Summary
The hypnagogic state refers to a transitional stage between wakefulness and sleep, in which sensory perceptions can be experienced. In this review, we compile and discuss the recent scientific literature on hypnagogia research regarding the future directions proposed by Schacter (1976; Psychological Bulletin, 83, 452). After a short introduction discussing the terminology used in hypnagogia research and the differentiation of hypnagogic states with other related phenomena, we review the reported prevalence of hypnagogic states. Then, we evaluate the six future directions suggested by Schacter and we propose three further future directions. First, a better understanding of the emotional quality of hypnagogic states is needed. Second, a better understanding of why hypnagogic states occur so frequently in the visual and kinaesthetic modalities is needed. Lastly, a better understanding of the purpose of hypnagogic states is needed. In conclusion, research has made great progress in recent years, and we are one step closer to demystifying the hypnagogic state.
Keywords: hypnagogia, hypnagogic hallucinations, hypnagogic states, hypnopompic hallucinations
1. THE HYPNAGOGIC STATE: AN UPDATE
Over four decades have elapsed since Schacter (1976) reviewed the literature on the hypnagogic state. The hypnagogic state can be defined as “spontaneously appearing visual, auditory and kinaesthetic images; qualitatively unusual thought processes and verbal constructions; tendencies towards extreme suggestibility; symbolic representations of ongoing mental and physiological processes; and so on” (Schacter, 1976, 452–453). Schacter noted that the most common factor of these phenomena was their occurrence in the drowsy interval between the waking state and sleeping. This factor is still one of the critical characteristics defining hypnagogic experiences. Schacter provided valuable and coherent insight into the hypnagogic state, which is briefly summarized first. Next, we follow up on Schacter's review and present an update on the state of the field.
2. MAIN FINDINGS OF SCHACTER (1976)
Earlier research focused on the prevalence of hypnagogic states. Surveys quickly suggested that the hypnagogic state was relatively common, with prevalences ranging from 72% up to 77%. Particular reference was made to a possible age effect, according to which age was negatively related to the occurrence of hypnagogic states. Research in children between the age of 3 and 15 years resulted in ambiguous findings. Differences in hypnagogic states between childhood and adulthood, however, were not investigated. Thus, a decline in hypnagogic states over the course of life had yet to be examined. In addition, a cultural influence was suspected but remained unassessed. Overall, the phenomenology of hypnagogic state was described extensively, but little emphasis was put on factors that influence the likelihood of hypnagogic states.
One of the most indicative characteristics of hypnagogic states is their spontaneous emergence into consciousness. Several authors noted the lack of active involvement in the hypnagogic state, as the experience resembled the act of passively spectating a play or a movie. Hypnagogic states were reported to occur most frequently in the visual modality, followed by the auditory and tactile‐kinaesthetic modality. There were attempts to differentiate hypnagogic states from dreams: compared with dreams, hypnagogic states were described as being mostly emotionally flat. Moreover, hypnagogic states were described as disconnected snapshots, whereas dreams were described as usually longer and better organized. Regarding the content of hypnagogic states, some researchers noted that the content could be traced back to activities pursued during the day.
Data on the hypnagogic state were acquired through multiple approaches. First and foremost, researchers conducted either spontaneous or systematic self‐observation or trained subjects to do so. To gather a larger amount of data, researchers used questionnaire sampling. Other researchers attempted to induce and prolong the hypnagogic state, such as by using biofeedback or the ganzfeld approach (an approach that will be explained in detail later on in this review). Lastly, researchers investigated the physiological correlates of the hypnagogic state using electroencephalography (EEG) and electrooculography (EOG). The neurological origin of hypnagogic states, however, was not determined.
Schacter (1976) indicated several directions for future research. First and foremost, the need for further developed experimental techniques, which permit a systematic exploration of the phenomenology of hypnagogic experiences. Second, a more precise description of the phenomenology of hypnagogic imagery and auditory experiences. Can hypnagogic imagery be successfully categorized, to what extent is it consistent over time, and can it be differentiated from other imagery? Third, a more detailed understanding of the individual's capacity to process the environment in the hypnagogic state. To what extent does sensory stimulation experienced before the hypnagogic state influence hypnagogic imagery? Fourth, a better understanding of the influence of cognitive, perceptual and personality variables on the content hypnagogic states. How do these factors influence the likelihood of hypnagogic states and their content? Fifth, the need for fuller development of a psychophysiological approach. What are the physiological correlates of hypnagogic states and how do they vary? And lastly, a better understanding of the necessary conditions to enter the hypnagogic state. Can hypnagogic states be successfully induced? And, further, how do naturally induced hypnagogic states differ from artificially induced ones? Besides reviewing the more recent literature, we also evaluate the progress made regarding the future directions proposed by Schacter (1976).
3. METHODOLOGICAL NOTE
The search for literature began on 5 March 2020. We searched for scientific articles and chapters published in the last 20 years using the keywords “hypnagogia”, “hypnagogic state”, “hypnagogic hallucinations”, “hypnagogic imagery” and “sleep onset imagery” in PSYINDEX and PsycINFO. We included articles focusing on hypnagogic states themselves, and we excluded those articles in which hypnagogic states were a byproduct of another topic of interest (e.g. an article focusing on narcolepsy with a brief mention of hypnagogic states). Further, we focused on experimental and quasi‐experimental studies, and included English publications only. Reference lists of consulted articles and chapters were then searched for additional literature, which resulted in a total of 40 publications we reviewed. Further additional literature was searched independently in favour of supplementary information using Google Scholar.
4. TERMINOLOGY ISSUES
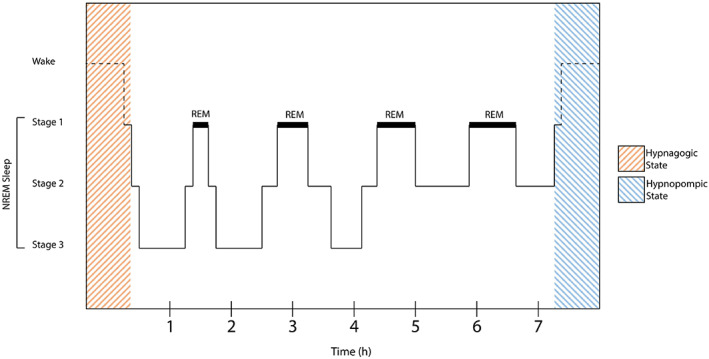
Before summarizing the scientific findings on the hypnagogic state in recent years, we must clarify the terminology used when discussing hypnagogia. The term hypnagogic, from the words ύπνος (“hypnos”), meaning “sleep”, and αγωγός (“agōgos”), meaning “conductor” or “leader”, was first coined by Maury (1848). Hypnagogic refers to the phenomena occurring in the transition from wakefulness to sleep. The term hypnopompic, with the word‐ending originating from the word πομπός (“pompos”), meaning “sender”, was later coined by Myers (1904). Hypnopompic, on the other hand, refers to the very same phenomena occurring at sleep‐offset. However, these states seem not to be limited to the transitional phases between wakefulness and sleep, but may also occur during the day (Gurstelle & de Oliveira, 2004; Steen, 2017). In this review, we use the term hypnagogic states to refer to the hypnagogic states at sleep‐onset and hypnopompic states at sleep‐offset (Figure 1).
FIGURE 1.
Localization of hypnagogic stages over the course of sleep
Hypnagogic states have sometimes been labelled as hypnagogic hallucinations. However, as there is great stigma surrounding hallucinations (Sacks, 2012), we believe that this stigma bound to the term hallucinations could be projected onto the commonly occurring hypnagogic state. In fact, the term hallucination, which describes a pathological phenomenon, is misleading because hypnagogic experiences occur in the normal range of perception. While hallucinations are described as a defining feature of schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders (5th edn; DSM‐5), those “hallucinations” occurring at sleep‐onset or ‐offset are not, and are mentioned explicitly as normal experiences (American Psychiatric Association, 2013).
Other important differences between hallucinations and hypnagogic states have been proposed: while hallucinations are reflected upon, reacted to and incorporated with internal representations, hypnagogic experiences are not; an individual's sense of self, its beliefs or personal narrative are not affected by hypnagogic experiences in comparison to hallucinations (Waters et al., 2016). At best, hypnagogic states could be classified as pseudo‐hallucinations, although the ambiguous nature of this term, as two different meanings of the term pseudo‐hallucinations have been proposed (Taylor, 1981).
Based on those arguments, we propose a distinction between hypnagogic states and hypnagogic hallucinations. The term hypnagogic states refers to the experiences of perception in the different modalities at sleep‐onset and ‐offset, which may also, although less frequently, occur during wakefulness. However, these experiences can become clinically relevant, that is, when a complaint is issued or when they result in discomfort in the affected individual. In accordance with the criteria defined in the third edition of the International Classification of Sleep Disorders (ICSD‐3; American Academy of Sleep Medicine, 2014), we then classify these phenomena as hypnagogic hallucinations. Consequently, we would expect hypnagogic states to be more prevalent in the general population than hypnagogic hallucinations.
5. DIFFERENTIATION
Several sleep‐related phenomena share similarities with hypnagogic states. The most difficult differentiation might be the one from dreams, especially at sleep‐offset. Whereas both can be differentiated on the level of electrophysiological signatures, their phenomenological differentiation appears more complex. Some differentiating characteristics, however, include dreams generally being longer and less thought‐like (Zadra & Domhoff, 2017), as well as containing fewer episodic memories (Baylor & Cavallero, 2001). Moreover, dream content appears to be rather consistent over time (Domhoff, 1996) and can occur repetitively (Van de Castel, 1995), whereas little is known about the consistency of hypnagogic experiences. Lastly, whereas dreams occur during sleep (Schredl, 2018), hypnagogic experiences at sleep‐offset are usually a continuation of dreams after waking up (Waters et al., 2016).
Another closely related phenomenon is the inability to move while maintaining the ability to breathe at sleep‐onset or ‐offset called sleep paralysis, a phenomenon often associated with hallucinations (Schiappa et al., 2018). Both sleep paralysis and hypnagogic states are features commonly associated with narcolepsy (D'Agostino & Limosani, 2010). Appropriately, the anomalous experiences during sleep paralysis have been labelled as hypnagogic and hypnopompic (Cheyne et al., 1999).
Another sleep‐related phenomenon sharing similarities with hypnagogic states is the explosive head syndrome (EHS). EHS describes the experience of a loud explosive noise at sleep‐onset or ‐offset, awakening the affected individual, with some reports of such an occurrence during the day (Green, 2001). This usually benign and infrequent experience, which can cause fear, confusion and distress, can manifest itself in a more chronic form (Sharpless, 2014). EHS generally has a lower prevalence than hypnagogic states (Fulda et al., 2008). However, one could argue that EHS‐related experiences are but amplified auditory hypnagogic experiences.
