Abstract
Isolated, seasonal wetlands within agricultural landscapes are important ecosystems. However, they are currently experiencing direct and indirect effects of agricultural management surrounding them. Because wetlands provide important ecosystem services, it is crucial to determine how these factors affect ecological communities. Here, we studied the long‐term effects of land‐use intensification, cattle grazing, prescribed fires, and their interactions on wetland plant diversity, community dynamics, and functional diversity. To do this, we used vegetation and trait data from a 14‐year‐old experiment on 40 seasonal wetlands located within seminatural and intensively managed pastures in Florida. These wetlands were allocated different grazing and prescribed fire treatments (grazed vs. ungrazed, burned vs. unburned). Our results showed that wetlands within intensively managed pastures have lower native plant diversity, floristic quality, evenness, and higher nonnative species diversity and exhibited the most resource‐acquisitive traits. Wetlands embedded in intensively managed pastures were also characterized by lower species turnover over time. We found that 14 years of cattle exclusion reduced species diversity in both pasture management intensities and had no effect on floristic quality. Fenced wetlands exhibited lower functional diversity and experienced a higher rate of community change, both due to an increase in tall, clonal, and palatable grasses. The effects of prescribed fires were often dependent on grazing treatment. For instance, prescribed fires increased functional diversity in fenced wetlands but not in grazed wetlands. Our study suggests that cattle exclusion and prescribed fires are not enough to restore wetlands in intensively managed pastures and further highlights the importance of not converting seminatural pastures to intensively managed pastures. Our study also suggests that grazing levels applied in seminatural pastures maintained high plant diversity and prevented tree and shrub encroachment and that in the absence of grazing, prescribed fire became crucial to maintaining higher species evenness.
Keywords: coefficient of conservatism, functional dispersion, long‐term experiment, management intensification, nonnative species, plant diversity, plant traits
INTRODUCTION
Wetland communities are important because they provide multiple ecosystem services whose economic value is immense and they are sensitive to anthropogenic management and land‐use change (Costanza et al., 1997, 2014; de Groot et al., 2012). In many parts of the world, small isolated wetlands comprise a significant part of the landscape, especially in grazing lands, which occupy 25% of the global land surface (Asner et al., 2004). Despite their importance, the world has lost a large percentage of wetlands, and the wetlands that remain are often heavily degraded by human activities (Brinson & Malvárez, 2002; Dahl, 2014; Junk et al., 2013; Zedler & Kercher, 2005). Partial or total drainage, eutrophication, pollution, conversion of surrounding uplands to urban landscape, and changes in disturbance regime are only a few factors threatening the integrity of natural wetlands. Wetlands embedded in grazing lands are particularly at risk because many of these factors can be at play, resulting in long‐lasting changes in plant composition and richness (Boughton et al., 2011, 2016), with considerable effects on wetland functioning (Cohen et al., 2016; Collins et al., 2014; Gerakis & Kalburtji, 1998).
Although the impact of livestock grazing on uplands has been studied in depth and synthesized (Cingolani, Noy‐Meir, & Diaz, 2005; Milchunas et al., 1988; Olff & Ritchie, 1998), the body of literature on livestock grazing impacts on wetlands is more limited (but see Boughton et al., 2011; Bovee et al., 2018; Marty, 2005), especially in subtropical regions. This is particularly the case when grazing is combined with other management practices, such as the fertilization of surrounding uplands, which may interact with grazing to affect wetlands (Boughton et al., 2016). For example, grazing has been shown to increase grassland diversity in nutrient‐rich ecosystems, but it may decrease diversity in nutrient‐poor ecosystems (Bakker et al., 2006; Koerner et al., 2018). As such, wetlands that receive nutrient runoff from fertilized pastures may respond differently to cattle grazing than wetlands that did not.
In Central Florida, ranchers rely on a matrix of intensively managed pastures (IMPs) and extensively managed seminatural pastures (SNPs) to remain economically viable (Swain et al., 2013), with important consequences for embedded wetlands. Typically, IMPs are fertilized, drained by a dense ditch network, and planted with nonnative productive grasses, with Bahiagrass (Paspalum notatum Flueggé) being the most common across Florida ranches. Wetlands embedded in IMPs are not directly fertilized but receive nutrient runoff as indicated by sediment enrichment and increased nutrients in water (Bohlen & Gathumbi, 2007; Jansen et al., 2019). In contrast, SNPs are never fertilized, include few drainage ditches, and are seldom planted with nonnative productive grasses. Thus, SNPs retain most of their native vegetation (Swain et al., 2013). SNPs usually experience a lower stocking rate than IMP.
