Abstract
Background
The number of patients with cardiac implantable electronic devices (CIEDs) undergoing radiotherapy (RT) for cancer treatment is growing. At present, prevalence and predictors of RT‐induced CIEDs malfunctions are not defined.
Methods
Systematic review and meta‐analysis conducted following the PRISMA recommendations. PubMed, Scopus and Google Scholar were searched from inception to 31/01/2022 for studies reporting RT‐induced malfunctions in CIEDs patients. Aim was to assess the prevalence of RT‐induced CIEDs malfunctions and identify potential predictors.
Results
Thirty‐two out of 3962 records matched the inclusion criteria and were included in the meta‐analysis. A total of 135 CIEDs malfunctions were detected among 3121 patients (6.6%, 95% confidence interval [CI]: 5.1%–8.4%). The pooled prevalence increased moving from pacemaker (PM) to implantable cardioverter defibrillator (ICD), and cardiac resynchronization therapy and defibrillator (CRT‐D) groups (4.1%, 95% CI: 2.9–5.8; 8.2% 95% CI: 5.9–11.3; and 19.8%, 95% CI: 11.4–32.2 respectively). A higher risk ratio (RR) of malfunctions was found when neutron‐producing energies were used as compared to non‐neutron‐producing energies (RR 9.98, 95% CI: 5.09–19.60) and in patients with ICD/CRT‐D as compared to patients with PM/CRT‐P (RR 2.07, 95% CI: 1.40–3.06). On the contrary, no association was found between maximal radiation dose at CIED >2 Gy and CIEDs malfunctions (RR 0.93; 95% CI: 0.31–2.76).
Conclusions
Radiotherapy related CIEDs malfunction had a prevalence ranging from 4% to 20%. The use of neutron‐producing energies and more complex devices (ICD/CRT‐D) were associated with higher risk of device malfunction, while the radiation dose at CIED did not significantly impact on the risk unless higher doses (>10 Gy) were used.
Keywords: cancer, cardiac implantable electronic devices, implantable cardioverter defibrillator, pacemaker, radiation therapy, radiotherapy
Highlights
This is the first meta‐analysis investigating the prevalence of RT‐related CIEDs malfunctions and its risk factors.
The pooled prevalence of RT‐related CIEDs malfunctions is variable (4%–20%).
The use of neutron‐producing energies is associated with a higher risk of CIED malfunctions as compared to non‐neutron‐producing energies.
Similarly, ICD/CRT‐D showed a higher risk of malfunctions as compared to PM/CRT‐P.
High radiation dose at CIED (>2 Gy) did not confer a significantly higher risk of CIED malfunctions.
1. INTRODUCTION
The total number of cardiac implantable electronic devices (CIEDs) implanted every year is constantly growing. 1 Similarly, the incidence of cancer patients is expected to increase with population aging. 2 In this context, a rising number of CIED patients will require radiotherapy (RT) for cancer treatment. Therefore, careful patient evaluation and appropriate planning of the RT course planning is crucial to prevent any possible interference with the device. RT‐induced CIED malfunctions have been reported with varying prevalence, ranging between 0% and 25%, 3 and can be life‐threatening particularly in pacemaker (PM)‐dependent and implantable cardioverter defibrillator (ICD) patients. 3 , 4 At present, only small‐scale studies addressed this issue and robust predictors of device malfunction or failure are lacking. Most national and international guidelines and consensus documents 3 , 4 , 5 , 6 on this topic suggest a personalized approach to patient management, based on classes of risk for device malfunction. In this regard, several factors related to patient profile (e.g., PM‐dependency, prior ICD interventions for ventricular tachyarrhythmias), device type (e.g., PM or ICD) and RT characteristics (e.g., beam energies and radiation dose at CIED) are usually being considered, but most of the recommendations are based on expert opinion, highlighting the need for more solid up‐to‐date scientific evidence.
The aim of the present systematic review and meta‐analysis is to describe the prevalence of RT‐induced CIEDs malfunction and to identify potential risk factors, based on data reported so far in the literature.
2. METHODS
We conducted the present metanalysis following the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) recommendations 7 , 8 and the study protocol was registered with PROSPERO (CRD42022308152).
2.1. Search strategy, study selection, data extraction and quality assessment
We searched multiple electronic records (Pubmed, Scholar and Scopus) for publications from inception to 31/01/2022. The following search terms were used: (“implantable cardioverter defibrillator” OR pacemaker OR CIED OR “cardiac implantable electronic device” OR “cardiac resynchronization therapy”) AND (radiotherapy OR radiation OR electromagnetic OR interference). The corresponding MeSH terms are reported in Appendix S1, no language restriction was applied. All titles and abstracts were screened by two independent reviewers (AT and VLM). Studies eligible for full‐text evaluation were identified and data extracted on a prespecified spreadsheet for subsequent statistical analysis. Potential disagreements were resolved by a third independent reviewer (JFI). Study inclusion criteria were as follows: (i) original studies including at least four CIED patients (only in vivo studies), (ii) patients implanted with PM or ICD or cardiac resynchronization therapy and pacing (CRT‐P)/cardiac resynchronization therapy and defibrillator (CRT‐D) before RT for cancer treatment, and (iii) availability of follow‐up data on CIEDs malfunctions. Case reports, paediatric populations and in vitro studies were excluded. Whenever available, the following data were extracted: baseline characteristics of the patients, cancer site, type of CIED implanted, beam energies (neutron‐ or non‐neutron‐producing radiation), radiation dose at CIED, and study design. Neutron‐producing energy was defined according to the definition provided in each single study. In case it was not clearly stated, the definition provided by Zecchin et al. 3 was used (photon beam energy >6 MV, electron beam energy ≥20 MeV). The risk of bias for each study was assessed using The Newcastle‐Ottawa scale for non‐randomized cohort studies. We evaluated the following domains: study group selection, study group comparability and outcome assessment; a score ≥7 identified high‐quality studies.
2.2. Endpoints
The endpoints of the present meta‐analysis were to describe the prevalence of RT‐induced CIED malfunctions and to identify potential predictors of malfunction.
2.3. Statistical analysis
Continuous variables are reported as mean and standard deviation (SD) or median and interquartile range (IQR). Categorical variables are reported as counts and percentages.