While synaesthesia and hypnagogic states seem to share similarities at first glance, they are distinguishable by clear characteristics. Synaesthesia, the automatic activation of a concurrent sensation induced by a specific stimulus, is amongst other things defined by its consistent association between the inducing stimulus and the synaesthetic experience (Meier et al., 2014; Ward, 2013). In contrast, in hypnagogic experiences there is no comparably consistent inducing stimulus. Nevertheless, it is an open question whether hypnagogic experiences may occur more frequently in synaesthetes (Steen, 2017).
The ICSD‐3 further suggests other phenomena that must be differentiated from hypnagogic states, such as epileptic seizures, visual loss (Charles Bonnet hallucinations), and midbrain and diencephalic pathology (peduncular hallucinosis). Since these phenomena have an organic cause, however, the differentiation, therefore, appears to be clearer and more convenient. We will thus not further elaborate on the differences between these phenomena and hypnagogic states, but acknowledge that they must be properly differentiated. In this section, we refrained from discussing all sleep‐related phenomena that are potentially associated with the hypnagogic state. Rather, we decided to focus on phenomena associated with evoked imagery. Conclusively, both sleep‐paralysis‐related experiences and the EHS can be classified as hypnagogic phenomena, while synaesthesia can clearly be delimited from such. However, sleep paralysis and the EHS are not the focus of this review.
6. PREVALENCE
Several studies have investigated the prevalence of hypnagogic experiences; however, the results differed markedly. In Table 1 we present an overview of the reported prevalences and definitions. Most notably, there has been great variability in the definitions of hypnagogic states used when assessing their prevalence. Thus, we describe the studies in greater detail, beginning with large‐scale prevalence studies, followed by smaller studies in favour of questionnaire validation, and proceed to discuss their differences.
TABLE 1.
Summary of prevalences and definitions of hypnagogic states
| Author | Year | Mean age | Sample size | Prevalence | Definition of hypnogogic states |
|---|---|---|---|---|---|
| Bosch et al. | 2012 | 39.7 years | 29 | 8% | Auditory or visual illusions that accompany falling asleep or waking in a distressing or threatening manner (e.g. hearing sounds or voices, or seeing people or things that are not in the room) |
| Fulda et al. | 2008 | 35.0 years | 65 | 6% | |
| Jones et al. | 2009 | 21.1 years | 365 | 85% | Feeling an evil presence in the room; hearing one's name being called; seeing a blurry human figure in the room; hearing the voice of a familiar person; seeing things or figures floating in the room; […] |
| Jones et al. | 2010 | 22.5 years | 325 | 33% a | |
| Ohayon | 1996 | N/A | 4972 | 37%/12% b | The realistic feeling that someone or something is present in the room; a vivid experience of being caught in a fire; a vivid experience that one is about to be attacked; the feeling that one will soon fall into an abyss; […] over the last year |
| Ohayon | 2000 | N/A | 13,057 | 25%/7% b | Seeing things, objects or persons other people cannot see; feeling something is under or on one's skin; having the feeling of being outside one's body watching oneself; hearing sounds, music or voices; […] |
| Ohayon & Shapiro | 2000 | N/A | 1832 | 22%/6% b | Questionnaire item according to Sleep‐EVAL (Ohayon et al., 1999) |
| Schacter | 1976 | 72%–77% | Individual definitions reviewed | ||
| Sherwood | 2012 | 27.0 years | 492 | 9%–82% c | Visual imagery, auditory sensations, smell or taste when falling asleep; the feeling of falling; the sensation of seeming to touch, or be touched by, someone or something. The feeling of a presence in the room; […] |
Note: Prevalence estimates are reported for healthy (non‐psychiatric) samples and the numbers were rounded to the nearest integer.
Reported prevalence for the auditory modality only.
Hypnagogic states at sleep‐onset/hypnagogic states at sleep‐offset.
Prevalences were reported for different modalities only, the most frequent being visual perceptions (82%), the lowest being perceptions of taste (9%).
Ohayon et al. (1996) assessed the prevalence of hypnagogic and hypnopompic states in a general population study based in the UK. Questionnaire items asked for specific experiences with a primarily negative emotional quality. Some examples include a vivid experience of being caught in a fire, a realistic feeling of someone or something being present, or a vivid experience of being attacked, limited to the auditory, the visual and the kinetic modality. From a total of 4972 participants, 37% reported experiencing hypnagogic states at least twice a week over the recent year, and 12.5% reported hypnopompic states at least twice a week over the recent year. Hypnagogic states were reported to be more common in participants who experienced sleep problems or insomnia (Ohayon et al., 1996).
In a later study with a general population sample based in the UK, Germany and Italy, lower prevalences of hypnagogic experiences were reported (Ohayon, 2000). In this study, however, hypnagogic experiences were assessed in a broader and emotionally neutral way. Participants were asked about hypnagogic experiences for each of the different modalities, including out‐of‐body experiences. For example, participants were asked whether they had smelled something other people could not smell or seen something others could not see when waking up or falling asleep. From a total of 13,057 participants, only 24.8% reported experiencing hypnagogic states, and 6.6% reported experiencing hypnopompic states. Moreover, a higher prevalence for women and younger adults was reported (Ohayon, 2000). Another survey conducted in the same year reported prevalences of hypnagogic states of 21.6% and hypnopompic states of 6% in healthy participants (Ohayon & Shapiro, 2000).
A study with the main aim of validating the Munich Parasomnia Screening (MUPS) reported a lifetime prevalence of hypnagogic states of only 6.2% in a general‐population‐based sample of 65 healthy participants from Germany (Fulda et al., 2008). The prevalence for hypnagogic states in this study was higher both in a sample of not nearer defined psychiatric patients (21.5%) and another sample of sleep‐disordered patients (20%). Hypnagogic states were assessed with one item only, and they were defined as “auditory or visual illusions that accompany falling asleep or waking in a distressing or threatening manner (e.g. hearing sounds or voices, or seeing people or things that are not in the room)”, thus rather targeting disturbing hypnagogic hallucinations than hypnagogic states per se.
In contrast, a validation study for the Durham Hypnagogic and Hypnopompic Hallucinations Questionnaire (DHQ) in 365 university students from the UK reported a much higher combined prevalence for both sleep‐onset and ‐offset hypnagogia of 85% (Jones et al., 2009). In this study, hypnagogic states were assessed with 14 different items, each asking for a specific hypnagogic experience in a mostly emotionally neutral way, such as seeing the image of a face or hearing the voice of a familiar person.
The reported studies differed in two key characteristics when assessing the prevalence of hypnagogic states: the emotional valence and the specificity of questionnaire items. Whereas some studies phrased questionnaire items in an emotionally negative way (Fulda et al., 2008; Ohayon et al., 1996), others included more emotionally neutral phrased items (Jones et al., 2009; Ohayon, 2000). Further, some studies used more broadly worded questionnaire items (Fulda et al., 2008; Ohayon, 2000), whereas others asked for more specific experiences (Jones et al., 2009; Ohayon et al., 1996). Finding common ground in assessing prevalences would be beneficial. We would argue for an emotionally neutral and broader definition of hypnagogic states while assessing their emotional nature and specific experiences separately.
Reported prevalences were generally high in Schacter's (1976) review, ranging from 72% to 77%. He noted that “figures representing the percentage of people who have experienced at least one hypnagogic image are likely to be misleading” (Schacter, 1976, p. 454), as individuals are less likely to admit experienced visions or hallucinations due to their negative connotation through societal views (Galton, 1883). This further supports our argument for a broader and emotionally neutral definition of hypnagogic states, and for the differentiation between both hypnagogic states and hypnagogic hallucinations.
Two studies reported a gender effect, with hypnagogic states occurring more frequently in women than men (Ohayon et al., 1996; Ohayon, 2000). However, no newer study has reported such a gender effect, as only one study reported insignificant findings (Larøi et al., 2019). Few studies reported an association between age and the prevalence of hypnagogic states: of those studies, only one reported a significant decrease in hypnagogic experiences with age (Larøi et al., 2019), in accordance with previous studies illustrating an age effect (Ohayon et al., 1996; Ohayon, 2000), and as described in Schacter's review. Overall, it is unclear whether studies did not investigate gender and age effects or whether they did not report them due to insignificant findings. We encourage future studies assessing the prevalence of hypnagogic states to investigate and report gender and age effects. Lastly, it remains yet to be further elaborated to what extent culture might account for differences in the prevalence of hypnagogic states, as no newer study has assessed cultural differences.
7. DIRECTION 1: THE DEVELOPMENT OF EXPERIMENTAL TECHNIQUES
As a future direction, Schacter (1976) proposed the further development of experimental techniques. While the methodological approach has not changed by a lot since Schacter's review, the newly acquired insight into the hypnagogic state is remarkable. In Table 2 we present a summary of these findings. In this section, we first discuss methods used for broad data collection, followed by methods used to assess the quality of hypnagogic states in recent times. Lastly, we discuss the linguistic analysis and its advantages over methodological approaches.
TABLE 2.
