Abstract
Infection with human papillomavirus (HPV) can cause cervical cancers in women, and vaccination against the virus is one of most effective ways to prevent these cancers. Two vaccines made of virus-like particles (VLPs) of HPV L1 proteins are currently commercially available. However, these HPV vaccines are highly expensive, and thus not affordable for women living in developing countries. Therefore, great demand exists to produce a cost-effective vaccine. Here, we investigate the production of self-assembled HPV16 VLPs in plants. We generated a chimeric protein composed of N-terminal 79 amino acid residues of RbcS as a long-transit peptide to target chloroplasts, the SUMO domain, and HPV16 L1 proteins. The chimeric gene was expressed in plants with chloroplast-targeted bdSENP1, a protein that specifically recognizes the SUMO domain and cleaves its cleavage site. This co-expression of bdSENP1 led to the release of HPV16 L1 from the chimeric proteins without any extra amino acid residues. HPV16 L1 purified by heparin chromatography formed VLPs that mimicked native virions. Moreover, the plant-produced HPV16 L1 VLPs elicited strong immune responses in mice without adjuvants. Thus, we demonstrated the cost-effective production of HPV16 VLPs in plants.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12374-023-09393-6.
Keywords: Human papillomavirus, Vaccines, Virus-like particles, Chloroplast, Cervical cancer, SUMO protein
Introduction
Cervical cancer, caused by human papillomaviruses (HPVs), is the second most common disease among adolescent women. Currently, more than 200 different genotypes of HPV have been sequenced and classified into low- and high-risk serotypes based on their ability to induce cancer. Among them, HPV16 is a high-risk strain that accounts for more incidents of cancer deaths (Zur Hausen 2002; Graham 2017; Fernandes et al. 2022). HPV16 is a non-enveloped double-stranded DNA virus, typically divided into three open reading frames (ORFs): early E1, E2, E4, E5, E6, and E7 regulatory genes, late L1 and L2 genes, and a noncoding region (NCR). The early (E1–7) genes encode the proteins required for viral replication and malignant transformation, while the L1 and L2 genes encode major and minor capsid proteins, essential for the assembly of mature virions. The complexity of the HPV16 virus life cycle enables it to evade the host's immune system and lead to infection of host cells, resulting in significant morbidity and mortality worldwide (Xia et al. 2021; Zayats et al. 2022).
Cervical cancer prevention depends on the availability of effective prophylactic vaccines against HPVs. Extensive studies on the HPV life cycle have found that the major capsid protein L1 is a promising candidate for creating a protective vaccine against HPV infection (Yousefi et al. 2021). The unique property of L1 is its assembly into virus-like particles (VLPs) without viral genomic DNA; these particles mimic the morphology of native virions (Chabeda et al. 2018). Moreover, similar to native virions, L1-based VLPs can display immunodominant epitopes. A great deal of effort has been devoted to developing vaccines against HPV infection. Two HPV vaccines, Gardasil and Cervarix, are commercially available (McNamara et al. 2016; Kombe Kombe et al. 2020). These vaccines were developed using VLPs produced in yeast and insect cells (Paavonen et al. 2007; Reisinger et al. 2007), and they have shown high efficacy in preventing cervical cancers. However, the highly sophisticated expression systems and downstream processes used to produce VLPs have increased the cost of the vaccines significantly. Therefore, these vaccines are not affordable to people in developing countries (Chen et al. 2013), despite the fact that high incidences of cervical cancers are reported in South Asia and Africa (Xia et al. 2021; Akumbom et al. 2022). Thus, the great demand to produce a cost-effective HPV vaccine is high.
Plants are alternative systems for expressing pharmaceutically important proteins (Nooraei et al. 2021), and they hold the potential to be cost-effective systems for recombinant proteins that enable production scalability. Additionally, plant-produced recombinant proteins are considered safer than those from bacteria and animal cells because they possess lower or no chance of contamination with endotoxin and animal viruses. Therefore, various target proteins have been expressed in plants and tested for their activities (Moon et al. 2019; Shanmugaraj et al. 2022). These studies have explored various organelles as a storage place of the target protein and have found that favorable organelles for recombinant protein storage are the ER and chloroplasts. These organelles have shown a high degree of stability for recombinant proteins and proper folding both of which are essential for high-level expression (Daniell et al. 2009; Scotti et al. 2013; Feng et al. 2022).
Several research groups have successfully expressed HPV16 L1 protein in chloroplasts and assembled L1 into VLPs and capsomeres (Millán et al. 2008; Pineo et al. 2013; Zahin et al. 2016; Naupu et al. 2020). These plant-produced HPV16 L1 VLPs strongly elicited neutralizing antibodies in animals (De la Rosa et al. 2009; Naupu et al. 2020). In these studies, a 59-amino-acid-encoded RbcS transit peptide (TP) was used to target the HPV16 L1 protein into the chloroplast. This TP includes only the N-terminal region of RbcS to the cleavage site. However, sequence motifs downstream from the cleavage site also play a role in the translocation efficiency of the target protein into the chloroplast (Lee et al. 2002; Lee et al. 2018). Therefore, utilizing a TP with sequence motifs that are downstream of the cleavage site can also enhance the accumulation of recombinant protein in plants (Gnanasambandam et al. 2007). However, these chloroplast-imported recombinant proteins contain some extra amino acids residues of TP that are not favorable for pharmaceutical products. On the other hand, previous reports (Kim et al. 2010; Pineo et al. 2013; Zahin et al. 2016), suggest that purified HPV16-VLPs contained contamination from host proteins, including Ribulose-1, 5-bisphosphate carboxylase/oxygenase (RuBisCO). However, it is possible to minimize these host protein contamination through a simple approach of treating total soluble proteins with phytate. Phytate molecules are electronegative and have been shown to precipitate abundant RuBisCO from plant soluble extracts in the presence of Ca2+ ion (Krishnan et al. 2009). Thus, using this approach would ensure the high-quality production of HPV16-VLPs, which is crucial for vaccine development.
In this study, we aimed to produce the target proteins without extra residues using the extended transit peptide. Previously, we showed that extra residues attached at the N-terminus of target proteins can be removed in vivo by incorporating a small ubiquitin-like modifier (SUMO) specific to a proteolytic cleavage site that is co-expressed with bdSENP1, a SUMO-specific proteolytic enzyme in plants (Islam et al. 2020; Khan et al. 2022). Here, we demonstrate that HPV16 L1 proteins without extra residues at the N-terminus can be produced in chloroplasts at a high level in N. benthamiana. We inserted the SUMO domain between a long-transit peptide of RbcS N-terminal 79 amino-acid residues from Arabidopsis and the N-terminus of HPV16 L1. Co-expression of this recombinant gene and the chloroplast-targeted bdSENP1 led to the production of L1 protein without extra amino acid residues. Moreover, we found that purified HPV16 L1 proteins assembled into VLPs and elicited a strong immune response in mice.
