Abstract
Secukinumab demonstrated high efficacy and favorable safety profile in patients with moderate‐to‐severe plaque psoriasis (PsO) in clinical trials. However, understanding of patient characteristics and clinical outcomes in real world in Thailand is still limited. To describe patient characteristics, effectiveness and safety of secukinumab in Thai PsO patients. This retrospective study analyzed data from medical records of adult PsO patients who initiated secukinumab at 7 dermatology centers from September 2017 to April 2021. Study outcomes included patient characteristics and changes in Psoriasis Area and Severity Index (PASI) score from baseline at weeks 4 and 16 after secukinumab initiation. Adverse events were recorded. Subgroup analyses by adherence rate and completeness of loading dose were performed. Of 163 patients, the mean (SD) age was 44.0 (14.0) years. Most patients (84.7%) were previously treated with topical therapy while 62.0% and 21.5% of patients had received systemic and biologic therapy, respectively. The mean baseline PASI score was 15.4 (9.3). Overall, the mean PASI score improved by 58.0% at week 4 and 78.4% at week 16. Statistically significant differences in PASI approvement were revealed among subgroups of patients with different loading dose and adherence rate. Adverse effects were reported in 8.0% of patients. The characteristics of patients in this study were slightly different from clinical trials in terms of demographic and clinical characteristics, as well as PsO treatment. Secukinumab was effective and safe in Thai patients with PsO, especially among those with complete loading dose and a higher adherence rate.
Keywords: biologics, IL‐17, psoriasis, real world, secukinumab
1. INTRODUCTION
Psoriasis is a chronic systemic immune‐mediated inflammatory disease with prominent skin manifestations. Its physical burdens substantially impact psychosocial well‐being and socioeconomic status of affected individuals and their caregivers. 1 , 2 , 3 , 4 , 5 , 6 The prevalence of psoriasis varies across different ethnics and geographic regions, ranging from 0.09% to 8%. 7 , 8 The prevalence of psoriasis in Thailand is 0.13%, less than those in western countries but is similar to other east Asian countries. 9
Psoriasis is a multifactorial disease dominated by the interplay between multiple genetic and environmental factors resulting in an abnormal exaggerated immune response and associated with several comorbidities. Recent insights into psoriasis immunopathogenesis reshaped the facet of therapeutic approaches to novel, highly selective biologic therapies aiming to achieve better outcomes and minimize adverse effects. 8 , 10 , 11
Secukinumab, a fully human IgG1 monoclonal antibody against interleukin (IL)‐17A, has been approved by the US Food and Drug Administration (FDA) for adult plaque psoriasis since 2015. Secukinumab neutralizes IL‐17A, a highly potent effector cytokine of IL‐23/Th‐17 pathway, leading to diminished psoriatic inflammatory response. 12 , 13 , 14 , 15 The efficacy and tolerability of secukinumab for moderate‐to‐severe plaque psoriasis have been proven in multiple randomized controlled trials. Since its launch, the use of secukinumab continues to expand globally due to its favorable effectiveness and safety.
Despite the number of real‐world evidences of secukinumab, 16 only few studies focused on patient characteristics and clinical outcomes of secukinumab in Thai patients. As the treatment outcomes in patients treated with various treatment patterns in the real world may differ from those observed in clinical trials, this study aimed to describe patient characteristics, effectiveness, and safety of secukinumab in Thai real‐world practice.
2. METHODS
2.1. Study designs and settings
This was a 16‐week non‐interventional, multi‐center, retrospective medical record review study conducted in 7 dermatology centers in Thailand.
This study was approved by the Central Research Ethics Committee (CREC: COA‐CREC092/2020) and the local Institutional Review Board (IRB) of each participating institution. This study was conducted in accordance with the Declaration of Helsinki and adhered to Good Clinical Practice guidelines. Due to retrospective nature of the study, informed consent was exempted by the IRB.
2.2. Study population
This study included all patients aged at least 18 years who were diagnosed with moderate‐to‐severe plaque psoriasis with available baseline Psoriasis Area and Severity Index (PASI) at index date (date 0 to date −7 prior to index date) and initiated secukinumab treatment for psoriasis from September 2017 to April 2021. The first documented secukinumab injection in the medical record was defined as the index date.
2.3. Data collection
Medical records of eligible patients were reviewed to obtain data from the date of first psoriasis diagnosis to the most recent dose of secukinumab as of April 2021.