Summary of publications, their methods used and their main findings regarding this review
| Author | Year | Method | Findings |
|---|---|---|---|
| Bódizs et al. | 2005 | ECoG | 1.5–3.0 Hz activity in the parahippocampus increases at sleep‐onset, thought to be REM‐sleep specific |
| Bódizs et al. | 2008 | EEG | EEG activity at sleep‐onset resembles REM sleep activity more closely than stage 2 sleep activity |
| Bosch et al. | 2012 | Questionnaire‐Study | Patients with depression experience hypnagogic states more often than healthy controls |
| Cancelli et al. | 2004 | Review | Tricyclic antidepressants are linked with increased hypnagogic states |
| Del Prete & Tressoldi | 2005 | Experimental Study | Hypnosis can induce hypnagogic states successfully |
| Fortuyn et al. | 2009 | Questionnaire‐Study | Patients with narcolepsy experience hypnagogic states and daytime hypnagogia more often than healthy controls |
| Fulda et al. | 2008 | Questionnaire‐Study | The prevalence for hypnagogic states was higher both in a sample of not nearer defined psychiatric patients and another sample of sleep‐disordered patients, compared with healthy controls |
| Germain & Nielsen | 2001 | Systematic Self‐Observation, EEG | Kinaesthetic images were accompanied by prefrontal and frontal delta activation, visual images were accompanied by activation in left‐central and temporal regions at sleep‐onset |
| Haar Horowitz et al. | 2020 | Serial Awakening, Dormio | The content of hypnagogic experiences can successfully be induced through instruction to think about a specific target before going to sleep |
| Hayashi et al. | 1999 | EEG | Hypnagogic experiences of landscapes occur earlier during sleep‐onset, followed by dream‐like images, whereas static objects and colour patterns occur later during sleep‐onset |
| Hinton et al. | 2019 | Questionnaire‐Study | Individuals with PTSD report being bothered more by hypnagogic states (“ghost attacks”) than healthy controls |
| Horikawa et al. | 2013 | Machine Learning (fMRI data) | Brain areas that process specific stimuli show activation during the perception of corresponding stimuli in the hypnagogic state |
| Jones et al. | 2009 | Questionnaire‐Study | Intrusive thoughts and undertaking thought suppression are associated with hypnagogic experiences |
| Jones et al. | 2010 | Questionnaire‐Study | Compared with REM dreams, verbal hypnagogic experiences are less likely to be commanding, but more likely to contain a single clear word rather than sentences, sound more like familiar voices than unknown persons, and talk directly to the individual |
| Kjaer et al. | 2002 | PET | In stage 1 sleep, relative blood flow increases in visual association cortices, while relative blood flow decreases in the frontal and parietal cortex, the cerebellum and the thalamus |
| Kussé et al. | 2012 | Serial Awakening, EEG | Playing TETRIS results in hypnagogic images related to the game Anticipating to play the game after sleep results in fewer reports related to the game |
| Larøi et al. | 2019 | Questionnaire‐Study | Age is associated with a decrease in hypnagogic experiences. Depression and anxiety are associated with an increase in hypnagogic experiences over the lifespan |
| Lewis‐Hanna et al. | 2011 | fMRI | fMRI measures indicate that individuals with a history of auditory hypnagogic experiences exhibit a significantly greater speech‐evoked activation during wakefulness in the left posterior temporoparietal cortex |
| McCarthy‐Jones et al. | 2011 | Questionnaire‐Study | Auditory hypnagogic experiences are more prevalent in individuals who report a higher susceptibility to intrusive thoughts |
| Nielsen et al. | 2005 | Serial Awakening, EEG | The dream‐like quality of hypnagogic states increases after preceding REM sleep deprivation |
| Nielsen | 2017 | Sleep‐Onset Observations | The content of hypnagogic states may be influenced by self‐generated external stimuli (autosensory imagery) and non‐generated external stimuli (exosensory imagery) |
| Noreika et al. | 2015 | Systematic Self‐Observation, EEG | Linguistic intrusions are associated with higher alpha and gamma power in the left hemisphere, whereas visual images are associated with higher beta power in the right hemisphere |
| Ohayon et al. | 1996 | Questionnaire‐Study | Hypnagogic states are much more common than expected |
| Ohayon | 2000 | Questionnaire‐Study | The most frequent hypnagogic experienced mentioned is the feeling of falling down an abyss, followed by felt‐presence experiences |
| Ohayon & Shapiro | 2000 | Questionnaire‐Study | Individuals with PTSD classify the emotional quality of their hypnagogic experiences as more terrifying than healthy controls |
| Pizzagalli et al. | 2000 | Questionnaire‐Study EEG | Individuals with strong beliefs in paranormal phenomena report having hypnagogic experiences more often |
| Schacter | 1976 | Review | An extensive review on the hypnagogic state |
| Schmidt & Gendolla | 2008 | Serial Awakening, EEG | Suppressed thoughts during the day are likely to rebound into the hypnagogic state |
| Sherwood | 2012 | Questionnaire‐Study | Visual experiences, the feeling of falling and feeling a presence in the room are the most frequent hypnagogic experiences, followed by auditory, tactile, bodily and movement sensations. The rarest experiences are olfactory and gustatory |
| Siclari et al. | 2017 | Serial Awakening, EEG | A bilateral parieto‐occipital “hot zone” correlates with the likelihood of individuals reporting hypnagogic experiences. High activity in this area is associated with dream‐like experiences |
| Siclari et al. | 2013 | Serial Awakening, EEG | Hypnagogic experiences are less rich and complex, and have less continuity than REM dreams |
| Soffer‐Dudek & Shahar | 2011 | Questionnaire‐Study | Stress is associated with increased hypnagogic states when sleep quality is poor |
| Speth et al. | 2013 | Linguistic Analysis, Serial Awakening, EEG | Compared with REM dreams, hypnagogic states are experienced less from a first, second or third perspective. Moreover, there were fewer instances in which the individual or others were involved in the act of speaking |
| Speth et al. | 2016 | Linguistic Analysis, Serial Awakening, EEG | Memories of the past and thoughts about the present remain fairly constant, while memories of future events decrease throughout sleep‐onset |
| Speth et al. | 2017 | Linguistic Analysis, Serial Awakening, EEG | Hypnagogic experiences involve fewer acts of speaking and of hearing speech compared with REM dreams |
| Speth & Speth | 2016 | Linguistic Analysis, Serial Awakening, EEG | Cognitive agencies decrease throughout sleep‐onset, while motor agencies increase |
| Stenstrom et al. | 2012 | Serial Awakening, EEG | The content of hypnagogic experiences can relate to previous episodic memories |
| Stickgold et al. | 2000 | Serial Awakening (Nightcap), EEG | Playing TETRIS results in hypnagogic images related to the game. This effect also occurs in patients suffering from dense amnesia |
| Szklo‐Coxe et al. | 2007 | Questionnaire‐Study | Depression and anxiety are both associated with increased reports of hypnagogic states |
| Wackermann et al. | 2002 | Ganzfeld Method, EEG, EOG, EMG | Experiences in the ganzfeld and hypnagogic states do not differ in their phenomenology. Vigilance, however, does not decrease in the ganzfeld compared with the hypnagogic state |
| Wamsley et al. | 2010 | Serial Awakening (Nightcap), EEG | Playing a highly engaging visuomotor video game or observing someone else play results in hypnagogic images related to the game |
Abbreviations: ECoG, electrocorticography; EEG, electroencephalogram; EMG, electromyography; EOG, electrooculography; fMRI, functional magnetic resonance imaging; PET, positron emission tomography; PTSD, post‐traumatic stress disorder; REM, rapid eye movement.
First and foremost, studies aiming at investigating the prevalence and presence of hypnagogic states in the general population were most commonly conducted as telephone surveys (Ohayon et al., 1996; Ohayon, 2000; Ohayon & Shapiro, 2000), used questionnaires sent by mail (Larøi et al., 2019) or were conducted online (Sherwood, 2012). These methods allow researchers to gather a much larger quantity of reports than before, provide a broad overview about the occurrence of hypnagogic states, and invoke new theories and future research directions.
When assessing the qualitative content of hypnagogic experiences, researchers either applied the method of self‐observation (Steen, 2017) or collected data through questionnaires (Sherwood, 2012). More prominently, however, researchers relied on subject's reports at sleep‐onset combined with EEG measures to determine and assign sleep phases to the respective reports (Nielsen et al., 2005; Siclari et al., 2013; C. Speth & Speth, 2016; J. Speth et al., 2013, 2016, 2017; Wackermann et al., 2002). In “serial awakening paradigms”, participants are woken up at certain intervals during the night, and are asked about the presence or characteristics of specific experiences (Siclari et al., 2013; J. Speth et al., 2013, 2017). The reports are then contrasted among the sleep stages. Other studies focusing on the period of sleep‐onset specifically recorded measures closer in time to wakefulness, generally from a few seconds after sleep‐onset to a couple of minutes (Germain & Nielsen, 2001; Michida et al., 2005; Nielsen et al., 2005; Noreika et al., 2015; Schmidt & Gendolla, 2008; C. Speth & Speth, 2016; J. Speth et al., 2016; Stenstrom et al., 2012). In some instances, these measures were combined with an approach of systematic self‐observation (Germain & Nielsen, 2001; Nielsen, 1995; Noreika et al., 2015).
Recently, a new and more refined method of quantifying verbal reports has re‐emerged. The “linguistic analysis” allows the classification of report content to predefined categories. The number of classified elements during sleep‐onset is then statistically contrasted with those from rapid eye movement (REM) dreams reports. This method is particularly interesting as it allows a systematic comparison of hypnagogic experiences: different phenomenological characteristics of hypnagogic states and REM sleep can thus be investigated and distinguished (J. Speth et al., 2013, 2017). This procedure can further be applied to sleep‐onset by analysing reports during wakefulness and a couple of seconds to a couple of minutes after sleep‐onset (C. Speth & Speth, 2016; J. Speth et al., 2016). Linguistic analysis possesses one major advantage over the other mentioned research methods: participants are free to report their experiences and are not limited by being asked about specific experiences, as the classification occurs after reports are collected. Thus, the provided report of hypnagogic experiences remains unbiased but can still be examined and contrasted systematically. An example of this procedure is provided in the following section.
8. DIRECTION 2: THE PHENOMENOLOGY OF HYPNAGOGIC STATES
Schacter (1976) suggested that a more precise phenomenology of imagery and verbal experiences in the hypnagogic state is needed. First, we investigate the modalities in which hypnagogic states occur. Next, we report how hypnagogic states are experienced in comparison to REM dreams, how their phenomenology changes over the course of falling asleep, and end up discussing the emotional quality of hypnagogic states.
Subjectively, hypnagogic experiences have been described as crisp, detailed, non‐transparent, as well as changing unexpectedly and rapidly (Steen, 2017), and can consist of multiple elements integrated in a coherent three‐dimensional scene (Stenstrom et al., 2012). Questionnaire studies reported different findings regarding the frequency of the modality in which hypnagogic states are experienced. In the survey conducted by Ohayon (2000), hypnagogic experiences in the haptic modality were by far the most prominent, such as the feeling of falling, followed by the feeling of someone or something being present in the room, while visual and auditory experiences were the least reported. Similarly, in the study of Jones et al. (2009), the feeling of a presence in the room was the most frequently reported hypnagogic experience, followed by auditory experiences such as participants hearing their names or familiar voices. However, a more recent survey conducted by Sherwood (2012) suggested hypnagogic experiences in the visual modality to be the most prominent, followed by the feeling of falling, the sense of a presence and auditory experiences, with the perception of taste and smell being the rarest.
Generally, hypnagogic experiences occurring at sleep‐onset have been described as more vivid and hallucinatory than dreams (Chokroverty, 2017). On the other hand, they have been described as less rich and complex, and to have less continuity than REM dreams (Siclari et al., 2013). More unique characteristics of hypnagogic experiences at sleep‐onset and ‐offset emerge with the application of the linguistic analysis, particularly when contrasting these experiences with REM dreams. Compared with REM dreams, participants reported experiencing the hypnagogic state significantly less from a first (e.g. I ran; We ran), second or third (e.g. You/She/They ran) perspective, with less reported instances in which the individuals themselves (e.g. I said: “Run!”) or others (e.g. You said: “Run!”) were involved in the act of speaking (J. Speth et al., 2013). We would thus expect individuals to experience the hypnagogic state less from a specific perspective as an involved participant in an ongoing narrative but much more so as a passive spectator of internal events. In REM dreams, however, the individual acts as a direct participant (Waters et al., 2016), contrary to hypnagogic states.
