Summary
Background
Nirmatrelvir/ritonavir treatment decreases the hospitalisation rate in immunocompetent patients with COVID-19, but data on efficacy in patients with haematological malignancy are scarce. Here, we describe the outcome of nirmatrelvir/ritonavir treatment in a large cohort of the latter patients.
Methods
This is a retrospective cohort study from the multicentre EPICOVIDEHA registry (NCT04733729) on patients with haematological malignancy, who were diagnosed with COVID-19 between January and September 2022. Patients receiving nirmatrelvir/ritonavir were compared to those who did not. A logistic regression was run to determine factors associated with nirmatrelvir/ritonavir administration in our sample. Mortality between treatment groups was assessed with Kaplan–Meier survival plots after matching all the patients with a propensity score. Additionally, a Cox regression was modelled to detect factors associated with mortality in patients receiving nirmatrelvir/ritonavir.
Findings
A total of 1859 patients were analysed, 117 (6%) were treated with nirmatrelvir/ritonavir, 1742 (94%) were treated otherwise. Of 117 patients receiving nirmatrelvir/ritonavir, 80% had received ≥1 anti-SARS-CoV-2 vaccine dose before COVID-19 onset, 13% of which received a 2nd vaccine booster. 5% were admitted to ICU. Nirmatrelvir/ritonavir treatment was associated with the presence of extrapulmonary symptoms at COVID-19 onset, for example anosmia, fever, rhinitis, or sinusitis (aOR 2.509, 95%CI 1.448–4.347) and 2nd vaccine booster (aOR 3.624, 95%CI 1.619–8.109). Chronic pulmonary disease (aOR 0.261, 95%CI 0.093–0.732) and obesity (aOR 0.105, 95%CI 0.014–0.776) were not associated with nirmatrelvir/ritonavir use. After propensity score matching, day-30 mortality rate in patients treated with nirmatrelvir/ritonavir was 2%, significantly lower than in patients with SARS-CoV-2 directed treatment other than nirmatrelvir/ritonavir (11%, p = 0.036). No factor was observed explaining the mortality difference in patients after nirmatrelvir/ritonavir administration.
Interpretation
Haematological malignancy patients were more likely to receive nirmatrelvir/ritonavir when reporting extrapulmonary symptoms or 2nd vaccine booster at COVID-19 onset, as opposed to chronic pulmonary disease and obesity. The mortality rate in patients treated with nirmatrelvir/ritonavir was lower than in patients with targeted drugs other than nirmatrelvir/ritonavir.
Funding
EPICOVIDEHA has received funds from Optics COMMIT (COVID-19 Unmet Medical Needs and Associated Research Extension) COVID-19 RFP program by GILEAD Science, United States (Project 2020-8223).
Keywords: Nirmatrelvir, SARS-CoV-2, Haematology, Malignancy, COVID-19
Research in context.
Evidence before this study
Nirmatrelvir/ritonavir is a new antiviral targeting the SARS-CoV-2 3Cl protease. It is widely recommended for patients with mild symptoms to prevent severe episodes of COVID-19. A web search was performed on November 12th for articles in English, French, German, Italian, or Spanish using strings combining the terms “haemato∗, “hemato∗”, “nirmatrelvir∗”, “paxlovid” with “administration”, “mortality”, “outcome”, and “treatment". No studies focusing specifically on nirmatrelvir/ritonavir administration in haematological patients were found. Single publications targeting the general population or immunosuppressed patients were retrieved, but provided no detailed information on patients with haematological malignancy.
Added value of this study
To the best of our knowledge, this is the first publication describing the factors associated with nirmatrelvir/ritonavir administration and investigating factors associated with mortality in patients with haematological malignancy. Our results show that nirmatrelvir/ritonavir was administered more frequently to patients with extrapulmonary symptoms and those who had received a 2nd vaccine booster dose against SARS-CoV-2. On the contrary, patients with chronic pulmonary disease or obesity were less likely to receive nirmatrelvir/ritonavir. Mortality was significantly lower than in patients with other directed treatments.
Implications of all the available evidence
Clinical management of COVID-19 patients with baseline haematological malignancies remains challenging two years after onset of the pandemic. Although vaccines prevent hospitalization and reduce mortality rates, patients with haematological malignancy may not mount protective immune response. In this vulnerable immunosuppressed patient group nirmatrelvir/ritonavir treatment results in lower mortality rates as compared to other treatment approaches. Still not all patients qualifying for nirmatrelvir/ritonavir received that treatment. Reasons for underuse remain unclear at this point.
Introduction
Since the coronavirus disease 2019 (COVID-19) pandemic was declared in March 2020,1 unprecedented efforts by all stakeholders led to effective treatment options that can ameliorate the disease. Vaccines have proved to be a most effective method preventing hospitalisation and mortality.2,3 Immunocompromised patients, for instance those with haematological malignancy, have been at increased risk for severe courses of COVID-19 and fatal outcome. Because of impaired immune response to vaccination, they are still at high-risk and need other approaches than current vaccines.4, 5, 6
Such approaches have been implemented with the antivirals molnupiravir,7 nirmatrelvir/ritonavir,8 and remdesivir,9 and with the monoclonal antibodies targeting viral antigens.10, 11, 12, 13, 14 The common goal of these drugs is reducing rates of hospitalisation, severe disease, and death. Approved for administration in 2022,15,16 during the initial moments of the omicron wave, nirmatrelvir/ritonavir is an oral protease inhibitor administered in high-risk patients with mild symptoms early in the course of COVID-19.15,16 Although the phase 3 development programme addressed high-risk patients, only few patients with cancer were enrolled.8 Current consensus guidelines recommend nirmatrelvir/ritonavir in patients with haematological malignancy,17 although there is a lack of available data on this patient group.18
This EPICOVIDEHA study compares epidemiology and outcome of patients with haematological malignancy receiving nirmatrelvir/ritonavir treatment versus those who did not.
Methods
All data included in this analysis were exported from the EPICOVIDEHA registry. EPICOVIDEHA (NCT04733729) is an online registry open for patients with haematologic malignancy and SARS-CoV-2 infection. Cases from various regions of the world are documented in an electronic case report form (eCRF) accessible via www.clinicalsurveys.net (EFS Summer 2021, TIVIAN, Cologne, Germany). The eCRF comprises epidemiological data, such as baseline pre-COVID-19 conditions, previous clinical management of the haematologic malignancy, anti-SARS-CoV-2 vaccination history, COVID-19 diagnosis and management, and outcome. All patients are included in a validation process for data coherence and completeness performed by experts in haematologic malignancy and infectious diseases.19 In this validation process, data missing completely at random were reduced as possible, contacting contributors were to solve pending queries. Exclusions from the database only happened if a patient was not fulfilling all the inclusion criteria (no haematological malignancy, not active within the last 5 years prior to COVID-19, no adult, no laboratory-based COVID-19 diagnosis).
For the present analysis, patients needed to fulfil all inclusion criteria to be eligible: active haematologic malignancy within the last five years prior to COVID-19, including patients at onset or watch and wait; age ≥18 years; SARS-CoV-2 infection confirmed by either polymerase chain reaction (PCR) or antigen test; and SARS-CoV-2 diagnosis between January 1st and September 30th, 2022.
Selected patients were grouped according to treatment specifically received for COVID-19. Thus, we formed the following categories: patients treated with nirmatrelvir/ritonavir ± other directed or non-directed treatments, patients receiving other SARS-CoV-2 directed antivirals or monoclonal antibodies ± other non-directed treatments, and finally patients receiving neither directed antivirals or monoclonal antibodies, nor corticosteroids or convalescent plasma.
Statistical analysis
Consecutive data from participating institutions were summarised with frequencies and percentages for categorical variables and with median, interquartile range (IQR) and absolute range for continuous variables. Proportion comparisons were performed using Fisher's exact or Pearson's chi (X) squared tests, respectively. Logistic regression was utilised to determine which independent variables were associated with subsequent nirmatrelvir/ritonavir administration. For comparison analyses, patients were matched with a propensity score based on a logistic regression. The model included the variables sex, age (±10 years), baseline haematological malignancy, haematological malignancy status at COVID-19 onset, and origin continent (Europe). In order to determine the power and robustness of the performed propensity score, variables used for the matching were confronted with a median difference (age), Phi coefficient (sex and origin continent [Europe]), or Cramér's V (baseline haematological malignancy and haematological malignancy status at COVID-19 onset), as appropriate (Supplementary Table S1). A log-rank test was used to compare the survival probability of the patients based on the treatment received for COVID-19, which was graphically represented with a Kaplan–Meier survival plot. Additionally, Cox regression was used to analyse which factor could be associated with mortality both in every patient and in nirmatrelvir/ritonavir recipients who had data on duration of follow up. Variables with a p value < 0.1 in the univariable models were considered for the respective multivariable model. P value < 0.05 was considered statistically significant.
Ethics statement
The central ethics committee is at Fondazione Policlinico Universitario Agostino Gemelli - IRCCS, Università Cattolica del Sacro Cuore of Rome, Italy (Study ID: 3226). Additionally, each participating institution may also have a local approval for the research initiative as appropriate. The anonymized data that do not contain any personally identifiable information from any sources implies that the informed consent is not applicable.
Role of the funding source
The funders had no role in the study design, the collection, analysis, and interpretation of data, writing of the report and the decision to submit for publication. JSG, FM, LP, and OAC had access to and verified all raw data sets and made the decision to submit the manuscript.
Results
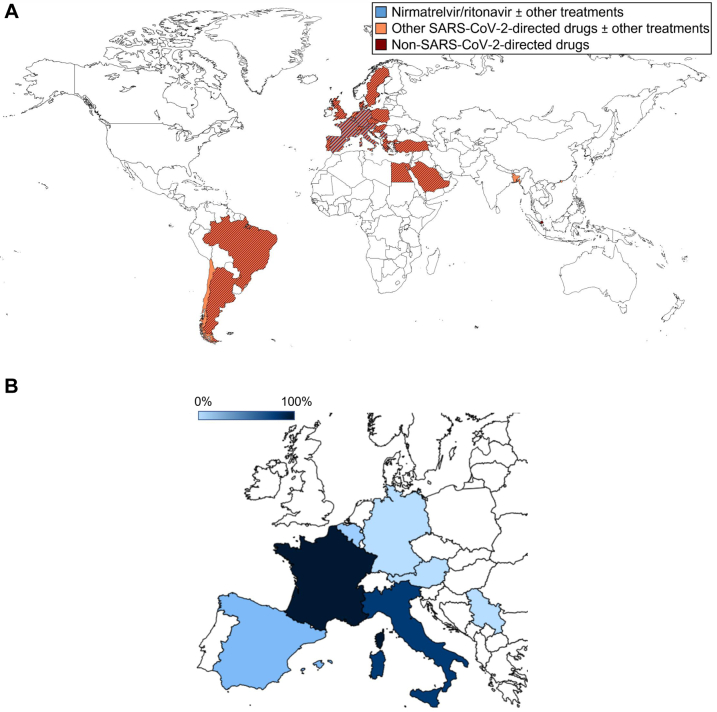
This EPICOVIDEHA data set comprises 1859 patients with haematological malignancy from 84 centres in 28 countries, who were diagnosed with COVID-19 between January and September 2022 (Fig. 1A).
