Abstract
Skin aging goes beyond a chronological process and also results from extrinsic factors referred to as the exposome. Hyaluronic acid (HA) is an important component of the extracellular matrix, with loss starting at 25 years old. While many studies of HA concern topical use, few literature reviews only address the use of topical HA in dermatology. This review describes the different characteristics of HA‐containing cosmeceuticals, with a focus on skin aging and the impact of exposome factors on HA synthesis and degradation. A review was performed using the terms HA, hyaluronan, topical, dermatology, cosmetic, aging treatment, exposome, and cosmeceuticals. Results are also presented from a recent randomized controlled trial (RCT), which investigated the additional benefit of using a HA epidermic filler (HA‐filler serum) combined with Botulinum toxin type A (BoNTA) to treat signs of skin aging. Subjects were randomized to two groups: HA‐filler serum starting 24 h after the BoNTA injection then twice daily for 24 weeks, or the control group, which received BoNTA. HA is a key ingredient used in cosmeceuticals for its hydration/antiaging properties (hygroscopic, rheological, and viscoelastic). Several clinical studies indicate that HA is both well tolerated and effective, adjuvant to both post‐surgical and facial rejuvenation procedures. In the RCT, one of few studies to combine BoNTA and HA with a 6‐month follow‐up, the HA‐filler serum lengthened the duration of BoNTA's effect in reducing wrinkles. Numerous studies support HA‐based cosmeceuticals as a noninvasive, effective solution for improving skin hydration and rejuvenation.
Keywords: cosmeceutical, dermatology, hyaluronic acid, skin, topical application
1. INTRODUCTION
Recent advances in the understanding of skin aging indicate that it is not only due to a chronological process, but also due to the result of multiple extrinsic factors. 1 These factors are collectively referred to as the “exposome,” a term originally proposed in the field of oncology research in 2005 by Professor Wild in order to draw as much attention to it as the genome deserves. 2 The exposome overlaps with external factors affecting perceived age, 3 yet this extends beyond the aesthetic level. One or several of these extrinsic factors, from environment and lifestyle to chronic diseases, can accelerate the aging process and should be assessed by dermatologists and other clinicians caring for patients who may be concerned about skin aging, and how to minimize or delay it. 4 , 5 , 6
Staying informed about evidence‐based treatments is essential for the clinician, however this may be challenging as many individuals often turn to antiaging cosmetics that are not necessarily supported by rigorous studies. “Cosmeceuticals,” on the other hand, provide effects beyond mere cosmetic enhancement. According to Dr. Kligman, the instigator of this term in 1984 when he experimented on the antiaging effects of tretinoin, the performance of cosmeceuticals can suggest pharmaceutical action. 7
The main molecule involved in skin moisture is hyaluronic acid (HA), and loss of skin moisture as HA shifts to deeper layers is implicated in skin aging. 8 Studies with HA drew interest when the first clinical application of HA was developed during the 1970s and 1980s for use in ophthalmic surgery. Since then, the applicability of HA in dermatology has increased significantly due to its hygroscopic, rheological, and viscoelastic properties. HA has been developed into filler injections as well as included in cosmeceuticals for topical use. 9
Although the literature highlights HA fillers as the most frequent procedure used to improve the appearance of aging skin, not all patients are ready to start injectables. Indeed, topical HA may provide complementary benefits. Several authors contend that topically applied HA‐based cosmeceuticals have their place in the antiaging armamentarium of clinicians, not only to improve skin moisturization, but also skin aging signs and elasticity. 8 , 10 , 11 However, few comprehensive reviews have addressed the use of topical HA in dermatology.
In this review, we describe the different applications of HA‐containing cosmeceuticals, with a focus on skin aging and the impact of exposome factors on HA synthesis and degradation.
2. METHODS
2.1. Literature search
A literature review was conducted of the PubMed, Google Scholar, and Cochrane databases for English literature among adults (from 1992 through July 2021) using the terms HA, hyaluronan, topical, dermatology, cosmetic, aging treatment, exposome, and cosmeceuticals. Injectable HA was excluded from the search. Starting with biochemistry, the subsequent sections outline clinical study results of topical HA, from its versatile role in dermatology to both preventing harm from exposome factors and improving postprocedural outcomes.
2.2. Clinical study
A prospective, single‐blind, 24‐week RCT investigated the additional benefit of using a HA epidermic filler (Liftactiv HA epidermic filler [HA‐filler serum], Vichy, Paris, France) combined with BoNTA in the treatment of facial skin aging. 12 The HA‐filler serum, with 1.5% multimolecular weight HA, contains ingredients including Vitamin C glucoside (Cg), peptides, and volcanic mineralizing water that are intended to provide antioxidant and anti‐aging effects as well as strengthen the skin barrier. 12 , 13 Subjects were randomized to two groups: HA‐filler serum starting 24 h after the botulinum toxin injection followed by twice daily, or the control group, which received BoNTA. Both treatment groups received SPF 50+ sunscreen for use throughout the study.
Evaluations were performed before the BoNTA injection at day 0, then once at days 14, 84, and 168. Deep wrinkles, fine lines, Crow's feet wrinkles, skin tone, skin texture, radiance, and skin elasticity were assessed by a dermatologist, and safety (i.e., tolerability and any adverse event) was monitored throughout the study. Instrumental evaluations were carried out to investigate skin barrier integrity using Tewameter® readings. Subject satisfaction questionnaires were also performed regarding the HA‐filler serum. Subjects signed informed consent for participation.
