Abstract
Background
Previous studies have shown that several agents that stimulate heptahelical G-protein coupled receptors activate the extracellular signal regulated kinases ERK1 (p44mapk) and ERK2 (p42mapk) in hepatocytes. The molecular pathways that convey their signals to ERK1/2 are only partially clarified. In the present study we have explored the role of Ca2+ and Ca2+-dependent steps leading to ERK1/2 activation induced by norepinephrine and prostaglandin (PG)F2α.
Results
Pretreatment of the cells with the Ca2+ chelators BAPTA-AM or EGTA, as well as the Ca2+ influx inhibitor gadolinium, resulted in a partial decrease of the ERK response. Furthermore, the calmodulin antagonists W-7, trifluoperazine, and J-8 markedly decreased ERK activation. Pretreatment with KN-93, an inhibitor of the multifunctional Ca2+/calmodulin-dependent protein kinase, had no effect on ERK activation. The Src kinase inhibitors PP1 and PP2 partially diminished the ERK responses elicited by both norepinephrine and PGF2α.
Conclusion
The present data indicate that Ca2+ is involved in ERK activation induced by hormones acting on G protein-coupled receptors in hepatocytes, and suggest that calmodulin and Src kinases might play a role in these signaling pathways.
Background
The extracellular signal regulated kinases ERK1 (p44mapk) and ERK2 (p42mapk) are activated in response to stimulation of receptor tyrosine kinases (RTKs) as well as heptahelical G protein coupled receptors (GPCR) and transmit signals which regulate cell differentiation and growth [1-3]. The molecular steps involved in signaling from GPCRs to ERK are incompletely understood. Data obtained in various cell systems have provided evidence in support of several signaling pathways including protein kinase C (PKC) [4], Ca2+-mediated mechanisms [5-12], and transactivation of receptor tyrosine kinases [13,14]. In hepatocytes several hormones, including vasopressin, angiotensin II, norepinephrine, and PGF2α, that bind to GPCRs activate ERK [15-17]. The mechanisms mediating the ERK activation by GPCR agonists are not clarified; there is evidence that protein kinase C is involved [15,18], but a role for Ca2+ also appears likely, since all the agents above activate phospholipase C and elevate intracellular Ca2+ in hepatocytes [19,20]. Furthermore, agents that elevate intracellular Ca2+ through mechanisms bypassing receptors have been found to activate ERK [15,21]. However, agonist-stimulated phospholipase C activity is rapidly down-regulated upon culturing of hepatocytes [22,23], and we recently reported that norepinephrine and PGF2α activate ERK under conditions where the level of inositol 1,4,5-trisphosphate (InsP3) was only slightly, and transiently elevated [17]. In the present study we have, therefore, examined more closely the role of Ca2+ in ERK activation induced by norepinephrine and PGF2α and mechanisms downstream of elevated [Ca2+]i.
Results
Agents that elevate [Ca2+]i activate ERK
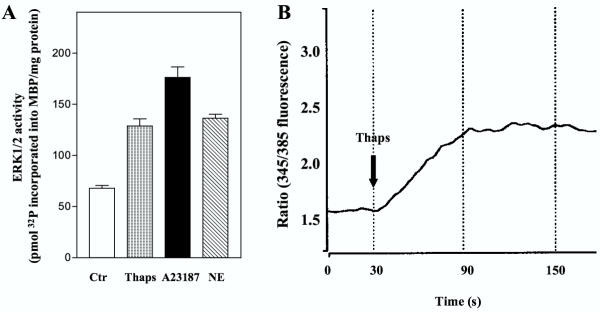
In agreement with previous observations [15,21] treatment of hepatocytes with thapsigargin, which inhibits Ca2+ reuptake to endoplasmatic reticulum [24], and A23187, which induces Ca2+ influx, stimulated ERK1/2 activity 2–2.5 fold (Fig. 1A). The elevation of intracellular Ca2+ resulting from stimulation with thapsigargin is shown in Fig. 1B. These observations are compatible with a role for Ca2+-elevating mechanisms in the events that trigger ERK1/2 activation in hepatocytes.
Figure 1.
ERK1/2 activation and Ca2+ response in hepatocytes. A: At 3 h after the time of seeding hepatocytes were preincubated with timolol (10 μM) for 30 min prior to stimulation with thapsigargin (1 μM), A23187 (10 μM) or norepinephrine (10 μM) for 5 min before they were harvested and ERK 1/2 activity assessed. Results represent mean ± S.E.M. of five different experiments. B: Single cell measurement of [Ca2+]i as described in Materials and Methods. Results given as ratio (345/385 fluorescence) represent a typical single cell response after stimulation with thapsigargin (10 μM) in a fura-2 AM loaded hepatocyte.
Activation of ERK by norepinephrine and PGF2α involves Ca2+
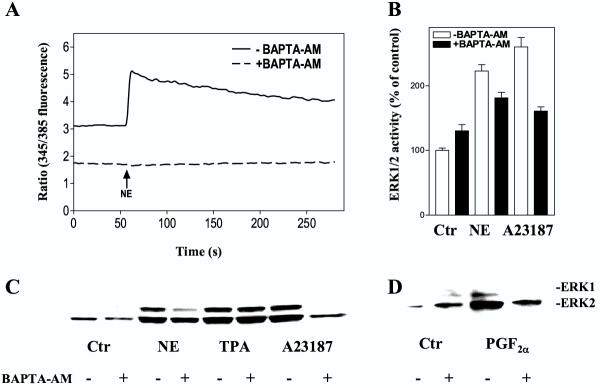
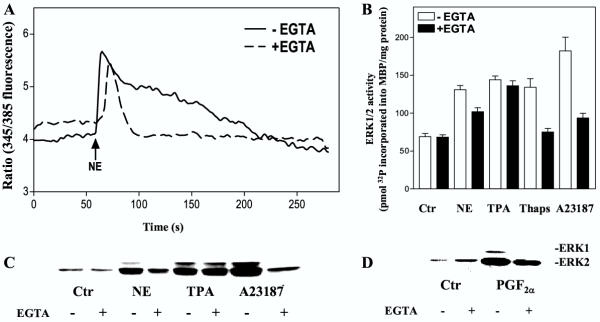
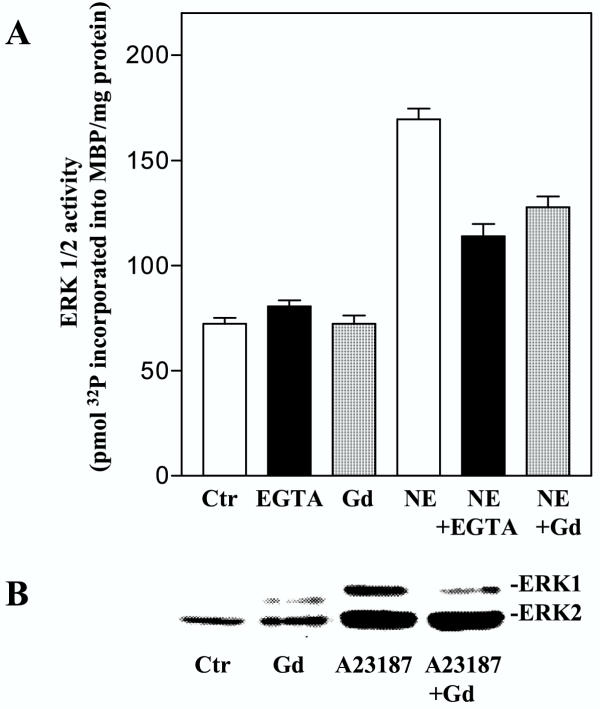
