Abstract
Several phase‐1 clinical trials have been performed to evaluate the safety and efficacy of candidate anti‐Zika vaccines. In this systematic review, we systematically evaluated the safety and immunogenicity of candidate vaccines, which would aid researchers in formulating an effective vaccination strategy for phase‐2 trials based on current evidence. A literature search was conducted using the electronic databases MEDLINE through Pubmed, Web of Science, and Cochrane Database for relevant studies on candidate anti‐zika vaccines. Studies on animal models were excluded from our study. Healthy individuals who were administered candidate Zika vaccines to evaluate the immune response and adverse events (AEs) compared to placebo were considered. Data were extracted, tabulated, and analysed using Microsoft Excel, while the risk of bias plots were generated using tidyverse and Robvis packages in R‐studio. A total of five phase‐1 clinical trials were included in our analysis comprising of studies on inactivated, viral vector, and DNA vaccines. Immunogenicity ranged from 10% to 100% after vaccination with the lowest seroconversion rate (10%) and geometric mean titre (GMT) (6.3; 95% confidence interval (CI):3.7–10.8) observed among recipients of single‐dose inactivated anti‐zika vaccine (ZPIV). For DNA vaccines, the seroconversion rate ranged from 60% to 100% with the highest seroconversion rate (100%) and GMT (2871; 95% CI:705.3–11688) observed among recipients of three shots of high dose GLS‐5700 vaccine. For viral vector vaccine (Ad26.ZIKV.001) seroconversion rate (100%) and GMT peaked after two shots with both low and high‐dose vaccines. In all those studies AEs were mostly local including injection site pain, erythema, and itching. The most common systemic AEs included fever, myalgia, nausea, and fatigue. In phase‐1 clinical trials, all candidate vaccines were found to be highly immunogenic and relatively safe, especially when administered in higher doses and with the help of needle‐free devices.
Keywords: clinical trial, vaccine, vaccine safety, zika virus
1. INTRODUCTION
Zika virus, a mosquito‐borne flavivirus, responsible for congenital Zika syndrome (CZS) was first isolated from rhesus monkeys in the Zika forest in Uganda in 1947. 1 After small outbreaks in 2007 and 2013 in the Federal State of Micronesia and French Polynesia respectively, there was an epidemic outbreak of the virus in Brazil in 2015. 2 The number of cases continued to increase late into 2016, spreading to many countries throughout the world. But since 2017, incidences of Zika virus infection have decreased significantly and no further epidemic outbreaks have been documented. 3
Zika virus is a positive sense, single‐stranded RNA virus with a genome length of 11 kilobases. The virus contains three structural proteins including E protein, a major target for virus‐elicited immune response and vaccine development, and seven non‐structural proteins. 4 The virus is transmitted primarily through bites from infected Aedes Ageypti mosquitoes. But there is evidence that the virus is transmitted through sexual contact, vertical transmission from mother to foetus, and blood transfusion too. 5 The infection, in most cases, is a self‐limiting one. Often, the infected individual develops headache, fever, rash, conjunctivitis, and malaise that can last between two to seven days. 6 In a small number of cases, approximately 6%–11% of total cases, Zika virus infection is responsible for CZS in children comprising of microcephaly and other craniofacial and musculoskeletal abnormalities. 7 In adults, the virus is responsible for Guillain Barrie Syndrome (GBS). 7
Since no drug is yet available against the virus, preventive vaccines remain the preferred tool against the complications of Zika virus infection. Several candidate vaccines have been tested in animal models and small‐scale phase‐1 clinical trials since that outbreak back in 2015 and 2016. 8 These include DNA and RNA vaccines, subunit vaccines, inactivated whole virus vaccines, viral‐vector vaccines, live attenuated vaccines, and protein antigen vaccines. 8 After completing trials in animal models, several of those candidate vaccines have progressed to and completed phase‐1 clinical trials, producing encouraging results.
To our knowledge, there is no systematic review available that discuss the safety and efficacy of Zika virus vaccines that have undergone phase‐1 clinical trials. Although, there is one systematic review that discusses the safety and efficacy of Zika virus vaccines on non‐human primate models (NHP). 9 This is specifically important since vaccine acceptance by people, often in addition to other factors, depends on published safety and efficacy statistics of candidate vaccines. 10 , 11 Hence, we decided to perform a systematic review of published results from phase‐1 clinical trials of Zika virus candidate vaccines to better understand the safety and efficacy of each vaccine.
2. METHODS
This systematic review was conducted maintaining the guidelines provided in ‘Preferred Reporting Items for Systematic Review and Meta‐Analysis (PRISMA)’ and registered in PROSPERO (CRD42021235009).
