Abstract
Problem
Tumors compromise the patients’ immune system to promote their own survival. We have previously reported that HGSC exosomes play a central role, downregulating NKG2D cytotoxicity. Primary surgery's effect on tumor exosomes and NKG2D cytotoxicity in HGSC patients has not been studied before. The overall objective of this study was to explore the effect of surgery on the exosome‐induced impairment of NKG2D cytotoxicity in HGSC.
Method of study
Paired pre‐ and post‐operative blood samples were subjected to cell and exosome analyses regarding the NKG2D receptor and ligands, and NKG2D‐mediated cytotoxicity. Lymphocytes were phenotyped by immunoflow cytometry. Exosomes, isolated by ultracentrifugation, and characterized by nanoparticle tracking analysis, transmission and immune electron microscopy and western blot were used in functional cytotoxic experiments. HGSC explant culture‐derived exosomes, previously studied by us, were used for comparison.
Results
HGSC exosomes from patients’ sera downregulated NKG2D‐mediated cytotoxicity in NK cells of healthy donors. In a subgroup of subjects, NKG2D expression on CTLs and NK cells was upregulated after surgery, correlating to a decrease in the concentration of exosomes in postoperative sera. An overall significantly improved NKG2D‐mediated cytotoxic response of the HGSC patients’ own NK cells in postoperative compared to preoperative samples was noted.
Conclusions
Surgical removal of the primary tumor has a beneficial effect, relieving the exosome‐mediated suppression of NKG2D cytotoxicity in HGSC patients, thus boostering their ability to combat cancer.
Keywords: cytotoxicity, EOC/HGSC, epithelial ovarian cancer, exosomes, immune suppression, NKG2D, NKG2D ligands, surgery
1. INTRODUCTION
Cancers develop various strategies to subvert host immune defense and compromise immune surveillance, thus promoting tumor immune escape and survival. These strategies include the ability to create a cancer‐tolerant tumor microenvironment (TME) and activation of mechanistic pathways that counteract the effect of anti‐tumor immune responses, reviewed in. 1 Ovarian cancer (OC), a highly heterogeneous tumor group, has the highest mortality rate of all gynecological malignancies. Epithelial ovarian cancer (EOC) comprises 90% of malignant ovarian tumors and can be further classified according to histopathology and genetic alterations. We chose to investigate the most common and highly malignant type, high‐grade serous cancer (HGSC), which constitutes 70% of EOC cases. 2 The establishment, metastatic spread, and poor outcome of the disease are strongly associated with tumor‐induced derangement of the immune system. 3 , 4 , 5 This is facilitated by intrinsic tumor cell changes, such as uncontrolled growth signaling, limitless replication, sustained angiogenesis and invasiveness; and by a variety of mechanisms suppressing host immunity and creating an immunosuppressive TME. 6 We have previously reported that the cytokine mRNA profile in the HGSC TME is dominated by immunoinhibitory and pro‐inflammatory cytokines, favoring tumor survival and spread. 7
Furthermore, it is well known that HGSC, and other cancers, constitutively secrete extracellular vesicles (EV) that in various ways promote cancer establishment and survival. Extracellular vesicles, produced by living cells, are characterized by their origin and size into small and large EV. Small EVs (30–150 nm in size) of endosomal origin are called exosomes, reviewed in. 8 According to the guidelines by the International Society for Extracellular Vesicles (ISEV), MISEV2018, 9 the term small EVs should be used when no specific isolation method and morphological analysis of the vesicles on the ultrastructural level has been applied. We have a well‐documented long‐term experience in exosome research and isolation methodology. 10 , 11 , 12 , 13 Here, since we have used an exosome‐specific isolation method based on ultrafiltration and ultracentrifugation and applied nanoparticle tracking analysis (NTA) and electron‐ and immunoelectron microscopy methods to prove the exosomal origin and morphology of the isolated vesicles, we have chosen to use the term exosomes.
We and others have studied tumor‐derived exosomes, including exosomes from HGSC. 11 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 Exosomes are released by virtually all cell types and participate in the intercellular communication in normal and pathological processes. Exosomes carry on their surface, and inside, proteins, DNA, mRNA, and non‐coding RNAs such as micro‐ and long non‐coding RNAs. 22 HGSC cells’ exosomes are secreted in ascites and peripheral blood. 14 , 15 , 16 , 17 These exosomes carry immunosuppressive cytokines like IL‐10 and TGF‐β1, 18 known to induce formation of immunosuppressive immune cells, for example, T regulatory (Treg) cells 18 and tumor‐promoting M2 macrophages. 19 They can induce secretion of pro‐inflammatory cytokines, such as IL‐6, TNF‐α and IL‐1β, 20 suppress the CD3 ζ‐chain expression 21 and enhance apoptosis by expressing FasL and TRAIL. 14 Tumor‐derived exosomes have been shown to express NKG2D ligands, interacting with the major NK cell activating receptor NKG2D and impairing its function, 12 , 23 , 24 reviewed in. 25 Our group showed for the first time that HGSC exosomes had the ability to downregulate cytotoxicity in a differential way. 11 Studying isolated HGSC exosomes from ascites, tissue explant cultures and cell‐line supernatants, we proved that these exosomes carried NKG2D ligands on their surface and thus could inhibit the NKG2D receptor‐mediated cytotoxicity while the exosomal surface was deprived of DNAM‐1 ligands, leaving the accessory DNAM‐1 receptor‐mediated pathway unaffected. 11 Our results 11 gave a mechanistic explanation to the previous observation that the cytotoxic immune response in HGSC patients is carried by the accessory DNAM‐1/PVR mediated cytotoxic pathway and the NKG2D ligand pathway is only complementary. 26
Surgery is a cornerstone of ovarian cancer treatment and the importance of maximal primary debulking surgery for the patient's prognosis is well substantiated. 27 , 28 It has been suggested that some of the beneficial effects of surgery are due to improvements of the host immune defense. 27 , 29
Prompted by these reports we wanted to investigate if surgery improved the NKG2D‐mediated cytotoxicity, known to constitute the anti‐tumor immune surveillance in cancer patients. In the current mechanistic proof‐of‐concept study, we collected peripheral blood samples from HGSC patients before and 3–14 weeks after surgery and for the first time evaluated the effect of surgery on the cytotoxic potency of the patients’ NK cells and circulating HGSC exosomes.
