Dear Editor,
Erythrodermic psoriasis (EP) is a rare variant of psoriasis, accounting for 1%–2% of all cases of plaque psoriasis. 1 EP is characterized by generalized erythema and scaling affecting more than 75% of body surface area, with or without systemic symptoms. 1 Due to both the rarity and the severity of this condition, which make it difficult to carry out randomized controlled clinical trials, the treatment of EP is challenging. 1 , 2 The latest National Psoriasis Foundation Consensus Guidelines recommend cyclosporine or infliximab as first‐line therapy due to their rapid onset of action. 2 However, these guidelines do not take into account the most recent biologic drugs, as only limited experience was available on anti‐IL‐17 and anti‐IL‐23 at that time. Here we report a case of sub‐erythrodermic psoriasis successfully treated with bimekizumab, a humanized anti‐IL‐17A/F monoclonal antibody. 3
A 44‐year‐old patient, with a 20‐year history of plaque psoriasis, came to our emergency department referring to a worsening of his cutaneous disease. He was previously treated with acitretin 20 mg/day, with only partial improvement. On physical examination, we observed widespread erythema and desquamation to almost the entire skin area including hands, feet, scalp, and auricles (Figure 1A–C). Psoriasis Area and Severity Index (PASI) score was 39, body surface area (BSA) was 55, while the Dermatology Life Quality Index (DLQI) of the patient was 30. As the patient had already undergone screening tests in anticipation of biologic therapy, we decided to prescribe bimekizumab 160 mg, 2 subcutaneous injections every 4 weeks up to week 16. At week 4, after only one administration of bimekizumab, the patient came back to our Dermatology Unit for the first follow‐up visit, showing already a consistent improvement of the cutaneous lesions with PASI = 2.6 and DLQI = 5. At week 12, after the third administration of bimekizumab, we observed almost complete skin clearance, as PASI was 1, BSA was 1, and DLQI was 2 (Figure 1D–F). The patient is still under treatment with bimekizumab and he has reported no adverse events, to date.
FIGURE 1.
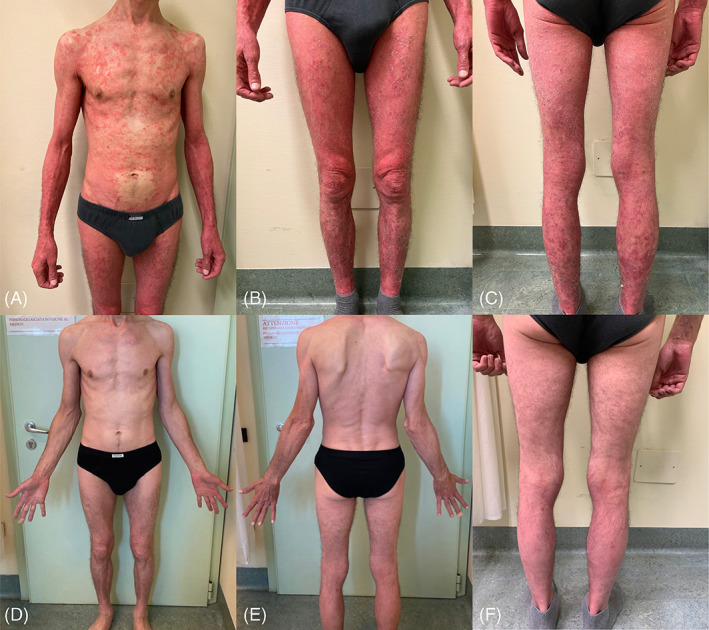
Erythematous and scaly patches involving the trunk and the four limbs of a 44‐year‐old patient at the baseline visit (A–C). Almost complete resolution of skin lesions after 12 weeks of treatment with bimekizumab (D–F)
Bimekizumab is a humanized antibody that selectively binds and neutralizes the biologic functions of interleukin (IL)‐17A and IL‐17F. 3 It has been approved for the treatment of moderate‐to‐severe plaque psoriasis after four Phase‐3 studies (BE VIVID, BE READY, BE RADIANT, BE SURE) demonstrated the high levels of efficacy of bimekizumab. 4 , 5 , 6 , 7 At week 4, a reduction of at least 75% in the PASI (PASI75) was observed in a percentage of patients between 71% and 76% among the four clinical trials, showing superiority compared with adalimumab, 5 ustekinumab 6 and secukinumab. 4 This rapid onset of action could be due to the double neutralization of both IL‐17A and IL‐17F compared with targeting IL‐17A alone. 3 At week 16, PASI 100 was observed in at least 59% of the patients among the four Phase‐3 studies. 4 , 5 , 6 , 7 However, no data are available regarding the efficacy of bimekizumab in EP, because of the very recent approval of this drug. We decided to start bimekizumab because of encouraging data from clinical trials regarding efficacy, rapidity and safety, given the severity of the cutaneous symptoms of our patient. Notably, bimekizumab is administered with two subcutaneous injections at weeks 0, 4, 8, 12, and 16, and then every 8 weeks. 3 Our patient experienced almost complete skin clearance at week 12, even before completing the induction phase.