3. RESULTS
3.1. The biochemistry of HA
Photoaging affects all skin compartments, and although the majority of skin HA is found in the dermal layer, it can also be found in the epidermis. 14 This is interesting in terms of maintaining the stratum corneum structure and epidermal barrier function. 8 The epidermis consists of keratinocytes forming the stratum corneum, with melanocytes interspersed in the basal layer. The dermis predominantly comprises the extracellular matrix, which is made up of collagen and elastic fibers, proteoglycans, and glycosaminoglycans (GAGs). 15 , 16
GAGs are large linear polysaccharides and are a major component of the extracellular matrix. There are six types of GAGs: chondroitin sulfate, dermatan sulfate, keratan sulfate, heparan sulfate, heparin, and HA. 16 Unlike other GAGs, HA is nonsulfated and occurs in a vast number of configurations and shapes, depending on size, salt concentration, pH, and associated cations. 8 , 9
In mammals, HA is synthesized by three types of HA synthases (HAS): HAS1, HAS2, and HAS3. HAS1 and HAS2 proteins exert moderate activity and form high molecular weight HA (HMW‐HA; around 600–1200 daltons [kDa]), while the HAS3 protein possesses the highest activity and polymerizes into low molecular weight HA (LMW‐HA; around 5–50 kDa). HA has a half‐life of less than 1 day in the skin, and is degraded by hyaluronidases and can also be degraded via non‐enzymatic reactions such as acid/alkaline hydrolysis and oxidant decomposition (i.e., free radicals). 8 , 9
Despite its simple structure, HA has many biological functions that depend on size and result from their interaction with certain binding proteins (“hyaladerins”) and surface receptors. HA binds to extracellular matrix molecules and cell surface receptors, thereby regulating cellular behavior via control of the tissue's macro‐ and microenvironments. HA can bind to three main classes of cell surface receptors: (1) CD44 (a membrane glycoprotein), (2) receptor for hyaluronate‐mediated motility (RHAMM), and (3) intercellular adhesion molecule 1 (ICAM‐1), which perform different functions. CD44 is the most widely distributed cell surface receptor recognized for HA binding, and regulates cell adhesion, migration, lymphocyte activation and homing, and cancer metastasis. Two vital roles of CD44 in skin are projected: the regulation of keratinocyte proliferation in response to change in stimuli, and the maintenance of native HA. The interactions of HA with RHAMM control cell growth and migration by a complex network of signal transduction events and interactions with the cytoskeleton. 8 , 9
Transforming growth factor (TGF)‐β1 stimulates cell motility, elicits the synthesis and expression of RHAMM and HA, and thereby initiates cell locomotion. ICAM‐1 is considered to be a metabolic cell surface receptor for HA. This supramolecule could also be responsible for the clearance of HA from body fluid and plasma that accounts for most of its turnover in the whole body. In addition, ICAM‐1 may function as a cell adhesion molecule, and the binding of HA to ICAM‐1 may therefore contribute to regulating ICAM‐1‐mediated inflammatory activation. 8 , 9
Commercially available HA can be isolated from animal sources or bacterial fermentation; and can be applied in many indications and pharmaceutical forms. HA has a good biocompatibility because its molecular structure is similar between different species, resulting in its biodegradable characteristics. 11
3.2. High versus low molecular weight topical HA
The size of HA appears to be of critical importance because the permeability of HA is predominantly related to its molecular weight. HMW‐HA has very limited permeability through the skin and primarily stays on the skin's surface, forming a thin protective hydration layer, while LMW‐HA can permeate the stratum corneum, epidermis, and deeper dermal layers (Figure 1). 8 , 9 , 11 Indeed, Raman spectroscopy, which is molecularly specific in evaluating the permeation of actives, has demonstrated that LMW‐HA (20–300 kDa) diffuses past the stratum corneum. 17
FIGURE 1.
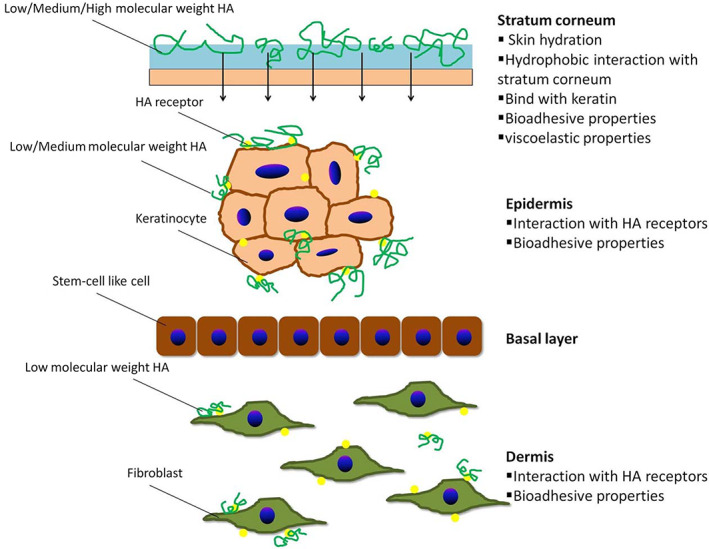
Skin permeability of hyaluronic acid with various molecular weights. Printed from reference 11 with permission from Elsevier
HMW‐HA is usually added in cosmetic formulations to increase formulation viscosity and improve the stability of composition film when applied on skin. In this way, HMW‐HA has a positive effect on hydration of upper epidermis layers, which translates into a lower transepidermal water loss (TEWL). 18
3.3. Hydrating
Hydration of the skin is an important indicator of the maintenance of a proper skin barrier both in aesthetic dermatology and in pathological skin diseases. Hydration of the skin critically depends on HA‐bound water in the dermis and in the vital area of the epidermis, while maintenance of hydration essentially depends on the stratum granulosum. 8
HA is a hygroscopic molecule with the ability to bind 1000 times its volume in water. Due to this exceptionally strong water absorption property, HA is able to hydrate both the stratum corneum and the dermis. It has typically been classified as a humectant moisturizer, since it draws water from the dermis to epidermis. 8 , 9 , 11 HA in the dermis regulates water balance, osmotic pressure, and ion flow, and functions as a sieve, excluding certain molecules, enhancing the extracellular domain of cell surfaces, and stabilizing skin structures by electrostatic interactions. Dermal fibroblasts provide the synthetic machinery for dermal HA and are considered to be the target of pharmacologic attempts to enhance skin hydration.