We then examined the role of Ca2+ in activation of ERK1/2 induced by stimulation of α1-adrenoceptors (with norepinephrine in the presence of timolol) and prostaglandin receptors (using PGF2α) [21,25,26]. The hepatocytes were pretreated with BAPTA-AM, which is activated intracellularly to bind Ca2+, EGTA, which binds extracellular Ca2+ and eventually may deplete intracellular Ca2+[27,28], or gadolinium, a competitive inhibitor of Ca2+ influx [29-31]. BAPTA-AM completely attenuated the norepinephrine-induced rise of [Ca2+]i (Fig. 2A), while the ERK1/2 activity in response to norepinephrine was partially decreased (Fig. 2B,2C). ERK1/2 activity induced by PGF2α and the Ca2+ ionophore A23187 was also inhibited by BAPTA-AM, while the TPA response was unaffected (Fig. 2B,2C,2D). When the cells were pretreated with EGTA, the initial peak of the Ca2+ elevation was only slightly affected, while the prolonged phase of the Ca2+-response was abolished (Fig. 3A). The activation of ERK1/2 by norepinephrine or PGF2α was partly decreased by EGTA (Fig. 3B,3C,3D). EGTA also markedly decreased the ERK1/2 response induced by A23187 and thapsigargin, while the TPA-induced ERK1/2 activation was unaffected (Fig. 3B,3C). Pretreatment with gadolinium decreased the adrenergic activation almost to the level obtained by EGTA (Fig. 4A). Gadolinium also decreased the A23187-induced activation of ERK1/2 (Fig. 4B). Taken together, the results suggest a role for Ca2+ in the activation of ERK by norepinephrine and PGF2α and that this involves Ca2+ influx as well as release from internal pools.
Figure 2.
Effect of BAPTA-AM on [Ca2+]i and ERK1/2 activation. A: Measurement of [Ca2+]i. Hepatocytes were preincubated with 0.55 % DMSO or BAPTA-AM (40 μM) during the last 25 minutes of the fura-2 AM loading. After 60 seconds of registration the cells were stimulated with norepinephrine (10 μM) in the presence of timolol (10 μM). Results show a typical single cell response. B-D: ERK1/2 responses. Hepatocytes cultured for 3 h were pretreated for 30 min with BAPTA-AM (40 μM) in the presence of timolol (10 μM) prior to stimulation with norepinephrine (10 μM), A23187 (10 μM), TPA (1 μM) or PGF2α (10 μM) for 5 min before cells were harvested. All cultures contained DMSO at a concentration of 0.5 % during the preincubation and a final concentration of 1 % DMSO during incubation with agonist. B: Activity measurements of ERK1/2 representing mean ± S.E.M. of three experiments. C, D: Immunoblots using antibody against dually phosphorylated ERK1/2.
Figure 3.
Effect of EGTA on [Ca2+]i and ERK 1/2 activation. A: [Ca2+]i measurements. Hepatocytes were preincubated in Krebs-Ringer-Hepes buffer with or without 5 mM EGTA for 15 min after fura-2 AM loading. After 60 seconds of registration the cells were stimulated with norepinephrine (10 μM) in the presence of timolol (10 μM). Results show a typical single cell response. B-D: ERK1/2 responses. Hepatocytes cultured for 3 h were pretreated with timolol (10 μM) for 30 min and EGTA (5 mM) for 15 min before stimulation with norepinephrine (10 μM), TPA (1 μM), thapsigargin (1 μM), A23187 (10 μM) or PGF2α (10 μM) for 5 min (in the presence of 0.5 % DMSO). B: Activity measurements of ERK1/2 representing the mean ± S.E.M. of three experiments. C, D: Immunoblots using antibody against double phosphorylated, i.e. activated, forms of ERK1/2.
Figure 4.
Effect of gadolinium on ERK1/2 responses. A: Hepatocytes cultured for 3 h were pretreated with gadolinium (100 μM) or EGTA (5 mM) for 15 min in the presence of timolol (10 μM) prior to stimulation with norepinephrine (10 μM) for 5 min before cells were harvested. Results are activity measurements of ERK1/2 given as pmol 32P incorporated into MBP/mg protein representing mean ± S.E.M. of three experiments. B: Immunoblot showing the effect of pretreatment with gadolinium (100 μM) for 15 min on A23187 (10 μM) induced ERK1/2 response. Antibody against dually phosphorylated ERK1/2 was used.
Effect of antagonists of calmodulin and the multifunctional Ca2+/calmodulin-dependent protein kinase in ERK activation in hepatocytes
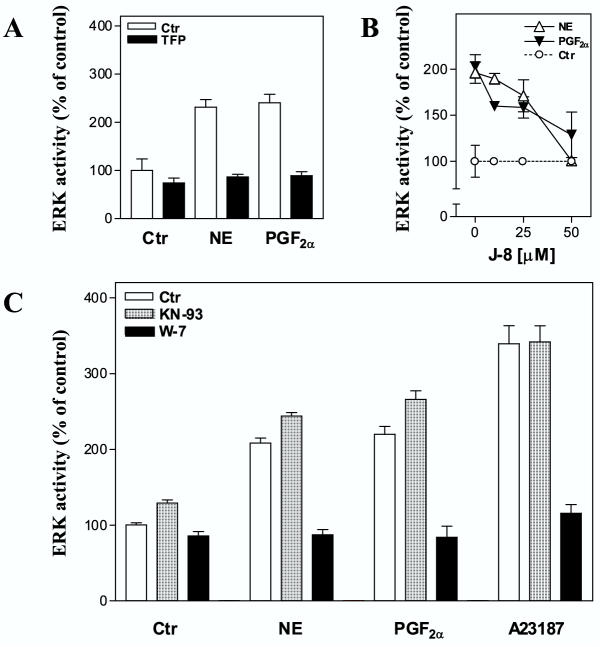
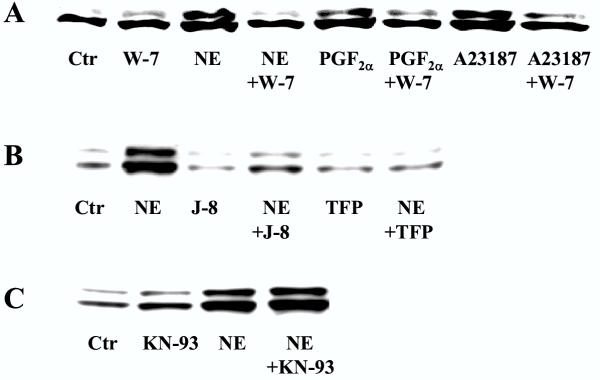
A major mechanism for Ca2+-induced signaling is through formation of a complex with calmodulin [32,33]. Calmodulin has been found to stimulate as well as inhibit ERK1/2 activity [12,34,35]. We therefore examined the role of calmodulin in these pathways. Pretreatment of hepatocytes with the calmodulin inhibitors trifluoperazine, J-8, and W-7 markedly inhibited the ERK1/2 activation after stimulation with norepinephrine and PGF2α (Fig. 5). The results were confirmed with immunoblots (Fig. 6). Activation of ERK1/2 by A23187 was also markedly sensitive to pretreatment with W-7 (Fig. 5, 6).