2. MATERIALS AND METHODS
2.1. Study population
The main characteristics of the study patients are summarized in Table 1. Eighteen women having surgery for primary HGSC were recruited from the Department of Gynecology and Obstetrics at Norrland's University Hospital, Umeå, Sweden. Exclusion criteria was a history of any other tumor disease. The investigation was approved by the Human Ethics Committee of the Medical Faculty, Umeå University (d.nr 09–108 M). All patients donated samples after written informed consent. Information on histopathological diagnosis according to the World Health Organization Classification, 30 was extracted from their medical records and verified via the local Pathology Registry and a senior consultant in Gynecological Pathology (EL). The majority of women (n = 14) received chemotherapy during the sample collection period (Table 1), according to guidelines. 31 These women were given 1 up to 3 cycles, three weeks apart, of carboplatin and paclitaxel before the postoperative blood sample was collected (Table 1). One patient received an addition of bevacizumab (Avastin®) to her second cycle (the last before collection of the postoperative sample).
TABLE 1.
Patient characteristics (n = 18)
| Characteristics | Value |
|---|---|
| Age, years, median (range) | 60.5 (42–76) |
| FIGO stage, n | |
| II | 2 |
| III | 15 |
| IV | 1 |
| Chemotherapy treatment prior to postoperative sample, n | |
|
0 cycles 1 cycle 2 cycles 3 cycles |
4 10 2 2 |
| Postoperative residual tumor, n | |
| Macroscopically radical | 9 |
| Residual tumor mass | 9 |
| Time‐point for sample collection, weeks, median (range a ) | |
| Preoperative sample | 0 (0–2) |
| Postoperative sample | 6 (3–14) |
In relation to surgery.
2.2. Rationale for the division of the HGSC patients into Group 1 and Group 2
Our previous work concentrated on in vitro studies of suppression of the NKG2D cytotoxicity by HGSC‐derived exosomes. 11 Here, we wanted to test this finding in clinical settings as a proof‐of‐concept. We asked the questions: (1) Exosomes are extremely short‐lived in the peripheral blood ‐ can we trace NKG2D ligand bearing exosomes in the blood of HGSC patients? (2) Are those downregulating the receptor? (3) Is the patients’ cytotoxic response downregulated? (4) Surgery will remove the tumor, that is, the major source of NKG2D ligand bearing exosomes – will that mean less amount of NKG2D ligand‐bearing exosomes and upregulation of cytotoxicity? There were no quantitative differences in the phenotype of PBMCs of the patients before and after surgery, but differences in the NKG2D receptor expression. We used the receptor expression differences to divide the patients into two groups: one where the NKG2D receptor expression was upregulated after surgery, named Group 1, and one where the NKG2D receptor expression was downregulated after surgery, named Group 2. We followed these groups individually pre‐ and post‐operatively in all tests done (Supplementary Figure 1).
2.3. Blood sample collection and isolation of peripheral blood mononuclear cells (PBMC)
Venous blood was collected in tubes with and without EDTA supplementation from all women (n=18) before and 3–14 weeks (median 6 weeks) after surgery. PBMCs were isolated using Ficoll–Isopague gradient centrifugation (Lymphoprep,Nycomed Pharma A/S,Oslo,Norway), as previously described. 32 One portion was used directly after isolation for lymphocyte phenotyping by immunoflow cytometry, and one portion was frozen at −80°C for later use. Serum was frozen and stored at −80°C. In addition, PBMCs from healthy donors (non–smokers without medication) were used in phenotypic studies and for in vitro studies of receptor downregulation and cytotoxicity experiments with exosomes isolated from the pre‐ and post‐operative HGSC patients’ sera.
2.4. Antibodies used in the study
The following antibodies were used: anti‐CA125 (OV185:1, Imgenex), anti‐MICA (159227, R&D Systems), anti‐MICB (236511, R&D‐bs‐6933R, Bioss), anti‐ULBP1 (H‐46), anti‐ULBP2 (H‐48; N‐16), anti‐ULBP3 (H‐45), anti‐CD63 (MX‐49.129.5), anti‐CD81 (5A6) all from Santa Cruz; anti‐DNAM‐1 (102511, R&D); anti‐NKG2D (BD Biosciences); Alexa 647‐conjugated CD56 (B159), all from BD Pharmingen; FITC‐conjugated anti‐CD3 (SK7, BioLegend); FITC‐conjugated anti‐CD63 (CLBGran/12, Beckman Coulter); PE‐conjugated anti‐CD8 (DK25) all from, DAKO A/S; subclass control antibodies anti‐mouse IgG1 and IgG2b (DAK‐GO1 and DAK‐GO9), rabbit IgG, FITC‐conjugated goat anti‐mouse IgG, all from DAKO.
2.5. Phenotypic analysis of HGSC patients’ peripheral blood lymphocytes before and after surgery
Single and double immunofluorescence staining were used to determine the subpopulations of lymphocytes, and their expression of the NKG2D receptor, MICA/B, and the DNAM‐1 receptor. mAbs for the following markers were used: CD19, CD4, CD8, CD56, CD16, CD161, NKG2D, MICA, MICB, DNAM‐1, NKG2D/CD56, NKG2D/CD8, DNAM‐1/CD56, DNAM‐1/CD8. In brief, three hundred thousand cells/well, suspended in PBS containing .2 % BSA and .02% NaN3, were incubated with appropriate concentrations of primary mAbs/Abs for 30 min with gentle shaking. After wash, the cells were incubated with FITC‐ and/or PE‐conjugated antibodies for 30 min in darkness. Isotype‐matched primary IgG antibodies (DAKO) were used in negative controls. CD45/CD14 staining was used for lymphocyte gating. Ten thousand events per marker were collected by Accuri 6C Flow Cytometer (BD Biosciences) and analyzed with CFlow Plus program (BD Biosciences).
2.6. Exosome isolation
Exosomes were isolated from HGSC patients’ serum collected before and 3–14 weeks after surgery. In addition, HGSC exosomes for control purposes were isolated from HGSC ascites, peritoneal lavage and supernatants of HGSC tumor explant cultures following the method previously described. 10 For ascites and supernatants, after sequential centrifugation steps, removing cellular debris and larger EV and particles, a filtration through a .2 μm‐filter was applied, followed by sucrose gradient ultracentrifugation at 110 000 × g for 2 h. For the serum samples, due to limited available material and the need for higher exosomal yield for various receptor expression‐ and functional experiments, the density gradient step, which is a critical step where exosomal yield is lost, was excluded. All samples were reconstituted in PBS containing protease inhibitor cocktail (Roche Diagnostics) and diluted in Milli‐Q water to a total volume of 1 ml and kept frozen at −20°C until further use. The yield, purity, size distribution, and concentration of EV isolated was assessed by NTA, using ZetaView instrument (Particle Metrix, Germany). The average of five measurements/sample of size distribution, average size, and concentration (number/ml) was calculated. The exosome morphology and purity from other vesicles and debris were estimated by negative contrast staining and transmission electron microscopy (TEM), and the expression of exosomal and other markers by immune electron microscopy (IEM). Exosomes isolated with or without density gradient ultracentrifugation had similar size and size distributions and were comparable in NTA analyses and EM/IEM (data not shown).