When auditory experiences are further differentiated into an auditory–verbal agency, meaning the act of speaking, and auditory–verbal experiences, describing the act of hearing speech, both are reported to be significantly less frequent in hypnagogic states than REM dreams (J. Speth et al., 2017). Verbal experiences were described as less likely to be commanding, more likely to contain single clear words than clear sentences, sound more like familiar voices than unknown persons, and talk directly to the individual (Jones et al., 2010). In summary, speech seems less present in hypnagogic states than REM dreams, independent of the speaker's perspective. However, if speech is present, it is more likely to sound like single words from a familiar voice addressing the individual directly in a non‐commanding way.
When inspecting sleep‐onset, the linguistic analysis further provides insight into the phenomenology of hypnagogic states: while memories of the past and thoughts about the present remain fairly constant, it seems that memories of events that will occur in the future decrease while falling asleep (J. Speth et al., 2016). This suggests a decrease in prospective hypnagogic content to a focus on present and past events. Over the process of falling asleep, hypnagogic experiences decrease in their reported cognitive agencies (e.g. thoughts or thinking about performing an action), while reported motor agencies (e.g. imagery of performing an action) increase (C. Speth & Speth, 2016). These results indicate a transition from a predominantly thought‐filled wake state to a higher presence of motor imagery in the hypnagogic state. Conclusively, phenomenological differences in the hypnagogic state can already be observed as early as a few seconds up to a few minutes after sleep‐onset.
Rarely, the emotional valence of hypnagogic experiences has been reported in studies assessing the phenomenology of the hypnagogic states. However, one study questioned Cambodian refugees with post‐traumatic stress disorder (PTSD) about their experiences with so‐called “ghost attacks”, as it had been noted that Cambodians often attribute their auditory and visual hallucinations during wakefulness and experiences in states of partial wakefulness to spirits (Hinton et al., 2019): during the hypnagogic state, individuals most prominently reported seeing a human‐shaped shadow at the foot of their bed, heard someone calling out to them and beckoning them. Regarding the emotional nature of such an experience, individuals with PTSD reported being bothered more by these encounters on average than individuals without PTSD. Thus, hypnagogic states can very much be experienced negatively and as bothersome, which supports our differentiation of hypnagogic states and hypnagogic hallucinations.
In conclusion, there are differences between hypnagogic imagery and dreams. Individuals are much less actively involved in their hypnagogic experiences than in their dreams, both in physical actions and verbal interaction. Over the course of sleep‐onset, hypnagogic experiences regarding future events decrease, and a shift away from a thought‐filled state towards motor imagery occurs. Lastly, regarding the emotional quality of hypnagogic states, these experiences appear more bothersome in individuals with PTSD.
9. DIRECTION 3: THE INDIVIDUAL'S CAPACITY TO PROCESS THE ENVIRONMENT
Schacter (1976) proposed the need for a better understanding of the individual's capacity to process environmental stimuli in the hypnagogic state. Does the stimulation experienced prior to the hypnagogic state influence hypnagogic imagery?
Throughout sleep, humans cycle through the three non‐REM (NREM) stages (N1–N3) of increasing sleep depth, and end up in the last stage of sleep named REM sleep (Patel et al., 2020). As hypnagogic stages occur during the transition from wakefulness to sleep, they are by definition assigned to the first stage of sleep (Vaitl et al., 2005), as seen in Figure 1. During this same cycle of sleep, the processing of external stimuli (in the different modalities of perception) is still present in NREM sleep but vanishes during REM sleep (Waters et al., 2016). While perceptions of sounds during wakefulness elicit a response in the thalamus and the primary auditory cortex, these responses persist in NREM sleep and show cortically enhanced activation when sounds induce K‐complexes, suggesting a continued response of the brain to auditory stimuli during NREM sleep (Dang‐Vu et al., 2011). This cortical responsivity was proposed to be enhanced in individuals commonly experiencing hypnagogic states (Waters et al., 2016).
The capacity of an individual to process environmental information in the hypnagogic state seems to have been investigated in the auditory modality only: the ability of an individual to process auditory stimuli from the environment persists in early phases of sleep. Further, the likelihood of hypnagogic experience might be linked to an increased cortical responsivity. Interestingly, however, there is evidence that the processing of hypnagogic experiences might impair the simultaneous processing of external stimuli at sleep‐onset (Michida et al., 1998, 2005). Nonetheless, the individual's ability to process stimuli during the hypnagogic state is maintained, albeit less pronounced. Thus, the content of hypnagogic states could be influenced by external stimuli. These findings provide significant directions towards identifying the neural correlates of hypnagogic states and, consequently, determining individuals' proneness to experience them.
Recently, Nielsen (2017) proposed two distinct processes on how external stimuli might shape the content of hypnagogic experiences, expanding on Silberer's (1951) study of autosymbolic images: autosensory imagery and exosensory imagery. Autosensory imagery arises through self‐generated stimuli, such as muscle twitches or jerks, or snoring. Such self‐generated stimuli may be caused by a previous image and merge with a subsequent one. On the other hand, exosensory imagery is caused by environmental stimuli, such as turbulations on an airplane or noise from the nearby tennis court. Although based on anecdotal evidence, this distinction provides a valuable perspective on the possible interplay between internal and external stimuli and the content of hypnagogic states.
The second element of this future direction proposed by Schacter (1976), that is, to advance the capacity to process the environment in the hypnagogic state, revolves around how sensory stimulation experienced before entering the hypnagogic state influences its occurrence and content. Several activities before sleep have been reported to influence the likelihood of hypnagogic experiences: for instance, activities such as reading in bed, the engagement in intellectual activities, and the consumption of alcohol in bed were associated with more frequently occurring hypnagogic experiences (Ohayon et al., 1996). However, these findings do not disclose to what extent the activities performed before the hypnagogic state influence its content.
In a study by Stickgold et al. (2000), participants who played the game TETRIS before going to sleep reported hypnagogic images related to the game. Interestingly, this effect also occurred in patients suffering from dense amnesia. These findings suggest independence of hypnagogic states from declarative memory, as the patients had no recollection of playing the game (Stickgold et al., 2000). In a similar study by Kussé et al. (2012), playing TETRIS before sleep resulted in hypnagogic images being related to the game (10% of total reports), while anticipating to play the game after sleep resulted in fewer reports related to the game (3% of total reports). Thus, the sole anticipation of playing the game was less reliable to produce related hypnagogic images compared with the actual exposure to visual input from the game. Moreover, Wamsley et al. (2010) had their participants play a highly engaging visuomotor video game named Alpine Racer™ II, and collected subsequent reports at sleep‐onset and wakefulness. Reports related to the task were present, independently of whether participants were actively engaged in the task or passively observed another person playing the video game. A recent study, however, questioned the independence of hypnagogic imagery from declarative memory, and suggested that the hippocampus was involved when perceiving more complex scenes (Stenstrom et al., 2012).
These studies demonstrate that the consumption of video games or visual media before going to sleep can influence the content of hypnagogic states. As the study of Wamsley et al. (2010) demonstrated that passively observing a task is enough to influence the content of hypnagogic states, the consumption of visual media (such as TV) before bed may already be sufficient to influence hypnagogic imagery. Conclusively, it seems that stimulation experienced before going to bed is likely to find its way into our hypnagogic states.
10. DIRECTION 4: INDIVIDUAL AND CLINICAL DIFFERENCES
In his fourth future direction, Schacter (1976) emphasized the need for a better understanding of the influence of cognitive, perceptual and personality variables on the content hypnagogic states. Thus, this section revolves around the multiple individual and clinical differences associated with hypnagogic states. We discuss individual differences, followed by their associated clinical diagnosis, respectively. How are these differences associated with an increased likelihood of hypnagogic states, and can these differences determine the content of these experiences?
Indicators of insomnia, such as difficulties falling asleep, maintaining sleep, early awakening, difficulties maintaining sleep and an overall non‐restorative sleep, as well as daytime sleepiness, were reported to be associated with a higher likelihood of reporting hypnagogic states (Ohayon et al., 1996). This influence of sleep quality further appears to be moderated by stress, with more hypnagogic states occurring in stressed individuals when their overall sleep quality is poor (Soffer‐Dudek & Shahar, 2011). Moreover, the quality of hypnagogic states was reported to increase in dream‐like qualities when individuals were deprived from REM sleep prior to entering the hypnagogic state (Nielsen et al., 2005). As such, we would emphasize the role of sleep in the emergence and quality of hypnagogic states.
In a clinical context, hypnagogic states might be most known to be associated with narcolepsy, a chronic sleep disorder characterized by excessive daytime sleepiness (Schiappa et al., 2018). Hypnagogic states are not only an associated feature of narcolepsy, but they also are favourable for a diagnosis. Accordingly, the prevalence of hypnagogic states was higher in patients with narcolepsy than controls (Bosch et al., 2012; Fortuyn et al., 2009). Moreover, patients with narcolepsy are also more prone to experience daytime hypnagogia (Fortuyn et al., 2009), most likely due to excessive daytime sleepiness.
The DSM‐5 further suggests that vivid hypnagogic hallucinations, mistaken as real experiences, might indicate schizophrenia. Thus, one would expect a higher prevalence of hypnagogic states in individuals at risk of schizophrenia. Accordingly, hypnagogic states were more frequent in individuals who experienced dissociations (both clinical and non‐clinical), such as absorption, amnesia and derealization/depersonalization (Soffer‐Dudek & Shahar, 2011). Moreover, individuals who exhibited strong beliefs in paranormal phenomena reported having more hypnagogic experiences than those who did not (Pizzagalli et al., 2000). Interestingly, individuals with schizophrenia reported hypnagogic states more often than healthy controls (Bosch et al., 2012; Fortuyn et al., 2009), but this difference did not yield significance (Bosch et al., 2012). This insignificance, however, might be caused by a lack of statistical power due to small sample sizes of 29 participants in each group.
Further, the suppression of thoughts and the susceptibility to intrusive thoughts seem to be associated with hypnagogic states. When instructed to suppress a specific thought content during the day, subjects reported more target thoughts when falling asleep than controls, an effect that has been demonstrated in dreams previously (Schmidt & Gendolla, 2008; Wegner et al., 1987). Similarly, auditory hypnagogic experiences were associated with the tendency to undertake thought suppression and the self‐reported susceptibility to intrusive thoughts (Jones et al., 2009; McCarthy‐Jones et al., 2011).
