Abstract
Flint, Michigan reignited the public discourse surrounding lead contamination in drinking water with Newark, New Jersey recently experiencing its own lead‐in‐water crisis. Following Flint's experience, the Environmental Protection Agency proposed changes to the Lead and Copper Rule (LCR), but these changes may not produce better detection of contamination. LCR testing requirements were evaluated for their ability to predict or identify problems from the recent (2015–2019) Newark lead exceedance data. LCR compliance and water quality data were obtained from the New Jersey Department of Environmental Protection (NJDEP) website. Between 2002 and 2015, Newark sampled on a reduced sampling plan (50 samples once every 3 years), as required, for lead and copper. These samples were divided between Newark's two water sources with uneven sampling distribution across the city, further limiting the potential to identify a risk of lead in drinking water. Results suggest a more rigorous testing requirement may have identified the problem sooner. Limitations related to the LCR that prevented Newark water suppliers from earlier detection of lead risk will continue under the revised LCR.
This article is categorized under:
Engineering Water > Water, Health, and Sanitation
Science of Water > Water Quality
Keywords: lead, Lead and Copper Rule, water
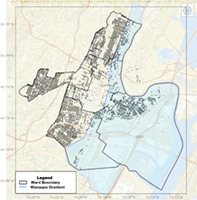
This figure shows Newark has two different water sources, with uneven distribution of lead service lines across the service areas. These two water sources are treated as one system under the Lead and Copper Rule (LCR) leading to insufficient sampling and potential misclassification of exposures to lead in drinking water. The Wanaque gradient is shaded in blue, while the Pequannock gradient is the unshaded portion of the City of Newark.
1. INTRODUCTION
1.1. Lead in drinking water
Lead is an elemental contaminant not expected to be present in water leaving treatment plants (Brown & Margolis, 2012). Drinking water contamination usually occurs from lead pipes or lead bearing plumbing parts (Triantafyllidou & Edwards, 2012). Dissolved lead (soluble lead) and particulate lead are the two types of lead present in drinking water. Dissolved lead is usually found in improperly treated water or in water from lead bearing plumbing (B. Clark et al., 2014). Particulate lead occurs when pipe scales (material build up on the inner pipe surfaces) are no longer resistant or adhesive (B. Clark et al., 2014). Spikes in particulate lead can occur when water flow changes or physical/chemical disturbances occur, releasing particulate lead from pipe scales (B. Clark et al., 2014). The total lead concentration is dependent on multiple elements including lead sources, such as lead or brass plumbing fixtures, leaded solder, or a lead service line (LSL), and water characteristics, such as corrosion control, water flow rates, and pipe scale characteristics (B. N. Clark et al., 2015).
1.2. The safe drinking water act
In 1974, The Safe Drinking Water Act (SDWA) was established by congress. This act protects the quality of all U.S. water sources designed for drinking with the exception of private wells. In 1986, the SDWA was amended, prohibiting the use of leaded plumbing components including LSLs and goosenecks in new construction requiring all plumbing to be “lead free” (EPA, 2020). “Lead free” was defined as “solder and flux with no more than 0.2% lead by weight and pipes and fixtures with no more than 8% lead by weight” (EPA., 2020). This “lead free” definition was further reduced by the 2011 SDWA amendment to “no more than a weighted average of 0.25% calculated across the wetted surfaces of pipes, pipe fittings, plumbing fittings, and fixtures, and 0.2% for solder and flux” (EPA, 2020). LSLs may contribute 50%–75% of lead in drinking water when present (Triantafyllidou & Edwards, 2012). There are an estimated 6.1–10.2 million LSL across the United States (Cornwell et al., 2016).
1.3. Health effects of lead
Lead is a bio accumulative toxicant, which effects multiple organ systems. (ATSDR, 2020). The neurodevelopmental effects of even low‐level lead exposure disproportionately effects children, and there is no known safe exposure level (NTP, 2012; Ruckart et al., 2021; WHO, 1995). Drinking water is estimated to make up at least 20% of an individual's total lead exposure (EPA, 2020). Tap water can contribute 40%–60% of an infant's exposure when used in formula (EPA, 2020). In children, blood lead levels have been associated with lead in drinking water (Ngueta et al., 2016). Modeling conducted using the U.S. Environmental Protection Agency's (EPA) Integrated Exposure Uptake Biokinetic (IEUBK) model, indicates that at a water lead level of 7 ppb, 25% of 1–2‐year‐old children drinking 500 ml/day of tap water would exceed a blood lead level of 5 μg/dl (Triantafyllidou et al., 2014). For 0–1‐year‐old infants consuming reconstituted baby formula at a rate of 800 ml/day, a water lead level of 4 ppb would result in 5% of those infants having a blood lead level above 5 μg/dl (Triantafyllidou et al., 2014).
2. THE LEAD AND COPPER RULE
2.1. Lead and copper rule: History/current
The U.S. Environmental Protection Agency (EPA) established the Lead and Copper Rule (LCR) in 1991. The LCR established treatment techniques to reduce lead and copper in drinking water systems when levels exceed action limits. An action level of 15 μg/L (15 parts per billion [ppb]) for lead and 1.3 mg/L (1.3 parts per million [ppm]) for copper were established. If lead or copper concentrations exceed these levels in at least 10% of tap water samples, mitigative action is required. These actions include optimizing corrosion control, replacing LSLs, and informing the public on steps individuals can take to protect health. The LCR requires utilities to develop and maintain records of LSLs in its distribution system (141.84[a]). The action level is based on “technical feasibility” using available data on corrosion control rather than on health effects from exposure to lead or copper (EPA, 2020). The maximum contaminant level goal (MCLG) for lead was set to zero (0 ppb) based on emerging science (EPA, 2020). The Natural Resources Defense Council (NRDC) estimates that approximately 5.5 million people were supplied water by drinking water systems in exceedance of 15 ppb for lead between 2015 and 2018 (NRDC, 2018).
The LCR dictates that first‐draw 1‐liter samples are to be collected from residential homes at the kitchen or bathroom tap using cold water after at least 6 h of stagnation. Homes within a water system are divided into three tiers. Tier 1 sites are single‐family homes built between 1983 and 1988 containing copper pipes with lead solder, lead pipes, or have an LSL. Multiple‐family residences with the same criteria as Tier 1 are designated as Tier 2. Tier 3 sites were homes built before 1983 and contain lead soldered copper pipes. When multiple‐family residences comprise at least 20% of the structures served by the water system, the system may include these types of structures in its Tier 1 sampling pool (141.86[a]), otherwise. Tier 2 and 3 sites may only be used if insufficient Tier 1 sites exist.