Prescribed fire is another common practice used by ranch managers in Florida. Ranch managers usually use it to stimulate forage production, forage digestibility, and weed control and apply it in both uplands and wetlands. For conservationists, it is viewed as a tool to mimic historical lightning‐ignited wildfires, which occur regularly in Central and South Florida ecosystems (Main & Barry, 2002; Menges & Kohfeldt, 1995; Slocum et al., 2003). Frequent fires promote fire‐adapted species with high conservative value and have been shown to enhance wetland species diversity and reproductive output, at least in the short term (Main & Barry, 2002; Marty, 2015a). However, in a recent study, Boughton et al. (2016) found that the effects of prescribed fire on plant diversity depended on pasture management intensity and grazing treatment. For example, they found that prescribed fire was important to maintain evenness in fenced wetland in SNPs, but it did not significantly affect the diversity of wetlands in IMPs. However, this study analyzed shifts in wetland plant communities in the short term (3 years of cattle exclusion 2007–2009) and following a single prescribed fire event (2008).
Our study built upon the short‐term analysis conducted by Boughton et al. (2016). Here, we extended this study to include vegetation data collected up to year 2020, representing 14 years of cattle exclusion and multiple prescribed fire events (wetlands were burned every 2–3 years). We also extended this study to include multiple responses of the plant community, utilizing species number, multivariate metrics, and functional diversity and composition to provide a comprehensive picture of community responses to multiple management factors. To our knowledge, this represents one of the longest running experiments testing the effects of cattle removal, management intensity, and prescribed fires on 40 whole wetlands. Using this expanded data set, we determined the long‐term changes in plant diversity (native and nonnative), evenness (Pielou, 1974), and floristic quality (measured as the mean coefficient of conservatism) (Spyreas, 2019) and assessed whether these differed from their short‐term response. In agreement with Boughton et al. (2016), we expected cattle exclusion to decrease evenness and floristic quality in SNPs. Contrary to Boughton et al. (2016), we expected cattle exclusion to decrease the evenness and floristic quality of wetlands in IMPs over the long term. The short‐term study found no main effect of cattle exclusion on native richness. Here, we expected that, over the long term, excluding cattle would increase native plant diversity in wetlands in SNPs but decrease it in wetlands in IMPs, where higher nutrient levels may promote invasive, nonnative plants and a few tall plant species without grazing (Cingolani, Noy‐Meir, & Diaz, 2005; Milchunas et al., 1988). Because our data set spanned 14 years, we were able to assess species compositional changes through time by measuring the nondirectional species' reordering (mean rank shift [MRS]) and the directional rate of community change (Collins et al., 2008; Jones et al., 2017). We hypothesized that species' reordering would be higher in wetlands within SNPs compared to wetlands in IMPs because stochastic factors in relation to dispersal were more important in wetlands within SNPs (Boughton et al., 2010; Medley et al., 2015). We expected the rate of community change to be higher in fenced wetlands compared to grazed wetlands because plant communities switched from vegetation dominated by unpalatable species to vegetation dominated by palatable grasses following fencing (Sonnier et al., 2020). Finally, using trait data, we determined whether pasture management, grazing, and fire affected functional composition and functional diversity. We hypothesized that removing cattle would decrease functional diversity independently of its effect on species richness by selecting for species expressing traits associated with high competitive ability (i.e., high specific leaf area [SLA] and low leaf dry matter content [LDMC]) (Peco et al., 2012).
METHODS
Study system
We studied seasonal wetlands on Buck Island Ranch (BIR), a 4249‐ha cattle ranch located in South Central Florida. BIR is a full‐scale commercial cow–calf operation run by Archbold Biological Station used as a platform for ecological research. The climate is subtropical humid (Köppen climate classification) with a clear dry season (from November to May), with 60% of the 132‐cm/year mean precipitation falling during the summer rainy season. BIR is a part of Archbold University of Florida Long‐Term Agroecosystem Research (LTAR), one of 18 sites within the USDA's LTAR network, and this experiment is a component of BIR's LTAR Common Experiment with data contributed to a national‐scale analysis of sustainable agriculture (Kleinman et al., 2018; Spiegal et al., 2018).
