Abstract
Background
The influenza viruses pose a threat to human health and medical services, and vaccination is an important way to prevent infection. However, the effectiveness of influenza vaccines is affected by various aspects. This study aimed to explore factors related to the immune response to influenza vaccines.
Methods
The study was conducted from September 2019 to September 2021, and a total of 593 volunteers were recruited from the Center for Disease Control and Prevention in 3 provinces in China. The hemagglutination inhibition assay was used to measure antibody levels. The Chi-square test, multivariable logistic regression analysis, and sum-rank test were used to analyze the factors associated with influenza vaccine immune response.
Results
The Chi-square test showed that seroconversion rates and response rate were associated with age group, vaccination history, chronic conditions, the frequency of colds, and region (P < 0.05). The multivariable logistic regression analysis showed that age was an important factor that affected participants’ seroconversion rates for A/H1N1, A/H3N2, B/Victoria, and response status (18–64 vs. ≤5: OR = 2.77, P < 0.001; ≥65 vs. ≤5: OR = 0.38, P = 0.01; 18–64 vs. ≤5: OR = 2.64, P = 0.03). Vaccination history was also an affecting factor for A/H1N1, B/Victoria, and response status (yes vs. no: OR = 0.4 / 0.44 / 0.25, P < 0.001). The frequency of colds and chronic conditions were also affecting factors for participants’ seroconversion rates and response levels to different degrees. The sum-rank test showed that the fold changes for A/H1N1, B/Victoria, and B/Yamagata were associated with age group and vaccination history (P < 0.01). The fold changes for A/H3N2 were associated with the frequency of colds (P < 0.05), and those for B/Victoria were associated with gender and chronic conditions (P < 0.05).
Conclusions
Vaccination history, age, health condition, and frequency of colds were important factors affecting the seroconversion rate of the influenza vaccine in human. There is a need for developing optimized vaccination strategies for vulnerable groups to improve the efficacy of influenza vaccines in human.
Keywords: Influenza, Influenza vaccine, Vaccine effectiveness, Immune response, Immunogenicity
Introduction
The influenza viruses are enveloped negative-sense single-strand RNA viruses with a segmented genome, including types A, B, C, and D. [1] Influenza A and influenza B viruses cause seasonal epidemics annually. [2] Among them, the major circulating strains include influenza A H1N1, A H3N2, B/Victoria and B/Yamagata lineages. [1] There are an estimated 1 billion influenza cases worldwide each year, including 3 to 5 million severe cases and 290, 000 to 650, 000 deaths, [3] of which pose a threat to human health and medical services.
Vaccination is the most effective way to reduce human influenza disease burden. [2] The risk of seeking treatment will decrease by 40–60% if influenza vaccine viruses match circulating viruses. [4] However, such protection effectiveness may be lower for some reasons, especially when the vaccine strains are mismatched with circulating viruses. [5] The immunogenicity of the vaccine is also one of the most important factors influencing vaccine effectiveness. [6] Previous studies indicate that the immunogenicity of the vaccine can be affected by repeated vaccination; [7] vaccine factors, such as vaccine types, dosage, and delivery mode of vaccine; [8–10] and host factors, such as age, gender, and health conditions. [6, 11]
In this study, 557 volunteers were recruited from three provinces in China and then vaccinated with the influenza vaccines to explore the factors associated with the vaccine immunogenicity. Several factors associated with responsiveness to influenza vaccination were identified. The results may provide supporting data for identifying influenza vaccination low responders and optimizing the vaccination strategies, thereby improving the effectiveness of the influenza vaccine in human.
Materials and methods
Participants and data Collection
Based on our previous research, [12] 593 volunteers were recruited by the staff of the Center for Disease Control (CDC) and Prevention of Yunnan Province, Shaanxi Province, and Xinjiang Uygur Autonomous Region from September 2019 to September 2021. We enrolled volunteers who were: (1) Han Chinese, (2) and had not already received the northern hemisphere formulation of influenza vaccine for the corresponding year. Volunteers were excluded if they: (1) reported medical conditions not suitable to receive influenza vaccines such as any allergic reaction to egg protein or previous dose of influenza vaccine; (2) reported medical conditions not suitable for intramuscular injection or venous blood collection such as the use of anticoagulant medication. As a result, 36 volunteers were excluded, and 557 were eligible for further research.
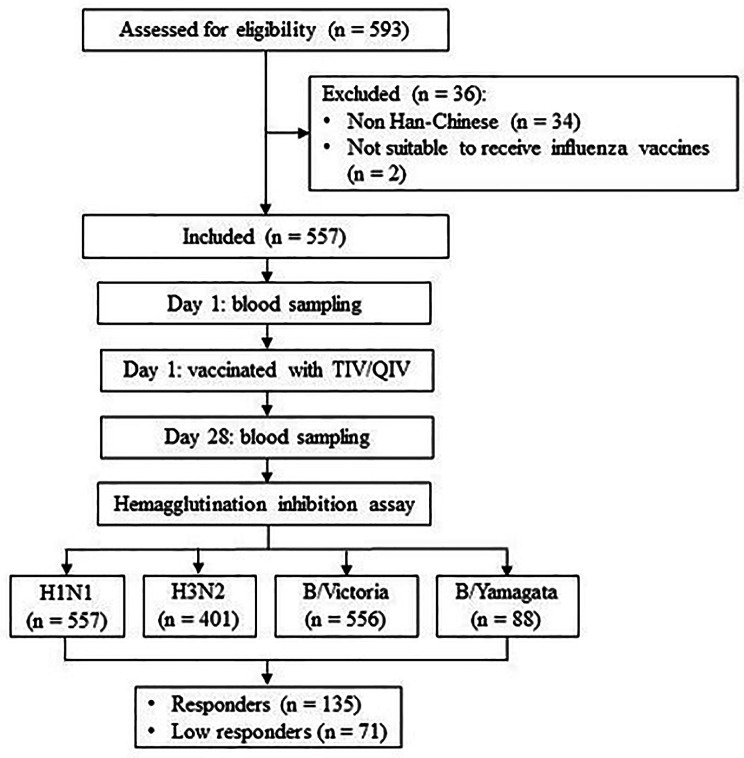
The following information of volunteers was collected by the staff of CDC via questionnaire: gender, age, height, weight, region, vaccine type, frequency of colds, vaccination history, smoking and alcohol consumption, and health conditions. A total of 10 ml peripheral venous blood was collected by the staff of CDC before (day 1) and 28 days after the vaccination (day 28). The serum was isolated after blood samples setting for 4 h and stored in a − 80 °C ultra-low temperature freezer (Thermo Fisher Scientific, USA). The flow chart was described in Fig. 1.