Unfortunately, exogenous HA is cleared from the dermis and is rapidly degraded. 8 Even though the mechanism of skin aging is not yet fully elucidated, it has been described that the most dramatic histochemical change observed is the marked disappearance of HA at the epidermal level as HA shifts to the dermis. 8 Thus, the epidermis loses the principle molecule responsible for binding and retaining water molecules, resulting in loss of skin moisture.
Even in the dermis, variations of HA due to intrinsic aging may explain the observed decrease in skin turgor and microvessel support, and presence of wrinkling and modified elasticity. 19 Furthermore, intrinsic aging involves other phenomena such as the degraded and even absent connection between collagen and elastic fibers. 19
3.4. Skin rejuvenating
Maintaining a youthful and pleasant appearance of the face in today's culture impacts the quality of life for many patients. Facial aging is a complex and dynamic process. All people age differently as a result of imbalance, lack of harmony, and disproportion of the aging process between the overlying skin and subcutaneous soft tissues as well as the underlying bony frameworks.
In the dermis, for intrinsically aged skin, HA binding proteins (HABPs) are reduced compared with young skin, while the level of HA itself is not significantly different. HABPs are known to trigger several intracellular signaling pathways regulating proliferation, migration, and differentiation. In contrast, dermal HA content in photoaged skin is significantly increased, particularly in regions of solar elastosis. Although UV irradiation induces HA synthase activation, HAS mRNA levels in aged sun‐exposed skin are significantly reduced compared to those in sun‐protected skin. Similar to solar elastosis, increased HA in photoaged skin might be the result of abnormal accumulation of nonfunctional proteins. 8 , 16 , 20 Increased dermal HA may be a recovery response in order to compensate for the inflicted damage. All of the above age‐related phenomena contribute to the apparent dehydration, atrophy, and loss of elasticity that characterizes aged skin.
3.5. Healing
HA possesses healing properties and thus is an important tool in the treatment of acute wounds such as burns and diabetic foot. 21 , 22 Postsurgical scars may also benefit from the healing properties (e.g., faster wound closure) of topical HA, as shown in a RCT among 21 volunteers (the majority female) which evaluated different skin repair creams by investigating the reliability of laser‐induced wound induction. 23
3.6. Impact of exposome factors on HA
As previously mentioned, the exposome involves multiple intrinsic and extrinsic factors (e.g., UV light, infrared and radiation exposure, air pollution, smoking, tanning beds, sun exposure, alcohol and drugs, stress, and poor diet) as well as acute stressors that can negatively affect skin functions and lead to cell damage (Figure 2). 24 , 25 HA can be degraded by these factors due to oxidative stress, therefore HA‐containing products counteract this by restoring HA loss.
FIGURE 2.
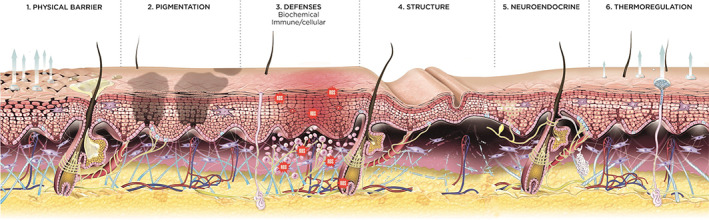
Skin functions affected by acute stressors. Skin functions affected by acute stressors: (1) skin physical barrier (stratum corneum), (2) melanocytes and interacting keratinocytes involved in skin pigmentation, (3) skin biochemical immune/cellular defenses, (4) skin structure including the extracellular matrix and adnexa, (5) skin neuroendocrine delivery, and (6) the thermoregulation function. Used under the terms of the Creative Commons Attribution‐Non‐Commercial License from reference 25
A prospective study revealed that oxidative stress may affect women over 40 years old similarly, regardless of age, possibly due to lifestyle changes made by older women such as decreased alcohol/smoking. 26 A study from Harvard Medical School found shorter telomere length, a sign of cellular aging, to be related to modifiable factors including trunk fat and lack of physical activity. 27
In addition to behavior change, formulations including topical antioxidants, such as vitamin C, vitamin E, carotenoids, or polyphenols, or the stimulation of natural antioxidant pathways can reduce reactive oxygen species and protect against both environmental toxins and UV damage. 14 , 28 , 29 Results of a recent in vivo study describing skin contamination from pollution corroborates this. 30 Age‐related changes may already impact growth of the papillary dermis, which may trigger degradation of components of the extracellular matrix (which includes HA) and impaired the upper dermis and basement membrane. 31
However, the exposome can also be harnessed for its beneficial effects. In a cross‐sectional study of Spanish adults, age, smoking, and the use of sunscreen and cosmetics independently predicted skin aging, and thus the exposome exerted either a preventive or a detrimental effect in terms of aging. 32
3.7. Clinical study results of HA‐containing products: how topical HA application can improve skin aging signs, even postprocedure
Topical HA can be found alone or in association with other active ingredients, such as vitamins, ceramide, thermal or volcanic water, glycerin, and herbal extracts, among others. In the literature, clinical studies of HA‐containing products for facial aging benefits range from dermatological to aesthetic or procedure focused.
