAE of interest | 74 (47.1) | 59 (38.1) | 133 (42.6) |
|
| |||
| Hypoglycemia | 39 (24.8) | 38 (24.5) | 77 (24.7) |
| Diuretic Effects | 18 (11.5) | 5 (3.2) | 23 (7.4) |
| UTI | 11 (7.0) | 5 (3.2) | 16 (5.1) |
| Acute Kidney Injury | 8 (5.1) | 6 (3.9) | 14 (4.5) |
| Falls and Fractures | 7 (4.5) | 6 (3.9) | 13 (4.2) |
| Hypotension Episode | 6 (3.8) | 5 (3.2) | 11 (3.5) |
| Malignancies | 3 (1.9) | 4 (2.6) | 7 (2.2) |
| Genital Mycotic Infection | 5 (3.2) | 0 | 5 (1.6) |
| Rash | 0 | 3 (1.9) | 3 (1.0) |
| Acid-Base Disorder | 1 (0.6) | 0 | 1 (0.3) |
| Syncope | 1 (0.6) | 0 | 1 (0.3) |
| Amputation | 1 (0.6) | 0 | 1 (0.3) |
| MACE (adjudicated) | 2 (1.3) | 0 | 2 (0.6) |
Key: TEAE=Treatment-Emergent Adverse Events; AE=Adverse Event. UTI=Urinary Tract Infection; MACE=Major Adverse Cardiac Event
AEs are considered treatment-related if the causality is definite, probable, possible or missing.
Permanent discontinuation of study drug due to AE.
Subject withdrew due to AE.
Hypoglycemia was the most common adverse event of interest (39 [25%] bexagliflozin vs. 38 [25%] placebo), followed by diuretic effects (18 [11%] bexagliflozin vs. 5 [3%] placebo), urinary tract infections (11 [7%] bexagliflozin vs. 5 [3%] placebo) and AKI (8 [5%] bexagliflozin vs. 6 [4%] placebo). All AKI events were stage I AKI. Stage II/III AKI or a need for kidney replacement therapy was not reported. AKI events did not lead to any withdrawals. New malignancies were uncommon (3 [2%] bexagliflozin vs. 4 [3%] placebo). There were five genital mycotic infections in the bexagliflozin arm, one amputation in the bexagliflozin arm, and seven falls/fractures in the bexagliflozin arm (compared to six in the placebo arm). There were 2 adjudicated MACE events, both in the bexagliflozin arm. There were no cases of diabetic ketoacidosis (DKA) in either arm (Table 3).