Figure 5.
Effect of inhibitors of calmodulin (Trifluoperazine, J-8, W-7) and the multifunctional Ca2+/calmodulin dependent protein kinase (KN-93). Hepatocytes were cultured for 3 h before preincubation with timolol (10 μM) for 30 min in the presence or absence of indicated inhibitors prior to stimulation for 5 min with norepinephrine (10 μM), PGF2α (10 μM) or A23817 (10 μM), before cells were harvested and ERK1/2 activation assessed. A: Pretreatment with trifluoperazine (50 μM) in 0.5 % DMSO. The results represent one typical experiment out of three and are expressed as percent of untreated control. B: Pretreatment with J-8 (10, 25, and 50 μM) in 0.5 % DMSO. The results represent mean ± S.E.M of three experiments and are expressed as percent of corresponding control values. C: Pretreatment with W-7 (100 μM) or KN-93 (20 μM) in 0.5 % DMSO, while the final DMSO concentration during incubation with agonist was 1 %. Results represent mean ± S.E.M. of five experiments and are expressed as percent of untreated control.
Figure 6.
Immunoblots showing the effect of inhibitors of calmodulin (W-7, J-8, trifluoperazine) and the multifunctional Ca2+/calmodulin dependent protein kinase (KN-93) on ERK1/2 activation (A-C). Hepatocytes were cultured for 3 h before preincubation for 30 min with timolol (10 μM) A: in the presence or absence of W-7 (25 μM) before stimulation for 5 min with norepinephrine (10 μM), PGF2α (10 μM) or A23187 (10 μM) or B, C: with or without J-8 (40 μM), trifluoperazine (50 μM) or KN-93 (20 μM) before 5 min of stimulation with norepinephrine (10 μM). Western analyses were based on the use of antibody against dually phosphorylated ERK1/2.
Calmodulin may act on several regulatory enzymes [32,36-40], including the Ca2+/calmodulin-dependent protein kinases, which have been implicated in the activation of ERK1/2 [7-9]. We explored a possible role for the multifunctional Ca2+/calmodulin-dependent protein kinase in ERK1/2 activation in hepatocytes stimulated by norepinephrine and PGF2α. Pretreatment of the cells with KN-93, an inhibitor of the multifunctional Ca2+/calmodulin-dependent protein kinase [41], did not decrease the activation of ERK1/2 either by norepinephrine, PGF2α, or A23187 (Fig. 5, 6). In supplementary experiments we examined the effects of higher concentrations of KN-93 (up to 100 μM) or prolonged exposure times (up to 24 hours) which in none of the cases resulted in a decreased ERK1/2 activation (data not shown). These results suggest that calmodulin might be involved in hormone-induced activation of ERK1/2 in hepatocytes, however the data do not support a role for the multifunctional Ca2+/calmodulin-dependent protein kinase in Ca2+/calmodulin-mediated activation of ERK1/2 in hepatocytes
Inhibitors of src kinases attenuate ERK activation in hepatocytes
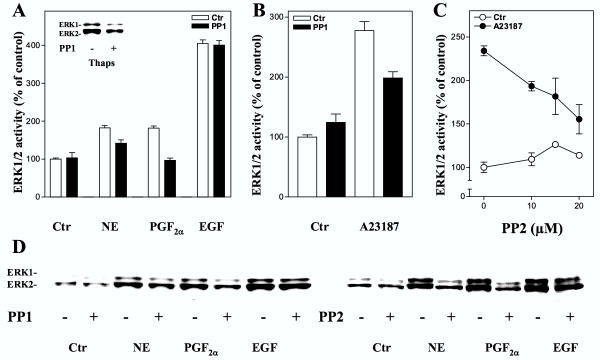
Src kinases [42] have been implicated in the mechanisms resulting in ERK1/2 activation in response to stimulation of both Gi- and Gq- coupled heptahelical receptors [43-45], and several observations suggest that activation of Src in these pathways involves Ca2+[46,47]. Data obtained in this study showed that the Src inhibitors PP1 and PP2, which are reported to primarily inhibit the Lck, Fyn, and Hck subtypes of Src kinases [48], markedly decreased the PGF2α-induced ERK1/2 activation and led to partial inhibition of the effect of norepinephrine, while the EGF induced ERK1/2 response was not reduced (Fig. 7). Furthermore, ERK1/2 activation induced by A23187 and thapsigargin was also decreased after Src inhibition (Fig. 7). The results suggest a role for Src kinases in the mechanisms leading to ERK1/2 activation both by PGF2α and norepinephrine, and that this step at least in part may be located distal to increases in the intracellular level of Ca2+.
Figure 7.
Effect of inhibition of Src kinases on ERK1/2 activation. A-D: Hepatocytes were cultured for 3 h prior to preincubation for 30 min with timolol (10 μM) and the Src kinase inhibitors PP1 or PP2 at indicated concentrations in 0.5 % DMSO before stimulation for 5 min with norepinephrine (10 μM), PGF2α (10 μM), EGF (10 nM), thapsigargin (1 μM), or A23187 (10 μM). A: ERK1/2 activity, given as percent of untreated control, induced by hormonal agents expressed as the mean ± S.E.M. of four experiments after treatment with PP1 (10 μM). The inset shows an immunoblot of the effect of PP1 (20 μM) on thapsigargin-induced ERK1/2 response. B: A23187-induced ERK1/2 response after PP1 (10 μM) treatment. Results represent mean ± S.E.M of three experiments. C: Dose-response curve for the effect of PP2 on ERK1/2 activity induced by A23187. Results represent mean ± S.E.M of three experiments. All activity measurements (A-C) are expressed as percent of untreated control. D: Immunoblots showing the effect of PP1 (10 μM) or PP2 (10 μM) on ERK1/2 responses. Antibody against dually phosphorylated ERK1/2 was used.
Discussion
The present findings confirm previous reports of a role for Ca2+ in ERK1/2 activation in hepatocytes [15,21] and suggest that release of Ca2+ from intracellular stores as well as influx of extracellular Ca2+ is of importance for the hormone-induced activation of ERK1/2. Furthermore, the results suggest that calmodulin and Src kinases might be involved in the Ca2+-dependent activation of ERK1/ERK2.