Fig. 1.
Geographical distribution of patients documented in 2022 in EPICOVIDEHA according to the treatment received for COVID-19. A) Overall sample. This figure includes patients from institutions worldwide. Countries with patterns of more than one colour indicate that more than one type of treatment has been administered in the respective country. Blue indicates patients with nirmatrelvir/ritonavir ± other treatments: Italy (n = 59), Spain (n = 31), Belgium (n = 13), Germany (n = 7), France (n = 5), and Austria and Serbia (n = 1, each). Orange indicates patients with other SARS-CoV-2-directed drugs ± other treatments: Italy (n = 284), Spain (n = 162), Denmark (n = 73), Netherlands (n = 59), Germany (n = 51), Croatia (n = 36), Egypt (n = 33), Hungary (n = 32), Austria (n = 31); Belgium and France (n = 29, each), North Macedonia (n = 23), Serbia (n = 20), Czech Republic (n = 19), Greece (n = 18), Argentina, Poland, and Portugal (n = 16, each), Switzerland (n = 13), United Kingdom (n = 11), Sweden (n = 8), Turkey (n = 7), Brazil and Saudi Arabia (n = 6, each), and Bangladesh, Chile, and Hong Kong SAR (n = 1, each). Brown indicates patients with non-SARS-CoV-2-directed drugs: Spain (n = 192), Italy (n = 166), Netherlands (n = 105), Portugal (n = 80), Belgium (n = 35), Croatia (n = 26), France (n = 23), Argentina (n = 20), Austria (n = 15), Egypt (n = 14), Hungary (n = 11), Germany (n = 10), North Macedonia (n = 9), Switzerland (n = 7), Brazil (n = 6), Greece (n = 5), Turkey (n = 4), Serbia (n = 3), Denmark, Poland, and Sweden (n = 2, each), and Czech Republic, Saudi Arabia, Singapore, and United Kingdom (n = 1, each). B) Proportional ambulatory administration of nirmatrelvir/ritonavir. This figure includes patients from European institutions. The darker the blue, the higher proportion of patients receiving ambulatory nirmatrelvir/ritonavir: France (5/5) 100.0%, Italy (48/59) 81.4%, Spain (5/29) 17.2%, Belgium (1/13) 7.7%, and Austria (0/1), Germany (0/7), and Serbia (0/1) 0.0%, each.
Overall cohort
Almost 60% of the patients were male (n = 1070, 57.6%), and often with no other comorbidities (n = 766, 41.2%) besides haematological malignancy. Non-Hodgkin lymphoma (n = 602, 32.5%) was the most frequent haematological malignancy, followed by multiple myeloma (n = 337, 18.1%). Only one in four documented patients (n = 501, 26.9%) had active malignancy when COVID-19 was diagnosed. Overall, 69.0% (n = 1282) had received antineoplastic treatment within the preceding three months, or hematopoietic stem-cell transplantation or chimeric antigen receptor T cell administration within the last six months before COVID-19 diagnosis. The overall vaccination rate was 76.9% (n = 1430). Of 1118/1859 (60.1%) patients receiving directed treatment for COVID-19, 117/1118 (10.5%) received nirmatrelvir/ritonavir, either as monotherapy (n = 93/117, 79.5%) or in combination with other recommended drugs (n = 24/117, 20.5%), while 1001/1118 (89.5%) patients received treatment schemes without nirmatrelvir/ritonavir. A total of 741/1859 (39.9%) patients did not receive SARS-CoV-2 directed treatment. In total, intensive care was delivered to 148 (8.0%) patients (Table 1, Table 2).
Table 1.
Baseline characteristics of EPICOVIDEHA patients after licensing of nirmatrelvir/ritonavir.
| Directed treatment other than N/R |
p value N/R vs other directed treatment |
N/R |
p value N/R vs no/other non-directed tx |
No treatment/non-SARS-CoV-2 directed treatment |
Overall |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |||
| Sex | 0.112 | 0.618 | ||||||||
| Female | 400 | 40.0 | 56 | 47.9 | 333 | 44.9 | 789 | 42.4 | ||
| Male | 601 | 60.0 | 61 | 51.7 | 408 | 55.1 | 1070 | 57.6 | ||
| Age | 66 (53–75) [18–95] | 0.858 | 66 (55–75) [22–88] | 0.165 | 63 (51–74) [19–97] | 65 (52–75) [18–97] | ||||
| Comorbidities at COVID-19 onset | ||||||||||
| No comorbidities | 371 | 37.1 | 0.007 | 53 | 45.3 | 0.618 | 342 | 46.2 | 766 | 41.2 |
| 1 comorbidity | 320 | 32.0 | 40 | 34.2 | 248 | 33.5 | 608 | 32.7 | ||
| 2 comorbidities | 192 | 19.2 | 22 | 18.8 | 102 | 13.8 | 316 | 17.0 | ||
| 3 or more comorbidities | 118 | 11.8 | 2 | 1.7 | 49 | 6.6 | 169 | 9.1 | ||
| Chronic cardiopathy | 405 | 40.5 | 0.921 | 48 | 41.0 | 0.176 | 255 | 34.4 | 708 | 38.1 |
| Chronic pulmonary disease | 116 | 11.6 | 0.017 | 5 | 4.3 | 0.077 | 70 | 9.4 | 191 | 10.3 |
| Diabetes mellitus | 130 | 13.0 | 0.187 | 10 | 8.5 | 0.623 | 77 | 10.4 | 217 | 11.7 |
| Liver disease | 44 | 4.4 | 0.469 | 3 | 2.6 | 0.789 | 28 | 3.8 | 75 | 4.0 |
| Obesity | 73 | 7.3 | 0.005 | 1 | 0.9 | 0.072 | 33 | 4.5 | 107 | 5.8 |
| Renal impairment | 67 | 6.7 | 0.039 | 2 | 1.7 | 0.298 | 31 | 4.2 | 100 | 5.4 |
| Smoking history | 136 | 13.6 | 0.387 | 12 | 10.3 | 0.472 | 60 | 8.1 | 208 | 11.2 |
| Baseline malignancy at COVID-19 onset | 0.432 | 0.635 | ||||||||
| Leukemia | 393 | 39.3 | 49 | 41.9 | 282 | 38.1 | 724 | 38.9 | ||
| Acute myeloid leukemia | 152 | 15.2 | 17 | 14.5 | 62 | 8.4 | 231 | 12.4 | ||
| Chronic myeloid leukemia | 15 | 1.5 | 4 | 3.4 | 36 | 4.9 | 55 | 3.0 | ||
| Acute lymphoid leukemia | 58 | 5.8 | 8 | 6.8 | 35 | 4.7 | 101 | 5.4 | ||
| Chronic lymphoid leukemia | 107 | 10.7 | 5 | 4.3 | 97 | 13.1 | 209 | 11.2 | ||
| Myelodisplastic syndrome | 58 | 5.8 | 14 | 12.0 | 50 | 6.7 | 122 | 6.6 | ||
| Hairy cell leukemia | 3 | 0.3 | 1 | 0.9 | 2 | 0.3 | 6 | 0.3 | ||
| Lymphoma | 392 | 39.2 | 38 | 32.5 | 257 | 34.7 | 687 | 37.0 | ||
| Hodgkin lymphoma | 39 | 3.9 | 1 | 0.9 | 45 | 6.1 | 85 | 4.6 | ||
| Non-Hodgkin lymphoma | 353 | 35.3 | 37 | 31.6 | 212 | 28.6 | 602 | 32.4 | ||
| PH negative myeloproliferative diseases | 28 | 2.8 | 5 | 4.3 | 51 | 6.9 | 84 | 4.5 | ||
| Essential thrombocythemia | 2 | 0.2 | 0 | 0.0 | 19 | 2.6 | 21 | 1.1 | ||
| Myelofibrosis | 20 | 2.0 | 3 | 2.6 | 15 | 2.0 | 38 | 2.0 | ||
| Polycythemia vera | 4 | 0.4 | 1 | 0.9 | 14 | 1.9 | 19 | 1.0 | ||
| Systemic mastocytosis | 2 | 0.2 | 1 | 0.9 | 3 | 0.4 | 6 | 0.3 | ||
| Plasma cell disorders | 179 | 17.9 | 25 | 21.4 | 143 | 19.3 | 347 | 18.7 | ||
| Multiple myeloma | 175 | 17.5 | 25 | 21.4 | 137 | 18.5 | 337 | 18.1 | ||
| Amyloid light-chain amyloidosis | 4 | 0.4 | 0 | 0.0 | 6 | 0.8 | 10 | 0.5 | ||
| Other hematological malignancies | 9 | 0.9 | 0 | 0.0 | 10 | 1.3 | 19 | 1.0 | ||
| Aplastic anemia | 9 | 0.9 | 0 | 0.0 | 10 | 1.3 | 19 | 1.0 | ||
| Status malignancy at COVID-19 onset | 0.234 | 0.065 | ||||||||