Two different meta‐analysis techniques were used. With the aim to describe the standardized prevalence of malfunction, we conducted a meta‐analysis of proportions. Prevalences were transformed using logit transformation and were pooled with the inverse variance method; tau was estimated with the restricted maximum‐likelihood (REML) method. To evaluate the presence of potential confounders, we performed a subgroup analysis based on the type of CIED implanted (PM/CRT‐P, ICD, CRT‐D). We also conducted a multivariable meta‐regression using study‐level year of publication, device investigated (PM/CRT‐P, ICD, CRT‐D) and sample size as covariates.
In order to explore potential risk factors for CIEDs failure, we made three pairwise meta‐analyses comparing the type of CIED (ICD/CRT‐D vs. PM/CRT‐P), beam energy (neutron‐ vs. non‐neutron‐producing energy) and radiation dose at CIED (>2 Grey [Gy] vs. ≤2 Gy). In order to evaluate also higher dose limits, we performed a proportion and a binary meta‐analysis comparing ≤5 Gy vs. >5 Gy and ≤ 10 Gy vs. > 10 Gy doses to the device. Subgroup analysis comparing retrospective and prospective studies was performed. Results were reported as risk ratio (RR) and 95% confidence interval (CI). All meta‐analyses were modelled with a random‐effect approach and results were graphically reported by forest plots. The I 2 statistic was employed to measure heterogeneity among the studies for each analysis. The following thresholds were applied: low heterogeneity if I 2 < 25%, moderate if I 2 between 25% and 75% and high if I 2 > 75%. If I 2 was >25% we performed a sensitivity analysis using the “leave‐one‐out” technique. Meta‐regression analyses according to study‐level year of publication and publication bias was assessed by visual inspection of funnel plots and also using the Egger's test. Data were analysed using R v.4 (R Core Team [2021]. R: A language and environment for statistical computing. R Foundation for Statistical Computing. URL http://www.R‐project.org/) with the packages meta 9 and metafor. 10 A p‐value < .05 was considered significant. Reporting of the study conforms to broad EQUATOR guidelines. 11
3. RESULTS
3.1. Study selection and quality of study assessment
A total of 3962 records were obtained through the literature search. Relevant citations were assessed following the Patient/Population, Intervention, Comparison, Outcomes (PICO) process. Thirty‐two papers matched the inclusion/ exclusion criteria and were included in the present meta‐analysis. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 Figure 1 summarizes the study selection process. The quality assessment performed using the Newcastle‐Ottawa scale showed an overall high quality of the studies included (Table S1). Eighteen (56%) studies scored ≥7/9 points, 14 , 15 , 19 , 21 , 22 , 23 , 24 , 26 , 28 , 30 , 32 , 34 , 36 , 37 , 38 , 39 , 40 , 41 12 (38%) studies scored 4–6 points, 12 , 16 , 17 , 18 , 20 , 25 , 29 , 31 , 33 , 35 , 42 , 43 and only 2 (6%) studies scored ≤3 points. 13 , 27
FIGURE 1.
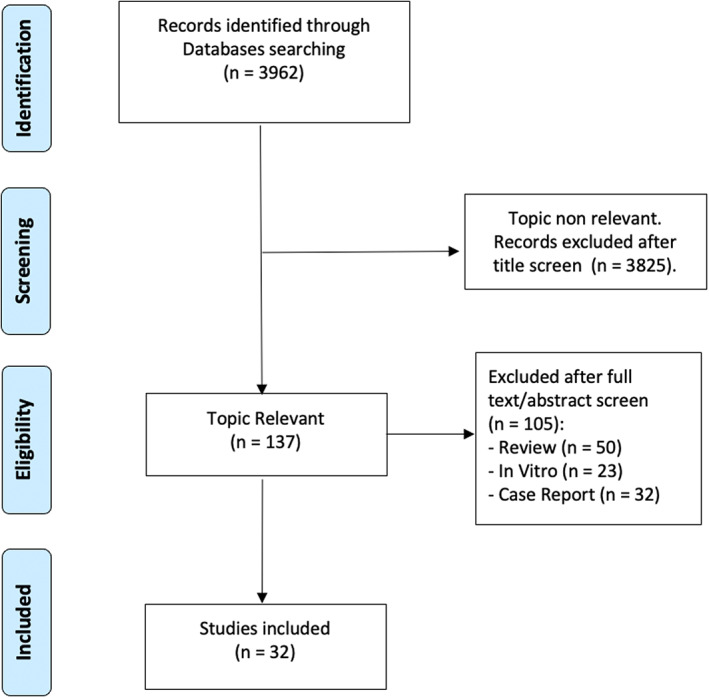
Flow diagram showing the study selection process
3.2. Studies and patient characteristics
Among the 32 studies considered, 13 (41%) were prospective 12 , 14 , 15 , 16 , 17 , 18 , 19 , 21 , 22 , 24 , 33 , 37 , 40 and 19 (59%) were retrospective. 13 , 20 , 23 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 34 , 35 , 36 , 38 , 39 , 41 , 42 , 43 With regard to CIED type, PM/CRT‐P were considered in 28 studies, 13 , 14 , 16 , 17 , 18 , 19 , 20 , 21 , 23 , 24 , 25 , 26 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 ICD in 29 studies 12 , 13 , 15 , 16 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 and CRT‐D in 12 studies. 22 , 24 , 25 , 28 , 29 , 30 , 32 , 33 , 34 , 37 , 39 , 43 Publication years ranged from 2002 to 2021. The main characteristics of the included studies are summarized in Table 1.
TABLE 1.