The study endpoints were patient characteristics, including baseline demographics, co‐morbidities and treatment history, and the proportion of patients achieving at least 75%, 90%, and 100% improvement in PASI score from baseline (PASI 75, PASI 90, and PASI 100, respectively) at week 4 and week 16 after secukinumab initiation. Adherence rate was denoted as the completeness of dosing by measuring the total number of injections at each time point regarding the standard dose of secukinumab (300 mg of secukinumab = 2 injections), that is, 10 injections for week 4 and 16 injections for week 16.
2.4. Statistical analysis
All analyses were performed using the SAS 9 platform. Categorical variables were reported as counts (N) and proportions (percentage), whereas continuous variables were summarized with either mean and standard deviation (SD) or median and interquartile range (IQR), depending on data distribution. If applicable, confidence intervals (CI) were derived. Subgroup analyses were performed in patients receiving secukinumab as the first biologic agent for treating plaque psoriasis and those with different adherence rate (100%, 75%–99%, 50%–74%, and <50%), using either Chi‐square or Fisher's exact test. p‐values of <0.05 were considered statistically significant.
3. RESULTS
A total of 163 patients were enrolled and included in patient characteristic analyses. PASI score was available in 94 (57.7%) and 62 (38.0%) patients at week 4 and week 16, respectively.
Patient demographics and baseline characteristics are summarized in Table 1. Their median (IQR) duration from the first symptom of psoriasis was 11.0 (14.3) years while the duration of moderate to severe PsO diagnosis was 6.1 (10.9) years. Nearly half of patients were covered by private insurance or self‐payment. Metabolic syndrome and psoriatic arthritis were the most common comorbidities.
TABLE 1.
Demographics and baseline clinical characteristics
| Characteristics | N | n (%) unless otherwise stated |
|---|---|---|
| Sex | ||
| Male | 80 (49.1) | |
| Female | 83 (50.9) | |
| Age (year), mean ± SD | 163 | 44.0 ± 14.0 |
| Weight (kg), mean ± SD | 130 | 73.2 ± 18.9 |
| Height (cm), mean ± SD | 99 | 164.3 ± 8.9 |
| BMI (kg/m2), mean ± SD | 98 | 26.7 ± 5.6 |
| Health insurance scheme | ||
| Universal Coverage Scheme | 21 (13.0%) | |
| Social Security Scheme | 30 (18.5%) | |
| Civil Servant Medical Benefits Scheme | 33 (20.4%) | |
| Private Insurance or Self‐Pay | 78 (48.2%) | |
| Comorbidity | ||
| None | 60 (36.8%) | |
| Hypertension | 46 (28.2%) | |
| Dyslipidemia | 40 (24.5%) | |
| Hepatic disease | 24 (14.7%) | |
| Diabetes mellitus | 21 (12.9%) | |
| Psoriatic arthritis | 20 (12.3%) | |
| Latent tuberculosis | 7 (4.3%) | |
| Chronic kidney disease | 6 (3.7%) | |
| Hepatitis B virus infection | 5 (3.1%) | |
| Hepatitis C virus infection | 2 (1.2%) | |
| Others | 67 (41.1%) |
Abbreviations: BMI, body mass index; cm, centimeter; kg, kilogram; m2, square meter; SD, standard deviation.
Regarding previous therapy for psoriasis, topical steroids and methotrexate were the most commonly used topical and systemic therapy, respectively (94.9% and 87.1%). Only 21.5% of patients were biologic experienced (Table 2).
TABLE 2.
Treatment history for psoriasis
| Treatment history | N | n (%) unless otherwise stated |
|---|---|---|
| Topical therapy | ||
| Number of topical therapy use | ||
| 1 topical therapy | 12 (7.4%) | |
| >1 topical therapies | 126 (77.3%) | |
| None/No data | 25 (15.3%) | |
| Duration of topical therapy use (month), median (IQR) | 56 | 73.4 (118.8) |
| Previously used topical therapy | ||
| Topical corticosteroid | 131 (94.9%) | |
| Coal tar | 114 (82.6%) | |
| Vitamin D analogues | 82 (59.4%) | |
| Salicylic acid | 33 (23.9%) | |
| Mineral oil | 18 (13.0%) | |
| Calcineurin inhibitors | 14 (10.1%) | |
| Anthralin | 4 (2.9%) | |
| Olive oil | 3 (2.2%) | |
| Systemic therapy | ||
| Number of systemic therapy use | ||
| 1 systemic therapy | 39 (23.9%) | |
| 2 systemic therapies | 38 (23.3%) | |
| 3 systemic therapies | 24 (14.7%) | |
| None/No data | 62 (38.0%) | |
| Duration of systemic therapy use (month), median (IQR) | 55 | 60.0 (132.7) |
| Previously used systemic therapy | ||
| Methotrexate | 88 (87.1%) | |
| Acitretin | 54 (53.5%) | |
| Cyclosporine | 42 (41.6%) | |
| Sulfasalazine | 3 (3.0%) | |
| Biologic therapy | ||
| Number of systemic therapy use | ||
| 1 biologic therapy | 29 (17.8%) | |
| 2 biologic therapies | 5 (3.1%) | |
| 3 biologic therapies | 1 (0.7%) | |
| None/No data | 128 (78.5%) | |
| Duration of biologic therapy use (month), median (IQR) | 25 | 9.3 (28.2) |
| Previously used biologic therapy | 35 | |
| Etanercept | 10 (28.6%) | |
| Ixekizumab | 10 (28.6%) | |
| Infliximab | 8 (22.9%) | |
| Secukinumab | 6 (17.1%) | |
| Ustekinumab | 6 (17.1%) | |
| Brodalumab | 1 (2.9%) | |
| Golimumab | 1 (2.9%) |
Abbreviation: IQR, interquartile range.
