Summary
Disrupted hormonal appetite signaling plays a crucial role in obesity as it may lead to uncontrolled reward‐related eating. Such disturbances can be induced not only by weight gain itself but also by glucocorticoid overexposure, for example, due to chronic stress, disease, or medication use. However, the exact pathways are just starting to be understood. Here, we present a conceptual framework of how glucocorticoid excess may impair hormonal appetite signaling and, consequently, eating control in the context of obesity. The evidence we present suggests that counteracting glucocorticoid excess can lead to improvements in appetite signaling and may therefore pose a crucial target for obesity prevention and treatment. In turn, targeting hormonal appetite signals may not only improve weight management and eating behavior but may also decrease detrimental effects of glucocorticoid excess on cardio‐metabolic outcomes and mood. We conclude that gaining a better understanding of the relationship between glucocorticoid excess and circulating appetite signals will contribute greatly to improvements in personalized obesity prevention and treatment.
Keywords: appetite regulation, eating behavior, gut‐brain axis, HPA axis
1. INTRODUCTION
Over the past years, obesity (BMI ≥ 30 kg/m2) has emerged as a pandemic that poses a major health problem and a challenge to health care providers worldwide. 1 Environmental catalysts such as the omnipresence of palatable high‐calorie foods and sedentary lifestyles are major steering wheels in the development of an obese phenotype, especially in Western societies. 2 , 3 , 4 One might therefore argue that an intact physiological and psychological control over one's eating behavior might be needed more than ever in a world full of obesogenic temptations.
However, disturbances in this control are a key problem in obesity. The tendency for uncontrolled hedonic overeating is frequently reported in people with a high BMI along with altered signaling of appetite‐regulating hormones. 5 Prominent dysfunctional eating behaviors in this context include disinhibited/binge eating, emotional eating (comfort eating as a coping strategy in response to negative emotions), and eating impulsivity or external eating (opportunistic, often reward‐related eating that is triggered by food cues). All are characterized by the tendency to eat for the hedonic value of food in the absence of homeostatic need. 6 While the capability to overeat at times when food is abundant was probably an evolutionary advantage in the human past, our current obesogenic environment promotes overeating and weight gain. Unsurprisingly, uncontrolled hedonic eating behaviors were found to predict future increases in BMI. 7 , 8 In fact, a prospective epidemiological study (1,562 subjects, 38.4% women) showed that high emotional eating was a better predictor for subsequent weight gain than lifestyle behaviors such as low physical activity, high alcohol consumption, or low intake of healthy food (vegetables, fruits). 7 Meanwhile, a large body of evidence indicates that uncontrolled overeating is related to the signaling of hormones regulating appetite and metabolism. 9 , 10 Prominent support for this idea comes from genome‐wide association studies showing strong links between BMI and common variants of genes involved in brain‐appetite regulation, indicating that there is a neurohormonal basis for an individual's susceptibility to weight gain in an obesogenic environment. 11 Moreover, patients with obesity often show altered peripheral and central signaling of appetite‐regulating hormones compared to healthy‐weight controls. 9 Thus, the (neuro‐)hormonal connection between peripheral sites of appetite regulation (e.g., the gastrointestinal tract, adipose tissue, the pancreas) and the brain is seen as a promising target for obesity treatment. 12 , 13 Indeed, the first anti‐obesity drugs became available, which target circulating regulators of hedonic and homeostatic food intake and thereby successfully induce weight loss and metabolic improvements. 14 , 15
Intriguingly, the hormonal gut‐brain connection works in close interaction with the hypothalamic‐pituitary‐adrenal (HPA) axis and its major downstream effector cortisol, an endogenous glucocorticoid. In addition to their profound peripheral gluconeogenic and anti‐inflammatory effects, glucocorticoids can induce an increase in food intake and a preference for highly caloric foods. 16 , 17 , 18 , 19 As a result, chronically increased levels of glucocorticoids, e.g. due to medication or chronic stress, may give rise to weight gain and, eventually, obesity. 16 , 20 , 21 , 22 Previous research suggests that this effect is at least partially mediated by glucocorticoid‐induced alterations in the levels and/or signaling of appetite‐regulating hormones. 16 , 19 , 23 Notably, many patients with obesity have cortisol levels in the high‐physiological range and/or use glucocorticoid medication, 24 , 25 indicating that there may be a large subgroup of patients suffering from glucocorticoid effects on appetite and metabolism.
Altogether, altered neuroendocrine appetite signaling may be a crucial mediator in the relationship between glucocorticoid excess and overeating. Here, we provide a conceptual framework of how glucocorticoid‐induced alterations of hormonal appetite signaling may induce uncontrolled eating and thereby contribute to the development and/or maintenance of obesity. In this context, we present evidence building the hypothesis that circulating appetite signals may serve as highly valuable biomarkers for the prevention, diagnostics and treatment success evaluation in obesity, particularly for patients suffering from chronic glucocorticoid excess. Moreover, the presented findings suggest the utmost value of further investigating the potential use of pharmacological therapeutics targeting circulating appetite regulators in such patients.
2. GLUCOCORTICOIDS, APPETITE‐REGULATING HORMONES, AND EATING BEHAVIOR IN OBESITY
Homeostatic and hedonic aspects of eating behavior and appetite are mainly regulated via two central systems. Concerning the physiological need to eat, the hypothalamus plays a crucial role as the main integrator of peripheral and central signals for hunger and satiety. Specifically, orexigenic neuropeptide‐Y (NPY) and agouti‐related peptide (AgRP) neurons as well as anorexigenic pro‐opiomelanocortin (POMC) neurons in the hypothalamic arcuate nucleus orchestrate appetite signals in order to maintain energy homeostasis. 26 Circulating appetite‐regulating hormones are of major importance in this process, not only as regulators of short‐term food intake initiation or termination (e.g., ghrelin, PYY, and GLP‐1), but also as long‐term sensors of energy status and fat storage (e.g., leptin and insulin). 13 , 27 Meanwhile, the hedonic desire to consume highly palatable foods (which are high in sugar and fat) is mediated by mesolimbic reward circuits in response to food cues such as sight, smell, and taste. Mainly acting through projections from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) and the prefrontal cortex, dopaminergic signals induce a feeling of reward in response to the intake of highly palatable foods and thereby act reinforcing. 28 The VTA, however, also receives input from peripheral appetite signals (e.g., insulin, leptin, and ghrelin), thereby serving as a major site of integration of hedonic and homeostatic appetite–regulating signals. 10 , 28
Glucocorticoids can act directly on the hypothalamus and mesolimbic regions (e.g., the VTA), but also indirectly via interactions with circulating appetite regulators. Overall, this may result in diminished homeostatic control and an increased hedonic drive for food intake. 16 , 19 , 22 , 28 , 29 Interactions of appetite‐regulating hormones with the HPA axis have been implicated in various psychoneuroendocrine models of stress and obesity, which has been addressed in previous reviews. 16 , 19 , 22 , 29 , 30 , 31 , 32 , 33 Notably, the eating response to stress can vary not only between different individuals, but also depending on stimulus‐dependent characteristics, such as the duration of stress. Acute stress, especially, can also cause suppression of appetite via effects of corticotrophin‐releasing hormone and urocortin release leading to an inhibition of orexigenic NPY/AgRP neurons. 22
Here, we focus on effects on hormonal appetite regulation in the context of obesity and how this may translate into decreased eating control, especially under conditions of chronically increased glucocorticoid exposure. We give a brief overview of the recent advances in this field and highlight the relevance of further progress. A summary is depicted in Table 1.
TABLE 1.
Proposed interactions of appetite hormones and the HPA axis
| Hormone | Major site of synthesis | Central effect on appetite | Major appetite‐ regulating receptor(s) | Major site(s) of appetite‐regulating actions | Proposed effects of chronic glucocorticoid excess | Other comments (explanations in text) |
|---|---|---|---|---|---|---|
| Leptin | White adipose tissue1 | Anorexigenic2 | LepR3 | ArcN & VTA3 | Increase, fosters central resistance2 | / |
| Insulin | Pancreatic β cells4 | Anorexigenic4 | Insulin receptor4 | Hypothalamus & VTA4 | Increase, fosters central resistance5 | / |
| Ghrelin | Stomach6 | Orexigenic6 | GHSR1a7 | Vagus nerve, ArcN & VTA6 | Increase8, 9 | Potential moderator of the stress response |
| NPY | NPY/AgRP neurons10 | Orexigenic11 | Y1 & Y511 | ArcN10, 12 | Increase12 | Potential moderator of stress + diet effects on obesity outcomes |
| Adiponectin | White and brown adipose tissue13 | Inconsistent14–16 | AdipoR114–16 | ArcN & VTA14–17 | Decrease18, 19 |
Potential moderator of the stress response Target for future pharmacotherapy |
| PYY | Intestinal L cells6 | Anorexigenic20 | Y220 | Gut, vagus nerve& ArcN6 | Inconsistent | Target for future pharmacotherapy |
| GLP‐1 | Intestinal L cells6 | Anorexigenic21, 22 | GLP‐1R21, 22 | Gut, vagus nerve, brainstem, hypothalamus, VTA21–23 | Inconsistent |
Potential moderator of stress + diet effects on obesity outcomes Already target for pharmacotherapy |
Note: (1) Considine, R.V., et al, N Engl J Med, 1996; (2) Zakrzewska, K.E., et al, Diabetes, 1997; (3) Myers, M.G., M.A. Cowley, and H. Münzberg, Annu. Rev. Physiol., 2008; (4) Kullmann, S., et al, Lancet Diabetes Endocrinol, 2020; (5) Baura, G.D., et al, Diabetes, 1996; (6) Cummings, D.E. and J. Overduin, J Clin Invest, 2007; (7) Steinert, R.E., et al, Physiol. Rev., 2017; (8) Lutter, M., et al, Nat. Neurosci., 2008; (9) Yousufzai, M., et al, Transl Psychiatry, 2018; (10) la Fleur, S.E., et al, Int J Obes (Lond), 2010; (11) Duhault, J., et al, Can J Physiol Pharmacol, 2000; (12) Konno, J., et al, Neurosci. Res., 2008; (13) Nigro, E., et al, Biomed Res. Int., 2014; (14) Kubota, N., et al, Cell Metab, 2007; (15) Suyama, S., et al, Sci. Rep., 2016; (16) Coope, A., et al, FEBS Lett, 2008; (17) Sun, F., et al, Mol. Psychiatry, 2019; (18) Roerink, S.H.P.P., et al, Obesity (Silver Spring), 2017; (19) Babinska, A., et al, Steroids, 2018; (20) Batterham, R.L., M.A. Cowley, and C.J. Small, Nature, 2002; (21) Holt, M.K., et al, Diabetes, 2019; (22) Alhadeff, A.L., L.E. Rupprecht, and M.R. Hayes, Endocrinology, 2012; (23) Brierley, D.I., et al, Nat Metab, 2021.