Main characteristics of the included studies and baseline population characteristics
| Author, year | Study design | Study period | Patients | Mean age | Females% | Cancer site | Devices n | Malfunctions |
|---|---|---|---|---|---|---|---|---|
| Hoecht, 2002 | Prospective | NR | 8 | NR | NR | Pelvic | ICD 8 | 2 |
| Kapa, 2008 | Retrospective | 2002–2007 | 13 | NR | NR | Lung, Skin, Lymphoma, Pancreas, Thyroid | PM 8 ICD 5 | 0 |
| Oshiro, 2008 | Prospective | 2001–2008 | 8 | 79 | 12.5 | Liver, Lung | PM 8 | 2 |
| Gelblum, 2008 | Prospective | 2005–2007 | 33 | NR | NR | Head and neck, Thorax, Abdomen, Pelvis, Legs | ICD 33 | 2 |
| Ferrara, 2010 | Prospective | 1999–2007 | 45 | 71.3 | 18 | Head and neck, Thorax, Abdomen, Pelvis | PM 37 ICD 8 | 0 |
| Wadasadawala, 2010 | Prospective | 2005–2009 | 8 | 67 | 37.5 | Head and neck, Lung, Breast | PM 8 | 0 |
| Menard, 2010 | Prospective | 2008–2010 | 7 | 68 | 100 | Breast | PM 7 | 0 |
| Soejima, 2011 | Prospective | 2006–2008 | 62 | 76 | 27 | Lung, Prostate, Oesophageal, Breast, Head and Neck | PM 60 ICD 2 | 1 |
| Croshaw, 2011 | Retrospective | 2007–2010 | 8 | 79 | NR | Breast | PM 5 ICD 3 | 0 |
| Makkar, 2012 | Prospective | 2005–2011 | 69 | 74.1 | 27 | Head and Neck, Breast, Abdomen, Thorax, Extremities, Oesophagus | PM 50 ICD 19 | 2 |
| Elders, 2013 | Prospective | NR | 15 | 72 | 13 | Head and neck, lung, Abdomen, Pelvis, Legs | ICD 8 CRT‐D 7 | 4 |
| Gomez, 2013 | Retrospective | 2009–2012 | 42 | NR | NR | Thorax, Prostate, Liver, Skull | PM 28 ICD 14 | 5 |
| Brambatti, 2015 | Prospective | 2008–2012 | 261 | 78 | 30 | Lung, Skin, Breast, Oesophagus, Others | PM 206 CRT‐P 1 ICD 51 CRT‐D 3 | 4 |
| Zaremba, 2015 | Retrospective | 2003–2012 | 453 | 74.7 | NR | Head and Neck, Thorax, Abdomen, Pelvis ‐ Oesophagus ‐ Other | PM 370 CRT‐P 24 ICD 48 CRT‐D 11 | 14 |
| Grant, 2015 | Retrospective | 2005–2014 | 215 | 73 | 29 | Abdomen, Brain, Chest, Head and Neck, Pelvis | PM 123 ICD 92 | 16 |
| Hudson, 2017 | Retrospective | 2008–2012 | 25 | NR | NR | Lung, Rectum, Prostate, Bladder | ICD 25 | 2 |
| Bagur, 2017 | Retrospective | 2007–2013 | 230 | 78 | 30 | Lung, Skin, Prostate, Lymphoma, Brain | PM 199 ICD 21 CRT‐D 10 | 17 |
| Riva, 2018 | Retrospective | 2010–2016 | 63 | 74.1 | 11.1 | Lung, Prostate, Bladder, Breast, Lymphoma, Uterin | PM 52 ICD 7 CRT‐D 4 | 2 |
| Bravo‐Jaimes, 2018 | Retrospective | 2000–2015 | 109 | 79.3 | 35 | Thorax, Abdomen, Pelvis, Other | PM 60 ICD 35 CRT‐D 14 | 6 |
| Yeung, 2019 | Retrospective | 2007–2018 | 189 | 78 | 29.1 | Head and Neck, Abdomen, Pelvis, Thorax | PM 159 ICD 29 | 4 |
| Brouillard, 2019 | Retrospective | 2007–2013 | 230 | 77.6 | 30.4 | Head and Neck, Abdomen, Pelvis, Thorax, Brain | PM 199 ICD 21 CRT‐D 10 | 18 |
| Steger, 2019 | Prospective | 2014–2018 | 51 | 75 | 23.5 | Head and Neck, Abdomen, Pelvis, Thorax, Brain | PM 41 CRT‐P 1 ICD 7 CRT‐D 2 | 3 |
| Malavasi, 2019 | Retrospective | 2004–2018 | 127 | 77.8 | 33 | Thorax, Abdomen, Pelvis | PM 99 ICD 14 CRT‐D 13 NR 1 | 3 |
| Seidensaal, 2019 | Retrospective | 2001–2013 | 31 | 72 | 39 | Head and Neck, Thorax, Abdomen, Pelvis, Bone | PM 28 ICD 3 | 0 |
| Sharifzadehgan, 2020 | Retrospective | 2006–2017 | 90 | 78 | 27 | Head and Neck, Abdomen, Pelvis, Thorax | PM 82 ICD 8 | 5 |
| Niedziela, 2020 | Prospective | 2016–2018 | 157 | 73 | 33 | Brain, Thorax, Abdomen, Pelvis, Head and Neck | PM 113 ICD 36 CRT‐D 8 | 1 |
| López‐Honrubia, 2020 | Retrospective | 2006–2017 | 56 | 78.2 | 23.2 | Head and Neck, Abdomen, Pelvis, Thorax, Brain | PM 49 ICD 7 | 6 |
| Levis, 2020 | Retrospective | 2007–2019 | 34 | 78 | 14.7 | Lung | PM 24 ICD 6 CRT‐D 4 | 0 |
| Gauter‐Fleckenstein, 2020 | Prospective | 2007–2011 | 200 | 73 | NR | Head and neck, Thorax, Abdomen, Pelvis, Extremities | PM 108 ICD 92 | 8 |
| Hamza, 2021 | Retrospective | 2000–2018 | 193 | 76 | 35 | Head and Neck, Abdomen, Pelvis, Thorax, Brain | PM 125 ICD 68 | 2 |
| Okano, 2021 | Retrospective | 2010–2019 | 22 | 72 | 31.8 | Thorax, Abdomen, Prostate, Liver, Bones | PM 21 ICD 1 | 0 |
| Hashimoto, 2021 | Retrospective | 2012–2019 | 69 | 81 | 21.8 | Pelvic | PM 64 ICD 4 CRT‐D 1 | 6 |
Note: References 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 .
Abbreviations: CRT‐D, cardiac resynchronization therapy and defibrillator; CRT‐P, cardiac resynchronization therapy and pacing; ICD, implantable cardioverter defibrillator; NR, not reported; PM, pacemakers.
3.3. RT‐induced CIED malfunctions
3.3.1. Proportion of malfunctions
A total of 135 CIED malfunctions were detected among 3121 patients (pooled prevalence: 6.6%, 95% CI: 5.1%–8.4%), with a moderate degree of heterogeneity (I 2 = 41%). When analysing data according to the type of CIED, 84 malfunctions were observed among 2359 PM/CRT‐P patients across 28 studies (pooled prevalence: 4.1%, 95% CI: 2.9–5.8), 39 malfunctions among 675 ICD patients in 29 studies (pooled prevalence: 8.2% 95% CI: 5.9–11.3) and 12 malfunctions among 87 CRT‐D patients in 12 studies (pooled prevalence:19.8%, 95% CI 11.4–32.2), with statistical significance for the difference among subgroups (p = .0003) (Figure 2).