2.7. Electron microscopy of isolated exosomes
Negative contrast staining and TEM were used for analyses of exosome morphology and purity. IEM was used for assessment of exosomal surface markers (tetraspanins CD63 and CD81), the OC‐associated marker CA‐125, and the NKG2D ligands MICA/B and ULBP 1–3. The negative contrast staining procedure was performed as previously described. 13 In brief, after adsorption to formvar/carbon‐coated nickel grids, the exosomes were fixed with 2 % paraformaldehyde and stained with 1.9 % methyl cellulose containing .3 % uranyl acetate. For IEM, the exosomes were incubated with primary mAbs and isotype‐matched IgG as negative controls for 1 h in wet chamber. After washing, 5 or 10 nm gold‐conjugated secondary antibodies were applied for 16 h, followed by washing and negative contrast staining as described above. All samples were analyzed in JEOL1600 electron microscope.
2.8. NKG2D receptor downregulation experiments
Receptor downregulation of NKG2D expression with exosomes was done as described. 13 In brief, PBMCs were incubated in 5 % CO2, at 37°C for 24 h in absence/presence of equal concentration (40 μg/ml) of native OC‐exosomes isolated from HGSC patient serum, or after treatment with specific mAbs against NKG2D ligands or CD63. After incubation, the cells were stained for NKG2D receptor expression; the events were collected by Accuri 6C Flow Cytometer (BD Biosciences) and analyzed in CFlow Plus program (BD Biosciences) with a gate on CD56+/CD3− NK‐cells.
2.9. Cytotoxicity assay
NKG2D‐mediated cytotoxicity was assessed using PBMCs from healthy donors or HGSC patients as effector cells and the erythroleukemic cell line K562 as target cells in an effector: target ratio of 40:1, as previously described. 13 , 33 Cell death was assessed by CytoTox 96 Non‐Radioactive Cytotoxicity Assay (Promega), which measures lactate dehydrogenase release. The assay was performed according to the manufacturer's instructions. K562 cells were chosen as target cells to measure specifically and only cytotoxicity of NK cells, mediated by NKG2D receptors, without mixing MHC compatibility and TCR activation in the experimental set‐up. K562 targets and effector PBMCs from patients before and after surgery and from healthy donors were incubated at 37°C for 4 h to assess baseline cytotoxicity. In parallel, for the experiments with HGSC exosomes, K562 targets and healthy donor effector cells were incubated with native‐ or mAb‐blocked exosomes at a concentration of 40 μg/ml. Anti‐NKG2D antibodies (clone 1D11, 10 μg/ml, BD Biosciences) were used for receptor blocking. Anti‐CD63 mAbs and a cocktail of four antibodies against MICA/B and ULBP1‐3, at a concentration of 5 μg/ml of each antibody, were used for blocking. Specific lysis was calculated with a standard formula according to the manufacturer's instructions.
2.10. Statistics
Statistical analyses were performed using SPSS (IBM SPSS Statistics 26, IBM, New York, NY, US). The comparison between pre‐ and post‐operative samples from the same patients, was carried out with Wilcoxon signed‐rank test and p ≤ .05 was considered significant. The comparison between independent samples (i.e., the postoperative exosome concentrations in Group 1 and 2) was carried out with Mann–Whitney U test and p ≤ .05 was considered significant. The results from NKG2D receptor downregulation and cytotoxicity experiments are presented as quotas, normalized to the respective control experiments.
3. RESULTS
3.1. Lymphocyte phenotyping reveals differential response to surgery regarding NKG2D receptor expression on PBMCs from HGSC patients
We analyzed the lymphocyte phenotype by double immunofluorescence staining and flow cytometry in paired pre‐ and post‐operative samples of freshly isolated PBMCs from HGSC patients. We concluded that no statistically significant difference was seen in the percentage of B (CD19+) cells, helper (CD4+) and cytotoxic/suppressor (CD8+) T cells, NK cells (CD56+), cells expressing the CD16/Fcγ receptor and cells expressing the cytotoxic NKG2D receptor in the peripheral blood of age‐matched healthy controls and patients (n = 18) before and after surgery (Figure 1A). Next, we analyzed the NKG2D receptor expression on the same lymphocytes by measuring mean fluorescence intensity (MFI) and found that the patients could be divided into two groups ‐ Group 1 (n = 9) and Group 2 (n = 9) depending on the NKG2D expression level. The groups had similar number (%) of CD56+ NKG2D+ and CD8+ NKG2D+ cells before and after surgery (Figure 1B). As can be seen in Group 1 (Figure 1C), there was a significant upregulation of the NKG2D receptor expression after surgery, measured as MFI (p = .005). In Group 2 (Figure 1D) there was a significant downregulation (p = .008). In addition, graphs presenting pre‐ and post‐operative NKG2D expression as an MFI ratio, where the preoperative MFI value is set to 1, are shown in Figure 1C and D. In Group 1, the NKG2D expression, measured as an MFI ratio, was upregulated 2.6 times following surgery (Figure 1C) and in Group 2 it was downregulated .7 times (Figure 1D). The characteristics of Group 1 and Group 2 patients are presented in Table 2.
FIGURE 1.
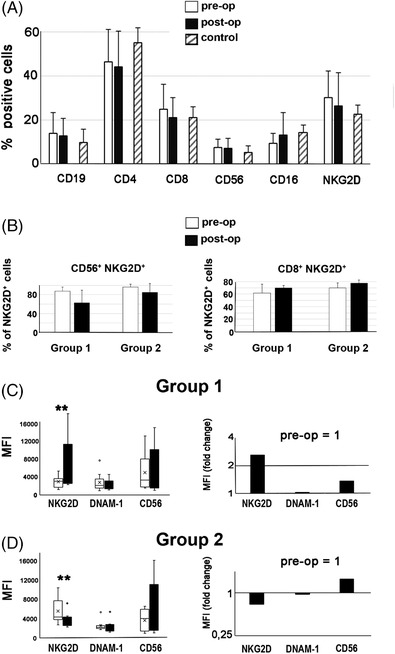
Assessment of the peripheral blood mononuclear cell phenotype in high‐grade serous ovarian cancer patients before and after surgery, using flow cytometry. (A) Percentage of cells positive for different phenotype markers (n = 18 patients). (B) Percentage of CD56+ NKG2D + cells and CD8+ NKG2D+ cells (C), (D), NKG2D, DNAM‐1 and CD56 expression pre‐ and post‐operatively, using mean fluorescent intensity (MFI). Stars (*p ≤ .05, **p < .01) indicate a significant difference in the NKG2D receptor expression for Group 1 and Group 2 (p = .005 and p = .008, respectively).