3.1. Effectiveness of secukinumab in real‐world practice
Overall, the mean PASI score improvement was reported as 58.0% at week 4 and 78.4% at week 16 (Table 3). A similar trend was reported in BSA. However, worsening PASI scores were observed in five patients during this study.
TABLE 3.
Clinical improvement from baseline at week 4 and week 16 after secukinumab therapy initiation
| Week 0 (N = 163) | Week 4 (N = 94) | Week 16 (N = 62) | |
|---|---|---|---|
| PASI score | |||
| N | 163 | 94 | 62 |
| Mean (SD) | 15.4 (9.3) | 6.3 (5.8) | 1.8 (4.0) |
| % Change, mean (SD) | 58.0 (35.2) | 78.4 (44.9) | |
| BSA involvement, % | |||
| N | 82 | 60 | 58 |
| Mean (SD) | 37.2 (28.6) | 18.0 (20.1) | 3.4 (9.6) |
| % Change, mean (SD) | 34.3 (49.4) a | 76.3 (51.0) b | |
| DLQI score | |||
| N | 30 | 3 | 4 |
| Mean (SD) | 12.4 (7.2) | 6.0 (4.6) | 4.5 (7.1) |
| % Change, mean (SD) | 66.7 (23.7) a | 25.0 (75.0) b |
Abbreviations: BSA, body surface area; DLQI, dermatology life quality index; IQR, interquartile range; PASI, Psoriasis Area and Severity Index.
53 and 3 Patients with BSA/DLQI score at both baseline and week 4, respectively.
39 and 3 Patients with BSA/DLQI score at both baseline and week 16, respectively.
At baseline, the most common concomitant therapies with secukinumab were topical therapy (69.2%), systemic therapy (31.9%) and phototherapy (16.0%). Almost 40% of patients received more than one concomitant therapy. However, concomitant therapy use was gradually withdrawn at week 4 and week 16, respectively (Table 4).
TABLE 4.
Concomitant therapy
| Concomitant therapy | Week 0 (N = 163) | Week 4 (N = 94) | Week 16 (N = 62) |
|---|---|---|---|
| Topical therapy | |||
| Topical corticosteroid | 98 (60.1%) | 53 (56.4%) | 26 (42.0%) |
| Coal tar | 53 (32.5%) | 26 (27.7%) | 14 (22.6%) |
| Vitamin D analogues | 46 (28.2%) | 18 (19.2%) | 6 (9.7%) |
| Salicylic acid | 13 (8.0%) | 8 (8.5%) | 1 (1.6%) |
| Mineral oil | 7 (4.3%) | 5 (5.3%) | 2 (3.2%) |
| Calcineurin inhibitors | 5 (3.1%) | 2 (2.1%) | 0 (0.0%) |
| Anthralin | 2 (1.2%) | 0 (0.0%) | 0 (0.0%) |
| Phototherapy | |||
| Yes | 26 (16.0%) | 6 (6.4%) | 3 (4.9%) |
| Systemic therapy | |||
| Methotrexate | 27 (16.6%) | 11 (11.7%) | 6 (9.7%) |
| Acitretin | 16 (9.9%) | 9 (9.6%) | 3 (4.8%) |
| Cyclosporine | 14 (8.6%) | 4 (4.3%) | 2 (3.2%) |
| Sulfasalazine | 3 (1.8%) | 2 (2.1%) | 1 (1.6%) |
| Leflunomide | 2 (1.2%) | 1 (1.1%) | 0 (0.0%) |
| Combination therapy | |||
| Topical therapy + Systemic therapy | 39 (24.0%) | 17 (18.1%) | 10 (16.1%) |
| Topical therapy + Phototherapy | 16 (9.8%) | 4 (4.3%) | 1 (1.6%) |
| Topical therapy + Phototherapy + Systemic therapy | 6 (3.7%) | 2 (2.1%) | 0 (0.0%) |
Note: Value is presented as n (%).