FIGURE 2.
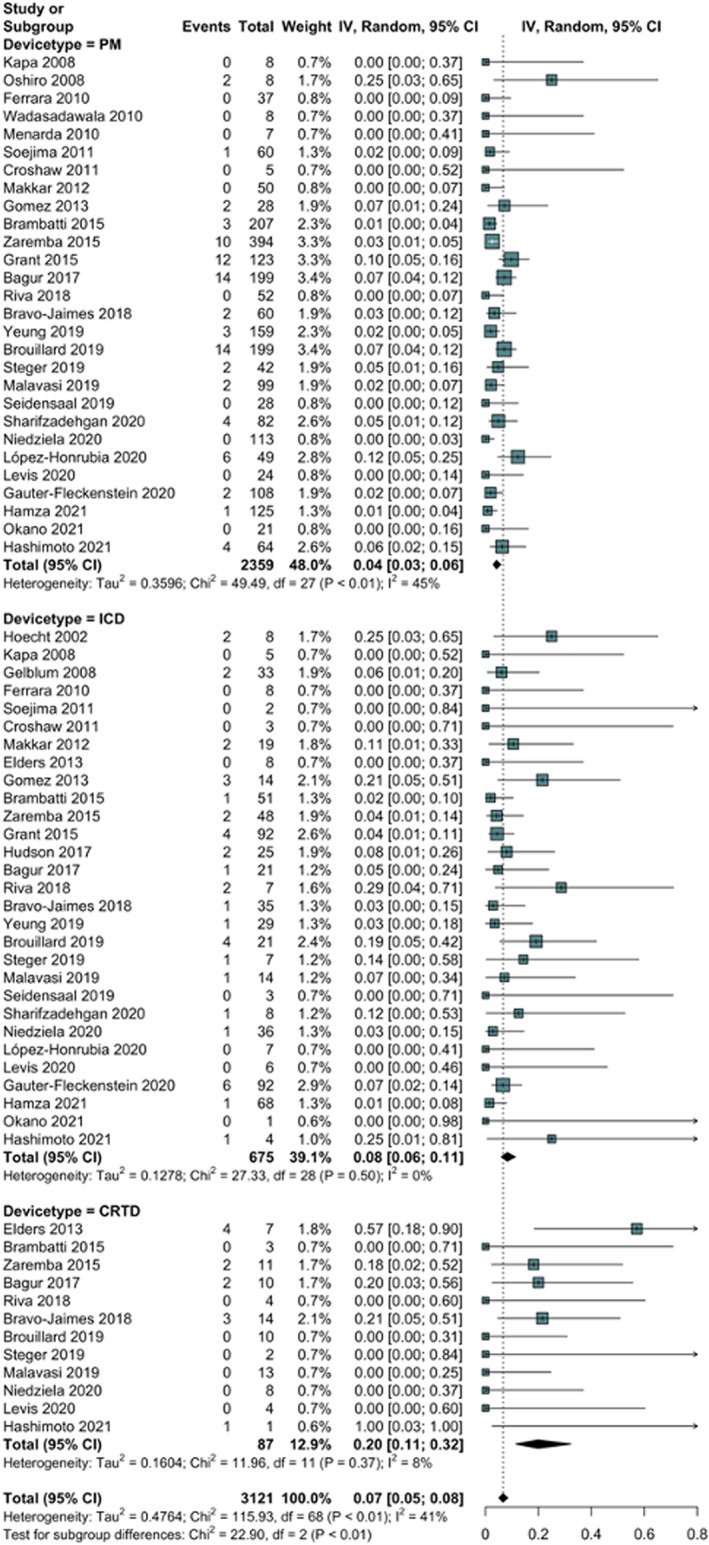
Forest plot showing the pooled prevalence of radiation therapy‐induced malfunctions in patients with pacemaker, implantable cardioverter defibrillator, and cardiac resynchronization therapy and defibrillator. CRT‐D, cardiac resynchronization therapy and defibrillator; ICD, implantable cardioverter defibrillator, PM, pacemaker.
Only three malfunctions were reported as potentially life‐threatening (two inappropriate shocks and one ventricular tachycardia). Meta‐regression showed that the prevalence of device malfunction was associated with device type, particularly CRT‐D (p = .0012), but not with the sample size (p = .2031) or year of publication (p = .1417); after the meta‐regression, the unaccounted heterogeneity (I 2 residual) was 30%. Among subgroups, we found the highest heterogeneity in the PM/CRT‐P group (I 2 = 45%), followed by CRT‐D (I 2 = 8%), and ICD (I 2 = 0%). Sensitivity analysis showed a low influence of single studies on pooled prevalence or heterogeneity (Figure S1). Visual inspection of the funnel plots and Egger's test did not show a significant publication bias (Figure S2).
3.3.2. Analysis of risk factors for RT‐associated device malfunctions
The association of RT using neutron‐producing energy and CIEDs malfunctions was evaluated in 13 studies including 1350 patients. 17 , 19 , 21 , 22 , 26 , 27 , 29 , 32 , 34 , 36 , 39 , 40 , 41 The meta‐analysis showed that the use of neutron‐producing energies was associated with a risk ratio of 9.98 (95% CI: 5.09–19.60) for device malfunctions when compared to non‐neutron‐producing energies (Figure 3, Panel A). Low heterogeneity was observed among studies (I 2 = 4%).
FIGURE 3.
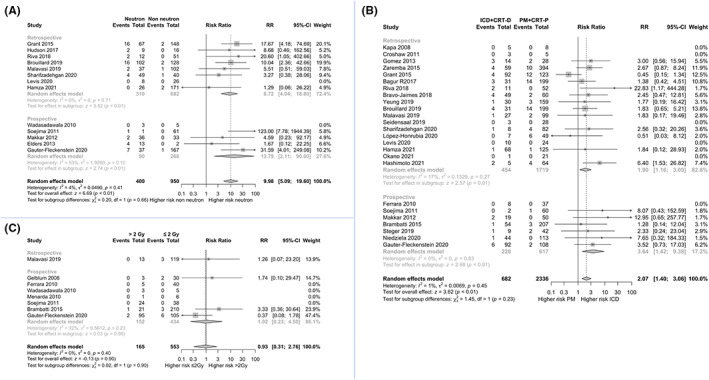
Forest plots showing the meta‐analysis of risk factors for cardiac implantable electronic device malfunctions associated with radiation therapy. Panel (A) comparison of neutron‐producing energies versus non‐neutron‐producing energies. Panel (B) comparison of PM/ CRT‐P versus ICD/ CRT‐D. Panel (C) comparison of maximal radiation dose at device >2 Gy versus ≤2 Gy. CI, confidence interval; CRT‐D, cardiac resynchronization therapy and defibrillator; CRT‐P, cardiac resynchronization therapy and pacing; Dmax, maximal radiation dose at device; Gy, greygray; ICD, implantable cardioverter defibrillator; PM, pacemakers; RR, risk ratio.