2.2. Lead and copper rule: Proposed revisions
The following relevant changes to the LCR have been proposed by the EPA to take effect on October 16, 2024 (EPA, 2020). First, in addition to an action level set at 15 ppb for lead, a “trigger level” will be placed at 10 ppb. If a lead action level exceedance occurs, the water system must replace 3% of LSLs annually until the system measures below 15 ppb for four consecutive monitoring periods (40 CFR 141.84[g]). Exceedance of the trigger level results in public notification and “goal‐based” LSL replacement until two consecutive monitoring periods are below 10 ppb (40 CFR 141.84[f]). After replacement of an LSL, the water system must provide a pitcher filter certified to remove lead along with 3 months of replacement cartridges to the resident (40 CRF 141.84[e][iii]). The water system must also collect one follow up sample post replacement, between three and 6 months after replacement (141.84[e][iv]).
Community Water Systems (CWS) that are above the action level for lead or copper are required to monitor semi‐annually (40 CFR 141.86[d][A]). Systems that exceed 10 ppb for lead but are below 15 ppb, will be required to monitor for lead annually and copper triennially (40 CFR 141.86[d][B]). Systems below 10 ppb for lead and 1.3 ppm for copper in consecutive monitoring periods are eligible for reduced monitoring (40 CFR 141.86[d][4]).
The tap sampling strategy is also changing. In systems with LSLs, any home that has an LSL will be sampled for the first and fifth liter of water. The first liter will be analyzed for copper and the fifth liter will be analyzed for lead. For homes without an LSL, the first liter will be analyzed for both lead and copper.
Finally, any water system that is currently utilizing corrosion control treatment (CCT), will be required to conduct a CCT study if the trigger level is exceeded (40 CFR 141.81[a][1]). Water systems that currently do not use CCT may be required to conduct a CCT study by the Primacy Agency upon a trigger level exceedance. If an action level exceedance occurs, water systems must complete CCT installation if they currently do not use CCT, or the water system must re‐optimize their CCT. Utilities may also be required to conduct a CCT study prior to a source water or treatment change, or if the EPA or state regulatory agency determines the current CCT to be not optimal (141.81[b][3][iii]). These new requirements will increase the number of water systems needing to evaluate CCT and potentially change their current CCT.
3. CASE STUDY: NEWARK, NEW JERSEY
3.1. Sociodemographic characteristics
Newark has the highest population of any city in New Jersey with 311,549 residents: 49.5% are African American or Black, 36.7% are Hispanic, and 6.6% identify as two or more races (U.S. Census, 2020). The median household income is $37,476 with 26.3% of residents living in poverty and only 23.5% of households are owner‐occupied. Given its disadvantaged socioeconomic status, Newark is a vulnerable community susceptible to poor health status and increased rates of disease (NJHA, 2019).
3.2. Drinking water sources
Newark's drinking water distribution system uses two geographically distinct sources. The Pequannock Water Treatment Plant (WTP) serves customers in the West Ward and portions of the North, Central, and South Wards, and the Wanaque WTP serves the East Ward and the remaining portions of the North, Central, and South Wards. These two WTPs have their own source waters, dedicated customers, corrosion control strategies, and differing water parameters (e.g., pH, flow rate, etc.; CDM Smith, 2019a; Lytle et al., 2020). Each system serves more than 50,000 persons but operate as one CWS in Newark (Figure 1).
FIGURE 1.
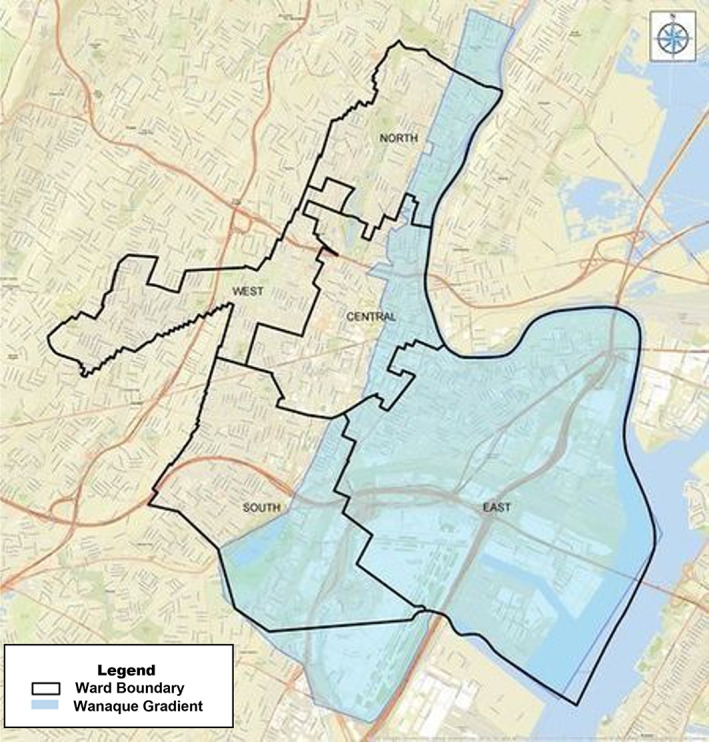
Newark water system boundary, Wanaque gradient in blue, Pequannock gradient is the section not shaded within the city (obtained from City of Newark).
3.3. Lead service lines
The City of Newark's Water and Sewer System includes more than 1000 miles of pipes that are more than 100 years old. In 2019, the City of Newark identified approximately 23,800 LSLs within Newark; therefore, approximately 60% of the City's 39,255 service lines were LSLs at that time (Newark, 2019). The locations of these LSLs are shown in Figure 2.
FIGURE 2.
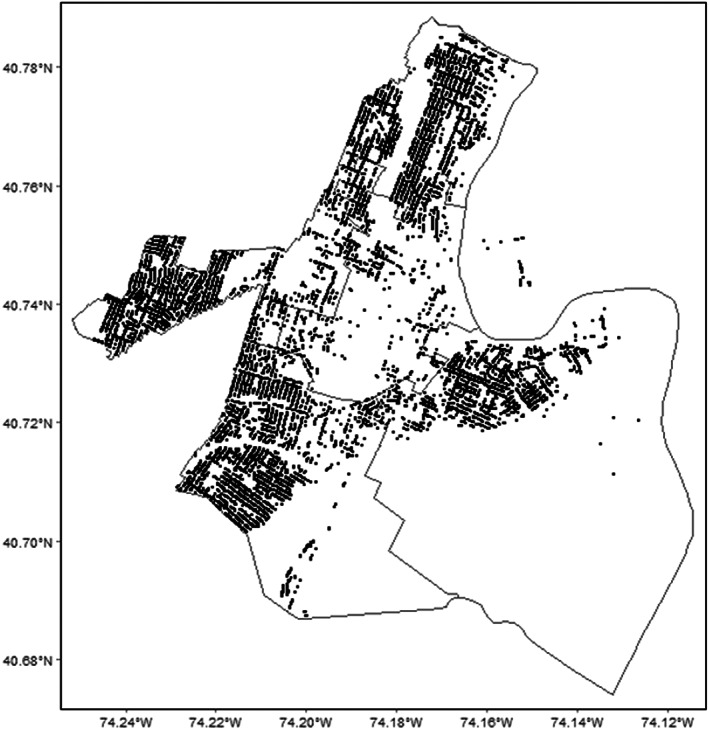
Newark's LSL locations in 2019 (locations obtained through Open Public Record Act request).