Abbreviations: ArcN, arcuate nucleus (hypothalamus); VTA, ventral tegmental area (mesolimbic system).
2.1. Leptin
Leptin is an adipocyte‐derived hormone that primarily acts as a long‐term sensor for the amount of body fat. 34 , 35 Low levels of leptin induce a physiological starvation response including feelings of hunger and decreases of energy expenditure as well as increases in the hedonic value of food. 36 , 37 Normal and high levels of leptin reduce food intake by inhibiting orexigenic NPY/AgRP neurons, activating anorexigenic POMC neurons in the hypothalamus and diminishing food reward by reducing the firing rate of dopaminergic VTA neurons. 37 , 38 Thus, leptin poses a tonic background signal which informs the brain about the state of fat (i.e., energy) stores in the body. As such, it also has a permissive effect for the mainly gut‐derived satiation signals involved in meal termination. This modulating (or enhancing) effect of circulating leptin on gut‐derived signals is mediated (1) by descending projections from the hypothalamus to the brainstem and (2) by a direct effect of leptin on the brainstem, where these satiation signals are integrated. 39 , 40 , 41 , 42 Serum leptin levels correlate positively with BMI and elevated circulating leptin levels are observed in obesity. 35 , 43 The paradoxical lack of anorectic effects in individuals with a high BMI has been attributed to leptin resistance—a state of altered leptin‐mediated hypothalamic and VTA functioning that renders individuals insensitive for leptin's satiating and reward‐reducing actions. 34 , 44 , 45 Understanding of this phenomenon is still limited, and the term “leptin resistance” is still being discussed. However, accumulating evidence indicates that obesity‐related attenuation of central leptin actions (which some would summarize under the term “leptin resistance”) involve altered intra‐neuronal signaling of leptin receptor‐expressing neurons, neuro‐inflammation, gliosis and endoplasmic reticulum stress. 34 , 39 , 45 It has been proposed previously that, in the context of obesity, even enhanced leptin signaling itself (caused by elevated leptin levels) may attenuate downstream leptin action, thus imposing a functional ceiling for leptin action and thereby allowing for an excessive accumulation of body fat. 39 Nevertheless, this innate propensity to overeat may be more strongly pronounced in some individuals than others, possibly depending on environmental influences.
Accumulating evidence points towards a role of glucocorticoid excess in attenuating central leptin actions. 16 , 22 For example, adrenalectomized rodents show strongly increased sensitivity to leptin injections along with weight loss; an effect which is dose‐dependently reversed upon glucocorticoid supplementation using dexamethasone. 46 , 47 Partially in line with this, prednisolone treatment induced increased food intake despite strongly increased circulating leptin levels in women. 48 Treatment with dexamethasone inhibited leptin‐mediated IL‐1β expression in the murine hypothalamus and glia cell cultures (a pathway assumed to mediate leptin's anorexigenic effects). 49 Hypothalamic leptin receptor expression was not affected, suggesting another, possibly intra‐neuronal signaling pathway. Additionally, rodent studies support the hypothesis that deficits in leptin signaling (e.g., resistance or deficiency) pose a mediating link between obesity and the common occurrence of comorbid depression, a disease that, notably, is characterized by HPA axis dysregulation and weight change. 50 , 51 , 52 In patients with major depressive disorder leptin levels correlated positively with disordered eating, for example, emotional eating and inability to quit. 53 However, this correlation was not corrected for BMI which strongly limits the interpretation of these results. Other studies suggest that leptin may directly affect HPA axis activity and could even act as a protective factor in response to acute stress: raised leptin levels, either at baseline or in response to an acute stressor, have been associated with decreased stress‐induced snack intake. 32 , 50 , 54 , 55 Although this is in line with leptin's antidepressant effects, 50 it demonstrates the need for technological progress to distinguish the effects of leptin resistance from increased leptin levels themselves on behavioral phenotypes in humans.
Overall, attenuations or defects of central leptin actions may lead to increased susceptibility for overeating via (1) direct effects due to altered signaling in the hypothalamus and VTA and (2) indirect effects such as altered satiety signaling of gut‐derived hormones in hindbrain. This may be induced by glucocorticoid excess and may be especially of relevance in the context of negative affect. To pinpoint the role of (glucocorticoid‐induced) leptin signaling deficits in hedonic overconsumption, and specifically emotional eating, quantitative biomarkers for an individual's leptin signaling deficits are needed.
2.2. Insulin
Secreted from the pancreas, insulin is an anabolic agent that is not only essential for glucose metabolism but is also a crucial regulator of homeostatic and hedonic eating. Insulin highly resembles leptin concerning its role in central appetite regulation, including its anorexigenic and reward‐diminishing effects on food intake via actions on the hypothalamus and dopaminergic neurons of the VTA, respectively. 10 , 56 Boosting brain insulin action has therefore been suggested as a target to improve eating behavior by reducing food cravings. 56 Similar to leptin, plasma insulin levels correlate closely with fat accumulation, suggesting that insulin poses another long‐term marker for energy stores rather than an acute satiety signal. 57 However, insulin levels in the cerebrospinal fluid (CSF) are negatively correlated with BMI 58 and the ratio of CSF/blood insulin decreases with whole‐body insulin resistance, 58 , 59 indicating disrupted signaling. This seems at least partially mediated by impaired insulin transport across the blood‐brain‐barrier (BBB) via reduced insulin receptor sensitivity and may result in brain insulin resistance. 59 , 60 , 61
Importantly, chronically elevated glucocorticoid levels seem to interfere with insulin signaling on multiple levels, including the development of whole‐body insulin resistance 16 , 22 , 61 and impaired insulin transport across the BBB. 62 While central infusions of insulin lead to reduced food intake in adrenalectomized rats, this was not observed in intact counterparts. 63 This supports the idea of glucocorticoid‐induced suppression of insulin's ability to exert its anorexigenic effects in the hypothalamus, although other studies also report anorectic effects of central insulin infusions in ‘intact’ animals (as noted above). 64 , 65 Intriguingly, disruptions in brain insulin signaling may also pose a pathological link between type 2 diabetes (i.e., insulin resistance) and depressive symptomatology. 52 , 66 Indeed, the incidence of depression in diabetic patients is two times higher than in the general population. 67 Improving brain insulin signaling has therefore been proposed as an interesting target to reduce mood disturbances, particularly in type 2 diabetes, via insulin's beneficial effects on emotional regulation and HPA axis activity in response to stress. 52 Indeed, intranasal insulin administration can reduce symptoms of depression and anxiety as well as HPA axis activity in animals and humans, as reviewed previously. 68 Altogether, counteracting glucocorticoid‐induced defects in insulin signaling (such as steroid‐induced diabetes) may pose an important treatment target to reduce (emotional) overconsumption and mood disturbances in obesity.
2.3. Ghrelin
Ghrelin is a gut peptide that is mainly produced in the stomach and acts as a potent stimulator of appetite and food intake. 10 , 69 , 70 It is known that ghrelin heightens reward sensitivity and promotes the intake of highly palatable foods by acting on the mesolimbic dopaminergic pathway, thereby opposing the effects of leptin and insulin. 28 , 69 , 71 , 72 In obesity, altered circulating ghrelin levels have been reported (both upregulation and downregulation compared to healthy controls), although most evidence points towards lower levels in obesity. 73 Notably, ghrelin is present in two forms: acylated and unacylated ghrelin. Since only acylated ghrelin binds to the appetite‐regulating GHSR1a receptor, it was originally assumed that unacylated ghrelin is merely an inactive degradation product. However, more recent evidence suggests that both forms have distinct functional roles which may even oppose each other. 70 , 74 Although the value of measuring them separately (instead of total ghrelin) is therefore becoming increasingly evident, total ghrelin is still often measured. 31 , 74
Intriguingly, studies in animals and humans have demonstrated that levels of acylated and total ghrelin rise under chronic and acute stress. 75 , 76 , 77 , 78 , 79 Since ghrelin seems to have anxiolytic and antidepressant actions, 75 , 78 this has been proposed as a protective mechanism which may come at the expense of increased food intake. 22 , 23 , 30 , 31 , 75 , 78 Indeed, it was repeatedly shown that ghrelin mediates chronic‐stress‐induced increases in food intake, food‐reward behavior and weight gain in mice. 75 , 80 In humans, emotional eaters show lower baseline ghrelin levels and a lack of postprandial ghrelin decreases when presented with a snack during or after stress (although both emotional and non‐emotional eaters show a stress‐induced increase in ghrelin levels), 77 , 79 indicating that ghrelin signaling likely plays an important role in determining inter‐individual differences of eating behavior in response to stress. Notably, diet‐induced obesity is often associated with central resistance to the effects of ghrelin (e.g., in the hypothalamus and the VTA). 72 , 81 This led to the hypothesis that a reduced ability to mobilize central ghrelin mediates impaired coping and an increased susceptibility to reward‐related eating in response to negative emotions in the context of obesity. 31 However, our understanding of how peripheral ghrelin acts on deeper brain areas such as the VTA is still very limited (although upstream mechanisms likely play a role, including, e.g., decreased hypothalamic GHSR expression and actions via GHSR1a heterodimers with dopamine receptors). 71 , 81 Intriguingly, chronic stress‐induced elevations of circulating acyl ghrelin levels seem to cause ghrelin resistance in the amygdala (a key region for ghrelin to exert its anxiolytic effects) via decreased amygdala GHSR1a expression, 82 supporting the idea that ghrelin poses a link between chronic stress, obesity, mood dysregulation and overeating. 82
Altogether, disrupted ghrelin signaling may pose a functional link between chronic stress, emotional dysregulation and reward‐related eating behavior in obesity. However, more evidence is needed to support this hypothesis. When trying to unravel ghrelin's role in (glucocorticoid‐induced) hedonic overeating, we need to gain a better understanding of ghrelin's central targets, central ghrelin sensitivity and the role of glucocorticoids in this context. It would also be useful to establish distinct acylated and unacylated ghrelin measurements as the regular approach to assess ghrelin's function.