| Controlled disease | 473 | 47.3 | 64 | 54.7 | 397 | 53.6 | 934 | 50.2 | ||
| Stable disease | 188 | 18.8 | 19 | 16.2 | 175 | 23.6 | 382 | 20.5 | ||
| Active disease | 314 | 31.4 | 29 | 24.8 | 158 | 21.3 | 501 | 26.9 | ||
| Unknown | 26 | 2.6 | 5 | 4.3 | 11 | 1.5 | 42 | 2.3 | ||
| Last haematological malignancy treatment immediately before COVID-19 onset | ||||||||||
| No treatment | 85 | 8.5 | 4 | 3.4 | 95 | 12.8 | 184 | 9.9 | ||
| alloHSCT | 65 | 6.5 | 6 | 5.1 | 32 | 4.3 | 103 | 5.5 | ||
| In the last 6 months | 32 | 3.2 | 1 | 0.9 | 14 | 1.9 | 47 | 2.5 | ||
| >6 months | 32 | 3.2 | 5 | 4.3 | 18 | 2.4 | 55 | 3.0 | ||
| Unknown | 1 | 0.1 | 0 | 0.0 | 0 | 0.0 | 1 | 0.1 | ||
| autoHSCT | 16 | 1.6 | 5 | 4.3 | 5 | 0.7 | 26 | 1.4 | ||
| In the last 6 months | 13 | 1.3 | 3 | 2.6 | 4 | 0.5 | 20 | 1.1 | ||
| >6 months | 3 | 0.3 | 2 | 1.7 | 1 | 0.1 | 6 | 0.3 | ||
| CAR-T | 11 | 1.1 | 1 | 0.9 | 3 | 0.4 | 15 | 0.8 | ||
| In the last 6 months | 4 | 0.4 | 0 | 0.0 | 2 | 0.3 | 6 | 0.3 | ||
| >6 months | 7 | 0.7 | 1 | 0.9 | 1 | 0.1 | 9 | 0.5 | ||
| Conventional chemotherapy | 300 | 30.0 | 26 | 22.2 | 209 | 28.2 | 535 | 28.8 | ||
| In the last month | 129 | 12.9 | 10 | 8.5 | 82 | 11.1 | 221 | 11.9 | ||
| In the last 3 months | 146 | 14.6 | 13 | 11.1 | 92 | 12.4 | 251 | 13.5 | ||
| >3 months | 20 | 2.0 | 3 | 2.6 | 35 | 4.7 | 58 | 3.1 | ||
| Unknown | 5 | 0.5 | 0 | 0.0 | 0 | 0.0 | 5 | 0.3 | ||
| Demethylating agents | 57 | 5.7 | 7 | 6.0 | 41 | 5.5 | 105 | 5.6 | ||
| In the last month | 43 | 4.3 | 5 | 4.3 | 34 | 4.6 | 82 | 4.4 | ||
| In the last 3 months | 11 | 1.1 | 0 | 0.0 | 6 | 0.8 | 17 | 0.9 | ||
| >3 months | 3 | 0.3 | 0 | 0.0 | 1 | 0.1 | 4 | 0.2 | ||
| Unknown | 0 | 0.0 | 2 | 1.7 | 0 | 0.0 | 2 | 0.1 | ||
| Immuno-chemotherapy | 351 | 35.1 | 49 | 41.9 | 238 | 32.1 | 638 | 34.3 | ||
| In the last month | 229 | 22.9 | 34 | 29.1 | 156 | 21.1 | 419 | 22.5 | ||
| In the last 3 months | 42 | 4.2 | 5 | 4.3 | 24 | 3.2 | 71 | 3.8 | ||
| >3 months | 79 | 7.9 | 10 | 8.5 | 57 | 7.7 | 146 | 7.9 | ||
| Unknown | 1 | 0.1 | 0 | 0.0 | 1 | 0.1 | 2 | 0.1 | ||
| Immunotherapy | 46 | 4.6 | 1 | 0.9 | 34 | 4.6 | 81 | 4.4 | ||
| In the last month | 25 | 2.5 | 1 | 0.9 | 17 | 2.3 | 43 | 2.3 | ||
| In the last 3 months | 10 | 1.0 | 0 | 0.0 | 6 | 0.8 | 16 | 0.9 | ||
| >3 months | 9 | 0.9 | 0 | 0.0 | 11 | 1.5 | 20 | 1.1 | ||
| Unknown | 2 | 0.2 | 0 | 0.0 | 0 | 0.0 | 2 | 0.1 | ||
| Supportive measures | 16 | 1.6 | 5 | 4.3 | 19 | 2.6 | 40 | 2.2 | ||
| In the last month | 3 | 0.3 | 1 | 0.9 | 1 | 0.1 | 5 | 0.3 | ||
| In the last 3 months | 2 | 0.2 | 0 | 0.0 | 0 | 0.0 | 2 | 0.1 | ||
| >3 months | 1 | 0.1 | 0 | 0.0 | 0 | 0.0 | 1 | 0.1 | ||
| Unknown | 10 | 1.0 | 4 | 3.4 | 18 | 2.4 | 32 | 1.7 | ||
| Targeted therapy | 183 | 18.3 | 23 | 19.7 | 147 | 19.8 | 353 | 19.0 | ||
| In the last month | 138 | 13.8 | 21 | 17.9 | 116 | 15.7 | 275 | 14.8 | ||
| In the last 3 months | 19 | 1.9 | 0 | 0.0 | 9 | 1.2 | 28 | 1.5 | ||
| >3 months | 17 | 1.7 | 0 | 0.0 | 16 | 2.2 | 33 | 1.8 | ||
| Unknown | 9 | 0.9 | 2 | 1.7 | 6 | 0.8 | 17 | 0.9 | ||
| Neutrophils at COVID-19 onset | 0.704 | 0.708 | ||||||||
| <501 | 91 | 9.1 | 8 | 6.8 | 29 | 3.9 | 128 | 6.9 | ||
| 501–999 | 58 | 5.8 | 6 | 5.1 | 33 | 4.5 | 97 | 5.2 | ||
| >999 | 758 | 75.7 | 91 | 77.8 | 459 | 61.9 | 1308 | 70.4 | ||
| Lymphocytes at COVID-19 onset | 0.044 | 0.180 | ||||||||
| <201 | 124 | 12.4 | 9 | 7.7 | 22 | 3.0 | 155 | 8.3 | ||
| 201–499 | 166 | 16.6 | 12 | 10.3 | 57 | 7.7 | 235 | 12.6 | ||
| >499 | 618 | 61.7 | 84 | 71.8 | 436 | 58.8 | 1138 | 61.2 | ||
| SARS-CoV-2 vaccination status at COVID-19 onset | ||||||||||
| Overall days from last dose administration to COVID-19 onset | 127 (73–203) [2–532] | <0.001 | 174 (121–235) [14–482] | <0.001 | 116 (70–191) [3–372] | 126 (75–201) [2–532] | ||||
| Number of doses | 0.003 | 0.040 | ||||||||
| Not vaccinated | 258 | 25.8 | 23 | 19.7 | 148 | 20.0 | 429 | 23.1 | ||
| One dose | 32 | 3.2 | 3 | 2.6 | 27 | 3.6 | 62 | 3.3 | ||
| Days from last dose administration to COVID-19 onset | 231 (123–275) [5–461] | 110 (23–394) [23–394] | 163 (108–247) [6–368] | 195.5 (110–258) [5–461] | ||||||
| Two doses | 208 | 20.8 | 15 | 12.8 | 165 | 22.3 | 388 | 20.9 | ||
| Days from last dose administration to COVID-19 onset | 233 (161–276) [8–425] | 305 (166–365) [39–482] | 198 (141–244) [7–355] | 212 (153–267) [7–482] | ||||||
| Three doses | 454 | 45.4 | 61 | 52.1 | 351 | 47.4 | 866 | 46.6 | ||
| Days from last dose administration to COVID-19 onset | 97 (57–134) [2–532] | 155 (111–190) [14–286] | 90 (56–123) [3–372] | 98 (59–135) [2–532] | ||||||
| Four doses | 49 | 4.9 | 15 | 12.8 | 50 | 6.7 | 114 | 6.1 | ||
| Days from last dose administration to COVID-19 onset | 188 (155–216) [94–297] | 234 (197–260) [104–295] | 197 (164.5–234) [84–293] | 193 (161–234) [84–297] | ||||||
| Type of last vaccine | ||||||||||
| mRNA | 683 | 68.2 | 92 | 78.6 | 547 | 73.8 | 1322 | 71.1 | ||
| BioNTech/Pfizer | 548 | 54.7 | 75 | 64.1 | 408 | 55.1 | 1031 | 55.5 | ||
| Moderna COVE | 135 | 13.5 | 17 | 14.5 | 139 | 18.8 | 291 | 15.7 | ||
| Vector-based | 38 | 3.8 | 1 | 0.9 | 39 | 5.3 | 78 | 4.2 | ||
| AstraZeneca Oxford | 29 | 2.9 | 1 | 0.9 | 18 | 2.4 | 48 | 2.6 | ||
| Sputnik | 5 | 0.5 | 0 | 0.0 | 6 | 0.8 | 11 | 0.6 | ||
| J&J - Janssen | 4 | 0.4 | 0 | 0.0 | 15 | 2.0 | 19 | 1.0 | ||
| Inactivated | 18 | 1.8 | 1 | 0.9 | 5 | 0.7 | 24 | 1.3 | ||
| CoronaVac | Sinovac | 5 | 0.5 | 0 | 0.0 | 1 | 0.1 | 6 | 0.3 | ||
| Sinopharm | 13 | 1.3 | 1 | 0.9 | 4 | 0.5 | 18 | 1.0 | ||
This table includes patients from institutions worldwide.
alloHSCT, allogeneic hematopoietic stem-cell transplantation; autoHSCT, autologous hematopoietic stem-cell transplantation; CAR-T, chimeric antigen receptor T cells; COVE, coronavirus efficacy; COVID-19, coronavirus 2019 disease; J&J, Johnson and Johnson; mRNA, messenger ribonucleic acid; N/R, nirmatrelvir/ritonavir; PH, Philadelphia; tx, treatment.
Reasons for no vaccination in nirmatrelvir/ritonavir: unknown (n = 23, 100.0%). Reasons for no vaccination in other SARS-CoV-2-directed drugs: unknown (n = 216, 83.7%), patient refusal (n = 24, 9.3%), ongoing malignancy treatment (n = 14, 5.4%), and other reasons (n = 4, 1.6%). Reasons for no vaccination in non-SARS-CoV-2-directed drugs: unknown (n = 129, 87.2%), patient refusal (n = 14, 9.5%), ongoing malignancy treatment (n = 4, 2.7%), and other reasons (n = 1, 0.7%).
Table 2.
Characteristics of COVID-19 episodes.