Results
Design of the HPV16 L1 Recombinant Construct for Expression in Chloroplasts of N. benthamiana
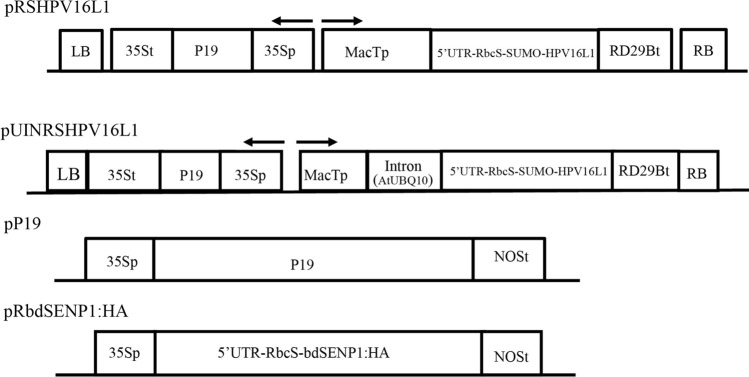
To target proteins into chloroplasts, a transit peptide is necessary. The transit peptide is cleaved off during the import process at a specific site. However, the import efficiency of a transit peptide depends not only on the sequence motifs upstream of the cleavage site but also on those downstream from the cleavage site (Jeong et al. 2021). After the transit peptide is imported into chloroplasts, the sequence motifs located downstream of the cleavage site remain attached to the mature protein. This can be a serious problem for producing pharmaceutical proteins because, in most cases, addition of extra residues to the target proteins is not allowed or is less preferable. Thus, we aimed to design a system in which chloroplast import efficiency was maximized using a long-transit peptide containing the sequence motifs downstream of the cleavage site and in which no extra residues from the transit peptide remained attached to the target protein after import into chloroplasts. For this system, we used the RbcS N-terminal region with 79 amino acid residues as a transit peptide; this region has shown a high efficiency (Supplementary Fig. 1) of protein import into chloroplasts (Lee et al. 2006). However, in this case, 25 amino acid residues downstream of the processing site after the 54 aa position, remained attached to the target protein (Fig. 1). Previous studies have shown that the N-terminal region of the HPV16 L1 is critical for the formation of virus-like particles (VLPs), and the presence of extra residues at the N-terminal region would impede the assembly of these VLPs (Chen et al. 2000). To remove the extra residues from the L1 protein, we explored the system comprising the SUMO domain and SUMO-specific protease (Frey et al. 2014). The bdSENP1 recognizes the SUMO domain and cleaves at a precise cleavage site after Gly-Gly of the SUMO domain. Thus, we generated an expression construct, RSHPV16, which consisted of RbcS79, a SUMO domain of Brachypodium distachyon, and the full-length L1 protein of HPV16. Then we added a translationally enhancing 5’ UTR to the 5’ end of the fusion construct (Kim et al. 2014). Finally, we placed the recombinant construct under the promoter of MacT. We generated MacT from Mac with a substitution of the 3’ end with T base (Song et al. 2021). In addition, to enhance the transcription level, we generated another construct by inserting the first intron of ubiquitin 10 (UBQ10) of Arabidopsis at the 5’ untranslated region. Previous studies have shown that certain introns at the 5’ region of a gene increase transcription efficiency (Rose 2004). We also generated another construct, RbcS:bdSENP1:HA, to target HA-tagged bdSENP1 to chloroplasts. Once these proteins are imported into chloroplasts, bdSENP1:HA should cleave off the SUMO domain as well as the remaining residues of transit peptide from RbcS:SUMO:HPV16L1 in vivo, thereby releasing L1 protein with no extra residues. In the same binary vector, we included CaMV35S::P19:35S-ter (in short, P19) for the co-expression of a gene-silencing suppressor P19 (Chapman et al. 2004). Moreover, we generated another P19 construct, 35S::P19:Nos-t, in an independent binary vector (Fig. 1).
Fig. 1.
Schematic diagram of HPV16 L1 protein constructs for Agrobacterium-mediated transient expression in N. benthamiana. pRSHPV16L1, pUINRSHPV16L1, pP19, and pRbdSENP1:HA binary plasmids were generated. CaMVp cauliflower mosaic virus promoter; NOSt nopaline synthase terminator; HPV human papillomavirus; RbcS rubisco small subunit transit peptide; MacTp MacT promoter; RD29Bt RD29B terminator; UBQ10 UBQ10 intron; HA hemagglutinin epitope tag
Agrobacterium-Mediated Transient Expression of Recombinant Fusion L1 Protein in N. benthamiana
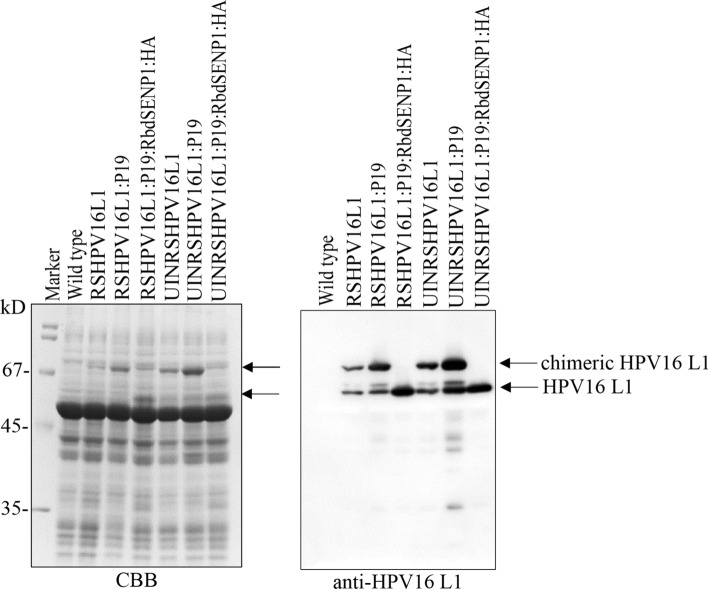
To examine the expression of the recombinant gene for HPV16 L1 in N. benthamiana, we syringe-infiltrated leaf tissues with Agrobacterium-harboring plasmids, including pRSHPV16L1 or pUINRSHPV16L1 alone and together with additional gene-silencing suppressor: P19 or P19 and pRbdSENP1:HA. We prepared the total soluble extracts from the leaf tissues harvested 4 days post infiltration (dpi) using 10% SDS-PAGE, and we analyzed them with western blot analysis using anti-HPV16L1 antibodies. Both RSHPV16L1 and UINRSHPV16L1 showed two bands at the positions of ~ 67 kD and 56 kD (Fig. 2). When RSHPV16L1 and UINRSHPV16L1 were co-expressed with P19, the intensity of the upper band at the 67 kD position was significantly increased, indicating that P19 acts as a gene-silencing suppressor for RSHPV16L1 or UINRSHPV16L1. When pRbdSENP1:HA-harboring Agrobacteria were included in the infiltration, a protein band was detected only at the position of 56 kD, indicating that the 67 kD band was processed to the 56 kD band by co-expressed RbdSENP1:HA. In addition, UINRSHPV16L1 showed a higher level of proteins than RSHPV16L1, indicating that the intron of UBQ10 at the 5’ UTR region of the recombinant gene construct led to an increase in the expression level. To further assess the expression level of these constructs, we separated the total protein extracts using 10% SDS/PAGE and stained them with CBB. We detected the protein bands of RSHPV16L1 or UINRSHPV16L1 by CBB-stained gels upon co-expression of P19; thus, our constructs for HPV16 L1 recombinant genes were expressed at high levels. Moreover, the L1 protein expressed at 4 dpi was slightly higher than that at 7 dpi (Supplementary Fig. 2).