2.4. Neuropeptide Y (NPY)
NPY is a highly orexigenic peptide, which is produced by neurons in the hypothalamic arcuate nucleus, but in the periphery it can also act directly on adipose tissue where it promotes lipogenesis (i.e., energy storage in fat). 83 , 84 , 85 , 86 , 87 , 88 Similar to ghrelin, NPY increases (food‐)reward‐seeking behavior but also acts as an anxiolytic. 88 , 89 , 90 , 91
It is therefore not surprising that NPY seems to play an important role in the relationship of obesity and glucocorticoid excess. First, animal models have shown that elevated glucocorticoid levels directly stimulate hypothalamic NPY release, which may in turn directly lead to increased food intake and fat accumulation. 84 , 92 , 93 Second, evidence from animal and human studies suggests that the role of NPY in the relationship between increased glucocorticoid levels and obesity is that of a modulator whereby high NPY levels, in concert with increased glucocorticoid exposure, lead to more detrimental obesity outcomes. 84 , 86 , 94 , 95 Indeed, central amygdala NPY neurons mediate the combined negative effects of stress and an unhealthy calorie‐dense diet (resulting e.g. in accelerated weight gain). 96 This is in line with human studies that demonstrated a gene‐by‐psychosocial stress interaction effect of NPY polymorphisms to be linked to overall obesity and the metabolic syndrome. 94
Altogether, NPY may augment the detrimental effect of increased glucocorticoid levels in the context of obesity. Notably, Glucagon‐like peptide‐1 (GLP‐1) may directly or indirectly inhibit hypothalamic NPY expression 97 , 98 while GLP‐1 receptor (GLP‐1R) agonists are already available for obesity treatment (see below). Consequently, these drugs might have beneficial effects in the treatment of obesity associated with combined glucocorticoid and NPY excess which should be further investigated in future studies.
2.5. Adiponectin
Adiponectin is a circulating adipokine and well known for its insulin‐sensitizing effects. 99 Its role in appetite regulation is not yet fully unraveled as studies indicate both anorexigenic and orexigenic actions in the hypothalamus, possibly depending on blood glucose levels. 100 , 101 , 102 , 103 , 104 However, altered adiponectin levels in human eating disorders (e.g., anorexia nervosa and bulimia nervosa) point towards a direct link with appetite. 105 Moreover, adiponectin was recently found to decrease the activity of dopaminergic VTA neurons, suggesting a potential role in hedonic eating. 106 It is noteworthy that adiponectin circulates in three isoforms while only the smaller trimeric and hexameric oligomers, but not the larger high‐molecular‐weight adiponectin, have been found in the CSF. 107 Future studies should therefore investigate potential differences regarding the signaling pathways and central effects of different adiponectin isoforms to shed light on adiponectin's role in the regulation of energy homeostasis.
In obesity, adiponectin is often decreased and, in contrast to other adipokines (such as leptin), levels are inversely correlated with BMI. 108 , 109 Due to its various protective properties (including anti‐inflammatory, anti‐hyperglycemic and anti‐atherogenic actions), adiponectin is seen as a highly interesting pharmaceutical target for the treatment of obesity and related diseases. 99 , 110 , 111 Notably, lower adiponectin levels are not only associated with the metabolic syndrome, but also with depressive symptomatology independently of weight status. 99 , 110 , 112 , 113 , 114 Adiponectin deficits may therefore pose a vulnerability factor linking mood disturbances, metabolic dysfunction, and appetite regulation.
Although findings regarding adiponectin‐HPA axis‐interactions are not without controversy, 115 , 116 strong evidence points towards glucocorticoid‐induced suppression of adiponectin. In vitro treatment with dexamethasone (16–48 h) promotes decreased adiponectin expression in adipocytes. 115 , 117 In vivo, adrenalectomy increases adiponectin expression and serum levels in obese mice. 118 In line with these findings, studies in patients with clinical or subclinical Cushing's syndrome (“CS,” a disease characterized by chronic cortisol excess) reported decreased adiponectin levels compared to age‐, BMI,‐ and gender‐matched healthy controls. 119 , 120 , 121 Intriguingly, such glucocorticoid‐induced reductions may even persist years after remission as has been shown when comparing adiponectin levels of former CS patients to those of control subjects, also matched for age‐, sex‐ and BMI. 122 , 123 However, the presence of adiponectin receptors in human and rodent adrenal glands suggests that the adiponectin‐glucocorticoid relation is not unidirectional. 124 , 125 Indeed, in vitro adiponectin administration has been reported to acutely reduce basal corticosterone production and ACTH‐induced steroidogenesis in mouse adrenocortical cells. 125 Partly in line with this, blockade of central adiponectin action (via receptor deletion or haploinsufficiency) led to increased stress‐induced anxiety‐ and depressive‐like behavior in mice while intra‐VTA adiponectin administration prevented stress‐induced increases of dopaminergic VTA signaling and anxiety‐like behavior. 106 , 126 Adiponectin might therefore represent a protective factor in the stress response.
In conclusion, evidence suggests that adiponectin poses a highly interesting target to induce improvements in metabolism, insulin sensitivity, mood, and, possibly, eating control; especially in the context of glucocorticoid excess. Future studies investigating these links should measure the different isoforms separately, instead of only assessing total adiponectin, as they may differ in their effects.
2.6. Peptide tyrosine‐tyrosine (PYY)
Peptide tyrosine‐tyrosine (PYY) is a potent anorexigenic peptide hormone produced by intestinal L cells which circulates in two forms; PYY1–36 and the active form PYY3–36. 13 , 70 PYY is released postprandially and acts in the gut to induce fullness via delayed gastric emptying, which is also referred to as the “ileal brake.” 70 Nevertheless, PYY exerts its appetite‐reducing effects mainly through binding to the inhibitory neuropeptide Y2 receptor (Y2R) of orexigenic NPY/AgRP neurons in the hypothalamic arcuate nucleus. 13 , 127 , 128 Moreover, PYY may play a role in emotional regulation as it has been reported that PYY deficiency leads to increased depressive‐like behavior in mice. 129
Compared to healthy controls, patients with obesity often show decreased basal PYY plasma levels 130 , 131 , 132 and a blunted postprandial increase. 132 , 133 , 134 In genetically manipulated mice, PYY deletion led to hyperphagia and weight gain, especially when fed a high fat diet, 130 , 135 indicating that PYY deficiencies may be involved in the development and maintenance of obesity by reducing satiety signaling and thereby promoting overeating. 131 , 132 , 134 , 136 Indeed, a blunted postprandial PYY increase has been associated with more disinhibited/uncontrolled eating in healthy humans. 136 Conversely, injection of PYY3–36 acutely suppresses appetite and, subsequently, an ~30% decrease in calorie intake both in patients with obesity and healthy volunteers, suggesting that patients are not resistant to the actions of PYY3–36. 128 , 132 , 137 Thus, the field of pharmacological PYY treatment is advancing to overcome adverse side effects (including nausea and vomiting) and to provide PYY‐analog‐based options for therapeutic use. 138 , 139
The possibility of future PYY drug treatment could have special relevance in the context of glucocorticoid‐related obesity. As reviewed previously, exposure to acute or chronic stressors can modulate circulating PYY, possibly resulting in decreased levels. 23 , 140 , 141 However, evidence is inconclusive so far and especially studies regarding long‐term glucocorticoid exposure in humans are missing. One study in former CS patients revealed no change in PYY levels after remission, but unfortunately the study did not include a healthy control group. 142
Altogether, disruption of PYY's satiety signal seems to be involved in obesity pathology and may be induced/worsened under conditions of stress. This would make PYY3–36 and Y2R agonistic drugs interesting targets in the context of glucocorticoid‐related obesity. Future research should continue studying PYY's involvement in eating (dis‐)inhibition and emotional regulation as well as the effects of chronic glucocorticoid overexposure on PYY signaling in humans. Future studies should also assess the effects of long‐term glucocorticoid or PYY administration on the respective counterpart.