Accordingly, hypnagogic experiences are more likely to occur in individuals with PTSD (Hinton et al., 2019; Ohayon & Shapiro, 2000), as intrusive thoughts are a hallmark of PTSD and are more likely following traumatic events (Shipherd & Salters‐Pedneault, 2008). In fact, hypnagogic experiences are intrusive as they occur unwillingly. Individuals with PTSD judge the emotional quality of their hypnagogic states as more terrifying than healthy controls (Ohayon & Shapiro, 2000). Moreover, the severity of being bothered by these experiences is highly correlated with the severity of the diagnosis (Hinton et al., 2019).
Two further clinical diagnoses associated with the frequency of hypnagogic states are depression and anxiety. The prevalence of hypnagogic states was significantly higher in patients diagnosed with depression (Bosch et al., 2012), although medication was not controlled for. A control for medication would be especially crucial, as, for example, tricyclic antidepressants have been linked with an increase in hypnagogic states (Cancelli et al., 2004). Further, both depression and anxiety were associated with increased reports of hypnagogic states both overall (Szklo‐Coxe et al., 2007) and over the lifespan (Larøi et al., 2019).
In conclusion, multiple factors can influence the occurrence of hypnagogic states, such as narcolepsy, PTSD, anxiety and depression, as well as other factors, such as sleep, stress and strong beliefs in paranormal phenomena. Regarding hypnagogic imagery, certain individual differences, such as the suppression of specific thoughts that rebound into hypnagogic states and the consumption of video games, were shown to influence the content of hypnagogic states.
11. DIRECTION 5: THE PSYCHOPHYSIOLOGICAL APPROACH
Schacter (1976) requested a fuller development of the psychophysiological approach. This approach has made great strides in recent years and was complemented by newer methodological approaches. Thus, we discuss the different attempts made to identify the neural correlates of hypnagogic states.
Horikawa et al. (2013) used machine learning to predict the neural correlates of visual images during the hypnagogic state. Words describing visual content from participant reports were added into a lexical database, which grouped semantically similar words. Then, every functional magnetic resonance imaging (fMRI) dataset recorded before participants' awakenings was labelled with information about the presence or absence of each of the semantically similar word groups. Next, the content of visual experiences in the hypnagogic state based on the brain activity at sleep‐onset was predicted using machine learning. Results indicated that higher visual areas such as the fusiform face area performed better with human‐associated word groups. In contrast, the parahippocampal place area performed better with word groups associated with scenes. The authors concluded that brain areas processing specific stimuli show activation during the perception of corresponding stimuli in the hypnagogic state.
Extending these findings, Siclari et al. (2017) used a serial awakening paradigm to investigate the neural correlates of dreaming in REM and NREM sleep. Participants were awoken repeatedly during the night and questioned about their experiences: if participants reported having experienced something, they were asked to describe the most recent content of their experience. Further, they were asked to rate their experience from exclusively thought‐like to exclusively perceptive. EEG measures indicated a bilateral parieto‐occipital “hot zone” correlating with the likelihood of participants reporting dream experiences both in REM and NREM sleep: When EEG activity was high in this area, subjects reported having dream‐like experiences. When EEG activity was low, however, subjects reported no experiences. The authors suggested that, as slow waves relatively spare sensory parieto‐occipital regions in the early part of the sleep‐onset process (Siclari et al., 2014), this circumstance may account for hypnagogic experiences at sleep‐onset (Siclari & Tononi, 2017). Hypnagogic states could thus be associated with higher activity in this very parietal‐occipital “hot zone”.
Kjaer et al. (2002) investigated the cerebral blood flow during light sleep. Specifically, they used positron emission tomography (PET) to identify changes in regional cerebral blood flow during subjectively reported hypnagogic experiences at sleep‐onset. In stage 1 sleep, a relative blood flow increase in visual association cortices compared with the state of wakefulness was found. Interestingly, this did not apply to the primary visual cortex. Concurrently, a relative blood flow decrease in the frontal and parietal cortex, the cerebellum, and the thalamus was measured. According to the authors, the relative blood flow increase in visual association cortices accounts for the visual imagery experienced at sleep‐onset.
Lewis‐Hanna et al. (2011) investigated differences in cortical activation in individuals who had experienced auditory hypnagogic states compared with individuals who did not. The fMRI measures indicated that individuals with a history of auditory hypnagogic experiences exhibited a significantly greater speech‐evoked activation in the left posterior temporoparietal cortex during wakefulness, which peaked in the left supramarginal gyrus. The authors suggested that the hypersensitivity of the left supramarginal gyrus might play a role in whether individuals experience auditory hypnagogic states or not. Although these results must be interpreted with caution due to their small sample size, they further indicate differences in cortical activation that might be accountable for a disposition towards hypnagogic experiences.
Several studies assessed the electrophysiological correlates of specific content of hypnagogic experiences at sleep‐onset. This line of research was guided by the distinction between different stages during sleep‐onset by Hori et al. (1994). They defined nine distinct stages of sleep‐onset, which were distinct in their electrophysiological characteristics (Table 3). Hori et al. (1994) found that most hypnagogic experiences were recalled when awoken from sleep‐onset stage 5. Moreover, kinaesthetic imagery decreased over the course of the sleep‐onset stages, whereas visual and auditory imagery increased. This subdivision of sleep‐onset into different stages marks a major improvement since Schacter's (1976) review, as it allows the investigation of hypnagogic states on a smaller and more precise scale.
TABLE 3.
Sleep‐onset stages defined by Hori et al. (1994)
| Sleep stage | EEG stage | Characteristics |
|---|---|---|
| Wake | 1 | Alpha wave train |
| 2 | Alpha wave intermittent (> 50% alpha activity) | |
| Stage 1 | 3 | Alpha wave intermittent (< 50% alpha activity) |
| 4 | EEG flattening | |
| 5 | Ripples | |
| 6 | Hump solitary | |
| 7 | Humps train | |
| 8 | Humps with incomplete spindles | |
| Stage 2 | 9 | Spindles |
Abbreviation: EEG, electroencephalogram.
Hayashi et al. (1999) also awakened participants during different sleep‐onset stages (Hori et al., 1994) and asked them to report the content of their experiences. The results suggested that different contents coincided with specific EEG signatures: landscapes were most prevalent early over sleep‐onset during the presence of alpha waves (sleep‐onset stage 3), whereas dream‐like images and images of people occurred later when EEG flattening (sleep‐onset stage 5) and vertex sharp waves (sleep‐onset stage 6) occurred. On the other hand, static objects and colour patterns occurred the latest over the course of sleep‐onset when sleep spindles were present. Thus, the content of hypnagogic states was associated with different electrophysiological characteristics and was temporally distributed over the course of sleep‐onset.
Germain and Nielsen (2001) compared EEG signals over the course of sleep‐onset for both reported unimodal visual and kinaesthetic hypnagogic experiences. Most hypnagogic experiences were reported during Hori stages 4 and 5 of sleep‐onset. Changes in topographical power were different for visual and kinaesthetic reports. Overall, visual and kinaesthetic reports were preceded by a decrease in alpha and theta power. Kinaesthetic experiences coincided with increased frontal delta power, while visual experiences coincided with increased power in left central and temporal regions. Germain and Nielsen concluded that kinaesthetic experiences occur earlier than visual experiences over the course of sleep‐onset, consistent with Hori et al. (1994).
Noreika et al. (2015) were interested in how linguistic intrusions differed from the more common hypnagogic experiences in a single case study. Linguistic intrusions, which could be classified as auditory hypnagogic experiences, were described as words or phrases that occasionally occurred and were unrelated to imagery perceived at sleep‐onset. In accordance with Germain and Nielsen (2001), the authors found a decrease in alpha and theta power right before reports of visual hypnagogic experiences. However, these so‐called linguistic intrusions differed from reports of visual experiences in that they were preceded by a significant increase in theta power. Moreover, linguistic intrusions were associated with higher alpha and gamma power in the left hemisphere, whereas visual hypnagogic experiences were associated with higher beta power in the right hemisphere.
Lastly, two studies assessed similarities between REM sleep and sleep‐onset activity. According to the covert‐REM sleep hypothesis of dreaming, elements of REM sleep that emerge during sleep‐onset are suspected to be causal for hypnagogic experiences (Nielsen, 2000). To examine this hypothesis, Bódizs et al. (2005) used electrocorticography (ECoG) to measure parahippocampal activity. The authors reported an increase in 1.5–3.0 Hz activity in the parahippocampus at sleep‐onset, which was reported to be specific to REM sleep. In a second study, Bódizs et al. (2008) used EEG measures to compare sleep‐onset‐activity with stage 2 and REM sleep. EEG activity during sleep‐onset shared more similarities with REM sleep activity than with stage 2 sleep activity. Further, REM‐like EEG activity increased during alpha dropout at sleep‐onset. The authors concluded their results to be in favour of the covert‐REM sleep hypothesis. The interplay between REM‐like activity and hypnagogic states, however, remains to be further inspected.
Since Schacter (1976) proposed a fuller development of the psychophysiological approach to assessing hypnagogic states, research has progressed. While EEG measures still represent an integral part of hypnagogia research, they were complemented with other imaging techniques. Attempts at understanding the neural correlates of specific contents during hypnagogic experiences are heading in a promising direction.
12. DIRECTION 6: THE INDUCTION OF HYPNAGOGIC STATES
The last proposed future direction suggested by Schacter (1976) revolves around the necessary conditions to enter the hypnagogic state. Two questions are of interest in this section, namely, whether hypnagogic states can successfully be induced, and whether these artificially induced hypnagogic states differ from naturally occurring ones.
One study tried to induce hypnagogic states using the multi‐modal ganzfeld technique (Wackermann et al., 2002), an approach generally used to induce altered states of consciousness (Wackermann et al., 2008). Participants sitting in a reddish illuminated room were instructed to sit with their eyes open while having their eyes covered with anatomically shaped halves of ping‐pong balls. Concurrently, the sound of a waterfall was projected onto headphones as white noise, and reports were acquired on‐demand, as initiated by the experimenter. A qualitative analysis of reports did not yield a difference between hypnagogic experiences and experiences in the ganzfeld. However, the EEG signal indicated that a decrease in vigilance was absent in ganzfeld‐induced states, unlike when falling asleep. The authors concluded that, although different from the waking state, the ganzfeld‐induced state was more comparable to the waking state than the hypnagogic state, deeming this approach imprecise. However, even if different from sleep‐onset hypnagogia, this method may be useful to investigate daytime hypnagogia.