3.4. LCR data
Individual sample locations and locations of LSL were obtained through Open Public Record Act (OPRA) requests to the New Jersey Department of Environmental Protection (NJDEP). Digital shapefiles were obtained from the City of Newark's Open Data website (https://data.ci.newark.nj.us/). Sampling addresses were validated using both the LSL locations provided by OPRA, and Newark's Check Your Address website (https://www.newarkleadserviceline.com/check-your-address) to determine if an LSL was ever present at the location. Additional data was obtained from engineering reports (CDM Smith, 2019a, 2019b). LCR compliance data was obtained through NJDEP's New Jersey Drinking Water Watch website (https://www9.state.nj.us/DEP_WaterWatch_public/).
3.4.1. Corrosion control treatment
Both Newark WTPs implemented corrosion control using different treatments in the mid to late 1990s (CDM Smith, 2019a). Based upon a Corrosion Control Study conducted by the City of Newark in the 1990's, sodium silicate was ultimately chosen as the corrosion inhibitor for the Pequannock WTP (Newark, 1994). The study found that at the time, in lead pipes, sodium silicate treatment produced better results than orthophosphate or blended phosphate. Zinc orthophosphate has been used as the corrosion inhibitor for the Wanaque WTP since the mid‐1990s (CDM Smith, 2019b). On May 7, 2019, zinc orthophosphate became the new corrosion inhibitor for the Pequannock WTP (CDM Smith, 2021).
Lead release into drinking water can be reduced, but not necessarily eliminated by CCT, (S. V. Masters et al., 2021). CCT is more successful at reducing soluble lead than particulate lead (Betanzo et al., 2021). However, it does not control other conditions, unrelated to corrosivity (e.g., plumbing age, prolonged stagnation, low water use, high water flow, and physical disturbance of plumbing; Del Toral et al., 2013; Lytle & Schock, 2000; S. Masters et al., 2016). LCR revisions focus heavily on CCT over LSL replacement, even allowing small community water systems to substitute CCT for LSL replacement when the action level is exceeded. Further, LSL replacement after an exceedance will be reduced from a mandatory 7% to 3% per year. The 1991 LCR allowed utilities to recognize replacement if only the utility‐owned portion was removed for systems with joint responsibilities with homeowner for service lines. If the homeowner did not want to pay for the replacement, a partial LSL replacement was permitted and met the 7% replacement requirement.
3.4.2. pH measurements
Limited pH data for Newark's water distribution system is available in the NJDEP database; Table 1 is a summary of the Newark pH data from 1992 to 2021. A statistically significant decrease (p < 0.01) in average pH from 8.08 (in 2015) to 7.33 (in 2016) was observed; the data set was not available between 1993 and 2015 so it is not possible to determine if pH varied significantly during that period. According to a CDM Smith report, “the average pH ranged from 7.6 to 8.0 from 2005 to 2012 with a few sustained periods above pH 8.0. After 2012, pH dropped to an average range of 7.5–7.7” (CDM Smith, 2019a). A notable drop in pH beginning in 2016 was observed in CDM Smith, 2019a, figs. 3–18.
TABLE 1.
Newark water pH data for the Pequannock distribution system
| Sample year | pH samples (N) | Average pH | Max pH | Min pH |
|---|---|---|---|---|
| 2021 | 288 | 7.39 | 7.85 | 6.90 |
| 2020 | 209 | 7.45 | 8.52 | 6.79 |
| 2019 | 122 | 7.57 | 8.96 | 6.88 |
| 2018 | 52 | 7.32 | 8.16 | 6.69 |
| 2017 | 52 | 7.58 | 8.73 | 7.17 |
| 2016 | 26 | 7.33 | 7.81 | 6.90 |
| 2015 | 25 | 8.08 | 8.67 | 7.45 |
| 1993 | 2 | 7.97 | 8.45 | 7.48 |
| 1992 | 101 | 8.11 | 9.20 | 6.90 |
| 1991 | 3 | 7.97 | 8.50 | 7.40 |
3.4.3. LCR compliance sampling
Newark's LCR compliance data is summarized in Table 2. Newark first exceeded the action level for lead in 1992 (90th percentile = 26.4 ppb) shortly after the LCR was implemented in 1991 (CDM Smith, 2019a). Limited data exists for the 1990s but, during the 1998 monitoring period, Newark tested below the action level (90th percentile = 12.4 ppb). At some point after 1998, but before 2003, Newark was placed on reduced triennial sampling, requiring only 50 samples to be collected once every 3 years, instead of 100 samples twice a year. Newark remained on reduced triennial sampling from 2003 to 2016.
TABLE 2.
Newark water system LCR compliance sampling data: 90th percentile for lead (ppb)
| Sample year | 90th percentile for lead (ppb) | Number of samples |
|---|---|---|
| 2021 (1st) | 6.6 | 116 |
| 2020 (2nd) | 13 | 136 |
| 2020 (1st) | 13.9 | 140 |
| 2019 (2nd) | 31 | 495 |
| 2019 (1st) | 57 | 348 |
| 2018 (2nd) | 47.9 | 246 |
| 2018 (1st) | 17.8 | 129 |
| 2017 (2nd) | 26.7 | 153 |
| 2017 (1st) | 27 | 129 |
| 2015 | 10 | 52 |
| 2012 | 9 | 53 |
| 2009 | 11 | 52 |
| 2006 | 7.9 | 52 |
| 2003 | 8.3 | 57 |
| 1998 | 12.4 | 102 |
| 1992 | 26.4 | 229 |
Beginning on January 1, 2017, the NJDEP moved all large community water systems to standard monitoring (100 samples every 6 months). In 2017, Newark exceeded the 15‐ppb lead action level and continued to exceed the action level until the first monitoring period of 2020.
Table 3 shows the distribution of Newark's LCR compliance data sampling across wards and water sources by monitoring periods from 2005 to 2015. Each of the four triennial monitoring periods between these dates were below the 15‐ppb action level with approximately half of the samples taken from the Pequannock system and half from the Wanaque system. Overall, 25% or more of the sample locations did not contain an LSL, it is also unclear whether they met Tier 1 criteria.