Fig. 1.
Flow Chart
All volunteers signed informed consent. The study was approved by the Ethics Review Committee of the National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention (NIVDC, assurance number, 202023).
Vaccines
All volunteers received trivalent inactivated vaccine (TIV) or quadrivalent inactivated vaccine (QIV) by intramuscular injection. The vaccines strains consisted of the Northern Hemisphere vaccine components recommended by the World Health Organization (WHO), and details were shown in Table 1. The TIV contained 15 µg hemagglutination (HA) for 3 strains, including A H1N1, A H3N2, and either B/Victoria or B/Yamagata lineage, and was provided by Shenzhen Sanofi Pasteur Biological Products Co., Ltd. The QIV contained 15 µg HA for 4 strains above and was provided by HUALAN BIOLOGICAL ENGINEERING, INC. Volunteers aged 5 years or older got 1 dose of vaccine containing 15 µg HA for each strain on day 1, and volunteers younger than 5 years got 2 doses of vaccine containing 7.5 µg HA for each strain with an interval of 30 days. All the vaccines were free for volunteers.
Table 1.
Vaccine strains recommended by WHO for northern hemisphere from 2019–2021 and vaccination details
| Influenza season | Vaccine strains |
Vaccine type | Vaccinated number |
|---|---|---|---|
| 2019–2020 | A/Brisbane/02/2018 (H1N1)pdm09-like virus | TIV | 179 |
| A/Kansas/14/2017 (H3N2)-like virus | |||
| B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage) | |||
| B/Phuket/3073/2013-like virus (B/Yamagata/16/88 lineage) | |||
| 2020–2021 | A/Guangdong-Maonan/SWL1536/2019 (H1N1)pdm09-like virus | TIV | 214 |
| A/Hong Kong/2671/2019 (H3N2)-like virus | |||
| B/Washington/02/2019 (B/Victoria lineage)-like virus | |||
| B/Phuket/3073/2013 (B/Yamagata lineage)-like virus. | |||
| 2021–2022 | A/Victoria/2570/2019 (H1N1)pdm09-like virus | QIV | 164 |
| A/Cambodia/e0826360/2020 (H3N2)-like virus | |||
| B/Washington/02/2019 (B/Victoria lineage)-like virus | |||
| B/Phuket/3073/2013 (B/Yamagata lineage)-like virus |
Hemagglutination inhibition assay
Hemagglutination inhibition (HAI) assay was used to measure the serum antibody titers against vaccine strains. The influenza virus strains used in the study were provided by the Chinese National Influenza Center. They were cultured by specified pathogen-free (SPF) chicken embryos (Xin Xing Da Hua Nong Poultry and Egg Co., LTD, China). 1% red blood cells from turkey (Guangzhou Hongquan Biotechnology Co., LTD, HQ80085-2, China) were used for influenza A/H1N1 and B HAI assays, and those from guinea pigs (Guangzhou Hongquan Biotechnology Co., LTD, HQ80077-4, China) were used for influenza A/H3N2 HAI assays. Before the assay, the serum was mixed with receptor-destroying enzyme (RDE) (Denka Seiken Co., Ltd., 340016, Japan) in a ratio of 1:3 in order to remove the non-specific inhibitor. The mixture was bathed in a 37 °C water bath (Jiangsu Keyi Instrument Co., LTD, China) for 16–18 h and then in a 56 °C water bath (Jiangsu Keyi Instrument Co., LTD, China) for 30 min for inactivation. The mixture was diluted to a final dilution of 1:5 in phosphate-buffered saline (Biosharp, BL302A, China) and then serially diluted 2-fold down a U-bottom 96-well plate for A/H3N2 (V-bottom 96-well plate for A/H1N1, B/Victoria, and B/Yamagata). 25 µl live virus containing 4 HAU was added to each well for a final 50 µl/well volume. After being incubated for 15–30 min at room temperature, 50 µl of 1% red blood cell was added to each well. After incubating for 30–60 min, the lowest dilution ratio leading to complete hemagglutination inhibition was recorded as the antibody titer. Operations involving the influenza virus were conducted in the Biosafety Level II laboratory.
The seroconversion rate referred to the proportion of participants showing a four-fold increase in post-immunization compared with pre-immunization titers, or titers < 10 pre-immunization to at least 40 post-immunization. [13] The seroprotection rate referred to the proportion of participants with a post-vaccination titer ≥ 40.13 The logarithm of antibody titer post-vaccination was divided by the logarithm pre-vaccination to obtain the fold change for antibody titer. Responders were defined as participants whose HAI titers achieved seroconversion to all influenza vaccine strains. Low responders were those whose HAI titers failed to achieve seroconversion to all influenza vaccine strains. The response rate referred to the proportion of responders to the sum of responders and low responders.
Statistical analysis
Descriptive statistics were used to describe the participants’ characteristics, including mean (M), standard deviation (SD), frequency, and percentage. According to the HAI assay, the seroconversion status for each vaccine strain was categorized into “seroconversion” and “non - seroconversion”. For further analysis, the participants were categorized into “responders” and “low responders”. Independent variables include gender, age group, region, vaccine type, frequency of colds, vaccination history, body mass index (BMI) group, smoking, and alcohol consumption, and health conditions. The Chi-square test was used to compare the proportions of participants among different groups first. Associations of independent variables with the seroconversion were analyzed by multivariable logistic regression models adjusted for gender and age. Mann-Whitney U test or Kruskal-Wallis H test was used to explore the associations of independent variables with the fold changes since the fold changes among different groups were not normally distributed according to the results of the Kolmogorov-Smirnov test and Shapiro-Wilk test. Statistical significance was defined as P < 0.05. SPSS Statistics for Windows, Version 25.0 was used for statistical analysis. GraphPad Prism for Windows, Version 9.3.1 was used for processing graphs.