Evidence from several experimental models suggest that activation of ERK1/2 may occur through Ca2+-dependent as well as Ca2+-independent mechanisms [5,28,49-54]. The present data suggest that Ca2+ is involved in activation of ERK1/2 in hepatocytes in response to norepinephrine and PGF2α. The ERK1/2 response was decreased by chelation of intracellular and extracellular Ca2+ with BAPTA-AM and EGTA, respectively, as well as by gadolinium, which competitively inhibits Ca2+ influx. It may appear that extracellular and intracellular Ca2+ act in a concerted, possibly sequential manner in the mechanisms involved in activation of ERK1/2 by norepinephrine and PGF2α. An integration of Ca2+ signals from the extracellular and intracellular environment is presumably due to store-operated Ca2+ influx [31,55,56]. The mechanisms that initiate Ca2+ influx subsequent to depletion of intracellular stores are incompletely understood, but recent studies have suggested that direct interaction between InsP3 receptors and calcium channels in the plasma membrane may lead to activation of the calcium channels [57]. A diffusible Ca2+ influx factor may also be involved [58]. Previous studies have suggested that hormone-induced Ca2+ influx involves heterotrimeric Gi proteins in hepatocytes [59,60]. It is notable that norepinephrine and PGF2α activate ERK1/2 in the presence of a barely detectable increase in intracellular InsP3[17]. This may suggest either the occurrence of local elevations of InsP3 which do not affect global InsP3, or that Ca2+ pools are regulated by other mechanisms such as generation of sphingosine-1-phosphate [61]. A role for ryanodine-sensitive Ca2+ stores in the endoplasmatic reticulum has also been proposed in hepatocytes [62] and EGTA-sensitive pools that are located in plasma membrane micro villar structures have been described [63].
Our data further suggest that calmodulin, which has previously been implicated in growth regulation in liver [64], is involved in activation of ERK1/2. The ERK1/2 responses induced by norepinephrine and PGF2α were markedly decreased after pretreatment with the calmodulin inhibitors trifluoperazine, J-8, or W-7. Besides calmodulin, it is conceivable that the effect of Ca2+ is mediated through other Ca2+-binding proteins [39,65]. Calmodulin may also act in a Ca2+-independent manner [38,66], which might account for the more pronounced inhibition of hormone-stimulated ERK activity by calmodulin antagonists than by agents inhibiting the Ca2+ signal. Alternatively, nonspecific effects produced by calmodulin antagonists in higher doses might explain their relatively stronger inhibition. Among the downstream targets of calmodulin, the Ca2+/calmodulin-dependent protein kinases have been implicated in ERK1/2 activation in smooth muscle cells [8,9], but not in other cells [67,68]. Furthermore, the multifunctional Ca2+/calmodulin-dependent protein kinase was located downstream of ERK1/2 activation by platelet-derived growth factor (PDGF) in vascular smooth muscle cells [69]. Pretreatment of hepatocytes with KN-93 did not decrease ERK1/2 activation induced by hormones or the Ca2+ ionophore A23187. Thus, while the multifunctional Ca2+ /calmodulin-dependent protein kinase exerts several effects in hepatocytes, including growth inhibition under certain conditions [70-72], it does not appear to be involved in ERK activation. It is of interest that the α1-adrenoceptor-induced c-fos expression in fibroblasts was also observed to involve calmodulin, but not the multifunctional Ca2+/calmodulin-dependent protein kinase [73].
Increasing evidence suggest a role of Src kinases downstream of Ca2+/calmodulin in ERK1/2 signaling [10,14,46]. The present results suggest that Src kinases may be involved in ERK1/2 activation induced by PGF2α and norepinephrine, while the EGF induced ERK1/2 response appears to be independent of these Src kinases. Furthermore, the ERK1/2 activation induced by the Ca2+ ionophore A23187 or by thapsigargin was partially decreased by Src inhibition suggesting a role of Src distal to increases in intracellular Ca2+. Of the possible downstream targets for Ca2+/calmodulin in ERK signaling in hepatocytes our findings thus lend support to a role of Src kinases, although the results do not exclude the possibility that Src kinases and calmodulin act in parallel pathways leading to ERK activation.
While the present results show a role for Ca2+ in ERK1/2 activation by norepinephrine and PGF2α, it is notable that even complete inhibition of Ca2+ signaling only partially inhibited ERK1/2 activity. Taken together with previous observations that inhibition of PKC almost completely inhibited ERK1/2 activation by norepinephrine, vasopressin, and angiotensin II [18], the results suggest that several mechanisms contribute to and may act in concert in the hormonal stimulation of ERK1/2 in hepatocytes.
Conclusion
Our present data indicate that both extracellular and intracellular Ca2+ is involved in hormone-induced ERK1/2 activation in cultured hepatocytes, and suggest that calmodulin and Src kinases might play a role in these signaling pathways, while the multifunctional Ca2+/calmodulin-dependent protein kinase does not appear to be involved.
Materials and Methods
Materials
Dulbecco's modified Eagle's medium, Waymouth's medium MAB 87/3, penicillin and streptomycin were from Gibco, Grand Island, NY, U.S.A. Adenosine 5'-triphosphate, collagen, collagenase, EGTA, phenylmethylsulfonyl fluoride, benzamidine, leupeptin, pepstatin A, myelin basic protein (MBP), norepinephrine, prostaglandin F2α, epidermal growth factor, insulin, timolol, gadolinium chloride (hexahydrate), sulfinpyrazone, and 2-mercaptoethanol were from Sigma, St. Louis, MO, USA. A23187, 12-O-tetradecanoyl phorbol-13-acetate, thapsigargin, N-(6-aminohexyl)-5-chloro-1-naphtalenesulfonamide (W-7), trifluoperazine dimaleate, 2-[N-(2-hydroxyethyl)-N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine (KN-93), and l,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid tetra(acetoxymethyl)ester (BAPTA-AM), PP1, PP2 were from Calbiochem, La Jolla, CA, USA. (N-8-Aminooctyl)-5-iodo-1-naphtalenesulfonamide (J-8) was from Alexis Biochemicals, Lausen, Switzerland. Fura-2 AM and Pluronic F-127 were from Molecular Probes, Eugene, OR, USA. Sodium(meta)vanadate was from Fluka Chemie AG, Buchs, Switzerland. Phenyl Sepharose CL-4B was from Pharmacia Biotech., Uppsala, Sweden. Dexamethasone was from Norwegian Medicinal Depot, Oslo, Norway, [γ-32P] Adenosine 5'-triphosphate (3000 Ci/mol) was from Amersham International, Buckinghamshire, England.
Isolation and culture of hepatocytes
Male Wistar rats (170–220 g) fed ad libitum were used. Parenchymal liver cells were isolated by in vitro collagenase perfusion and low-speed centrifugation [74] with modifications as previously described [75]. Cell viability was at least 95 %, measured as the ability to exclude trypan blue. The cells were suspended in medium and plated in Costar wells at 20.000 cells/cm2, unless otherwise specified. The culture medium (0.2 ml/cm2) was a 1:1 mixture of Dulbecco's modified Eagle's medium and Waymouth's medium MAB 87/3 containing 16.8 mM glucose [76], supplemented with penicillin (100 U/ml), streptomycin (0.1 mg/ml), dexamethasone (25 nM) and insulin (100 nM). The cultures were gassed with 95 % air/5 % CO2 and kept at 37°C.