TABLE 3.
Newark water system LCR compliance data: distribution across wards and water sources, 2005–2015
| Sampling period | LCR 90th percentile for lead (ppb) | Total samples (N) | Non‐LSL samples (copper with lead solder) (N [%]) | North ward (N) | Central ward (N) | South ward (N) | East ward (N) | West ward (N) | Pequannock (N) | Wanaque (N) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2005–2007 | 7.9 | 52 | 13 (25%) | 15 | 1 | 8 | 26 | 2 | 25 | 27 |
| 2007–2009 | 11 | 52 | 13 (25%) | 13 | 2 | 9 | 26 | 2 | 25 | 27 |
| 2010–2012* | 9 | 53 | 14 (26.4%) | 13 | 1 | 9 | 26 | 2 | 24 | 27 |
| 2013–2015 | 10 | 52 | 16 (30.8%) | 13 | 1 | 10 | 26 | 2 | 25 | 27 |
Two sampling locations could not be determined.
Existing data (Figure 3) shows what the Pequannock and Wanaque system's 90th percentile for lead would be if their 90th percentiles were calculated independently for the years 1992–2018. Both systems would have exceeded the action level in 1992. After 1992, the Pequannock system generally had higher lead levels. The Wanaque system, analyzed independently, would never have exceeded the action level after 1992, while the Pequannock system would have exceeded the action level in 2015.
FIGURE 3.
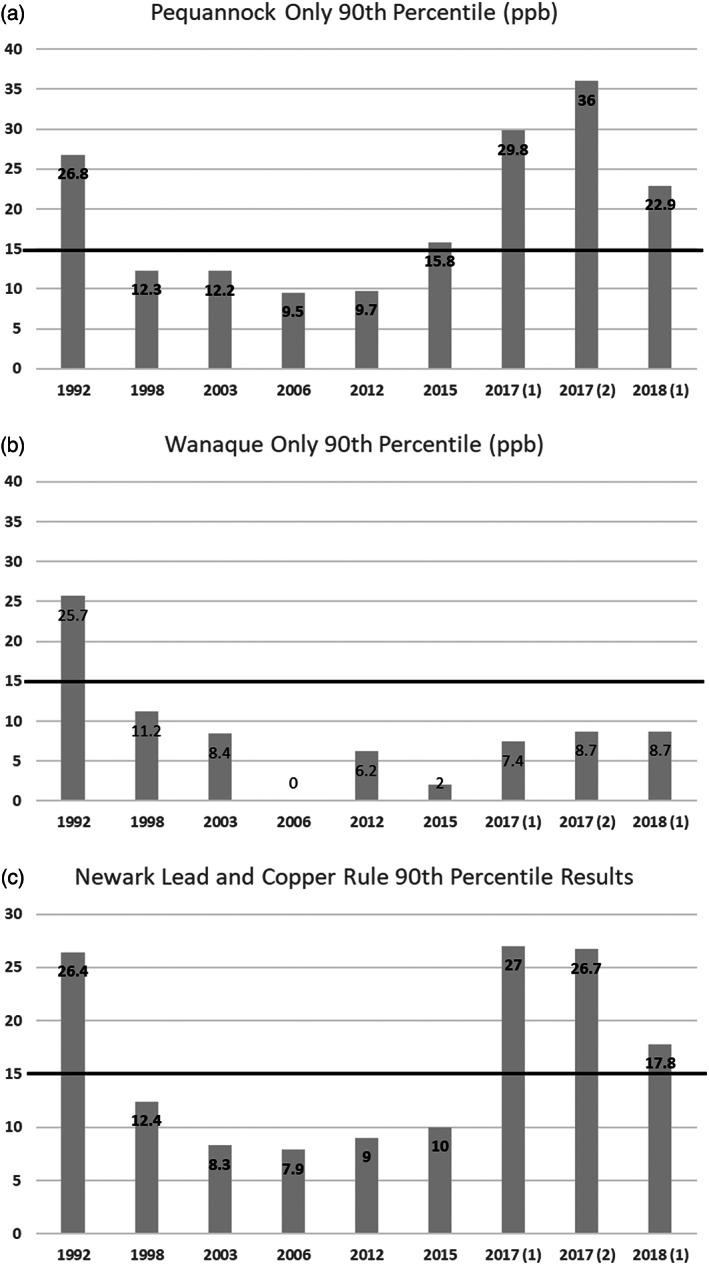
Newark water system LCR compliance sampling data (1992–2018): (a) Pequannock 90th percentile for lead (ppb). (b) Wanaque 90th percentile for lead (ppb). (c) Newark's reported LCR results.
3.5. Ineffective CCT as a potential cause of increased lead in drinking water
EPA along with the assistance of CDM Smith used x‐ray diffraction analysis, scanning electron microscopy (SEM), and energy dispersive spectroscopy (EDS) to characterize Newark pipe scales in 2018. This analysis indicated that silicate complexation with lead was not occurring, possibly resulting in higher soluble lead levels (CDM Smith, 2019a). A silica crust (SiO2) was found on all pipes; however, it is likely that the crust did not act as a barrier against lead release from the pipe as it was porous (CDM Smith, 2019a).
Tetravalent lead (Pb[IV]) compounds can form scales on pipes in highly oxidizing waters with elevated oxidation reduction potential (Boyd et al., 2008). These Pb(IV) scales are effective at reducing lead corrosion when appropriate water chemistry (high pH and high ORP) is maintained. If water chemistry changes (e.g., pH is lowered or ORP is lowered due to change in disinfection), Pb(IV) scales will be disrupted, resulting in release of particulate lead (Boyd et al., 2008). Additionally, the newly exposed surface is vulnerable to lead leaching from water with low pH. With a change in redox conditions, the highly insoluble PbO2 reduces back to a more soluble Pb(II) when ORP is not maintained. If ORP is maintained, but the pH decreases, then previously stable PbO2 can covert to more soluble Pb(II) carbonate compounds and soluble Pb, increasing both particulate and soluble water lead levels (CDM Smith, 2019a). In Newark, the EPA and CDM Smith determined that LSL scales primarily consisted of hydrocerussite, cerussite, and plattnerite (CDM Smith, 2019a). Plattnerite (PbO2) is a Pb(IV) compound while hydrocerussite and cerussite are divalent Pb(II) compounds that are carbonate‐based scales. Cerussite is stable at pH 8–8.5 while hydrocerussite is stable at higher pH (≥9). Since both Pb(II) and Pb(IV) scales were found on Newark's LSLs, the reduction of pH within Newark's distribution system may have been the primary cause for elevated lead levels by increasing the solubility of the present scales (CDM Smith, 2019a).