2.7. Glucagon‐like peptide 1 (GLP‐1)
GLP‐1 is an incretin hormone that is released postprandially and known to reduce food intake, food reward, and food‐directed motivation. 143 While peripheral GLP‐1 is secreted by intestinal L‐cells and acts mainly on the vagus nerve to transmit signals to the brainstem and hypothalamus, central GLP‐1 is mainly produced by brainstem preproglucagon neurons and exerts its anorexigenic effects via actions on the hypothalamus and mesolimbic regions (e.g. the VTA and the NAc). 144 , 145 Notably, recent evidence suggests that these two innate GLP‐1 circuits operate rather independently of each other. 146
Due to GLP‐1's potent food intake‐suppressing properties along with beneficial effects on glycemic control, GLP‐1R agonists have already been successfully developed into FDA‐ and EMA‐approved drugs for the treatment of diabetes and obesity (e.g., semaglutide and liraglutide). 15 Importantly, the anorexigenic effects of these drugs are mediated by the central, rather than peripheral, endogenous GLP‐1 system. 98 , 147 In addition, targeting GLP‐1 and its receptor GLP‐1R is seen as a promising approach for the development of novel antidepressants, specifically for patients suffering from obesity and/or diabetes with comorbid mood disorders. 148 , 149 Intriguingly, emotional eaters show less GLP‐1R agonism‐induced reductions in mesolimbic reward signaling compared to non‐emotional eaters, indicating that emotional eating could be (at least partially) mediated by disrupted GLP‐1 signaling in the reward pathway. 150
In view of the major advances in GLP‐1‐targeted drug development, research investigating possible GLP‐1‐HPA axis‐interactions is surprisingly limited. Glucocorticoids may suppress GLP‐1 signaling as in vitro 48‐h exposure to dexamethasone decreased GLP‐1 secretion in murine L cells. Likewise, 1‐week in vivo dexamethasone treatment of rats decreases circulating GLP‐1 levels along with inducing insulin resistance. Altogether, the authors interpreted these findings as a possible pathway of “steroid diabetes.” 151 In turn, other studies have shown that acute and subchronic treatment (≤2 weeks) with GLP‐1R agonists can induce HPA axis stimulation in rodents and humans. 152 , 153 , 154 However, more recent findings in healthy human subjects do not indicate any effects of 3‐week treatment with a GLP‐1R agonist. 155 The originally observed increases in HPA axis stimulation may reflect an acute treatment response which declines on the long term; an idea which is supported by the beneficial effects of chronic GLP‐1R agonist treatment on cardiovascular outcomes and glycemic control. 155 , 156 Remarkably, a rodent study showed that limiting endogenous glucocorticoid secretion enhances the anorectic effect of an GLP‐1R agonist, 157 suggesting that glucocorticoids may interfere with the appetite‐suppressing actions of endogenous GLP‐1. Undoubtedly, more research is needed to pinpoint the relationship of GLP‐1R agonism and HPA axis activation. Ideally, future studies should include long‐term measurements of glucocorticoid exposure such as scalp hair cortisol/corticosterone in addition to the widely used short‐term measures (e.g., from blood, urine, and saliva).
A different line of evidence from animal studies suggests that GLP‐1 may have multiple protective effects under conditions of stress, including reduced inflammation, food intake and depressive‐like behavior (as has been reviewed in detail before). 23 Indeed, this possibility deserves further investigation, especially in view of a study which demonstrated that GLP‐1R agonists successfully prevented glucocorticoid‐induced glucose intolerance in 8 healthy human volunteers. 158 Nevertheless, evidence is still very limited and further studies in humans, especially patients with obesity, are needed to unravel potential protective actions of GLP‐1 analogs to counteract glucocorticoid excess and its physiological effects.
Altogether, GLP‐1R agonists may be readily available pharmaceutical targets to modulate not only overeating, overweight and glycemic control, but also related comorbidities such as mood dysregulation, especially in the context of glucocorticoid excess. Gaining a better understanding of the interactions between (especially chronic) GLP‐1 receptor agonism and (long‐term) HPA axis activity may therefore be of utmost value towards development of more personalized medicine, for example, for patients suffering from obesity and related disorders such as steroid‐induced diabetes and mood dysregulations.
3. LIMITATIONS
Here, we provide a brief overview of well‐known appetite‐regulating hormones and their interactions with the HPA‐axis. We want to highlight the therapeutic potential of further research. We do not claim to give a comprehensive description of all mechanisms involved that are potentially of relevance (e.g., neuropsychological factors and peripheral energy metabolism). On this note, there are two important limitations to be mentioned regarding this paper: (1) For some of the hormones described above, prominently, for example, leptin and insulin, profound sex differences have been described regarding their central actions (likely driven by estrogens). 159 , 160 This could be of high relevance, especially in view of sex differences in eating such as stress‐related and emotional eating being more prevalent in women than men. 5 , 161 , 162 (2) The list of (potential) appetite‐regulating hormones is long. We decided to focus on those which are of special interest due to recent advances in understanding their interactions with the HPA‐axis/therapeutic targeting.
4. CONCLUSION
Altogether, the evidence we present in this review indicates that chronic glucocorticoid excess may lead to disrupted signaling of major appetite‐regulating hormones. These hormones are needed not only for maintaining energy homeostasis, but also emotional functioning. The resulting increases in reward sensitivity and mood dysregulation may induce an increased hedonic drive to eat while simultaneously diminishing homeostatic control. Together, this may lead to hedonic overeating (see Figure 1). In turn, targeting hormonal signals of appetite may enable researchers and clinicians to counteract the vicious circle of glucocorticoid excess and weight gain as well as comorbid detrimental effects on eating control, metabolism and mood. This idea seems especially relevant in view of the advances in pharmacological anti‐obesity therapeutics targeting the hormones discussed in this review.
FIGURE 1.
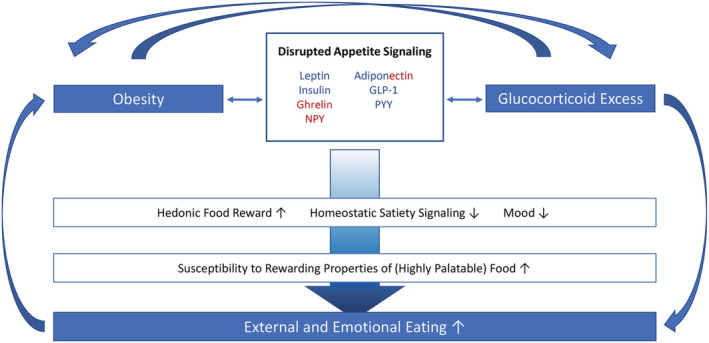
Proposed conceptual framework. Dysregulations of hormonal appetite signaling are observed in obesity, which can foster uncontrolled eating, especially of highly palatable foods. HPA‐axis hyperactivity, for example, due to chronic stress or medication, can also dysregulate the highly sensitive system of appetite regulation, thereby fueling an orexigenic hormonal profile, but also detrimental metabolic processes and mood impairments. These disturbances can, in turn, promote further weight gain along with HPA‐axis alterations and result in a vicious circle. Orexigenic, anorexigenic
We conclude that hormonal appetite signals represent promising biomarkers and treatment targets for obesity, which may be especially useful in the context of glucocorticoid excess. Nevertheless, current knowledge is still very limited, and extending it will be crucial on the way towards the development of more personalized and efficient obesity treatment. Physiological markers for individual signal functioning will be needed as we are trying to objectively quantify (glucocorticoid‐induced) dysregulation of appetite signals and their psycho‐behavioral consequences manifested, for example, in uncontrolled overeating.
CONFLICT OF INTEREST
All authors declare that they have no conflict of interest.
ACKNOWLEDGMENTS
EFCvR is supported by a Vidi grant from the Netherlands Organization of Scientific Research, Netherlands Organization of Scientific Research (NWO), grant number: 91716453. EFCvR and BvdV are also funded by the Elisabeth Foundation.