Another method to induce the hypnagogic state is hypnosis. So far, only one study attempted to induce hypnagogic experiences through hypnosis (Del Prete & Tressoldi, 2005). In this study, a hypnotist induced the hypnagogic state through a modified Jacobson technique. The individual is instructed on how to relax the principal muscle groups of the body (Jacobson, 1925) for about 20–30 min. Then, the participant was induced into hypnosis through indirect flight suggestion for 15–20 min. Different indices, such as “deep muscular relaxation, slow and regular breath, reports of spontaneous images, slow ocular movements, and a sensation of hand paralysis” (Del Prete & Tressoldi, 2005, p. 332), were interpreted as the attainment of the hypnagogic state.
Thus, hypnosis seems to be promising. In fact, it has been stated that the induction of drowsiness during hypnosis can increase the vividness of imagery through a reduction of internal and external distractors (Robazza & Bortoli, 1995). The increasing internal awareness associated with hypnosis (Demertzi et al., 2015) could thus facilitate the occurrence of hypnagogic experiences during this state of drowsiness, and improve the ability to monitor and report these experiences in greater detail. Interestingly, hypnosis has not only been used to induce hypnagogic states, but also to treat hypnagogic hallucinations (Gathercole, 2008).
Recently, Haar Horowitz et al. (2020) proposed a new approach to intentionally induce the content of hypnagogic states, a targeted dream incubation procedure called Dormio. Before sleep‐onset, participants were prompted with a target stimulus to be induced, for example, “remember to think of a tree”. Heart rate, finger flexion and electrodermal activity were then tracked to identify sleep‐onset. Once asleep, participants were woken up and asked about the content of their thoughts and whether they were asleep. After collecting reports, participants were reminded to think of the target stimulus. This procedure was then repeated for a total of 45 min. The procedure successfully induced the target stimulus into the content of hypnagogic experiences, in contrast to a control condition where no target stimulus was presented. However, as admitted by the authors, it remains questionable whether reports from subjects can be trusted due to influence from demand characteristics. Nevertheless, the results demonstrate the malleability of hypnagogic experiences through external stimuli and/or demands.
In summary, the artificial induction of hypnagogic states has not been sufficiently tested, but hypnosis seems to be a promising method to induce hypnagogic states at sleep‐onset. The ganzfeld method, on the other hand, could be used to assess daytime hypnagogia. Hypnagogia has generally been investigated when naturally occurring, such as by using self‐observation or sleep‐onset measures. Importantly, these artificially induced hypnagogic states do not seem to differ from naturally occurring ones.
13. CONCLUSIONS
The purpose of this review was to provide an update on hypnagogic states since the review of Schacter (1976). We evaluated the proposed future directions suggested by Schacter (1976) based on the more recent literature.
Multiple methods were used to assess both the prevalence and quality of hypnagogic states. Two approaches emerged as the most promising to examine hypnagogic states: the serial awakening paradigm (Siclari et al., 2013, 2017); and the linguistic analysis (C. Speth & Speth, 2016; J. Speth et al., 2013, 2016, 2017). Both approaches assess hypnagogic states when they naturally occur in close temporal proximity to the experience. Moreover, both allow hypnagogic states to be contrasted with dreams. The linguistic analysis also allows a systematic exploration of hypnagogic experiences without limiting reports to predefined categories, as the classification occurs after collected reports.
Previously, literature reported hypnagogic states to occur most frequently in the visual modality, followed by the auditory and tactile‐kinaesthetic modality (Schacter, 1976). However, our review suggests otherwise: kinaesthetic experiences, such as the feeling of a presence in the room and the feeling of falling, as well as visual experiences were the most frequent, followed by experiences in the auditory modality (Jones et al., 2009; Ohayon, 2000; Sherwood, 2012). When comparing hypnagogic states with dreams, both can be differentiated by clear characteristics. Most notably, individuals experience less active involvement in hypnagogic states than in dreams (J. Speth et al., 2013, 2017).
Both stimuli prior to and during the hypnagogic state can influence an individual's experiences. There is evidence for a continued response of the individuum to external stimuli during the hypnagogic state (Dang‐Vu et al., 2011; Michida et al., 2005; Waters et al., 2016) and, thus, these stimuli could shape the content of our hypnagogic experiences. However, it remains to be assessed how stimuli in modalities other than the auditory modality are accessible while falling asleep. An influence on the content of hypnagogic states from self‐generated and environmental external stimuli appears plausible (Nielsen, 2017). Regarding experiences prior to the hypnagogic states, three studies successfully demonstrated that playing or watching someone other play a game during wakefulness dictated the content of hypnagogic experiences (Kussé et al., 2012; Stickgold et al., 2000; E. J. Wamsley et al., 2010). Conclusively, visual activities before bedtime, such as watching movies or playing video games, are likely to influence the content we experience during the hypnagogic state.
Likewise, several clinical and individual differences are associated with the occurrence of hypnagogic states. Sleep quality and narcolepsy, traits of dissociation and schizophrenia, intrusive thoughts and PTSD, and depression and anxiety were linked to more frequent hypnagogic states. Most notably, however, suppressing a thought throughout the day resulted in its re‐emergence during the hypnagogic state, as it was previously observed in dreams, in accordance with the theory of dream rebound (Wegner et al., 2004; Wegner et al., 1987).
We have seen further progress in the development of the psychophysiological approach, with results providing insight as to how hypnagogic states may occur. First, three possible correlates of hypnagogic states were identified: a blood flow increase in visual association areas, a decrease in alpha and theta power, and an increased activity in a parieto‐occipital zone. Second, the content of hypnagogic experiences coincides with specific patterns of electrophysiological activity and might be determined by activation in the very same cortical areas that process specific stimuli during wakefulness, as machine learning suggests. Third, findings suggest that individuals with higher cortical activity are more likely to experience hypnagogic states.
Lastly, hypnagogic states can be induced with the ganzfeld method and hypnosis, and their content can be induced with procedures through targeted dream incubation. Hypnagogic reports did not differ qualitatively in the ganzfeld from naturally occurring ones. As EEG signals in the ganzfeld relate more to wakefulness than sleep‐onset, we would suggest that the ganzfeld procedure may be useful to assess daytime hypnagogia. So far, hypnosis was barely used to investigate hypnagogic states, but seems to be a promising approach to induce hypnagogic experiences and eventually manipulate their content. On the other hand, procedures such as Dormio can be used to induce specific experiences in the hypnagogic state (Haar Horowitz et al., 2020).
A barely addressed aspect of hypnagogic experiences is their emotional quality. Previously, researchers assumed hypnagogic experiences to have either a negative emotional quality or be emotionally flat. However, our review states that hypnagogic experiences can be positive and negative or have no emotional quality at all. We thus proposed the differentiation between hypnagogic states and hypnagogic hallucinations, with the latter having a negative emotional quality, being clinically relevant. However, their interplay has yet to be examined. When do hypnagogic states become hypnagogic hallucinations? And what factors determine the emotional quality of these experiences?
Moreover, the question as to why hypnagogic states occur most frequently in the kinaesthetic and visual modality requires further investigation. The hypnagogic state differs from wakefulness in two ways regarding sensory perception. First, there is a lack of visual input due to generally closed eyes at sleep‐onset, which might account for the visual experiences in the hypnagogic state. Second, vestibular input differs due to a lying position when going to sleep. This circumstance might account for the often occurring kinaesthetic experiences, such as the feeling of falling. Hypnagogic states could be a byproduct from adaptational processes of the vestibular system, which could explain the frequent kinaesthetic experiences at sleep‐onset.
Another, far more central question, is that of their purpose: do hypnagogic states fulfil a specific purpose or are they but a phenomenon without meaning occurring due to the loss of volitional control? We now know that memories of future events significantly decrease during the hypnagogic state, while memories of the past persist (J. Speth et al., 2016). During dreams, however, more than a quarter of dreams relate to future events (Wamsley, 2021). As suppressed thoughts have been shown to rebound both into the hypnagogic state and dreams, it could be that both phenomena help us cope with defining experiences, be it in different ways: hypnagogic states perhaps help us process and consolidate our past experiences, while dreams could allow us to process these experiences and prepare us for future events. This form of prospective coding in dreams has been discussed recently (Llewellyn, 2016). However, future research is needed to understand the purpose of hypnagogic states.
Hypnagogic states occur quite frequently and can occur in all modalities. They are distinguishable from similar phenomena by clear characteristics and are generally benign. They can, however, manifest as hypnagogic hallucinations, where they are perceived as bothersome and have a negative emotional quality. As hypnagogia research progresses, a better understanding of the hypnagogic state will be achieved. Although some uncertainties remain at present, this phenomenon becomes more and more tangible.
AUTHOR CONTRIBUTIONS
Romain Ghibellini and Beat Meier wrote the manuscript and approved the final manuscript for submission.
CONFLICTS OF INTEREST
The authors declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
ACKNOWLEDGMENTS
Open access funding provided by Universitat Bern.