Measurement of ERK activity
The measurement of ERK1/2 activity was performed as previously described [17,77]. In brief, the hepatocyte cultures were exposed to agonists for 5 minutes before rinsing and scraping the cells into a 10 % ethylene glycol buffer. The lysate was centrifugated (15,800 × g) for 10 min, and the supernatant was mixed with phenyl-Sepharose which was washed twice in a 10 %, twice in a 35 % ethylene glycol buffer, and finally ERK1/2 was eluted with a 60 % ethylene glycol buffer [78]. The eluate was assayed for ERK1/2 activity with MBP as substrate, thereafter spotted onto P81 paper (Whatman, Maidstone, UK), which was washed, dried and counted in a liquid scintillation counter. Protein content was determined with the BCA Protein Assay (Pierce, Rockford, IL, U.S.A.).
Immunoblotting
Aliquots with 20 μg cell protein (total cell lysate prepared in Laemmli buffer) were electrophoresed on 10 % polyacrylamide gels (acrylamide:N'N'-bis-methylene acrylamide 30:0.8) followed by protein electrotransfer to nitrocellulose membranes and immunoblotting with a polyclonal ERK1/2 antibody against the dually threonine- and tyrosine phosphorylated forms of ERK1 and ERK2 (Promega Corporation, Madison, WI). Assessment of the multifunctional Ca2+/calmodulin dependent protein kinase was performed by immunoblotting using an antibody against the phosphorylated from of the enzyme. Immunoreactive bands were visualised with ECL Western blotting detection reagents (Amersham International).
Measurement of cytosolic Ca2+ in single hepatocytes
The calcium measurements were done as described previously [20,79]. Freshly isolated hepatocytes (50.000/cm2) were plated onto glass coverslips coated with collagen and kept in the culture medium for 30 minutes in an atmosphere of 95 % air/5 % CO2 at 37°C. The cells were loaded in Krebs-Ringer-Hepes buffer (KRH) with 1 % albumin and 16.8 mM glucose, supplemented with 5 μM fura-2 AM, 0.25 % (v/v) DMSO, 0.025 % Pluronic F-127 and 250 μM sulfinpyrazone for 90 minutes at 37°C. After loading, the cells were washed once and incubated with 400 μl KRH buffer with 16.8 mM glucose. Cells were preincubated with timolol (10 μM) in the presence or absence of EGTA (5 mM) for 15 minutes after loading and the experiments were performed in the same buffer. Preincubation with timolol (10 μM) and DMSO or BAPTA-AM (40 μM) was performed within the last 25 minutes of the loading period. 100 μl norepinephrine was injected after 60 seconds of registration to a final concentration of 10 μM. The experiments were carried out at 37°C. Single cell Ca2+ measurements were based on the ratios of the fluorescence with excitation at 345 and 385 nm. Because fura-2 AM is partly compartmentalized in hepatocytes after loading, we did not calculate apparant values for [Ca2+]i from the ratios. The equipment consisted of a PTI-Δ-scan excitation device, a Nikon inverted microscope, a Hamamatsu CCD video camera and a Sony video recorder.
List of abbreviations
BAPTA-AM, l,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid tetra (acetoxymethyl)ester; EGF, epidermal growth factor; EGTA, ethyleneglycol-bis(β-aminoethyl)-N,N,N',N'-tetraacetic acid; ERK1/2, extracellular signal-regulated kinase 1 and 2; Gd, gadolinium chloride (hexahydrate); GPCR, G protein-coupled receptor; J-8; (N-8-Aminooctyl)-5-iodo-1-naphtalenesulfonamide; InsP3, inositol (l,4,5)-trisphosphate; KN-93, 2-[N-(2-hydroxyethyl)-N-(4-methoxybenzenesulfonyl)] amino-N-(4-chlorocinnamyl)-N-methylbenzylamine; MBP, myelin basic protein; NE, norepinephrine; PGF2α, prostaglandin F2α ; PP1, 4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo [3,4-d]pyrimidine; PP2, 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo [3,4-d]pyrimidine; RTK, receptor tyrosine kinase. TFP, trifluoperazine dimaleate; Thaps, thapsigargin; TPA, 12-O-tetradecanoyl phorbol-13-acetate; W-7, N-(6-aminohexyl)-5-chloro-1-naphtalenesulfonamide.
Acknowledgments
Acknowledgements
This work was supported by The Norwegian Cancer Society, The Research Council of Norway, and the Novo Nordisk Foundation. We thank Eva Østby and Ellen Johanne Johansen for technical help and John-Arne Røttingen for helpful discussions
Contributor Information
Øyvind Melien, Email: oyvind.melien@c2i.net.
Laila S Nilssen, Email: l.d.s.nilssen@labmed.uio.no.
Olav F Dajani, Email: olav.dajani@labmed.uio.no.
Kristin Larsen Sand, Email: k.s.larsen@basalmed.uio.no.
Jens-Gustav Iversen, Email: i.g.h.iversen@basalmed.uio.no.
Dagny L Sandnes, Email: d.l.sandnes@labmed.uio.no.
Thoralf Christoffersen, Email: thoralf.christoffersen@labmed.uio.no.
References
- Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/er.22.2.153. [DOI] [PubMed] [Google Scholar]
- Talarmin H, Rescan C, Cariou S, Glaise D, Zanninelli G, Bilodeau M, Loyer P, Guguen-Guillouzo C, Baffet G. The mitogen-activated protein kinase kinase/extracellular signal-regulated kinase cascade activation is a key signalling pathway involved in the regulation of G(1) phase progression in proliferating hepatocytes. Mol Cell Biol. 1999;19:6003–6011. doi: 10.1128/mcb.19.9.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkind JS. Cell growth control by G protein-coupled receptors: from signal transduction to signal integration. Oncogene. 1998;17:1331–1342. doi: 10.1038/sj.onc.1202186. [DOI] [PubMed] [Google Scholar]
- Chao TS, Byron KL, Lee KM, Villereal M, Rosner MR. Activation of MAP kinases by calcium-dependent and calcium-independent pathways. Stimulation by thapsigargin and epidermal growth factor. J Biol Chem. 1992;267:19876–19883. [PubMed] [Google Scholar]
- Schliess F, Sinning R, Fischer R, Schmalenbach C, Haussinger D. Calcium-dependent activation of Erk-1 and Erk-2 after hypo-osmotic astrocyte swelling. Biochem J. 1996;320:167–171. doi: 10.1042/bj3200167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enslen H, Tokumitsu H, Stork PJ, Davis RJ, Soderling TR. Regulation of mitogen-activated protein kinases by a calcium/calmodulin-dependent protein kinase cascade. Proc Natl Acad Sci USA. 1996;93:10803–10808. doi: 10.1073/pnas.93.20.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthalif MM, Benter IF, Uddin MR, Harper JL, Malik KU. Signal transduction mechanisms involved in angiotensin-(1-7)-stimulated arachidonic acid release and prostanoid synthesis in rabbit aortic smooth muscle cells. J Pharmacol Exp Ther. 1998;284:388–398. [PubMed] [Google Scholar]