TABLE 2.
Patient characteristics, Group 1 and Group 2
| Group 1 (n = 9) | Group 2 (n = 9) | |
|---|---|---|
| Characteristics | Value | Value |
| Age, years, median (range) | 63 (54–76) | 58 (42–74) |
| FIGO stage, n | ||
| II | 2 | |
| III | 8 | 7 |
| IV | 1 | |
| Chemotherapy treatment, n | ||
| Prior to postoperative sample | 7 | 7 |
| Surgical outcome, n | ||
|
Primary tumor removed Macroscopically radical |
8 3 |
8 6 |
| Residual tumor mass | 6 | 3 |
| Time‐point for sample collection, weeks, median (range a ) | ||
| Preoperative sample | 0 (−1 to 0) | 0 (−2 to 0) |
| Postoperative sample | 6 (3–10) | 5 (4–14) |
In relation to surgery.
3.2. Group 1 patients had a significantly lower concentration of exosomes in serum after surgery compared to Group 2
The upregulation of NKG2D expression after surgery (Group 1) and our previous finding 11 that EOC produces and secretes NKG2D ligand‐expressing exosomes that downregulate the NKG2D receptor in vitro on PBMCs from healthy donors, prompted us to find out if the downregulation of the NKG2D receptor by exosomes might take place in vivo in the blood of EOC patients and if it could be alleviated by surgically removing the primary tumor. For this purpose, we isolated exosomes from patient sera to use in NKG2D receptor studies and in functional cytotoxic assays. Figure 2A‐C summarizes results from representative experiments of size distribution curves, mean exosomal size and concentration obtained by NTA with ZetaView instrument, and morphology and exosomal marker expression from representative photomicrographs obtained by TEM and IEM analyses with JEOL1600 electron microscope.
FIGURE 2.
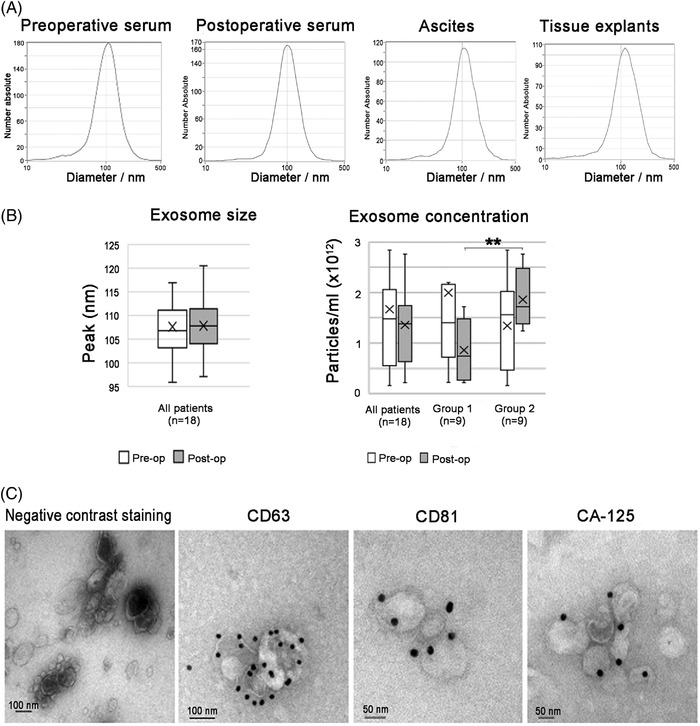
Experiments showing size, morphology and purity of the exosome isolation from serum samples of high‐grade serous ovarian cancer patients. Nanoparticle tracking analysis using ZetaView, illustrating: (A) size distribution of representative samples; (B) median exosome size and concentration and; (C) negative contrast staining and transmission electron microscopy showing exosome morphology and immune electron microscopy of the tetraspanins CD63 and CD81, and the ovarian cancer marker CA‐125. Stars (*p ≤ .05, **p < .01) indicate a significant difference (p = .009) in the postoperative exosome concentration between Group 1 and Group 2.
In Figure 2A, normal size distribution curves of exosomal yields from pre‐ and post‐operative sera, which were comparable to size distribution curves of exosomes isolated from HGSC ascites and supernatants from HGSC tumor explant cultures used as positive controls. There was no difference in median exosome size of exosomes from pre‐ (106.8 nm, Figure 2B) and post‐operative sera (107.8 nm, Figure 2B). The morphology of the isolated EVs from patients’ sera was estimated by negative contrast staining and TEM, and expression of exosomal tetraspanin markers and the OC marker CA‐125 was revealed by immunogold staining and IEM. The obtained EV had a typical cup‐shaped morphology (Figure 2C), characteristic for exosomes analyzed in TEM. They expressed the tetraspanin markers CD63 and CD81 and the OC marker CA‐125, as shown by IEM. The electron microscopy photomicrographs showed a high isolation purity as almost all EV observed had the morphology of exosomes.
The median exosome concentration for all samples (n = 18), assessed by NTA, was preoperatively 1.48 × 1012 and postoperatively 1.38 × 1012 particles/ml. In Group 1 (n = 9), the median preoperative exosome concentration was 1.40 × 1012 particles/ml before surgery and decreased to .76 × 1012 particles/ml after surgery (p = .066). In Group 2 (n = 9), the median exosome concentration was preoperatively 1.56 × 1012 and postoperatively 1.72 × 1012. The postoperative serum exosome concentration in Group 1 patients was significantly lower than the postoperative concentration in Group 2 (p = .009).
From these experiments we conclude that the exosomes isolated from pre‐ and post‐operative sera from Group 1 and Group 2 did not differ in size, morphology, expression of tetraspanin and OC markers or in purity of isolation. The only statistically significant difference between these groups was the lower exosome concentration in the postoperative sera of Group 1 compared to Group 2 patients.