Fig. 2.
Transient expression of the chimeric fusion genes of HPV16 L1 protein in N. benthamiana. The indicated constructs were transiently expressed in N. benthamiana by Agrobacterium-mediated infiltration. Total soluble protein extracts were prepared from leaf tissues harvested at 4 dpi and separated by 10% SDS-PAGE. The gel was stained with Coomassie brilliant blue (CBB). Alternatively, the proteins on the gel were transferred onto a PVDF membrane for western blot analysis using anti-rabbit HPV16 L1 antibodies. M molecular weight standard; the arrow indicates chimeric fusion protein and cleaved L1
Purification of HPV16 L1 Protein Using Heparin Resin
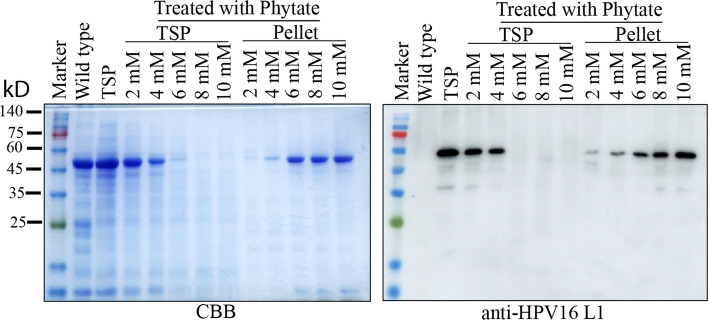
We aimed to create a protocol for purifying HPV16 L1 proteins using affinity column chromatography. A previous study showed that heparin resin can be used to purify HPV VLPs from protein extracts (Pineo et al. 2013). First, we decided to remove high-molecular-weight complexes, such as the Rbc complex, as much as possible from the total soluble protein extracts. To this end, we used sodium phytate and Ca2+ to induce aggregation of a high-molecular-weight complex. We then tested varying concentrations of sodium phytate to determine which would remove the high-molecular-weight complexes but not HPV16 L1 recombinant proteins. After we added sodium phytate and Ca2+ at varying concentrations, we centrifuged the mixture to separate the pellet and supernatant and analyzed these fractions by western blotting using anti-HPV16 L1 antibodies. At the condition of 2 mM sodium phytate and 2 mM Ca2+, the pellet contained little HPV16 L1 recombinant proteins, whereas higher sodium phytate concentrations induced co-precipitation of HPV16 L1 recombinant proteins into the pellet. Thus, we used 2 mM sodium phytate and 2 mM Ca2+ to remove Rbc complexes from the total soluble protein extracts before purifying the HPV16 L1 recombinant proteins using heparin resin (Fig. 3).
Fig. 3.
Optimization for the condition of RuBisCO removal by sodium phytate and Ca2+ treatment. Total soluble protein was extracted from 4-dpi infiltrated N. benthamiana leaves, which were treated with different concentrations of sodium phytate and calcium chloride. These clarified proteins were separated using 10% SDS-PAGE and analyzed by western blot analysis using anti-HPV16 L1 antibodies. M protein size marker; CBB Coomassie brilliant blue staining
Next, we identified a buffer condition for binding HPV16 L1 proteins to heparin resins. In particular, we changed the NaCl concentration in the buffer to optimize binding of HPV16 L1 protein to heparin, which depends on the charges of arginine residues in the protein (Kim et al. 2010). We used 0.33, 0.4, 0.5, or 0.65 M NaCl to bind HPV16 L1 proteins to heparin resins. Subsequently, we eluted HPV16 L1 proteins from heparin resin using 0.8 M NaCl. We analyzed proteins in the flow-through and eluents using SDS/PAGE followed by CBB staining or western blot analysis with anti-HPV16 L1 antibodies. The western blot analysis showed that HPV16 L1 proteins bind to heparin resin at nearly equal levels in NaCl concentrations. Thus, the binding of HPV16 L1 to heparin resin did not show any dependency on the NaCl concentration. However, the eluents contained almost no non-specific bands at 0.5 and 0.65 M NaCl conditions, whereas many non-specific proteins were contained in the eluents at 0.33 and 0.4 NaCl conditions (Supplementary Fig. 3), indicating that high concentration of NaCl reduces binding of host proteins to the resin. This result suggested that the salt concentration is critical for large-scale purification of VLPs.
After establishing the purification conditions, we purified HPV16 L1 proteins at a pilot scale. We prepared the total soluble protein extracts from leaf tissues that had been vacuum infiltrated with Agrobacteria-harboring UINRSHPV16L1, P19, or RbdSENP1:HA at 4 DPI. We then passed the protein extracts through the heparin column via gravitational force to bind the HPV16 L1 protein to heparin resin, after which we washed the heparin resin with PBS containing 300 mM NaCl. Subsequently, we eluted the HPV16 L1 proteins with 0.8 M NaCl. We measured the protein concentration of the eluents using a Bradford assay. Using SDS-PAGE, we separated 0.25 and 0.5 μg of the proteins and then performed CBB staining or western blot analysis using anti-HPV16 L1 antibodies. CBB-stained gel showed a protein band at the position of 56 kD with a weakly stained band at the position of 45 kD (Fig. 4), indicating that the HPV16 L1 proteins were purified at a high degree of purity. Moreover, the 56 kD band was detected by the anti-HPV16 L1 antibody, which confirmed the presence of HPV16 L1 protein. We estimated the yield of HPV16 L1 proteins with SDS/PAGE using BSA as reference protein. We found that the final yielded amount was approximately 6 to 8 µg/g of fresh weight.
Fig. 4.
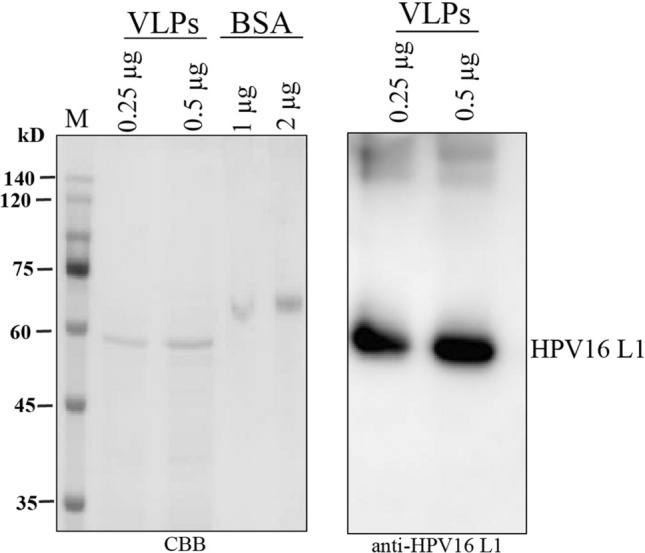
Purification of HPV16 L1 protein by heparin chromatography. The total soluble proteins were extracted from leaf tissues of N. benthamiana at 4 dpi, and HPV16 L1 protein was purified using heparin resins. Purified HPV16 L1 proteins were separated by SDS-PAGE followed by CBB staining or western blot analysis using anti-rabbit HPV16 L1 antibodies. M, protein size marker; CBB, Coomassie brilliant blue staining
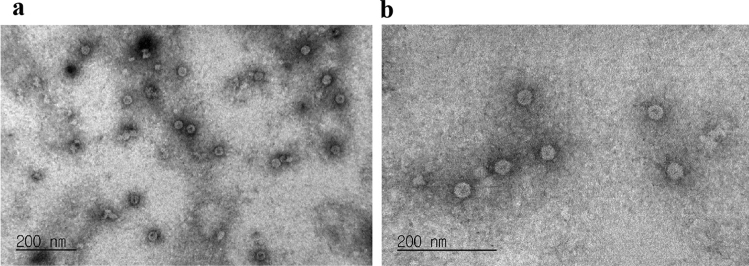
To examine whether the HPV16 L1 protein produced in chloroplasts properly assembles into VLPs, we analyzed purified HPV16 L1 proteins using electron microscopy after negative staining. The EM data showed that HPV16 L1 proteins were observed as VLPs. The diameter of the HPV16 L1 VLPs was above 30 nm with little variation (Fig. 5).