Kuckuck S, van der Valk ES, Scheurink AJW, et al. Glucocorticoids, stress and eating: The mediating role of appetite‐regulating hormones. Obesity Reviews. 2023;24(3):e13539. doi: 10.1111/obr.13539
Funding information Netherlands Organization of Scientific Research (NWO), Grant/Award Number: 91716453; Elisabeth Foundation
REFERENCES
- 1. Collaborators GBDO, Afshin A, Forouzanfar MH, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13‐27. doi: 10.1056/NEJMoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kopp W. How Western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab Syndr Obes. 2019;12:2221‐2236. doi: 10.2147/dmso.S216791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hill JO, Wyatt HR. Role of physical activity in preventing and treating obesity. J Appl Physiol. 2005;99(2):765‐770. doi: 10.1152/japplphysiol.00137.2005 [DOI] [PubMed] [Google Scholar]
- 4. Prentice SA, Jebb S. Fast foods, energy density and obesity: a possible mechanistic link. Obes Rev. 2003;4(4):187‐194. doi: 10.1046/j.1467-789X.2003.00117.x [DOI] [PubMed] [Google Scholar]
- 5. Nagl M, Hilbert A, Zwaan MD, Braehler E, Kersting A. The German version of the Dutch eating behavior questionnaire: psychometric properties, measurement invariance, and population‐based norms. PLoS One. 2016;11(9):1‐15. doi: 10.1371/journal.pone.0162510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vainik U, Neseliler S, Konstabel K, Fellows LK, Dagher A. Eating traits questionnaires as a continuum of a single concept. Uncontrolled eating. Appetite. 2015;90:229‐239. doi: 10.1016/j.appet.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 7. Koenders PG, van Strien T. Emotional eating, rather than lifestyle behavior, drives weight gain in a prospective study in 1562 employees. J Occup Environ Med. 2011;53(11):1287‐1293. doi: 10.1097/JOM.0b013e31823078a2 [DOI] [PubMed] [Google Scholar]
- 8. Boggiano MM, Wenger LE, Turan B, Tatum MM, Morgan PR, Sylvester MD. Eating tasty food to cope. Longitudinal association with BMI. Appetite. 2015;87:365‐370. doi: 10.1016/j.appet.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lean MEJ, Malkova D. Altered gut and adipose tissue hormones in overweight and obese individuals: cause or consequence? IJO. 2016;40(4):622‐632. doi: 10.1038/ijo.2015.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murray S, Tulloch A, Gold MS, Avena NM. Hormonal and neural mechanisms of food reward, eating behaviour and obesity. Nat Rev Endocrinol. 2014;10(9):540‐552. doi: 10.1038/nrendo.2014.91 [DOI] [PubMed] [Google Scholar]
- 11. Speliotes EK, Willer CJ, Berndt SI, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42(11):937‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Monteiro MP, Batterham RL. The importance of the gastrointestinal tract in controlling food intake and regulating energy balance. Gastroenterology. 2017;152(7):1707‐1717.e2. doi: 10.1053/j.gastro.2017.01.053 [DOI] [PubMed] [Google Scholar]
- 13. Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117(1):13‐23. doi: 10.1172/jci30227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pilitsi E, Farr OM, Polyzos SA, et al. Pharmacotherapy of obesity: available medications and drugs under investigation. Metabolism. 2019;92:170‐192. doi: 10.1016/j.metabol.2018.10.010 [DOI] [PubMed] [Google Scholar]
- 15. Yu M, Benjamin MM, Srinivasan S, et al. Battle of GLP‐1 delivery technologies. Adv Drug Deliv Rev. 2018;130:113‐130. doi: 10.1016/j.addr.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91(4):449‐458. doi: 10.1016/j.physbeh.2007.04.011 [DOI] [PubMed] [Google Scholar]
- 17. Gibson EL. Emotional influences on food choice: sensory, physiological and psychological pathways. Physiol Behav. 2006;89(1):53‐61. doi: 10.1016/j.physbeh.2006.01.024 [DOI] [PubMed] [Google Scholar]
- 18. Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress‐induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26(1):37‐49. doi: 10.1016/s0306-4530(00)00035-4 [DOI] [PubMed] [Google Scholar]
- 19. Maniam J, Morris MJ. The link between stress and feeding behaviour. Neuropharmacology. 2012;63(1):97‐110. doi: 10.1016/j.neuropharm.2012.04.017 [DOI] [PubMed] [Google Scholar]
- 20. Dallman MF. Stress‐induced obesity and the emotional nervous system. Trends Endocrinol Metab. 2010;21(3):159‐165. doi: 10.1016/j.tem.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Van der Valk E, Savas M, van Rossum EFCV. Stress and obesity: are there more susceptible individuals? Curr Obes Rep. 2018;7(2):193‐203. doi: 10.1007/s13679-018-0306-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sominsky L, Spencer SJ. Eating behavior and stress: a pathway to obesity. Front Psychol. 2014;5:434‐434. doi: 10.3389/fpsyg.2014.00434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stengel A, Taché Y. Gut‐brain neuroendocrine signaling under conditions of stress—focus on food intake‐regulatory mediators. Front Endocrinol. 2018;9:498‐498. doi: 10.3389/fendo.2018.00498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wester VL, Staufenbiel SM, Veldhorst MAB, et al. Long‐term cortisol levels measured in scalp hair of obese patients. Obesity (Silver Spring, Md). 2014;22(9):1956‐1958. doi: 10.1002/oby.20795 [DOI] [PubMed] [Google Scholar]
- 25. van der Valk E, Abawi O, Mohseni M, et al. Cross‐sectional relation of long‐term glucocorticoids in hair with anthropometric measurements and their possible determinants: a systematic review and meta‐analysis. Obes Rev. 2022;23(3):e13376. doi: 10.1111/obr.13376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rossi MA, Stuber GD. Overlapping brain circuits for homeostatic and hedonic feeding. Cell Metab. 2018;27(1):42‐56. doi: 10.1016/j.cmet.2017.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science. 2005;307(5717):1909 doi: 10.1126/science.1109951 [DOI] [PubMed] [Google Scholar]
- 28. Meye FJ, Adan RAH. Feelings about food: the ventral tegmental area in food reward and emotional eating. Trends Pharmacol Sci. 2014;35(1):31‐40. doi: 10.1016/j.tips.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 29. Lee CY, Abizaid A. The gut–brain‐Axis as a target to treat stress‐induced obesity. Front Endocrinol. 2014;5:117‐117. doi: 10.3389/fendo.2014.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abizaid A. Stress and obesity: the ghrelin connection. J Neuroendocrinol. 2019;31(7):e12693. doi: 10.1111/jne.12693 [DOI] [PubMed] [Google Scholar]
- 31. Labarthe A, Fiquet O, Hassouna R, et al. Ghrelin‐derived peptides: a link between appetite/reward, GH axis, and psychiatric disorders? Front Endocrinol. 2014;5:163‐163. doi: 10.3389/fendo.2014.00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tomiyama AJ. Stress and obesity. Annu Rev Psychol. 2019;70:703‐718. doi: 10.1146/annurev-psych-010418-102936 [DOI] [PubMed] [Google Scholar]
- 33. Keegan R, Naumovski N. Insulin resistance, glucose regulation, obesity and mood: a review of the literature. J Nutr Intermed Metab. 2016;4:37‐38. doi: 10.1016/j.jnim.2015.12.292 [DOI] [Google Scholar]
- 34. Myers MG, Cowley MA, Münzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70(1):537‐556. doi: 10.1146/annurev.physiol.70.113006.100707 [DOI] [PubMed] [Google Scholar]
- 35. Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive‐leptin concentrations in normal‐weight and obese humans. N Engl J Med. 1996;334(5):292‐295. doi: 10.1056/nejm199602013340503 [DOI] [PubMed] [Google Scholar]
- 36. Rosenbaum M, Leibel RL. 20 years of leptin: role of leptin in energy homeostasis in humans. J Endocrinol. 2014;223(1):T83‐T96. doi: 10.1530/JOE-14-0358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hommel JD, Trinko R, Sears RM, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51(6):801‐810. doi: 10.1016/j.neuron.2006.08.023 [DOI] [PubMed] [Google Scholar]
- 38. Cowley MA, Smart JL, Rubinstein M, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480‐484. doi: 10.1038/35078085 [DOI] [PubMed] [Google Scholar]
- 39. Pan WW, Myers MG Jr. Leptin and the maintenance of elevated body weight. Nat Rev Neurosci. 2018;19(2):95‐105. doi: 10.1038/nrn.2017.168 [DOI] [PubMed] [Google Scholar]
- 40. Williams DL, Baskin DG, Schwartz MW. Leptin regulation of the anorexic response to glucagon‐like peptide‐1 receptor stimulation. Diabetes. 2006;55(12):3387‐3393. doi: 10.2337/db06-0558 [DOI] [PubMed] [Google Scholar]
- 41. Morton GJ, Blevins JE, Williams DL, et al. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest. 2005;115(3):703‐710. doi: 10.1172/JCI22081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Flak JN, Patterson CM, Garfield AS, et al. Leptin‐inhibited PBN neurons enhance responses to hypoglycemia in negative energy balance. Nat Neurosci. 2014;17(12):1744‐1750. doi: 10.1038/nn.3861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cohen SS, Fowke JH, Cai Q, et al. Differences in the association between serum leptin levels and body mass index in black and white women: a report from the southern community cohort study. Ann Nutr Metab. 2012;60(2):90‐97. doi: 10.1159/000336180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matheny M, Shapiro A, Tumer N, Scarpace PJ. Region‐specific diet‐induced and leptin‐induced cellular leptin resistance includes the ventral tegmental area in rats. Neuropharmacology. 2011;60(2‐3):480‐487. doi: 10.1016/j.neuropharm.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. El‐Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet‐induced obesity. J Clin Invest. 2000;105(12):1827‐1832. doi: 10.1172/JCI9842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zakrzewska KE, Cusin I, Sainsbury A, Rohner‐Jeanrenaud F, Jeanrenaud B. Glucocorticoids as counterregulatory hormones of leptin: toward an understanding of leptin resistance. Diabetes. 1997;46(4):717‐719. doi: 10.2337/diab.46.4.717 [DOI] [PubMed] [Google Scholar]