Kidney Function Tests
Kidney function tests and changes from baseline values are displayed in Figure 4. A slight increase in serum creatinine concentration and decrease in eGFR affected subjects assigned to the bexagliflozin arm throughout the treatment period. An increase in creatinine of 0.08 mg dL−1 and a decrease of eGFR of 2.41 mL min−1 per 1.73 m2 were observed at week 24. Two weeks after the end of the treatment period, the eGFR for the bexagliflozin arm had increased to near-baseline values (net increase from baseline 1.37 mL min−1 per 1.73 m2).
Figure 4:
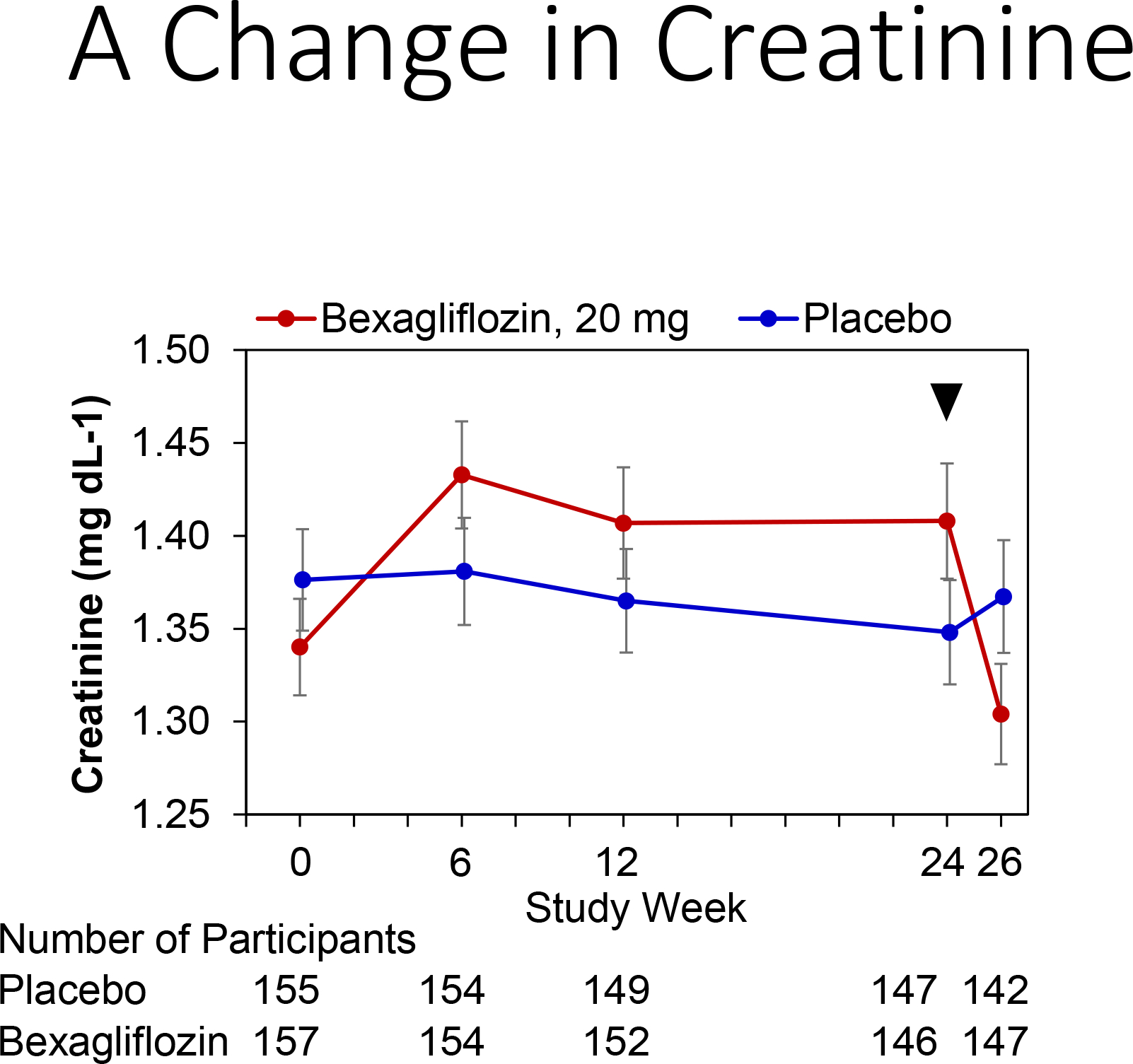
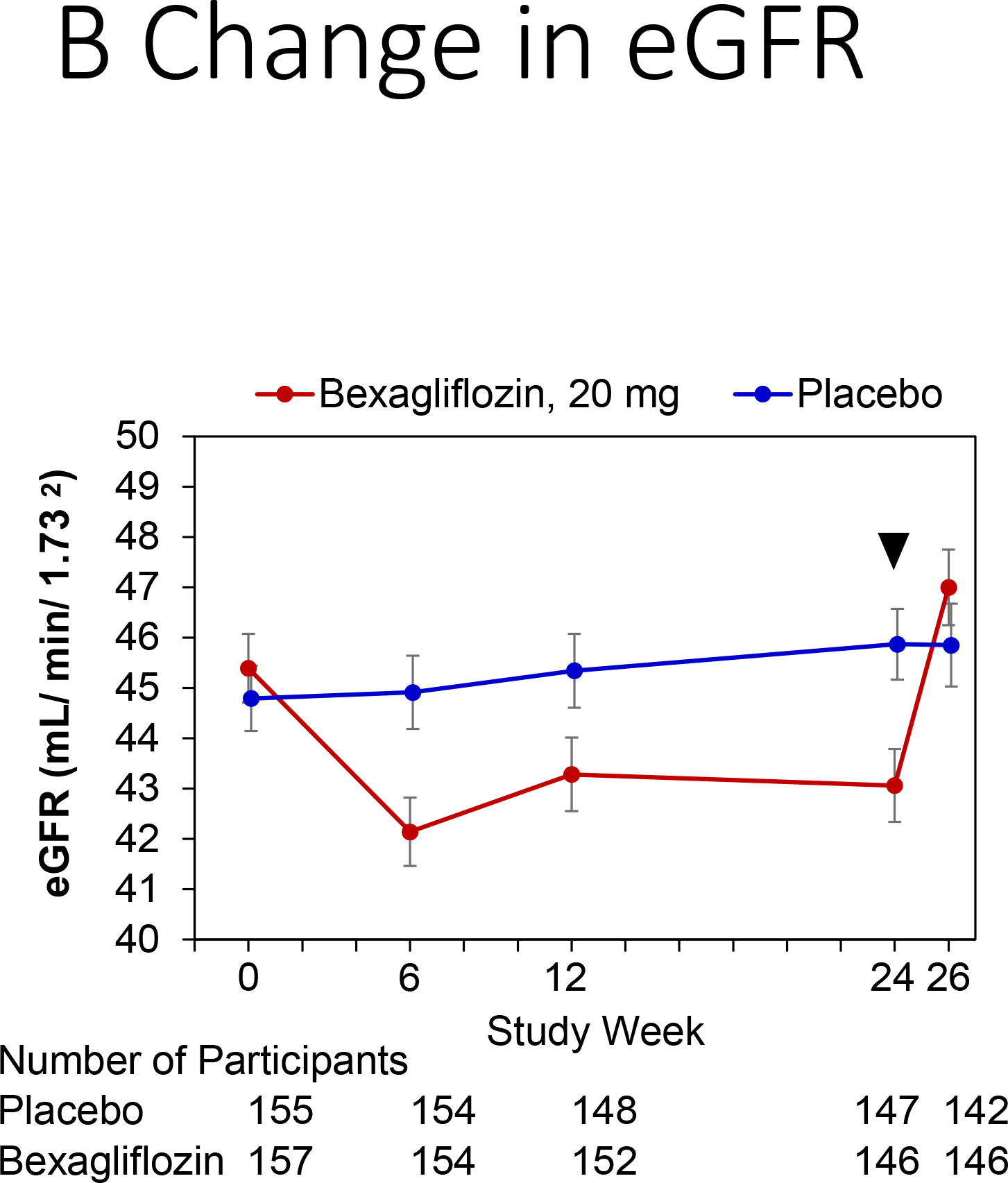
Change in kidney function by treatment group.
Panels: (A) Change in creatinine, (B) Change in estimated glomerular filtration rate (eGFR). Bexagliflozin/placebo was dosed through week 24, with an arrow denoting last dose.
Discussion
Bexagliflozin is a novel member of the SGLT2 inhibitor class currently in phase 3 development. In earlier studies in patients with eGFR > 60 mL min−1 per 1.73 m2, bexagliflozin demonstrated good safety and efficacy, with hemoglobin A1c reductions comparable to those of the already approved members of the class (ClinicalTrials.gov NCT02715258, NCT02769481, NCT02956044). In the current study, we aimed to examine the impact of bexagliflozin in patients with significant kidney impairment to determine if hemoglobin A1c lowering efficacy was preserved, despite a reduction in eGFR. In a study population consisting of predominantly older individuals with inadequately controlled diabetes and CKD stage 3a/3b, exposure to bexagliflozin produced a statistically significant and clinically meaningful decrease in hemoglobin