Topical HA has been studied in the reduction of aging signs. Also, an increasing number of studies focus on the association of HA with various facial rejuvenation procedures. This section provides an overview of current evidence of such interventions including in combination with BoNTA, photodynamic therapy (PDT), fractional CO2 laser skin resurfacing, fillers, microneedling, and chemical peeling.
3.7.1. Topical treatment of aging skin
A RCT including 40 females with mild‐to‐moderate clinical signs of skin aging demonstrated improvement in skin appearance versus placebo after 30 days of using six types of topical HA of different molecular weights according to wrinkle volume. 10 Another RCT in 65 females with periocular wrinkles showed significant improvement in skin hydration and elasticity versus placebo after 60 days of using 0.1% sodium hyaluronate formulations of different molecular weights (50, 130, 300, 800, and 2000 kDa, respectively). 33 Moreover, two of the lowest molecular weight HA formulations led to significantly reduced wrinkle depth; the authors speculate that this may be explained by the greater anti‐inflammatory and penetration potential of LMW‐HA.
A 12‐week clinical study among 59 women with mild‐to‐moderate photodamage evaluated the tolerance and efficacy of a multimodal facial serum containing HA, Proxylane (C‐Xyloside), purple rice extract, and dipotassium glycyrrhizate. 34 The combination of the actives stimulated HA and skin extracellular matrix components including HAS2 and collagen type 1a1, and improved skin hydration. In 20 females with moderate facial skin aging, significant improvement was observed after 2 months of treatment with a HA serum (mixed with acetyl glucosamine and gamma‐amino butyric acid) for the following: hydration (via corneometry), moisture, elasticity, and lightening of the skin. 35 Finally, topical HA with growth factor has been found to diminish periorbital wrinkles in a 8‐week trial, 36 as well as to reduce fine lines and other signs of facial skin aging in a 12‐week study. 37
3.7.2. HA used with procedures
Photodynamic therapy
While PDT has been unexpectedly found to impact cosmetic rejuvenation, standard treatment guidelines have not been developed for this indication. Results from a 12‐week pilot study (n = 6) of PDT (three sessions using a 2% 5‐aminolevulinic acid [ALA] gel along with HA) suggest that the procedure is well tolerated and can lead to both clinical and subject‐assessed improvements in skin damage. 38
Lasers for skin rejuvenation
Few studies were identified using topical HA as adjunct to fractional CO2 laser skin resurfacing, yet topical HA was either used in a standard care/control 39 or comparator group. 40 In one study aiming to reduce the inflammatory reaction associated with laser treatment, a probiotic‐derived experimental cream was applied post‐treatment twice daily to 42 consecutively enrolled subjects, and a topical antibiotic cream 3 times daily for 3 days followed by a HA‐based cream twice daily for 15 days was administered to the control group of 20 subjects. 39 There were between 1 to 4 laser sessions for the face or hands every 2–3 months. Researchers found that the experimental cream decreased the average time of expected side effects (erythema and swelling) compared to control, and suspected that HA's optimal protection could reduce fluid drainage and augment infection risk. In a different prospective study, laser treatment of the perioral area was performed in 50 subjects. 40 Postprocedure, 25 subjects self‐applied topical platelet‐rich plasma (Prp) containing growth factors twice daily for 12 weeks, and 25 subjects applied both a topical antibiotic and topical steroid for 7 days followed by HA gel for 12 weeks. There was no mention of randomization in the methods section. Based on a digital skin analyzer and the clinician's/subject's assessments, moisture, quantity of collagen fiber, elasticity, wrinkle reduction, and satisfaction improved for both groups from baseline to week 12 (significantly for the Prp group).
Other procedures (fillers, peels, etc.)
