Abstract
Introduction
As the science base around the potential benefits of a reduced-nicotine standard for cigarettes grows, information on the potential effects on adolescent smokers is a high priority. The aim of this randomized trial was to test the influence of 3-week exposure to reduced nicotine cigarettes in a sample of adolescent daily smokers.
Aims and Methods
In this double-blind, two-arm, randomized controlled trial (NCT0258731), following a 1-week baseline, adolescent daily smokers not currently intending to quit (ages 15–19 years, n = 66 randomized) were urn randomized to use either very low nicotine content (VLNC; 0.4 mg/g; n = 33) or normal nicotine content (NNC, 15.8 mg/g; n = 33) research cigarettes for 3 weeks. Participants attended five study sessions at our clinical laboratory. The primary outcome was average total cigarettes smoked per day (CPD; including both study and non-study cigarettes) at week 3.
Results
Stepwise regression results demonstrated that compared with NNC cigarettes (n = 31), assignment to VLNC cigarettes (n = 29), was associated with 2.4 fewer CPD on average than NNC assignment (p < .05) week 3 when controlling for covariates (p < .01, Cohen’s d = 0.52 n = 60 completed all procedures). VLNC cigarettes were also associated with lower levels of craving reduction than NNC cigarettes (Questionnaire on Smoking Urges Factor 2, p < .05). No group differences were found for secondary outcomes.
Conclusions
Adolescent participants assigned to VLNC use for 3 weeks smoked fewer total CPD relative to the NNC group. Overall, data suggest that a VLNC policy would reduce cigarette smoking in adolescents who smoke, but high rates of incomplete adherence suggest that youth may seek alternative sources of nicotine in this scenario.
Implications
The US Food and Drug Administration may enact a reduced-nicotine product standard that would affect all commercially available cigarettes. One important population affected by this policy would be adolescents who smoke. This study, the first clinical trial of VLNC cigarettes in adolescents, demonstrates that adolescents switched to VLNC cigarettes for 3 weeks reduced their CPD relative to the normal-nicotine cigarette control group, without leading to increased respiratory symptoms or increased withdrawal. Biomarkers indicated the use of other sources of nicotine, suggesting that such a policy will need to consider approaches to assist in transitioning away from smoking.
Introduction
In 2018, the US Food and Drug Administration, which has the authority to regulate cigarettes, released an Advanced Notice of Proposed Rulemaking that would reduce the public health burden of combustible cigarette smoking by setting a nicotine product standard that reduces allowable levels of nicotine in cigarettes and some other finished tobacco products to nonaddictive levels.1 In 2022, the Biden Administration announced an intention to pursue this policy, indicating a greater likelihood that a nicotine reduction standard could become a reality.2 Such a policy would impact not only current smokers by reducing reinforcement from cigarettes but also would potentially reduce smoking initiation rates.3 This policy, if enacted, would fundamentally change the tobacco product landscape and would be likely to greatly impact public health.4 The empirical evidence base for the potential effects of such a policy lies in laboratory studies and clinical trials where participants are asked to switch to researcher-provided very low nicotine content (VLNC) or normal nicotine content (NNC) control cigarettes, and effects on cigarette use are measured.
Nearly all studies of VLNC cigarettes have been conducted with adults. In several studies in adult daily cigarette smokers who were not trying to quit smoking, those who were switched to VLNC cigarettes reduced their smoking rate, nicotine exposure, and cigarette toxicant exposure.5–7 These results have been replicated in vulnerable populations of adults, including individuals with serious mental illness and socioeconomically disadvantaged women, without leading to exacerbation of underlying conditions.8,9 Studies have also shown that an immediate switch to VLNC cigarettes led to less overall toxicant exposure than a gradual approach in which nicotine levels were stepped down over time.10,11 Furthermore, across studies, concerns about the potential for VLNC cigarette use to result in harmful compensatory smoking—that is, smoking low-nicotine cigarettes more intensely or more frequently—have not been supported.12,13 Overall, this large and growing body of evidence suggests that a low-nicotine product standard would likely lead to improvements in health outcomes for current adult smokers. However, little research has been conducted in adolescent smokers.
There are several reasons why adolescent smokers should be studied as an independent population, as results from adult studies may not generalize to youth. First, adolescent smokers (variously defined as age 13–17 years, or 6th- to 12th-grade students which encompass roughly ages 11 to 18 years) in the United States have different patterns of smoking: they tend to be lighter and more intermittent smokers,14 and therefore experience less nicotine exposure on average than adult smokers.15 While adolescent smokers do develop nicotine dependence symptoms,16,17 their smoking is also influenced by non-nicotine factors such as peer context18,19 and endorsement of smoking identity.20 Furthermore, despite a similar prevalence of intentions to quit as reported in adults, adolescents rarely use evidence-based treatments to quit,21–23 and many adolescent daily smokers in the United States, will continue to smoke into adulthood.24 Finally, the vast majority of American current smokers began smoking before the age of 20 years,25 and therefore reducing smoking among youth is essential to reducing the overall burden of smoking-related disease. These important factors have led to a growing body of work on the potential effect of a nicotine reduction policy on youth.26
In general, experimental data on the potential effects of cigarette nicotine reduction in youth in the United States have shown that such a policy would likely lead to reduced abuse liability of cigarettes. In a laboratory study of adolescent smokers’ response to nicotine in cigarettes, adolescent smokers aged 15–19 years sampled research cigarettes containing 15.8, 5.2, 1.3, and 0.4 mg nicotine/g of tobacco in the laboratory following overnight abstinence.27 These results showed that VLNC cigarettes reduced withdrawal symptoms and led to lower indices of abuse liability (positive subjective evaluations and cigarette craving) relative to NNC cigarettes. These findings comport with results from other acute laboratory studies showing that VLNC cigarettes reduced negative affect (NA) and cravings in youth.28
However, unlike in adults, acute studies in adolescents who smoke have shown that the reinforcing value of VLNC cigarettes was not lower than that of NNC cigarettes. In a laboratory study in which adolescent smokers were asked to sample cigarettes varying in nicotine content, in separate sessions, and to estimate their hypothetical demand for each cigarette using a hypothetical purchase task, no differences were found between NNC and VLNC cigarettes.29 However, demand for all study cigarettes was significantly lower than demand for their usual brand cigarettes. These results were in contrast to what has been found with adults, who have shown greater sensitivity to nicotine content in cigarette purchase tasks,30,31 suggesting that perhaps NNC study cigarettes were less reinforcing for youth than for adults. The laboratory study was limited, however, by its acute exposure protocol; more differences in reinforcing efficacy may be expected to emerge over time.
To date, the available data on adolescent (age 15–19 years) response to nicotine reduction has been limited to acute exposure. Herein, we report results from the first clinical trial of extended exposure to VLNC cigarettes in adolescents. We hypothesized that adolescents assigned to the VLNC condition would smoke fewer cigarettes per day and have lower total nicotine exposure at the end of the 3-week intervention than those assigned to NNC cigarettes. We also explored the effects of VLNC use on respiratory symptoms but did not hypothesize changes due to the short exposure period.