| Directed treatment other than N/R |
p value N/R vs other directed treatment |
N/R |
p value N/R vs no/other non-directed tx |
No treatment/non-SARS-CoV-2 directed treatment |
Overall |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | % | n | |||
| SARS-CoV-2 variant of concern | 0.139 | 1.000 | ||||||||
| Wild type | 1 | 0.1 | 0 | 0.0 | 1 | 0.1 | 2 | 0.1 | ||
| Delta | 7 | 0.7 | 0 | 0.0 | 1 | 0.1 | 8 | 0.4 | ||
| Omicron | 479 | 47.9 | 44 | 37.6 | 281 | 37.9 | 804 | 43.2 | ||
| Not tested | 514 | 51.3 | 73 | 62.4 | 458 | 61.8 | 1045 | 56.2 | ||
| Symptoms at COVID-19 onset | 0.001 | <0.001 | ||||||||
| Pulmonary | 145 | 14.5 | 34 | 29.1 | 220 | 29.7 | 399 | 21.5 | ||
| Pulmonary + extrapulmonary | 176 | 17.6 | 32 | 27.4 | 121 | 16.3 | 329 | 17.7 | ||
| Extrapulmonary | 556 | 55.5 | 38 | 32.5 | 178 | 24.0 | 772 | 41.5 | ||
| Screening | 124 | 12.4 | 13 | 11.1 | 222 | 30.0 | 359 | 19.3 | ||
| COVID-19 infection | <0.001 | <0.001 | ||||||||
| Asymptomatic | 145 | 14.5 | 13 | 11.1 | 211 | 28.5 | 369 | 19.8 | ||
| Mild infection | 176 | 17.6 | 38 | 32.5 | 185 | 25.0 | 399 | 21.5 | ||
| Severe infection | 556 | 55.5 | 60 | 51.3 | 327 | 44.1 | 943 | 50.7 | ||
| Critical infection | 124 | 12.4 | 6 | 5.1 | 18 | 2.4 | 148 | 8.0 | ||
| Stay during COVID-19 episode | <0.001 | <0.001 | ||||||||
| Home | 264 | 26.4 | 59 | 50.4 | 554 | 74.8 | 877 | 47.2 | ||
| Hospital | 736 | 73.5 | 56 | 47.9 | 182 | 24.6 | 974 | 52.4 | ||
| Overall days of hospital stay | 12 (7–21) [1–135] | <0.001 | 8 (1–15) [1–57] | 0.975 | 7 (2–15) [1–118] | 11 (6–20) [1–135] | ||||
| ICU admission | 124 | 12.4 | 0.021 | 6 | 5.1 | 0.124 | 18 | 2.4 | 148 | 8.0 |
| Overall days in ICU | 9 (5–14) [1–68] | 0.609 | 7 (3–11) [2–32] | 0.323 | 3 (2–11) [1–24] | 9 (4–14) [1–68] | ||||
| COVID-19 treatment | ||||||||||
| Days under COVID-19 treatment | 2 (1–4) [1–34] | 4 (4–5) [1–24] | 4 (1–4) [1–34] | |||||||
| Days under nirmatrelvir/ritonavir treatment | 4 (4–5) [1–10] | 4 (4–5) [1–10] | ||||||||
| Days from COVID-19 onset to nirmatrelvir/ritonavir treatment | 1 (0–3) [0–151] | 1 (0–3) [0–151] | ||||||||
| Type of treatment | ||||||||||
| No treatment administered | 0 | 0.0 | 0 | 0.0 | 741 | 100.0 | 741 | 39.9 | ||
| Antiviral + monoclonal antibody | ||||||||||
| ± corticosteroids ± plasma | 141 | 14.1 | 0 | 0.0 | 0 | 0.0 | 141 | 7.6 | ||
| Antiviral ± corticosteroids ± plasma | 293 | 29.3 | 0 | 0.0 | 0 | 0.0 | 293 | 15.8 | ||
| Corticosteroids | 242 | 24.2 | 0 | 0.0 | 0 | 0.0 | 242 | 13.0 | ||
| Monoclonal antibodies | ||||||||||
| ± plasma ± corticosteroids | 305 | 30.5 | 0 | 0.0 | 0 | 0.0 | 305 | 16.4 | ||
| Nirmatrelvir combination therapy | ||||||||||
| ± corticosteroids ± plasma | 0 | 0.0 | 24 | 20.5 | 0 | 0.0 | 24 | 1.3 | ||
| 1st line | 0 | 0.0 | 7 | 6.0 | 0 | 0.0 | 7 | 0.4 | ||
| Other line | 0 | 0.0 | 17 | 14.5 | 0 | 0.0 | 17 | 0.9 | ||
| Nirmatrelvir monotherapy | ||||||||||
| ± corticosteroids ± plasma | 0 | 0.0 | 93 | 79.5 | 0 | 0.0 | 93 | 5.0 | ||
| 1st line | 0 | 0.0 | 93 | 79.5 | 0 | 0.0 | 93 | 5.0 | ||
| Plasma ± corticosteroids | 20 | 2.0 | 0 | 0.0 | 0 | 0.0 | 20 | 1.1 | ||
| Outcome | 0.027 | 0.016 | ||||||||
| Days from COVID-19 onset to final day of follow up/death | 26 (12–47) [0–219] | 35 (16–64) [0–191] | 27 (12–46) [0–214] | 27 (13–48) [0–219] | ||||||
| Alive | 853 | 85.2 | 0.023 | 109 | 93.2 | 0.666 | 701 | 94.6 | 1663 | 89.5 |
| Dead | 148 | 14.8 | 8 | 6.8 | 40 | 5.4 | 196 | 10.5 | ||
| Days from COVID-19 onset to final day of follow up | 29 (14–51) [0–219] | 35 (15–63) [0–191] | 28 (13–46) [0–214] | 29 (14–50) [0–219] | ||||||
| Days from COVID-19 onset to death | 16 (8–28) [0–109] | 48.5 (28.5–83) [14–156] | 12 (4–25) [0–127] | 16 (7–30) [0–156] | ||||||
| Reason for death | 0.177 | 0.588 | ||||||||
| COVID-19 | 84 | 8.4 | 2 | 1.7 | 17 | 2.3 | 103 | 5.5 | ||
| COVID-19 | ||||||||||
| + hematological malignancy | 46 | 4.6 | 4 | 3.4 | 11 | 1.5 | 61 | 3.3 | ||
| Hematological maligancies | ||||||||||
| +/− other reasons | 14 | 1.4 | 2 | 1.7 | 9 | 1.2 | 25 | 1.3 | ||
| Other reasons | 4 | 0.4 | 0 | 0.0 | 3 | 0.4 | 7 | 0.4 | ||
This table includes patients from institutions worldwide.
COVID-19, coronavirus 2019 disease; ICU, intensive care unit; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
In univariable logistic regression, nirmatrelvir/ritonavir treatment was associated with a patient without history of chronic pulmonary disease (odds ratio [OR] 0.341, 95% confidence interval [CI] 0.136–0.852), without obesity (OR 0.110, 95% CI 0.015–0.796), with extrapulmonary (i.e., anosmia, fever, rhinitis, or sinusitis) symptoms at COVID-19 onset (OR 2.102, 95% CI 1.278–3.457), and receipt of a second booster/4th dose (OR 3.434, 95% CI 1.674–7.045). In the multivariable model, patients with extrapulmonary symptoms (aOR 2.509, 95% CI 1.448–4.347) and those having received a 4th vaccine dose (aOR 3.624, 95% CI 1.619–8.109) were more likely to be treated with nirmatrelvir/ritonavir, while patients with chronic pulmonary disease (adjusted OR [aOR] 0.261, 95% CI 0.093–0.732) and obesity (aOR 0.105, 95% CI 0.014–0.776) were not (Table 3).
Table 3.
Factors associated with a potential nirmatrelvir/ritonavir administration.
| Univariable analysis |
Multivariable analysis |
|||||||
|---|---|---|---|---|---|---|---|---|
| p value | OR | 95% CI |
p value | OR | 95% CI |
|||
| Lower limit | Upper limit | Lower limit | Upper limit | |||||
| Sex | ||||||||
| Female | – | – | – | – | ||||
| Male | 0.101 | 0.725 | 0.494 | 1.065 | ||||
| Age | 0.521 | 1.004 | 0.992 | 1.016 | ||||
| Comorbidities at COVID-19 onset | ||||||||
| Chronic cardiopathy | 0.906 | 1.024 | 0.694 | 1.511 | ||||
| Chronic pulmonary disease | 0.021 | 0.341 | 0.136 | 0.852 | 0.011 | 0.261 | 0.093 | 0.732 |
| Diabetes mellitus | 0.173 | 0.626 | 0.319 | 1.228 | ||||
| Liver disease | 0.356 | 0.572 | 0.175 | 1.873 | ||||
| Obesity | 0.029 | 0.110 | 0.015 | 0.796 | 0.027 | 0.105 | 0.014 | 0.776 |
| Renal failure | 0.050 | 0.242 | 0.059 | 1.003 | 0.071 | 0.265 | 0.063 | 1.120 |
| Smoking history | 0.316 | 0.727 | 0.389 | 1.357 | ||||
| No comorbidity | 0.261 | 1.250 | 0.847 | 1.845 | ||||
| Neutrophils at COVID-19 onset | ||||||||
| <501 | – | – | – | – | ||||
| 501–999 | 0.774 | 1.177 | 0.388 | 3.565 | ||||
| >999 | 0.418 | 1.366 | 0.642 | 2.905 | ||||
| Lymphocytes at COVID-19 onset | ||||||||
| <201 | – | – | – | – | – | – | – | – |
| 201–499 | 0.993 | 0.996 | 0.407 | 2.438 | 0.672 | 1.219 | 0.487 | 3.052 |
| >499 | 0.085 | 1.873 | 0.917 | 3.824 | 0.050 | 2.080 | 1.000 | 4.329 |
| Status malignancy at COVID-19 onset | ||||||||
| Controlled disease | – | – | – | – | ||||
| Stable disease | 0.289 | 0.747 | 0.436 | 1.281 | ||||
| Active disease | 0.105 | 0.683 | 0.430 | 1.083 | ||||
| Unknown | 0.487 | 1.421 | 0.527 | 3.833 | ||||
| Baseline malignancy at COVID-19 onset | ||||||||
| Leukaemia | – | – | – | – | ||||
| Lymphoma | 0.269 | 0.777 | 0.498 | 1.215 | ||||
| PH negative myeloproliferative diseases | 0.480 | 1.432 | 0.529 | 3.881 | ||||
| Plasma cell disorders | 0.665 | 1.120 | 0.671 | 1.871 | ||||
| Other haematological malignancies | 0.999 | 0.000 | 0.000 | – | ||||
| Symptoms at COVID-19 onset | ||||||||
| Pulmonary | – | – | – | – | – | – | – | – |
| Pulmonary + extrapulmonary | 0.908 | 0.970 | 0.585 | 1.611 | 0.995 | 1.002 | 0.570 | 1.762 |
| Extrapulmonary | 0.003 | 2.102 | 1.278 | 3.457 | 0.001 | 2.509 | 1.448 | 4.347 |
| Screening | 0.357 | 0.732 | 0.376 | 1.423 | 0.740 | 0.888 | 0.441 | 1.789 |
| SARS-CoV-2 vaccination status at COVID-19 onset | ||||||||
| Not vaccinated | – | – | – | – | – | – | – | – |
| One dose | 0.937 | 1.052 | 0.299 | 3.700 | 0.654 | 0.706 | 0.154 | 3.233 |
| Two doses | 0.539 | 0.809 | 0.412 | 1.590 | 0.292 | 0.673 | 0.322 | 1.406 |
| Three doses | 0.110 | 1.507 | 0.911 | 2.493 | 0.081 | 1.618 | 0.942 | 2.780 |
| Four doses | <0.001 | 3.434 | 1.674 | 7.045 | 0.002 | 3.624 | 1.619 | 8.109 |
This table includes patients from institutions worldwide.