Fillers, microneedling, and chemical peeling were found to benefit from a topical HA when used postprocedurally, according to a randomized, investigator‐blinded, split‐face, parallel‐arm trial conducted among 24 adult females with dry facial skin (skin hydration level ≤ 60 arbitrary units [A.U.] via corneometry) and aging signs. 41 Starting 2 days post‐procedure and for 28 days, the HA serum was applied twice daily, combined with a HA‐based balm for 2 days after the procedure. The HA serum was well tolerated, and three‐dimensional in vivo imaging of the lateral canthal areas, bioinstrumental including corneometry and cutometry (i.e., increased skin hydration, firmness, tonicity, and elasticity), and clinical assessments showed significantly greater improvement than untreated areas. A meta‐analysis in eight countries among 2363 subjects corroborates that a HA‐containing product can be applied topically with improved efficacy and tolerability following a variety of procedures: peels, fractional ablative lasers and/or continuous (CO2), pigment lasers, laser tattoo removal, intense pulsed light, cryotherapy, injections, and minor surgery. 42 In addition, these authors present pediatric cases where topical HA use resulted in high efficacy after thermal burns to prevent scarring. 42
Use of topical HA in combination with BoNTA
BoNTA is frequently used in clinical practice for relaxing facial muscles of the upper face, which in turn gives the perception that there are fewer wrinkles. 43 BoNTA is injected into muscle, binding at the nerve terminal, which prevents release of the neurotransmitter, acetylcholine, from the nerve synapse. Thus, muscle contraction (and hyperdynamic wrinkle) is prevented. Its duration of effect is considered to last 4–6 months, however, authors of a recent Cochrane review note that defining its duration of effect needs further study. 43
Benefits of using topical HA in conjunction with BoNTA injection are also present in the literature. An open‐label, before‐and‐after, interventional study highlighted the possible benefits post‐BoNTA of a topical HA, which led to significant improvement in facial fine lines and wrinkles after 8 weeks of treatment when compared to baseline. 44
As previously described in Section 2, a prospective, single‐blind, 24‐week RCT was recently performed using an innovative cosmeceutical with 1.5% multimolecular weight HA, Vitamin Cg, peptides, and volcanic mineralizing water (Liftactiv HA epidermic filler [HA‐filler serum]) in combination with BoNTA (Botulinum Toxin A 300 U [Dysport®], Galderma Laboratories, L.P.) to treat facial skin aging. 12 To our knowledge, this is the first RCT to combine BoNTA and HA with a 6‐month follow up.
This study included adult females with moderate symmetrical facial wrinkles graded Scale 3 to 4 by the Skin Aging Atlas (forehead, glabellar, and Crow's feet wrinkles). 45 Wrinkles and fine lines were by far their main skin concern (approximately 94%), however only 11%–13% used a serum or eye care products, 71% used sunscreen, and nearly 20% smoked. Subjects were randomized to two groups: 31 subjects used HA‐filler serum 24 h after the BoNTA injection then twice daily (over the entire face including the eye contour) for 24 weeks, and 32 in the control group received the procedure alone. Both treatment groups received SPF 50+ sunscreen (UV Age daily photoprotection [SPF50+, UVA‐PF 46] with Netlock technology, Vichy Laboratories) for use during the study. The primary objective was to evaluate the benefit of HA‐filler serum with BoNTA injection compared to the procedure alone in improving signs of facial skin aging (graded on a 0–10 visual analog scale by a dermatologist) and subject satisfaction.
The maximum effect of BoNTA was reached at day 14 for all clinical parameters. Additionally, a significant benefit in terms of skin radiance was already observed at day 14 in favor of HA‐filler serum (p = 0.018 vs. BoNTA only). Significant improvement with a plateau effect was sustained after 24 weeks of HA‐filler serum use in terms of fine lines, Crow's feet wrinkles, skin tone, skin texture, radiance, and skin elasticity compared to the control group with differences of approximately 16%, 17%, 14%, 24%, 24%, and 20%, respectively (p < 0.05; Figure 3). Tewameter® results showed significant reduction in TEWL, and thus improved skin barrier integrity, after 12 weeks of HA‐filler serum use when compared to baseline (p < 0.001); a trend toward better skin barrier function favored the serum throughout the study. There were no reported local adverse events related to HA‐filler serum. Subjects were satisfied with their skin appearance in both groups, and while nonsignificant, a trend toward greater satisfaction was found in favor of HA‐filler serum at week 24 (mean percentage improvement of 66% vs. 41% for procedure only). Moreover, all subjects were willing to reuse the HA‐filler serum. Therefore, this study supports that adding a 1.5% HA‐containing cosmeceutical along with BoNTA improves signs of aging and skin quality compared to BoNTA alone and is well tolerated, even when used around eye contours. Indeed, while using sunscreen was required as key to a daily skin care routine, added benefits of an HA‐containing cosmeceutical were observed in this study which may be partly due to the effects of the HA‐filler serum's ingredients (notably Vitamin Cg, peptides, and volcanic mineralizing water). 12 , 13 Additionally, this HA‐filler serum appears to prolong the duration of effect of BoNTA injections in reducing wrinkles.
FIGURE 3.