- Muthalif MM, Benter IF, Uddin MR, Malik KU. Calcium/calmodulin-dependent protein kinase IIα mediates activation of mitogen-activated protein kinase and cytosolic phospholipase A2 in norepinephrine-induced arachidonic acid release in rabbit aortic smooth muscle cells. J Biol Chem. 1996;271:30149–30157. doi: 10.1074/jbc.271.47.30149. [DOI] [PubMed] [Google Scholar]
- Della Rocca GJ, van Biesen T, Daaka Y, Luttrell DK, Luttrell LM, Lefkowitz RJ. Ras-dependent mitogen-activated protein kinase activation by G protein-coupled receptors. Convergence of Gi- and Gq-mediated pathways on calcium/calmodulin, Pyk2, and Src kinase. J Biol Chem. 1997;272:19125–19132. doi: 10.1074/jbc.272.31.19125. [DOI] [PubMed] [Google Scholar]
- Munsch N, Gavaret JM, Pierre M. Ca2+ dependent purinergic regulation of p42 and p44 MAP kinases in astroglial cultured cells. Biomed & Pharmacother. 1998;52:180–186. doi: 10.1016/S0753-3322(98)80208-4. [DOI] [PubMed] [Google Scholar]
- Egea J, Espinet C, Comella JX. Calmodulin modulates mitogen-activated protein kinase activation in response to membrane depolarization in PC 12 cells. J Neurochem. 1998;70:2554–2564. doi: 10.1046/j.1471-4159.1998.70062554.x. [DOI] [PubMed] [Google Scholar]
- Daub H, Wallasch C, Lankenau A, Herrlich A, Ullrich A. Signal characteristics of G protein-transactivated EGF receptor. EMBO J. 1997;16:7032–7044. doi: 10.1093/emboj/16.23.7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi S, Iwasaki H, Inagarni T, Nurnaguchi K, Yarnakawa T, Motley ED, Owada KM, Marurno F, Hirata Y. Involvement of PYK2 in angiotensin II signaling of vascular smooth muscle cells. Hypertension. 1999;33:201–206. doi: 10.1161/01.hyp.33.1.201. [DOI] [PubMed] [Google Scholar]
- Romanelli A, van de Werve G. Activation of mitogen-activated protein kinase in freshly isolated rat hepatocytes by both a calcium- and a protein kinase C-dependent pathway. Metabolism. 1997;46:548–555. doi: 10.1016/s0026-0495(97)90193-1. [DOI] [PubMed] [Google Scholar]
- Spector MS, Auer KL, Jarvis WD, Ishac EJ, Gao B, Kunos G, Dent P. Differential regulation of the mitogen-activated protein and stress-activated protein kinase cascades by adrenergic agonists in quiescent and regenerating adult rat hepatocytes. Mol Cell Biol. 1997;17:3556–3565. doi: 10.1128/mcb.17.7.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melien Ø, Thoresen GH, Sandnes D, Østby E, Christoffersen T. Activation of p42/p44 mitogen-activated protein kinase by angiotensin II, vasopressin, norepinephrine, and prostaglandin F2α in hepatocytes is sustained, and like the effect of epidermal growth factor, mediated through pertussis toxin-sensitive mechanisms. J Cell Physiol. 1998;175:348–358. doi: 10.1002/(SICI)1097-4652(199806)175:3<348::AID-JCP13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Dajani OF, Sandnes D, Melien Ø, Rezvani F, Nilssen LS, Thoresen GH, Christoffersen T. Role of diacylglycerol (DAG) in hormonal induction of S phase in hepatocytes: the DAG-dependent protein kinase C pathway is not activated by epidermal growth factor (EGF), but is involved in mediating the enhancement of responsiveness to EGF by vasopressin, angiotensin II, and norepinephrine. J Cell Physiol. 1999;180:203–214. doi: 10.1002/(SICI)1097-4652(199908)180:2<203::AID-JCP8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Lynch CJ, Blackmore PF, Charest R, Exton JH. The relationships between receptor binding capacity for norepinephrine, angiotensin II, and vasopressin and release of inositol trisphosphate, Ca2+ mobilization, and phosphorylase activation in rat liver. Mol Pharmacol. 1985;28:93–99. [PubMed] [Google Scholar]
- Dajani OF, Røttingen JA, Sandnes D, Horn RS, Refsnes M, Thoresen GH, Iversen JG, Christoffersen T. Growth-promoting effects of Ca2+-mobilizing agents in hepatocytes: lack of correlation between the acute activation of phosphoinositide-specific phospholipase C and the stimulation of DNA synthesis by angiotensin II, vasopressin, norepinephrine, and prostaglandin F2α. J Cell Physiol. 1996;168:608–617. doi: 10.1002/(SICI)1097-4652(199609)168:3<608::AID-JCP13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Melien Ø, Sandnes D, Johansen EJ, Christoffersen T. Effects of pertussis toxin on ERK activation by hormones and receptor-independent agents: evidence suggesting a stimulatory role of Gi proteins at a level distal to receptor coupling. J Cell Physiol. 2000;184:27–36. doi: 10.1002/(SICI)1097-4652(200007)184:1<27::AID-JCP3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Bouscarel B, Augert G, Taylor SJ, Exton JH. Alterations in vasopressin and angiotensin-II receptors and responses during culture of liver cells. Biochim Biophys Acta. 1990;1055:265–272. doi: 10.1016/0167-4889(90)90042-C. [DOI] [PubMed] [Google Scholar]
- Sandnes DL, Dajani OF, Bjørneby A, Christoffersen T. The relationship between activation of phosphoinositide-specific phospholipase C and growth stimulation by Ca2+-mobilizing hormones in hepatocytes. Pharmacol Toxicol. 1999;84:234–240. doi: 10.1111/j.1600-0773.1999.tb01488.x. [DOI] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuschäfer-Rube F, DeVries C, Hänecke K, Jungermann K, Püschel GP. Molecular cloning and expression of a prostaglandin E2 receptor of the EP3β subtype from rat hepatocytes. FEBS Lett. 1994;351:119–122. doi: 10.1016/0014-5793(94)00837-X. [DOI] [PubMed] [Google Scholar]
- Neuschäfer-Rube F, Püschel GP, Jungermann K. Characterization of prostaglandin-F2α-binding sites on rat hepatocyte plasma membranes. Eur J Biochem. 1993;211:163–169. doi: 10.1111/j.1432-1033.1993.tb19883.x. [DOI] [PubMed] [Google Scholar]
- Bánhegyi G, Bellomo G, Fulceri R, Mandl J, Benedetti A. Intraluminal calcium of the liver endoplasmic reticulum stimulates the glucuronidation of p-nitrophenol. Biochem J. 1993;292:99–104. doi: 10.1042/bj2920099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney JA, Tsygankova OM, Yang L, Li Q, Szot A, Baysal K, Williamson JR. Activation of ERK by Ca2+ store depletion in rat liver epithelial cells. Am J Physiol. 1999;276:C221–C230. doi: 10.1152/ajpcell.1999.276.1.C221. [DOI] [PubMed] [Google Scholar]