CI, confidence interval; COVID-19, coronavirus 2019 disease; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
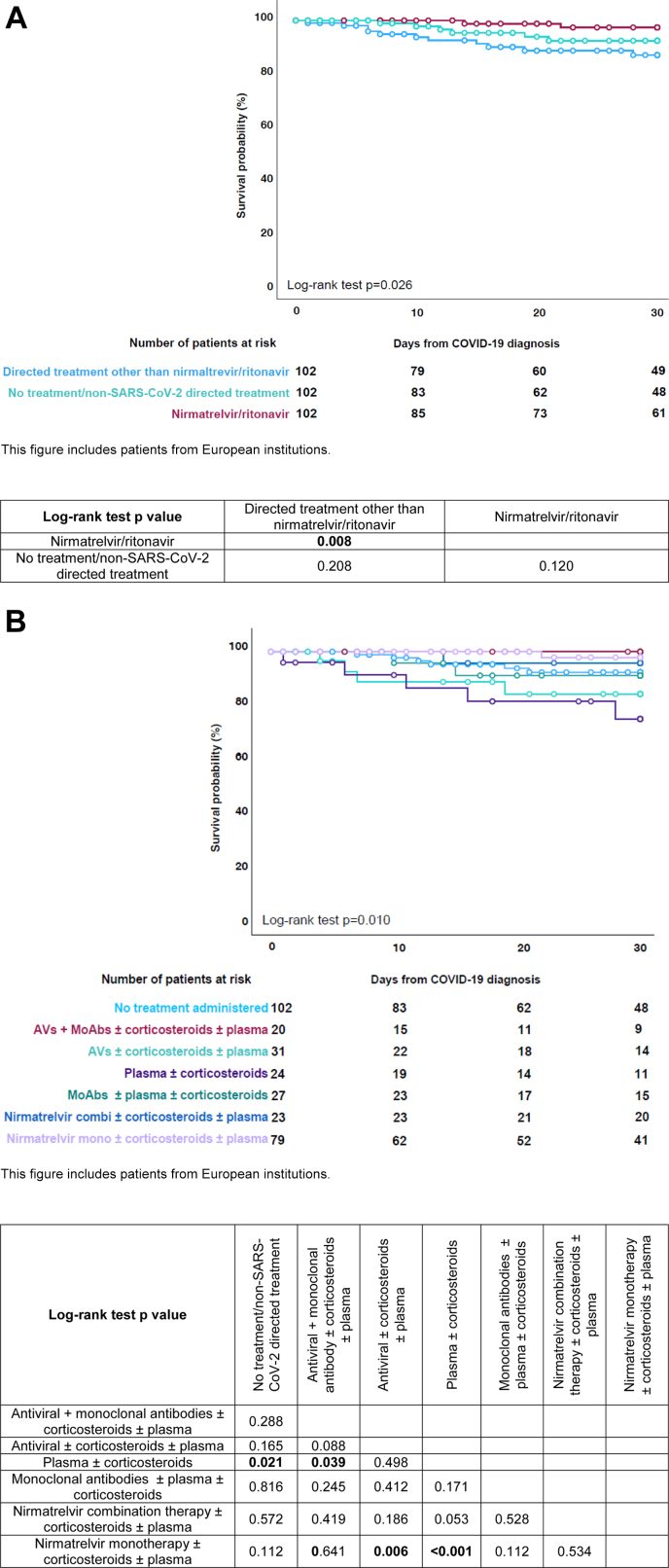
After matching the patients from the three treatment groups by sex, age (±10 years), baseline haematological malignancy, haematological malignancy status at COVID-19 onset, and origin continent (Europe), the day-30 mortality rate was 2.0% (n = 2/102) after nirmatrelvir/ritonavir administration, 10.8% (n = 11/102) after the administration of directed treatment options other than nirmatrelvir/ritonavir, and 5.9% (n = 6/102) in patients without treatment administration (p = 0.036, Table 4, Supplementary Table S2). Survival probability was significantly higher in patients treated with nirmatrelvir/ritonavir as compared to those with directed treatment options other than nirmatrelvir/ritonavir (p = 0.008, Fig. 2A and B).
Table 4.
Characteristics of patients and COVID-19 episodes after matching by propensity score.
| Directed treatment other than N/R |
N/R |
No treatment/non-SARS-CoV-2 directed treatment |
p value |
||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Sex | 1.000 | ||||||
| Female | 44 | 43.1% | 44 | 43.1% | 44 | 43.1% | |
| Male | 58 | 56.9% | 58 | 56.9% | 58 | 56.9% | |
| Age | 66 (57–74) [21–89] | 67 (56–75) [22–88] | 67 (58–75) [22–89] | 0.913 | |||
| Comorbidities at COVID-19 onset | |||||||
| No comorbidities | 39 | 38.2% | 46 | 45.1% | 36 | 35.3% | 0.429 |
| 1 comorbidity | 32 | 31.4% | 33 | 32.4% | 35 | 34.3% | |
| 2 comorbidities | 22 | 21.6% | 21 | 20.6% | 24 | 23.5% | |
| 3 or more comorbidities | 9 | 8.8% | 2 | 2.0% | 7 | 6.9% | |
| Chronic cardiopathy | 41 | 40.2% | 42 | 41.2% | 38 | 37.3% | 0.874 |
| Chronic pulmonary disease | 15 | 14.7% | 5 | 4.9% | 12 | 11.8% | 0.062 |
| Diabetes mellitus | 9 | 8.8% | 10 | 9.8% | 9 | 8.8% | 1.000 |
| Liver disease | 8 | 7.8% | 3 | 2.9% | 7 | 6.9% | 0.397 |
| Obesity | 5 | 4.9% | 1 | 1.0% | 5 | 4.9% | 0.245 |
| Renal impairment | 9 | 8.8% | 2 | 2.0% | 5 | 4.9% | 0.097 |
| Smoking history | 9 | 8.8% | 10 | 9.8% | 16 | 15.7% | 0.281 |
| Baseline malignancy at COVID-19 onset | 1.000 | ||||||
| Leukemia | 43 | 42.2% | 43 | 42.2% | 43 | 42.2% | |
| Acute myeloid leukemia | 12 | 11.8% | 15 | 14.7% | 11 | 10.8% | |
| Chronic myeloid leukemia | 3 | 2.9% | 3 | 2.9% | 8 | 7.8% | |
| Acute lymphoid leukemia | 9 | 8.8% | 8 | 7.8% | 5 | 4.9% | |
| Chronic lymphoid leukemia | 9 | 8.8% | 4 | 3.9% | 9 | 8.8% | |
| Myelodisplastic syndrome | 10 | 9.8% | 13 | 12.7% | 10 | 9.8% | |
| Hairy cell leukemia | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | |
| Lymphoma | 34 | 33.3% | 34 | 33.3% | 34 | 33.3% | |
| Hodgkin lymphoma | 2 | 2.0% | 1 | 1.0% | 4 | 3.9% | |
| Non-Hodgkin lymphoma | 32 | 31.4% | 33 | 32.4% | 30 | 29.4% | |
| PH negative myeloproliferative diseases | 3 | 2.9% | 2 | 2.0% | 7 | 6.9% | |
| Essential thrombocythemia | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | |
| Myelofibrosis | 2 | 2.0% | 2 | 2.0% | 3 | 2.9% | |
| Polycythemia vera | 1 | 1.0% | 0 | 0.0% | 1 | 1.0% | |
| Systemic mastocytosis | 0 | 0.0% | 0 | 0.0% | 3 | 2.9% | |
| Plasma cell disorders | 21 | 20.6% | 21 | 20.6% | 21 | 20.6% | |
| Multiple myeloma | 19 | 18.6% | 21 | 20.6% | 19 | 18.6% | |
| Amyloid light-chain amyloidosis | 2 | 2.0% | 0 | 0.0% | 2 | 2.0% | |
| Other hematological malignancies | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | |
| Aplastic anemia | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | |
| Status malignancy at COVID-19 onset | 1.000 | ||||||
| Controlled disease | 62 | 60.8% | 62 | 60.8% | 62 | 60.8% | |
| Stable disease | 13 | 12.7% | 13 | 12.7% | 13 | 12.7% | |
| Active disease | 27 | 26.5% | 27 | 26.5% | 27 | 26.5% | |
| Last hematological malignancy treatment immediately before COVID-19 onset | |||||||
| No treatment | 12 | 11.8% | 4 | 3.9% | 11 | 10.8% | |
| alloHSCT | 10 | 9.8% | 5 | 4.9% | 5 | 4.9% | |
| In the last 6 months | 5 | 4.9% | 1 | 1.0% | 3 | 2.9% | |
| >6 months | 5 | 4.9% | 4 | 3.9% | 2 | 2.0% | |
| Unknown | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | |
| autoHSCT | 2 | 2.0% | 5 | 4.9% | 1 | 1.0% | |
| In the last 6 months | 2 | 2.0% | 3 | 2.9% | 0 | 0.0% | |
| >6 months | 0 | 0.0% | 2 | 2.0% | 1 | 1.0% | |
| CAR-T | 0 | 0.0% | 1 | 1.0% | 0 | 0.0% | |
| In the last 6 months | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | |
| >6 months | 0 | 0.0% | 1 | 1.0% | 0 | 0.0% | |
| Conventional chemotherapy | 12 | 11.8% | 14 | 13.7% | 16 | 15.7% | |
| In the last month | 10 | 9.8% | 8 | 7.8% | 10 | 9.8% | |
| In the last 3 months | 2 | 2.0% | 3 | 2.9% | 0 | 0.0% | |
| >3 months | 0 | 0.0% | 3 | 2.9% | 6 | 5.9% | |
| Unknown | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | |
| Demethylating agents | 10 | 9.8% | 6 | 5.9% | 6 | 5.9% | |
| In the last month | 8 | 7.8% | 4 | 3.9% | 6 | 5.9% | |
| In the last 3 months | 2 | 2.0% | 0 | 0.0% | 0 | 0.0% | |
| >3 months | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | |
| Unknown | 0 | 0.0% | 2 | 2.0% | 0 | 0.0% | |
| Immuno-chemotherapy | 38 | 37.3% | 43 | 42.2% | 37 | 36.3% | |
| In the last month | 27 | 26.5% | 29 | 28.4% | 27 | 26.5% | |
| In the last 3 months | 5 | 4.9% | 4 | 3.9% | 5 | 4.9% | |
| >3 months | 6 | 5.9% | 10 | 9.8% | 5 | 4.9% | |
| Unknown | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | |
| Immunotherapy | 0 | 0.0% | 1 | 1.0% | 4 | 3.9% | |
| In the last month | 0 | 0.0% | 1 | 1.0% | 3 | 2.9% | |
| In the last 3 months | 0 | 0.0% | 0 | 0.0% | 1 | 1.0% | |
| >3 months | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | |
| Unknown | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | |
| Supportive measures | 0 | 0.0% | 5 | 4.9% | 0 | 0.0% | |
| In the last month | 0 | 0.0% | 1 | 1.0% | 0 | 0.0% | |
| In the last 3 months | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | |
| >3 months | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | |
| Unknown | 0 | 0.0% | 4 | 3.9% | 0 | 0.0% | |
| Targeted therapy | 17 | 16.7% | 18 | 17.6% | 24 | 23.5% | |
| In the last month | 15 | 14.7% | 17 | 16.7% | 22 | 21.6% | |
| In the last 3 months | 2 | 2.0% | 0 | 0.0% | 1 | 1.0% | |
| >3 months | 0 | 0.0% | 0 | 0.0% | 1 | 1.0% | |
| Unknown | 0 | 0.0% | 1 | 1.0% | 0 | 0.0% | |
| Neutrophils at COVID-19 onset | 0.866 | ||||||
| <501 | 4 | 3.9% | 6 | 5.9% | 3 | 2.9% | |
| 501–999 | 4 | 3.9% | 5 | 4.9% | 3 | 2.9% | |
| >999 | 88 | 86.3% | 80 | 78.4% | 76 | 74.5% | |
| Lymphocytes at COVID-19 onset | <0.001 | ||||||
| <201 | 15 | 14.7% | 8 | 7.8% | 0 | 0.0% | |
| 201–499 | 21 | 20.6% | 11 | 10.8% | 9 | 8.8% | |
| >499 | 60 | 58.8% | 72 | 70.6% | 73 | 71.6% | |
| SARS-CoV-2 vaccination status at COVID-19 onset | |||||||
| Overall days from last dose administration to COVID-19 onset | 112 (47–173) [2–425] | 173 (121–235) [14–482] | 104 (57–170) [6–368] | <0.001 | |||
| Number of doses | 0.003 | ||||||
| Not vaccinated | 27 | 26.5% | 20 | 19.6% | 15 | 14.7% | 0.047 § |
| One dose | 2 | 2.0% | 2 | 2.0% | 4 | 3.9% | |
| Days from last dose administration to COVID-19 onset | 175 (112–237) [112–237] | 67 (23–110) [23–110] | 191 (55–323) [6–368] | ||||
| Two doses | 21 | 20.6% | 14 | 13.7% | 20 | 19.6% | |
| Days from last dose administration to COVID-19 onset | 235 (83–276) [20–425] | –482 | 304 (166–364) [39] | 208 (135–238) [7–272] | |||
| Three doses | 49 | 48.0% | 52 | 51.0% | 58 | 56.9% | |
| Days from last dose administration to COVID-19 onset | 81 (37–125) [2–250] | 154 (110–189) [14–286] | 84 (51–113) [10–260] | ||||
| Four doses | 3 | 2.9% | 14 | 13.7% | 5 | 4.9% | |
| Days from last dose administration to COVID-19 onset | 152 (125–161) [125–161] | 231 (197–260) [104–295] | 189 (158–193) [127–196] | ||||
| Type of last vaccine | |||||||
| mRNA | 74 | 72.5% | 80 | 78.4% | 81 | 79.4% | |