2.1. Eligibility criteria
Clinical trials (phase 1‐3) evaluating the immunogenicity and safety of candidate Zika virus vaccine on human subjects were initially considered for this systematic review. Pre‐clinical studies and clinical trials on NHP were excluded. Only articles in English were considered. No timeline restriction was applied as regards the study eligibility for systematic review.
2.2. Search methodology
A comprehensive literature search was conducted in different databases including MEDLINE (through PubMed), Web of Science, and Cochrane database. Articles dating from the inception of record‐keeping to May 2021 were considered for this systematic review. All databases were searched on a single day. Besides, bibliographies of selected articles were hand‐searched for relevant articles.
Following primary keywords were used in isolation or in combination using Boolean operators – ‘Zika’, ‘vaccine’, and ‘immunisation’. The comprehensive search strategy for all the databases has been provided in Supplementary File 1.
2.3. Screening process
Once all duplicate entries were removed using Rayyan QCRI software, article titles and abstracts were screened by two independent researchers (HMAAM and MMAM) for suitability. If any of those initial entries were deemed suitable, full‐text articles were downloaded for further analysis. HMAAM and MMAM again conducted full‐text screening of each downloaded article independently using prioritisation and sequential exclusion technique, 12 and if there was confusion regarding the suitability of any of the articles, senior author KMSUR was consulted for expert advice.
2.4. Data extraction
Relevant information from each included article was extracted by MY including the first author, year of study, study location, age of study population, the vaccine being studied, dosage, the clinical outcome of the trial, number of participants, and time points when vaccines were administered. Regarding discrepancies in extracted data, expert advice was sought from senior author KMSUR.
2.5. Assessment of risk of bias
For this systematic review, the risk of bias was evaluated by two independent researchers (MY and MMAM) using the Cochrane risk of bias tool (ROB) for randomised controlled trials. In case of discrepancy, senior author KMSUR was consulted.
2.6. Data analysis
Extracted data were stored in Microsoft Excel. Computational analysis and graphical representations for risk of bias assessment were prepared using tidyverse and Robvis packages in R‐studio. Since the number of studies included in this systematic review was low and considering the heterogenicity (e.g., type of vaccine administered, dosage, immunogenicity measurement, and outcome varied across studies) of each article, a meta‐analysis could not be performed for this systematic review. We did not assess the publication bias as the test of funnel plot asymmetry or assessment of publication bias is not recommended if there is no meta‐analysis. 13
3. RESULTS
3.1. Characteristics of included studies
According to pre‐determined search criteria, we initially retrieved 812 records from three databases and based on the above eligibility criteria five clinical trials that included 369 subjects were finally included in the analysis. The flowchart of the literature screening for this study is provided in Figure 1.
FIGURE 1.
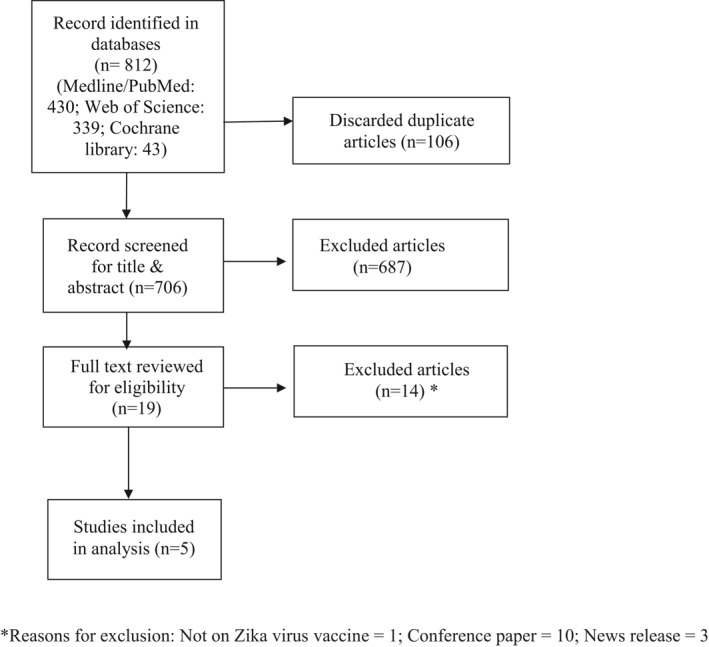
Systematic review flow diagram
The baseline characteristics of the included 5 clinical trials are summarised in Table 1. All the selected studies in the phase‐1 trial assessed the safety and immunogenicity of the candidate anti‐Zika virus vaccine. Among 5 clinical trials, 4 mentioned randomisations, and 1 was non‐randomised trial (Tebes et al). Anti‐Zika virus candidate vaccines were further classified into three groups: DNA vaccines (GLS‐5700, VRC5288, and VRC5283), inactivated viral vaccines (ZPIV), and viral vector vaccines (Ad26.ZIKV.001)
TABLE 1.