A1c. The effect was more marked in the sub-population with eGFR between 30 and < 45 (CKD stage 3b), an outcome that was ascribed to a larger placebo effect on hemoglobin A1c in participants with an eGFR between 45 and 60 (CKD stage 3a). Similar positive, short-term effects on body weight, systemic blood pressure, albuminuria and eGFR have been reported for other drugs in the class.13,15 However, to the best of our knowledge, no previous study has reported a statistically significant A1c lowering effect in diabetic patients with stage 3b CKD.
As eGFR declines, less serum glucose is filtered by the glomerulus. SGLT2 inhibitors are expected to have diminished capacity to increase urinary glucose excretion and lower plasma glucose concentration in the setting of kidney failure.12,17 In the current study, the absolute decrease in hemoglobin A1c observed for subjects in the bexagliflozin arm was consistent with or better than expectations based on eGFR,25 and persisted after being controlled for placebo effect and the contribution of rescue medications. In contrast to the data of the current study, data from studies of other SGLT2 inhibitors in subjects with CKD stage 3a/3b have failed to show A1c lowering effects.19,20 Further study is needed to determine the properties of SGLT2 inhibitors that allow for persistent effectiveness in lowering hemoglobin A1c at lower eGFR. There may also be other transport mechanisms that influence SGLT2 inhibitor action. For example, in mice, the glucosuric effect of empagliflozin has been shown to be dependent on active transport of empagliflozin into the proximal tubule by the organic anion transporter OAT3.26 The structure-activity relationship for SGLT2 inhibitors and OAT3 transport has not been elucidated in humans.