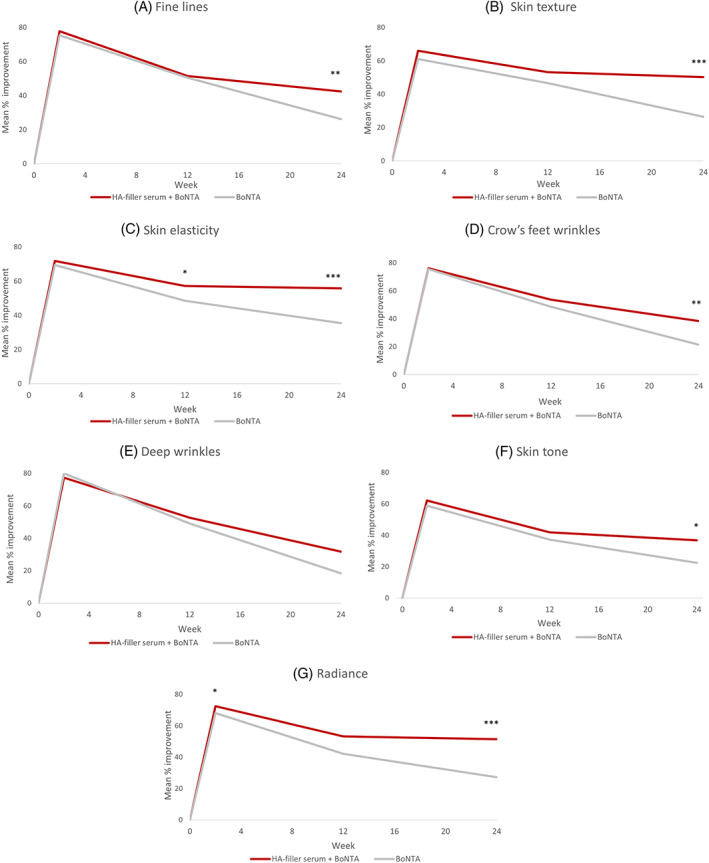
Improved clinician‐assessed signs of facial aging with HA‐filler serum for (A) fine lines, (B) skin texture, (C) skin elasticity, (D) Crow's feet wrinkles, (E) deep wrinkles, (F) skin tone, and (G) radiance. p‐Values versus control (BoNTA): *p < 0.05; **p < 0.01; ***p < 0.001
4. CONCLUSIONS
HA is present in many cosmetic formulations due to excellent hygroscopic, rheological, and viscoelastic properties, and some literature highlights its benefits when used topically in dermatological practice. Indeed, several clinical studies indicate that HA is both well tolerated and effective, adjuvant to both postsurgical and facial rejuvenation procedures.
As described in this manuscript, the body of evidence is growing in terms of studies which associate HA with various facial rejuvenation procedures, including in combination with BoNTA, PDT, fractional CO2 laser skin resurfacing, fillers, microneedling, and chemical peeling. The recent RCT we presented, combining BoNTA and HA‐filler serum, was robustly designed with a 6‐month follow‐up and control group. The findings support that HA‐filler serum lengthened the duration of BoNTA's effect in reducing wrinkles, was well tolerated, and led to high patient satisfaction. Despite the limitations of certain other studies including short duration and lack of a control group, we consider these results to be pertinent and applicable to patients who may benefit from using HA‐based cosmeceuticals as part of their daily skin care routine. From the literature to clinical evidence, clinicians can integrate this information with both a science‐based and practical approach.
In conclusion, a greater number of long, well‐conducted, controlled studies that provide objective data are warranted. Nevertheless, results from several trials generally support that HA‐based cosmeceuticals are a non‐invasive, effective solution for improving skin hydration, rejuvenation, and healing. Furthermore, in addition to educating about healthy lifestyle habits and skin protection including the regular use of sunscreen, 24 clinicians can advise patients that HA‐based cosmeceuticals may help to counterbalance the negative impact of the exposome.
AUTHOR CONTRIBUTIONS
Bruna Bravo, Priscila Correia, José Euzébio Gonçalves Junior, Beatriz Sant'Anna, and Delphine Kerob had full access to all of the data in this study. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. Priscila Correia wrote the manuscript, and all authors have read and approved the final manuscript.
FUNDING INFORMATION
Cosmetic Active International, L'Oréal, funded the Liftactiv HA epidermic filler study and provided the study products.
CONFLICT OF INTEREST
Dr. Bravo is a paid consultant for L'Oréal Brasil. Priscila Correia, José Euzébio Gonçalves Junior and Beatriz Sant'Anna are employees of L'Oréal Brazil, and Dr. Delphine Kerob is an employee of L'Oréal Paris, the manufacturer of the formulation under study.
ETHICS STATEMENT
The study was conducted in accordance with the principles of Resolution 466/2012 of the National Health Council Brazil, and ethical principles stemming from the Declaration of Helsinki (and subsequent modifications) defined by ICH E65 ref. EMA/CHMP/ICH/135/1995, 2016. Following the guidelines of the Brazilian legislation, the study protocol was submitted and approved by the Ethical Committee of the Pro‐Cardiac Hospital, Opinion Number 4.258.397 issued on September 4th, 2020.
ACKNOWLEDGMENTS
The authors would like to thank Galadriel Bonnel, PhD, RN, FNP, Resonance Medical Writing & Consulting, for editorial assistance.
Bravo B, Correia P, Gonçalves Junior JE, Sant'Anna B, Kerob D. Benefits of topical hyaluronic acid for skin quality and signs of skin aging: From literature review to clinical evidence. Dermatologic Therapy. 2022;35(12):e15903. doi: 10.1111/dth.15903
Funding information Cosmetic Active International, L'Oréal
DATA AVAILABILITY STATEMENT
New data not previously published are available on request due to privacy/ethical restrictions.