Results
Characteristics of participants
A total of 593 volunteers were recruited from 3 provinces of China, and 557 volunteers were included in the final analysis after 36 were excluded, whose details were shown in Table 2. The participants ranged from 2 to 89 years old, with a mean age of 49.4 ± 28. 6 years, and 60.7% (n = 338) were female. 42.9% (n = 239) were from Yunnan Province, 39.9% (n = 222) of participants were from Shaanxi Province, and 17.2% (n = 96) were from Xinjiang Uygur Autonomous Region. A total of 179 (32.6%) reported having a vaccination history, while 370 (67.4%) hadn’t been vaccinated. In the 2019–2020 and 2020–2021 season, 179 (32.2%) and 214 (38.4%) participants were vaccinated with TIV, respectively. In the 2021–2022 season, 164 (29.4%) were vaccinated with QIV. 34.0% (n = 188) of participants’ BMI was between 18.5 and 23.9, 30.7% (n = 170) was < 18.5, 23.9% (n = 132) was between 24 and 27.9, and only 11.4% (n = 63) was ≥ 28. When asked about the frequency of colds, 67.1% (n = 308) of participants reported 2–3, 17.2% (n = 79) reported 4–6, followed by ≤ 1 (11.3%, n = 52), and only 4.4% (n = 20) reported >6. Most participants reported not smoking (85.8%, n = 442) and not drinking (81.9%, n = 420). 76.6% (n = 422) of participants didn’t report chronic diseases. The seroconversion rates for A/H1N1, A/H3N2, B/Victoria, and B/Yamagata were 57.1%, 58.6%, 57.0%, and 52.3%, respectively. There were 135 (24.2%) responders and 71 (12.7%) low responders. The fold changes for A/H1N1, A/H3N2, B/Victoria, and B/Yamagata were 1.47(1.00-2.72), 1.60(1.23–2.20), 1.60(1.00-2.72), and 1.38(1.00-1.92), respectively.
Table 2.
Characteristics of Participants in Different Groups (n = 557)
| Variable | Category | Frequency/Mean/Median | Percentage/ Standard Deviation/ (P25, P75) |
|---|---|---|---|
| Gender | Male | 219 | 39.3% |
| Female | 338 | 60.7% | |
| Age Group | ≤ 5 | 141 | 25.3% |
| 6–17 | 36 | 6.5% | |
| 18–64 | 226 | 40.6% | |
| ≥ 65 | 154 | 27.6% | |
| Region | Yunnan | 239 | 42.9% |
| Xinjiang | 96 | 17.2% | |
| Shaanxi | 222 | 39.9% | |
| Frequency of Colds | ≤ 1 | 52 | 11.3% |
| 2–3 | 308 | 67.1% | |
| 4–6 | 79 | 17.2% | |
| >6 | 20 | 4.4% | |
| Vaccination History | No | 370 | 67.4% |
| Yes | 179 | 32.6% | |
| BMI Group | < 18.5 | 170 | 30.7% |
| 18.5–23.9 | 188 | 34.0% | |
| 24-27.9 | 132 | 23.9% | |
| ≥ 28 | 63 | 11.4% | |
| Smoking | No | 442 | 85.8% |
| Yes | 73 | 14.2% | |
| Alcohol Drinking | No | 420 | 81.9% |
| Yes | 93 | 18.1% | |
| Chronic Diseases | No | 422 | 76.6% |
| Yes | 129 | 23.4% | |
| Vaccine Type | TIV | 393 | 70.6% |
| QIV | 164 | 29.4% | |
| Seroconversion for A/H1N1 | No | 239 | 42.9% |
| Yes | 318 | 57.1% | |
| Seroconversion for A/H3N2 | No | 166 | 41.4% |
| Yes | 235 | 58.6% | |
| Seroconversion for B/Victoria | No | 239 | 43.0% |
| Yes | 317 | 57.0% | |
| Seroconversion for B/Yamagata | No | 42 | 47.7% |
| Yes | 46 | 52.3% | |
| Response status | Low Responder | 71 | 12.7% |
| Responder | 135 | 24.2% | |
| Neither | 351 | 63.1% | |
| Age | - | 40.4 | 28.6 |
| Height/cm | - | 144.3 | 28.0 |
| Weight/kg | - | 49.1 | 23.0 |
| BMI | - | 21.5 | 4.8 |
| The fold change for A/H1N1 | - | 1.47 | 1.00-2.72 |
| The fold change for A/H3N2 | - | 1.60 | 1.23–2.20 |
| The fold change for B/Victoria | - | 1.60 | 1.00-2.72 |
| The fold change for B/Yamagata | - | 1.38 | 1.00-1.92 |
BMI: body mass index; TIV: trivalent inactivated vaccine; QIV: quadrivalent inactivated vaccine; P25: Lower quartile; P75: Upper quartile
Frequency of Colds: The frequency of common colds caused by adenovirus, parainfluenza virus, rhinovirus, respiratory syncytial virus (RSV), enterovirus, and coronavirus, etc. with mild upper respiratory syndromes per year; Vaccination history: Influenza vaccination history in the past 5 years. BMI Group: The grouping critiria was WS/T 428–2013. Smoking: Smoking in the past 5 years. Alcohol Drinking: Alcohol drinking in the past 5 years. Chronic Diseases: Diagnosed or treated for chronic medical condition during the past 5 years
Associations between independent variables and responsiveness to influenza vaccination
The results of the Chi-square test were shown in Table 3. Seroconversion rates for A/H1N1, B/Victoria, B/Yamagata, and response rate were associated with the age group (P < 0.05), and participants aged between 6 and 18 years old had better responsiveness to the influenza vaccines. Seroconversion rates for A/H1N1, A/H3N2, and B/Victoria were associated with the region (P < 0.05). In addition, the seroconversion rate for A/H3N2 and response rate were related to the frequency of colds (P < 0.05). Participants from Xinjiang Uygur Autonomous Region or with a lower frequency of colds had worse responsiveness. Besides, seroconversion rates for A/H1N1, B/Victoria, and response rate were associated with repeated vaccination (P < 0.05). Furthermore, seroconversion rates for B/Victoria, and B/Yamagata were associated with chronic conditions (P < 0.05). Those without a vaccination history or chronic conditions had better responsiveness to the influenza vaccine.
Table 3.