All pastures have been grazed for at least 75 years, but not all at the same intensity. The history of the ranch's management is well documented. Ranch pastures are divided into IMPs and SNPs. To sustain high productivity in IMPs, the pastures were fertilized annually or semiannually with N, P, and K from the early 1970s to 1987 (56 kg/ha as NH4SO4 or NH4NO3 and 34–90 kg/ha of P2O5 and K2O). P fertilization was stopped after 1987 to reduce P runoff downstream. SNPs have been neither fertilized nor heavily seeded. As such, the SNPs retained a large proportion of their native species. Rotational grazing is implemented throughout the ranch with on average 2.3 months of rest in IMPs and 6.6 months of rest in SNPs. Cattle‐stocking density is on average lower in SNPs (mean 0.47 cow–calf pairs/ha) compared to IMPs (mean 1.11 cow–calf pairs/ha) (average 2014–2020).
Experimental design
The ranch has about 600 seasonally flooded wetlands of varying size and shape and with hydroperiods ranging from 2 to 10 months (Steinman et al., 2003). They are all embedded within pastures, so they are heavily influenced by the management of the pastures surrounding them. In 2006, we selected 40 seasonally flooded wetlands (Boughton et al., 2011, 2016; Kelly et al., 2015; Medley et al., 2015). Twenty wetlands were within IMPs and 20 within SNPs. In early 2007, we fenced 20 wetlands (10 within IMPs and 10 within SNPs) to prevent cattle grazing while allowing access by deer and feral pigs. In early 2008, we exposed 20 of the 40 wetlands to prescribed fire and subsequently burned them every 2–3 years during the dry season depending on burning permit authorization. It was sometimes not possible to burn all 20 wetlands within the same year, in which case remaining wetlands were burned the following year (Appendix S1: Table S1). We also noted when a wetland had been accidentally burned. Wetland allocated to the different grazing and prescribed fire treatments did not differ significantly in terms of size (~0.79 ha), hydroperiod (~4.65 months), and water depth (~61.92 cm) prior to the onset of the experiment (Appendix S1: Tables S2–S4). This resulted in a 2 × 2 × 2 complete factorial design, where treatments were all combinations of management intensity (IMP vs. SNP), cattle exclosure (grazed vs. fenced), and prescribed fire (burned vs. unburned). Each of the eight treatments were replicated five times. Note that management intensity also implies different stocking rates between IMPs (mean 1.11 cow–calf pairs/ha) and SNPs (mean 0.47 cow–calf pairs/ha). Additionally, although these wetlands may be used as watering resources for cattle, all pastures also included one or more water troughs with water pumped from the aquifer belowground. Cattle preferentially use water troughs over wetlands for water resources.
Plant community surveys
We surveyed plant communities in each of these wetlands at the end of the growing season (September–October) every year between 2006 and 2016 and every 2 years after 2016. The only exception was 2011 when only a subset of the wetlands were surveyed. Thus, we excluded 2011 from the analyzes. We separated each wetland into five sectors of equal size (Center, South, North, East, and West sectors), and we placed three 1‐m2 circular plots at random locations within each sector, resulting in 15 plots per wetland. The location of the plots was reshuffled every year; hence, our survey does not rely on permanent plots. We recorded the presence of all vascular plant species in each of the 15 plots. We identified individuals to the species level or, when not possible, to the genus level. Any occurrence that could not be identified to species or genus level were removed from the analysis. We standardized species name to account for changes in nomenclature through time. We compiled native/nonnative status from the literature (https://plants.usda.gov, http://florida.plantatlas.usf.edu). Based on these surveys, we calculated species richness (native and nonnative) at both plot and wetland levels each sampling year. We also computed the relative incidence of each species in each wetland (i.e., the number of plots a particular species was observed in) and calculated the exponential of Shannon diversity (Jost, 2010), and species evenness (Pielou, 1974).