REFERENCES
- 1. Russell‐Goldman E, Murphy GF. The pathobiology of skin aging: new insights into an old dilemma. Am J Pathol. 2020;190(7):1356‐1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wild CP. Complementing the genome with an "exposome": the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomark Prev. 2005;14(8):1847‐1850. [DOI] [PubMed] [Google Scholar]
- 3. Kasprzak D, Wnorowski A. A variety of processes that affect the perception of skin aging. Curr Issues Pharm Med Sci. 2019;32(3):146‐153. [Google Scholar]
- 4. Addor FAS. Beyond photoaging: additional factors involved in the process of skin aging. Clin Cosmet Investig Dermatol. 2018;11:437‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Araviiskaia E, Berardesca E, Bieber T, et al. The impact of airborne pollution on skin. J Eur Acad Dermatol Venereol. 2019;33(8):1496‐1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vierkötter A, Schikowski T, Ranft U, et al. Airborne particle exposure and extrinsic skin aging. J Invest Dermatol. 2010;130(12):2719‐2726. [DOI] [PubMed] [Google Scholar]
- 7. Pandey A, Jatana GK, Sonthalia S. Cosmeceuticals. [Updated August 11, 2021]. StatPearls [Internet]. StatPearls Publishing; 2021. Accessed December 27, 2021. https://www.ncbi.nlm.nih.gov/books/NBK544223/ [Google Scholar]
- 8. Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid. A key molecule in skin aging. Dermato‐Endocrinology. 2012;4(3):253‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vasvani S, Kulkarni P, Rawtani D. Hyaluronic acid: a review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int J Biol Macromol. 2020;151:1012‐1029. [DOI] [PubMed] [Google Scholar]
- 10. Nobile V, Buonocore D, Michelotti A, Marzatico F. Anti‐aging and filling efficacy of six types hyaluronic acid based dermo‐cosmetic treatment: double blind, randomized clinical trial of efficacy and safety. J Cosmet Dermatol. 2014;13(4):277‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu J, Tang X, Jia Y, Ho CT, Huang Q. Applications and delivery mechanisms of hyaluronic acid used for topical/transdermal delivery ‐ a review. Int J Pharm. 2020;578:119127. [DOI] [PubMed] [Google Scholar]
- 12. Bravo B, Correia P, Gonçalves Júnior JE, et al. Randomized controlled study to assess the additional benefit of a dermocosmetic with hyaluronic acid and Vitamin C combined with Botulinum toxin for skin aging signs. Poster presented at: EADV Congress; September 29–October 2, 2021.
- 13. Rasmont V, Valois A, Gueniche A. Vichy volcanic mineralizing water has unique properties to strengthen the skin barrier and skin defenses against exposome aggressions. J Eur Acad Dermatol Venereol. 2022;36(Suppl. 2):5‐15. [DOI] [PubMed] [Google Scholar]
- 14. Krutmann J, Bouloc A, Sore G, Bernard BA, Passeron T. The skin aging exposome. J Dermatol Sci. 2017;85(3):152‐161. [DOI] [PubMed] [Google Scholar]
- 15. Chambers EA, Vukmanovic‐Stejic M. Skin barrier immunity and ageing. Immunology. 2020;160(2):116‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shin JW, Kwon SH, Choi JH, et al. Molecular mechanisms of dermal aging and antiaging approaches. Int J Mol Sci. 2019;20(9):2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Essendoubi M, Gobinet C, Reynaud R, Angiboust JF, Manfait M, Piot O. Human skin penetration of hyaluronic acid of different molecular weights as probed by Raman spectroscopy. Skin Res Technol. 2016;22(1):55‐62. [DOI] [PubMed] [Google Scholar]
- 18. Smejkalova D, Huerta‐Angeles G, Ehlova T. Hyaluronan (hyaluronic acid) a natural moisturizer for skin care. Harry's. Vol 2, Part 4.1.3. 9th ed. Contipro Pharma; 2015. [Google Scholar]
- 19. Ghersetich I, Lotti T, Campanile G, et al. Hyaluronic acid in cutaneous intrinsic aging. Int J Dermatol. 1994;33(2):119‐122. [DOI] [PubMed] [Google Scholar]
- 20. Keen MA. Hyaluronic acid in dermatology. Skinmed. 2017;15:462‐469. [PubMed] [Google Scholar]
- 21. Longinotti C. The use of hyaluronic acid based dressings to treat burns: a review. Burns Trauma. 2014;2(4):162‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Voigt J, Driver VR. Hyaluronic acid derivatives and their healing effect on burns, epithelial surgical wounds, and chronic wounds: a systematic review and meta‐analysis of randomized controlled trials. Wound Repair Regen. 2012;20(3):317‐331. [DOI] [PubMed] [Google Scholar]
- 23. Sabadotto M, Theunis J, Black D, Mengeaud V, Schmitt AM. In vivo assessment of the effect of a cream containing Avena Rhealba(®) extract and hyaluronic acid on the restoration of the skin barrier in de‐epidermised skin produced with an erbium‐YAG laser. Eur J Dermatol. 2014;24(5):583‐588. [DOI] [PubMed] [Google Scholar]
- 24. Baumann L. How to use oral and topical cosmeceuticals to prevent and treat skin aging. Facial Plast Surg Clin N Am. 2018;26:407‐413. [DOI] [PubMed] [Google Scholar]