While there is no consensus in the literature, many studies have determined silicate CCT is ineffective for controlling for lead release within water systems (Li et al., 2021). When sodium silicates dissolves in water, pH is increased. Alternatively, orthophosphate controls lead solubility through the formation of hydroxl‐pyromorphite (Pb5[PO4]3OH) or other poorly soluble lead phosphate minerals (Schock et al., 1996). A mechanism other than based on pH control, for soluble lead reduction by silicate CCT has not been identified (Li et al., 2021). Generally, a pH increase will decrease lead solubility (Schock et al., 1996) but the specific role of silicate outside of any other additive to increase pH is still unknown. Despite this, sodium silicate continues to be listed as a corrosion inhibitor in the LCR revisions.
It is possible that when Newark began using sodium silicate in the 1990s for the Pequannock system, the pH adjustment from the added sodium silicate was enough to form the hydrocerussite, cerussite, and plattnerite scales, preventing large soluble lead release. However, when pH decreased, the scales could not be maintained which resulted in soluble and particulate lead release. Due to limited/split sampling, it is unclear if the increased lead levels were present prior to 2017 when Newark was required to conduct standard monitoring, rather than reduced monitoring.
3.5.1. Two distinct water systems, regulated as one
During sampling periods from 2005 to 2015, Newark split their total samples equally between the Pequannock and Wanaque systems. The locations and breakdown of samples by Ward can be seen in Figure 4 and Table 3. Between 13% and 17% of sampling locations (representing 6–9 homes) changed each sampling period. However, neither their location nor their measured concentrations created a significant variance in the reported results. These samples were taken from areas with high densities of LSLs but only 75% of samples were from homes containing an LSL (Table 3). Newark's sampling plan met the LCR requirements but was insufficient to identify the increase in lead. Splitting the total sample pool between two water systems diminished the ability to detect a problem. Regulating these two systems with different source waters, CCT, and water chemistry as two separate systems would have been a better option. Figure 3 demonstrates that the problem in the Pequannock system probably would have been detected 2 years earlier if the 90th percentiles were calculated separately. LCR revisions do not include guidance on regulating or monitoring the numerous water utilities that have multiple distinct treatment facilities or water sources and blending zones.
FIGURE 4.
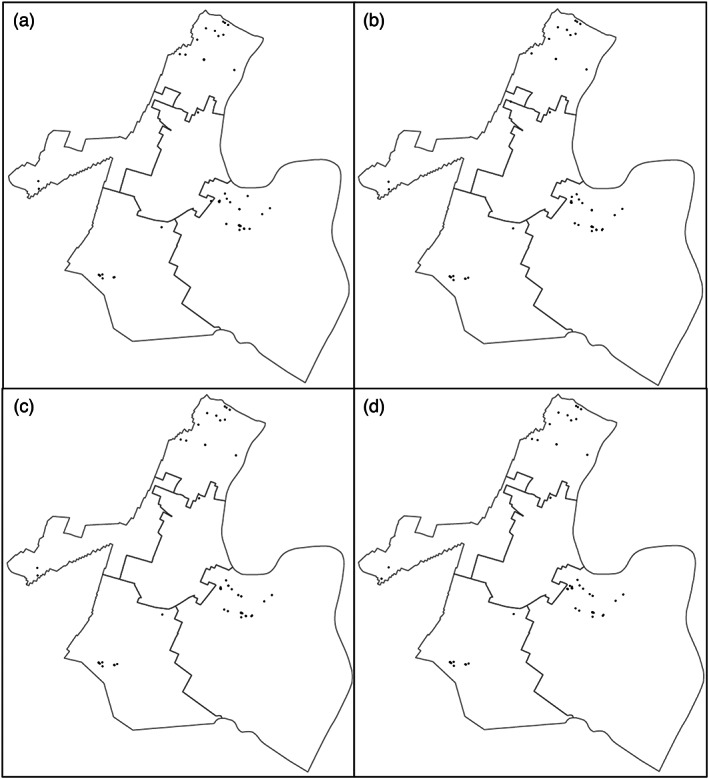
Sampling locations for LCR compliance: (a) sampling locations for LCR compliance 2005–2007. (b) Sampling locations for LCR compliance 2007–2009. (c) Sampling locations for LCR compliance 2010–2012. (d) Sampling locations for LCR compliance 2013–2015.
3.5.2. Other Newark lead and copper rule limitations
While Newark was compliant with the LCR, this was not enough to detect a lead issue due to the uniqueness as a two “system” purveyor. The reduced triennial sampling and split sample pool resulted in only 25 samples collected from the Pequannock system once every 3 years. Of the total sample, approximately 13 samples per sampling period or at least a quarter of the samples were from homes without LSLs, possibly biasing toward lower results. In a city with more than 23,000 LSLs, a more cautionary approach may have been to collect all samples from homes with LSLs as required by the LCR revisions.
First draw sampling protocols do not capture water that was stagnant within the LSL. Review and modeling of Michigan data indicated that collecting samples that were stagnant within the LSL increases the chance of exceeding the action level (Betanzo et al., 2021; S. V. Masters et al., 2021). The LCR revisions for water systems with LSLs will require sampling only from homes with an LSL and collection of only a fifth liter sample. First draw samples are more representative of contamination that occurred within the home (copper pipes used in conjunction of lead solder, ore lead containing fixtures), whereas samples collected from water stagnant within the LSL is more indicative of lead leaching from the service lines. Collecting first draw samples from homes with LSLs may underestimate leaching from the LSL, while fifth liter samples may miss contributions from the home's pipes and plumbing fixtures.
3.6. Water utility response: LSL replacement
The City of Newark operated on the belief that mass replacement of LSLs was the most effective means of lead reduction and was feasible given substantial investments, even though new corrosion control (zinc orthophosphate) brought the Newark Water System compliance data under the drinking water lead action level. In 3 years, Newark replaced 23,800 LSLs, at a cost of about $7200 per LSL (Kutzing, 2021), arguably the most aggressive LSL replacement program in the U.S. Starting in 2022, Newark is back to sampling homes with copper with lead solder as there are no longer sufficient LSLs to fill a sampling pool.
4. LESSONS LEARNED FROM NEWARK LEAD IN DRINKING WATER
Water chemistry is complex, and the LCR, including the proposed revisions, is limited in its ability to protect from transient or sustained spikes in water lead levels. If zero lead is the ultimate goal, then there are four concerns that need to be addressed, (1) older homes have interior copper plumbing that most likely contains lead solder if constructed before 1987, (2) pipe fittings purchased from other countries may contain higher lead content than the more stringent U.S. rule, (3) millions of LSLs still exist across the country, and (4) new plumbing fixtures that meet the U.S. “lead free” definition can still yield lead water results greater than 1 ppb (Parks et al., 2018).