Baseline characteristics of included articles
| Publication year [Ref] | Clinical trial identifying no | Location | Population (age in years) | Vaccine studied | Dosage | Outcome | No of participants | Time points |
|---|---|---|---|---|---|---|---|---|
| 2017 14 | NCT02809443 | USA and Canada | Healthy adult (18–65) | GLS‐5700 | 1 mg; 2 mg | Safety and immunogenicity | 40 | 0, 4, and 12 weeks |
| 2018 15 | NCT02840487 | USA | Healthy adult (18–50) | VRC5288 and VRC5283 | 4mg | Safety and immunogenicity | 125 | VRC5288: |
| G‐1 (0 and 8 weeks) | ||||||||
| G‐2 (0 and 12 weeks) | ||||||||
| G‐3 (0,4 and 8 weeks) | ||||||||
| G‐4 (0,4 and 20 weeks) | ||||||||
| VRC5283: | ||||||||
| G‐1,2,3 (0,4 and 8 weeks) | ||||||||
| *(G‐group) | ||||||||
| 2018 16 | NCT02963909, NCT02952833,NCT02937233 | USA | Healthy adult (18–49) | ZPIV | 5 μg | Safety and immunogenicity | 68 | 0 and 4 weeks |
| 2020 17 | NCT02937233 | USA | Healthy adult (18–50) | ZPIV | 5 μg | Safety and immunogenicity | 36 | Standard regimen (0 and 4 weeks); |
| Accelerated regimen (0 and 2 weeks); single‐dose regimen (0 weeks alone) | ||||||||
| 2021 18 | NCT03356561 | USA | Healthy adult (18–50 years) | Ad26.ZIKV.001 | 5 × 1010; 1 × 1011 viral particles | Safety and immunogenicity | 100 | 0 and 8 weeks (Day 1 and day 57) |
3.2. Risk of bias assessment of clinical trials
The risk of bias assessment of the included studies was performed and their detailed assessments are shown in Figure 2. And Figure 3. Most studies were dominated by a low risk of bias as a result of proper implementation of clinical trials. Three papers presented a high risk of bias for outcome assessments due to the non‐blinding of data to the analyst, two papers for allocation concealment and one paper for random sequence generation. We did not find any issue to report ‘other bias’ while assessing the ROB.
FIGURE 2.
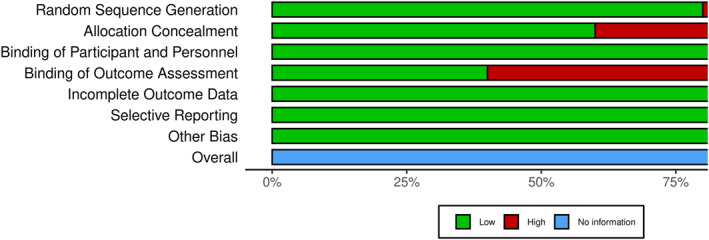
Risk of bias summary
FIGURE 3.
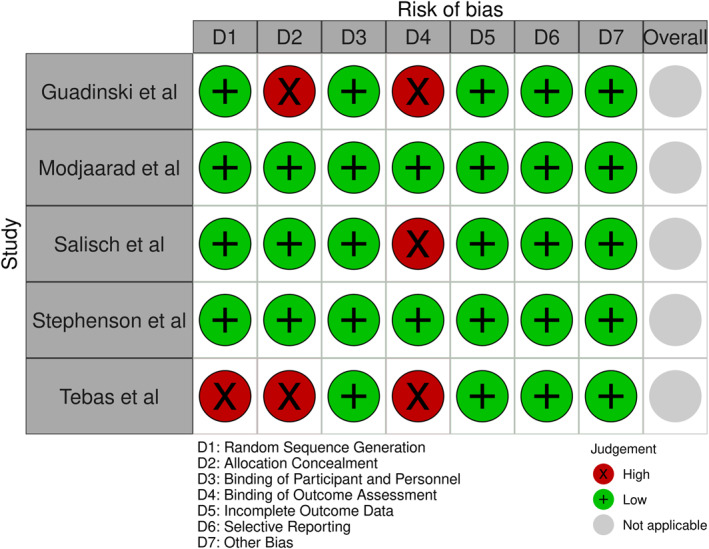
Risk of bias assessment
3.3. Immunogenicity
Healthy adults including both men and women aged 18–65 years with normal findings in laboratory tests, medical history, and physical examinations were selected for these five clinical trials. No prior history or serological evidence of dengue/flavivirus infection or vaccination was considered as inclusion criteria for 3 studies (Tebes et al.; Stephenson et al.; salisch et al.), whereas participants irrespective of their previous flavivirus infection status were included in the study conducted by Modjarrad et al. Guadinski et al. did not mention prior flavivirus status of study participants.