| BioNTech/Pfizer | 58 | 56.9% | 65 | 63.7% | 59 | 57.8% | |
| Moderna COVE | 16 | 15.7% | 15 | 14.7% | 22 | 21.6% | |
| Vector-based | 0 | 0.0% | 1 | 1.0% | 5 | 4.9% | |
| AstraZeneca Oxford | 0 | 0.0% | 1 | 1.0% | 4 | 3.9% | |
| Sputnik | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | |
| J&J - Janssen | 0 | 0.0% | 0 | 0.0% | 1 | 1.0% | |
| Inactivated | 1 | 1.0% | 1 | 1.0% | 0 | 0.0% | |
| CoronaVac | Sinovac | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | |
| Sinopharm | 1 | 1.0% | 1 | 1.0% | 0 | 0.0% | |
| SARS-CoV-2 variant of concern | 0.147 | ||||||
| Wild type | 1 | 1.0% | 0 | 0.0% | 0 | 0.0% | |
| Delta | 1 | 1.0% | 0 | 0.0% | 0 | 0.0% | |
| Omicron | 50 | 49.0% | 38 | 37.3% | 40 | 39.2% | |
| Not tested | 50 | 49.0% | 64 | 62.7% | 62 | 60.8% | |
| Symptoms at COVID-19 onset | 0.001 | ||||||
| Pulmonary | 35 | 34.3% | 28 | 27.5% | 25 | 24.5% | |
| Pulmonary + extrapulmonary | 21 | 20.6% | 30 | 29.4% | 15 | 14.7% | |
| Extrapulmonary | 21 | 20.6% | 34 | 33.3% | 30 | 29.4% | |
| Screening | 25 | 24.5% | 10 | 9.8% | 32 | 31.4% | |
| COVID-19 infection | <0.001 | ||||||
| Asymptomatic | 20 | 19.6% | 10 | 9.8% | 29 | 28.4% | |
| Mild infection | 22 | 21.6% | 34 | 33.3% | 32 | 31.4% | |
| Severe infection | 41 | 40.2% | 52 | 51.0% | 40 | 39.2% | |
| Critical infection | 19 | 18.6% | 6 | 5.9% | 1 | 1.0% | |
| Stay during COVID-19 episode | <0.001 | ||||||
| Home | 29 | 28.4% | 53 | 52.0% | 72 | 70.6% | |
| Hospital | 73 | 71.6% | 48 | 47.1% | 29 | 28.4% | |
| Overall days of hospital stay | 14 (7–28) [1–135] | 8 (1–15) [1–57] | 6 (1–11) [1–52] | <0.001 | |||
| ICU admission | 19 | 18.6% | 6 | 5.9% | 1 | 1.0% | <0.001 |
| Overall days in ICU | 14 (9–20) [3–54] | 7 (3–11) [2–32] | (−) [-] | 0.160 | |||
| COVID-19 treatment | |||||||
| Days under COVID-19 treatment | 2 (1–4) [1–10] | 4 (4–5) [1–24] | |||||
| Days under nirmatrelvir/lopinavir treatment | 4 (4–5) [1–10] | ||||||
| Days from COVID-19 onset to nirmatrelvir/lopinavir treatment | 1 (0–3) [0–151] | ||||||
| Type of treatment | |||||||
| No treatment administered | 0 | 0.0% | 0 | 0.0% | 102 | 100.0% | |
| Antiviral + monoclonal antibody ± corticosteroids ± plasma | 20 | 19.6% | 0 | 0.0% | 0 | 0.0% | |
| Antiviral ± corticosteroids ± plasma | 31 | 30.4% | 0 | 0.0% | 0 | 0.0% | |
| Corticosteroids | 21 | 20.6% | 0 | 0.0% | 0 | 0.0% | |
| Monoclonal antibodies ± plasma ± corticosteroids | 27 | 26.5% | 0 | 0.0% | 0 | 0.0% | |
| Nirmatrelvir combination therapy ± corticosteroids ± plasma | 0 | 0.0% | 23 | 22.5% | 0 | 0.0% | |
| 1st line | 0 | 0.0% | 6 | 5.9% | 0 | 0.0% | |
| Other line | 0 | 0.0% | 17 | 16.7% | 0 | 0.0% | |
| Nirmatrelvir monotherapy ± corticosteroids ± plasma | 0 | 0.0% | 79 | 77.5% | 0 | 0.0% | |
| 1st line | 0 | 0.0% | 79 | 77.5% | 0 | 0.0% | |
| Plasma ± corticosteroids | 3 | 2.9% | 0 | 0.0% | 0 | 0.0% | |
| Outcome | |||||||
| Days from COVID-19 onset to final day of follow up/death | 28 (10–30) [1–30] | 30 (16–30) [0–30] | 28 (12–30) [0–30] | 0.197 | |||
| Day 30 mortality rate | 11 | 10.8% | 2 | 2.0% | 6 | 5.9% | 0.036 |
| Days from COVID-19 onset to alive | 30 (14–30) [1–30] | 30 (16–30) [0–30] | 30 (13–30) [0–30] | ||||
| Days from COVID-19 onset to death | 10 (6–16) [1–28] | 18 (14–22) [14–22] | 13 (10–19) [7–21] | ||||
This table includes patients from European institutions. § Sensitivity analyses on vaccination coverage proportions after propensity score-based matched paired analyses are depicted in Supplementary Table S2.
alloHSCT, allogeneic hematopoietic stem-cell transplantation; autoHSCT, autologous hematopoietic stem-cell transplantation; CAR-T, chimeric antigen receptor T cells; COVE, coronavirus efficacy; COVID-19, coronavirus 2019 disease; ICU, intensive care unit; J&J, Johnson and Johnson; mRNA, messenger ribonucleic acid; N/R, nirmatrelvir/ritonavir; PH, Philadelphia; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; tx, treatment.
Fig. 2.
Survival probability by COVID-19 treatment strategy. A) Summarised treatment strategies. This table includes patients from European institutions. B) Detailed treatment strategies. This table includes patients from European institutions.
Sensitivity analyses we performed to determine which factors were associated to mortality in all the matched patients. Thus, we could observe how, in the univariable analyses, administration of directed drugs other than nirmatrelvir/ritonavir was associated with an increased mortality (p = 0.046), as compared to nirmatrelvir/ritonavir. Nevertheless, the factor more greatly associated with mortality in the analysed patients, either in the univariable or in multivariable analyses was the hospital, especially to ICU (Supplementary Tables S3 and S4). Nirmatrelvir/ritonavir administration remained as a protective factor in the coupled multivariable analyses performed considering the COVID-19 treatment and the variables observed to have a p < 0.1 in the univariable models when including in couples COVID-19 treatment and age, comorbidities, neutrophils, status of the malignancy at COVID-19 onset and symptoms at COVID-19 onset, respectively (Supplementary Tables S5 and S6).
Nirmatrelvir/ritonavir recipients
The majority of the 117 patients receiving nirmatrelvir/ritonavir was male (n = 61, 51.7%). Non-Hodgkin lymphoma (n = 37/117, 31.6%) and multiple myeloma (n = 25/117, 21.4%) were the baseline haematological malignancy in more than half of the patients, 24.8% (n = 29/117) of which had active malignancy at COVID-19 onset. Immuno-chemotherapy (n = 49/117, 41.9%), targeted therapy (n = 23/117, 19.7%) and conventional chemotherapy (n = 16/117, 13.7%) were the most common HM treatment strategies administered immediately before infection diagnosis. Only 3.4% (n = 4/117) of the patients received no haematological malignancy treatment any time before COVID-19, all due to a contemporaneous diagnosis of malignancy and infection (Table 1).
In the unmatched populations, the proportion of patients without any additional comorbidity at COVID-19 onset was higher in patients with nirmatrelvir/ritonavir (n = 53/117, 45.3%) than in those receiving other directed drugs (n = 371/1001, 37.1%, p = 0.007), with no difference between nirmatrelvir/ritonavir patients and those with no treatment/non-SARS-CoV-2 directed treatment (n = 342/741, 46.2%, p = 0.618). Among the patients with at least one comorbidity, chronic cardiopathy was the most frequent (n = 48/117, 41.0%), similar proportion to other patients (other directed drugs n = 405/1001, p = 0.921; no targeted drugs n = 255/741, p = 0.176). As compared to patients receiving other directed drugs against SARS-CoV-2, patients receiving nirmatrelvir/ritonavir had a lower proportion of chronic pulmonary diseases (n = 5/117, 4.3% versus n = 116/1001, 11.6%, p = 0.017), obesity (n = 1/117, 0.9% versus n = 73/1001, 7.3%, p = 0.005) and renal impairment (n = 2/117, 1.7% versus n = 67/1001, 6.7%, p = 0.039). No significant differences were observed between patients with nirmatrelvir/ritonavir and those with no treatment/non-SARS-CoV-2 directed treatment. Up to 80.3% (n = 94/117) had received at least one anti-SARS-CoV-2 vaccine dose preceding infection onset, a higher frequency than the 74.2% (n = 743/1001) in patients with other directed drugs (p = 0.003). Compared to patients with no treatment/non-SARS-CoV-2 directed treatment, patients with nirmatrelvir/ritonavir had received more frequently four vaccine doses (n = 15/117, 12.8% versus n = 50/741, 6.7%, p = 0.040, Table 1, Table 2). After matching, statistically significant differences were observed between patients in lymphocyte levels at COVID-19 onset (p < 0.001), number of vaccine doses (p = 0.047), and symptoms at COVID-19 onset (p = 0.001, Table 4, Supplementary Table S2).