Ghibellini, R. , & Meier, B. (2023). The hypnagogic state: A brief update. Journal of Sleep Research, 32(1), e13719. 10.1111/jsr.13719
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- American Academy of Sleep Medicine . (2014). International classification of sleep disorders—Third edition (ICSD‐3) online version. American Academy of Sleep Medicine. [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders: DSM‐5 (5th ed.). American Psychiatric Association. [Google Scholar]
- Baylor, G. W. , & Cavallero, C. (2001). Memory sources associated with REM and NREM dream reports throughout the night: A new look at the data. Sleep, 24(2), 165–170. [PubMed] [Google Scholar]
- Bódizs, R. , Sverteczki, M. , Lázár, A. S. , & Halász, P. (2005). Human parahippocampal activity: Non‐REM and REM elements in wake–sleep transition. Brain Research Bulletin, 65(2), 169–176. 10.1016/j.brainresbull.2005.01.002 [DOI] [PubMed] [Google Scholar]
- Bódizs, R. , Sverteczki, M. , & Mészáros, E. (2008). Wakefulness–sleep transition: Emerging electroencephalographic similarities with the rapid eye movement phase. Brain Research Bulletin, 76(1–2), 85–89. 10.1016/j.brainresbull.2007.11.013 [DOI] [PubMed] [Google Scholar]
- Bosch, P. , van Luijtelaar, G. , Groetelaers, L. , van den Noort, M. , Lim, S. , Egger, J. , & Coenen, A. (2012). The Munich parasomnia screening in psychiatry. Somnologie ‐ Schlafforschung und Schlafmedizin, 16(4), 257–262. 10.1007/s11818-012-0587-4 [DOI] [Google Scholar]
- Cancelli, I. , Marcon, G. , & Balestrieri, M. (2004). Factors associated with complex visual hallucinations during antidepressant treatment. Human Psychopharmacology: Clinical and Experimental, 19(8), 577–584. 10.1002/hup.640 [DOI] [PubMed] [Google Scholar]
- Cheyne, J. A. , Rueffer, S. D. , & Newby‐Clark, I. R. (1999). Hypnagogic and hypnopompic hallucinations during sleep paralysis: Neurological and cultural construction of the night‐mare. Consciousness and Cognition, 8(3), 319–337. 10.1006/ccog.1999.0404 [DOI] [PubMed] [Google Scholar]
- Chokroverty, S. (2017). Basic science technical considerations and clinical aspects. In Sleep Disorders Medicine. Springer. 10.1007/978-1-4939-6578-6 [DOI] [Google Scholar]
- D'Agostino, A. , & Limosani, I. (2010). Hypnagogic hallucinations and sleep paralysis. In Goswami M., Pandi‐Perumal S. R., & Thorpy M. J. (Eds.), Narcolepsy. Springer. 10.1007/978-1-4419-0854-4_8 [DOI] [Google Scholar]
- Dang‐Vu, T. T. , Bonjean, M. , Schabus, M. , Boly, M. , Darsaud, A. , Desseilles, M. , Degueldre, C. , Balteau, E. , Phillips, C. , Luxen, A. , Sejnowski, T. J. , & Maquet, P. (2011). Interplay between spontaneous and induced brain activity during human non‐rapid eye movement sleep. Proceedings of the National Academy of Sciences, 108(37), 15438–15443. 10.1073/pnas.1112503108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete, G. , & Tressoldi, P. E. (2005). Anomalous cognition in hypnagogic state with OBE induction: An experimental study. Journal of Parapsychology, 69(2), 329. [Google Scholar]
- Demertzi, A. , Vanhaudenhuyse, A. , Noirhomme, Q. , Faymonville, M.‐E. , & Laureys, S. (2015). Hypnosis modulates behavioural measures and subjective ratings about external and internal awareness. Journal of Physiology‐Paris, 109(4–6), 173–179. 10.1016/j.jphysparis.2015.11.002 [DOI] [PubMed] [Google Scholar]
- Domhoff, G. W. (1996). Finding meaning in dreams: A quantitative approach (Vol. 14, p. 356). Plenum Press. 10.1007/978-1-4899-0298-6 [DOI] [Google Scholar]
- Fortuyn, H. A. D. , Lappenschaar, G. A. , Nienhuis, F. J. , Furer, J. W. , Hodiamont, P. P. , Rijnders, C. A. , Lammers, G. J. , Renier, W. O. , Buitelaar, J. K. , & Overeem, S. (2009). Psychotic symptoms in narcolepsy: Phenomenology and a comparison with schizophrenia. General Hospital Psychiatry, 31(2), 146–154. 10.1016/j.genhosppsych.2008.12.002 [DOI] [PubMed] [Google Scholar]
- Fulda, S. , Hornyak, M. , Müller, K. , Cerny, L. , Beitinger, P. A. , & Wetter, T. C. (2008). Development and validation of the Munich parasomnia screening (MUPS): A questionnaire for parasomnias and nocturnal behaviors. Somnologie ‐ Schlafforschung Und Schlafmedizin, 12(1), 56–65. 10.1007/s11818-008-0336-x [DOI] [Google Scholar]
- Galton, F. (1883). Inquiries into human faculty and its development. Macmillan. [Google Scholar]
- Gathercole, M. (2008). The use of hypnosis for the treatment of hypnagogic hallucinations. Australian Journal of Clinical & Experimental Hypnosis, 36(2), 169–175. [Google Scholar]
- Germain, A. , & Nielsen, T. A. (2001). EEG power associated with early sleep onset images differing in sensory content. Sleep Research Online, 4(3), 83–90. [Google Scholar]
- Green, M. W. (2001). The exploding head syndrome. Current Pain and Headache Reports, 5(3), 279–280. 10.1007/s11916-001-0043-9 [DOI] [PubMed] [Google Scholar]
- Gurstelle, E. B. , & de Oliveira, J. L. (2004). Daytime parahypnagogia: A state of consciousness that occurs when we almost fall asleep. Medical Hypotheses, 62(2), 166–168. 10.1016/S0306-9877(03)00306-2 [DOI] [PubMed] [Google Scholar]
- Haar Horowitz, A. , Cunningham, T. J. , Maes, P. , & Stickgold, R. (2020). Dormio: A targeted dream incubation device. Consciousness and Cognition, 83, 102938. 10.1016/j.concog.2020.102938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, M. , Katoh, K. , & Hori, T. (1999). Hypnagogic imagery and EEG activity. Perceptual and Motor Skills, 88(2), 676–678. 10.2466/pms.1999.88.2.676 [DOI] [PubMed] [Google Scholar]
- Hinton, D. E. , Reis, R. , & de Jong, J. (2019). Ghost encounters among traumatized cambodian refugees: Severity, relationship to PTSD, and phenomenology. Culture, Medicine, and Psychiatry., 44, 333–359. 10.1007/s11013-019-09661-6 [DOI] [PubMed] [Google Scholar]
- Hori, T. , Hayashi, M. , & Morikawa, T. (1994). Topographical EEG changes and the hypnagogic experience. In Sleep onset: Normal and abnormal processes (pp. 237–253). American Psychological Association. 10.1037/10166-014 [DOI] [Google Scholar]
- Horikawa, T. , Tamaki, M. , Miyawaki, Y. , & Kamitani, Y. (2013). Neural decoding of visual imagery during sleep. Science, 340(6132), 639–642. 10.1126/science.1234330 [DOI] [PubMed] [Google Scholar]
- Jacobson, E. (1925). Progressive relaxation. The American Journal of Psychology, 36, 73–87. [Google Scholar]
- Jones, S. R. , Fernyhough, C. , & Larøi, F. (2010). A phenomenological survey of auditory verbal hallucinations in the hypnagogic and hypnopompic states. Phenomenology and the Cognitive Sciences, 9(2), 213–224. 10.1007/s11097-010-9158-y [DOI] [Google Scholar]
- Jones, S. R. , Fernyhough, C. , & Meads, D. (2009). In a dark time: Development, validation, and correlates of the Durham hypnagogic and hypnopompic hallucinations questionnaire. Personality and Individual Differences, 46(1), 30–34. 10.1016/j.paid.2008.08.021 [DOI] [Google Scholar]
- Kjaer, T. W. , Law, I. , Wiltschiotz, G. , Paulson, O. B. , & Madsen, P. L. (2002). Regional cerebral blood flow during light sleep—A H215O‐PET study. Journal of Sleep Research, 11(3), 201–207. 10.1046/j.1365-2869.2002.00303.x [DOI] [PubMed] [Google Scholar]
- Kussé, C. , Shaffii‐Le Bourdiec, A. , Schrouff, J. , Matarazzo, L. , & Maquet, P. (2012). Experience‐dependent induction of hypnagogic images during daytime naps: A combined behavioural and EEG study. Journal of Sleep Research, 21(1), 10–20. [DOI] [PubMed] [Google Scholar]
- Larøi, F. , Bless, J. J. , Laloyaux, J. , Kråkvik, B. , Vedul‐Kjelsås, E. , Kalhovde, A. M. , Hirnstein, M. , & Hugdahl, K. (2019). An epidemiological study on the prevalence of hallucinations in a general‐population sample: Effects of age and sensory modality. Psychiatry Research, 272, 707–714. 10.1016/j.psychres.2019.01.003 [DOI] [PubMed] [Google Scholar]
- Lewis‐Hanna, L. L. , Hunter, M. D. , Farrow, T. F. D. , Wilkinson, I. D. , & Woodruff, P. W. R. (2011). Enhanced cortical effects of auditory stimulation and auditory attention in healthy individuals prone to auditory hallucinations during partial wakefulness. NeuroImage, 57(3), 1154–1161. 10.1016/j.neuroimage.2011.04.058 [DOI] [PubMed] [Google Scholar]
- Llewellyn, S. (2016). Dream to predict? REM dreaming as prospective coding. Frontiers in Psychology, 6. 10.3389/fpsyg.2015.01961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury, A. (1848). Des hallucinations hypnagogiques, ou des erreurs des sens dans l'état intermédiaire entre la veille et le sommeil.