Under the LCR revisions, the number of homes required to be sampled remains the same. The current and proposed LCR requires 100 samples, (50 samples, if the system is under reduced monitoring) to determine whether lead concerns exist within a given large CWS (serves >50,000 persons). Given the inherent variability in lead sources and the diverse conditions across water distribution systems, resulting in dramatically different patterns of lead corrosion and release, the current sampling requirements are not sufficient to capture high lead in drinking water to prevent human exposure. For Newark, to ensure that the 95% confidence interval for the estimate of the mean water lead concentration is within 2.5 ppb of the true mean, 272 samples would have been required to be collected per sampling period (this assumes a standard deviation of 21 ppb, and a margin of error of 2.5 ppb). The required number of high‐risk homes sampled each monitoring cycle should be increased to achieve effective protective predictions. Planned or unplanned changes in the distribution system, (e.g., source water, treatment methods, chemicals, or operations) may have unintended consequences on tap water lead levels that may go unnoticed. The LCR's goal, “reducing the lead and copper levels at consumer's taps to as close to the MCLG,” should require routine tap water monitoring even after optimized corrosion control treatment is implemented. Tap water monitoring not only assesses the effectiveness of CCT, but also provides an ongoing protective measure to help ensure that inadvertent increases in lead are promptly detected. For homes with an LSL, the sample analyzed for lead will change from a first draw to a fifth draw, which may be a more effective method at detecting lead contamination in homes with an LSL (S. V. Masters et al., 2021). The LCR should adopt Michigan's sampling plan of analyzing both a first draw and 5th liter sample for lead to capture both interior and service line lead contribution. The required number of high‐risk homes sampled each monitoring cycle should be increased to achieve effective protective predictions. Additional requirements for identifying transient changes to source water and CCT protocols, and safeguards on insufficient sampling methods should be considered.
In March 2016, Newark recorded water samples above 15 ppb for lead from water fountains in public schools. These schools were served water from the same system (Pequannock) that serves homes and businesses (NJDEP, 2019) triggering a closer examination of Newark's LCR compliance data by concerned stakeholders. These events highlighted the limited federal regulations that exist for schools and child day‐care centers across the United States under the current LCR, leaving the nation's most vulnerable population of children at risk for exposure during the time spent in those environments (Lambrinidou et al., 2010). Only 11 states require licensed childcare facilities to test drinking water for lead (EDF, 2020). For schools, 23 states have state‐wide voluntary lead testing programs, and 18 states have mandatory testing for schools. Of the states requiring testing, only 13 require mitigation if lead is found in drinking water (NASBE, 2021). The LCR revisions will provide guidelines for testing schools and day cares. Additionally, many water systems blend water from multiple sources. Blending water post CCT can have a potential dilution effect and can compromise CCT.
Under the LCR and the proposed revisions, sodium silicates are regarded as a CCT option as a class that is different from a pH adjustment chemical. Yet studies indicate that sodium silicates reduce lead release through the increase of pH (Li et al., 2021). As previously mentioned, in Newark, sodium silicates were initially effective in controlling pH and lead release; however, this CCT method was not successful at controlling lead release under lower pH conditions as described above (Section 3.5). When pH in the water system decreased, soluble lead levels rose. All CCT are not equal in efficacy, as reduction in lead is a complicated titration involving multiple approaches. Therefore, clear CCT guidelines should be adopted including characterization of multiple parameters (e.g., pH, lead species, and concomitant ions) to prevent water utilities from facing similar issues in the future.
The revised LCR bans partial LSL replacement unless required for emergency repairs (e.g., broken pipe), or the homeowner refuses to pay for their portion of the LSL. Partial LSL replacement leaves a potential source of lead exposure for both current and future residents. Partial LSL replacement may raise environmental justice concerns, as those who cannot afford to replace may bear a larger burden. Washington, D.C. children living in homes with partial LSLs were 2–3 times more likely to have high blood lead levels compared to children living in homes without LSLs (Brown et al., 2011). Due to the potential increase in water lead levels from a partial LSL replacement (Trueman et al., 2016), we recommend partial LSL replacements not be conducted. One potential solution may be to require full replacement when the property changes hands (Renner, 2010). With government funding, Newark conducted full replacement without charge to the homeowner.
5. CONCLUSIONS AND POLICY IMPLICATIONS
Prior to Newark, the 21st century has had two defining lead in water events. These included Washington, D.C. and Flint, Michigan (Roy & Edwards, 2019). The November 2000 switch from chlorine to chloramine in Washington, D.C., triggered releases of lead in water. (Roy & Edwards, 2019). In April 2014, the change in water source from Lake Huron to the Flint River coupled with absence of CCT, triggered the Flint Water Crisis (S. Masters et al., 2016; Pauli, 2020). A key lesson learned from these crises is that compliance with the LCR does not ensure lead‐safe water within a given water system (A. L. Katner et al., 2018; Roy & Edwards, 2019).
These crises have led to renewed scrutiny of the LCR and motivated the proposed LCR revisions seeking to address deficiencies in the sampling methods. Sampling practices used in Flint, including the use of bottles that restrict flow rate, pre‐flushing pipes, and biased selection of sampling sites were identified as potentially minimizing measured lead concentrations (Goovaerts, 2017; A. Katner et al., 2016). The EPA has acknowledged that water utilities have collected samples in a manner that can miss high lead levels (Del Toral et al., 2013; Edwards & Dudi, 2004; A. Katner et al., 2016).
In the wake of the Flint Water Crisis, the Michigan Department of Environment, Great Lakes, and Energy (EGLE), developed a revised LCR for the state of Michigan (MDEGLE, 2018). The Michigan LCR modified the sample collection protocols for water systems that contain LSLs to collect samples only from homes with an LSL and requires the collection of both a first draw and 5th liter sample (Betanzo et al., 2021). The Michigan LCR calculates the 90th percentile using the highest value from each sampling location. Unfortunately, a comparison for Newark's historical LCR data with the new Michigan LCR cannot be done because 5th liter samples were not historically collected in Newark. Michigan's LCR sampling method provides a higher resolution then the LCR revisions, as it analyses water that has been stagnant within the home's plumbing system and water that was potentially stagnant within an LSL.
Of primary concern in a country with aging infrastructure, there remain 6.1–10.2 million LSLs across the United States that potentially pose a health risk (Cornwell et al., 2016). Washington, D.C., Flint, and now Newark are the three most recent examples and emphasize environmental justice concerns related to lead in drinking water (A. L. Katner et al., 2018). Additionally, Flint has shown the potential health risks as illustrated by the increase in children's blood lead levels attributed to exposure to lead in drinking water (Hanna‐Attisha et al., 2016).