- 47. Solano JM, Jacobson L. Glucocorticoids reverse leptin effects on food intake and body fat in mice without increasing NPY mRNA. Am J Physiol Endocrinol Metab. 1999;277(4):E708‐E716. doi: 10.1152/ajpendo.1999.277.4.E708 [DOI] [PubMed] [Google Scholar]
- 48. Udden J, Bjorntorp P, Arner P, Barkeling B, Meurling L, Rossner S. Effects of glucocorticoids on leptin levels and eating behaviour in women. J Intern Med. 2003;253(2):225‐231. doi: 10.1046/j.1365-2796.2003.01099.x [DOI] [PubMed] [Google Scholar]
- 49. Hosoi T, Okuma Y, Wada S, Nomura Y. Inhibition of leptin‐induced IL‐1beta expression by glucocorticoids in the brain. Brain Res. 2003;969(1‐2):95‐101. doi: 10.1016/s0006-8993(03)02282-0 [DOI] [PubMed] [Google Scholar]
- 50. Lu X‐Y. The leptin hypothesis of depression: a potential link between mood disorders and obesity? Curr Opin Pharmacol. 2007;7(6):648‐652. doi: 10.1016/j.coph.2007.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yamada N, Katsuura G, Ochi Y, et al. Impaired CNS leptin action is implicated in depression associated with obesity. Endocrinology. 2011;152(7):2634‐2643. doi: 10.1210/en.2011-0004 [DOI] [PubMed] [Google Scholar]
- 52. Roberts RE, Deleger S, Strawbridge WJ, Kaplan GA. Prospective association between obesity and depression: evidence from the Alameda County study. IJO. 2003;27(4):514‐521. doi: 10.1038/sj.ijo.0802204 [DOI] [PubMed] [Google Scholar]
- 53. Mills JG, Larkin TA, Deng C, Thomas SJ. Weight gain in major depressive disorder: linking appetite and disordered eating to leptin and ghrelin. Psychiatry Res. 2019;279:244‐251. doi: 10.1016/j.psychres.2019.03.017 [DOI] [PubMed] [Google Scholar]
- 54. Appelhans BM. Circulating leptin moderates the effect of stress on snack intake independent of body mass. Eat Behav. 2010;11(3):152‐155. doi: 10.1016/j.eatbeh.2010.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tomiyama AJ, Schamarek I, Lustig RH, et al. Leptin concentrations in response to acute stress predict subsequent intake of comfort foods. Physiol Behav. 2012;107(1):34‐39. doi: 10.1016/j.physbeh.2012.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kullmann S, Kleinridders A, Small DM, et al. Central nervous pathways of insulin action in the control of metabolism and food intake. Lancet Diabetes Endocrinol. 2020;8(6):524‐534. doi: 10.1016/S2213-8587(20)30113-3 [DOI] [PubMed] [Google Scholar]
- 57. Seidell JC, Björntorp P, Sjöström L, Kvist H, Sannerstedt R. Visceral fat accumulation in men is positively associated with insulin, glucose, and C‐peptide levels, but negatively with testosterone levels. Metabolism. 1990;39(9):897‐901. doi: 10.1016/0026-0495(90)90297-P [DOI] [PubMed] [Google Scholar]
- 58. Kern W, Benedict C, Schultes B, et al. Low cerebrospinal fluid insulin levels in obese humans. Diabetologia. 2006;49(11):2790‐2792. doi: 10.1007/s00125-006-0409-y [DOI] [PubMed] [Google Scholar]
- 59. Heni M, Schöpfer P, Peter A, et al. Evidence for altered transport of insulin across the blood–brain barrier in insulin‐resistant humans. Acta Diabetol. 2014;51(4):679‐681. doi: 10.1007/s00592-013-0546-y [DOI] [PubMed] [Google Scholar]
- 60. Arnold SE, Arvanitakis Z, Macauley‐Rambach SL, et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. 2018;14(3):168‐181. doi: 10.1038/nrneurol.2017.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther. 2012;136(1):82‐93. doi: 10.1016/j.pharmthera.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Baura GD, Foster DM, Kaiyala K, Porte D Jr, Kahn SE, Schwartz MW. Insulin transport from plasma into the central nervous system is inhibited by dexamethasone in dogs. Diabetes. 1996;45(1):86‐90. doi: 10.2337/diab.45.1.86 [DOI] [PubMed] [Google Scholar]
- 63. Chavez M, Seeley RJ, Green PK, Wilkinson CW, Schwartz MW, Woods SC. Adrenalectomy increases sensitivity to central insulin. Physiol Behav. 1997;62(3):631‐634. doi: 10.1016/s0031-9384(97)00188-1 [DOI] [PubMed] [Google Scholar]
- 64. Air EL, Benoit SC, Blake Smith KA, Clegg DJ, Woods SC. Acute third ventricular administration of insulin decreases food intake in two paradigms. Pharmacol Biochem Behav. 2002;72(1–2):423‐429. doi: 10.1016/s0091-3057(01)00780-8 [DOI] [PubMed] [Google Scholar]
- 65. Chavez M, Kaiyala K, Madden LJ, Schwartz MW, Woods SC. Intraventricular insulin and the level of maintained body weight in rats. Behav Neurosci. 1995;109(3):528‐531. doi: 10.1037//0735-7044.109.3.528 [DOI] [PubMed] [Google Scholar]
- 66. Lyra e Silva NDM, Lam MP, Soares CN, Munoz DP, Milev R, de Felice FG. Insulin resistance as a shared pathogenic mechanism between depression and type 2 diabetes. Front Psych. 2019;10:57 doi: 10.3389/fpsyt.2019.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Roy T, Lloyd CE. Epidemiology of depression and diabetes: a systematic review. J Affect Disord. 2012;142(Suppl):S8‐S21. doi: 10.1016/S0165-0327(12)70004-6 [DOI] [PubMed] [Google Scholar]
- 68. Zou XH, Sun LH, Yang W, Li BJ, Cui RJ. Potential role of insulin on the pathogenesis of depression. Cell Prolif. 2020;53(5):e12806. doi: 10.1111/cpr.12806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Abizaid A, Liu ZW, Andrews ZB, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116(12):3229‐3239. doi: 10.1172/JCI29867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Steinert RE, Feinle‐Bisset C, Asarian L, Horowitz M, Beglinger C, Geary N. Ghrelin, CCK, GLP‐1, and PYY(3‐36): secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol Rev. 2017;97(1):411‐463. doi: 10.1152/physrev.00031.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Navarro G, Rea W, Quiroz C, et al. Complexes of ghrelin GHS‐R1a, GHS‐R1b, and dopamine D1 receptors localized in the ventral tegmental area as main mediators of the dopaminergic effects of ghrelin. J Neurosci. 2022;42(6):940‐953. doi: 10.1523/JNEUROSCI.1151-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lockie SH, Dinan T, Lawrence AJ, Spencer SJ, Andrews ZB. Diet‐induced obesity causes ghrelin resistance in reward processing tasks. Psychoneuroendocrinology. 2015;62:114‐120. doi: 10.1016/j.psyneuen.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 73. Wang Y, Wu Q, Zhou Q, et al. Circulating acyl and des‐acyl ghrelin levels in obese adults: a systematic review and meta‐analysis. Sci Rep. 2022;12(1):2679. doi: 10.1038/s41598-022-06636-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Delhanty PJ, Neggers SJ, van der Lely A. Mechanisms in endocrinology: Ghrelin: the differences between acyl‐ and des‐acyl ghrelin. Eur J Endocrinol. 167(5):601‐608 (1479‐683X [Electronic]). doi: 10.1530/EJE-12-0456 [DOI] [PubMed] [Google Scholar]
- 75. Lutter M, Sakata I, Osborne‐Lawrence S, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11(7):752‐753. doi: 10.1038/nn.2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yousufzai M, Harmatz ES, Shah M, Malik MO, Goosens KA. Ghrelin is a persistent biomarker for chronic stress exposure in adolescent rats and humans. Transl Psychiatry. 2018;8(1):74. doi: 10.1038/s41398-018-0135-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Raspopow K, Abizaid A, Matheson K, Anisman H. Psychosocial stressor effects on cortisol and ghrelin in emotional and non‐emotional eaters: Influence of anger and shame. Horm Behav. 2010;58(4):677‐684. doi: 10.1016/j.yhbeh.2010.06.003 [DOI] [PubMed] [Google Scholar]
- 78. Huang HJ, Zhu XC, Han QQ, et al. Ghrelin alleviates anxiety‐ and depression‐like behaviors induced by chronic unpredictable mild stress in rodents. Behav Brain Res. 2017;326:33‐43. doi: 10.1016/j.bbr.2017.02.040 [DOI] [PubMed] [Google Scholar]
- 79. Raspopow K, Abizaid A, Matheson K, Anisman H. Anticipation of a psychosocial stressor differentially influences ghrelin, cortisol and food intake among emotional and non‐emotional eaters. Appetite. 2014;74:35‐43. doi: 10.1016/j.appet.2013.11.018 [DOI] [PubMed] [Google Scholar]
- 80. Chuang J‐C, Perello M, Sakata I, et al. Ghrelin mediates stress‐induced food‐reward behavior in mice. J Clin Invest. 2011;121(7):2684‐2692. doi: 10.1172/jci57660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Briggs DI, Enriori PJ, Lemus MB, Cowley MA, Andrews ZB. Diet‐induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology. 2010;151(10):4745‐4755. doi: 10.1210/en.2010-0556 [DOI] [PubMed] [Google Scholar]
- 82. Harmatz ES, Stone L, Lim SH, et al. Central ghrelin resistance permits the overconsolidation of fear memory. Biol Psychiatry. 2017;81(12):1003‐1013. doi: 10.1016/j.biopsych.2016.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. la Fleur SE, van Rozen AJ, Luijendijk MC, Groeneweg F, Adan RA. A free‐choice high‐fat high‐sugar diet induces changes in arcuate neuropeptide expression that support hyperphagia. Int J Obes (Lond). 2010;34(3):537‐546. doi: 10.1038/ijo.2009.257 [DOI] [PubMed] [Google Scholar]