3.4. Immunogenicity of DNA vaccines
GLS‐5700 contains plasmid pGX7201 encodes pre‐membrane and envelope proteins of pre‐2016 human infectious ZIKV strain, cloned into a modified pVax1 expression vector, pGX0001. 14 Findings of the clinical trial revealed that all the recipients (100%) of both 1 and 2 mg dose groups developed anti‐Zika specific binding antibodies by week 14 after completion of the third dose (Table 2). Vero cell assay showed neutralising antibodies against Zika virus had developed in 62% of the vaccine recipients with titres ranging from 1:18 to 1:317 and had no correlation with dosage. Antigen‐specific IFN‐ secreting T‐cells showed higher median responses after third doses than baseline on ELISPOT assay in both groups with no significant differences. Neuronal cell assay at week 14 on human glioblastoma cells (U87 MG) revealed that serum samples from 95% of participants neutralised infection by 50% whereas more than 70% of samples showed 90% inhibition relative to baseline. 14
TABLE 2.
Summary of immunogenicity (B cell response) and adverse events (AEs) of the included studies
| Publication year [Ref] | Vaccine type | Group | Immunogenicity (B cell response) | Side effects | |||
|---|---|---|---|---|---|---|---|
| Peak GMT (geometric mean titre) (95% CI) | Seroconversion rate | Local adverse events (%) | Systemic adverse events (%) | ||||
| 2017 14 | GLS‐5700 (DNA vaccine) | Lower dose‐1 mg | 1642 (347.3–7760) | 100% | Injection site erythema (47.5%), itching (37.5%) and pain (22.5%) | Different kinds of infection (32.5%), headache (15%), myalgia (10%), malaise (7.5%) and nausea (5%). | |
| Higher dose‐2 mg | 2871 (705.3–11688) | 100% | |||||
| 2018 15 | VRC5288 (DNA vaccine) | Group‐1 (2 doses‐ by needle and syringe) | 67 (40–114) | 60% | Injection site pain and tenderness (46%), injection site redness (6%), swelling (1%) | Malaise (28%), headache (23%), myalgia (22%), nausea (9%), chills (7%) and joint pain (5%) | |
| Group‐2 (2 doses‐ by needle and syringe) | 55 (39–78) | 75% | |||||
| Group −3 (3 doses‐ by needle and syringe) | 81 (51–127) | 80% | |||||
| Group −4 (3 doses‐ by needle and syringe) | 120 (73–197) | 89% | |||||
| VRC5283 (DNA vaccine) | Group −1 (3 doses via single‐dose needle and syringe) | 48 (28–83) | 77% | Injection site pain and tenderness (73%), injection site redness (2%) and swelling (7%) | Malaise (37%), headache (33%), myalgia (20%), joint pain (18%), nausea (4%) and chills (4%) | ||
| Group −2 (3 doses via split dose needle and syringe) | 150 (99–226) | 93% | |||||
| Group −3 (3 doses by needle‐free straight device) | 304 (215–430) | 100% | |||||
| 2018 16 | ZPIV (Inactivated viral vaccine) | Standard regimen (2 doses) | 286.7 (170.6–481.6) | 92% | Injection site pain (60%), tenderness (47%), redness (4.5%), swelling (4.5%), itching (3%) | Malaise (22. 4%), myalgia (16.4%), nausea (9%), rash (3%), vomiting (1.5%), temperature elevation (1.5%) | |
| 2020 17 | ZPIV (Inactivated viral vaccine) | Standard regimen (2 doses) | 1153.9 (455.2–2925.2) | 100% | Injection site pain (80%), erythema (23%), induration (13%), pruritus (3%) | Fatigue (53%), headache (46%), myalgia (44%), malaise (34%), nausea (23%), diarrhoea (17%), abdominal pain (10%), vomiting (7%), arthralgia (7%), rash (3%), fever (3%) | |
| Accelerated regimen (2 doses) | 517.7 (142.9–1875.6) | 100% | |||||
| Single‐dose regimen | 6.3 (3.7–10.8) | 10% | |||||
| 2021 18 | Ad26.ZIKV.001 (viral vector vaccine) | Lower dose‐5 × 1010 vp | 2 doses | 1065.6 (494.9–2294.5) | 100% | Injection site pain or tenderness, injection site erythema and swelling. | Fatigue, headache, myalgia, nausea, chills, and pyrexia |
| Single‐dose | 76.4 (42.5–137.4) | 88.9% | |||||
| Higher dose‐1 × 1011 vp | 2 doses | 956.6 (595.8–1535.8) | 100% | ||||
| Single‐dose | 142.8 (67–304.5) | 94.4% | |||||
VRC5288 (Zika and Japanese encephalitis chimera) and VRC5283 (wild‐type Zika virus), another two DNA vaccines were investigated in a phase‐1, open‐label, randomised trial. In the case of VRC5288, positive antibody responses varied from 60% to 89% depending on the total number and time gap between the doses. The highest geometric mean titre (GMT) (120) and antibody response and titres were observed in three doses (group 4) of VRC5288 where the vaccine was given with an extended time between the second and third doses. Considering VRC5283, 100% antibody response and the highest GMT (304) across all groups in both studies were achieved in group 3 (Table 2). Splitting the doses improved overall antibody response and GMT. Both CD4 and CD8 responses in group‐4 and only CD8 responses in group‐3 to pooled peptides increased significantly after 4 weeks of vaccination with VRC5288 compared to baseline, measured by intracellular cytokine staining. 15