In 25 studies (3018 patients), it was possible to compare PM/CRT‐P versus ICD/CRT‐D. 13 , 16 , 19 , 20 , 21 , 23 , 24 , 25 , 26 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 The meta‐analysis showed a risk ratio of 2.07 (95% CI: 1.40–3.06) for malfunctions in ICD/CRT‐D patients when compared to PM/CRT‐P patients (Figure 3, Panel B), with low heterogeneity (I 2 = 1%). Subgroup analysis confirmed the statistical significance of both associations in retrospective and prospective studies.
The association between the maximal radiation dose at CIED (Dmax) and malfunctions was explored in eight studies for a total of 718 patients. 15 , 16 , 17 , 18 , 19 , 24 , 34 , 40 No association was found between Dmax >2 Gy and CIEDs malfunctions as compared to Dmax ≤2 Gy (risk ratio 0.93; 95% CI: 0.31–2.76) (Figure 3, Panel C), with no heterogeneity (I 2 = 0%). At subgroup analysis, such an association was neither observed in retrospective nor in prospective studies. Table 2 summarizes the results of the aforementioned pairwise comparisons. Sensitivity analysis are shown in Figures S3–S5. No significant publication bias was detected (Figures S6–S8) Analysing the 5 Gy cut‐off, no differences in the proportion of malfunctions were found nor any difference was found in a pairwise comparison (Figures S9 and S10). Some differences were found when taking into consideration the 10 Gy threshold, where the proportion of malfunctions became significantly different (5%, 95% CI: 2–9 in the group ≤10Gy vs. 37%, 95% CI: 8–79 in >10 Gy; p = .02, I 2 = 72%), with a risk ratio in the >10 Gy vs. ≤10 Gy group of 13.91 (95% CI: 3.3–58.5; p = .0003; I 2 = 41%) (Figures S11 and S12). These results should be taken with caution because in the >5 Gy and > 10 Gy groups few patients were actually treated, and the number of malfunctions was small (1/70 in >5 Gy and 5/32 in >10 Gy group).
TABLE 2.
Meta‐analysis of risk factors for cardiac implantable electronic devices malfunctions associated with radiation therapy
| Studies (n) | Patients (n) | Malfunctions (n) | Risk ratio (95% CI) | p | I 2% (95% CI) |
Egger's test p |
|
|---|---|---|---|---|---|---|---|
| Neutron‐producing vs. non‐neutron‐producing energy | 13 | 1350 | 65 | 9.98 (5.09–19.60) | <.0001 | 4 (0–61.7) | .472 |
| PM/ CRT‐P vs. ICD/ CRT‐D | 25 | 3018 | 123 | 2.07 (1.40–3.06) | .0003 | 1 (0–49.4) | .074 |
| Dmax ≤2Gy vs. Dmax >2Gy | 8 | 718 | 17 | 0.93 (0.31–2.76) | .8983 | 0 (0–84.7) | .282 |
Abbreviations: CRT‐D, cardiac resynchronization therapy and defibrillator; CRT‐P, cardiac resynchronization therapy and pacing; Dmax, maximal radiation dose at device; Gy, grey; ICD, implantable cardioverter defibrillator; PM, pacemakers.
Specific details on the type of malfunctions, follow‐up time, need for relocation, actions needed to sort the malfunctions out and RT energies/doses at cancer/CIED are reported in Tables S2 and S3, respectively.
4. DISCUSSION
To the best of our knowledge, this is the first meta‐analysis investigating the prevalence of RT‐related CIEDs malfunctions and its risk factors. The main findings of the present paper are the following: (i) the pooled prevalence of RT‐related CIEDs malfunctions is variable, ranging from around 4% to 20%; (ii) the use of neutron‐producing energies is associated with a higher risk of CIED malfunctions as compared to non‐neutron‐producing energies; similarly, (iii) ICD/CRT‐D showed a higher risk of malfunctions as compared to PM/CRT‐P. On the contrary, (iv) a higher radiation dose, that is, Dmax >2 Gy did not confer a significantly higher risk of CIED malfunctions.
Our study shows that the prevalence of RT‐associated CIEDs malfunctions is variable, and this suggests the need for risk stratification on the basis of factors related to the patient, the type of CIED, and the RT procedure. However, it is noteworthy that according to literature only three reported CIED malfunctions were classified as potentially life‐threatening, thus highlighting the overall safety profile of RT even in this subset of patients. 38 Our analysis showed a pooled proportion of malfunction probably higher than what reported in a European Heart Rhythm Association survey 44 where approximately 1/3 of the 36 respondent centres reported no malfunction, 1/3 reported malfunction in 2% of the irradiated patients and 11% malfunction in 5%. This could depend on radiation and risk stratification protocols, volume of the centres or other not specified factors. Moreover, the type of feedback that can be obtained through a survey may be quite different from what measured in dedicated studies, either prospective or retrospective.
The use of neutron‐producing energies was the strongest predictor of CIEDs malfunctions. This finding is substantiated by experimental data showing that at high beam energies (≥10 MeV), secondary neutrons are produced in the head of the linear accelerator.
We found that the prevalence of malfunctions increased in parallel with the complexity of implanted devices (4.1%, 8.2%, 19.8% for PM/CRT‐P, ICD and CRT‐D, respectively) and ICD/ CRT‐D had a nearly double risk of malfunctions as compared to PM/CRT‐P. This association has not yet been fully understood, but it can be speculated that more complex devices (e.g., ICD and CRT‐D) contain more complex integrated circuits producing ionizing particles, potentially interacting with secondary neutrons. 45 Nowadays, the circuitry of CIEDs is based on complementary metal‐oxide semiconductor (CMOS) technology. It is known that such technology is sensitive to radiation beams employed in RT and secondary neutrons can interact with the CMOS materials. 3 , 46 , 47 It has been hypothesized that ICD may be more sensitive to radiation damage due to the larger amount of software and hardware technology inside the device, with higher probability of software errors. 45 The year of study's publication was not associated with malfunctions prevalence, suggesting that modern therapies are as risky as older ones.