The Seroconversion Rate and Response Rate among Different Groups (n = 557)
| Variable | Category | A/H1N1 | A/H3N2 | B/Victoria | B/Yamagata | Response Status | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| The seroconv-ersion rate | χ2 | P | The seroconv-ersion rate | χ2 | P | The seroconv-ersion rate | χ2 | P | The seroconv-ersion rate | χ2 | P | The Response rate | χ2 | P | ||
| Age Group | ≤ 5 | 51.1% | 13.27 | 0.004 | 66.0% | 4.03 | 0.26 | 53.2% | 11.27 | 0.01 | 42.9% | 8.50 | 0.04 | 60.0% | 8.68 | 0.03 |
| 6–17 | 72.2% | 63.3% | 71.4% | 57.1% | 88.2% | |||||||||||
| 18–64 | 63.7% | 56.7% | 62.8% | 67.6% | 70.8% | |||||||||||
| ≥ 65 | 49.4% | 53.3% | 48.7% | 30.4% | 53.3% | |||||||||||
| Region | Yunnan | 61.1% | 29.40 | < 0.001 | 80.7% | 85.21 | < 0.001 | 62.3% | 17.48 | < 0.001 | - | - | - | 71.0% | 2.57 | 0.28 |
| Xinjiang | 32.3% | 86.5% | 37.9 | - | 64.9% | |||||||||||
| Shaanxi | 63.5% | 38.3% | 59.5% | 52.3% | 59.2% | |||||||||||
| Frequency of Colds | ≤ 1 | 63.5% | 3.62 | 0.31 | 9.1% | 34.34 | < 0.001 | 51.9% | 6.23 | 0.10 | 50.0% | 2.88 | 0.41 | 50.0% | 5.66 | 0.13 |
| 2–3 | 63.3% | 50.7% | 64.6% | 59.1% | 71.7% | |||||||||||
| 4–6 | 54.4% | 77.5% | 51.9% | 30.0% | 54.5% | |||||||||||
| >6 | 75.0% | 55.6% | 60.0% | 50.0% | 60.0% | |||||||||||
| Vaccination History | No | 64.3% | 27.23 | < 0.001 | 58.1% | 0.13 | 0.72 | 64.8% | 28.29 | < 0.001 | 56.9% | 1.72 | 0.19 | 72.7% | 14.62 | < 0.001 |
| Yes | 40.8% | 60.0% | 40.8% | 41.9% | 43.1% | |||||||||||
| Chronic Diseases | No | 60.3% | 1.10 | 0.29 | 53.9% | 0.07 | 0.79 | 63.6% | 11.48 | 0.001 | 64.1% | 5.19 | 0.02 | 67.8% | 1.18 | 0.28 |
| Yes | 54.7% | 55.7% | 45.3% | 33.3% | 58.1% | |||||||||||
The results of the multivariable logistic regression analysis are presented in Table 4. Age (18–64 vs. ≤5: OR = 2.77, P < 0.001) and repeated vaccination (yes vs. no: OR = 0.4, P < 0.001) were important factors that affected participants’ seroconversion rates against A/H1N1. As for A/H3N2, age (≥ 65 vs. ≤5: OR = 0.38, P < 0.05) and frequency of colds (4–6 vs. ≤1: OR = 10.71, P < 0.05) affected the seroconversion rate. In the case of B/Victoria, age (18–64 vs. ≤5: OR = 2.45, P < 0.001), vaccination history (yes vs. no: OR = 0.44, P < 0.001), and chronic conditions (yes vs. no: OR = 0.48, P < 0.01) affected the seroconversion rate to influenza vaccines. Regarding B/Yamagata, chronic conditions (yes vs. no: OR = 0.18, P < 0.05) also affected the seroconversion rate. Similarly, age (18–64 vs. ≤5: OR = 2.64, P < 0.05) and vaccination history (yes vs. no: OR = 0.25, P < 0.001) were factors influencing participants’ response status to vaccine strains.
Table 4.
Multivariable Logistic Regression Analysis of Responsiveness to Influenza Vaccine
| Variable | Category | A/H1N1 | A/H3N2 | B/Victoria | B/Yamagata | Response Status | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR(95%CI) | P | OR(95%CI) | P | OR(95%CI) | P | OR(95%CI) | P | OR(95%CI) | P | ||
| Age Group | ≤ 5 | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - |
| 6–17 | 2.83(0.98–8.12) | 0.05 | 1.51(0.38–6.08) | 0.56 | 2.30(0.87–6.11) | 0.09 | 1.91(0.36–10.12) | 0.45 | 4.31(0.43–43.07) | 0.21 | |
| 18–64 | 2.77(1.68–4.57) | < 0.001 | 0.80(0.42–1.54) | 0.51 | 2.45(1.51-4.0) | < 0.001 | 2.45(0.58–10.28) | 0.22 | 2.64(1.08–6.46) | 0.03 | |
| ≥ 65 | 1.32(0.76–2.28) | 0.32 | 0.38(0.18–0.81) | 0.01 | 1.46(0.86–2.47) | 0.16 | 0.36(0.07–1.89) | 0.23 | 1.16(0.42–3.25) | 0.78 | |
| Frequency of Colds | ≤ 1 | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - |
| 2–3 | 1.17(0.56–2.46) | 0.68 | 4.00(0.75–21.58) | 0.11 | 1.28(0.63–2.60) | 0.50 | 1.39(0.45–4.33) | 0.57 | 1.38(0.33–5.69) | 0.66 | |
| 4–6 | 0.74(0.31–1.78) | 0.50 | 10.71(1.68–68.05) | 0.01 | 0.70(0.30–1.63) | 0.41 | 0.30(0.04–2.14) | 0.23 | 0.50(0.10–2.50) | 0.40 | |
| >6 | 2.15(0.63–7.36) | 0.22 | 7.11(0.75–67.68) | 0.09 | 1.16(0.38–3.54) | 0.79 | 1.06(0.11–9.89) | 0.96 | 0.80(0.08–8.08) | 0.85 | |
| Vaccination History | No | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - |
| Yes | 0.40(0.26,0.62) | < 0.001 | 0.73(0.38–1.41) | 0.35 | 0.44(0.29–0.67) | < 0.001 | 0.87(0.30–2.52) | 0.79 | 0.25(0.11–0.56) | < 0.001 | |