3.5. Immunogenicity of inactivated viral vaccines
Modjarrad et al. observed that, after receiving 2 doses (day 0 and day 29) of the vaccine, 92% (42/52) of participants seroconverted by peak titre when the GMT threshold was 1:10% and 77% (40/52) seroconverted when GMT threshold was 1:100 at 2–4 weeks since second doses. 16 GMTs slightly declined after day 43 (2 weeks after second dose) albeit they remained higher than 1:60 (the threshold for protection in previous mouse and non‐human primate studies) at day 57 (immunological endpoint). Neutralising antibody titres showed a significant negative correlation with age and these values exceeded baseline early (at day 15) and persisted at higher levels throughout the follow‐up period in this age group. 16
Zika purified inactivated virus (ZPIV) vaccine was also trialed by Stephenson et al. and participants were divided into three groups (standard regimen, accelerated, and single‐dose regimen). 17 Results demonstrated that geometric mean MN50 (50% microneutralisation) neutralising antibody titre was highest at the 6‐week time point (983.3, 95% CI 425.5–2272.5) for the standard regimen group whereas the accelerated regimen group showed the highest level at the 4‐week time point (477.4, 95% CI 111.7–2039.8) and only one participant of single‐dose regimen group had detectable antibody level which peaked at 2 weeks (GMT 53) since the first dose. In the standard regimen group, the geometric mean MN50 neutralising antibody titre declined to 13.9 (CI 3.5–55.1) and in the accelerated regimen group, this titre was 6.9 (CI 4–11.9) by week 28. 17 The observed peak value of the GMT of all individual participants of different regimen groups is shown in Table 2.
All vaccine recipients (100%, 10/10) of the standard regimen group and 80% (8/10) vaccinees of the accelerated regimen group had an MN50 titre of 100 or higher (considered as a protective level based on animal study) at their observed peak response, but by week 28 and at the end of the study follow up (week 52), only 13% (1/8) participants of both standard and accelerated regimen group had an MN50 titre of 100 or higher. No participant from the single‐dose regimen group developed an MN50 titre of 100 or higher and the placebo group had an MN50 titre of 10 or higher at their peak response. 17
3.6. Immunogenicity of viral vector vaccine
Both lower and higher dosages of Ad26.ZIKV.001 vaccine was evaluated in phase‐1 trial and demonstrated that 88% (35/40) of lower doses (those who received 2 lower doses‐LD/LD or 1 lower dose and 1 placebo‐LD/PL combined) and 94% (36 out of 38) of a higher dose (those who received 2 higher doses‐HD/HD or 1 higher dose and 1 placebo‐ HD/PL combined) receiving vaccinees seroconverted after 28 days of first dose. 18 100% of Both LD/LD and HD/HD group seroconverted after second dose and had developed peaked ZIKV MN50 GMT titres on day 71 (Table 2) and it persisted in about 80% of participants up to day 365 with a titre of 68.7 for LD/LD and 87.0 for HD/HD. All the participants (100%) of the single higher dose group (HD/PL) and 90% of the single lower dose group (LD/PL) also developed potent MN50 GMT titres of 103.4 and 74.3 respectively on days 57 and peaked on day 71 (Table 2), and then gradually declined, but titre was more durable in HD/PL group on day 365 (88% had detectable titres of 90.2). Higher MN50 titres were observed in women at days 57 and 85. After 28 days of first vaccination of Env‐specific INF‐gamma, ELISPOT responses were observed in all groups with group medians of 100 to 428 spot forming units per 106 cells except the placebo group (PL/PL) and responses increased after second dose (day 85) which persisted in most participants for at least 1 year. 18
3.7. Safety and reactogenicity
All these phase‐1 trials assessed Zika virus vaccine safety by observing solicited local and systemic reactogenicity for 7 days, unsolicited local and systemic adverse events (AEs) for 28 days after each vaccination, and serious AEs and immediate reportable events such as neurologic and neuroinflammatory disorders (such as GBS) throughout the study period. No vaccine‐related serious AEs and neurological or neuroinflammatory AEs of special interest following anti‐Zika vaccination were mentioned in these five clinical trials. In those studies participants mostly experienced mild local AEs including injection site pain, redness, swelling, fatigue, etc (Table 2). Systemic AEs included abdominal swelling, nausea, fever, joint pain and myalgia. In the case of viral vector vaccine seven participants reported grade‐3 systemic AEs after the first dose, but none of them reported AEs above grade‐2 after the second dose. 18