In conclusion, we reported a case of sub‐erythrodermic psoriasis which was successfully treated with bimekizumab, highlighting the rapid onset of action of this drug. Other experiences are needed to further establish the role of bimekizumab on the treatment of severe cases, including erythrodermic psoriasis.
AUTHOR CONTRIBUTIONS
Mario Valenti collected the clinical data, wrote the draft of the manuscript, adapted the clinical image, and reviewed the manuscript. Luigi Gargiulo, Luciano Ibba, and Giulia Pavia collected the clinical data and wrote a draft of the manuscript. Alessandra Narcisi collected the clinical data and critically reviewed the manuscript. Antonio Costanzo collected the clinical data, obtained the figures, and critically reviewed the manuscript.
CONFLICT OF INTEREST
Mario Valenti has been a consultant and/or speaker for Sanofi, Leo Pharma, Eli Lilly, Boehringer. Alessandra Narcisi has been a consultant and/or speaker for Abb‐Vie, Almirall, Amgen, Janssen, Leo Pharma, Eli Lilly, Boehringer, Novartis, Pfizer and UCB Antonio Costanzo has been a consultant and/or speaker for Abb‐Vie, Almirall, Amgen, Janssen, Leo Pharma, Eli Lilly, Galderma, Boehringer, Novartis, Pfizer, Sandoz, and UCB. The other authors have nothing to disclose.
INFORMED CONSENT
The patient in this manuscript has given written informed consent to publication of his case details.
ACKNOWLEDGMENTS
Open access funding provided by BIBLIOSAN.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Lo Y, Tsai TF. Updates on the treatment of erythrodermic psoriasis. Psoriasis (Auckl). 2021;11:59‐73. doi: 10.2147/PTT.S288345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carrasquillo OY, Pabón‐Cartagena G, Falto‐Aizpurua LA, et al. Treatment of erythrodermic psoriasis with biologics: a systematic review. J Am Acad Dermatol. 2020;83(1):151‐158. doi: 10.1016/j.jaad.2020.03.073 [DOI] [PubMed] [Google Scholar]
- 3. Freitas E, Blauvelt A, Torres T. Bimekizumab for the treatment of psoriasis. Drugs. 2021;81(15):1751‐1762. doi: 10.1007/s40265-021-01612-z [DOI] [PubMed] [Google Scholar]
- 4. Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385(2):142‐152. doi: 10.1056/NEJMoa2102383 [DOI] [PubMed] [Google Scholar]
- 5. Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385(2):130‐141. doi: 10.1056/NEJMoa2102388 [DOI] [PubMed] [Google Scholar]
- 6. Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52‐week, multicentre, double‐blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397(10273):487‐498. [published correction appears in Lancet. 2021 Feb 20;397(10275):670]. doi: 10.1016/S0140-6736(21)00125-2 [DOI] [PubMed] [Google Scholar]
- 7. Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double‐blind, placebo‐controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397(10273):475‐486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