The changes in eGFR and albuminuria observed in this study were consistent with reports from studies of other SGLT2 inhibitors and of renin-angiotensin-aldosterone axis inhibitors that have demonstrated kidney protective effects.5,13,15 Long term assessments of the effects of empagliflozin and canagliflozin have reported an initial reduction in eGFR within weeks of exposure to study drug, followed by a prolonged plateau in eGFR and an increase in eGFR following the end of the treatment period. The net effect was a reduction in long-term GFR decline compared to placebo. We observed a similar initial decrease in eGFR followed by a plateau over 24 weeks, with an appropriate rebound after the end of the treatment period in this study. A plausible mechanism for eGFR reduction invokes tubulo-glomerular feedback mediated by an increased delivery of solute in the form of sodium chloride to the macula densa, resulting from the osmotic diuresis produced by SGLT2 inhibition. The effect of the reduced filtration rate, combined with the possible beneficial influence of lower glucotoxicity, may account for the renoprotective action of SGLT2 inhibitors. A sustained reduction of eGFR has been documented in studies of ACE inhibitors and angiotensin receptor blockers that have since been incorporated in practice guidelines for the management of diabetic patients with CKD.22 Other SGLT2 inhibitors have shown the prompt reduction in albuminuria16 observed in this study. Whether this effect on albuminuria is secondary to reduced filtration has not been established.
Reduction in body weight and SBP were observed in patients treated with bexagliflozin. These findings may be due to caloric wasting and proximal tubular diuretic effects that result from increased urinary glucose excretion. Other proposed mechanisms involve concurrent increased urinary sodium excretion, uric acid excretion and effects attributable to interactions with the renin-aldosterone and sympathetic nervous systems.25 While outside the direct scope of this trial, long-term study of other SGLT2 inhibitors have shown a cardiovascular mortality benefit.13,14 Further study is needed to elucidate the interactions and mechanisms behind this clinical observation, given the positive effects SGLT2 inhibitors have demonstrated towards cardiac risk factors, such as weight, blood pressure, kidney function, and glycemic control.
In general, bexagliflozin was well-tolerated but increased adverse events in the categories of urinary tract infection and genital mycotic infections were found in this study. These findings have been previously attributed to SGTL2 inhibition. The findings of mild volume depletion, weight loss and reduction in systolic blood pressure reported here are consistent with the expected osmotic diuresis and caloric wasting induced by SGLT2 inhibition. Similarly, the elevation of serum creatinine concentration, described as acute kidney injury, is consistent with the pharmacology of the SGLT2 inhibitor class and the expected effect of natriuresis on glomerular filtration rate. Other serious adverse events were rare and included two MACE events in the bexagliflozin arm, one amputation in the bexagliflozin arm, and seven falls/fractures in the bexagliflozin arm (compared to six in the placebo arm).
This trial should be interpreted in the context of its limitations. The number of patients in this trial was too small to evaluate changes in frequency of rare adverse events. Although the short-term kidney and blood pressure findings are encouraging, this study was not specifically powered or designed to evaluate reno- or vaso-protective effects. Larger trials evaluating cardiovascular outcomes of bexagliflozin are ongoing.
Patients with diabetes and mild to moderate kidney failure have fewer treatment options compared to those with preserved kidney function. Bexagliflozin appears to be beneficial for intensification of glycemic control for patients in this vulnerable condition. Additional therapeutic advantages of bexagliflozin include reduction in body weight, systolic blood pressure and albuminuria. The results of this study support the conduct of additional investigations on the renoprotective potential of bexagliflozin for the management of diabetic kidney disease.
Supplementary Material
Acknowledgements:
The authors would like to thank all study participants, clinical investigators and study staff for their dedication and effort, as well as the Translational Medicine Group at Massachusetts General Hospital for their logistical and academic support.
Support/Financial Disclosures:
Trial was funded by Theracos Sub, LLC. The study funders did not have a role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication. ASA, TT, MWF, and YDH have received research support from Theracos Sub, LLC.
List of Abbreviations:
- CKD
chronic kidney disease
- SGLT2
sodium-glucose cotransporter-2
- eGFR
estimated glomerular filtration rate
- IRB
institutional review board
- BMI
body mass index
- SBP
systolic blood pressure
- UACR
urine albumin to creatinine ratio
- AKI
acute kidney injury
- MACE
major adverse cardiac event
- MMRM
mixed-effect model repeated measures
- ANCOVA
analysis of covariance model
- KDIGO
kidney disease improving global outcomes
- DKA
diabetic ketoacidosis
- MDRD
modification of diet in renal disease
Footnotes
Prior Presentation: Results from this trial were presented as an oral abstract during the High Impact Clinical Trials session at ASN Kidney Week 2018 in San Diego, CA.