4. DISCUSSION
4.1. Summary of findings
This systematic review includes five phase‐1 clinical trials of three broadly classified types of anti‐Zika vaccines on human subjects. There is a systematic review of anti‐Zika vaccine safety and efficacy on NHP but, for the first time, we have performed a systematic review of anti‐Zika vaccine phase‐1 clinical trials conducted in different countries. 9 Interestingly, all vaccines had different dosages and schedules with participants receiving one to three dosages of the anti‐Zika vaccine at a defined time interval. In all included studies participants developed antibody response after a scheduled dosage of anti‐Zika vaccine (60%–100%), excluding one study from Stephenson et al., where a single dose regimen resulted in 10% seroconversion. Regarding safety and reactogenicity, all included studies reported mild to moderate local and systemic AEs. Most participants complained of local erythema and swelling with few participants reporting systemic AEs including headache, abdominal pain, nausea, fever, fatigue, myalgia, etc. None of the participants experienced symptoms that forced them to withdraw from the study.
4.2. Comparison with pre‐clinical studies
Results of Zika vaccine trial in mice and NHP produced encouraging results. But the reproductive system of mice is different to human reproductive system and hence the results obtained in mice could not be extrapolated to human. On the other hand, NHP share a distinctive similarity to human reproductive system. 19
Limited number of studies that were conducted in NHPs showed that candidate Zika vaccines produced neutralising antibodies in tested subjects, demonstrated by lack of viraemia post Zika virus challenge. 20 Besides, several vaccine candidates were able to maintain a sustained level of neutralising antibodies for a long period of time. Regarding side effects, most candidate vaccines were well tolerated in NHPs, with few of them experiencing mild symptoms. 21
4.3. The implication of findings
In a previous study, Lund reviewed pre‐clinical studies of anti‐Zika candidates on NHP. 9 But a suitable review comprising immunogenicity and efficacy of anti‐Zika vaccine candidates on human subjects was lacking. This review would aid in future policy making regarding phase‐2 and phase‐3 clinical trials of candidate vaccines, and subsequent authorisation of a suitable vaccine. Since there is no Zika virus outbreak currently ongoing around the world and due to the limited number of cases, conducting phase‐2 and a phase‐3 clinical trial is proving to be troublesome with many vaccine developers suspending their trials. Nevertheless, in case of possible future outbreaks, this systematic review of phase‐1 clinical trials would act as useful starting guidelines for all the stakeholders.
Another important aspect of potential Zika virus vaccine development is the cross‐reactivity of the Zika vaccine with that of four serotypes of the dengue virus since these are antigenically similar. A previous murine model study found that the Zika vaccine provides cross‐protection against all four dengue serotypes. 22 Hence, findings from these studies could have ramifications for developing a dengue vaccine in the future.
4.4. Lessons to learn from vaccine development during the COVID‐19 pandemic
Starting in December 2019, the world has been going through a pandemic caused by the rapid spread of the Severe Acute Respiratory Syndrome Coronavirus‐2 (SARS‐CoV‐2) virus. 23 But unlike many viral vaccines, candidate vaccines for SARS‐CoV‐2 were developed and trialed at an unprecedented speed. Apart from a novel mRNA‐based vaccine (developed by Pfizer and Moderna), technologies used in developing Coronavirus disease 2019 (COVID‐19) vaccines are like the one being considered for anti‐Zika vaccine development. 23 While the low number of Zika cases around the world hampers the development of an effective vaccine, strategies could be implemented to fast‐track the development of an anti‐Zika vaccine including approval of a Zika vaccine for high‐risk groups based on phase‐1 or phase‐2 clinical trial data, as happened with COVID‐19 vaccine where emergency use authorisation was provided based on data from a small pool of participants. 24 While the development of an anti‐Zika vaccine, at least on paper, is a less lucrative one compared to COVID‐19 vaccines, where a significant portion of the world population was affected, government subsidies could facilitate the late‐stage clinical trials of candidate vaccines.