3.1.1. Achievement in PASI score
The proportions of patients achieving PASI 75, PASI 90, and PASI 100 significantly increased from week 4 to week 16 as shown in Figure 1.
FIGURE 1.
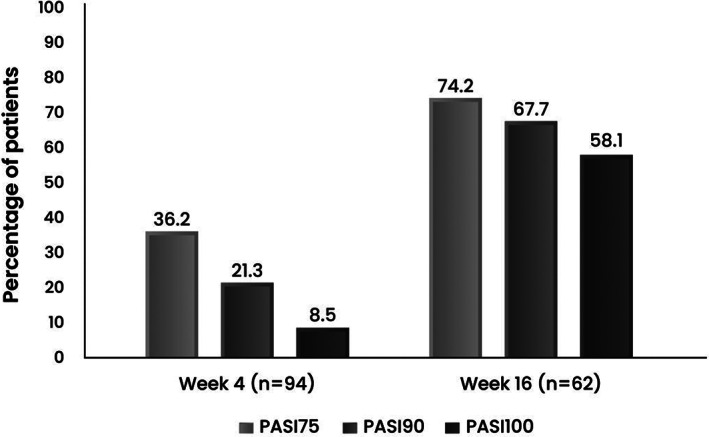
Percentage of patients achieving PASI 75, PASI 90, and PASI 100 at week 4 and week 16 after secukinumab initiation
3.1.2. Subgroup analyses for patients who received secukinumab as the first biologic agent
As shown in Figure 2, a numerically higher proportion of patients achieving PASI 75 and PASI 90 was observed in biologic‐naïve patients at week 4 and week 16.
FIGURE 2.
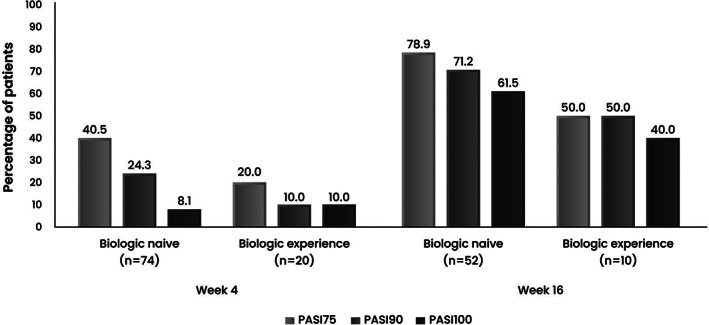
Percentage of patients achieving PASI 75, PASI 90, and PASI 100 at week 4 and week 16 after secukinumab initiation in biologic‐naïve and biologic‐experienced patients
3.1.3. Subgroup analyses for patients who received secukinumab with different adherence rates
According to adherence rate, there were 11.7%, 21.5%, 27.6%, and 39.3% of patients with 100%, 75%–99%, 50%–74%, and <50% adherence, respectively. The percentage of patients who achieved clear/almost clear skin was higher in the group with 100% adherence rate (Table 5).
TABLE 5.
Proportion of patients achieving PASI 75, PASI 90, and PASI 100 at week 4 and week 16 after secukinumab initiation categorized by adherence rate
| PASI improvement (%) | Adherence rate | p‐value | |||
|---|---|---|---|---|---|
| 100% | 75%–99% | 50%–74% | <50% | ||
| n (%) (95% CI) | n (%) (95% CI) | n (%) (95% CI) | n (%) (95% CI) | ||
| Week 4 | |||||
| N | 13 | 30 | 30 | 21 | |
| PASI 75 | 10 (76.9%) | 14 (46.7%) | 7 (23.3%) | 3 (14.3%) | 0.001 † , * |
| (54.0–99.8) | (28.8–64.5) | (8.2–38.5) | (0.0–29.3) | ||
| PASI 90 | 7 (53.9%) | 8 (26.7%) | 2 (6.7%) | 3 (14.3%) | 0.005 ‡ , * |
| (26.8–81.0) | (10.8–42.5) | (0.0–15.6) | (0.0–29.3) | ||
| PASI 100 | 2 (15.4%) | 3 (10.0%) | 2 (6.7%) | 1 (4.7%) | 0.726 ‡ |
| (0.0–35.0) | (0.0–20.7) | (0.0–15.6) | (0.0–13.9) | ||
| Week 16 | |||||
| N | 15 | 13 | 16 | 18 | |
| PASI 75 | 14 (93.3%) | 12 (92.3%) | 11 (68.8%) | 9 (50.0%) | 0.014 ‡ , * |
| (80.7–100.0) | (77.8–100.0) | (46.1–91.5) | (26.9–73.1) | ||
| PASI 90 | 13 (86.7%) | 11 (84.6%) | 9 (56.3%) | 9 (50.0%) | 0.054 ‡ |
| (69.5–100.0) | (65.0–100.0) | (31.9–80.6) | (26.9–73.1) | ||
| PASI 100 | 11 (73.3%) | 11 (84.7%) | 7 (43.8%) | 7 (38.9%) | 0.026 † , * |
| (51.0–95.7) | (65.0–100.0) | (19.4–68.1) | (16.4–61.4) | ||
p‐value <0.05, indicating a statistically significant difference.