Methods
Participants
Eligibility criteria included being 15–19 years old, having smoked at least one cigarette per day on ≥28 of the previous 30 days, having smoked daily for ≥6 months, and providing a breath carbon monoxide (CO) of ≥6 ppm (or a NicAlert urinary cotinine reading of greater than 3 if the CO criterion was not met). Participants who were pregnant or breastfeeding, currently intending to quit smoking, used other tobacco products more than 9 out of the last 30 days, reported daily alcohol or drug use (excluding marijuana), or who exclusively used roll-your-own tobacco, were excluded. Participants who reported past 30-day suicide plan or attempt were excluded after speaking with the study clinician. Participants who reported a lifetime suicide attempt were also excluded to reduce the possibility of exacerbating underlying mood conditions during nicotine withdrawal.32 Sample size was determined based on group effects found in previous work in adults.7
Recruitment, Screening, and Consent
Participants were recruited in the United States between October 2016 and August 2019 from Rhode Island and Southeastern Massachusetts area using websites and social media, college newspapers and listservs, and community and school in-person events. Schools were chosen from the Providence, RI and surrounding cities based on previous relationships and willingness of school administrators to hold an in-person event; these consisted of setting up a table at lunchtime with relevant information. Interested participants who called the study were given information about participating, and if still interested they completed a phone screener to establish initial eligibility based on recent smoking. Adolescents recruited in person could self-administer an identical screening questionnaire using an iPad. If the participant was eligible at phone screen and was under 18 years, the staff asked permission to call the adolescent’s parent or guardian. If the parent verbally consented to their child’s participation over the phone, a parental consent form was either mailed to the parent to sign and for their child to bring to their first session or was signed electronically via an emailed Qualtrics link. At the beginning of the first session, participants under 18 years completed and signed a written informed assent form, and participants over 18 years provided written informed consent. All procedures were approved by the Brown University Institutional Review Board. The office of the Attorney General of the state in which the study was conducted provided a waiver of prosecution for the research, allowing for the distribution of study tobacco products to participants.
Research Cigarettes
Spectrum research cigarettes (22nd Century Group, Inc.; produced for NIDA; NOT-DA-14-004) were used. The NNC cigarette contained 15.8 mg nicotine/g tobacco, and 10.5 ± 1.5 mg tar; and the VLNC contained 0.4 mg/g nicotine, 9 ± 1.5 mg tar. Participants were assigned menthol or non-menthol research cigarettes based on their usual brand preference.
Procedures
Screening/Baseline 1 Session
At the initial session, phone screen eligibility criteria were reassessed. If participants remained eligible, baseline procedures commenced. During this session, participants were asked to smoke one of their own usual brand cigarettes in the research laboratory. This was done to compare the acute effects of study cigarettes with those of usual brand under controlled conditions. Participants also provided a urine sample to test for total nicotine equivalents (TNEs) and pregnancy if female, and a saliva sample to test for cotinine Participants were compensated $25 for completing the in-person screening/baseline session, regardless of eligibility. Participants were also reimbursed for travel to the study site; either via a bus ticket provided by the study to our centrally located site, a prepaid taxi pickup and dropoff, or parking validation in our attached parking garage.
Randomization
All cigarette cartons from both conditions were assigned condition codes upon shipment by the Principal Investigator (PI). Then, an unblinded research assistant (RA) (who was not involved in assessing participants) created random codes for each carton, which were kept in a database and sorted by condition and menthol status. Following a successful baseline, an unblinded RA would use an urn randomization procedure33 to stratify participants on gender, cigarettes smoked per day (≥8 CPD) and minor status (≤ or ≥18 years old) and make a condition assignment (equal assignment) and provide the study RA with a carton code corresponding to the condition and menthol status to dispense to the participant. The study was triple masked such that during the trial, neither the participant, the investigators, nor the outcomes assessor was aware of condition assignment.
Baseline 2 (Randomization Session)
Following a battery of assessments, participants were asked to smoke their first study cigarette in the laboratory. Participants were dispensed either VLNC or NNC research cigarettes, totaling 125% of their usual weekly number of cigarettes smoked (calculated from the 30-day Timeline Follow Back at baseline [TLFB] administered at Baseline 1), under double-blind conditions. Participants were instructed to smoke only those assigned cigarettes for the remainder of the study, were encouraged to report any non-study cigarette or other tobacco product use honestly and that they would not be penalized for nonadherence, and not to share the cigarettes with anyone else. To encourage product accountability, participants were compensated $1.00 per empty pack returned. To discourage selling or losing unsmoked cigarettes, participants were offered a nominal amount for unsmoked cigarettes ($5.00 for returning 50%–75%, $2.50 for 25%–49%); total possible compensation from returned packs of cigarettes was $5.00 per week. To discourage nonadherent use of usual brand cigarettes, participants were also told that if they ran out of cigarettes prior to their next session, they could come into the lab and obtain a supply to last them until their next session. This was rare, with only five participants requesting additional cigarettes throughout the study; the study site was available for appointments in the evening (6:30 pm) and on weekends as needed.
Study Session Weeks 1–3
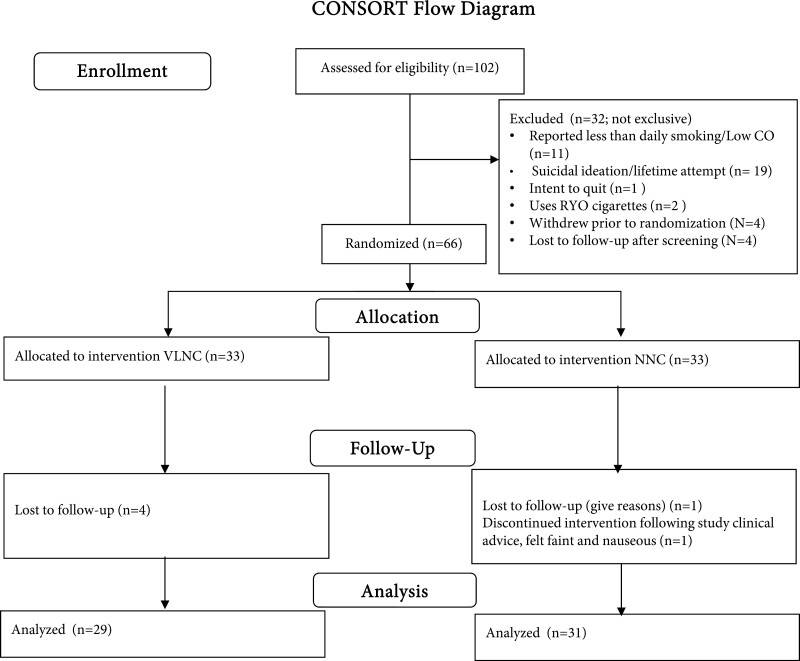
Participants returned to the lab on three subsequent occasions. Participants completed a battery of pre-smoking assessments, including respiratory symptoms, craving, withdrawal, and then were instructed to smoke a single study cigarette in the lab using a handheld smoking topography measurement device (Borgwaldt, KC, Richmond, VA). After completing the cigarette, participants completed post-smoking measures of craving, withdrawal, and subjective response to the cigarettes. Participants were not asked to be abstinent prior to the session. At each session, participants were reminded to bring back their unsmoked cigarettes and empty packs and to account for the cigarettes that they smoked. At the week 3 session, participants provided urine samples that were assayed for TNEs. Following the week 3 session, participants were given resources on quitting smoking and advised to do so. Participants were paid $30 for Baseline 2, $40, $50, and $60 for Sessions 1–3 respectively, and a $50 bonus for completing all study sessions. Figure 1 depicts the flow of participants through the study.