COVID-19 was diagnosed after a median of 174 days (IQR 121–235) since last vaccination. Thirteen (11.1%) patients out of 117 remained asymptomatic during the entire COVID-19 episode, whereas 38/117 (32.5%) had mild symptoms, 60/117 (51.3%) progressed to severe disease, and 6/117 (5.1%) to a critical condition (Table 2).
In unmatched patients with directed drugs other than nirmatrelvir/ritonavir, there were similar percentages of asymptomatic (n = 145/1001, 14.5%) and severely sick patients (556/1001, 55.5%), but fewer mild infections (176/1001, 17.6%) and more critical evolutions (124/1001, 12.4%) (p < 0.001). Patients with no treatment or no SARS-CoV-2 directed drugs, had a higher rate of asymptomatic courses (n = 211/741, 28.5%), and lower rates of mild (n = 185/741, 25.0%), severe (n = 327/741, 44.1%) and critical infections (n = 18/741, 2.4%) than patients with nirmatrelvir/ritonavir (p < 0.001). After matching the patients by sex, age (±10 years), baseline haematological malignancy, haematological malignancy status at COVID-19 onset, and origin continent (Europe), those with directed drugs other than nirmatrelvir/ritonavir remained with a higher prevalence of critical COVID-19 episodes (p < 0.001, Table 1, Table 2, Table 4, Supplementary Table S2).
Overall, nirmatrelvir/ritonavir was administered with similar frequencies to hospital in-patients (n = 59/117, 50.4%) and out-patients (n = 56/117, 47.9%). However, when analysing results by country, this significantly differed, being more common for outpatients in Italy (n = 48/59, 81.4%) versus (n = 11/59, 18.6%) in-patients rather than in Spain (24/29, 82.8% hospital versus n = 5/29, 17.2% home, p < 0.001, Fig. 1B). Of the 56/117 (47.9%) patients admitted to hospital, 6/117 (10.7%) required intensive care. In patients with other directed drugs, both hospitalization (n = 736/1001, 73.5%, p < 0.001) and intensive care (n = 124/1001, 12.4%, p = 0.02) were significantly more frequent. On the contrary, patients with no treatment/non-SARS-CoV-2 directed treatment were less frequently in-hospital (n = 182/741, 24.6%, p < 0.001), although similarly treated in intensive care (n = 18/741, 2.4%, p = 0.12, Table 2, Table 4, Supplementary Table S2).
Nirmatrelvir/ritonavir treatment commenced the day after COVID-19 diagnosis (median 1, IQR 0–3) and lasted a median of 4 days (IQR 4–5). Among the 24/117 (20.5%) patients receiving nirmatrelvir/ritonavir in combination with other drugs (9/24 with other antivirals, 7/24 with monoclonal antibodies and 8/24 with both) only 17/24 received it as salvage treatment (Table 2).
The overall mortality rate in nirmatrelvir/ritonavir recipients was 6.8% (n = 8/117), death was attributed to COVID-19 in 5.1% (n = 6/8) cases. In the Cox regression models, no factor stood out to be associated with mortality after administration of nirmatrelvir/ritonavir (Table 2, Table 5).
Table 5.
Factors associated with mortality in patients with nirmatrelvir/ritonavir administration.
| Univariable analysis |
Multivariable analysis |
|||||||
|---|---|---|---|---|---|---|---|---|
| p value | HR | 95% CI |
p value | HR | 95% CI |
|||
| Lower limit | Upper limit | Lower limit | Upper limit | |||||
| Sex | ||||||||
| Female | – | – | – | – | ||||
| Male | 0.234 | 2.644 | 0.533 | 13.116 | ||||
| Age | 0.332 | 1.029 | 0.972 | 1.089 | ||||
| Comorbidities at COVID-19 onset | ||||||||
| Chronic cardiopathy | 0.063 | 7.479 | 0.900 | 62.169 | 0.092 | 6.761 | 0.731 | 62.532 |
| Chronic pulmonary disease | 0.619 | 0.044 | 0.000 | . | ||||
| Diabetes mellitus | 0.056 | 5.001 | 0.957 | 26.126 | 0.167 | 4.229 | 0.547 | 32.698 |
| Liver disease | 0.720 | 0.046 | 0.000 | . | ||||
| Obesity | . | . | . | . | ||||
| Renal failure | 0.799 | 0.048 | 0.000 | . | ||||
| Smoking history | 0.626 | 0.044 | 0.000 | . | ||||
| No comorbidity | 0.122 | 0.191 | 0.023 | 1.555 | ||||
| Neutrophils at COVID-19 onset | ||||||||
| <501 | – | – | – | – | ||||
| 501–999 | 0.642 | 0.512 | 0.030 | 8.614 | ||||
| >999 | 0.172 | 0.210 | 0.022 | 1.970 | ||||
| Lymphocytes at COVID-19 onset | ||||||||
| <201 | – | – | – | – | ||||
| 201–499 | 0.640 | 0.563 | 0.051 | 6.249 | ||||
| >499 | 0.149 | 0.266 | 0.044 | 1.607 | ||||
| Status malignancy at COVID-19 onset | ||||||||
| Controlled disease | – | – | – | – | – | – | – | – |
| Stable disease | 0.980 | 0.000 | 0.000 | . | 0.950 | 0.000 | 0.000 | . |
| Active disease | 0.064 | 4.721 | 0.915 | 24.354 | 0.311 | 2.462 | 0.431 | 14.060 |
| Unknown | 0.993 | 0.000 | 0.000 | . | 0.995 | 0.000 | 0.000 | . |
| Baseline malignancy at COVID-19 onset | ||||||||
| Leukaemia | – | – | – | – | ||||
| Lymphoma | 0.837 | 0.863 | 0.213 | 3.492 | ||||
| PH negative myeloproliferative diseases | 0.989 | 0.000 | 0.000 | . | ||||
| Plasma cell disorders | 0.971 | 0.000 | 0.000 | . | ||||
| Symptoms at COVID-19 onset | ||||||||
| Pulmonary | – | – | – | – | ||||
| Pulmonary + extrapulmonary | 0.966 | 0.968 | 0.212 | 4.419 | ||||
| Extrapulmonary | 0.305 | 0.299 | 0.030 | 3.001 | ||||
| Screening | 0.988 | 0.000 | 0.000 | . | ||||
| SARS-CoV-2 vaccination status at COVID-19 onset | ||||||||
| Not vaccinated | – | – | – | – | ||||
| One dose | 0.940 | . | 0.000 | . | ||||
| Two doses | 0.920 | . | 0.000 | . | ||||
| Three doses | 0.924 | . | 0.000 | . | ||||
| Four doses | 0.980 | 1735.991 | 0.000 | . | ||||
| ICU admission | 0.106 | 3.614 | 0.760 | 17.178 | ||||
| Nirmatrelvir/ritonavir line | ||||||||
| Other line | – | – | – | – | ||||
| First line | 0.997 | 1.003 | 0.188 | 5.349 | ||||
| Days from COVID-19 onset to nirmatrelvir/ritonavir treatment | 0.771 | 1.003 | 0.980 | 1.027 | ||||
| Stay during COVID-19 episode | ||||||||
| Home | – | – | – | – | ||||
| Hospital | 0.171 | 4.387 | 0.528 | 36.477 | ||||
This table includes patients from European institutions.
CI, confidence interval; COVID-19, coronavirus 2019 disease; HR, hazard ratio; ICU, intensive care unit; PH, Philadelphia; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Discussion
Of 1859 patients with haematological malignancy registered in EPICOVIDEHA since the licensing of nirmatrelvir/ritonavir, 117 (6.3%) received the drug to prevent complicated courses of COVID-19.8 Patients with extrapulmonary symptoms at COVID-19 onset and those who had received a 2nd booster dose of an mRNA vaccine were more likely to receive nirmatrelvir/ritonavir. Recipients of nirmatrelvir/ritonavir had a significantly lower mortality rate than patients with other treatment approaches. Additionally, we aimed to discover factors associated with mortality in patients receiving nirmatrelvir/ritonavir for the COVID-19 treatment, although none was observed as significant, potentially linked to the reduced sample size and overall mortality rate.
Patients with chronic pulmonary diseases and obesity were less likely to receive nirmatrelvir/ritonavir, although precisely these are patients at risk of developing more severe COVID-19,20 and therefore being the most appropriate candidates for nirmatrelvir/ritonavir administration even in the absence of haematological malignancy.15, 16, 17 This apparent underuse of nirmatrelvir/ritonavir may have multiple reasons explaining such results: lack of stock in hospital pharmacies and clinical practices,21 unawareness on when and how to administer it, unidentified obstacles in prescription, or already described adverse events, such us dysgeusia, diarrhoea or emboli, or drug–drug interactions.8,22 The performance of a similar analyses once the drug has been longer available may show broader use and may provide results more in line with the drug recommendations. In parallel, the presence of only extrapulmonary symptoms at COVID-19 was associated with nirmatrelvir/ritonavir use, following the prescription recommendations regarding target patients: mild COVID-19 episodes with high-risk for SARS-CoV-2 progression.15, 16, 17 Those fully vaccinated including a 2nd booster dose were more likely to receive nirmatrelvir/ritonavir. Interpretations range from pockets of patients with access to all management options being very well taken care of (patients receiving nirmatrelvir/ritonavir were concentrated in Western Europe, as opposed to a wider geographical spread of patients in the other groups), to effects of different vaccination schemes alleviating COVID-19 course.4,6,23
We observed an overall vaccination rate of 76.9%; 80.3% in patients receiving nirmatrelvir/ritonavir, 80.0% in those with no treatment/non-SARS-CoV-2 directed treatment, and 74.2% in patients with directed drugs other than nirmatrelvir/ritonavir. These vaccination rates are similar to those in the general population.24 In line with previous experiences in immunosuppressed settings,25 the absence of vaccination in our patients was related to an ongoing haematological malignancy treatment or to patient refusal. However, in the majority of them it was unknown the rationale. Specific studies analysing the vaccination coverage in patients with an increased risk for COVID-19-associated mortality could help to understand and overcome this situation, facilitating a close-to-full vaccination in haematological malignancy patients. With these data, one could elucidate that the variables included in the propensity score-based matching performed in our analysis (sex, age (±10 years), baseline haematological malignancy, haematological malignancy status at COVID-19 onset, and origin continent [Europe]) has made the treatment groups very homogeneous and comparable, not only in the matching variables, but also in the vaccination coverage. Additionally, the fact that all the patients were diagnosed in 2022 has facilitated a higher coverage. In order to see more detailed reasons, we may need to look patient to patient and thus, the heterogenicity of the results to be potentially obtained could have a very poor significance. Further analysis may need to focus on this aspect and we will definitely keep in mind in our prospective research.