With regard to RT doses, we found that a Dmax >2 Gy was not associated with higher risk of device malfunctions. The cut‐off value of 2 Gy was chosen according with current guidelines, 3 , 48 but our sub‐analysis failed to reach statistical significance (RR 0.93, 95% CI: 0.31–2.76). In a recent in vitro study, 49 19 explanted ICDs were irradiated with a 6‐MV photon beam reaching an increasing cumulative dose at ICD sites of 0.5, 1, 2, 3, 5 and 10 Gy. After radiation, the authors showed no CIED malfunctions or electromagnetic interferences. These data were confirmed by another even more recent study where, again with 6‐MV flattened and flattening‐filter‐free beams, CIED malfunctions were not related to total dose but seemed to be correlated with instantaneous local dose rate. 50 About the value of 2 Gy as a limit of the energy delivered to the CIED, an aforementioned European Heart Rhythm Association survey 44 reports that only 14% of centres involved in management of patients with CIED undergoing RT considered 2 Gy as risky limit, while 7% of respondent centres considered safe a limit of 5 Gy and another 7% did not take into account safety limits. It is noteworthy that, according to our meta‐analysis, the type of CIED and the use of neutron‐producing beam energies appear to be actually more important than these safety levels of RT dose. Taken together, these findings highlight that the radiation dose at CIED should not be considered as the most crucial variable during the risk stratification process for CIED patients undergoing RT unless higher doses (> 10 Gy) are used. However, in this latest subset of patients, our assessment of the risk could actually be imprecise (with regard to the actual risk), since the values of doses in patients without malfunction were often omitted, resulting in wide confidence intervals and moderate heterogeneity. Nevertheless, overall low exposure is usually delivered at a low dose rate, therefore doses should always be as low as achievable without compromising therapy goals.
In any case, the use of neutron‐producing energies should be avoided whenever possible as this dramatically reduces the risk of CIED malfunctions, especially when ICD and CRT‐D devices are involved. This should be possible in most patients, as energies >6MV are hardly needed when advanced techniques such as static intensity‐modulated radiation therapy (IMRT)/ volumetric modulated arc therapy (VMAT) are used. The synergy and close collaboration between cardiologists and radiation oncologists is essential to provide the best management for CIEDs patients undergoing RT. 3 , 51 Additional risk factors have been hypothesized such as distance between the radiation beam and CIED or sub‐diaphragmatic site of the cancer, 25 but either inconclusive (RT‐beam and CIED distance) or not confirmed results were found. Further research in this field is needed to identify additional predictors of RT‐related CIEDs malfunctions, which can be used to create personalized pathways for patient care, with a potentially positive impact not only on clinical outcomes, but also on resource allocation. 52 , 53 In this perspective, remote monitoring of CIEDs may represent a valuable tool to regularly check the devices' status during the whole duration of the RT course. Remote monitoring has a well‐established role in the early detection of CIED technical issues 54 and, integrated with in‐office visits, may be useful for a more effective and less expensive patient management.
4.1. Limitations
The present study has several limitations that need to be acknowledged. The studies included in the meta‐analysis were observational and approximately 60% were retrospective, thus data presented do not imply causality, rather they report associations. Nevertheless, for the comparisons associated with a high degree of malfunction, we found a very low heterogeneity and then we think that the signal detected by our analysis is hardly doubtful. Moreover, it is not likely that prospective controlled randomized trials will be performed in this field in the near future. It was not possible to meta‐analyse some variables potentially associated with device malfunction such as different Dmax cut‐offs, cancer location and distance between radiation beam and the device. Moreover, in all the papers analysed, the time‐to‐malfunction or follow‐up period were not sufficiently detailed to allow a punctual analysis. However, the time‐to‐event was comparatively reported in Table S2. In most studies, a standardized patient management protocol was not provided, and may have differed between studies. Finally, given the chance that some data were underreported or missed from the literature search, a certain degree of publication bias cannot be ruled out.
5. CONCLUSIONS
In a systematic review of the current literature, RT‐related CIED malfunction had a prevalence ranging from around 4% to 20%. The use of neutron‐producing energies and more complex devices (ICD/CRT‐D) were associated with higher risk of device malfunction, while the radiation dose at CIED did not significantly impact on the risk of CIED malfunctions unless higher doses (>10 Gy) were used in the RT. Further research is needed to further improve patient risk stratification.
AUTHOR CONTRIBUTIONS
VLM involved in conceptualization, formal analysis, data curation, writing—review and editing. JFI involved in conceptualization, methodology, writing—original draft, and writing—review and editing. AT involved in data curation and investigation. GFR involved in methodology, formal analysis, review and editing. MV, MZ, EM, and GDM involved in investigation, writing—review and editing. FL and GB involved in investigation, writing—review and editing, supervision. TLF involved in writing—review and editing, supervision.
CONFLICT OF INTEREST
GB: small speaker fee from Medtronic, Boston, Boehringer Ingelheim and Bayer. No fees are received personally. TL small speaker fees from Philips, Janssen and Incyte not related with the current work. The other authors declare no conflicts of interest.
Supporting information
Appendix S1 Supporting Information.
ACKNOWLEDGEMENT
Open Access Funding provided by Universita degli Studi di Modena e Reggio Emilia within the CRUI‐CARE Agreement.
Malavasi VL, Imberti JF, Tosetti A, et al. A systematic review and meta‐analysis on oncological radiotherapy in patients with a cardiac implantable electronic device: Prevalence and predictors of device malfunction in 3121 patients. Eur J Clin Invest. 2023;53:e13862. doi: 10.1111/eci.13862
Vincenzo Livio Malavasi and Jacopo Francesco Imberti Joint first authors.