4.5. Study limitations
This systematic review has several limitations. First, the number of included studies and the sample size were low with a total of five studies being included for review comprising 369 study participants. Hence, making policy decisions based on such a low number of studies would be inappropriate. Moreover, due to the extreme heterogenicity of included studies, conducting a meta‐analysis was not possible, which is a significant limitation of this study. In addition, we could not review articles in languages other than English, which might have been attributed to such low numbers of included studies. We could not assess the publication bias. However, it is evident with vaccine trials for other infectious diseases that developers often do not publish results in case of negative or unexpected results.
5. CONCLUSION
In conclusion, included studies reported enhanced immunogenicity and relatively low‐side effects among vaccine recipients. But due to the low number of study participants in each phase‐1 trial, it would be inappropriate to provide conclusive remarks on the safety and immunogenicity of each candidate vaccine. For better understanding and study precision, more participants should be recruited for future studies including planned follow‐ups for a longer period.
Abbreviations
- AE
Adverse Event
- COVID‐19
Coronavirus Disease 2019
- CZS
Congenital Zika Syndrome
- ELISPOT
Enzyme‐linked Immune Absorbent Spot
- GBS
Gullain Barrie Syndrome
- GMT
Geometric Mean Titre
- HD
Higher dose
- LD
Lower Dose
- MN50
50% Microneutralisation
- NHP
Nonhuman primtaes
- PL
Placebo
- PRISMA
Preferred Reporting Project for Systematic Evaluation and Meta‐Analysis
- RCT
Randomised Controlled Trial
- SARS‐CoV‐2
Severe Acute Respiratory Syndrome Coronavirus‐2
- ZPIV
Zika Virus Purified Inactivated Vaccine
AUTHOR CONTRIBUTIONS
KM Saif‐Ur‐Rahman conceptualised and designed this systematic review. KM Saif‐Ur‐Rahman led the search strategy. Mahmuda Yeasmin, Md. Maruf Ahmed Molla, H. M. Abdullah Al Masud, and KM Saif‐Ur‐Rahman obtained and apprised data. Mahmuda Yeasmin and Md. Maruf Ahmed Molla developed the first draft. All authors reviewed the manuscript and provided intellectual inputs and approved the final version. KM Saif‐Ur‐Rahman critically reviewed, edited, and finalised the manuscript. KM Saif‐Ur‐Rahman is the guarantor of this review.
CONFLICT OF INTEREST
No conflict of interest declared.
ETHICS STATEMENT
This is a systematic review of published articles. Ethical approval is not applicable.
PATIENT CONSENT STATEMENT
Not applicable for this study.
PERMISSIONS TO REPRODUCE MATERIALS FROM OTHER SOURCES
Not applicable.
Supporting information
Supporting Information S1
ACKNOWLEDGEMENTS
The author (KMSUR) would like to acknowledge the contribution of the current donors providing unrestricted support to icddr,b that include: the Governments of Bangladesh, Canada, Sweden, and the UK. We gratefully acknowledge these donors for their support and commitment to icddr,b's research efforts. This research received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors.
Open access funding provided by IReL.
Yeasmin M, Molla MMA, Masud HMAA, Saif‐Ur‐Rahman KM. Safety and immunogenicity of Zika virus vaccine: a systematic review of clinical trials. Rev Med Virol. 2023;33(1):e2385. 10.1002/rmv.2385
Mahmuda Yeasmin, Md. Maruf Ahmed Molla contributed equally.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in online repositories.