Figure 1.
CONSORT flow diagram.
Measures
Demographic Variables and Covariates
CO Level. Expired breath CO was measured using a Smokerlyzer ED50 CO meter (Bedfont Instruments).
Cotinine Level. Saliva samples were analyzed by an outside laboratory (Salimetrics, LLC, Carlsbad, CA) for cotinine, a measure of recent nicotine exposure, to characterize the sample at baseline using a widely used biomarker.34,35
Demographics and Smoking History. Participants self-reported their age, self-identified gender (male or female), race and ethnicity, age they first smoked a whole cigarette and age at which they became a daily smoker, and menthol preference. Participants were also asked about their sexual identity and sexual attraction.
Primary Outcomes
Total and Study Cigarettes per Day. Participants reported their past-month cigarette and other tobacco use (including their use of smokeless tobacco, little cigars and cigarillos, hookah, e-cigarettes, snus, and nicotine replacement therapies) using a TLFB,36 and reported on use between visits at each study session. Total cigarettes per day (number of study cigarettes smoked plus number of noncompliant non-study cigarettes smoked) was calculated at week 3 from the previous week’s TLFB.
Total Nicotine Equivalents. From urine, we extracted a measure of total nicotine exposure from all nicotine-containing products.37 TNEs were natural log transformed due to skewness.
American Thoracic Society Questionnaire. The American Thoracic Society Questionnaire (ATSQ) asks about eight respiratory symptoms along a frequency scale of (1) (never), (2) less than once per week, (3) 1–2 times per week, (4) several times per week, and to 5 (daily) scale: morning cough, daily cough, wheezing, shortness of breath when walking, shortness of breath during exercise, phlegm production, chest pain, and fatigue onset. ATSQ scores range from 8 to 40. Participants report the frequency of experiencing each of eight respiratory symptoms (eg, morning cough, wheezing, shortness of breath when walking). We have demonstrated that cigarette smoking is associated with elevated respiratory symptoms measured by the ATSQ, with symptoms emerging in adolescent smokers; ATSQ scores in young smokers are significantly related to CPD and smoking dependence.38
Cigarette Dependence. Dependence was assessed at baseline and week 3 using a test modified for adolescent populations from the Fagerström Test for Cigarette Dependence, the Modified Fagerström Tolerance Questionnaire.39,40
Pre- and Post-smoking Measures
Before and after the study smoking a cigarette in the laboratory at baseline and week 3, the following outcomes were assessed and characterized as difference scores (postscore minus prescore), to be compared with the same measures assessed at baseline using a usual brand cigarette.
Withdrawal. The Minnesota Nicotine Withdrawal Scale,41 which assesses seven symptoms (anger/irritability/frustration, anxiety/nervousness, difficulty concentrating, impatience/restlessness, hunger, depression, cigarette craving) was used to assess this construct.
Craving. Using the Brief Questionnaire on Smoking Urges (QSU), we assessed two factors of craving: positive reinforcement aspects of smoking (Factor 1; eg, “A cigarette would taste good right now”) and negative reinforcing aspects of smoking (Factor 2; eg, “Smoking would make me less depressed”)42,43 according to previously published factor analyses.
Negative Affect. NA was assessed using the Positive and Negative Affect Scale 10-item negative affect scale with a range of possible scores from 10 to 50.44
Smoking Topography. From the handheld topography device, several indices of smoking topography were generated: total puff volume (mL), average puff volume (mL), average puff duration (ms), and number of puffs.
Subjective Responses to Cigarettes
Cigarette Evaluation Scale. The 12-item CES45 asks participants to rate the subjective effects of the cigarette on a 0–6 point Likert scale from “not at all” to “extremely” and comprises five subscales: Smoking Satisfaction, Psychological Reward, Enjoyment of Respiratory Symptoms, Craving Reduction, and Aversion. The CES was administered after smoking a usual brand cigarette at baseline and after smoking an assigned study cigarette at weeks 1, 2, and 3.
Sample Size
We based our sample size on power analyses for hypothesis tests using effect sizes derived from a similar study of comparable duration with adult smokers7. We aimed to retain a final sample of 32 participants per group, which were comparable to those in the referenced trial and would allow us to detect the between-groups effect of VLNC cigarettes on cigarettes per day given an effect size as small as 0.99.
Data Analysis Plan
Prespecified analyses included comparing the primary outcome across groups at week 3; all other analyses were post hoc. We did not specify any secondary outcomes in the original trial registration but identified secondary outcomes later to compare our findings to similar studies of adults, but as these were not pre-registered they should be considered exploratory. All demographic and pertinent smoking variables were summarized using descriptive statistics. Next, we used stepwise linear regression to determine the effect of treatment group (0 = NNC, 1 = VLNC) on week 3 total CPD, TNEs, ATSQ score, withdrawal, craving, NA, and CES outcomes after entering baseline values of the outcome variable. This was to isolate the potential effects of group assignment in the presence of what were likely to be the strongest predictors of the outcome. We then added stratification variables as covariates in the second block: age (dichotomized as 18 years and over or under 18 years, to account for potential differences stemming from legal access to cigarettes prior to Tobacco 21 law implementation) and gender, a stratification variable as boys tend to smoke more heavily than girls. Residual analyses were conducted to test for normality and heteroscedasticity; we found no evidence for violations of these assumptions. We did not include corrections for multiple comparisons as our work is focused on the potential for negative effects of VLNC assignment in a vulnerable population, so avoidance of type I error was judged to be less important than avoiding type II error. Predictor variables were judged significant at alpha < 0.05, and all analyses were conducted in SPSS version 26 (IBM). We hypothesized that the VLNC group would report smoking fewer CPD than the NNC group. We further hypothesized that the VLNC group would have their withdrawal symptoms reduced to a lesser extent by the VLNC cigarettes relative to the NNC group. As further exploratory analyses, we conducted a regression model to test whether treatment condition would be associated with self-reported use of non-study (usual brand) cigarettes at week 3, and compared means of reported days of e-cigarette use and other tobacco use using t tests to test for the possibility of a group difference in noncompliance with study cigarettes and/or use of other tobacco products. We also compared smoking topography variables at week 3 across groups using t tests; further analyses were not conducted due to intermittent equipment failure and unequal data loss across groups limiting interpretability. The trial was preregistered on ClinicalTrials.gov (NCT02587312).