Overall p‐value (2‐sided) based on Chi‐square test.
Overall p‐value (2‐sided) based on Fisher's exact test.
Moreover, significant difference in PASI score improvement was observed between patients with complete and incomplete loading dose of secukinumab (Table 6). At both weeks 4 and 16, the proportions of patients achieving PASI 75, PASI 90, and PASI 100 were higher in patients with complete loading dose.
TABLE 6.
Proportion of patients achieving PASI 75, PASI 90, and PASI 100 at week 4 and week 16 after secukinumab initiation for patients with complete and incomplete loading dose of secukinumab
| PASI improvement (%) | Week 4 (N = 94) | Week 16 (N = 62) | ||||
|---|---|---|---|---|---|---|
| Loading dose of secukinumab | Loading dose of secukinumab | |||||
| Complete (N = 45) | Incomplete (N = 49) | p‐value | Complete (N = 26) | Incomplete (N = 36) | p‐value | |
| n (%) (95% CI) | n (%) (95% CI) | n (%) (95% CI) | n (%) (95% CI) | |||
| PASI 75 | 23 (51.1%) | 11 (22.5%) | 24 (92.3%) | 22 (61.1%) | ||
| (36.5–65.7) | (10.8–34.1) | 0.004 † , * | (82.1–100.0) | (45.2–77.0) | 0.006 † , * | |
| PASI 90 | 15 (33.3%) | 5 (10.2%) | 22 (84.6%) | 20 (55.6%) | ||
| (19.6–47.1) | (1.7–18.7) | 0.006 † , * | (70.8–98.5) | (39.3–71.8) | 0.016 † , * | |
| PASI 100 | 5 (11.1%) | 3 (6.1%) | 19 (73.1%) | 17 (47.2%) | ||
| (1.9–20.3) | (0.0–12.8) | 0.473 ‡ | (56.0–90.1) | (30.9–63.5) | 0.042 † , * | |
p‐value <0.05, indicating a statistically significant difference.
Overall p‐value (2‐sided) based on Chi‐square test.
Overall p‐value (2‐sided) based on Fisher's exact test.
3.2. Safety
Adverse events (AEs) possibly relevant to secukinumab therapy were reported in 13 (8.0%) patients. Seven adverse events were of mild severity, and the reported AEs led to secukinumab discontinuation in four patients (Table 7). A patient developed pancytopenia, which was considered of moderate severity. Nevertheless, the condition spontaneously improved after discontinuing secukinumab.
TABLE 7.
Summary of specific adverse events (N = 163)
| Adverse events | n (%) | Treatment discontinuation n (%) |
|---|---|---|
| Eczema | 2 (1.2%) | 2 (1.2%) |
| Injection site reaction | 1 (0.6%) | 0 (0.0%) |
| Pancytopenia | 1 (0.6%) | 1 (0.6%) |
| Pruritus | 1 (0.6%) | 0 (0.0%) |
| Seborrheic dermatitis | 1 (0.6%) | 0 (0.0%) |
| Dermatophyte infection | 1 (0.6%) | 1 (0.6%) |
| Upper respiratory tract infection | 1 (0.6%) | 0 (0.0%) |
| Worsening PASI score | 5 (3.1%) | ‐ a |
No specified detail.
4. DISCUSSION
Our study reports patient and disease characteristics and asserts real‐world effectiveness and safety of secukinumab in Thai psoriasis patients. At baseline, the patient demographics and disease characteristics were comparable to phase 3 randomized controlled trial populations (ERASURE, FIXTURE, and CLEAR) in terms of age, but of lower proportion of males, lower BMI, shorter duration of psoriasis diagnosis, and lower proportion of concomitant psoriatic arthritis. Gender ratio. 17 , 18 The high proportion of patients with metabolic comorbidities was congruent with previous real‐world studies in other countries. 19 , 20
The majority of patients in this study were biologic naïve and covered by private insurance or self‐payment. Most of them had been treated with topical and systemic therapies for at least 6 years before receiving biologics. In addition, almost half of patients still had nail and scalp psoriasis at baseline. These treatment patterns may indicate the patients' psoriasis severity and long cycling of conventional therapies for psoriasis. This study also revealed that, in the real practice, patients received secukinumab with various treatment patterns (66.7% with incomplete loading and 67.0% with <75% adherence rate) which differed from clinical trials. This finding could express the availability and accessibility of biologics in Thailand, which possibly lead to the compromised treatment outcomes.