Every water system with LSLs potentially faces future lead exceedance events due to changes in water chemistry, (e.g., pH) improper characterization of distinct water sources, or insufficient sampling methods. LCR limitations that prevented Newark water suppliers from detecting their lead in water problems, including ineffective CCT within the Pequannock system, reduced monitoring, and pooling of samples between two distinct water systems, will be allowed to continue under the LCR revisions. Newark demonstrated that with substantial resources, full LSL replacement can be accomplished quickly, setting an example for other systems. If the U.S. EPA is going to win its “war on lead,” substantial investment from public and private sources will be required. The events of Washington, D.C., Flint, and Newark, among others, demonstrate the need for regulations that are truly protective of human health.
AUTHOR CONTRIBUTIONS
Sean A. Stratton: Conceptualization (lead); data curation (lead); formal analysis (lead); investigation (equal); methodology (equal); project administration (lead); software (lead); validation (equal); visualization (lead); writing – original draft (lead); writing – review and editing (supporting). Adrienne S. Ettinger: Conceptualization (supporting); investigation (equal); methodology (equal); validation (equal); visualization (supporting); writing – original draft (supporting); writing – review and editing (lead). Cathleen L. Doherty: Conceptualization (supporting); data curation (supporting); formal analysis (supporting); methodology (equal); validation (equal); writing – original draft (supporting); writing – review and editing (supporting). Brian T. Buckley: Conceptualization (supporting); data curation (supporting); formal analysis (supporting); funding acquisition (lead); methodology (equal); project administration (supporting); resources (lead); supervision (lead); validation (equal); visualization (supporting); writing – original draft (supporting); writing – review and editing (supporting).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
RELATED WIREs ARTICLES
ACKNOWLEDGMENT
The authors would like to acknowledge Sandra Kutzing and CDM Smith for providing their technical reports on the City of Newark Pequannock and Wanaque Treatment Systems.
Stratton, S. A. , Ettinger, A. S. , Doherty, C. L. , & Buckley, B. T. (2023). The lead and copper rule: Limitations and lessons learned from Newark, New Jersey . WIREs Water, 10(1), e1620. 10.1002/wat2.1620
Edited by: Jan Seibert, Co‐Editor‐in‐Chief
Funding information National Institute of Environmental Health Sciences, Grant/Award Number: ES05022
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
FURTHER READING
- Masten, S. J. , Davies, S. H. , & Mcelmurry, S. P. (2016). Flint water crisis: What happened and why? Journal American Water Works Association, 108(12), 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
REFERENCES
- Agency for Toxic Substances and Disease Registry (ATSDR) . (2020). Toxicological profile for lead. U.S. Department of Health and Human Services, Public Health Service. 10.15620/cdc:95222 [DOI] [Google Scholar]
- Betanzo, E. , Rhyan, C. , & Hanna‐Attisha, M. (2021). Lessons from the first year of compliance sampling under Michigan's revised lead and copper rule and national lead and copper rule implications. AWWA Water Science, 3(6), e1261. [Google Scholar]
- Boyd, G. R. , Dewis, K. M. , Korshin, G. V. , Reiber, S. H. , Schock, M. R. , Sandvig, A. M. , & Giani, R. (2008). Effects of changing disinfectants on lead and copper release. Journal‐American Water Works Association, 100(11), 75–87. [Google Scholar]
- Brown, M. J. , & Margolis, S. (2012). Lead in drinking water and human blood lead levels in the United States. Morbidity and Mortality Weekly Report, 61, 1–9. [PubMed] [Google Scholar]
- Brown, M. J. , Raymond, J. , Homa, D. , Kennedy, C. , & Sinks, T. (2011). Association between children's blood lead levels, lead service lines, and water disinfection, Washington, DC, 1998–2006. Environmental Research, 111, 67–74. [DOI] [PubMed] [Google Scholar]
- CDM Smith . (2019a). Pequannock WTP corrosion control review and recommendations. Final City of Newark, Lead and Copper Rule Compliance Study.
- CDM Smith . (2019b). Wanaque gradient corrosion control review. City of Newark Lead and Copper Rule Compliance Study Wanaque Gradient.
- CDM Smith . (2021). Pequannock gradient corrosion control treatment effectiveness evaluation and pipe loop study results. Final Report, City of Newark.
- City of Newark . (1994). City of Newark report on corrosion optimization study .
- City of Newark . (2019). City of Newark's lead service line replacement program . https://www.newarkleadserviceline.com/
- Clark, B. , Masters, S. , & Edwards, M. (2014). Profile sampling to characterize particulate lead risks in potable water. Environmental Science & Technology, 48(12), 6836–6843. [DOI] [PubMed] [Google Scholar]
- Clark, B. N. , Masters, S. V. , & Edwards, M. A. (2015). Lead release to drinking water from galvanized steel pipe coatings. Environmental Engineering Science, 32(8), 713–721. [Google Scholar]
- Cornwell, D. A. , Brown, R. A. , & Via, S. H. (2016). National survey of lead service line occurrence. Journal‐American Water Works Association, 108(4), E182–E191. [Google Scholar]
- Del Toral, M. A. , Porter, A. , & Schock, M. R. (2013). Detection and evaluation of elevated lead release from service lines: A field study. Environmental Science & Technology, 47(16), 9300–9307. [DOI] [PubMed] [Google Scholar]
- Edwards, M. , & Dudi, A. (2004). Role of chlorine and chloramine in corrosion of lead‐bearing plumbing materials. Journal‐American Water Works Association, 96(10), 69–81. [Google Scholar]
- Environmental Defense Fund (EDF) . (2020). Child care lead in water requirements . https://www.edf.org/health/child-care-lead-water-requirements
- Environmental Protection Agency (EPA) . (2020). Basic information about lead in drinking water . https://www.epa.gov/ground-water-and-drinking-water/basic-information-about-lead-drinking-water
- Goovaerts, P. (2017). Monitoring the aftermath of Flint drinking water contamination crisis: Another case of sampling bias? Science of the Total Environment, 2017(590), 139–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna‐Attisha, M. , LaChance, J. , Sadler, R. C. , & Champney Schnepp, A. (2016). Elevated blood lead levels in children associated with the Flint drinking water crisis: A spatial analysis of risk and public health response. American Journal of Public Health, 106(2), 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katner, A. , Pieper, K. J. , Lambrinidou, Y. , Brown, K. , Hu, C.‐Y. , Mielke, H. W. , & Edwards, M. A. (2016). Weaknesses in federal drinking water regulations and public health policies that impede lead poisoning prevention and environmental justice. Environmental Justice, 9(4), 109–117. [Google Scholar]
- Katner, A. L. , Brown, K. , Pieper, K. , Edwards, M. , Lambrinidou, Y. , & Subra, W. (2018). America's path to drinking water infrastructure inequality and environmental injustice: The case of Flint, Michigan. In The Palgrave handbook of sustainability (pp. 79–97). Springer. [Google Scholar]
- Kutzing, S. (2021). Ortho implementation in a system with destabilized tetravalent lead scales. Water quality technology conference. Tacoma, Washington.