Results
A total of 66 participants were randomized, and 60 (91%) participants completed all study sessions. No participants tested positive for pregnancy at any session. The characteristics of the sample can be found in Table 1. On average, the sample was a little over 18 years old, and 49% was female. The sample was 71% white, 12% Hispanic, 3% black, 9% Asian or Pacific Islander, and 14% reporting more than one race. The participants did not differ by group on any measure at baseline. Results of stepwise regression analyses that determined whether treatment group was a significant predictor when including the baseline level of the outcome only (Block 1) and stratification variables (Block 2) are shown in Table 2.
Table 1.
Participant Characteristics and Outcome Variables Measured at Baseline as a Function of Group Assignment
| Participant Characteristics (n=66) | ||
|---|---|---|
| Variable | NNC group (n = 33) M (SD) |
VLNC group (n = 33) M (SD) |
| Age | 18.5 (0.6) | 18.5 (0.6) |
| Gender | 42% Female | 54% Female |
| Race | 18% nonwhite | 36% nonwhite |
| Menthol status | 45% Menthol | 45% Menthol |
| Age at first whole cigarette | 14.4 (2.2) | 14.2 (2.1) |
| Age first started daily smoking | 16.7 (1.5) | 16.2 (2.1) |
| mFTQ score | 4.0 (1.7) | 3.3 (1.6) |
| Salivary cotinine (ng/mL) | 225.0 (199.2) | 217.0 (173.2) |
| CO (ppm) | 11.8 (5.9) | 10.0 (5.1) |
| Average Cigarettes per Day | 9.2 (5.5) | 7.1 (6.0) |
| ATSQ | 18.7 (7.9) | 17.3 (7.1) |
| Ln TNE | 3.2 (1.4) | 3.6 (1.2) |
| NWQ Diff | −3.0 (3.7) | −2.5 (2.6) |
| QSUF1 Diff | −2.7 (1.7) | −2.8 (1.7) |
| QSUF2 Diff | −1.2 (1.2) | −1.1 (1.2) |
| PANAS Neg Diff | −0.8 (2.0) | −2.0 (3.1) |
| CES SS | 3.4 (1.4) | 3.6 (1.6) |
| CES PR | 2.2 (1.2) | 2.2 (1.2) |
| CES ERTS | 2.8 (1.8) | 2.8 (1.8) |
| CES CR | 3.6 (1.7) | 3.2 (1.3) |
| CES AV | 0.6 (0.6) | 0.6 (0.8) |
“Diff” refers to difference scores (post-smoking minus pre-smoking score, negative scores indicate a reduction in symptoms post-smoking). At baseline, both groups smoked a usual brand cigarette.
ATSQ = American Thoracic Society Questionnaire; AV = aversion subscale; CES = Cigarette Evaluation Scale; CO = Expired breath carbon monoxide; CR = craving reduction subscale; Diff = difference score; ERTA = Enjoyment of Respiratory Tract Sensations; Ln TNEs = Total Nicotine Equivalents, natural log transofrmed; mFTQ = Modified Fagerström Tolerance Questionnaire; NWQ = Nicotine Withdrawal Questionnaire; PANAS Neg = Positive and Negative Affect Scale, Negative Affect subscale; PR = Psychological Reward Subscale; QSUF1/F2 = Questionnaire on Smoking Urges Factor 1/2; SS = Smoking Satisfaction subscale.
Table 2.
Hierarchical Regression Results Reporting Unstandardized Beta Coefficients for Treatment Group Assignment, 95% CI for Beta Coefficients, and p-values Referring to the Significance of the Group Assignment Predictor in Each Model
| Unadjusted models | Adjusted models | |||
|---|---|---|---|---|
| Mean difference Beta (95% CI) |
p | Mean difference Beta (95% CI) |
p | |
| Total CPD | −2.4 (−4.2 to −0.62) | .01* | −2.4 (−4.3 to −.61) | .01* |
| Study CPD | −1.8 (−3.7 to 0.10) | .05 | −1.8 (−3.8 to 0.04) | .05 |
| Ln TNEs | −0.44 (−0.96 to 0.07) | .09 | −0.40 (−0.90 to .009) | .11 |
| ATSQ | 0.14 (−2.7 to 3.0) | .91 | −0.07 (−3.0 to 2.8) | .95 |
| mFTQ | −0.42 (−1.2 to 0.43) | .32 | 0.26 (−0.22 to 0.75) | .28 |
| MNWS Diff | 0.50 (−1.0 to 2.0) | .50 | 0.64 (−5.4 to 1.1) | .40 |
| QSUF1 Diff | 0.19 (−0.45 to 0.83) | .55 | 0.18 (−0.47 to 0.85) | .57 |
| QSUF2 Diff | 0.55 (0.12 to .98) | .01* | 0.56 (0.13 to 0.99) | .01* |
| PANAS Neg Diff | 0.71 (−0.31 to 1.7) | .17 | 0.67 (−0.37 to 1.7) | .20 |
| CES SS | −0.04 (−.75 to 0.67) | .91 | −0.07 (−0.79 to 0.65) | .84 |
| CES PR | −0.17 (−.75 to 0.41) | .56 | −0.17 (−0.77 to 0.42) | .42 |
| CES ERTS | −0.13 (−.93 to 0.66) | .74 | −0.2 (−1.0 to 0.59) | .60 |
| CES CR | −0.05 (−1.0 to 0.92) | .91 | −0.05 (1.0 to 0.94) | .92 |
| CES AV | −0.39 (−0.86 to 0.07) | .10 | −0.42 (−0.89 to 0.05) | .08 |
Unadjusted model included treatment condition and baseline level of the outcome. Adjusted models also included stratification variables gender (2-level, male and female) and age (2-level, over 18 years or under 18 years). Each row represents a model with the specified outcome variable.
ATSQ = American Thoracic Society Questionnaire; AV = aversion subscale; CES = Cigarette Evaluation Scale; CPD = Cigarettes Per Day; CR = craving reduction subscale; Diff = difference score, post- pre; ERTS = Enjoyment of Respiratory Tract Sensations; mFTQ = Modified Fagerstrom Tolerance Questionnaire; MNWS = Minnesota Nicotine Withdrawal Survey; PANAS Neg = Positive and Negative Affect Scale, Negative Affect subscale; PR = Psychological Reward Subscale; QSUF1/F2 = Questionnaire on Smoking Urges Factor 1/2; SS = Smoking Satisfaction subscale; TNEs = Total nicotine equivalents.