Fig. 5.
HPV16 L1 proteins produced in N. benthamiana are assembled into VLPs. Heparin-purified HPV16 L1 proteins were coated on a carbon grid and were then negatively stained with 2% uranyl acetate. The samples were examined by electron microscopy. Scale bar = 200 nm. Magnification of the image in (a) and (b) is 150,000 X and 250,000 X, respectively
HPV16 L1 VLPs Induce Strong Immune Responses in Mice upon Subcutaneous Administration
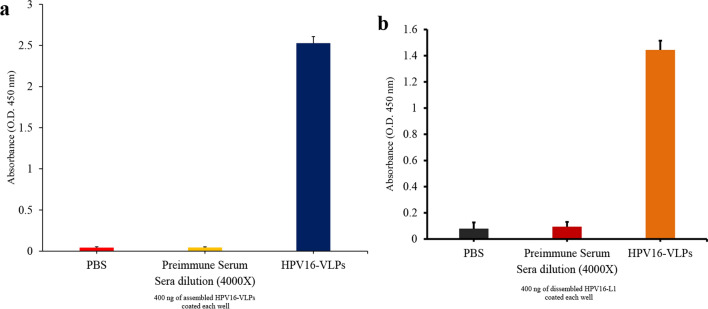
To determine whether the plant-produced HPV16 L1 VLPs elicited an immune response in mice without adjuvants, we injected 10 µg of purified HPV16 L1 VLPs subcutaneously into mice (4 mice/group) twice in a 2 week interval. As a control, we injected PBS alone. We collected sera 2 weeks after the second immunization and assessed the titer of IgG levels using ELISA with HPV16 L1 VLPs as antigens. We observed a high level of HPV16 L1-specific IgG in mice vaccinated with HPV16 L1 VLPs (Fig. 6a). Thus, plant-produced HPV16 L1 VLPs alone induced strong immune responses in mice without adjuvants. Next, we examined the same sera with HPV16 L1 VLPs that had been treated with 0.2 M NaHCO3. This treatment has been found to disassemble VLPs into monomeric proteins (McCarthy et al. 1998). The IgG titer was much lower than that of HPV16 L1 VLPs, indicating that a significant portion of IgG in the sera recognized the conformation epitopes of the VLPs of HPV16 L1 proteins (Fig. 6b). These results confirmed that plant-derived HPV16 VLPs elicited immune responses against conformation and non-conformation epitopes in mice (Fig. 6).
Fig. 6.
Plant-produced HPV16 L1 VLPs elicit strong immune response in mice. The healthy female BALB/c mice were injected subcutaneously with 10 µg of HPV16 L1 VLPs or PBS alone twice in a 2-week interval. Sera were collected at 42 dpi and used to assess the titer of IgG levels by ELISA. For ELISA, assembled (a) or 0.2 M NaHCO3-treated (b) HPV L1 VLPs were used for titration of IgG levels. Data indicate mean ± SEM (n = 4)
Discussion
In this study, we demonstrated the successful production of HPV16 L1 protein without any extra residues in chloroplasts of N. benthamiana plants. Moreover, we assembled purified L1 protein into VLPs. The plant-produced HPV L1 VLPs induced a strong immune response to both conformation and non-conformation epitopes in mice.
Previous studies have shown that HPV16 L1 proteins produced in chloroplasts of plants can assemble into VLPs (Zahin et al. 2016; Naupu et al. 2020). These studies employed a transit peptide containing amino acid residues up to the cleavage site. In our study, we used a 79-aa-residue long-transit peptide that showed maximal efficiency in chloroplast targeting. However, this long-transit peptide left a fragment of 25 aa residues of the mature protein after cleavage at the processing site in chloroplasts. Therefore, we inserted the SUMO domain in between the transit peptide and the target protein HPV16 L1 protein to remove the extra residues from the transit peptide, as well as the SUMO domain, from the HPV16 L1 protein by the co-expressed bdSENP1. This left no extra residues at the N-terminus of the target protein. In this approach, the amount of co-expressed bdSENP1 must be optimized to provide the maximal amount of HPV16 L1 protein; excess amounts of bdSENP1 lowered the amount of HPV16 L1 protein. A similar approach has been used to remove extra residues from the proteins expressed in the ER in plant cells (Islam et al. 2020; Khan et al. 2022).
Another important issue when producing recombinant proteins in plants is the production yield of the proteins. Over the past two decades, the plant-based expression system has been continually improved to produce therapeutic proteins and vaccines in lieu of conventional expression systems (Hefferon 2017). Thus, the success of the large-scale production of HPV16 L1 VLP is critically dependent on the yield. Recent studies have reported a significant improvement in the expression of HPV16 L1 assembled into VLPs. In these studies, the RNA virus-based vectors resulted in 250 mg/kg yield (Zahin et al. 2016). However, one drawback of the RNA virus-based vector is the potential to introduce mutations in the target protein due to error-prone replication of the target mRNA.
In this study, we improved the transient plant expression vector by focusing on three aspects during protein expression. The first was increasing the transcription level using the first intron of UBQ10 at the 5’ untranslated region (5’ UTR; (Rose 2004). The second was enhancing the translational efficiency using a translational enhancing 5’ UTR located immediately upstream of the AUG initiation codon (Kim et al. 2014). Finally, we maximized the targeting efficiency of protein into chloroplasts using the 79 aa residues of the RbcS N-terminal region as a transit peptide (Lee et al. 2006). We also used the P19 gene-silencing suppressor to prevent PTGS (Garabagi et al. 2012; Danielson et al. 2013; Muthamilselvan et al. 2016). These factors increased the accumulation of HPV16 L1 protein in plants; SDS/PAGE analysis showed that the HPV16 L1 protein band was visible by CBB staining (Fig. 2).