- Lambrinidou, Y. , Triantafyllidou, S. , & Edwards, M. (2010). Failing our children: Lead in US school drinking water. New Solutions, 20(1), 25–47. [DOI] [PubMed] [Google Scholar]
- Li, B. , Trueman, B. F. , Doré, E. , & Gagnon, G. A. (2021). Effectiveness of sodium silicates for lead corrosion control: A critical review of current data. Environmental Science & Technology Letters, 8(11), 932–939. [Google Scholar]
- Lytle, D. , & Schock, M. (2000). Impact of stagnation time on metal dissolution from plumbing materials in drinking water. Journal of Water Supply: Research and Technology‐AQUA, 49(5), 243–257. [Google Scholar]
- Lytle, D. A. , Schock, M. R. , Formal, C. , Bennett‐Stamper, C. , Harmon, S. , Nadagouda, M. N. , Williams, D. , DeSantis, M. K. , Tully, J. , & Pham, M. (2020). Lead particle size fractionation and identification in Newark, New Jersey's drinking water. Environmental Science & Technology, 54(21), 13672–13679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters, S. , Parks, J. , Atassi, A. , & Edwards, M. A. (2016). Inherent variability in lead and copper collected during standardized sampling. Environmental Monitoring and Assessment, 188(3), 177. [DOI] [PubMed] [Google Scholar]
- Masters, S. V. , Bradley, T. C. , Burlingame, G. A. , Seidel, C. J. , Schmelling, M. , & Bartrand, T. A. (2021). What can utilities expect from new lead fifth‐liter sampling based on historic first‐draw data? Environmental Science & Technology, 55(17), 11491–11500. [DOI] [PubMed] [Google Scholar]
- Michigan Department of Environment, Great Lakes, and Energy (MDEGLE) . (2018). Lead and Copper Rule . https://www.michigan.gov/egle/about/organization/drinking-water-and-environmental-health/community-water-supply/lead-and-copper-rule
- National Association of State Boards of Education (NASBE) . (2021, November 3). How states are handling lead in school drinking water . https://www.nasbe.org/how-states-are-handling-lead-in-school-drinking-water/
- National Toxicology Program (NTP) . (2012, June 13). NTP monograph on health effects of low‐level lead. Division of the National Toxicology Program, National Institute of Environmental Health Sciences, U.S. Department of Health and Human Services. https://ntp.niehs.nih.gov/ntp/ohat/lead/final/monographhealtheffectslowlevellead_newissn_508.pdf
- Natural Resources Defense Council (NRDC) . (2018, September 14). What's in your water? An updated analysis . https://www.nrdc.org/experts/kristi-pullen-fedinick/whats-your-water-updated-analysis
- New Jersey Department of Environmental Protection (NJDEP) . (2019, March 9). Joint release from DEP and Newark public schools on temporary use of alternate water sources after elevated levels of lead found in recent district sampling. https://www.nj.gov/dep/newsrel/2016/16_0012.htm
- New Jersey Hospital Association (NJHA) . (2019, November 2019). New Jersey's most vulnerable communities: A zip code analysis of social gaps and their impact on health . https://www.njha.com/media/578105/CHART-NJ-Most-Vulnerable-Communities.pdf
- Ngueta, G. , Abdous, B. , Tardif, R. , St‐Laurent, J. , & Levallois, P. (2016). Use of a cumulative exposure index to estimate the impact of tap water lead concentration on blood lead levels in 1‐to 5‐year‐old children (Montréal, Canada). Environmental Health Perspectives, 124(3), 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks, J. , Pieper, K. J. , Katner, A. , Tang, M. , & Edwards, M. (2018). Potential challenges meeting the American Academy of Pediatrics' lead in school drinking water goal of 1 μg/L. Corrosion, 74(8), 914–917. [Google Scholar]
- Pauli, B. J. (2020). The Flint water crisis. Wiley Interdisciplinary Reviews: Water, 7(3), e1420. [Google Scholar]
- Renner, R. (2010). Reaction to the solution: Lead exposure following partial service line replacement. National Institute of Environmental Health Sciences, 118, A202–A208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, S. , & Edwards, M. A. (2019). Preventing another lead (Pb) in drinking water crisis: Lessons from the Washington DC and Flint MI contamination events. Current Opinion in Environmental Science & Health, 7, 34–44. [Google Scholar]
- Ruckart, P. Z. , Jones, R. L. , Courtney, J. G. , LeBlanc, T. T. , Jackson, W. , Karwowski, M. P. , Cheng, P. Y. , Allwood, P. , Svendsen, E. R. , & Breysse, P. N. (2021). Update of the blood lead reference value—United States, 2021. MMWR. Morbidity and Mortality Weekly Report, 2021(70), 1509–1512. 10.15585/mmwr.mm7043a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schock, M. R. , Wagner, I. , & Oliphant, R. (1996). The corrosion and solubility of lead in drinking water. In Internal corrosion of water distribution systems (2nd ed., pp. 131–230). American Water Works Association Research Foundation and DVGW Technologiezentrum Wasser. [Google Scholar]
- Triantafyllidou, S. , & Edwards, M. (2012). Lead (Pb) in tap water and in blood: Implications for lead exposure in the United States. Critical Reviews in Environmental Science and Technology, 42(13), 1297–1352. [Google Scholar]
- Triantafyllidou, S. , Gallagher, D. , & Edwards, M. (2014). Assessing risk with increasingly stringent public health goals: The case of water lead and blood lead in children. Journal of Water and Health, 12(1), 57–68. [DOI] [PubMed] [Google Scholar]
- Trueman, B. F. , Camara, E. , & Gagnon, G. A. (2016). Evaluating the effects of full and partial lead service line replacement on lead levels in drinking water. Environmental Science & Technology, 50(14), 7389–7396. [DOI] [PubMed] [Google Scholar]
- U.S. Census . (2020, April 1). U.S. census bureau, population census. Newark, NJ. https://www.census.gov/quickfacts/fact/table/newarkcitynewjersey/POP010220#POP010220
- World Health Organization (WHO) . (1995). Lead in drinking water: Background document for development of who guidelines for drinking‐water quality . http://www.who.int/water_sanitation_health/dwq/chemicals/lead.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