*Significant predictor, p < .05.
Primary Outcome
Treatment group was a significant predictor of total CPD at week 3 in both blocks, such that VLNC assignment was associated with 2.4 fewer CPD on average than NNC assignment (p < .05). Expressed as a percentage of mean CPD in the NNC group at week 3, participants in the VLNC group smoked 22% fewer cigarettes on average.
Study Cigarette, Respiratory, and Biomarker Outcomes
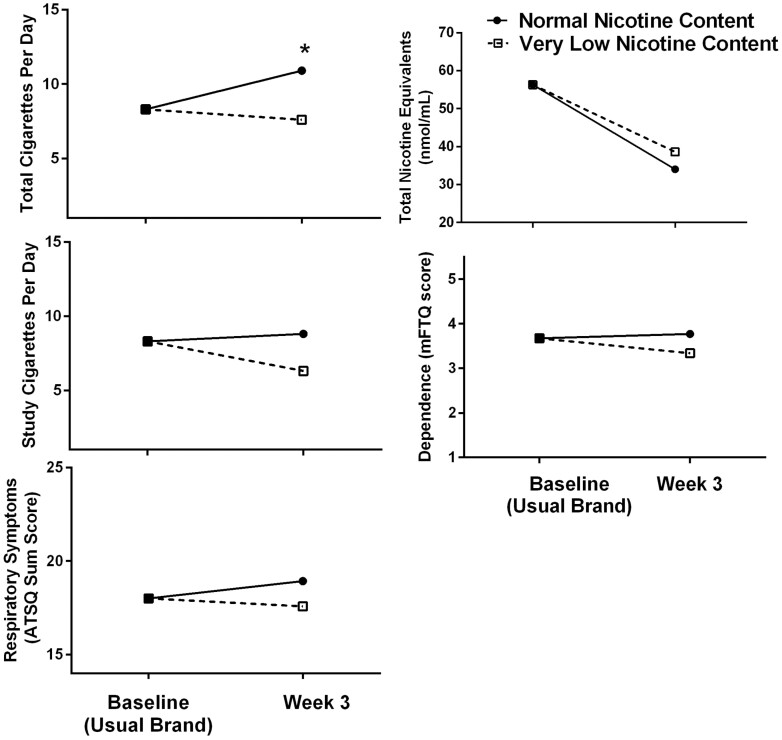
Treatment group was a marginally significant predictor of study CPD (p = .05), and the VLNC condition was associated with using 1.4 fewer study CPD on average relative to the NNC condition. Treatment group assignment was not a significant predictor of ATSQ (p = .95) scores, dependence (p = .28), or TNE (p = .11) at week 3. Primary outcomes are depicted in Figure 2.
Figure 2.
Main outcome means at baseline (usual brand) and at week 3 by group. Asterisks denote a significant effect of treatment group on the outcome at week 3.
Pre-post Smoking Measures of Withdrawal, Craving, and NA
At week 3, participants in the VLNC condition had less reduction in QSU Factor 2 craving scores after smoking a single study cigarette in the laboratory than those in the NNC condition, indicating that VLNC cigarettes were less effective at reducing negative reinforcement-related craving than NNC cigarettes (p < .05). No significant effects of treatment group assignment were found for post-smoking reductions in QSU F1, Minnesota Nicotine Withdrawal Scale withdrawal, or NA scores. These outcomes are depicted in Supplementary Figure 1.
Subjective Responses to Cigarettes
VLNC and NNC group assignment did not predict significantly different CES outcomes on any subscale at week 3. These outcomes are depicted in Supplementary Figure 2.
Other Tobacco Product Use
Group assignment was not a predictor of usual brand use (p = .49, M = 1.9 usual brand CPD in the NNC group, M = 1.2 non-study CPD in the VLNC group). Mean days of reported other combustible tobacco product use (p = .06, M = 0.87 days in the NNC group, M = 0.03 days in the VLNC group) was marginally significant. Mean days of e-cigarette use (p = .55, M = 0.12 days in the NNC group, M = .10 days in the VLNC group) were not significantly different across groups (ps > .05).
Smoking Topography
Equipment failure resulted in missing data for 10 of the 60 completers at week 3, and was unequal across groups (N = 8 missing in the VLNC group, N = 2 missing in the NNC group). Preliminary comparisons of means for total puff volume did not show any group differences: NNC group mean 631.1 mL (SD 489.7), VLNC group mean 595.0 mL (SD 324.9), t = .29, p = .77. Comparisons between groups were also nonsignificant for average puff volume (NNC group mean 44.5 mL [SD 30.9], VLNC group mean 43.6 mL [SD 18.3], t = 0.10, p = .91), average puff duration (NNC group mean 1430.1 ms [SD 1047], VLNC group mean 1536.5 ms [SD 592.5], t = −0.41, p = .67), and number of puffs (NNC group mean 15.1 [SD 5.3], VLNC group mean 13.7 [SD 4.7], t = 0.9, p = .33.
Discussion
This study presents the results of the first randomized controlled trial to model the potential effects of a nicotine reduction policy on young smokers, and as such provides important information to regulatory bodies that support the implementation of this policy. VLNC use across 3 weeks resulted in fewer total CPD relative to control cigarettes containing a normal amount of nicotine. Though this was the only primary outcome measure to show an effect of VLNC exposure, these results also do not indicate the potential for increased harm in this population. Overall, these data are in line with much of the reported literature on the effect of VLNCs on smoking behavior, and provide further evidence that such a policy would be likely to lead to decreased smoking in youth.5,26 Unlike studies of longer duration with adults, we did not see a decrease in dependence in the VLNC group (e.g.5); this may be due to the relatively short duration of product exposure (often 6 weeks or more in adults studies), or to the generally lower levels of nicotine dependence seen in these young smokers relative to adults.