Plant functional traits and coefficient of conservatism values
In 2016 and 2017, we measured plant height (cm), LDMC (mg/g), and SLA (cm2/g) on the most frequent and abundant species observed in the 40 wetlands. We ranked species in each wetland based on their biomass observed in 2016 biomass surveys (Sonnier et al., 2020) and tried to measure the traits of species that together made up at least 80% of the vegetation of each wetland (Pakeman & Quested, 2007). This threshold was reached in most wetlands. Formally, we sampled six individuals of each species in at least three wetlands and stratified the sampling to account for intraspecific variation due to treatment. For example, if a species occurred in both fenced and grazed wetlands within IMPs, then the species was collected in three fenced wetlands and in three grazed wetlands. We measured both vegetative plant height and maximum plant height in the field. We then collected young but mature leaves from each of the individuals to estimate SLA (= leaf area/dry mass) and LDMC (= dry mass/fresh mass). We followed standardized protocols (Pérez‐Harguindeguy et al., 2013) and made sure that leaves were fully hydrated before measurements of fresh mass. Trait data were submitted to the TRY database and are open access (Kattge et al., 2020). Based on these measurements, we calculated the mean value of each trait for each species.
We also compiled coefficient of conservatism values from the literature. Coefficient of conservatism is a measure of plant fidelity to specific habitats and plant tolerance to disturbance and it separates ubiquitous species (low coefficient of conservatism) from habitat specialists (high coefficient of conservatism). We used the classification proposed by Mortellaro et al. (2012) organized on a 1–10 scale and attributed a zero value to nonnative invasive species.
Statistical analyses
All analyses were performed in R (R Core Team, 2020) using the RStudio platform (RStudio Team, 2020) and tidyverse library (Wickham et al., 2019) for data manipulation formatting and plotting. We combined trait data and community surveys by calculating community weighted mean values of each trait in each wetland for each year of data. We also calculated the functional dispersion (Laliberté & Legendre, 2010) in each of the wetlands using the FD package (Laliberté et al., 2014). We used all three traits and Gower's distance to calculate the dissimilarity in trait attributes between species. Finally, we calculated the mean coefficient of conservatism in each wetland and for each year of data.
To investigate temporal shifts in species composition, we calculated the MRS (Collins et al., 2008) and the rate of community change through time (Collins et al., 2000) available in the codyn package (Hallett et al., 2016). The MRS indicates the degree of species reordering between time points. MRS is calculated as follows:
where N is the number of species in common at both time points, t is the time point, and R i,t is the relative rank of species i at time t. The rate of community change measures both the rate and direction of community change. It is based on pairwise distances across the entire time series, which are regressed against the time lag interval with the rate of change given by the slope of this relationship.
We related each species diversity metric (total species richness, native richness, nonnative richness, exponential of Shannon diversity, mean coefficient of conservatism), community‐weighted mean of each trait, and functional diversity to pasture management intensity, cattle exclusion, prescribed fires, and their interactions using general linear mixed models. For these models, we used a Gaussian family distribution, and we used wetland ID and year as a random intercept terms in each of the models. We built similar models to test the effect of treatments on nonnative incidence, forb, graminoid, and shrub relative frequencies as well as species‐specific responses (e.g., Panicum hemitomon Schult and Juncus effusus L. var. solutus Fernald & Wiegand). However, for these models, we used the beta family distribution. To test the effect of treatments on rate of community change and MRS (which combined compositional data from the entire time series), we used simple linear models with pasture type, grazing treatment, burning treatment, and their interactions as explanatory variables.
For each model, we started with the full model with all interactions and dropped interaction terms based on likelihood ratio test results, but we kept all three main effects in the final model. We used the lme4 package (Bates et al., 2015) for general linear mixed models with Gaussian distribution, the glmmTMB package for models with a beta distribution (Brooks et al., 2017), and the sjPlot package for visualization of the results and diagnostic plots (Lüdecke, 2021).