REFERENCES
- 1. Zecchin M, Torre M, Carrani E, et al. Seventeen‐year trend (2001‐2017) in pacemaker and implantable cardioverter‐defibrillator utilization based on hospital discharge database data: an analysis by age groups. Eur J Intern Med. 2021;84:38‐45. [DOI] [PubMed] [Google Scholar]
- 2. Rahib L, Wehner MR, Matrisian LM, Nead KT. Estimated projection of US cancer incidence and death to 2040. JAMA Netw Open. 2021;4(4):e214708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zecchin M, Severgnini M, Fiorentino A, et al. Management of patients with cardiac implantable electronic devices (CIED) undergoing radiotherapy: A consensus document from Associazione Italiana Aritmologia e Cardiostimolazione (AIAC), Associazione Italiana Radioterapia Oncologica (AIRO), Associazione Italiana Fisica Medica (AIFM). Int J Cardiol. 2018;255:175‐183. [DOI] [PubMed] [Google Scholar]
- 4. Indik JH, Gimbel JR, Abe H, et al. 2017 HRS expert consensus statement on magnetic resonance imaging and radiation exposure in patients with cardiovascular implantable electronic devices. Heart Rhythm. 2017;14(7):e97‐e153. [DOI] [PubMed] [Google Scholar]
- 5. Escande A, Frey P, Lacornerie T, et al. Radiotherapy for patient with cardiac implantable electronic device, consensus from French radiation oncology society. Cancer Radiother. 2022;26(1–2):404‐410. [DOI] [PubMed] [Google Scholar]
- 6. Miften M, Mihailidis D, Kry SF, et al. Management of radiotherapy patients with implanted cardiac pacemakers and defibrillators: a report of the AAPM TG‐203. Med Phys. 2019;46(12):e757‐e788. [DOI] [PubMed] [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta‐analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Viechtbauer W. Conducting meta‐analyses in R with the metafor package. J Stat Softw. 2010;36(3):1‐48. [Google Scholar]
- 11. Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40(1):35‐53. [DOI] [PubMed] [Google Scholar]
- 12. Hoecht S, Rosenthal P, Sancar D, Behrens S, Hinkelbein W, Hoeller U. Implantable cardiac defibrillators may be damaged by radiation therapy. J Clin Oncol. 2002;20(8):2212‐2213. [DOI] [PubMed] [Google Scholar]
- 13. Kapa S, Fong L, Blackwell CR, Herman MG, Schomberg PJ, Hayes DL. Effects of scatter radiation on ICD and CRT function. Pacing Clin Electrophysiol. 2008;31(6):727‐732. [DOI] [PubMed] [Google Scholar]
- 14. Oshiro Y, Sugahara S, Noma M, et al. Proton beam therapy interference with implanted cardiac pacemakers. Int J Radiat Oncol Biol Phys. 2008;72(3):723‐727. [DOI] [PubMed] [Google Scholar]
- 15. Gelblum DY, Amols H. Implanted cardiac defibrillator care in radiation oncology patient population. Int J Radiat Oncol Biol Phys. 2009;73(5):1525‐1531. [DOI] [PubMed] [Google Scholar]
- 16. Ferrara T, Baiotto B, Malinverni G, et al. Irradiation of pacemakers and cardio‐defibrillators in patients submit ted to radiotherapy: a clinical experience. Tumori. 2010;96(1):76‐83. [DOI] [PubMed] [Google Scholar]
- 17. Wadasadawala T, Pandey A, Agarwal JP, et al. Radiation therapy with implanted cardiac pacemaker devices: a clinical and dosimetric analysis of patients and proposed precautions. Clin Oncol (R Coll Radiol). 2011;23(2):79‐85. [DOI] [PubMed] [Google Scholar]
- 18. Menard J, Campana F, Kirov KM, et al. Radiothérapie pour uncancer du sein et stimulateur cardiaque. Cancer/Radiothérapie. 2011;15(3):197‐201. [DOI] [PubMed] [Google Scholar]
- 19. Soejima T, Yoden E, NIshimura Y, et al. Radiation therapy in patients with implanted cardiac pacemakers and implantable cardioverter defibrillators: a prospective survey in Japan. J Radiat Res. 2011;52(4):516‐521. [DOI] [PubMed] [Google Scholar]
- 20. Croshaw R, Kim Y, Lappinen E, Julian T, Trombetta M. Avoiding mastectomy: accelerated partial breast irradiation for breast cancer patients with pacemakers or defibrillators. Ann Surg Oncol. 2011;18(12):3500‐3505. [DOI] [PubMed] [Google Scholar]
- 21. Makkar A, Prisciandaro J, Agarwal S, et al. Effect of radiation therapy on permanent pacemaker and implantable cardioverter‐defibrillator function. Heart Rhythm. 2012;9(12):1964‐1968. [DOI] [PubMed] [Google Scholar]
- 22. Elders J, Kunze‐Busch M, Smeenk RJ, Smeets JL. High incidence of implantable cardioverter defibrillator malfunctions during radiation therapy: neutrons as a probable cause of soft errors. Europace. 2013;15(1):60‐65. [DOI] [PubMed] [Google Scholar]
- 23. Gomez DR, Poenisch F, Pinnix CC, et al. Malfunctions of implantable cardiac devices in patients receiving proton beam therapy: incidence and predictors. Int J Radiat Oncol Biol Phys. 2013;87(3):570‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brambatti M, Mathew R, Strang B, et al. Management of patients with implantable cardioverter‐defibrillators and pacemakers who require radiation therapy. Heart Rhythm. 2015;12(10):2148‐2154. [DOI] [PubMed] [Google Scholar]
- 25. Zaremba T, Jakobsen AR, Søgaard M, et al. Risk of device malfunction in cancer patients with implantable cardiac device undergoing radiotherapy: a population‐based cohort study. Pacing Clin Electrophysiol. 2015;38(3):343‐356. [DOI] [PubMed] [Google Scholar]
- 26. Grant JD, Jensen GL, Tang C, et al. Radiotherapy‐induced malfunction in contemporary cardiovascular implantable electronic devices: clinical incidence and predictors. JAMA Oncol. 2015;1(5):624‐632. [DOI] [PubMed] [Google Scholar]
- 27. Hudson FJ, Ryan EA. A review of implantable cardioverter defibrillator failures during radiation therapy in three Sydney hospitals. J Med Imaging Radiat Oncol. 2017;61(4):517‐521. [DOI] [PubMed] [Google Scholar]
- 28. Bagur R, Chamula M, Brouillard É, et al. Radiotherapy‐induced cardiac implantable electronic device dysfunction in patients with cancer. Am J Cardiol. 2017;119(2):284‐289. [DOI] [PubMed] [Google Scholar]
- 29. Riva G, Alessandro O, Spoto R, et al. Radiotherapy in patients with cardiac implantable electronic devices: clinical and dosimetric aspects. Med Oncol. 2018;35(5):73. [DOI] [PubMed] [Google Scholar]