When producing recombinant proteins, the most expensive step is the purification step. Thus, a cost-effective strategy for purification must be devised. Recently, heparin-based purification has been applied to purify HPV16 L1 VLPs from yeast and plant extracts (Kim et al. 2010, 2016; Pineo et al. 2013). However, previous studies have shown that when heparin resin is used for purification HPV16 L1 VLPs from plants or yeast, host proteins are still contaminated (Kim et al. 2010; Pineo et al. 2013). We included a step to remove a high-molecular-weight complex using sodium phytate and Ca2+ from the total soluble protein extracts before applying them to the heparin resin (Krishnan and Natarajan 2009). Through this step, the majority of Rbc complexes were removed, thereby facilitating the purification of HPV16 L1 proteins. We obtained 6 to 8 μg/g (fresh weight) of purified proteins. However, the purification step was not fully optimized, and the yield of purified proteins could be increased because a significant portion of HPV16 L1 protein was detected in the flow-through fraction.
As reported by previous studies (Zahin et al. 2016; Naupu et al. 2020), our HPV16 L1 protein as VLPs was highly immunogenic in mice. Furthermore, we showed that the IgG in the sera contained two types of IgG that recognized conformation and non-conformation epitopes.
Materials and Methods
Construction of Expression Cassettes
To determine the efficiency of RbcS transit peptide for targeting foreign protein into chloroplasts, we amplified different lengths of the N-terminal region of RbcS (a small subunit of rubisco complex, accession number, AY065101) by PCR using primers PF-1 and PR-2 (1–54 amino acids), PF-1 and PR-3 (1–60 amino acid), PF-1 and PR-4 (1–70 amino acids), and PF-1 and PR-5 (1–79 amino acids; Supplemental Table 1) with RbcS-nt:GFP vector as a template (Lee et al. 2006). Amplified PCR fragments were digested with XbaI and XhoI restriction endonucleases and ligated to the p326-RbcS-nt:GFP vector, which had been digested with XbaI and SalI restriction endonucleases (Lee et al. 2006, 2015).
The full-length HPV16 L1 gene was PCR amplified using primers PF-6 and FR-7 (Supplemental Table 1). These PCR products were further digested with SmaI and XhoI restriction endonucleases and ligated to vector p326-M:CBM3:SUMO:hIL6 (Islam et al. 2019), which had been digested by NaeI and XhoI restriction endonucleases to produce p326-M:CBM3:SUMO:HPV16L1. To target HPV16 L1 to chloroplasts, we amplified the DNA fragment encoding the first 79 aa residues of RbcS using PCR with primers PF-8 and PR-9 (Supplemental Table 1). We included the nucleotide sequence of the translationally enhancing 5’ UTR in PF-8. These PCR products were digested with XbaI and XmaI restriction endonucleases and ligated to p326-M:CBM3:SUMO:HPV16L1, which had been digested with the same restriction endonucleases to produce p326-5’UTR:RbcS:SUMO:HPV16L1.
To generate a vector for expressing P19, we fused pTEX19, the 35S promoter, and the DNA fragment encoding P19 silencing suppressor protein (Tobacco bushy stunt virus, accession number: AJ288943) by overlapping PCR using primers PF-10, PR-11, PF-12, and PR-13 (Supplemental Table 1). The PCR products were digested with restriction endonucleases BsrGI and SalI and ligated to the pTEX1 vector (Song et al. 2021), which had been digested with the same restriction endonucleases to produce pTEX19. The DNA fragment of 5’UTR:RbcS:SUMO:HPV16L1 was released from p326-5’UTR:RbcS:SUMO:HPV16L1 by restriction endonucleases XbaI and XhoI and ligated into pTEX19, which had been digested with restriction endonucleases XbaI and XhoI to generate the expression vector pRSHPV16L1.
To insert the UBQ10 intron at the 5’ UTR, the MacT and UBQ10 intron sequences were fused by overlapping PCR. First, we amplified the MacT fragment by PCR using primers PF-14 and PR-15, and we amplified the DNA fragment of UBQ10 intron (accession number: AT4G05320) by PCR using primers PF-16 and PR-17 (Supplemental Table 1). These two fragments were used for overlapping PCR with primers PF-14 and PR-17 (Supplemental Table 1). The resulting PCR products were digested with restriction endonucleases Pst1 and XbaI and ligated into pTEX19, which had been digested with the same restriction endonuclease to generate pTEXIN19. Subsequently, the 5’UTR:RbcS:SUMO:HPV16L1 fragment obtained by digestion using restriction endonucleases XbaI and XhoI was ligated to pTEXIN19, which had been digested with the same restriction endonucleases to generate pUINRSHPV16L1.
To generate p326-bdSENP1:HA, the DNA fragment encoding RbcS transit peptide was amplified by PCR using primers PF-8 and PR-18 (Supplemental Table 1). The PCR products were digested with restriction endonuclease XbaI and BamHI and ligated to p326-bdSENP1:HA (Islam et al. 2020), which had been digested with the same restriction endonucleases to produce p326-RbcS:bdSENP1:HA. Subsequently, RbcS:bdSENP1:HA was obtained by digestion using restriction endonucleases XbaI and EcoRI and was ligated to pCAMBIA1300 vector to produce pCAMBIA-RbcS:bdSENP1:HA (in short, pRbdSENP1:HA).
To generate pCAMBIA-P19 (in short, pP19), we chemically synthesized the P19 silencing suppressor gene. It was digested with restriction endonucleases XbaI and XhoI and ligated to pCAMBIA1300, which had been digested with XbaI and XhoI. We confirmed the nucleotide sequences of the constructs using DNA sequencing (Macrogen, Korea).
Subcellular Localization of GFP Reporter Protein
To test the competence of different length of RbcS transit peptide for targeting GFP reporter protein to chloroplast, the plasmids were purified using Qiagen midi prep columns (cat# 12145). These purified plasmids were transformed into protoplast by polyethylene glycol-mediated transformation, as reported previously (Lee et al. 2015).
Agrobacterium-Mediated Transient Expression in N. benthamiana Plant
The chimeric fusion gene of HPV16 L1 protein was transiently expressed in leaf cells of N. benthamiana. First, these constructs were transformed into EHA105 Agrobacterium strain. Agrobacteria harboring various plasmids, pRSHPV16L1, pUINRSHPV16L1, pP19 or pRbdSENP1:HA were grown in Luria broth liquid medium at 28 ℃ for overnight. The overnight cultures of Agrobacteria were centrifuged, and the pellets were resuspended in buffer (10 mM MgCl2, 10 mM MES-K, pH 5.6 and 200 μM acetosyringone). Subsequently, 6-week plants were infiltrated with Agrobacterium mixture at different combination using needless syringe or by vacuum infiltration. Plants were further grown in a greenhouse under the 16/8 light/dark condition for 4–7 days.
Western Blot Analysis of HPV16 L1 Protein
Total soluble protein extracts were prepared from N. benthamiana plant leaves harvested at 4 dpi using buffer (50 mM Tris–HCl, pH 8, 10 mM KCl, 10 mM MgCl2, 1 mM EDTA, 20% glycerol, 0.2% Triton-X 100 and 1X protease inhibitor). The total soluble proteins were analyzed by 10% SDS-PAGE gel electrophoresis (PAGE). Subsequently, the gel was stained with Coomassie brilliant blue or transferred onto a PVDF membrane (Millipore Korea, cat# 617203). The transferred membrane was incubated with primary anti-rabbit HPV16 L1 antibody (1: 10,000) after blocking with 5% non-fat milk-solution at 4 ℃ for overnight. Then the membrane was washed three times with PBST buffer. Immediately, the PVDF membrane was probed to HRP (horseradish peroxidase)-conjugated anti-rabbit IgG antibodies (1: 5000) at room temperature for 1 h. Immunoblot signal was developed using ECL solution (Amersham Pharmacia Biotech, Buckinghamshire, UK), and images were capture by LAS 4000 (FUJIFILM, Tokyo, Japan).