3.3. Exosomes derived from pre‐ and post‐operative sera of HGSC patients downregulate the NKG2D receptor on lymphocytes of healthy donors and impair cytotoxicity
Next, we wanted to see if exosomes isolated from pre‐ and post‐operative sera expressed NKG2D ligands and had the ability to downregulate the NKG2D receptor. In Figure 3A, four photomicrographs from a representative IEM experiment of preoperative serum exosomes isolated from a patient from Group 1 and stained with mAbs against the NKG2D ligands MICA/B and ULBP1‐3 and immunogold, are shown. The exosomes carried MICA/B and ULBP ligands on their surface, illustrated by the electron‐dense silver‐enhanced immunogold dots attached to the exosomal membrane (Figure 3A). From these experiments we concluded that the HGSC exosomes found in the peripheral blood of HGSC patients preserved their NKG2D ligand expression as exosomes isolated from HGSC ascites and tumor explant cultures, previously characterized. 11 Similar results were obtained with postoperative Group 1 exosomes and with pre‐ and post‐operative exosomes from Group 2 (data not shown). In all, pre‐ and post‐operative serum exosomes from three patients/group were investigated by IEM and gave similar results. We found HGSC‐derived NKG2D‐expressing exosomes in both groups. Thereafter, we tested if the HGSC exosomes suppressed the NKG2D receptor expression on PBMCs isolated from healthy donors. The receptor expression was assessed by MFI measured before and after incubation of PBMCs for 24 h with native HGSC exosomes or exosomes blocked with mAbs against NKG2D ligands or CD63. The results are summarized in Figure 3B and C. In Figure 3B, histograms from one representative experiment out of four in each group is shown. Black indicates the baseline NKG2D receptor expression in PBMCs and red indicates NKG2D receptor expression in PBMCs after treatment with native HGSC exosomes. CD3 is stained as a negative control, illustrating that the HGSC exosomes specifically downregulate the NKG2D receptor. The NKG2D receptor expression, measured by MFI, was shifted to the left in the presence of HGSC exosomes. Figure 3C illustrates that the downregulation of the NKG2D receptor expression by native HGSC exosomes (orange staples) is reversed when the exosomes are blocked by anti‐CD63 mAbs (gray staples). The downregulation of the NKG2D receptor expression in PBMCs after treatment with Group 1 preoperative native HGSC exosomes was approximately 35% and after treatment with Group 2 preoperative native exosomes approximately 25%. The effect of exosomes derived from postoperative serum samples was also investigated and gave similar results (data not shown).
FIGURE 3.
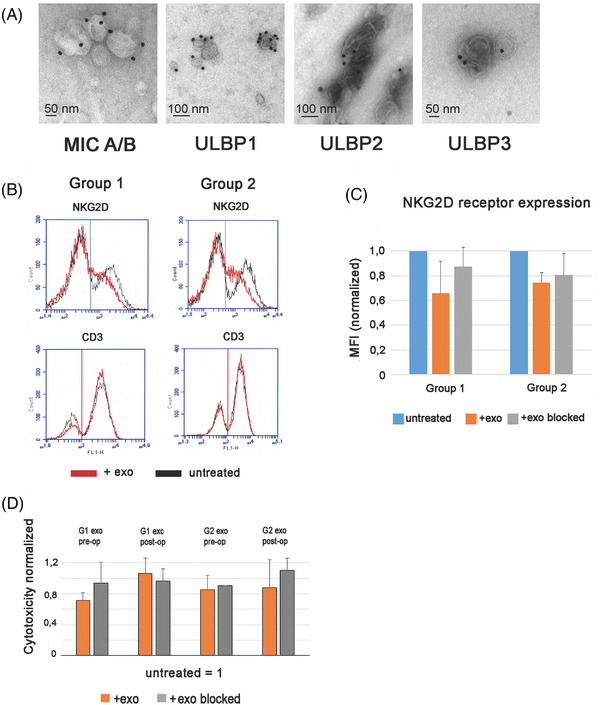
High‐grade serous ovarian cancer (HGSC) exosomes express NKG2D ligands, downregulate the cognate receptor and decrease NKG2D mediated cytotoxicity in peripheral blood mononuclear cells from healthy donors. (A) Immune electron microscopy showing exosomes isolated from HGSC patients’ serum expressing NKG2D ligands. (B) Representative experiment of NKG2D receptor expression assessed by mean fluorescent intensity (MFI) before and after incubation for 24 h with native preoperative serum‐derived HGSC exosomes from the two patient groups, or exosomes blocked with mAb against CD63. (C) Normalized MFI (fold change) of NKG2D receptor expression in PBMCs from healthy donors, before and after treatment with preoperatively serum‐derived HGSC exosomes, or exosomes blocked with mAb against CD63 (n = 4). (D) Inhibition of cytotoxicity against K562 in the presence of native pre‐ or post‐operative serum‐derived HGSC exosomes from Group 1 or Group 2 patients, or mAb‐treated exosomes, effector: target ratio 40:1 (n = 4), normalized to that of untreated cells.
Next, we tested the ability of HGSC exosomes, isolated from pre‐ or post‐operative sera, to suppress NKG2D–mediated NK cytotoxicity by functional experiments. The erythroleukemic K562 target cells, deprived of conventional MHC molecules, were suitable to measure the NK cell cytotoxicity as a model for the overall NKG2D‐mediated cytotoxicity. Experiments (n = 4) were performed with exosomes from individual serum samples collected pre‐ or post‐operatively from Group 1 and 2 patients (Figure 3D). In Figure 3D, cytotoxicity is presented normalized to that of NK cells from healthy donors, before incubation with HGSC exosomes. As can be seen, there was a downregulation of cytotoxicity by preoperatively derived serum exosomes from both groups, but more prominently by exosomes from Group 1 patients (approximately by 30%). When treated with postoperative exosomes from Group 1, the cytotoxicity was at the level of untreated cells. The cytotoxicity following treatment with pre‐ or post‐operative Group 2 exosomes remained more or less unchanged. From these results we conclude that HGSC exosomes in the sera of HGSC patients express NKG2D ligands, downregulate the cognate receptor on PBMCs from healthy donors and preserve their immunosuppressive function as previously shown by us in HGSC ascites and supernatants of tumor explant cultures. 11 In functional experiments, the Group 1 preoperative sera exosomes seem to be more potent than those isolated from Group 2, in reducing the cytotoxic function of NK cells. Interestingly, the NKG2D‐mediated cytotoxicity was unaffected when PBMCs were treated with exosomes isolated from postoperative serum samples of Group 1 patients.