Our patients' baseline PASI score (15.4) was comparable to those of previous real‐world studies in Asia‐Pacific and Middle‐east regions, Japan, China, and Italy. 21 , 22 , 23 , 24 In addition to baseline PASI score, several factors, that is, BSA, DLQI, area of involvement, course of disease, and so forth contribute to physician decision on biologic use. 17 , 18 The assessment of PASI, BSA, and DLQI will provide comprehensive information on the impact of psoriasis on the patients' life, and suggest early treatment step‐up. However, these assessments are not routinely assessed in real‐world clinical practice in Thailand.
Despite suboptimal adherence rate in real‐world setting, an impressive proportion of patients achieving PASI 75, PASI 90, and PASI 100 at weeks 4 and 16 was proven evidence of secukinumab effectiveness. These achievements were comparable to the results of a meta‐analysis of 7 phase 3 clinical trials and a meta‐analysis of 43 real‐world studies. 16 , 25 Our study reported that 5 patients (3.07%) had worsening PASI score during secukinumab treatment, with 4 of them received secukinumab with <50% adherence rate.
Regarding subgroup analyses of patients who received secukinumab as the first biologic agent, previous real‐world evidences revealed variations across different regions, which may be attributed to a discrepancy in biologic access. 26 , 27 Our findings revealed numerically higher proportions of patients achieving PASI improvement at weeks 4 and 16 in biologic‐naïve than biologic‐experienced groups. Compared to our finding, a higher proportion of biologic‐experienced patients was reported in REALIA and studies by Fujita et al. and Caldarola et al. while a lower proportion was observed in the study of Zhao et al. 21 , 22 , 23 , 24 A similar trend of a greater efficacy toward biologic‐naïve patients was observed in real‐world studies in Japan and China. 22 , 23
Moreover, significant differences in the proportion of patients with improved PASI score were demonstrated among different adherence rates. Of which, the greater percentage was observed in patients with 100% adherence rate compared to those with <75% adherence rate, and in patients who received a complete loading dose. Our findings reaffirm the association between initial weekly loading dose and treatment effectiveness. Gisondi et al. 28 reported significantly greater PASI 75 and PASI 90 response rates at week 12 in psoriatic patients who received the labeled loading dose versus those who did not. The absence of loading dose was also associated with a higher proportion of primary inefficiency.
Overall safety profile of secukinumab in our study was consistent with previous secukinumab trials. The use of biologic agents potentially raises the risk of infection due to their function in the inhibition of IL‐17, which plays an important role in innate and adaptive immune system. 29 A previous real‐world study reported patients receiving secukinumab experienced a slightly increased risk of infection compared to other biologics (e.g., anti‐TNF, anti‐IL12/23, and anti‐IL17A) (16.6% vs. 11.7%) which was, nevertheless, similar to that observed in clinical studies. 21 , 30 In our study, infection was reported in only 1.2% of our patients. During therapy, one patient experienced pancytopenia, which improved shortly after secukinumab discontinuation. However, no previous report of pancytopenia in secukinumab studies was available. In addition, eczema occurred in 1.2% of our patients, which was comparable to a previous study (1.6%). 31
We acknowledge a few limitations in this study. First, the retrospective design limited the access to some information that was not generally measured in real‐life practice. Second, the missing data in various variables, including the PASI scores at weeks 4 and 16, limited the number of patients included in our pre‐specified subgroup analyses.
In conclusion, most secukinumab‐treated patients were biologic‐naive and in private insurance or self‐payment. Baseline patient demographics and disease characteristics were similarly reported in other real‐world studies. According to the limited biologics access, patients were treated with various treatment patterns, and most of them received suboptimal dose of secukinumab. However, our results support good clinical outcomes and safety of secukinumab in Thai patients, especially among those with complete loading dose and a higher adherence rate.