REFERENCES
- 1. Dick G, Kitchen S, Haddow A. Zika Virus (I). Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46(5):509‐520. 10.1016/0035-9203(52)90042-4 [DOI] [PubMed] [Google Scholar]
- 2. Zanluca C, De Melo VCA, Mosimann ALP, Dos Santos GIV, Dos Santos CND, Luz KG. First report of autochthonous transmission of Zika virus in Brazil. Memórias do Inst Oswaldo Cruz. 2015;110(4):569‐572. 10.1590/0074-02760150192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weaver SC, Costa F, Blanco MAG, et al. Zika virus: history, emergence, biology, and prospects for control. Antivir Res. 2016;130:69‐80. 10.1016/j.antiviral.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sirohi D, Kuhn RJ. Zika virus structure, maturation, and receptors. J Infect Dis. 2017;216(Suppl l_10):s935‐s944. 10.1093/infdis/jix515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foy BD, Kobylinski KC, Chilson JL, et al. Probable non‐vector‐borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880‐882. 10.3201/eid1705.101939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pattanaik A, Sahoo BR, Pattanaik AK. Current status of Zika virus vaccines: successes and challenges. Vaccines. 2020;8(2):266. 10.3390/vaccines8020266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Panchaud A, Stojanov M, Ammerdorffer A, Vouga M, Baud D. Emerging role of Zika virus in adverse fetal and neonatal outcomes. Clin Microbiol Rev. 2016;29(3):659‐694. 10.1128/cmr.00014-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morabito KM, Graham BS. Zika virus vaccine development. J Infect Dis. 2017;216(Suppl l_10):S957‐S963. 10.1093/infdis/jix464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lund H. Efficacy of Vaccine Candidates against Zika Infection and Congenital Zika Syndrome: A Systematic Review and Meta‐Analysis. University of Nebraska Medical Center; 2021. Retrieved from. https://digitalcommons.unmc.edu/cgi/viewcontent.cgi?article=1138%26context=coph_slce [Google Scholar]
- 10. Yufika A, Anwar S, Maulana R, et al. Attitude towards Zika among frontline physicians in a dengue‐endemic country: a preliminary cross‐sectional study in Indonesia. Narrative J. 2021;1(1):e32. 10.52225/narraj.v1i1.e32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hassan W, Kazmi SK, Tahir MJ, et al. Global acceptance and hesitancy of COVID‐19 vaccination: a narrative review. Narrative J. 2021;1(3). 10.52225/narra.v1i3.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saif‐Ur‐Rahman KM, Hasan M, Hossain S, Anwar I, Hirakawa Y, Yatsuya H. Prioritization and sequential exclusion of articles in systematic reviews. Campbell Syst Rev. 2022;18(2):e1229. 10.1002/cl2.1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ioannidis JPA, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta‐analyses: a large survey. Can Med Assoc J. 2007;176(8):1091‐1096. 10.1503/cmaj.060410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tebas P, Christine C, Muthumani K, et al. Safety and immunogenicity of anti‐Zika virus DNA vaccine. N. Engl J Med. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaudinski MR, Houser KV, Morabito KM, et al. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: randomised, open‐label, phase 1 clinical trial. Lancet. 2017;391:552‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Modjarrad K, Lin L, George SL, et al. Preliminary aggregate safety and immunogenicity results from three trials of a purified inactivated Zika virus vaccine candidate: phase 1, randomised, double‐blind, placebo‐controlled clinical trials. Lancet. 2018;391(10120):563‐571. 10.1016/s0140-6736(17)33106-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stephenson KE, Tan CS, Walsh SR, et al. Safety and immunogenicity of a Zika purified inactivated virus vaccine given via standard, accelerated, or shortened schedules: a single‐centre, double‐blind, sequential‐group, randomised, placebo‐controlled, phase 1 trial. Lancet Infect Dis. 2020;20(9):1061‐1070. 10.1016/s1473-3099(20)30085-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salisch NC, Stephenson KE, Williams K, et al. A double‐blind, randomized, placebo‐controlled phase 1 study of Ad26.ZIKV.001, an Ad26‐Vectored anti–Zika virus vaccine. Ann Intern Med. 2021;174(5):585‐594. 10.7326/m20-5306 [DOI] [PubMed] [Google Scholar]
- 19. Larocca RA, Abbink P, Peron JP, et al. Vaccine protection against Zika virus from Brazil. Nature. 2016;536(7617):474‐478. 10.1038/nature18952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abbink P, Larocca RA, Visitsunthorn K, et al. Durability and correlates of vaccine protection against Zika virus in rhesus monkeys. Sci Transl Med. 2017;9(420). 10.1126/scitranslmed.aao4163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thomas SJ, Barrett A. Zika vaccine pre‐clinical and clinical data review with perspectives on the future development. Hum Vaccines Immunother. 2020;16(10):2524‐2536. 10.1080/21645515.2020.1730657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang R, Gao N, Li Y, et al. Cross‐Protection against four serotypes of dengue virus in mice conferred by a Zika DNA vaccine. Front Cell Infect Microbiol. 2019;9. 10.3389/fcimb.2019.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Surawan DP, Sumohadi D, Budhitresna AA, et al. Titer disparity of anti‐Spike receptor binding domain SARS‐CoV‐2 antibody between vaccinated and naturally infected individuals. Narrative J. 2022;2(1). 10.52225/narra.v2i1.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization . WHO Lists Additional COVID‐19 Vaccine for Emergency Use and Issues Interim Policy Recommendations; 2021. Retrieved from. https://www.who.int/news/item/07‐05‐2021‐who‐lists‐additional‐covid‐19‐vaccine‐for‐emergency‐use‐and‐issues‐interim‐policy‐recommendations
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
The data that support the findings of this study are openly available in online repositories.