- 84. Kuo LE, Czarnecka M, Kitlinska JB, Tilan JU, Kvetnanský R, Zukowska Z. Chronic stress, combined with a high‐fat/high‐sugar diet, shifts sympathetic signaling toward neuropeptide Y and leads to obesity and the metabolic syndrome. Ann N Y Acad Sci. 2008;1148:232‐237. doi: 10.1196/annals.1410.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Duhault J, Boulanger M, Chamorro S, et al. Food intake regulation in rodents: Y5 or Y1 NPY receptors or both? Can J Physiol Pharmacol. 2000;78(2):173‐185. [PubMed] [Google Scholar]
- 86. Kuo LE, Kitlinska JB, Tilan JU, et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress‐induced obesity and metabolic syndrome. Nat Med. 2007;13(7):803‐811. doi: 10.1038/nm1611 [DOI] [PubMed] [Google Scholar]
- 87. Park S, Fujishita C, Komatsu T, et al. NPY antagonism reduces adiposity and attenuates age‐related imbalance of adipose tissue metabolism. FASEB J. 2014;28(12):5337‐5348. doi: 10.1096/fj.14-258384 [DOI] [PubMed] [Google Scholar]
- 88. Pandit R, la Fleur SE, Adan RAH. The role of melanocortins and Neuropeptide Y in food reward. Eur J Pharmacol. 2013;719(1):208‐214. doi: 10.1016/j.ejphar.2013.04.059 [DOI] [PubMed] [Google Scholar]
- 89. Karlsson RM, Choe JS, Cameron HA, et al. The neuropeptide Y Y1 receptor subtype is necessary for the anxiolytic‐like effects of neuropeptide Y, but not the antidepressant‐like effects of fluoxetine, in mice. Psychopharmacology (Berl). 2008;195(4):547‐557. doi: 10.1007/s00213-007-0945-2 [DOI] [PubMed] [Google Scholar]
- 90. Brown CM, Fletcher PJ, Coscina DV. Neuropeptide Y‐induced operant responding for sucrose is not mediated by dopamine. Peptides. 1998;19(10):1667‐1673. doi: 10.1016/s0196-9781(98)00117-x [DOI] [PubMed] [Google Scholar]
- 91. Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38(4):213‐224. doi: 10.1016/j.npep.2004.05.002 [DOI] [PubMed] [Google Scholar]
- 92. Konno J, Yoshida S, Ina A, et al. Upregulated expression of neuropeptide Y in hypothalamic–pituitary system of rats by chronic dexamethasone administration. Neurosci Res. 2008;60(3):259‐265. doi: 10.1016/j.neures.2007.11.005 [DOI] [PubMed] [Google Scholar]
- 93. Zakrzewska KE, Cusin I, Zakrzewska KE, et al. Induction of obesity and hyperleptinemia by central glucocorticoid infusion in the rat. Diabetes. 1999;48(2):365‐370. doi: 10.2337/diabetes.48.2.365 [DOI] [PubMed] [Google Scholar]
- 94. Kim H‐J, Min K‐B, Min J‐Y. Neuropeptide Y gene‐by‐psychosocial stress interaction effect is associated with obesity in a Korean population. Psychoneuroendocrinology. 2016;69:10‐15. doi: 10.1016/j.psyneuen.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 95. Aschbacher K, Kornfeld S, Picard M, et al. Chronic stress increases vulnerability to diet‐related abdominal fat, oxidative stress, and metabolic risk. Psychoneuroendocrinology. 2014;46:14‐22. doi: 10.1016/j.psyneuen.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ip CK, Zhang L, Farzi A, et al. Amygdala NPY circuits promote the development of accelerated obesity under chronic stress conditions. Cell Metab. 2019;30(1):111‐128.e6. doi: 10.1016/j.cmet.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 97. Seo S, Ju S, Chung H, Lee D, Park S. Acute effects of glucagon‐like peptide‐1 on hypothalamic neuropeptide and AMP activated kinase expression in fasted rats. Endocr J. 2008;55(5):867‐874. doi: 10.1507/endocrj.k08e-091 [DOI] [PubMed] [Google Scholar]
- 98. Secher A, Jelsing J, Baquero AF, et al. The arcuate nucleus mediates GLP‐1 receptor agonist liraglutide‐dependent weight loss. J Clin Invest. 2014;124(10):4473‐4488. doi: 10.1172/jci75276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Nigro E, Scudiero O, Monaco ML, et al. New insight into adiponectin role in obesity and obesity‐related diseases. Biomed Res Int. 2014;2014:658913 doi: 10.1155/2014/658913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kubota N, Yano W, Kubota T, et al. Adiponectin stimulates AMP‐activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6(1):55‐68. doi: 10.1016/j.cmet.2007.06.003 [DOI] [PubMed] [Google Scholar]
- 101. Tang N, Li Y, Li Y, et al. Molecular cloning, expression and appetite regulation function of adiponectin in Siberian sturgeon (Acipenser baerii). Int J Biol Macromol. 2022;22:01301–0. doi: 10.1016/j.ijbiomac.2022.06.097 [DOI] [PubMed] [Google Scholar]
- 102. Coope A, Milanski M, Araújo EP, et al. AdipoR1 mediates the anorexigenic and insulin/leptin‐like actions of adiponectin in the hypothalamus. FEBS Lett. 2008;10:1471‐1476. doi: 10.1016/j.febslet.2008.03.037 [DOI] [PubMed] [Google Scholar]
- 103. Suyama S, Maekawa F, Maejima Y, Kubota N, Kadowaki T, Yada T. Glucose level determines excitatory or inhibitory effects of adiponectin on arcuate POMC neuron activity and feeding. Sci Rep. 2016;6:30796. doi: 10.1038/srep30796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lee TH‐Y, Cheng KK‐Y, Hoo RL‐C, Siu PM‐F, Yau S‐Y. The novel perspectives of adipokines on brain health. Int J Mol Sci. 2019;20(22):5638‐5638. doi: 10.3390/ijms20225638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Khalil RB, el Hachem C. Adiponectin in eating disorders. Eat Weight Disord. 2014;19(1):3‐10. doi: 10.1007/s40519-013-0094-z [DOI] [PubMed] [Google Scholar]
- 106. Sun F, Lei Y, You J, et al. Adiponectin modulates ventral tegmental area dopamine neuron activity and anxiety‐related behavior through AdipoR1. Mol Psychiatry. 2019;24(1):126‐144. doi: 10.1038/s41380-018-0102-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kusminski CM, McTernan PG, Schraw T, et al. Adiponectin complexes in human cerebrospinal fluid: distinct complex distribution from serum. Diabetologia. 2007;50(3):634‐642. doi: 10.1007/s00125-006-0577-9 [DOI] [PubMed] [Google Scholar]
- 108. Kaser S, Tatarczyk T, Stadlmayr A, et al. Effect of obesity and insulin sensitivity on adiponectin isoform distribution. Eur J Clin. 2008;38(11):827‐834. doi: 10.1111/j.1365-2362.2008.02028.x [DOI] [PubMed] [Google Scholar]
- 109. Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46(4):459‐469. doi: 10.1007/s00125-003-1074-z [DOI] [PubMed] [Google Scholar]
- 110. Phillips SA, Kung JT. Mechanisms of adiponectin regulation and use as a pharmacological target. Curr Opin Pharmacol. 2010;10(6):676‐683. doi: 10.1016/j.coph.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 111. Hara K, Horikoshi M, Yamauchi T, et al. Measurement of the high–molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care. 2006;29(6):1357 doi: 10.2337/dc05-1801 [DOI] [PubMed] [Google Scholar]
- 112. Cao B, Chen Y, Brietzke E, et al. Leptin and adiponectin levels in major depressive disorder: a systematic review and meta‐analysis. J Affect Disord. 2018;238:101‐110. doi: 10.1016/j.jad.2018.05.008 [DOI] [PubMed] [Google Scholar]
- 113. Taylor VH, Macqueen GM. The role of adipokines in understanding the associations between obesity and depression. J Obes. 2010;2010:748048. doi: 10.1155/2010/748048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Everson‐Rose SA, Clark CJ, Wang Q, et al. Depressive symptoms and adipokines in women: study of women's health across the nation. Psychoneuroendocrinology. 2018;97:20‐27. doi: 10.1016/j.psyneuen.2018.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sukumaran S, Dubois DC, Jusko WJ, Almon RR. Glucocorticoid effects on adiponectin expression. Vitam Horm. 2012;90:163‐186. doi: 10.1016/b978-0-12-398313-8.00007-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Valassi E, Biller BMK, Klibanski A, Misra M. Adipokines and cardiovascular risk in Cushing's syndrome. Neuroendocrinology. 2012;95(3):187‐206. doi: 10.1159/000330416 [DOI] [PubMed] [Google Scholar]
- 117. Kaikaew K, Steenbergen J, van Dijk TH, Grefhorst A, Visser JA. Sex difference in corticosterone‐induced insulin resistance in mice. Endocrinology. 2019;160(10):2367‐2387. doi: 10.1210/en.2019-00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Makimura H, Mizuno TM, Bergen H, Mobbs CV. Adiponectin is stimulated by adrenalectomy in ob/ob mice and is highly correlated with resistin mRNA. Am J Physiol Endocrinol Metab. 2002;283(6):1266‐1271. doi: 10.1152/ajpendo.00227.2002 [DOI] [PubMed] [Google Scholar]
- 119. Roerink SHPP, Wagenmakers MAEM, Langenhuijsen JF, et al. Increased adipocyte size, macrophage infiltration, and adverse local adipokine profile in perirenal fat in Cushing's syndrome. Obesity (Silver Spring). 2017;25(8):1369‐1374. doi: 10.1002/oby.21887 [DOI] [PubMed] [Google Scholar]
- 120. Babinska A, Kaszubowski M, Kmiec P, Sworczak K. Adipokine and cytokine levels in patients with adrenocortical cancer, subclinical Cushing's syndrome and healthy controls. Steroids. 2018;140:39‐44. doi: 10.1016/j.steroids.2018.08.011 [DOI] [PubMed] [Google Scholar]
- 121. Fallo F, Scarda A, Sonino N, et al. Effect of glucocorticoids on adiponectin: a study in healthy subjects and in Cushing's syndrome. Eur J Endocrinol. 2004;150(3):339‐344. doi: 10.1530/eje.0.1500339 [DOI] [PubMed] [Google Scholar]
- 122. Wagenmakers M, Roerink S, Gil L, et al. Persistent centripetal fat distribution and metabolic abnormalities in patients in long‐term remission of Cushing's syndrome. Clin Endocrinol (Oxf). 2015;82(2):180‐187. doi: 10.1111/cen.12639 [DOI] [PubMed] [Google Scholar]
- 123. Barahona M‐J, Sucunza N, Resmini E, et al. Persistent body fat mass and inflammatory marker increases after long‐term cure of Cushing's syndrome. J Clin Endocrinol Metab. 2009;94(9):3365‐3371. doi: 10.1210/jc.2009-0766 [DOI] [PubMed] [Google Scholar]