Purification of HPV16 L1 Using Heparin Column
First, we optimized the NaCl concentration for binding of HPV16 L1 to heparin resin. Total soluble protein extracts were treated with 2 mM phytate and 2 mM CaCl2. The mixture was immediately centrifuged for 13,000 g for 30 min. and the supernatant was collected separately. To optimize the NaCl concentration for binding of HPV16 L1 to heparin resin, the NaCl concentration was adjusted to final salt concentration to 0.33 M, 0.4, 0.5 or 0.65 M. These total soluble protein extracts were then applied to a column containing 12.5 mg heparin resin (POROS™ 50 HE Heparin Affinity Resin, cat# 4333410) and allowed to pass through by gravitational force. Subsequently, heparin resin were washed thoroughly with PBS with 300 mM NaCl buffer (20 mL). Fractions were eluted using PBS buffer supplemented with 0.8 M NaCl. The eluted fractions containing HPV16 L1 were concentrated using10-kD centricon (Millipore Korea, cat# UFC901024D). During this process, the elution buffer was changed with PBS buffer containing 20% glycerol and the concentrated proteins were stored at − 20 ℃ for further analysis.
To purify HPV16 L1 at a large scale, we prepared N. benthamiana leaf tissues expressing HPV16 L1 by vacuum infiltration. Leaf tissues (20 g) were harvested at 4 dpi and ground under liquid nitrogen. The extraction buffer (50 mM Tris-pH 7.2, 200 mM NaCl, and 0.2% Triton X-100) was added to the powder at 1:5 ratio of weight to volume, and homogenized in a shaker for 10 min. The homogenate was treated with 2 mM phytate and 2 mM CaCl2 after filtrated through three-layer of gauge-cloth. The mixture was immediately centrifuged for 13,000 g for 30 min. The supernatant was then collected, and NaCl concentration was adjusted to 500 mM. Protein samples were applied to heparin resin. The resin was washed with PBS containing 300 mM NaCl. Proteins were eluted from the resin using PBS solution containing 0.8 M NaCl. The purified protein was quantified by CBB staining of a gel after PAGE using BSA as a standard protein. The band intensity was measured using Multi Gauge V3.0 Software.
Morphology Analysis by Transmission Electron Microscopy
To examine the self-assembly of HPV16 L1 into VLPs, the grids were prepared using purified HPV16 L1 proteins. The grids were washed with PBS buffer and immediately subjected to negative staining with 2% uranyl acetate. The grids were examined under an electron microscope (BIO TEM JEM-1011, Jeol).
Immunization of Mice
The BLAB/C female mice were maintained under pathogen-free conditions. All experiments were performed in accordance with the guideline of the institutional animal care committee of POSTECH. The 6–8 weeks old female BLAB/C mice were divided to two group having four mice each. Then mice were subcutaneously injected with 10 μg of plant-derived HPV16 L1 VLPs or PBS alone twice in a 2-week interval. Pre-sera were collected at 7 day prior to vaccination. The sera were collected at day 42 from the first immunization.
Enzyme-Linked Immunosorbent Assay (ELISA)
To assess the titer of IgG in mice immunized with HPV16 L1 protein, we coated 400 ng of purified HPV16 L1 or 0.2 M NaHCO3-treated HPV16 L1 VLPs (disassembled HPV16 L1) onto the surface of a 96-well microtiter plate by overnight incubation at 4 ℃. Then we added 200 µL of blocking solution (5% non-fat dry milk in PBS) to the wells and incubated them at room temperature for 1 h, after which we washed them three times with PBST buffer (PBS containing 0.1% Tween-20). Subsequently, we added pre-immune sera, sera from mice immunized with HPV16 L1 VLPs (1: 4000), or PBS alone to the wells of the plate and incubated them in a microtiter plate shaker at room temperature for 2 h. We washed each plate three times with PBST and reacted it with the HRP-conjugated anti-mouse IgG secondary antibodies (Invitrogen, Cat# 32430, 1: 5000) for 2 h. Next, we added 50 µL TMB substrate (Thermo Fisher, Cat# 34025) and shook the mixture for a few minutes. We stopped the reaction by mixing it with 50 µL 0.18 M sulfuric acid, and signal intensity was read at 450 nm using an ELISA reader.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 The RbcS N-terminal transit peptide sequence downstream of the processing site also contributes to the efficiency of protein import into chloroplasts. The different lengths (54, 60, 70, and 79 amino acids) of the RbcS N-terminal region were N-terminally fused to GFP. These constructs were introduced into protoplasts and examined for their targeting efficiency into chloroplasts. Total protein extracts from the protoplasts were analyzed by western blotting using anti-mouse-GFP antibodies (JPG 282 KB)
Supplementary file2 Expression of chimeric fusion proteins of HPV16 L1 in N. benthamiana plant leaves. Total soluble protein extracts from leaf tissues of N. benthamiana at 4 and 7 dpi were separated by SDS-PAGE followed by CBB staining or western blot analysis using anti-HPV16 L1 antibodies. M protein size marker; CBB Coomassie brilliant blue staining; *, the chimeric fusion or cleaved HPV16 L1 protein (JPG 447 KB)
Supplementary file3 Optimization of salt concentration for HPV16 L1-binding to heparin resin. Total soluble protein extracts were adjusted to 0.33, 0.4, 0.5 and 0.65 M NaCl for final concentration and examined for binding HPV16 L1 proteins to heparin resins. Proteins bound to heparin resins were eluted with PBS containing 0.8 M NaCl. Proteins in the flow-through and eluents were separated by SDS-PAGE followed by CBB staining or western blot analysis using anti-HPV16 L1 antibodies. M protein size marker; lane 1, total soluble protein (TSP); lane 2, TSP treated with 2 mM phytate and 2 mM CaCl2; lanes 3–6, flow-through fractions of protein extracts containing 0.33, 0.4, 0.5 and 0.65 M NaCl; Lane 7-10, eluted proteins from total extracts containing 0.33, 0.4, 0.5, and 0.65 M NaCl; CBB coomassie brilliant blue; M standard protein marker (JPG 174 KB)
Acknowledgements
We thank Prof. Hong-Jin Kim for rabbit anti-sera and Dr. Hyoung Jin Kim for valuable suggestion. This work was supported by Korea Research Fellowship Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (grant number- 2016H1D3A1938045).
Data availability
The patant of HPV16 has been filed and yet to be published. Therefore, all data will be provided on request.
Declarations
Conflict of Interest
The patent has been filed.