- Fernando KC, Barritt GJ. Characterisation of the inhibition of the hepatocyte receptor-activated Ca2+ inflow system by gadolinium and SK&F 96365. Biochim Biophys Acta. 1994;1222:383–389. doi: 10.1016/0167-4889(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Benzeroual K, van de Werve G, Meloche S, Mathe L, Romanelli A, Haddad P. Insulin induces Ca2+ influx into isolated rat hepatocyte couplets. Am J Physiol. 1997;272:G1425–G1432. doi: 10.1152/ajpgi.1997.272.6.G1425. [DOI] [PubMed] [Google Scholar]
- Barritt GJ. Receptor-activated Ca2+ inflow in animal cells: a variety of pathways tailored to meet different intracellular Ca2+ signalling requirements. Biochem J. 1999;337:153–69. doi: 10.1042/0264-6021:3370153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel HJ. Calmodulin: a versatile calcium mediator protein. Biochem Cell Biol. 1994;72:357–376. [PubMed] [Google Scholar]
- Zhang M, Yuan T. Molecular mechanisms of calmodulin's functional versatility. Biochem Cell Biol. 1998;76:313–323. doi: 10.1139/bcb-76-2-3-313. [DOI] [PubMed] [Google Scholar]
- Eguchi S, Matsumoto T, Motley ED, Utsunomiya H, Inagami T. Identification of an essential signaling cascade for mitogen-activated protein kinase activation by angiotensin II in cultured rat vascular smooth muscle cells. Possible requirement of Gq-mediated p21ras activation coupled to a Ca2+/calmodulin-sensitive tyrosine kinase. J Biol Chem. 1996;271:14169–14175. doi: 10.1074/jbc.271.24.14169. [DOI] [PubMed] [Google Scholar]
- Bosch M, Gil J, Bachs O, Agell N. Calmodulin inhibitor W13 induces sustained activation of ERK2 and expression of p21cip1. J Biol Chem. 1998;273:22145–22150. doi: 10.1074/jbc.273.34.22145. [DOI] [PubMed] [Google Scholar]
- Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annu Rev Physiol. 1995;57:417–445. doi: 10.1146/annurev.physiol.57.1.417. [DOI] [PubMed] [Google Scholar]
- Yokokura H, Terada O, Naito Y, Sugita R, Hidaka H. Cascade activation of the calmodulin kinase family. Adv Second Messenger Phosphoprotein Res. 1997;31:151–157. doi: 10.1016/s1040-7952(97)80016-x. [DOI] [PubMed] [Google Scholar]
- Jurado LA, Chockalingam PS, Jarrett HW. Apocalmodulin. Physiol Rev. 1999;79:661–682. doi: 10.1152/physrev.1999.79.3.661. [DOI] [PubMed] [Google Scholar]
- MacKrill JJ. Protein-protein interactions in intracellular Ca2+-release channel function. Biochem J. 1999;337:345–361. doi: 10.1042/0264-6021:3370345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means AR. Regulatory cascades involving calmodulin-dependent protein kinases. Mol Endocrinol. 2000;14:4–13. doi: 10.1210/me.14.1.4. [DOI] [PubMed] [Google Scholar]
- Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, Hidaka H. The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem Biophys Res Commun. 1991;181:968–975. doi: 10.1016/0006-291x(91)92031-e. [DOI] [PubMed] [Google Scholar]
- Brown MT, Cooper JA. Regulation, substrates and functions of src. Biochim Biophys Acta. 1996;1287:121–149. doi: 10.1016/0304-419X(96)00003-0. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Hawes BE, van Biesen T, Luttrell DK, Lansing TJ, Lefkowitz RJ. Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gβγ subunit-mediated activation of mitogen-activated protein kinases. J Biol Chem. 1996;271:19443–19450. doi: 10.1074/jbc.271.21.12133. [DOI] [PubMed] [Google Scholar]
- Igishi T, Gutkind JS. Tyrosine kinases of the Src family participate in signaling to MAP kinase from both Gq and Gi-coupled receptors. Biochem Biophys Res Commun. 1998;244:5–10. doi: 10.1006/bbrc.1998.8208. [DOI] [PubMed] [Google Scholar]
- Della Rocca GJ, Maudsley S, Daaka Y, Lefkowitz RJ, Luttrell LM. Pleiotropic coupling of G protein-coupled receptors to the mitogen-activated protein kinase cascade. Role of focal adhesions and receptor tyrosine kinases. J Biol Chem. 1999;274:13978–13984. doi: 10.1074/jbc.274.20.13978. [DOI] [PubMed] [Google Scholar]
- Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- Murasawa S, Mori Y, Nozawa Y, Gotoh N, Shibuya M, Masaki H, Maruyama K, Tsutsumi Y, Moriguchi Y, Shibazaki Y, Tanaka Y, Iwasaka T, Inada M, Matsubara H. Angiotensin II type 1 receptor-induced extracellular signal-regulated protein kinase activation is mediated by Ca2+/calmodulin-dependent transactivation of epidermal growth factor receptor. Circ Res. 1998;82:1338–1348. doi: 10.1161/01.res.82.12.1338. [DOI] [PubMed] [Google Scholar]
- Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and src family-selective tyrosine kinase inhibitor. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- Lo YY, Luo L, McCulloch CA, Cruz TF. Requirements of focal adhesions and calcium fluxes for interleukin-1-induced ERK kinase activation and c-fos expression in fibroblasts. J Biol Chem. 1998;273:7059–7065. doi: 10.1074/jbc.273.12.7059. [DOI] [PubMed] [Google Scholar]
- Mulvaney JM, Zhang T, Fewtrell C, Roberson MS. Calcium influx through L-type channels is required for selective activation of extracellular signal-regulated kinase by gonadotropin-releasing hormone. J Biol Chem. 1999;274:29796–29804. doi: 10.1074/jbc.274.42.29796. [DOI] [PubMed] [Google Scholar]
- Schwarzschild MA, Cole RL, Meyers MA, Hyman SE. Contrasting calcium dependencies of SAPK and ERK activations by glutamate in cultured striatal neurons. J Neurochem. 1999;72:2248–2255. doi: 10.1046/j.1471-4159.1999.0722248.x. [DOI] [PubMed] [Google Scholar]
- Berts A, Zhong H, Minneman KP. No role for Ca2+ or protein kinase C in alpha-1A adrenergic receptor activation of mitogen-activated protein kinase pathways in transfected PC12 cells. Mol Pharmacol. 1999;55:296–303. doi: 10.1124/mol.55.2.296. [DOI] [PubMed] [Google Scholar]
- Wylie PG, Challiss RA, Blank JL. Regulation of extracellular-signal regulated kinase and c-Jun N-terminal kinase by G-protein-linked muscarinic acetylcholine receptors. Biochem J. 1999;338:619–628. doi: 10.1042/0264-6021:3380619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LJ, Guo YL, Trygankova O, Li QY, Maloney JA, Steinhauer M, Williamson JR. Epidermal growth factor and angiotensin II regulation of extracellular signal-regulated protein kinase in rat liver epithelial WB cells. Biochem Pharmacol. 1999;57:425–432. doi: 10.1016/S0006-2952(98)00308-6. [DOI] [PubMed] [Google Scholar]