| Chronic Diseases | No | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - |
| Yes | 0.83(0.51–1.34) | 0.44 | 0.77(0.92 − 0.51) | 0.77 | 0.48(0.30–0.78) | 0.003 | 0.18(0.04–0.76) | 0.02 | 0.66(0.28–1.57) | 0.66 | |
OR: odds ratio; CI: confidence interval
Associations between independent variables and antibody fold change after vaccination
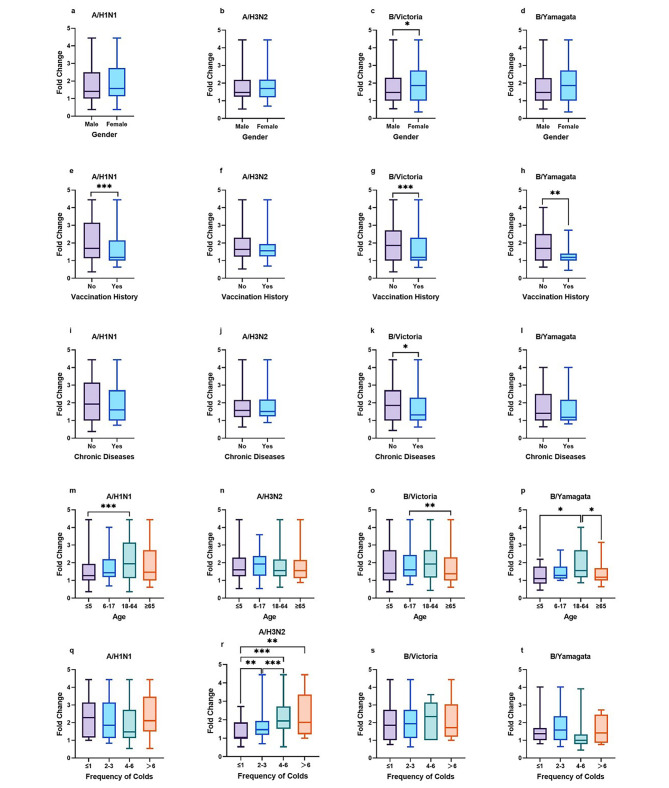
As shown in Table 5; Fig. 2, the fold changes for A/H1N1, B/Victoria, and B/Yamagata were associated with age group (P < 0.05) and vaccination history (P < 0.05). Consistent with the results above, participants aged from 18 to 65 or without vaccination history had higher antibody folds. The fold changes for B/Victoria were associated with gender (P < 0.05) and chronic conditions (P < 0.05), and those who were female or without chronic diseases had higher antibody folds. Besides, the fold changes for A/H3N2 were associated with the frequency of colds (P < 0.05), and participants with a higher frequency of colds had higher folds.
Table 5.
Rank-Sum Test of Antibody Fold Change
| Variable | Category | A/H1N1 | A/H3N2 | B/Victoria | B/Yamagata | ||||
|---|---|---|---|---|---|---|---|---|---|
| M(P25,P75) | P | M(P25,P75) | P | M(P25,P75) | P | M(P25,P75) | P | ||
| Gender | Male | 1.86(1.00-2.72) | 0.18 | 1.43(1.16–1.90) | 0.33 | 1.63(1.00-2.72) | 0.03 | 1.32(1.00-1.75) | 0.21 |
| Female | 1.88(1.16–3.15) | 1.62(1.16–2.20) | 2.29(1.12–2.74) | 1.38(1.00-2.39) | |||||
| Age Group | ≤ 5 | 1.27(1.00-2.14) | < 0.001 | 1.56(1.19–1.94) | 0.68 | 1.39(1.00-2.72) | 0.003 | 1.00(0.80–1.72) | 0.04 |
| 6–17 | 1.94(1.28–2.42) | 1.37(1.00-1.90) | 2.11(1.00-2.74) | 1.29(1.10–1.79) | |||||
| 18–64 | 2.34(1.27–3.26) | 1.69(1.23–2.20) | 2.29(1.19–3.15) | 1.60(1.19–2.72) | |||||
| ≥ 65 | 1.86(1.00-3.15) | 1.30(1.00-1.86) | 1.65(1.00-2.72) | 1.17(1.00-1.82) | |||||
| Frequency of Colds | ≤ 1 | 2.29(1.16–3.15) | 0.3 | 1.00(1.00-1.54) | < 0.001 | 1.86(1.00-2.72) | 0.58 | 1.38(1.00-1.69) | 0.09 |
| 2–3 | 1.86(1.14–3.15) | 1.47(1.19–1.94) | 1.94(1.12–2.72) | 1.56(1.00-2.39) | |||||
| 4–6 | 1.47(1.07–2.72) | 1.94(1.47–2.74) | 1.47(1.00-2.94) | 1.00(0.69–1.28) | |||||
| >6 | 2.12(1.50–3.48) | 1.86(1.22–3.37) | 1.71(1.20–3.05) | 1.43(0.86–2.47) | |||||
| Vaccination History | No | 2.16(1.14–3.15) | < 0.001 | 1.56(1.18–2.20) | 0.20 | 2.29(1.00-3.15) | < 0.001 | 1.69(1.00-2.51) | 0.005 |
| Yes | 1.38(1.09–2.29) | 1.43(1.09–1.90) | 1.29(1.00-2.29) | 1.19(1.00-1.41) | |||||
| Chronic Diseases | No | 2.25(1.14–3.15) | 0.20 | 1.60(1.19–2.20) | 0.98 | 1.94(1.16–2.74) | 0.02 | 1.49(1.05–2.67) | 0.34 |
| Yes | 1.86(1.16–3.05) | 1.53(1.23–2.36) | 1.63(1.14–2.67) | 1.31(1.00-2.02) | |||||
M: median; P25: lower quartile; P75: upper quartile
Fig. 2.
Rank-Sum Test of Antibody Fold Change. Comparison of antibody fold changes against 4 vaccine strains. (a ~ d) Comparison of antibody fold changes between male and female participants against 4 vaccine strains. (e ~ h) Comparison of antibody fold changes between participants with and without vaccination history against 4 vaccine strains. (i ~ l) Comparison of antibody fold changes between participants with and without chronic diseases against 4 vaccine strains. (m ~ p) Comparison of antibody fold changes among participants in 4 age groups against 4 vaccine strains. (q ~ t) Comparison of antibody fold changes among participants with 4 types of frequency of colds against 4 vaccine strains
Discussion
Immunogenicity response to the vaccine is a complicated process, which is affected by many factors, including vaccine and host factors. [6, 14] In this study, we identified several factors associated with the responsiveness to the influenza vaccine. The Chi-square test, multivariable logistic regression analysis, and sum-rank test showed that vaccination history, chronic condition, age, and frequency of colds were important factors affecting immunogenicity responsiveness to the influenza vaccine. These results will help optimize influenza vaccination strategies and improve the effectiveness of the vaccine in humans.