CONFLICT OF INTEREST
Dr. Asawanonda received honoraria for lecturing and research grant for Novartis, Zeullig Pharma, Kyowa Kirin, Janssen, and Leo Pharma. Dr. Pattamadilok received research grant and has served as a principal investigator, and an advisory board member for Novartis, Eli Lilly, Leo Pharma, and Boehringer Ingelheim. Dr. Chularojanamontri has served as paid speaker for Novartis, Janssen, and Zeullig Pharma. Dr. Chuamanochan received honoraria for lecturing for Novartis, Leo Pharma, and Janssen. Dr. Choonhakarn has served as paid speaker for Novartis, Janssen, Menarini, Beiersdof, Galderma, Zuellig Pharma, Takeda, MSD, GSK, and BeRich. Dr. Chakkavittumrong has served as paid speaker, and received research grant for Novartis, Zuellig Pharma, Janssen, Galderma, and IQVIA. Dr. Rajatanavin has served as paid speaker, and principal investigator for Novartis, Sanofi, Eli Lilly, and Boehringer Ingelheim. Ms. Sangob is a medical lead in Novartis.
ACKNOWLEDGMENTS
This study was funded by Novartis (Thailand) Limited. The authors would like to thank Dr. Ploysyne Rattanakaemakorn, Dr. Chanisada Wongpraparut, Dr. Narumol Silpa‐archa, Dr. Napatra Tovanabutra, Dr. Nunthanach Chuenboonngarm, Dr. Phumithep Phumariyapong, Dr. Surachanee Litkittanasombat, Dr. Pasita Palakornkitti, Dr. Tanaporn Anuntrangsee for help with the conduct of the study; Ms. Tipaporn Pongmesa (RWE lead) of Novartis, Thailand for advice regarding the development of study design and support the conduct of the study. All analyses were performed by Contracted Research Organization (CRO), Center of Excellence for Biomedical and Public Health Informatics (BIOPHICS) of Mahidol University, of which results were verified by authors. The authors take full responsibility for the accuracy and integrity of the data. Regarding the manuscript writing, all authors drafted, reviewed, provided feedback on subsequent versions, and finally agreed on the last version of manuscript before submitting for publication.
Asawanonda P, Pattamadilok B, Chularojanamontri L, et al. Real‐world experience of secukinumab in moderate to severe psoriasis patients in Thailand: Characteristics, effectiveness, and safety. Dermatologic Therapy. 2022;35(12):e15958. doi: 10.1111/dth.15958
Funding information Novartis Thailand
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Martínez‐Ortega JM, Nogueras P, Muñoz‐Negro JE, Gutiérrez‐Rojas L, González‐Domenech P, Gurpegui M. Quality of life, anxiety and depressive symptoms in patients with psoriasis: a case‐control study. J Psychosom Res. 2019;124:109780. [DOI] [PubMed] [Google Scholar]
- 2. Thakolwiboon S, Upala S, Geeratragool T, et al. The factors affecting quality of life in Thai psoriasis patients. J Med Assoc Thai. 2013;96(10):1344‐1349. [PubMed] [Google Scholar]
- 3. Unaeze J, Nijsten T, Murphy A, Ravichandran C, Stern RS. Impact of psoriasis on health‐related quality of life decreases over time: an 11‐year prospective study. J Invest Dermatol. 2006;126(7):1480‐1489. [DOI] [PubMed] [Google Scholar]
- 4. Augustin M, Krüger K, Radtke MA, Schwippl I, Reich K. Disease severity, quality of life and health care in plaque‐type psoriasis: a multicenter cross‐sectional study in Germany. Dermatology. 2008;216(4):366‐372. [DOI] [PubMed] [Google Scholar]
- 5. Salman A, Yucelten AD, Sarac E, Saricam MH, Perdahli‐Fis N. Impact of psoriasis in the quality of life of children, adolescents and their families: a cross‐sectional study. An Bras Dermatol. 2018;93(6):819‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tee SI, Lim ZV, Theng CT, Chan KL, Giam YC. A prospective cross‐sectional study of anxiety and depression in patients with psoriasis in Singapore. J Eur Acad Dermatol Venereol. 2016;30(7):1159‐1164. [DOI] [PubMed] [Google Scholar]
- 7. Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis. JAMA. 2020;323(19):1945. [DOI] [PubMed] [Google Scholar]
- 8. Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2016;31(2):205‐212. [DOI] [PubMed] [Google Scholar]
- 9. Chaiyamahapurk S, Warnnissorn P. Prevalence and characteristics of psoriasis patients in a primary care area in Thailand. J Med Assoc Thailand. 2021;104:610‐614. [Google Scholar]