However, there are features of this randomized controlled trial data that need to be taken into account when attempting to generalize to a larger population of adolescents who smoke. In the seminal paper on VLNC use in adults,5 at baseline participants smoked on average 15.6 cigarettes per day. Following 6 weeks of exposure, participants in the lowest nicotine groups (2.4, 1.3, and 0.4 mg/g nicotine) smoked on average 16.5, 16.3, and 14.9 CPD respectively, while the participants in the NNC group smoked 21.3 CPD on average. In other words, providing free cigarettes led to an increase in smoking, but this was not observed in the lowest nicotine groups. This same pattern of results was found here: at baseline, participants smoked an average of 8.3 CPD, while at the end of the study, those in the VLNC group smoked 7.6 CPD and those in the NNC group smoked 10.9. Thus, much like adults, we saw a modest increase in reported smoking in the NNC group and a generally flat to slightly decreased rate of CPD in the VLNC group. In a real-world regulatory environment in which cigarettes cost money and usual brand and NNC cigarettes are no longer available in the legal marketplace, we expect based on casual inference models that a nicotine product standard would result in decreases in overall smoking.46
We also found that participants in the VLNC and NNC conditions rated these cigarettes similarly in terms of their efficacy to reduce withdrawal or NA. As these sessions did not follow overnight abstinence, we did not expect that participants arriving at the lab would have been in significant withdrawal. We used these data to explore the hypothesis that those in the VLNC group may have been experiencing mild withdrawal overall that may have been reduced to a lesser degree by their VLNC cigarettes relative to the NNC condition; however, we did not find this. In terms of craving reduction, the effects of VLNC cigarettes were similar to NNC cigarettes in reducing craving as measured on one QSU subscale (indexing anticipated positive reinforcement from smoking) but led to smaller reductions in craving as measured by the second QSU subscale (indexing anticipated negative reinforcement from smoking). This indicates that the effects of the cigarettes measured pre- and post-smoking were largely similar in both groups. In the one measure where they differed, VLNC cigarettes had lower efficacy in reducing craving negative reinforcement-based than NNC; these findings are consistent with our acute study of adolescent response to nicotine dose in cigarettes,27 in which higher doses of nicotine significantly reduced the craving to a greater degree than lower doses, but this was not true for measures of withdrawal. However, when examining these outcomes relative to baseline when adolescents were rating their own usual brand cigarettes (ie, in Supplemental Figure 2, comparing baseline levels to week 3 in both groups), it is clear that while the effects of smoking the research cigarettes did not differ in most respects, both research cigarettes were generally rated as less subjectively satisfying and less able to reduce withdrawal and craving relative to their usual brand, regardless of nicotine content.
Biochemical data (ie, TNEs) indicated that there was incomplete adherence to the study cigarettes, given that those in the VLNC group did not differ from the NNC group at week 3. Differing degrees of incomplete adherence have also been found in adult studies,47 though those studies generally show a between-group effect on TNEs,11,48 which we did not find. Interestingly, while there was not a differential effect of condition on TNE, Figure 2 shows that both groups experienced a decrease in TNE relative to baseline. This indicates that despite self-reported similar or higher rates of smoking across groups, participants were exposed to less nicotine overall regardless of group, perhaps by smoking less of each cigarette, though topography analyses did not suggest this but are limited in their interpretability due to data loss. The exploratory analysis was conducted to test whether there was a group difference in “cheating”; however, the treatment group was not a predictor of usual brand use, e-cigarette use did not differ between groups, and reported days of other tobacco product use was marginally significant but showed greater use in the NNC group, suggesting that the difference in overall CPD across groups was driven primarily by less study cigarette use in the VLNC group. Because these data are self-reported and participants were encouraged not to use non-study cigarettes, they are likely not fully reliable; though it does suggest that patterns of use of other nicotine-containing products may not be driven primarily by nicotine level in this group. As mentioned above, adolescents may have perceived even NNC cigarettes to be not very reinforcing. In qualitative interviews with youth following the study, participants in both cigarette conditions reported that they did not like the study cigarettes; this provides further evidence that the research cigarettes in general were not as reinforcing as their usual brand regardless of nicotine content.49 We also excluded adolescents who used other nicotine products more than 9 days out of the last 30; therefore, this sample may have been less likely than other youth tobacco users to shift their behavior to other nicotine products in response to reduced satisfaction from the research cigarettes.
Federal Tobacco 21 laws were passed after the completion of data collection for this study, as well as after data collection for previous studies of the effects of VLNCs on young adults included in larger adult trials.10,50 While this allows for a comparison of the current data with extant data from young adults included in adult trials, the new laws are likely to change youth and young adult tobacco use patterns. While data on the impact of nationwide Tobacco 21 laws are still being collected, it is likely that Tobacco 21 will accelerate the shift in age of initiation upward from adolescence to young adulthood,51 making young adults and increasingly important population to focus on when studying young people early in their smoking trajectories. Such changes will be important to monitor when considering implementation of a nicotine reduction policy.
This study has several limitations. The current study was 3 weeks in duration; this is shorter than trials in adults, which have been as long as 20 weeks. We chose this duration as an important first step and due to concerns about potential retention; longer trials appear feasible and are warranted. As noted, this study excluded frequent users of other tobacco products to better isolate the effects of the research cigarettes on nicotine exposure; however, other product use, and specifically e-cigarette use, frequently co-occurs with cigarette smoking in adolescents, limiting the generalizability of the findings. The study also excluded those who want to quit; this is an important population to study but ethically problematic in the current study protocol, in which cigarettes were provided to participants. The generalizability of the study is also limited in that, as in all clinical trials in this area of research, participants were given free cigarettes and paid to participate; real-world conditions will differ greatly from this scenario. Further, while we collected data on participants’ sexual identity, participants could not identify as gender non-binary or gender diverse, which is a limitation; our ongoing studies now routinely include such questions, so future work on this topic will be more informative in this regard. The study also did not specifically focus on vulnerable populations that are disproportionately affected by tobacco, such as minoritized or socially disadvantaged populations. However, youth who smoke in the United States tend to be from vulnerable groups so studying ways to reduce smoking in youth is inherently a health equity issue. Future studies should include youth community advisory boards and increase diversity in sampling to address this. The study is also limited by the relatively small sample size; studies with larger sample sizes should be conducted. Furthermore, the study population was limited to daily-smoking youth; however, intermittent smoking is common in this population.52,53 In studies of adult intermittent smokers, reductions in CPD and nicotine dependence were seen following exposure to VLNC cigarettes54; whether this would be true in youth given their differing patterns of response is as yet unclear. The risks and benefits of testing the potential effects of a nicotine reduction policy in intermittently smoking youth should be weighed carefully given the need to provide relatively large quantities of cigarettes to model the policy. Finally, though our age inclusion criterion was between ages 15 and 19 years, the average age of our population was over 18 years. This reflects current trends showing that the average age of initiation of smoking is shifting to young adulthood from adolescence,51 and indicates that young adulthood should become a focus of future studies of smokers early in their smoking trajectory.
This study represents the first clinical trial of testing the effects of reduced nicotine cigarettes in adolescent smokers. The current study’s strengths include a randomized, blinded design, a comprehensive suite of measures, and high retention of a relatively challenging population. Our group is also working to address further limitations of duration excluding polytobacco users via a recently concluded clinical trial examining the effects of VLNC cigarettes on total harm from tobacco in adolescent polytobacco users across a longer time frame of 5 weeks (R01 DA047356); these results will give greater insight into how adolescents who use other products may respond to a nicotine reduction policy. Taken together with previous studies, the current results suggest that VLNC cigarettes at a minimum do not result in greater harm and may result in reduced smoking in adolescent smokers, bolstering the existing data that show similar benefits for adults. As the FDA continues to weigh the potential benefits and drawbacks of a policy, continued work on the effects of such a monumental policy shift on vulnerable young smokers will be needed.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Acknowledgments
We wish to thank Melissa Victor, Yeelen Edwards, and Jasminette DiLorenzo for their work as a research assistants on this project, and Tim Souza and Sue Sales for their data management assistance. Preliminary analyses of these data and a subset of the primary findings were presented as poster presentations at the Tobacco Regulatory Science Annual meeting in October 2019 and October 2020, respectively.