References
- Akumbom AM, Lee JJ, Reynolds NR, Thayer W, Wang J, Slade E. Cost and effectiveness of HPV vaccine delivery strategies: a systematic review. Prev Med Rep. 2022;26:101734. doi: 10.1016/j.pmedr.2022.101734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabeda A, Yanez RJR, Lamprecht R, Meyers AE, Rybicki EP, Hitzeroth II. Therapeutic vaccines for high-risk HPV-associated diseases. Papillomavirus Res. 2018;5:46–58. doi: 10.1016/j.pvr.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman EJ, Prokhnevsky AI, Gopinath K, Dolja VV, Carrington JC. Viral RNA silencing suppressors inhibit the microRNA pathway at an intermediate step. Genes Dev. 2004;18(10):1179–1186. doi: 10.1101/gad.1201204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Lai H. Plant-derived virus-like particles as vaccines. Hum Vaccin Immunother. 2013;9(1):26–49. doi: 10.4161/hv.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XS, Garcea RL, Goldberg I, Casini G, Harrison SC. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol Cell. 2000;5(3):557–567. doi: 10.1016/S1097-2765(00)80449-9. [DOI] [PubMed] [Google Scholar]
- Daniell H, Singh ND, Mason H, Streatfield SJ. Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci. 2009;14(12):669–679. doi: 10.1016/j.tplants.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson DC, Pezacki JP. Studying the RNA silencing pathway with the p19 protein. FEBS Lett. 2013;587(8):1198–1205. doi: 10.1016/j.febslet.2013.01.036. [DOI] [PubMed] [Google Scholar]
- De la Rosa GP, Monroy-García A, de Lourdes M-G, Peña CGR, Hernández-Montes J, Weiss-Steider B, Lim MAG. An HPV 16 L1-based chimeric human papilloma virus-like particles containing a string of epitopes produced in plants is able to elicit humoral and cytotoxic T-cell activity in mice. Virol J. 2009;6(1):2. doi: 10.1186/1743-422X-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Li X, Fan B, Zhu C, Chen Z. Maximizing the production of recombinant proteins in plants: from transcription to protein stability. Int J Mol Sci. 2022;23(21):13516. doi: 10.3390/ijms232113516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes A, Viveros-Carreno D, Hoegl J, Avila M, Pareja R. Human papillomavirus-independent cervical cancer. Int J Gynecol Cancer. 2022;32(1):1–7. doi: 10.1136/ijgc-2021-003014. [DOI] [PubMed] [Google Scholar]
- Frey S, Gorlich D. A new set of highly efficient, tag-cleaving proteases for purifying recombinant proteins. J Chromatogr A. 2014;1337:95–105. doi: 10.1016/j.chroma.2014.02.029. [DOI] [PubMed] [Google Scholar]
- Garabagi F, Gilbert E, Loos A, McLean MD, Hall JC. Utility of the P19 suppressor of gene-silencing protein for production of therapeutic antibodies in Nicotiana expression hosts. Plant Biotechnol J. 2012;10(9):1118–1128. doi: 10.1111/j.1467-7652.2012.00742.x. [DOI] [PubMed] [Google Scholar]
- Gnanasambandam A, Polkinghorne IG, Birch RG. Heterologous signals allow efficient targeting of a nuclear-encoded fusion protein to plastids and endoplasmic reticulum in diverse plant species. Plant Biotechnol J. 2007;5(2):290–296. doi: 10.1111/j.1467-7652.2007.00241.x. [DOI] [PubMed] [Google Scholar]
- Graham SV. The human papillomavirus replication cycle, and its links to cancer progression: a comprehensive review. Clin Sci (lond) 2017;131(17):2201–2221. doi: 10.1042/CS20160786. [DOI] [PubMed] [Google Scholar]
- Hefferon K. Reconceptualizing cancer immunotherapy based on plant production systems. Future Sci OA. 2017;3(3):FSO217. doi: 10.4155/fsoa-2017-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MR, Kwak JW, Lee JS, Hong SW, Khan MRI, Lee Y, Lee Y, Lee SW, Hwang I. Cost-effective production of tag-less recombinant protein in Nicotiana benthamiana. Plant Biotechnol J. 2019;17(6):1094–1105. doi: 10.1111/pbi.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MR, Choi S, Muthamilselvan T, Shin K, Hwang I. In vivo removal of N-terminal fusion domains from recombinant target proteins produced in Nicotiana benthamiana. Front Plant Sci. 2020;11:440. doi: 10.3389/fpls.2020.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J, Hwang I, Lee DW. Functional organization of sequence motifs in diverse transit peptides of chloroplast proteins. Front Physiol. 2021;12:795156. doi: 10.3389/fphys.2021.795156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MRI, Thangarasu M, Kang H, Hwang I. Plant produced endotoxin binding recombinant proteins effectively remove endotoxins from protein samples. Sci Rep. 2022;12(1):16377. doi: 10.1038/s41598-022-20776-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Kim SY, Lim SJ, Kim JY, Lee SJ, Kim HJ. One-step chromatographic purification of human papillomavirus type 16 L1 protein from Saccharomyces cerevisiae. Protein Expr Purif. 2010;70(1):68–74. doi: 10.1016/j.pep.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Kim Y, Lee G, Jeon E, Sohn EJ, Lee Y, Kang H, Lee DW, Kim DH, Hwang I. The immediate upstream region of the 5'-UTR from the AUG start codon has a pronounced effect on the translational efficiency in Arabidopsis thaliana. Nucleic Acids Res. 2014;42(1):485–498. doi: 10.1093/nar/gkt864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Kwag HL, Kim HJ. Characterization of human papillomavirus type 16 pseudovirus containing histones. BMC Biotechnol. 2016;16(1):63. doi: 10.1186/s12896-016-0296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombe Kombe AJ, Li B, Zahid A, Mengist HM, Bounda GA, Zhou Y, Jin T. Epidemiology and burden of human papillomavirus and related diseases, molecular pathogenesis, and vaccine evaluation. Front Public Health. 2020;8:552028. doi: 10.3389/fpubh.2020.552028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan HB, Natarajan SS. A rapid method for depletion of Rubisco from soybean (Glycine max) leaf for proteomic analysis of lower abundance proteins. Phytochemistry. 2009;70(17–18):1958–1964. doi: 10.1016/j.phytochem.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Lee DW, Hwang I. Evolution and design principles of the diverse chloroplast transit peptides. Mol Cells. 2018;41(3):161–167. doi: 10.14348/molcells.2018.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Kim DH, Lee SW, Kim ZH, Hwang I. In vivo import experiments in protoplasts reveal the importance of the overall context but not specific amino acid residues of the transit peptide during import into chloroplasts. Mol Cells. 2002;14(3):388–397. [PubMed] [Google Scholar]