In this study, participants with a vaccination history presented worse immunogenicity responsiveness to the influenza vaccines, indicating that continuous annual vaccination may lead to reduced vaccine efficacy, consistent with previous studies. A study evaluating the immunogenicity of trivalent inactivated influenza vaccine showed that the odds of seroconversion were strongly related to the baseline antibody titer, and the odds of seroconversion decreased dramatically when the baseline antibody level was higher. [15] Participants who received influenza vaccines in the previous year had significantly lower odds of seroconversion than those who did not. [15] One possible explanation was that repeated vaccination negatively interfered with immunogenicity. [16] Surender Khurana et al. [17] indicated that repeated vaccination negatively affected antibody binding, antibody affinity maturation, and hemagglutination inhibition responses to H1N1, H3N2, and B strains manufactured by three different vaccine platforms. At the same time, results showed that repeated vaccination failed to induce CD4 T cell activation. [7] Studies demonstrated that prior-year vaccination correlated with low production of antibody-secreting cells and reduced effector B-cell responses to new vaccine immunization. [18, 19] The concept of antigenic imprinting has been suggested to explain the negative effect of repeated vaccination. [20] When contacted with a novel virus strain similar to an individual exposed, the immune response to the original one was predominantly boosted at the expense of response to the subsequent one. These participants’ immune systems tended to induce a strong anamnestic response to the original strains, and the production of their memory cells stimulated by the subsequent strains was reduced.
Health conditions were important factors influencing the body’s immunity. [6] In this study, participants with chronic diseases had a higher risk of lower seroconversion rates. A similar study demonstrated that hypertensive subjects had lower antibody levels against the COVID-19 mRNA vaccine. [21] A recent study revealed that this association might result from a dysfunctional immune system. [22] A study on antibody responses following vaccination with adjuvanted influenza vaccine in immunocompromised children (including AIDS, congenital immunodeficiency, and autoimmune diseases) showed that their geometric mean titer (GMT) and seroprotection rates were lower compared with immunocompetent children. [23] This phenomenon could be due to the defects in their immune system, causing worse responses to the influenza vaccine. [24] However, diabetes status seemed to have less impact on the immunogenicity of the influenza vaccine. Sarah Spencer et al. [25] found that the serologic response to the influenza vaccine was similar in participants with and without type 2 diabetes mellitus. Daniela Frasca et al. [26] found that type 2 diabetes patients had normal in vivo and in vitro B cell responses to the influenza vaccine. The possible reasons for this were that the increase in their serum LPS and sCD14 would stimulate B cells, and the TLR4 expression level in their B cells also increased. [26] Therefore, the chronic underlying medical conditions affecting the immune response to the influenza vaccine were complex. These implied that vaccine boosters or different vaccine schedules should be used for patients with chronic diseases to increase vaccination effectiveness.
Participants of different ages also differed in immune responses to the influenza vaccine. In the current study, participants aged 18 to 65 had higher fold changes and seroconversion rates, and this was consistent with previous studies. A Polish study evaluating the influenza virus circulation in the 2015/2016 epidemic season reported that both GMT levels and seroprotection rates of participants aged 0 to 4 years and older than 65 years were lower, [27] suggesting the susceptibility of these two age groups to influenza virus. The immune system of infants is not completely developed, and the magnitude and activity of their antigen-presenting cells (APCs), immune cells, and cytokines are lower than in older children and adults. [28] For older people, Goodwin et al. [29] conducted a quantitative review of studies concerning antibody levels for the influenza vaccine. They reported that the elderly over 65 had significantly lower antibody levels than younger adults, as well as the seroconversion rate, seroprotection rate, and the GMT level. However, the underlying reason for the worse responsiveness to vaccines in the elderly was “immunosenescence”, referred to as age-related changes in the immune system. [30] Aging negatively affects the body’s immune cell repertoire and results in cell-intrinsic defects in lymphocytes. [30] These contribute to the deterioration of innate and adaptive immune responses. [31] Influenza vaccines can provide moderate protection against viruses, but such protection is reduced in younger children and the elderly, and these two groups of people become vulnerable. Optimized vaccination strategy and new vaccines with improved immunogenicity were needed to reduce influenza-related morbidity and mortality.
Intriguingly, there seemed to be a trend for A/H3N2 that the higher the frequency of common colds, the better the responsiveness. This was observed in both multivariable logistic regression analysis and the rank-sum test. We speculated that the common cold could generate cross-reactive binding antibodies against the influenza vaccine strain, [32] similar to Sealy’s study [33] et al. The study indicated that the pre-existing immunity conferred by an individual’s past exposures to common cold human coronaviruses might influence the body’s control for SARS-CoV-2. [33] The common cold is caused by well-adapted pathogens and is less harmful to their hosts. [34] Therefore, people who often catch a cold have relatively low immunity and are also vulnerable to the influenza virus. Influenza vaccination is necessary for this group of people, who can benefit more from it. However, more research is needed to explore the association between the common cold and the immune response to the influenza vaccines from various aspects, providing more implications for vaccination.
Gender is an important factor affecting the immunogenicity of influenza vaccines. [35] Engler et al. found that women had significantly higher GMT than men in the case of different doses and strains. [36] However, in the current study, only the antibody fold change for B/Victoria showed a significant difference between the male and female participants. In fact, the effect of gender on vaccine immunity is complex. Besides genetic factors, the difference can also be explained through the divergent levels of sex steroid hormones, which are changing with aging. [35] [37] Women seem to lose their immunological advantage after menopause. [35] Therefore, the composition and dosage of influenza vaccines need to be adjusted according to the sex-mediated immunity differences. Future studies need to explore more factors that mediate sex differences in the immune responses deeply and extensively.