Surprisingly, the patients in our sample received nirmatrelvir/ritonavir for a median of only four days, one day less than the manufacturer recommendations, which may hint towards general shortage of the drug.15,16 Other potential reasons may be drug–drug interactions,26,27 adverse effects22 or prompt recovery of symptoms. Additionally, storage and supply restrictions,21 which can potentially end in a distribution of available doses among more patients than recommended, and difficulties in the access to the compound,28 might have interfered with the correct number of administration days.
A retrospective study from Israel analysed factors associated with mortality in patients with high-risk of COVID-19 progression after receiving nirmatrelvir/ritonavir.29 Immunosuppressed patients showed increased mortality, adjusted by different comorbidities (i.e., cardiovascular disease, chronic kidney disease, chronic lung disease, diabetes mellitus, malignancy in the prior year, or neurological disease), but the authors did not differentiate haematologic malignancy from other causes of immunosuppression. Interestingly we did not identify factors associated with mortality. The high rate of vaccinated patients may have reduced death rates even in our immunosuppressed population. The 6.8% mortality rate in nirmatrelvir/ritonavir recipients, much lower than in previously reported populations without vaccination (31.2%)5 or including pre-nirmatrelvir/ritonavir cases4 (9.2%), jeopardises the performance of further mortality analyses.
Our study has some limitations. The retrospective design may intrinsically yield lower data quality, and the sample size is large by comparison to published studies, but still too small to allow additional subgroup comparisons. We do not capture the actual antineoplastic treatment days and doses limiting analyses of drug–drug interactions with ritonavir.26 Finally, we have been unable to detect which factors are associated with mortality. Further analyses with a larger sample size collecting more variables, including laboratory values, might overcome these limitations.
In conclusion, patients with extrapulmonary symptoms at COVID-19 onset and a 2nd vaccine dose are more prone to receive nirmatrelvir/ritonavir as opposed to those with chronic pulmonary disease and obesity. Despite mortality in patients with nirmatrelvir/ritonavir is lower as compared to that in other treatment schemes, no statistical significance was observed. Thus, further analyses are needed to depict the factors associated to this observation.
Contributors
JSG, FM, LP and OAC contributed to study design and study supervision. JSG did the statistical plan and analysis. JSG and OAC interpreted the data and wrote the paper. All the authors recruited, and documented participants, critically read, reviewed, and agreed to publish the manuscript.
Data sharing statement
Data are available upon reasonable request to the corresponding authors Dr. Jon Salmanton-García (jon.salmanton-garcia@uk-koeln.de) or Prof. Dr. Oliver A. Cornely (oliver.cornely@uk-koeln.de).
Declaration of interests
The authors do not declare conflicts of interest related to the submitted manuscript. The funder of the study had no role in study design, data analysis, interpretation, or writing of the report. All authors had full access to the data and had final responsibility for the decision to submit for publication.
Acknowledgments
The authors thank all participating institutions for their utmost contributions and support to the project during a pandemic situation. In addition, we would like to express our gratitude to Professor Francisco Javier Martín-Vallejo (Department of Statistics, Faculty of Medicine, University of Salamanca, Salamanca, Spain) for his guidance in performing the statistical analyses of this manuscript. EPICOVIDEHA has received funds from Optics COMMIT (COVID-19 Unmet Medical Needs and Associated Research Extension) COVID-19 RFP program by GILEAD Science, United States (Project 2020-8223).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101939.
Contributor Information
Jon Salmanton-García, Email: jon.salmanton-garcia@uk-koeln.de.
Oliver A. Cornely, Email: oliver.cornely@uk-koeln.de.
EPICOVIDEHA registry:
Klára Piukovics, Cristina De Ramón, François Danion, Ayel Yahya, Anna Guidetti, Carolina Garcia-Vidal, Uluhan Sili, Joseph Meletiadis, Elizabeth De Kort, Luisa Verga, Laura Serrano, Nurettin Erben, Roberta Di Blasi, Athanasios Tragiannidis, José-María Ribera-Santa Susana, Hans-Beier Ommen, Alessandro Busca, Nicola Coppola, Rui Bergantim, Giulia Dragonetti, Marianna Criscuolo, Luana Fianchi, Matteo Bonanni, Andrés Soto-Silva, Malgorzata Mikulska, Marina Machado, Chi Shan Kho, Nazia Hassan, Eleni Gavriilaki, Gregorio Cordini, Louis Yi Ann Chi, Matthias Eggerer, Martin Hoenigl, Juergen Prattes, María-Josefa Jiménez-Lorenzo, Sofia Zompi, Giovanni Paolo Maria Zambrotta, Gökçe Melis Çolak, Nicole García-Poutón, Tommaso Francesco Aiello, Romane Prin, Maria Stamouli, and Michail Samarkos
Appendix A. Supplementary data
References
- 1.Mahase E. Covid-19: WHO declares pandemic because of "alarming levels" of spread, severity, and inaction. BMJ. 2020;368:m1036. doi: 10.1136/bmj.m1036. [DOI] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pagano L., Salmanton-Garcia J., Marchesi F., et al. Breakthrough COVID-19 in vaccinated patients with hematologic malignancies: results from EPICOVIDEHA survey. Blood. 2022;140(26):2773–2787. doi: 10.1182/blood.2022017257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pagano L., Salmanton-Garcia J., Marchesi F., et al. COVID-19 infection in adult patients with hematological malignancies: a European Hematology Association Survey (EPICOVIDEHA) J Hematol Oncol. 2021;14(1):168. doi: 10.1186/s13045-021-01177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pagano L., Salmanton-Garcia J., Marchesi F., et al. COVID-19 in vaccinated adult patients with hematological malignancies: preliminary results from EPICOVIDEHA. Blood. 2022;139(10):1588–1592. doi: 10.1182/blood.2021014124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer W.A., 2nd, Eron J.J., Jr., Holman W., et al. A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci Transl Med. 2022;14(628) doi: 10.1126/scitranslmed.abl7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammond J., Leister-Tebbe H., Gardner A., et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386(15):1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottlieb R.L., Nirula A., Chen P., et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Brien M.P., Forleo-Neto E., Sarkar N., et al. Effect of subcutaneous Casirivimab and imdevimab antibody combination vs placebo on development of symptomatic COVID-19 in early asymptomatic SARS-CoV-2 infection: a randomized clinical trial. JAMA. 2022;327(5):432–441. doi: 10.1001/jama.2021.24939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J.Y., Sandulescu O., Preotescu L.L., et al. A randomized clinical trial of regdanvimab in high-risk patients with mild-to-moderate coronavirus disease 2019. Open Forum Infect Dis. 2022;9(8):ofac406. doi: 10.1093/ofid/ofac406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Group AC-TfIwC-S Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial. Lancet Infect Dis. 2022;22(5):622–635. doi: 10.1016/S1473-3099(21)00751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Group AC--TfIwC- S. Tixagevimab-cilgavimab for treatment of patients hospitalised with COVID-19: a randomised, double-blind, phase 3 trial. Lancet Respir Med. 2022;10(10):972–984. doi: 10.1016/S2213-2600(22)00215-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U.S. Food and Drug Administration Fact sheet for healthcare providers: emergency use authorization for paxlovid. 2022. https://www.fda.gov/media/155050/download
- 16.European Medicine Agency Paxlovid. 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/paxlovid
- 17.Cesaro S., Ljungman P., Mikulska M., et al. Recommendations for the management of COVID-19 in patients with haematological malignancies or haematopoietic cell transplantation, from the 2021 European Conference on Infections in Leukaemia (ECIL 9) Leukemia. 2022;36(6):1467–1480. doi: 10.1038/s41375-022-01578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun F., Lin Y., Wang X., Gao Y., Ye S. Paxlovid in patients who are immunocompromised and hospitalised with SARS-CoV-2 infection. Lancet Infect Dis. 2022;22(9):1279. doi: 10.1016/S1473-3099(22)00430-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salmanton-Garcia J., Busca A., Cornely O.A., et al. EPICOVIDEHA: a ready to use platform for epidemiological studies in hematological patients with COVID-19. Hemasphere. 2021;5(7):e612. doi: 10.1097/HS9.0000000000000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santos V.B.D., Stein A.T., Barilli S.L.S., et al. Adult patients admitted to a tertiary hospital for COVID-19 and risk factors associated with severity: a retrospective cohort study. Rev Inst Med Trop Sao Paulo. 2022;64 doi: 10.1590/S1678-9946202264020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmermann G.W. Paxlovid™ jetzt direkt an Patienten abgeben. MMW Fortschr Med. 2022;164(15):31. doi: 10.1007/s15006-022-1883-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birabaharan M., Martin T.C.S. Acute pulmonary emboli following rebound phenomenon after Nirmatrelvir/Ritonavir treatment for COVID-19. Am J Emerg Med. 2022;61:235.e5–235.e6. doi: 10.1016/j.ajem.2022.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McIntyre P.B., Aggarwal R., Jani I., et al. COVID-19 vaccine strategies must focus on severe disease and global equity. Lancet. 2022;399(10322):406–410. doi: 10.1016/S0140-6736(21)02835-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.European Centre for Disease Prevention and Control Uptake of the primary course of COVID-19 vaccination among the total population in EU/EEA countries as of 16 October 2022. 2022. https://covid19-country-overviews.ecdc.europa.eu/vaccination.html
- 25.Nguyen M., Bain N., Grech L., et al. COVID-19 vaccination rates, intent, and hesitancy in patients with solid organ and blood cancers: a multicenter study. Asia Pac J Clin Oncol. 2022;18(6):570–577. doi: 10.1111/ajco.13754. [DOI] [PubMed] [Google Scholar]
- 26.Fishbane S., Hirsch J.S., Nair V. Special Considerations for paxlovid treatment among transplant recipients with SARS-CoV-2 infection. Am J Kidney Dis. 2022;79(4):480–482. doi: 10.1053/j.ajkd.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berar Yanay N., Bogner I., Saker K., Tannous E. Paxlovid-tacrolimus drug-drug interaction in a 23-year-old female kidney transplant patient with COVID-19. Clin Drug Investig. 2022;42(8):693–695. doi: 10.1007/s40261-022-01180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gold J.A.W., Kelleher J., Magid J., et al. Dispensing of oral antiviral drugs for treatment of COVID-19 by zip code-level social vulnerability - United States, December 23, 2021-May 21, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(25):825–829. doi: 10.15585/mmwr.mm7125e1. [DOI] [PubMed] [Google Scholar]
- 29.Najjar-Debbiny R., Gronich N., Weber G., et al. Effectiveness of paxlovid in reducing severe COVID-19 and mortality in high risk patients. Clin Infect Dis. 2023;76(3):e342–e349. doi: 10.1093/cid/ciac443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.