- 10. Gao J, Shen X, Ko R, Huang C, Shen C. Cognitive process of psoriasis and its comorbidities: from epidemiology to genetics. Front Genet. 2021;26:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mahil SK, Capon F, Barker JN. Update on psoriasis immunopathogenesis and targeted immunotherapy. Semin Immunopathol. 2015;38(1):11‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Menter A, Strober BE, Kaplan DH, et al. Joint AAD‐NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029‐1072. [DOI] [PubMed] [Google Scholar]
- 13. Sanford M, McKeage K. Secukinumab: first global approval. Drugs. 2015;75(3):329‐338. [DOI] [PubMed] [Google Scholar]
- 14. Rønholt K, Iversen L. Old and new biological therapies for psoriasis. Int J Mol Sci. 2017;18(11):2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kirkham BW, Kavanaugh A, Reich K. Interleukin‐17A: a unique pathway in immune‐mediated diseases: psoriasis, psoriatic arthritis and rheumatoid arthritis. Immunology. 2014;141(2):133‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Augustin M, Jullien D, Martin A, Peralta C. Real‐world evidence of secukinumab in psoriasis treatment – a meta‐analysis of 43 studies. J Eur Acad Dermatol Venereol. 2020;34(6):1174‐1185. [DOI] [PubMed] [Google Scholar]
- 17. Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis – results of two phase 3 trials. N Engl J Med. 2014;371(4):326‐338. [DOI] [PubMed] [Google Scholar]
- 18. Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73(3):400‐409. [DOI] [PubMed] [Google Scholar]
- 19. Armstrong AW, Patil D, Levi E, et al. Real‐world satisfaction with secukinumab in clearing the skin of patients with plaque psoriasis through 24 months of follow‐up: results from US dermatology electronic medical records. Dermatol Ther. 2021;11(5):1733‐1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Galluzzo M, Talamonti M, De Simone C, et al. Secukinumab in moderate‐to‐severe plaque psoriasis: a multi‐center, retrospective, real‐life study up to 52 weeks observation. Expert Opin Biol Ther. 2018;18(7):727‐735. [DOI] [PubMed] [Google Scholar]
- 21. Foley P, Tsai T‐F, Rodins K, et al. Effectiveness and safety of secukinumab for psoriasis in a real‐world clinical setting in the Asia‐Pacific and Middle East regions: results from the REALIA study. Dermatol Ther. 2022;12(2):511‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fujita H, Ohtsuki M, Morita A, et al. Safety and effectiveness of secukinumab in psoriasis vulgaris and psoriatic arthritis: real‐world evidence in Japan. J Dermatol. 2020;48(2):175‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao Y, Cai L, Liu X‐Y, Zhang H, Zhang J‐Z. Efficacy and safety of secukinumab in Chinese patients with moderate‐to‐severe plaque psoriasis: a real‐life cohort study. Chin Med J. 2021;134(11):1324‐1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Caldarola G, Mariani M, Pirro F, et al. Comparison of short‐ and long‐term effectiveness of ixekizumab and secukinumab in real‐world practice. Expert Opin Biol Ther. 2021;21(2):279‐286. [DOI] [PubMed] [Google Scholar]
- 25. Ryoo JY, Yang H‐J, Ji E, Yoo BK. Meta‐analysis of the efficacy and safety of secukinumab for the treatment of plaque psoriasis. Ann Pharmacother. 2016;50(5):341‐351. [DOI] [PubMed] [Google Scholar]
- 26. Calara PS, Althin R, Carlsson KS, Schmitt‐Egenolf M. Regional differences in the prescription of biologics for psoriasis in Sweden: a register‐based study of 4168 patients. BioDrugs. 2017;31(1):75‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Calara PS, Norlin JM, Althin R, Carlsson KS, Schmitt‐Egenolf M. Healthcare provider type and switch to biologics in psoriasis: evidence from real‐world practice. BioDrugs. 2016;30(2):145‐151. [DOI] [PubMed] [Google Scholar]
- 28. Gisondi P, Rovaris M, Piaserico S, Girolomoni G. Efficacy of secukinumab without the initial weekly loading dose in patients with chronic plaque psoriasis. Brit J Dermatol. 2019;182(1):175‐179. [DOI] [PubMed] [Google Scholar]
- 29. Furst DE. The risk of infections with biologic therapies for rheumatoid arthritis. Semin Arthritis Rheum. 2010;39(5):327‐346. [DOI] [PubMed] [Google Scholar]
- 30. Körber A, Papavassilis C, Bhosekar V, Reinhardt M. Efficacy and safety of secukinumab in elderly subjects with moderate to severe plaque psoriasis: a pooled analysis of phase III studies. Drugs Aging. 2018;35(2):135‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Palakornkitti P, Nimmannitya K, Rattanakaemakorn P. Biological therapy in psoriasis: an emphasis on its dermatologic adverse events. Asian Pac J Allergy Immunol. 2021;39(4):215‐230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