- 30. Bravo‐Jaimes K, Samala V, Fernandez G, et al. CIED malfunction in patients receiving radiation is a rare event that could be detected by remote monitoring. J Cardiovasc Electrophysiol. 2018;29(9):1268‐1275. [DOI] [PubMed] [Google Scholar]
- 31. Yeung C, Hazim B, Campbell D, et al. Radiotherapy for patients with cardiovascular implantable electronic devices: an 11‐year experience. J Interv Card Electrophysiol. 2019;55:333‐341. [DOI] [PubMed] [Google Scholar]
- 32. Brouillard É, Chamula M, Lavoie C, Varfalvy N, Archambault L. Radiation therapy‐induced dysfunction in cardiovascular implantable electronic devices. Pract Radiat Oncol. 2019;9:266‐273. [DOI] [PubMed] [Google Scholar]
- 33. Steger F, Hautmann MG, Süß C, et al. Radiotherapy of patients with cardiac implantable electronic devices according to the DEGRO/DGK guideline—is the risk of relevant errors overestimated? Strahlenther Onkol. 2019;195(12):1086‐1093. [DOI] [PubMed] [Google Scholar]
- 34. Malavasi VL, De Marco G, Imberti JF, et al. Radiotherapy‐induced malfunctions of cardiac implantable electronic devices in cancer patients. Intern Emerg Med. 2020;15(6):967‐973. [DOI] [PubMed] [Google Scholar]
- 35. Seidensaal K, Harrabi SB, Scholz E, et al. Active‐scanned protons and carbon ions in cancer treatment of patients with cardiac implantable electronic devices: experience of a single institution. Front Oncol. 2019;9:798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sharifzadehgan A, Laurans M, Thuillot M, et al. Radiotherapy in patients with a cardiac implantable electronic device. Am J Cardiol. 2020;128:196‐201. [DOI] [PubMed] [Google Scholar]
- 37. Niedziela JT, Blamek S, Gadula‐Gacek E, et al. Radiation therapy in patients with cardiac implantable electronic devices. Kardiol Pol. 2021;79(2):156‐160. [DOI] [PubMed] [Google Scholar]
- 38. López‐Honrubia V, Hidalgo‐Olivares VM, Dobón‐Roux M, et al. Radiotherapy is safe in patients with implantable cardiac devices. Analysis of a systematic interrogation follow‐up. Clin Transl Oncol. 2020;22(12):2286‐2292. [DOI] [PubMed] [Google Scholar]
- 39. Levis M, Andreis A, Badellino S, et al. Safety of lung stereotactic ablative radiotherapy for the functioning of cardiac implantable electronic devices. Radiother Oncol. 2021;156:193‐198. [DOI] [PubMed] [Google Scholar]
- 40. Gauter‐Fleckenstein B, Barthel C, Büttner S, Wenz F, Borggrefe M, Tülümen E. Effectivity and applicability of the German DEGRO/DGK‐guideline for radiotherapy in CIED‐bearing patients. Radiother Oncol. 2020;152:208‐215. [DOI] [PubMed] [Google Scholar]
- 41. Hamza M, Rice S, Lamichhane N, Chen S, Mohindra P. Conformal radiation therapy in patients with cardiovascular Implantabl e electronic devices: proposed practical implementation of the 2019 american Association of Physicists in medicine task group no. 203 risk‐S tratified interrogation schedule. Pract Radiat Oncol. 2021;11(4):e402‐e414. [DOI] [PubMed] [Google Scholar]
- 42. Okano N, Sakai M, Shibuya K, et al. Safety verification of carbon‐ion radiotherapy for patients with cardiac implantable electronic devices (CIEDs). J Radiat Res. 2022;63(1):122‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hashimoto T, Demizu Y, Numajiri H, et al. Particle therapy using protons or carbon ions for cancer patients with cardiac implantable electronic devices (CIED): a retrospective multi‐ institutional study. Jpn J Radiol. 2022;40(5):525‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lenarczyk R, Potpara TS, Haugaa KH, et al. Approach to cardio‐oncologic patients with special focus on patients with cardiac implantable electronic devices planned for radiotherapy: results of the European heart rhythm association survey. Europace. 2017;19(9):1579‐1584. [DOI] [PubMed] [Google Scholar]
- 45. Zecchin M, Morea G, Severgnini M, et al. Malfunction of cardiac devices after radiotherapy without direct exposure to ionizing radiation: mechanisms and experimental data. Europace. 2016;18(2):288‐293. [DOI] [PubMed] [Google Scholar]
- 46. Sundar S, Symonds RP, Deehan C. Radiotherapy to patients with artificial cardiac pacemakers. Cancer Treat Rev. 2005;31(6):474‐486. [DOI] [PubMed] [Google Scholar]
- 47. Services GCCRMT . Impact of THERAPEUTIC Radiation and Guidant ICD/CRTD/CRT‐P/Pacing Systems. Guidant Corporation; 2004:1‐6. [Google Scholar]
- 48. Hurkmans CW, Knegjens JL, Oei BS, et al. Management of radiation oncology patients with a pacemaker or ICD: a new comprehensive practical guideline in The Netherlands. Dutch Society of Radiotherapy and Oncology (NVRO). Radiat Oncol. 2012;7:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zecchin M, Artico J, Morea G, et al. Radiotherapy and risk of implantable cardioverter‐defibrillator malfunctions: experimental data from direct exposure at increasing doses. J Cardiovasc Med (Hagerstown). 2018;19(4):155‐151. [DOI] [PubMed] [Google Scholar]
- 50. Gauter‐Fleckenstein B, Tülümen E, Rudic B, Borggrefe M, Polednik M, Fleckenstein J. Local dose rate effects in implantable cardioverter‐defibrillators with flattening filter free and flattened photon radiation. Strahlenther Onkol. 2022;198(6):566‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Glikson M, Nielsen JC, Kronborg MB, et al. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021;42(35):3427‐3520. [DOI] [PubMed] [Google Scholar]
- 52. Boriani G, Maniadakis N, Auricchio A, et al. Health technology assessment in interventional electrophysiology and device therapy: a position paper of the European heart rhythm association. Eur Heart J. 2013;34(25):1869‐1874. [DOI] [PubMed] [Google Scholar]
- 53. Sabater S, Montero A, López Fernández T, González Ferrer JJ, Arenas M. Management of patients with implanted cardiac devices during radiotherapy: results of a Spanish survey in radiation oncology departments. Clin Transl Oncol. 2018;20(12):1577‐1581. [DOI] [PubMed] [Google Scholar]
- 54. Imberti JF, Tosetti A, Mei DA, Maisano A, Boriani G. Remote monitoring and telemedicine in heart failure: implementation and benefits. Curr Cardiol Rep. 2021;23(6):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information.