- 124. Rossi GP, Sticchi D, Giuliani L, et al. Adiponectin receptor expression in the human adrenal cortex and aldosterone‐producing adenomas. Int J Mol Med. 2006;17(6):975‐980. doi: 10.3892/IJMM.17.6.975 [DOI] [PubMed] [Google Scholar]
- 125. Li P, Sun F, Cao H‐M, et al. Expression of adiponectin receptors in mouse adrenal glands and the adrenocortical Y‐1 cell line: adiponectin regulates steroidogenesis. Biochem Biophys Res Commun. 2009;390(4):1208‐1213. doi: 10.1016/j.bbrc.2009.10.122 [DOI] [PubMed] [Google Scholar]
- 126. Liu J, Guo M, Zhang D, et al. Adiponectin is critical in determining susceptibility to depressive behaviors and has antidepressant‐like activity. PNAS. 2012;109(30):12248‐12253. doi: 10.1073/pnas.1202835109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Batterham RL, Ffytche DH, Rosenthal JM, et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450(7166):106‐109. doi: 10.1038/nature06212 [DOI] [PubMed] [Google Scholar]
- 128. Batterham RL, Cowley MA, Small CJ. Gut hormone PYY 3‐36 physiologically inhibits food intake. Nature. 2002;418(August):728‐730. doi: 10.1038/nature00887 [DOI] [PubMed] [Google Scholar]
- 129. Painsipp E, Herzog H, Sperk G, Holzer P. Sex‐dependent control of murine emotional‐affective behaviour in health and colitis by peptide YY and neuropeptide Y. Br J Pharmacol. 2011;163(6):1302‐1314. doi: 10.1111/j.1476-5381.2011.01326.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Batterham RL, Heffron H, Kapoor S, et al. Critical role for peptide YY in protein‐mediated satiation and body‐weight regulation. Cell Metab. 2006;4(3):223‐233. doi: 10.1016/j.cmet.2006.08.001 [DOI] [PubMed] [Google Scholar]
- 131. Bartolomé MA, Borque M, Martinez‐Sarmiento J, et al. Peptide YY secretion in morbidly obese patients before and after vertical banded gastroplasty. Obes Surg. 2002;12(3):324‐327. doi: 10.1381/096089202321088084 [DOI] [PubMed] [Google Scholar]
- 132. Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY 3–36. N Engl J Med. 2003;349(10):941‐948. doi: 10.1056/NEJMoa030204 [DOI] [PubMed] [Google Scholar]
- 133. Stock S, Leichner P, Wong ACK, et al. Ghrelin, peptide YY, glucose‐dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J Clin Endocrinol Metab. 2005;90(4):2161‐2168. doi: 10.1210/jc.2004-1251 [DOI] [PubMed] [Google Scholar]
- 134. le Roux CW, Batterham RL, Aylwin SJB, et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147(1):3‐8. doi: 10.1210/en.2005-0972 [DOI] [PubMed] [Google Scholar]
- 135. Boey D, Lin S, Karl T, et al. Peptide YY ablation in mice leads to the development of hyperinsulinaemia and obesity. Diabetologia. 2006;49(6):1360‐1370. doi: 10.1007/s00125-006-0237-0 [DOI] [PubMed] [Google Scholar]
- 136. Martins C, Robertson MD, Morgan LM. Impact of restraint and disinhibition on PYY plasma levels and subjective feelings of appetite. Appetite. 2010;55(2):208‐213. doi: 10.1016/j.appet.2010.05.091 [DOI] [PubMed] [Google Scholar]
- 137. Degen L, Oesch S, Casanova M, et al. Effect of peptide YY3‐36 on food intake in humans. Gastroenterology. 2005;129(5):1430‐1436. doi: 10.1053/j.gastro.2005.09.001 [DOI] [PubMed] [Google Scholar]
- 138. Gantz I, Erondu N, Mallick M, et al. Efficacy and safety of intranasal peptide YY3–36 for weight reduction in obese adults. J Clin Endocrinol Metab. 2007;92(5):1754‐1757. doi: 10.1210/jc.2006-1806 [DOI] [PubMed] [Google Scholar]
- 139. Rangwala SM, D'Aquino K, Zhang Y‐M, et al. A long‐acting PYY3–36 analog mediates robust anorectic efficacy with minimal emesis in nonhuman primates. Cell Metab. 2019;29(4):837‐843. doi: 10.1016/j.cmet.2019.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Laessle R, Hilterscheid E. Stress‐induced release of eating‐related hormones in young women classified as restrained and unrestrained eaters. Neuropsychobiology. 2019;78(1):27‐30. doi: 10.1159/000498866 [DOI] [PubMed] [Google Scholar]
- 141. Kiessl GRR, Laessle RG. Stress inhibits PYY secretion in obese and normal weight women. Eat Weight Disord ‐ Studies Anorexia, Bulimia Obesity. 2016;21(2):245‐249. doi: 10.1007/s40519-015-0231-y [DOI] [PubMed] [Google Scholar]
- 142. Geer EB, Lalazar Y, Couto LM, et al. A prospective study of appetite and food craving in 30 patients with Cushing's disease. Pituitary. 2016;19(2):117‐126. doi: 10.1007/s11102-015-0690-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Muller TD, Finan B, Bloom SR, et al. Glucagon‐like peptide 1 (GLP‐1). Mol Metab. 2019;30:72‐130. doi: 10.1016/j.molmet.2019.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Holt MK, Richards JE, Cook DR, et al. Preproglucagon neurons in the nucleus of the solitary tract are the main source of brain GLP‐1, mediate stress‐induced hypophagia, and limit unusually large intakes of food. Diabetes. 2019;68(1):21‐33. doi: 10.2337/db18-0729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Alhadeff AL, Rupprecht LE, Hayes MR. GLP‐1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153(2):647‐658. doi: 10.1210/en.2011-1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Brierley DI, Holt MK, Singh A, et al. Central and peripheral GLP‐1 systems independently suppress eating. Nat Metab. 2021;3(2):258‐273. doi: 10.1038/s42255-021-00344-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Sisley S, Gutierrez‐Aguilar R, Scott M, D'Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP1R mediates liraglutide's anorectic but not glucose‐lowering effect. J Clin Invest. 2014;124(6):2456‐2463. doi: 10.1172/JCI72434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Pozzi M, Mazhar F, Peeters GGAM, et al. A systematic review of the antidepressant effects of glucagon‐like peptide 1 further link between metabolism and psychopathology: special section on “translational and neuroscience studies in affective disorders”. J Affect Disord. 2019;257:774‐778. doi: 10.1016/j.jad.2019.05.044 [DOI] [PubMed] [Google Scholar]
- 149. Anderberg RH, Richard JE, Hansson C, Nissbrandt H, Bergquist F, Skibicka KP. GLP‐1 is both anxiogenic and antidepressant; divergent effects of acute and chronic GLP‐1 on emotionality. Psychoneuroendocrinology. 2016;65:54‐66. doi: 10.1016/j.psyneuen.2015.11.021 [DOI] [PubMed] [Google Scholar]
- 150. van Bloemendaal L, Veltman DJ, ten Kulve JS, et al. Emotional eating is associated with increased brain responses to food‐cues and reduced sensitivity to GLP‐1 receptor activation. Obesity (Silver Spring). 2015;23(10):2075‐2082. doi: 10.1002/oby.21200 [DOI] [PubMed] [Google Scholar]
- 151. Kappe C, Fransson L, Wolbert P, Ortsäter H. Glucocorticoids suppress GLP‐1 secretion: possible contribution to their diabetogenic effects. Clin Sci. 2015;129(5):405‐414. doi: 10.1042/cs20140719 [DOI] [PubMed] [Google Scholar]
- 152. Gil‐Lozano M, Romaní‐Pérez M, Outeiriño‐Iglesias V, et al. Effects of prolonged exendin‐4 administration on hypothalamic‐pituitary‐adrenal axis activity and water balance. Am J Physiol Endocrinol Metab. 2013;304(10):E1105‐E1117. doi: 10.1152/ajpendo.00529.2012 [DOI] [PubMed] [Google Scholar]
- 153. Gil‐Lozano M, Pérez‐Tilve D, Alvarez‐Crespo M, et al. GLP‐1(7‐36)‐amide and Exendin‐4 stimulate the HPA axis in rodents and humans. Endocrinology. 2010;151(6):2629‐2640. doi: 10.1210/en.2009-0915 [DOI] [PubMed] [Google Scholar]
- 154. Krass M, Volke A, Rünkorg K, et al. GLP‐1 receptor agonists have a sustained stimulatory effect on corticosterone release after chronic treatment. Acta Neuropsychiatr. 2015;27(1):25‐32. doi: 10.1017/neu.2014.36 [DOI] [PubMed] [Google Scholar]
- 155. Winzeler B, da Conceição I, Refardt J, Sailer CO, Dutilh G, Christ‐Crain M. Effects of glucagon‐like peptide‐1 receptor agonists on hypothalamic‐pituitary‐adrenal axis in healthy volunteers. J Clin Endocrinol Metab. 2018;104(1):202‐208. doi: 10.1210/jc.2018-01420 [DOI] [PubMed] [Google Scholar]
- 156. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311‐322. doi: 10.1056/NEJMoa1603827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Lee SJ, Diener K, Kaufman S, et al. Limiting glucocorticoid secretion increases the anorexigenic property of Exendin‐4. Mol Metab. 2016;5(7):552‐565. doi: 10.1016/j.molmet.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Raalte D, Genugten R, Linssen L, Ouwens D, Diamant M. Glucagon‐like peptide‐1 receptor agonist treatment prevents glucocorticoid‐induced glucose intolerance and islet‐cell dysfunction in humans. Diabetes Care. 2011;34:412‐417. doi: 10.2337/dc10-1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55(4):978‐987. doi:10.2337/diabetes.55.04.06.db05–1339 [DOI] [PubMed] [Google Scholar]
- 160. Hallschmid M, Benedict C, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin reduces body fat in men but not in women. Diabetes. 2004;53(11):3024–9. doi: 10.2337/diabetes.53.11.3024 [DOI] [PubMed] [Google Scholar]
- 161. Meule A, Reichenberger J, Blechert J. Development and preliminary validation of the Salzburg stress eating scale. Appetite. 2018;120:442‐448. doi: 10.1016/j.appet.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 162. O'Connor D, Jones F, Conner M, McMillan B, Ferguson E. Effects of daily hassles and eating style on eating behavior. Health Psychol. 2008;27:S20‐S31. doi: 10.1037/0278-6133.27.1.S20 [DOI] [PubMed] [Google Scholar]