This study identified several factors relating to responsiveness to the influenza vaccine and suggested the need for more optimized vaccination strategies for susceptible groups to improve the efficacy of influenza vaccines, which is of great importance for public health. The findings in this study were a supplement to previous studies and may give some new insights into the immunogenicity response to the vaccine. However, there were some limitations in the current study. This study was descriptive, with some participants’ characteristics not collected comprehensively, and we did not perform mechanism studies based on the study cohort. The association between the immune response to different strains and host factors should be certain in the future. It is also necessary to perform larger cohort studies and experiments to explore the underlying mechanisms.
The updated position paper on the use of seasonal influenza vaccines published by WHO recommended that older adults, children, health workers, pregnant women, and individuals with comorbidities and underlying conditions be prioritized as target groups. [38] In the future, it is of significance to increase vaccination rates for these vulnerable populations and optimize vaccine components and vaccination strategies to improve vaccine efficacy and reduce the morbidity, mortality, and disease burden associated with influenza.
Conclusions
This study explored the factors associated with the immune response to influenza vaccines in China. The results showed that repeated vaccination, health condition, age, and frequency of colds were important factors affecting the immunogenicity responses to influenza vaccines. The results may provide supporting data for identifying low responders and optimizing the vaccination strategies to improve the effectiveness of influenza vaccines in human.
Acknowledgements
We are grateful for the contribution of the staff from the Center for Disease Control and Prevention of Yunnan Province, Shaanxi Province, and Xinjiang Uygur Autonomous Region for helping us to collect information. We thank all volunteers for participating in this study.
Abbreviations
- OR
Odds Ratio
- CI
Confidence Interval
- CDC
Center for Disease Control
- TIV
Trivalent Inactivated Vaccine
- QIV
Quadrivalent Inactivated Vaccine
- WHO
World Health Organization
- HA
Hemagglutination
- HAI
Hemagglutination Inhibition
- SPF
Specified Pathogen-Free
- RDE
Receptor Destroying Enzyme
- BMI
body mass index
- GMT
Geometric Mean Titer
- P25
Lower quartile
- P75
Upper quartile
Authors’ contributions
QX and HW are joint first authors. YS and DW designed the study. QX, SW, and HW managed the cohort. SW designed the questionnaire. YC and WH collected the questionnaire and samples and conducted the HAI assay. QX and JC analyzed the data. QX, SW, and YL drafted the manuscript. YS and DW are responsible for all the analyses and writing of this paper. All authors read and approved the final manuscript.
Funding
This study was supported financially by the National Key Research and Development Program of China (Grant number: 2021YFC2300100); Guangdong Basic and Applied Basic Research Foundation (Grant number: 2023A1515011767).
Data Availability
The datasets generated and analysed during the curret study are not publicly available due to existing general data protection rules and official secrecy, but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Review Committee of the National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention (NIVDC, assurance number, 202023). The study was performed in accordance with the principles of Declaration of Helsinki. Informed consent was obtained from all volunteers in the study (or their parent or legal guardian in the case of children under 16).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qiuyi Xu and Hejiang Wei were joint first authors.
Yuelong Shu and Dayan Wang were joint corresponding authors.
Contributor Information
Dayan Wang, Email: dayanwang@cnic.org.cn.
Yuelong Shu, Email: shuylong@mail.sysu.edu.cn.
References
- 1.Krammer F, Smith GJD, Fouchier RAM, Peiris M, Kedzierska K, Doherty PC, et al. Influenza Nat reviews Disease primers. 2018;4:3. doi: 10.1038/s41572-018-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. : Influenza (Seasonal) Ask the expert: Influenza Q&A. https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal). Accessed 07 Jul 2022.
- 3.Hswen Y, Brownstein JS, Liu J, Hawkins JB. Use of a Digital Health application for Influenza Surveillance in China. Am J Public Health. 2017;107:1130–6. doi: 10.2105/ajph.2017.303767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Vaccine Effectiveness: How Well Do Flu Vaccines Work? https://www.cdc.gov/flu/vaccines-work/vaccineeffect.htm#. Accessed 06 Jul 2022.
- 5.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. doi: 10.1016/s1473-3099(11)70295-x. [DOI] [PubMed] [Google Scholar]
- 6.Wen S, Wu Z, Zhong S, Li M, Shu Y. Factors influencing the immunogenicity of influenza vaccines. Hum Vaccin Immunother. 2021;17:2706–18. doi: 10.1080/21645515.2021.1875761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wild K, Smits M, Killmer S, Strohmeier S, Neumann-Haefelin C, Bengsch B, et al. Pre-existing immunity and vaccine history determine hemagglutinin-specific CD4 T cell and IgG response following seasonal influenza vaccination. Nat Commun. 2021;12:6720. doi: 10.1038/s41467-021-27064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowling BJ, Perera R, Valkenburg SA, Leung NHL, Iuliano AD, Tam YH, et al. Comparative immunogenicity of several enhanced influenza vaccine options for older adults: a Randomized, Controlled Trial. Clin Infect Dis. 2020;71:1704–14. doi: 10.1093/cid/ciz1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ambrose CS, Levin MJ, Belshe RB. The relative efficacy of trivalent live attenuated and inactivated influenza vaccines in children and adults. Influenza Other Respir Viruses. 2011;5:67–75. doi: 10.1111/j.1750-2659.2010.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorse GJ, Falsey AR, Fling JA, Poling TL, Strout CB, Tsang PH. Intradermally-administered influenza virus vaccine is safe and immunogenic in healthy adults 18–64 years of age. Vaccine. 2013;31:2358–65. doi: 10.1016/j.vaccine.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Potluri T, Fink AL, Sylvia KE, Dhakal S, Vermillion MS, Vom Steeg L, et al. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. NPJ Vaccines. 2019;4:29. doi: 10.1038/s41541-019-0124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen S, Wei H, Liao Q, Li M, Zhong S, Cheng Y, et al. Identification of two novel candidate genetic Variants Associated with the responsiveness to Influenza Vaccination. Front Immunol. 2021;12:664024. doi: 10.3389/fimmu.2021.664024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skowronski DM, De Serres G, Janjua NZ, Gardy JL, Gilca V, Dionne M, et al. Cross-reactive antibody to swine influenza A(H3N2) subtype virus in children and adults before and after immunisation with 2010/11 trivalent inactivated influenza vaccine in Canada, August to November 2010. Euro Surveill. 2012;17. 10.2807/ese.17.04.20066-en. [DOI] [PubMed]