- 25. Passeron T, Zouboulis CC, Tan J, et al. Adult skin acute stress responses to short‐term environmental and internal aggression from exposome factors. J Eur Acad Dermatol Venereol. 2021;35(10):1963‐1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gonçalves Mota MP, Santos Z, Soares J, et al. Oxidative stress function in women over 40 years of age, considering their lifestyle. Front Endocrinol. 2017;8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patel CJ, Manrai AK, Corona E, Kohane IS. Systematic correlation of environmental exposure and physiological and self‐reported behaviour factors with leukocyte telomere length. Int J Epidemiol. 2017;46(1):44‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burke KE. Mechanisms of aging and development ‐ a new understanding of environmental damage to the skin and prevention with topical antioxidants. Mech Ageing Dev. 2018;172:123‐130. [DOI] [PubMed] [Google Scholar]
- 29. Khmaladze I, Leonardi M, Fabre S, Messaraa C, Mavon A. The skin Interactome: a holistic "genome‐microbiome‐Exposome" approach to understand and modulate skin health and aging. Clin Cosmet Investig Dermatol. 2020;13:1021‐1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dimitrov A, Zanini M, Zucchi H, et al. Vitamin C prevents epidermal damage induced by PM‐associated pollutants and UVA1 combined exposure. Exp Dermatol. 2021;30(11):1693‐1698. [DOI] [PubMed] [Google Scholar]
- 31. Mine S, Fortunel NO, Pageon H, Asselineau D. Aging alters functionally human dermal papillary fibroblasts but not reticular fibroblasts: a new view of skin morphogenesis and aging. PLoS One. 2008;3(12):e4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buendía‐Eisman A, Prieto L, Abarquero M, et al. Study of the Exposome ageing‐related factors in the Spanish population [published correction appears in Acta Derm Venereol. 2020 Oct 6;100(17):adv00280]. Acta Derm Venereol. 2020;100(10):adv00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pavicic T, Gauglitz GG, Lersch P, et al. Efficacy of cream‐based novel formulations of hyaluronic acid of different molecular weights in anti‐wrinkle treatment. J Drugs Dermatol. 2011;10(9):990‐1000. [PubMed] [Google Scholar]
- 34. Raab S, Yatskayer M, Lynch S, Manco M, Oresajo C. Clinical evaluation of a multi‐modal facial serum that addresses hyaluronic acid levels in skin. J Drugs Dermatol. 2017;16(9):884‐890. [PubMed] [Google Scholar]
- 35. Ferrillo M, Vastarella M, Cantelli M, Mazzella C, Fabbrocini G. Instrumental, clinical and subjective evaluation of the efficacy of a cosmetic treatment for home use. J Cosmet Laser Ther. 2019;21(4):190‐195. [DOI] [PubMed] [Google Scholar]
- 36. Lee DH, Oh IY, Koo KT, et al. Improvement in skin wrinkles using a preparation containing human growth factors and hyaluronic acid serum. J Cosmet Laser Ther. 2015;17(1):20‐23. [DOI] [PubMed] [Google Scholar]
- 37. Draelos ZD. The effect of a combination of recombinant EGF cosmetic serum and a Crosslinked hyaluronic acid serum as compared to a fibroblast‐conditioned media serum on the appearance of aging skin. J Drugs Dermatol. 2016;15(6):738‐741. [PubMed] [Google Scholar]
- 38. Huang A, Nguyen JK, Austin E, et al. Facial rejuvenation using photodynamic therapy with a novel preparation of ALA and hyaluronic acid in young adults. Arch Dermatol Res. 2020;312(8):567‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zoccali G, Cinque B, La Torre C, et al. Improving the outcome of fractional CO2 laser resurfacing using a probiotic skin cream: preliminary clinical evaluation. Lasers Med Sci. 2016;31(8):1607‐1611. [DOI] [PubMed] [Google Scholar]
- 40. Araco A. A prospective study comparing topic platelet‐rich plasma vs. placebo on reducing superficial perioral wrinkles and restore dermal matrix. J Cosmet Laser Ther. 2019;21(6):309‐315. [DOI] [PubMed] [Google Scholar]
- 41. Sundaram H, Cegielska A, Wojciechowska A, Delobel P. Prospective, randomized, investigator‐blinded, Split‐face evaluation of a topical Crosslinked hyaluronic acid serum for post‐procedural improvement of skin quality and biomechanical attributes. J Drugs Dermatol. 2018;17(4):442‐450. [PubMed] [Google Scholar]
- 42. Saint Aroman M, Guillot P, Dahan S, et al. Efficacy of a repair cream containing Rhealba oat plantlets extract l‐ALA‐l‐GLU dipeptide, and hyaluronic acid in wound healing following dermatological acts: a meta‐analysis of >2,000 patients in eight countries corroborated by a dermatopediatric clinical case. Clin Cosmet Investig Dermatol. 2018;11:579‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Camargo CP, Xia J, Costa CS, et al. Botulinum toxin type a for facial wrinkles. Cochrane Database Syst Rev. 2021;7:CD011301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fabi SG, Zaleski‐Larsen L, Bolton J, Mehta RC, Makino ET. Optimizing facial rejuvenation with a combination of a novel topical serum and injectable procedure to increase patient outcomes and satisfaction. J Clin Aesthet Dermatol. 2017;10(12):14‐18. [PMC free article] [PubMed] [Google Scholar]
- 45. Bazin R, Doublet E. Skin aging atlas, Vol 1—Caucasian type. 1st ed. Med Com; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
New data not previously published are available on request due to privacy/ethical restrictions.