3.4. Surgery improves the cytotoxic potential of NK cells in HGSC patients
We performed cytotoxicity assays with K562 as target cells and the patient's own PBMCs as effectors. The cells were used in simultaneously performed cytotoxic experiments directly after thawing, that is, without any treatment, and after stimulation with IL‐2. In total, experiments were performed with cells from five patients in each group, collected before or after surgery. Figure 4 illustrates the cytotoxicity normalized (fold change) to the preoperative value ( = 1). The cytotoxic function was improved for all patients following surgery. The most prominent difference was found in Group 1, showing a 1.5 times improvement of NKG2D‐mediated cytotoxicity compared to preoperative values (p = .008). In Group 2, there was a lower but statistically significant (p = .05) improvement. The same tendency of improved NK cell cytotoxicity postoperatively was observed in experiments with stimulated cells (Figure 4)
FIGURE 4.
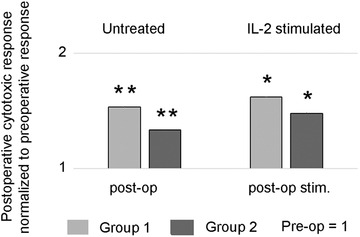
Surgery improves the cytotoxic response of NK cells from high‐grade serous ovarian cancer (HGSC) patients. Postoperative NKG2D‐mediated cytotoxic response of untreated and IL‐2 stimulated NK cells from HGSC patients was normalized to preoperative NKG2D‐mediated cytotoxic response ( = 1) of NK cells from the same patients. K562 cell line was used as target cells, effector: target ratio 40:1, n = 5 patients in each group. Stars (*p ≤ .05, **p < .01) indicate a significant upregulation of postoperative cytotoxic response seen in freshly isolated, untreated NK cells (Group 1: p = .008 and Group 2: p = .05). The response is only slightly further enhanced by IL‐2 treatment.
4. DISCUSSION
Constitutive secretion of exosomes is a common feature of the vast majority of tumors. We were first to identify the role of HGSC exosomes as differential regulators of cytotoxicity, downregulating the major NKG2D receptor‐ligand pathway and leaving the accessory killing via DNAM‐1 receptor as the major cytotoxic response. 11
We did mechanistic studies of the interactions between the HGSC exosomes and NKG2D receptor‐mediated cytotoxicity in the peripheral blood of a limited number of patients before and after surgical treatment, as a small proof‐of‐concept investigation on the role of surgery on the immune status of the patients. Our results can be summarized as follows: (1) Circulating HGSC exosomes, carrying the NKG2D ligands MICA/B and ULBP1‐3, were present in the patients’ sera and were able to downregulate the cognate receptor and impair the NKG2D‐mediated cytotoxic response. (2) In a group of patients, designated as Group 1, there was an increase in NKG2D receptor expression on NK cells following surgery, that correlated to the finding of a simultaneous decrease in concentration of circulating exosomes in the postoperative sera. (3) A significantly enhanced NKG2D‐mediated cytotoxicity was found in the HGSC patients’ own CD56+/NKG2D+ NK cells isolated from postoperative blood samples. Taken together, these results suggest that surgical treatment improves the cytotoxic anti‐tumor immune response in HGSC patients.
In our studies, we used exosomes derived from peripheral blood of the HGSC patients since to study the effect of surgery, blood samples have to be used. We are aware that EVs in sera can emanate from many sources, as well as HGSC, and to partly avoid that we have narrowed our investigation to exosomes expressing NKG2D ligands and compared them to HGSC explant cultures‐derived exosomes isolated from the same patients, characterized as in our previous investigation. 11 We can also assume that even if such exosomes in the peripheral blood can be produced by other cells, in patients suffering from HGSC, the majority of these exosomes would be tumor‐derived or upregulated by inflammation. The level of circulating exosomes is significantly higher in HGSC patients compared to women with benign ovarian conditions, suggesting that the tumor itself secrets higher amounts of exosomes, and/or influences other cells in its vicinity to exosome secretion. 34 Studies of the effect of tumor surgery on the serum levels of exosomes are scarce. We found only one small pilot study from 2019 35 on oral squamous cell carcinoma patients showing that surgery lowered the serum levels of exosomes after one week and correlated to increased survival. To our knowledge, this is the first report investigating the effect of surgery on exosome production and on cytotoxicity in HGSC patients.
Phenotyping the peripheral blood lymphocytes pre‐ and post‐operatively, we found that in some HGSC patients, the NKG2D receptor expression enhanced after surgery and we grouped the patients accordingly to investigate this further: Group 1 with a significant increase in NKG2D expression postoperatively and Group 2 with a significant decrease in NKG2D receptor expression postoperatively. These results correlated with the pre‐ to post‐operative exosome serum concentrations. Group 1 patients had a significantly lower exosome concentration postoperatively compared to Group 2 patients, confirming that the increase in NKG2D receptor expression in Group 1 after surgery could emanate from the decrease in exosome concentration in the postoperative sera of the same patients. The reason for the response difference in the patients regarding NKG2D receptor expression following surgery is at present not known. The majority, 6 out of 9 women, in Group 1, showing increased NKG2D receptor expression in PBMCs, lower serum exosome concentration and improved NK cell cytotoxicity after surgery, were not operated radically, suggesting that there could be several mechanisms at play. Besides exosome‐mediated, the difference might emanate from the heterogeneity of HGSC and the TME. 36 We have no information on the architecture of the TME and of the individual tumors, such as difference in resident cells, genomic changes in the HGSC cells and other immunosuppressive mechanisms operating at the same time. 7 , 14 , 18 It is interesting to observe that when we tested the cytotoxic capacity of the patients’ own NK cells before and after surgery, we found that their cytotoxic potential was enhanced postoperatively in both groups, although more prominent in Group 1 patients (Figure 4).
The single most important independent prognostic factor in advanced HGSC is surgical outcome, with the goal of no visible residual disease. 27 , 28 The positive effect of surgery on the immune system has previously been described as a decrease in circulating T regulatory cells and the immunoinhibitory cytokines IL‐10 and TGF‐β1 and an increase in CD8/CD4 ratio, 37 , 38 , 39 and is mainly seen when primary debulking surgery is performed, and not interval debulking surgery or surgery at recurrent disease. 37 We studied the effect of primary debulking surgery on exosome‐mediated suppression of cytotoxicity. We did not see a quantitative difference in the percentage of immune cell subsets in the pre‐ and post‐operative samples when phenotyping lymphocyte subpopulations. Our results confirmed the results of Nowak et al. who, similarly to us, did not find changes in the lymphocyte subpopulations before and after surgery. 40 They also compared the effect of radical versus non‐radical surgery and found that there was a beneficial effect even in patients that were not radically operated. This is in line with our finding that cytotoxicity was enhanced regardless of surgical radicality or extent.