- 14.Castrucci MR. Factors affecting immune responses to the influenza vaccine. Hum Vaccin Immunother. 2018;14:637–46. doi: 10.1080/21645515.2017.1338547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sperling RS, Engel SM, Wallenstein S, Kraus TA, Garrido J, Singh T, et al. Immunogenicity of trivalent inactivated influenza vaccination received during pregnancy or postpartum. Obstet Gynecol. 2012;119:631–9. doi: 10.1097/AOG.0b013e318244ed20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaglani M, Spencer S, Ball S, Song J, Naleway A, Henkle E, et al. Antibody response to influenza A(H1N1)pdm09 among healthcare personnel receiving trivalent inactivated vaccine: effect of prior monovalent inactivated vaccine. J Infect Dis. 2014;209:1705–14. doi: 10.1093/infdis/jit825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khurana S, Hahn M, Coyle EM, King LR, Lin TL, Treanor J, et al. Repeat vaccination reduces antibody affinity maturation across different influenza vaccine platforms in humans. Nat Commun. 2019;10:3338. doi: 10.1038/s41467-019-11296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews SF, Kaur K, Pauli NT, Huang M, Huang Y, Wilson PC. High preexisting serological antibody levels correlate with diversification of the influenza vaccine response. J Virol. 2015;89:3308–17. doi: 10.1128/jvi.02871-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasaki S, He XS, Holmes TH, Dekker CL, Kemble GW, Arvin AM, et al. Influence of prior influenza vaccination on antibody and B-cell responses. PLoS ONE. 2008;3:e2975. doi: 10.1371/journal.pone.0002975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang A, Stacey HD, Mullarkey CE, Miller MS. Original antigenic sin: how first exposure shapes lifelong Anti-Influenza Virus Immune responses. J Immunol. 2019;202:335–40. doi: 10.4049/jimmunol.1801149. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe M, Balena A, Tuccinardi D, Tozzi R, Risi R, Masi D, et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab Res Rev. 2022;38:e3465. doi: 10.1002/dmrr.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drummond GR, Vinh A, Guzik TJ, Sobey CG. Immune mechanisms of hypertension. Nat Rev Immunol. 2019;19:517–32. doi: 10.1038/s41577-019-0160-5. [DOI] [PubMed] [Google Scholar]
- 23.Meier S, Bel M, L’Huillier A, Crisinel PA, Combescure C, Kaiser L, et al. Antibody responses to natural influenza A/H1N1/09 disease or following immunization with adjuvanted vaccines, in immunocompetent and immunocompromised children. Vaccine. 2011;29:3548–57. doi: 10.1016/j.vaccine.2011.02.094. [DOI] [PubMed] [Google Scholar]
- 24.Brakemeier S, Schweiger B, Lachmann N, Glander P, Schönemann C, Diekmann F, et al. Immune response to an adjuvanted influenza a H1N1 vaccine (pandemrix(®)) in renal transplant recipients. Nephrol Dial Transplant. 2012;27:423–8. doi: 10.1093/ndt/gfr278. [DOI] [PubMed] [Google Scholar]
- 25.Spencer S, Chung JR, Belongia EA, Sundaram M, Meece J, Coleman LA, et al. Impact of diabetes status on immunogenicity of trivalent inactivated influenza vaccine in older adults. Influenza Other Respir Viruses. 2022;16:562–7. doi: 10.1111/irv.12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frasca D, Diaz A, Romero M, Mendez NV, Landin AM, Ryan JG, et al. Young and elderly patients with type 2 diabetes have optimal B cell responses to the seasonal influenza vaccine. Vaccine. 2013;31:3603–10. doi: 10.1016/j.vaccine.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kowalczyk D, Szymański K, Cieślak K, Hallmann-Szelińska E, Brydak LB. Circulation of Influenza Virus in the 2015/2016 epidemic season in Poland: serological evaluation of anti-hemagglutinin antibodies. Adv Exp Med Biol. 2019;1150:77–82. doi: 10.1007/5584_2018_271. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Zhivaki D, Lo-Man R. Unique aspects of the perinatal immune system. Nat Rev Immunol. 2017;17:495–507. doi: 10.1038/nri.2017.54. [DOI] [PubMed] [Google Scholar]
- 29.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–69. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 30.Sadighi Akha AA. Aging and the immune system: an overview. J Immunol Methods. 2018;463:21–6. doi: 10.1016/j.jim.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Crooke SN, Ovsyannikova IG, Poland GA, Kennedy RB. Immunosenescence and human vaccine immune responses. Immun Ageing. 2019;16:25. doi: 10.1186/s12979-019-0164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiu C, Wrammert J, Li GM, McCausland M, Wilson PC, Ahmed R. Cross-reactive humoral responses to influenza and their implications for a universal vaccine. Ann N Y Acad Sci. 2013;1283:13–21. doi: 10.1111/nyas.12012. [DOI] [PubMed] [Google Scholar]
- 33.Sealy RE, Hurwitz JL. Cross-reactive Immune responses toward the common Cold Human Coronaviruses and severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Mini-Review and a murine study. Microorganisms. 2021;9. 10.3390/microorganisms9081643. [DOI] [PMC free article] [PubMed]
- 34.Eccles R. Mechanisms of symptoms of the common cold and influenza. Br J Hosp Med (Lond) 2007;68:71–5. doi: 10.12968/hmed.2007.68.2.22824. [DOI] [PubMed] [Google Scholar]
- 35.Giefing-Kröll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14:309–21. doi: 10.1111/acel.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engler RJ, Nelson MR, Klote MM, VanRaden MJ, Huang CY, Cox NJ, et al. Half- vs full-dose trivalent inactivated influenza vaccine (2004–2005): age, dose, and sex effects on immune responses. Arch Intern Med. 2008;168:2405–14. doi: 10.1001/archinternmed.2008.513. [DOI] [PubMed] [Google Scholar]
- 37.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–38. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. WHO issues updated influenza vaccines position paper. https://www.who.int/news/item/01-06-2022-who-issues-updated-influenza-vaccines-position-paper. Accessed 20 Jul 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the curret study are not publicly available due to existing general data protection rules and official secrecy, but are available from the corresponding author on reasonable request.