- McCarthy‐Jones, S. , Barnes, L. J. , Hill, G. E. , Marwood, L. , Moseley, P. , & Fernyhough, C. (2011). When words and pictures come alive: Relating the modality of intrusive thoughts to modalities of hypnagogic/hypnopompic hallucinations. Personality and Individual Differences, 51(6), 787–790. 10.1016/j.paid.2011.07.003 [DOI] [Google Scholar]
- Meier, B. , Rothen, N. , & Walter, S. (2014). Developmental aspects of synaesthesia across the adult lifespan. Frontiers in Human Neuroscience, 8. 10.3389/fnhum.2014.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michida, N. , Hayashi, M. , & Hori, T. (1998). Comparison of event related potentials with and without hypnagogic imagery. Psychiatry and Clinical Neurosciences, 52(2), 145–147. 10.1111/j.1440-1819.1998.tb00997.x [DOI] [PubMed] [Google Scholar]
- Michida, N. , Hayashi, M. , & Hori, T. (2005). Effects of hypnagogic imagery on the event‐related potential to external Tone0Stimuli. Sleep, 28(7), 813–818. 10.1093/sleep/28.7.813 [DOI] [PubMed] [Google Scholar]
- Myers, F. W. H. (1904). Human personality and its survival of bodily death (Vol. 2). Longmans. [Google Scholar]
- Nielsen, T. A. (1995). Describing and modeling hypnagogic imagery using a systematic self‐observation procedure. Dreaming, 5(2), 75–94. 10.1037/h0094426 [DOI] [Google Scholar]
- Nielsen, T. A. (2000). A review of mentation in REM and NREM sleep: “Covert” REM sleep as a possible reconciliation of two opposing models. Behavioral and Brain Sciences, 23(6), 851–866. 10.1017/S0140525X0000399X [DOI] [PubMed] [Google Scholar]
- Nielsen, T. A. (2017). Microdream neurophenomenology. Neuroscience of Consciousness, 2017(1). 10.1093/nc/nix001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, T. A. , Stenstrom, P. , Takeuchi, T. , Saucier, S. , Lara‐Carrasco, J. , Solomonova, E. , & Martel, E. (2005). Partial REM‐sleep deprivation increases the dream‐like quality of mentation from REM sleep and sleep onset. Sleep, 28(9), 1083–1089. 10.1093/sleep/28.9.1083 [DOI] [PubMed] [Google Scholar]
- Noreika, V. , Canales‐Johnson, A. , Koh, J. , Taylor, M. , Massey, I. , & Bekinschtein, T. A. (2015). Intrusions of a drowsy mind: Neural markers of phenomenological unpredictability. Frontiers in Psychology, 6. 10.3389/fpsyg.2015.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon, M. M. (2000). Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Research, 97(2–3), 153–164. 10.1016/S0165-1781(00)00227-4 [DOI] [PubMed] [Google Scholar]
- Ohayon, M. M. , Guilleminault, C. , Zulley, J. , Palombini, L. , & Raab, H. (1999). Validation of the Sleep‐EVAL system against clinical assessments of sleep disorders and polysomnographic data. Sleep, 22(7), 925–930. 10.1093/sleep/22.7.925 [DOI] [PubMed] [Google Scholar]
- Ohayon, M. M. , Priest, R. G. , Caulet, M. , & Guilleminault, C. (1996). Hypnagogic and hypnopompic hallucinations: Pathological phenomena? British Journal of Psychiatry, 169(4), 459–467. 10.1192/bjp.169.4.459 [DOI] [PubMed] [Google Scholar]
- Ohayon, M. M. , & Shapiro, C. M. (2000). Sleep disturbances and psychiatric disorders associated with posttraumatic stress disorder in the general population. Comprehensive Psychiatry, 41(6), 469–478. 10.1053/comp.2000.16568 [DOI] [PubMed] [Google Scholar]
- Patel, A. K. , Reddy, V. , & Araujo, J. F. (2020). Physiology, sleep stages. StatPearls Publishing. [PubMed] [Google Scholar]
- Pizzagalli, D. , Lehmann, D. , Gianotti, L. , Koenig, T. , Tanaka, H. , Wackermann, J. , & Brugger, P. (2000). Brain electric correlates of strong belief in paranormal phenomena: Intracerebral EEG source and regional omega complexity analyses. Psychiatry Research: Neuroimaging, 100(3), 139–154. 10.1016/S0925-4927(00)00070-6 [DOI] [PubMed] [Google Scholar]
- Robazza, C. , & Bortoli, L. (1995). A case study of improved performance in archery using hypnosis. Perceptual and Motor Skills, 81(3), 1364–1366. 10.2466/pms.1995.81.3f.1364 [DOI] [PubMed] [Google Scholar]
- Sacks, O. (2012). Hallucinations. Pan Macmillan. [Google Scholar]
- Schacter, D. L. (1976). The hypnagogic state: A critical review of the literature. Psychological Bulletin, 83(3), 452–481. [PubMed] [Google Scholar]
- Schiappa, C. , Scarpelli, S. , D'Atri, A. , Gorgoni, M. , & De Gennaro, L. (2018). Narcolepsy and emotional experience: A review of the literature. Behavioral and Brain Functions, 14(1), 19. 10.1186/s12993-018-0151-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, R. E. , & Gendolla, G. H. E. (2008). Dreaming of white bears: The return of the suppressed at sleep onset. Consciousness and Cognition, 17(3), 714–724. 10.1016/j.concog.2007.09.002 [DOI] [PubMed] [Google Scholar]
- Schredl, M. (2018). Researching dreams: The fundamentals. Springer. [Google Scholar]
- Sharpless, B. A. (2014). Exploding head syndrome. Sleep Medicine Reviews, 18(6), 489–493. [DOI] [PubMed] [Google Scholar]
- Sherwood, S. J. (2012). A web survey of the content, sensory modalities, and interpretation of hypnagogic and hypnopompic experiences. Journal of Parapsychology, 76(1), 27. [Google Scholar]
- Shipherd, J. C. , & Salters‐Pedneault, K. (2008). Attention, memory, intrusive thoughts, and acceptance in PTSD: An update on the empirical literature for clinicians. Cognitive and Behavioral Practice, 15(4), 349–363. 10.1016/j.cbpra.2008.01.003 [DOI] [Google Scholar]
- Siclari, F. , Baird, B. , Perogamvros, L. , Bernardi, G. , LaRocque, J. J. , Riedner, B. , Boly, M. , Postle, B. R. , & Tononi, G. (2017). The neural correlates of dreaming. Nature Neuroscience, 20(6), 872–878. 10.1038/nn.4545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siclari, F. , Bernardi, G. , Riedner, B. A. , LaRocque, J. J. , Benca, R. M. , & Tononi, G. (2014). Two distinct synchronization processes in the transition to sleep: A high‐density electroencephalographic study. Sleep, 37(10), 1621–1637. 10.5665/sleep.4070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siclari, F. , LaRocque, J. J. , Postle, B. R. , & Tononi, G. (2013). Assessing sleep consciousness within subjects using a serial awakening paradigm. Frontiers in Psychology, 4. 10.3389/fpsyg.2013.00542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siclari, F. , & Tononi, G. (2017). Local aspects of sleep and wakefulness. Current Opinion in Neurobiology, 44, 222–227. 10.1016/j.conb.2017.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberer, H. (1951). 8. Report on a method of eliciting and observing certain symbolic hallucination‐phenomena. In Report on a method of eliciting and observing certain symbolic hallucination‐phenomena. In organization and pathology of thought (pp. 195–207). Columbia University Press. [Google Scholar]
- Soffer‐Dudek, N. , & Shahar, G. (2011). Daily stress interacts with trait dissociation to predict sleep‐related experiences in young adults. Journal of Abnormal Psychology, 120(3), 719–729. 10.1037/a0022941 [DOI] [PubMed] [Google Scholar]
- Speth, C. , & Speth, J. (2016). The borderlands of waking: Quantifying the transition from reflective thought to hallucination in sleep onset. Consciousness and Cognition, 41, 57–63. 10.1016/j.concog.2016.01.009 [DOI] [PubMed] [Google Scholar]
- Speth, J. , Frenzel, C. , & Voss, U. (2013). A differentiating empirical linguistic analysis of dreamer activity in reports of EEG‐controlled REM‐dreams and hypnagogic hallucinations. Consciousness and Cognition, 22(3), 1013–1021. 10.1016/j.concog.2013.07.003 [DOI] [PubMed] [Google Scholar]
- Speth, J. , Harley, T. A. , & Speth, C. (2017). Auditory verbal experience and agency in waking, sleep onset, REM, and non‐REM sleep. Cognitive Science, 41(3), 723–743. 10.1111/cogs.12363 [DOI] [PubMed] [Google Scholar]
- Speth, J. , Schloerscheidt, A. M. , & Speth, C. (2016). As we fall asleep we forget about the future: A quantitative linguistic analysis of mentation reports from hypnagogia. Consciousness and Cognition, 45, 235–244. 10.1016/j.concog.2016.08.014 [DOI] [PubMed] [Google Scholar]
- Steen, C. (2017). Synesthetic photisms and hypnagogic visions: A comparison. Multisensory Research, 30(3–5), 199–205. 10.1163/22134808-00002557 [DOI] [PubMed] [Google Scholar]
- Stenstrom, P. , Fox, K. , Solomonova, E. , & Nielsen, T. (2012). Mentation during sleep onset theta bursts in a trained participant: A role for NREM stage 1 sleep in memory processing? International Journal of Dream Research, 5, 37–46. 10.11588/ijodr.2012.1.9135 [DOI] [Google Scholar]
- Stickgold, R. , Malia, A. , Maguire, D. , Roddenberry, D. , & O'Connor, M. (2000). Replaying the game: Hypnagogic images in normals and amnesics. Science, 290(5490), 350–353. 10.1126/science.290.5490.350 [DOI] [PubMed] [Google Scholar]
- Szklo‐Coxe, M. , Young, T. , Finn, L. , & Mignot, E. (2007). Depression: Relationships to sleep paralysis and other sleep disturbances in a community sample. Journal of Sleep Research, 16(3), 297–312. 10.1111/j.1365-2869.2007.00600.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, F. K. (1981). On pseudo‐hallucinations. Psychological Medicine, 11(2), 265–271. 10.1017/S0033291700052089 [DOI] [PubMed] [Google Scholar]
- Vaitl, D. , Birbaumer, N. , Gruzelier, J. , Jamieson, G. A. , Kotchoubey, B. , Kübler, A. , Lehmann, D. , Miltner, W. H. R. , Ott, U. , Pütz, P. , Sammer, G. , Strauch, I. , Strehl, U. , Wackermann, J. , & Weiss, T. (2005). Psychobiology of altered states of consciousness. Psychological Bulletin, 131(1), 98–127. 10.1037/0033-2909.131.1.98 [DOI] [PubMed] [Google Scholar]
- Van de Castel, R. L. (1995). Our Dreaming Mind. Ballantine Books. [Google Scholar]
- Wackermann, J. , Putz, P. , & Allefeld, C. (2008). Ganzfeld‐induced hallucinatory experience, its phenomenology and cerebral electrophysiology. Cortex, 44(10), 1364–1378. 10.1016/j.cortex.2007.05.003 [DOI] [PubMed] [Google Scholar]
- Wackermann, J. , Pütz, P. , Büchi, S. , Strauch, I. , & Lehmann, D. (2002). Brain electrical activity and subjective experience during altered states of consciousness: Ganzfeld and hypnagogic states. International Journal of Psychophysiology, 46(2), 123–146. 10.1016/S0167-8760(02)00070-3 [DOI] [PubMed] [Google Scholar]
- Wamsley, E. (2021). Dreaming as constructive episodic future simulation. Sleep, 44(2), A15. 10.1093/sleep/zsab072.033 [DOI] [Google Scholar]
- Wamsley, E. J. , Perry, K. , Djonlagic, I. , Reaven, L. B. , & Stickgold, R. (2010). Cognitive replay of visuomotor learning at sleep onset: Temporal dynamics and relationship to task performance. Sleep, 33(1), 59–68. 10.1093/sleep/33.1.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, J. (2013). Synesthesia. Annual Review of Psychology, 64(1), 49–75. 10.1146/annurev-psych-113011-143840 [DOI] [PubMed] [Google Scholar]
- Waters, F. , Blom, J. D. , Dang‐Vu, T. T. , Cheyne, A. J. , Alderson‐Day, B. , Woodruff, P. , & Collerton, D. (2016). What is the link between hallucinations, dreams, and hypnagogic–hypnopompic experiences? Schizophrenia Bulletin, 42(5), 1098–1109. 10.1093/schbul/sbw076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner, D. M. , & Schneider, D. J. (1987). Paradoxical effects of thought suppression. Journal of Personality and Social Psychology, 53(1), 5–13. [DOI] [PubMed] [Google Scholar]
- Wegner, D. M. , Wenzlaff, R. M. , & Kozak, M. (2004). Dream rebound: The return of suppressed thoughts in dreams. Psychological Science, 15(4), 232–236. 10.1111/j.0963-7214.2004.00657.x [DOI] [PubMed] [Google Scholar]
- Zadra, A. , & Domhoff, G. W. (2017). Dream content. In Principles and practice of sleep medicine (pp. 515–522). Elsevier. 10.1016/B978-0-323-24288-2.00049-0 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