In contrast to the beneficial effects of surgery, there are opinions suggesting that primary tumor surgery might promote growth of micro‐ and distant metastasis, favoring recurrence. This has been explained by an initial postoperative state of immune suppression, caused by the surgical trauma, that could last up to 21 days postoperatively with a gradual recovery later on. 41 , 42 , 43 In the present study, to circumvent the initial surgical trauma‐induced immunosuppression and to give time for the immune system to adapt to the reduced tumor mass, we chose a longer interval (median 6 weeks) between collection of the pre‐ and post‐operative samples. Taking in consideration the contradictory reports on the effects of primary debulking surgery, we align with the opinion that removing the primary tumor has an overall beneficial effect on the patients’ immune defense in the long run.
We analyzed paired pre‐ and post‐operative blood samples obtained from 18 consecutively enrolled HGSC patients. Our healthcare district is sparsely populated and thus, we could expect a limited number of cases. This prohibited us from subdividing the material regarding to other parameters, such as radicality of operation and cytostatic drug treatment. No significant difference was found comparing progression‐free survival and overall survival between the two groups, probably due to the smaller number of patients. Despite the above‐mentioned weaknesses in this pilot study, we could still, for the first time, estimate the cytotoxic immune response before and after surgery, with a focus on the interference of NKG2D ligand‐bearing HGSC exosomes with the NKG2D receptor‐mediated major cytotoxic pathway, thus providing new insights into the role of cancer surgery. Additional future studies with larger and well‐defined patient cohorts, regarding different types of HGSC treatments and a longer follow‐up time, are needed to prove/disprove the results of this investigation.
In summary, we found that primary debulking surgery in HGSC patients improves their NKG2D‐mediated cytotoxic immune response and possibly their anti‐tumor immune surveillance and ability to fight cancer. One possible mechanistic explanation could be that removal of the tumor, a main source of exosome secretion, induces a systemic decrease in NKG2D ligand‐bearing immunosuppressive exosomes that otherwise could interfere with the major cytotoxic NKG2D receptor and impair the anti‐tumor immune response.
CONFLICT OF INTEREST
None.
Supporting information
SUPPORTING INFORMATION
ACKNOWLEDGMENTS
The authors are grateful to all donors, the staff at the Department of Obstetrics and Gynecology and the Biobank at Norrland's University Hospital, Umeå, for collecting blood samples. The Department of Medical Biosciences, Pathology is acknowledged for providing diagnosis and samples from the Biobank at Norrland's University Hospital, Umeå, Sweden. This work was supported by grants from the Swedish Research Council (18‐20 – 345240311 L.M‐N.), Swedish Cancer Society (CAN 2018/350; no. 18 07 17, L.M‐N.), Central ALF Funding (L.M‐N. and E.L.), Lion's Cancer Research Foundation (E.L., U.O. and P.I.), ALF Funding (U.O., E.L. and L.M‐N.) and the Faculty of Medicine, Umeå University. This study was performed at the Department of Clinical Microbiology/Infection and Immunology, Umeå University.
Israelsson P, Björk E, Nagaev I, et al. NKG2D‐mediated cytotoxicity improves after primary surgery for high‐grade serous ovarian cancer. Am J Reprod Immunol. 2023;89:e13647. 10.1111/aji.13647
Lucia Mincheva‐Nilsson and Ulrika Ottander shared last author position.
DATA AVAILABILITY STATEMENT
The author has provided the required Data Availability Statement, and if applicable, included functional and accurate links to said data therein.
REFERENCES
- 1. Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prat J, FCoG Oncology. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124(1):1‐5. [DOI] [PubMed] [Google Scholar]
- 3. Preston CC, Goode EL, Hartmann LC, Kalli KR, Knutson KL. Immunity and immune suppression in human ovarian cancer. Immunotherapy. 2011;3(4):539‐556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lavoué V, Thédrez A, Levêque J, et al. Immunity of human epithelial ovarian carcinoma: the paradigm of immune suppression in cancer. J Transl Med. 2013;11:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yigit R, Massuger LF, Figdor CG, Torensma R. Ovarian cancer creates a suppressive microenvironment to escape immune elimination. Gynecol Oncol. 2010;117(2):366‐372. [DOI] [PubMed] [Google Scholar]
- 6. Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6(10):715‐727. [DOI] [PubMed] [Google Scholar]
- 7. Israelsson LabaniM, Nagaev Dehlin, et al. Assessment of cytokine mRNA expression profiles in tumor microenvironment and peripheral blood mononuclear cells of patients with high‐grade serous carcinoma of the ovary. 2017;9(5):422‐429. [Google Scholar]
- 8. Taylor DD, Gercel‐Taylor C. Exosomes/microvesicles: mediators of cancer‐associated immunosuppressive microenvironments. Semin Immunopathol. 2011;33(5):441‐454. [DOI] [PubMed] [Google Scholar]
- 9. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mincheva‐Nilsson L, Baranov V, Nagaeva O, Dehlin E. Isolation and characterization of exosomes from cultures of tissue explants and cell lines. Curr Protoc Immunol. 2016;115:14.42.1‐14.42.21. [DOI] [PubMed] [Google Scholar]
- 11. Labani‐Motlagh A, Israelsson P, Ottander U, et al. Differential expression of ligands for NKG2D and DNAM‐1 receptors by epithelial ovarian cancer‐derived exosomes and its influence on NK cell cytotoxicity. Tumour Biol. 2016;37(4):5455‐5466. [DOI] [PubMed] [Google Scholar]
- 12. Hedlund M, Nagaeva O, Kargl D, Baranov V, Mincheva‐Nilsson L. Thermal‐ and oxidative stress causes enhanced release of NKG2D ligand‐bearing immunosuppressive exosomes in leukemia/lymphoma T and B cells. PLoS One. 2011;6(2):e16899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hedlund M, Stenqvist AC, Nagaeva O, et al. Human placenta expresses and secretes NKG2D ligands via exosomes that down‐modulate the cognate receptor expression: evidence for immunosuppressive function. J Immunol. 2009;183(1):340‐351. [DOI] [PubMed] [Google Scholar]
- 14. Peng P, Yan Y, Keng S. Exosomes in the ascites of ovarian cancer patients: origin and effects on anti‐tumor immunity. Oncol Rep. 2011;25(3):749‐762. [DOI] [PubMed] [Google Scholar]