- Putney JW. Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-N. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- Putneyr JW. TRP, inositol 1,4,5-trisphosphate receptors, and capacitative calcium entry. Proc Natl Acad Sci USA. 1999;96:14669–14671. doi: 10.1073/pnas.96.26.14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzigalinski BA, Willoughby KA, Hoffman SW, Faick JR, Ellis EF. Calcium influx factor, further evidence it is 5,6-epoxyeicosatrienic acid. J Biol Chem. 1999;274:175–182. doi: 10.1074/jbc.274.1.175. [DOI] [PubMed] [Google Scholar]
- Fernando KC, Barritt GJ. Evidence from studies with hepatocytes suspensions that store-operated Ca2+ inflow requires a pertussis toxin-sensitive trimeric G-protein. Biochem J. 1994;303:351–356. doi: 10.1042/bj3030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berven LA, Crouch MF, Katsis F, Kemp BE, Harland LM, Barritt GJ. Evidence that the pertussis toxin-sensitive trimeric GTP-binding protein Gi2 is required for agonist- and store-activated Ca2+ inflow in hepatocytes. J Biol Chem. 1995;270:25893–25897. doi: 10.1074/jbc.270.43.25893. [DOI] [PubMed] [Google Scholar]
- zu Heringdorf D Meyer, Lass H, Alemany R, Laser KT, Neumann E, Zhang C, Schmidt M, Rauen U, Jakobs KH, Koppen CJ. Sphingosine kinase-mediated Ca2+ signalling by G-protein-coupled receptors. EMBO J. 1998;77:2830–2837. doi: 10.1093/emboj/17.10.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus-Friedmann N. Hormonal regulation of cytosolic calcium levels in the liver. Braz J Med Biol Res. 1995;28:275–284. [PubMed] [Google Scholar]
- Lange J, Schlieps K, Lange K, Knoll-Köhler E. Activation of calcium signaling in isolated rat hepatocytes is accompanied by shape changes of microvilli. Exp Cell Res. 1997;234:486–497. doi: 10.1006/excr.1997.3652. [DOI] [PubMed] [Google Scholar]
- Piñol MR, Berchtold MW, Bachs O, Heizmann CW. Increased calmodulin synthesis in the pre-replicative phase of rat liver regeneration. FEBS Lett. 1988;231:445–450. doi: 10.1016/0014-5793(88)80868-8. [DOI] [PubMed] [Google Scholar]
- Niki I, Yokokura H, Sudo T, Kato M, Hidaka H. Ca2+ signaling and intracellular Ca2+ binding proteins. J Biochem (Tokyo) 1996;120:685–698. doi: 10.1093/oxfordjournals.jbchem.a021466. [DOI] [PubMed] [Google Scholar]
- Geiser JR, van Tuinen D, Brockerhoff SE, Neff MM, Davis TN. Can calmodulin function without binding calcium? Cell. 1991;65:949–959. doi: 10.1016/0092-8674(91)90547-c. [DOI] [PubMed] [Google Scholar]
- Goppelt-Struebe M, Hahn A, Stroebel M, Reiser CO. Independent regulation of cyclo-oxygenase 2 expression by p42/44 mitogen-activated protein kinases and Ca2+/calmodulin-dependent kinase. Biochem J. 1999;339:329–334. doi: 10.1042/0264-6021:3390329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Rocca GJ, Mukhin YV, Garnovskaya MN, Daaka Y, Clark GJ, Luttrell LM, Lefkowitz RJ, Raymond JR. Serotonin 5-HT1A receptor-mediated Erk activation requires calcium/calmodulin-dependent receptor endocytosis. J Biol Chem. 1999;274:4749–4753. doi: 10.1074/jbc.274.8.4749. [DOI] [PubMed] [Google Scholar]
- Lundberg MS, Curto KA, Bilato C, Monticone RE, Crow MT. Regulation of vascular smooth muscle migration by mitogen-activated protein kinase and calcium/calmodulin-dependent protein kinase II signaling pathways. J Mol Cell Cardiol. 1998;30:2377–2389. doi: 10.1006/jmcc.1998.0795. [DOI] [PubMed] [Google Scholar]
- Døskeland AP, Vintermyr OK, Flatmark T, Cotton RG, Døskeland SO. Phenylalanine positively modulates the cAMP-dependent phosphorylation and negatively modulates the vasopressin-induced and okadaic-acid-induced phosphorylation of phenylalanine 4-monooxygenase in intact rat hepatocytes. Eur J Biochem. 1992;206:161–170. doi: 10.1111/j.1432-1033.1992.tb16913.x. [DOI] [PubMed] [Google Scholar]
- Mellgren G, Bruland T, Døskeland AP, Flatmark T, Vintermyr OK, Døskeland SO. Synergistic antiproliferative actions of cyclic adenosine 3',5'-monophosphate, interleukin-1β, and activators of Ca2+/calmodulin-dependent protein kinase in primary hepatocytes. Endocrinology. 1997;138:4373–4383. doi: 10.1210/en.138.10.4373. [DOI] [PubMed] [Google Scholar]
- Velasco G, Guzman M, Zammit VA, Geelen MJ. Involvement of Ca2+/calmodulin-dependent protein kinase II in the activation of carnitine palmitoyltransferase I by okadaic acid in rat hepatocytes. Biochem J. 1997;321:211–216. doi: 10.1042/bj3210211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lin R, Hu ZW, Hoffman BB. α1-Adrenergic receptor activation of c-fos expression in transfected rat-1 fibroblasts: role of Ca2+. J Pharmacol Exp Ther. 1999;289:1376–1384. [PubMed] [Google Scholar]
- Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Christoffersen T, Refsnes M, Bronstad GO, Østby E, Huse J, Haffner F, Sand TE, Hunt NH, Sonne O. Changes in hormone responsiveness and cyclic AMP metabolism in rat hepatocytes during primary culture and effects of supplementing the medium with insulin and dexamethasone. Eur J Biochem. 1984;138:217–226. doi: 10.1111/j.1432-1033.1984.tb07904.x. [DOI] [PubMed] [Google Scholar]
- Dajani OF, Refsnes M, Guren T, Horn RS, Thoresen GH, Christoffersen T. Elevated glucose concentrations inhibit DNA synthesis and expression of c-myc in cultured hepatocytes. Biochem Biophys Res Commun. 1994;202:1476–1482. doi: 10.1006/bbrc.1994.2097. [DOI] [PubMed] [Google Scholar]
- Thoresen GH, Guren TK, Sandnes D, Peak M, Agius L, Christoffersen T. Response to transforming growth factor α (TGFα) and epidermal growth factor (EGF) in hepatocytes: Lower EGF receptor affinity of TGFα is associated with more sustained activation of p42/p44 mitogen-activated protein kinase and greater efficacy in stimulation of DNA synthesis. J Cell Physiol. 1998;175:10–18. doi: 10.1002/(SICI)1097-4652(199804)175:1<10::AID-JCP2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Anderson NG, Kilgour E, Sturgill TW. Activation of mitogen-activated protein kinase in BC3H1 myocytes by fluoroaluminate. J Biol Chem. 1991;266:10131–10135. [PubMed] [Google Scholar]
- Røttingen JA, Enden T, Camerer E, Iversen JG, Prydz H. Binding of human factor VIIa to tissue factor induces cytosolic Ca2+ signals in J82 cells, transfected COS-1 cells, Madin-Darby canine kidney cells and in human endothelial cells induced to synthesize tissue factor. J Biol Chem. 1995;270:4650–4660. doi: 10.1074/jbc.270.9.4650. [DOI] [PubMed] [Google Scholar]