Contributor Information
Rachel N Cassidy, Center for Alcohol and Addiction Studies, Brown University, Providence, RI, USA.
Jennifer W Tidey, Center for Alcohol and Addiction Studies, Brown University, Providence, RI, USA.
Kristina M Jackson, Center for Alcohol and Addiction Studies, Brown University, Providence, RI, USA.
Patricia A Cioe, Center for Alcohol and Addiction Studies, Brown University, Providence, RI, USA.
Sharon E Murphy, Masonic Cancer Center, University of Minnesota, Minneapolis, MN, USA.
Suchitra Krishnan-Sarin, Tobacco Center of Regulatory Science, Yale University, New Haven, CT, USA.
Dorothy Hatsukami, Masonic Cancer Center, University of Minnesota, Minneapolis, MN, USA.
Suzanne M Colby, Center for Alcohol and Addiction Studies, Brown University, Providence, RI, USA.
Funding
This research was supported by grant K01CA189300 (PI Cassidy). Research reported in this publication was supported by NCI and FDA Center for Tobacco Products (CTP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration. Research cigarettes were supplied by NIDA.
Declaration of Interests
The authors have no conflicts to declare.
Data Availability Statement
De-identified data will be made available to other qualified investigators upon request. The request will be evaluated by the corresponding author to ensure that it meets reasonable demands of scientific integrity. Study results will be uploaded to ClinicalTrials.gov.
References
- 1. Tobacco Product Standard for Nicotine Level of Combusted Cigarettes. Federal Register ; 2018. https://www.federalregister.gov/documents/2018/03/16/2018-05345/tobacco-product-standard-for-nicotine-level-of-combusted-cigarettes. Accessed May 5, 2022.
- 2. Food And Drug Administration. FDA Announces Plans for Proposed Rule to Reduce Addictiveness of Cigarettes and Other Combusted Tobacco Products. FDA; 2022. https://www.fda.gov/news-events/press-announcements/fda-announces-plans-proposed-rule-reduce-addictiveness-cigarettes-and-other-combusted-tobacco. Accessed October 19, 2022. [Google Scholar]
- 3. Apelberg BJ, Feirman SP, Salazar E, et al. Potential public health effects of reducing nicotine levels in cigarettes in the United States. N Engl J Med. 2018;378(18):1725–1733. [DOI] [PubMed] [Google Scholar]
- 4. Levy DT, Cummings KM, Heckman BW, et al. The public health gains had cigarette companies chosen to sell very low nicotine cigarettes. Nicotine Tob Res. 2021;23(3):438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Donny EC, Denlinger RL, Tidey JW, et al. Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med. 2015;373(14):1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Donny EC, Hatsukami DK, Benowitz NL, et al. Reduced nicotine product standards for combustible tobacco: building an empirical basis for effective regulation. Prev Med. 2014;68:17–22. doi: 10.1016/j.ypmed.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hatsukami DK, Kotlyar M, Hertsgaard LA, et al. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addict Abingdon Engl. 2010;105(2):343–355. doi: 10.1111/j.1360-0443.2009.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Higgins ST, Tidey JW, Sigmon SC, et al. Changes in cigarette consumption with reduced nicotine content cigarettes among smokers with psychiatric conditions or socioeconomic disadvantage: 3 Randomized Clinical Trials. JAMA Netw Open. 2020;3(10):e2019311e2019311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tidey JW, Colby SM, Denlinger-Apte RL, et al. Effects of 6-week use of very low nicotine content cigarettes in smokers with serious mental illness. Nicotine Tob Res. 2019;21(suppl 1):S38–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cassidy RN, Tidey JW, Cao Q, et al. Responses to gradual and immediate reduction of nicotine in cigarettes in young versus older adult smokers. Nicotine Tob Res. 2021;23(9):1559–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hatsukami DK, Luo X, Jensen JA, et al. Effect of immediate vs gradual reduction in nicotine content of cigarettes on biomarkers of smoke exposure: a Randomized Clinical Trial. JAMA. 2018;320(9):880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hatsukami DK, Donny EC, Koopmeiners JS, Benowitz NL.. Compensatory smoking from gradual and immediate reduction in cigarette nicotine content. Cancer Epidemiol Biomarkers. 2015;24(2):472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith TT, Koopmeiners JS, Hatsukami DK, et al. Mouth-level nicotine intake estimates from discarded filter butts to examine compensatory smoking in low nicotine cigarettes. Cancer Epidemiol Biomarkers. 2020;29(3):643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rubinstein ML, Rait MA, Sen S, Shiffman S.. Characteristics of adolescent intermittent and daily smokers. Addict Behav. 2014;39(9):1337–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Azagba S, Manzione L, Shan L, King J.. Trends in smoking behaviors among US adolescent cigarette smokers. Pediatrics. 2020;145(3):e20193047. [DOI] [PubMed] [Google Scholar]
- 16. Colby SM, Tiffany ST, Shiffman S, Niaura RS.. Are adolescent smokers dependent on nicotine? A review of the evidence. Drug Alcohol Depend. 2000;59(suppl 1):S83–S95. [DOI] [PubMed] [Google Scholar]
- 17. Selya AS, Dierker L, Rose JS, Hedeker D, Mermelstein RJ.. Early-emerging nicotine dependence has lasting and time-varying effects on adolescent smoking behavior. Prev Sci. 2016;17(6):743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mak KK, Ho SY, Day JR.. Smoking of parents and best friend--independent and combined effects on adolescent smoking and intention to initiate and quit smoking. Nicotine Tob Res. 2012;14(9):1057–1064. [DOI] [PubMed] [Google Scholar]
- 19. Seo DC, Huang Y.. Systematic review of social network analysis in adolescent cigarette smoking behavior. J Sch Health. 2012;82(1):21–27. [DOI] [PubMed] [Google Scholar]
- 20. Hertel AW, Mermelstein RJ.. Smoker identity and smoking escalation among adolescents. Health Psychol. 2012;31(4):467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Colby SM, Gwaltney CJ.. Pharmacotherapy for adolescent smoking cessation. JAMA. 2007;298(18):2182–2184. [DOI] [PubMed] [Google Scholar]
- 22. Solberg LI, Boyle RG, McCarty M, Asche SE, Thoele MJ.. Young adult smokers: are they different. Am J Manag Care. 2007;13(11):626–632. [PubMed] [Google Scholar]
- 23. Kahende J, Malarcher A, England L, et al. Utilization of smoking cessation medication benefits among medicaid fee-for-service enrollees 1999–2008. PLoS One. 2017;12(2):e0170381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bonnie RJ, Stratton K, Kwan LY, Committee on the Public Health Implications of Raising the Minimum Age for Purchasing Tobacco Products; Board on Population Health and Public Health Practice; Institute of Medicine. Patterns of Tobacco Use by Adolescents and Young Adults. Washington, DC: National Academies Press (US); 2015. https://www.ncbi.nlm.nih.gov/books/NBK310407/. Accessed December 7, 2021. [PubMed] [Google Scholar]