- Lee DW, Lee S, Lee GJ, Lee KH, Kim S, Cheong GW, Hwang I. Functional characterization of sequence motifs in the transit peptide of Arabidopsis small subunit of rubisco. Plant Physiol. 2006;140(2):466–483. doi: 10.1104/pp.105.074575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Woo S, Geem KR, Hwang I. Sequence motifs in transit peptides act as independent functional units and can be transferred to new sequence contexts. Plant Physiol. 2015;169(1):471–484. doi: 10.1104/pp.15.00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MP, White WI, Palmer-Hill F, Koenig S, Suzich JA. Quantitative disassembly and reassembly of human papillomavirus type 11 virus like particles in vitro. J Virol. 1998;72(1):32–41. doi: 10.1128/JVI.72.1.32-41.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara M, Batur P, Walsh JME, Johnson KM. HPV update: vaccination, screening, and associated disease. J Gen Intern Med. 2016;31(11):1360–1366. doi: 10.1007/s11606-016-3725-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millán FS, Ortigosa SM, Hervás-Stubbs S, Corral-Martínez P, Seguí-Simarro JM, Gaétan J, Coursaget P, Veramendi J. Human papillomavirus L1 protein expressed in tobacco chloroplasts self-assembles into virus-like particles that are highly immunogenic. Plant Biotechnol J. 2008;6(5):427–441. doi: 10.1111/j.1467-7652.2008.00338.x. [DOI] [PubMed] [Google Scholar]
- Moon KB, Park JS, Park YI, Song IJ, Lee HJ, Cho HS, Jeon JH, Kim HS. Development of systems for the production of plant-derived biopharmaceuticals. Plants (basel) 2019;9(1):30. doi: 10.3390/plants9010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthamilselvan T, Lee CW, Cho YH, Wu FC, Hu CC, Liang YC, Lin NS, Hsu YH. A transgenic plant cell-suspension system for expression of epitopes on chimeric Bamboo mosaic virus particles. Plant Biotechnol J. 2016;14(1):231–239. doi: 10.1111/pbi.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naupu PN, van Zyl AR, Rybicki EP, Hitzeroth II. Immunogenicity of plant-produced human papillomavirus (HPV) virus-like particles (VLPs) Vaccines (basel) 2020;8(4):740. doi: 10.3390/vaccines8040740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooraei S, Bahrulolum H, Hoseini ZS, Katalani C, Hajizade A, Easton AJ, Ahmadian G. Virus-like particles: preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J Nanobiotechnology. 2021;19(1):59. doi: 10.1186/s12951-021-00806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter DL, Kitchener HC, Castellsague X, de Carvalho NS, Skinner SR, Harper DM, Hedrick JA, Jaisamrarn U, Limson GA, Dionne M, Quint W, Spiessens B, Peeters P, Struyf F, Wieting SL, Lehtinen MO, Dubin G, Group HPs Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369(9580):2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- Pineo CB, Hitzeroth II, Rybicki EP. Immunogenic assessment of plant-produced human papillomavirus type 16 L1/L2 chimaeras. Plant Biotechnol J. 2013;11(8):964–975. doi: 10.1111/pbi.12089. [DOI] [PubMed] [Google Scholar]
- Reisinger KS, Block SL, Lazcano-Ponce E, Samakoses R, Esser MT, Erick J, Puchalski D, Giacoletti KE, Sings HL, Lukac S, Alvarez FB, Barr E. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J. 2007;26(3):201–209. doi: 10.1097/01.inf.0000253970.29190.5a. [DOI] [PubMed] [Google Scholar]
- Rose AB. The effect of intron location on intron-mediated enhancement of gene expression in Arabidopsis. Plant J. 2004;40(5):744–751. doi: 10.1111/j.1365-313X.2004.02247.x. [DOI] [PubMed] [Google Scholar]
- Scotti N, Rybicki EP. Virus-like particles produced in plants as potential vaccines. Expert Rev Vaccines. 2013;12(2):211–224. doi: 10.1586/erv.12.147. [DOI] [PubMed] [Google Scholar]
- Shanmugaraj B, Malla A, Bulaon CJI, Phoolcharoen W, Phoolcharoen N (2022) Harnessing the potential of plant expression system towards the production of vaccines for the prevention of human papillomavirus and cervical cancer. Vaccines 10(12):2064 [DOI] [PMC free article] [PubMed]
- Song SJ, Shin GI, Noh J, Lee J, Kim DH, Ryu G, Ahn G, Jeon H, Diao HP, Park Y, Kim MG, Kim WY, Kim YJ, Sohn EJ, Song CS, Hwang I. Plant-based, adjuvant-free, potent multivalent vaccines for avian influenza virus via Lactococcus surface display. J Integr Plant Biol. 2021;63(8):1505–1520. doi: 10.1111/jipb.13141. [DOI] [PubMed] [Google Scholar]
- Xia C, Li S, Long T, Chen Z, Chan PKS, Boon SS (2021) Current updates on cancer-causing types of human Papillomaviruses (HPVs) in east, southeast, and south Asia. Cancers (Basel) 13(11):2691 [DOI] [PMC free article] [PubMed]
- Yousefi Z, Aria H, Ghaedrahmati F, Bakhtiari T, Azizi M, Bastan R, Hosseini R, Eskandari N. An update on human papilloma virus vaccines: history, types, protection, and efficacy. Front Immunol. 2021;12:805695. doi: 10.3389/fimmu.2021.805695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahin M, Joh J, Khanal S, Husk A, Mason H, Warzecha H, Ghim SJ, Miller DM, Matoba N, Jenson AB. Scalable production of HPV16 L1 protein and VLPs from tobacco leaves. PLoS ONE. 2016;11(8):e0160995. doi: 10.1371/journal.pone.0160995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayats R, Murooka TT, McKinnon LR. HPV and the risk of HIV acquisition in women. Front Cell Infect Microbiol. 2022;12:814948. doi: 10.3389/fcimb.2022.814948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 The RbcS N-terminal transit peptide sequence downstream of the processing site also contributes to the efficiency of protein import into chloroplasts. The different lengths (54, 60, 70, and 79 amino acids) of the RbcS N-terminal region were N-terminally fused to GFP. These constructs were introduced into protoplasts and examined for their targeting efficiency into chloroplasts. Total protein extracts from the protoplasts were analyzed by western blotting using anti-mouse-GFP antibodies (JPG 282 KB)
Supplementary file2 Expression of chimeric fusion proteins of HPV16 L1 in N. benthamiana plant leaves. Total soluble protein extracts from leaf tissues of N. benthamiana at 4 and 7 dpi were separated by SDS-PAGE followed by CBB staining or western blot analysis using anti-HPV16 L1 antibodies. M protein size marker; CBB Coomassie brilliant blue staining; *, the chimeric fusion or cleaved HPV16 L1 protein (JPG 447 KB)
Supplementary file3 Optimization of salt concentration for HPV16 L1-binding to heparin resin. Total soluble protein extracts were adjusted to 0.33, 0.4, 0.5 and 0.65 M NaCl for final concentration and examined for binding HPV16 L1 proteins to heparin resins. Proteins bound to heparin resins were eluted with PBS containing 0.8 M NaCl. Proteins in the flow-through and eluents were separated by SDS-PAGE followed by CBB staining or western blot analysis using anti-HPV16 L1 antibodies. M protein size marker; lane 1, total soluble protein (TSP); lane 2, TSP treated with 2 mM phytate and 2 mM CaCl2; lanes 3–6, flow-through fractions of protein extracts containing 0.33, 0.4, 0.5 and 0.65 M NaCl; Lane 7-10, eluted proteins from total extracts containing 0.33, 0.4, 0.5, and 0.65 M NaCl; CBB coomassie brilliant blue; M standard protein marker (JPG 174 KB)
Data Availability Statement
The patant of HPV16 has been filed and yet to be published. Therefore, all data will be provided on request.