- 15. Keller S, König AK, Marmé F, et al. Systemic presence and tumor‐growth promoting effect of ovarian carcinoma released exosomes. Cancer Lett. 2009;278(1):73‐81. [DOI] [PubMed] [Google Scholar]
- 16. Runz S, Keller S, Rupp C, et al. Malignant ascites‐derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecol Oncol. 2007;107(3):563‐571. [DOI] [PubMed] [Google Scholar]
- 17. Taylor DD, Gercel‐Taylor C. MicroRNA signatures of tumor‐derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110(1):13‐21. [DOI] [PubMed] [Google Scholar]
- 18. Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL. Tumor‐derived microvesicles induce, expand and up‐regulate biological activities of human regulatory T cells (Treg). PLoS One. 2010;5(7):e11469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ying X, Wu Q, Wu X, et al. Epithelial ovarian cancer‐secreted exosomal miR‐222‐3p induces polarization of tumor‐associated macrophages. Oncotarget. 2016;7(28):43076‐43087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bretz NP, Ridinger J, Rupp AK, et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll‐like receptor signaling. J Biol Chem. 2013;288(51):36691‐36702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taylor DD, Gerçel‐Taylor C. Tumour‐derived exosomes and their role in cancer‐associated T‐cell signalling defects. Br J Cancer. 2005;92(2):305‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213‐228. [DOI] [PubMed] [Google Scholar]
- 23. Nilsson J, Skog J, Nordstrand A, et al. Prostate cancer‐derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer. 2009;100(10):1603‐1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lundholm M, Schröder M, Nagaeva O, et al. Prostate tumor‐derived exosomes down‐regulate NKG2D expression on natural killer cells and CD8+ T cells: mechanism of immune evasion. PLoS One. 2014;9(9):e108925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mincheva‐Nilsson L, Baranov V. Cancer exosomes and NKG2D receptor‐ligand interactions: impairing NKG2D‐mediated cytotoxicity and anti‐tumour immune surveillance. Semin Cancer Biol. 2014;28:24‐30. [DOI] [PubMed] [Google Scholar]
- 26. Carlsten M, Björkström NK, Norell H, et al. DNAX accessory molecule‐1 mediated recognition of freshly isolated ovarian carcinoma by resting natural killer cells. Cancer Res. 2007;67(3):1317‐1325. [DOI] [PubMed] [Google Scholar]
- 27.du Bois A, Reuss A, Pujade‐Lauraine E, Harter P, Ray‐Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO‐OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO). Cancer. 2009;115(6):1234‐1244. [DOI] [PubMed] [Google Scholar]
- 28. Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta‐analysis. J Clin Oncol. 2002;20(5):1248‐1259. [DOI] [PubMed] [Google Scholar]
- 29. Baumgartner JM, McCarter MD. Suppressing the suppressor: role of immunosuppressive regulatory T cells in cancer surgery. Surgery. 2009;145(4):345‐350. [DOI] [PubMed] [Google Scholar]
- 30. Meinhold‐Heerlein I, Fotopoulou C, Harter P, et al. The new WHO classification of ovarian, fallopian tube, and primary peritoneal cancer and its clinical implications. Arch Gynecol Obstet. 2016;293(4):695‐700. [DOI] [PubMed] [Google Scholar]
- 31. Regionalt cancercentrum i samverkan . Nationellt vårdprogram äggstockscancer. http://www.cancercentrum.se/samverkan/cancerdiagnoser/gynekologi/aggstock/vardprogram/: Regionalt cancercentrum i samverkan; 2019.
- 32. Nagaeva O, Jonsson L, Mincheva‐Nilsson L. Dominant IL‐10 and TGF‐beta mRNA expression in gammadeltaT cells of human early pregnancy decidua suggests immunoregulatory potential. Am J Reprod Immunol. 2002;48(1):9‐17. [DOI] [PubMed] [Google Scholar]
- 33. Mincheva‐Nilsson L, Nagaeva O, Chen T, et al. Placenta‐derived soluble MHC class I chain‐related molecules down‐regulate NKG2D receptor on peripheral blood mononuclear cells during human pregnancy: a possible novel immune escape mechanism for fetal survival. J Immunol. 2006;176(6):3585‐3592. [DOI] [PubMed] [Google Scholar]
- 34. Szajnik M, Derbis M, Lach M, et al. Exosomes in plasma of patients with ovarian carcinoma: potential biomarkers of tumor progression and response to therapy. Gynecol Obstet (Sunnyvale). 2013(Suppl4):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rodríguez Zorrilla S, Pérez‐Sayans M, Fais S, Logozzi M, Gallas Torreira M, García García A. A pilot clinical study on the prognostic relevance of plasmatic exosomes levels in oral squamous cell carcinoma patients. Cancers. 2019;11(3):429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Horowitz M, Esakov E, Rose P, Reizes O. Signaling within the epithelial ovarian cancer tumor microenvironment: the challenge of tumor heterogeneity. Ann Transl Med. 2020;8(14):905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Napoletano C, Bellati F, Landi R, et al. Ovarian cancer cytoreduction induces changes in T cell population subsets reducing immunosuppression. J Cell Mol Med. 2010;14(12):2748‐2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wicherek L, Jozwicki W, Windorbska W, et al. Analysis of Treg cell population alterations in the peripheral blood of patients treated surgically for ovarian cancer ‐ a preliminary report. Am J Reprod Immunol. 2011;66(5):444‐450. [DOI] [PubMed] [Google Scholar]
- 39. Wu M, Chen X, Lou J, et al. Changes in regulatory T cells in patients with ovarian cancer undergoing surgery: preliminary results. Int Immunopharmacol. 2017;47:244‐250. [DOI] [PubMed] [Google Scholar]
- 40. Nowak M, Głowacka E, Lewkowicz P, et al. Sub‐optimal primary surgery leads to unfavorable immunological changes in ovarian cancer patients. Immunobiology. 2018;223(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 41. Coffey JC, Wang JH, Smith MJ, Bouchier‐Hayes D, Cotter TG, Redmond HP. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol. 2003;4(12):760‐768. [DOI] [PubMed] [Google Scholar]
- 42. Bakos O, Lawson C, Rouleau S, Tai LH. Combining surgery and immunotherapy: turning an immunosuppressive effect into a therapeutic opportunity. J Immunother Cancer. 2018;6(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Menger MD, Vollmar B. Surgical trauma: hyperinflammation versus immunosuppression. Langenbecks Arch Surg. 2004;389(6):475‐484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION
Data Availability Statement
The author has provided the required Data Availability Statement, and if applicable, included functional and accurate links to said data therein.