- 25. National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention (US); 2012. http://www.ncbi.nlm.nih.gov/books/NBK99237/. Accessed December 7, 2021. [PubMed] [Google Scholar]
- 26. Colby SM, Cassidy RN, Denlinger-Apte R, et al. Anticipated effects of nicotine reduction on youth smoking initiation and maintenance. Nicotine Tob Res. 2019;21(suppl 1):S46–S48. doi: 10.1093/ntr/ntz101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cassidy RN, Colby SM, Tidey JW, et al. Adolescent smokers’ response to reducing the nicotine content of cigarettes: acute effects on withdrawal symptoms and subjective evaluations. Drug Alcohol Depend. 2018;188:153–160. doi: 10.1016/j.drugalcdep.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Faulkner P, Ghahremani DG, Tyndale RF, et al. Reduced-nicotine cigarettes in young smokers: impact of nicotine metabolism on nicotine dose effects. Neuropsychopharmacology. 2017;42(8):1610–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cassidy RN, Miller ME, Tidey JW, et al. The impact of nicotine dose on the reinforcing value of cigarettes in adolescents. Tob Regul Sci. 2019;5(2):105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Higgins ST, Heil SH, Sigmon SC, et al. Addiction potential of cigarettes with reduced nicotine content in populations with psychiatric disorders and other vulnerabilities to tobacco addiction. JAMA Psychiatry. 2017;74(10):1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith TT, Cassidy RN, Tidey JW, et al. Impact of smoking reduced nicotine content cigarettes on sensitivity to cigarette price: further results from a multi-site clinical trial. Addict Abingdon Engl. 2017;112(2):349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pergadia ML, Newcomer JW, Gilbert DG.. Depression and nicotine withdrawal associations with combustible and electronic cigarette use. Int J Environ Res Public Health. 2020;17(24):9334E9334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stout RL, Wirtz PW, Carbonari JP, Del Boca FK.. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Suppl. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- 34. Hukkanen J, Jacob P, Benowitz NL.. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1):79–115. [DOI] [PubMed] [Google Scholar]
- 35. Scherer G, Engl J, Urban M, et al. Relationship between machine-derived smoke yields and biomarkers in cigarette smokers in Germany. Regul Toxicol Pharmacol. 2007;47(2):171–183. [DOI] [PubMed] [Google Scholar]
- 36. Lewis-Esquerre JM, Colby SM, Tevyaw TO, et al. Validation of the timeline follow-back in the assessment of adolescent smoking. Drug Alcohol Depend. 2005;79(1):33–43. [DOI] [PubMed] [Google Scholar]
- 37. Hecht SS. Lung carcinogenesis by tobacco smoke. Int J Cancer. 2012;131(12):2724–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cassidy RN, Roberts ME, Colby SM.. Validation of a respiratory symptom questionnaire in adolescent smokers. Tob Regul Sci. 2015;1(2):121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO.. The fagerström test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 40. Prokhorov AV, Pallonen UE, Fava JL, Ding L, Niaura R.. Measuring nicotine dependence among high-risk adolescent smokers. Addict Behav. 1996;21(1):117–127. [DOI] [PubMed] [Google Scholar]
- 41. Hughes JR, Hatsukami D.. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–294. [DOI] [PubMed] [Google Scholar]
- 42. Tiffany ST, Drobes DJ.. The development and initial validation of a Questionnaire on Smoking Urges. Br J Addict. 1991;86(11):1467–1476. [DOI] [PubMed] [Google Scholar]
- 43. Cox LS, Tiffany ST, Christen AG.. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. [DOI] [PubMed] [Google Scholar]
- 44. Watson D, Clark LA, Tellegen A.. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. [DOI] [PubMed] [Google Scholar]
- 45. Arger CA, Heil SH, Sigmon SC, et al. Preliminary validity of the modified Cigarette Evaluation Questionnaire in predicting the reinforcing effects of cigarettes that vary in nicotine content. Exp Clin Psychopharmacol. 2017;25(6):473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Koopmeiners JS, Vock DM, Boatman JA, et al. The importance of estimating causal effects for evaluating a nicotine standard for cigarettes. Nicotine Tob Res. 2019;21(Suppl 1):S22–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Benowitz NL, Nardone N, Hatsukami DK, Donny EC.. Biochemical estimation of non-compliance with smoking of very low nicotine content cigarettes. Cancer Epidemiol Biomark Prev. 2015;24(2):331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Foulds J, Hobkirk A, Wasserman E, et al. Estimation of compliance with exclusive smoking of very low nicotine content cigarettes using plasma cotinine. Prev Med. 2018;117:24–29. doi: 10.1016/j.ypmed.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cassidy RN, DiLorenzo J, Tidey JW, Colby SM.. Adolescent response to using SPECTRUM cigarettes: qualitative results from an ongoing clinical trial. In: Poster Presentation, Society for Research on Nicotine & Tobacco Annual Conference, New Orleans, LA; 2020.
- 50. Cassidy RN, Tidey JW, Cao Q, et al. Age moderates smokers’ subjective response to very-low nicotine content cigarettes: evidence from a randomized controlled trial. Nicotine Tob Res. 2019;21(7):962–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barrington-Trimis JL, Braymiller JL, Unger JB, et al. Trends in the age of cigarette smoking initiation among young adults in the US from 2002 to 2018. JAMA Netw Open 2020;3(10):e2019022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. White HR, Bray BC, Fleming CB, Catalano RF.. Transitions into and out of light and intermittent smoking during emerging adulthood. Nicotine Tob Res. 2009;11(2):211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bover Manderski MT, Delnevo CD, Warner KE.. Toward a more comprehensive index of youth cigarette smoking: average number of cigarettes smoked per day among Students in the United States over two decades. Int J Environ Res Public Health. 2021;18(2):478E478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shiffman S, Scholl SM, Mao JM.. Very-low-nicotine-content cigarettes and dependence among non-daily smokers. Drug Alcohol Depend. 2019;197:1–7. doi: 10.1016/j.drugalcdep.2018.12.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data will be made available to other qualified investigators upon request. The request will be evaluated by the corresponding author to ensure that it meets reasonable demands of scientific integrity. Study results will be uploaded to ClinicalTrials.gov.