Peer Review: Received October 30, 2018. Evaluated by 2 external peer reviewers, with direct editorial input from a Statistics/Methods Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form March 7, 2019.
Data Sharing:
Individual participant data will not be publically available. Other supporting documents (study protocol, statistical analysis plan, informed consent form, clinical study report, analytic code) will not be available. Study design is available on clinicaltrials.gov (NCT02836873).
References
- 1.Emerging Risk Factors C, Sarwar N, Gao P, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes A 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S13–S27. [DOI] [PubMed] [Google Scholar]
- 3.National Kidney F KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60(5):850–886. [DOI] [PubMed] [Google Scholar]
- 4.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305(24):2532–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coll-de-Tuero G, Mata-Cases M, Rodriguez-Poncelas A, et al. Chronic kidney disease in the type 2 diabetic patients: prevalence and associated variables in a random sample of 2642 patients of a Mediterranean area. BMC Nephrol. 2012;13:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Group AC, Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. [DOI] [PubMed] [Google Scholar]
- 7.Roscioni SS, Heerspink HJ, de Zeeuw D. The effect of RAAS blockade on the progression of diabetic nephropathy. Nat Rev Nephrol. 2014;10(2):77–87. [DOI] [PubMed] [Google Scholar]
- 8.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 10.Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA. 2014;312(24):2668–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu WH, Hsiao PJ, Lin PC, Chen SC, Lee MY, Shin SJ. Effect of metformin on kidney function in patients with type 2 diabetes mellitus and moderate chronic kidney disease. Oncotarget. 2018;9(4):5416–5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheen AJ. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75(1):33–59. [DOI] [PubMed] [Google Scholar]
- 13.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377(7):644–657. [DOI] [PubMed] [Google Scholar]
- 14.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373(22):2117–2128. [DOI] [PubMed] [Google Scholar]
- 15.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375(4):323–334. [DOI] [PubMed] [Google Scholar]
- 16.Petrykiv S, Sjostrom CD, Greasley PJ, Xu J, Persson F, Heerspink HJL. Differential Effects of Dapagliflozin on Cardiovascular Risk Factors at Varying Degrees of Renal Function. Clin J Am Soc Nephrol. 2017;12(5):751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheen AJ. Pharmacokinetics, Pharmacodynamics and Clinical Use of SGLT2 Inhibitors in Patients with Type 2 Diabetes Mellitus and Chronic Kidney Disease. Clin Pharmacokinet. 2015;54(7):691–708. [DOI] [PubMed] [Google Scholar]
- 18.Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2(5):369–384. [DOI] [PubMed] [Google Scholar]
- 19.Grunberger G, Camp S, Johnson J, et al. Ertugliflozin in Patients with Stage 3 Chronic Kidney Disease and Type 2 Diabetes Mellitus: The VERTIS RENAL Randomized Study. Diabetes Ther. 2018;9(1):49–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85(4):962–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W, Welihinda A, Mechanic J, et al. EGT1442, a potent and selective SGLT2 inhibitor, attenuates blood glucose and HbA(1c) levels in db/db mice and prolongs the survival of stroke-prone rats. Pharmacol Res. 2011;63(4):284–293. [DOI] [PubMed] [Google Scholar]
- 22.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 23.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Annals of Internal Medicine. 2006;145(4):247–254. [DOI] [PubMed] [Google Scholar]
- 24.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney international supplements. 2012;2(1):6. [Google Scholar]
- 25.van Bommel EJ, Muskiet MH, Tonneijck L, Kramer MH, Nieuwdorp M, van Raalte DH. SGLT2 Inhibition in the Diabetic Kidney-From Mechanisms to Clinical Outcome. Clin J Am Soc Nephrol. 2017;12(4):700–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu Y, Breljak D, Onishi A, et al. Organic anion transporter OAT3 enhances the glucosuric effect of the SGLT2 inhibitor empagliflozin. Am J Physiol Renal Physiol. 2018;315(2):F386–F394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant data will not be publically available. Other supporting documents (study protocol, statistical analysis plan, informed consent form, clinical study report, analytic code) will not be available. Study design is available on clinicaltrials.gov (NCT02836873).