RESULTS
Wetland diversity
We observed 215 plant species throughout the 14 years of experiments with 189 species occurring more than once throughout the experiment. Among these 189 observations, 18 were only identified to the genus level and some genera corresponded to groups of species that were lumped together because they were hard to separate from each other when found in vegetative form. Forbs represented ~50% of all recorded species, whereas grasses and sedges together represented ~39%. The most diverse families were Poaceae (38), Cyperaceae (32), and Asteraceae (19). We recorded 23 nonnative species, the most common being Alternanthera philoxeroides (Mart.) Griseb., P. notatum, Paspalum accuminatum Raddi, and Panicum repens L. The plant species occurring most often across wetlands and sampling years were P. hemitomon, Centella asiatica (L.) Urb., Persicaria punctata (Elliott) Small, Leersia hexandra Sw., A. philoxeroides, Hydrocotyle umbellata L., Pontederia cordata L., and J. effusus. In IMP wetlands, species occurring the most often were J. effusus, A. philoxeroides, P. punctata, and P. hemitomon. In SNP wetlands, these were P. hemitomon, Rhynchospora inundata (Oakes) Fernald, C. asiatica, and Diodia virginiana L.
Species diversity in response to treatments
Species richness varied fivefold between wetlands ranging from 11 to 52 species, with, on average, 29.46 species per wetland. Using data from 2008 to 2020 (years when all treatments were in place), we observed significantly more species in wetlands embedded in SNPs (estimated mean 32.77, confidence interval at 95% [CI95%] [30.29, 35.25]) than in wetlands in IMPs (mean 25.99, CI95% [23.50, 28.47]) (Figure 1; Appendix S2: Table S1). We also observed significantly more species in grazed wetlands (mean 31.01, CI95% [28.53, 33.49]) than in fenced wetlands (mean 27.75, CI95% [25.26, 30.23]) (Figure 1, Appendix S2: Table S1). A similar pattern was observed with native species, which represent most of the species found in these wetlands (Figure 1). In contrast, we observed more nonnative species in wetlands within IMPs (mean 4.55, CI95% [4.06, 5.04]) than in SNPs (mean 2.50, CI95% [1.99, 3.00]) and no effect of grazing on nonnative species richness (Figure 1; Appendix S2: Table S1). Nonnative species were also more frequent in wetlands within IMPs (mean 23.93%, CI95% [20.72, 27.15]) compared to wetlands in SNPs (mean 6.42%, CI95% [3.21, 9.64]). Prescribed fire did not have a significant effect on native or nonnative species richness.
FIGURE 1.
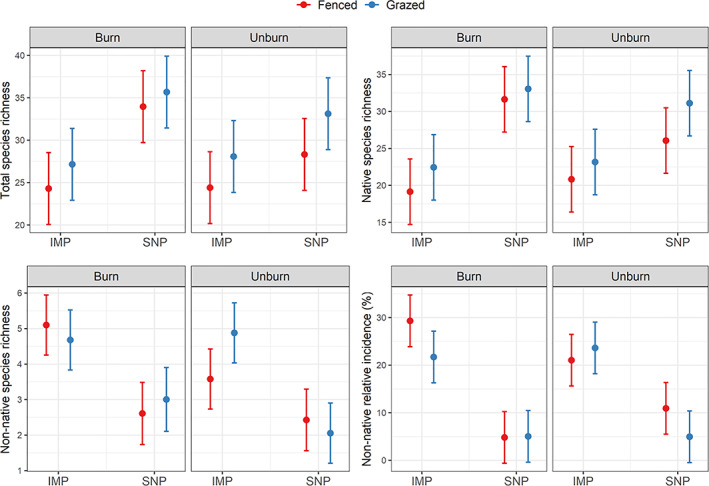
Effects of pasture type (intensively managed pasture [IMP] vs. seminatural pasture [SNP]), grazing (fenced vs. grazed), and prescribed fire (burn vs. unburn) on the total species richness, native richness, nonnative richness, and nonnative incidence (%) of Florida seasonal wetlands. Each panel provides the predicted mean (dot) and predicted confidence interval at 95% (error bar) associated with each combination of treatment and for each diversity metric.
The exponential Shannon diversity index (H′) calculated using incidence data (i.e., number of times a species was observed in a wetland out of 15 plots) varied from 7.42 to 39 equivalent species, with, on average, 21.34 species per wetland. H′ was higher in wetlands embedded in SNPs (mean 23.98, CI95% [22.08, 25.88]) than in wetlands in IMPs (mean 18.39, CI95% [16.49, 20.29]) and higher in grazed wetlands (mean 23.98, CI95% [22.08, 25.88]) compared to fenced wetlands (mean 19.81, CI95% [17.91, 21.72]) (Figure 2, Appendix S2: Table S2).
FIGURE 2.
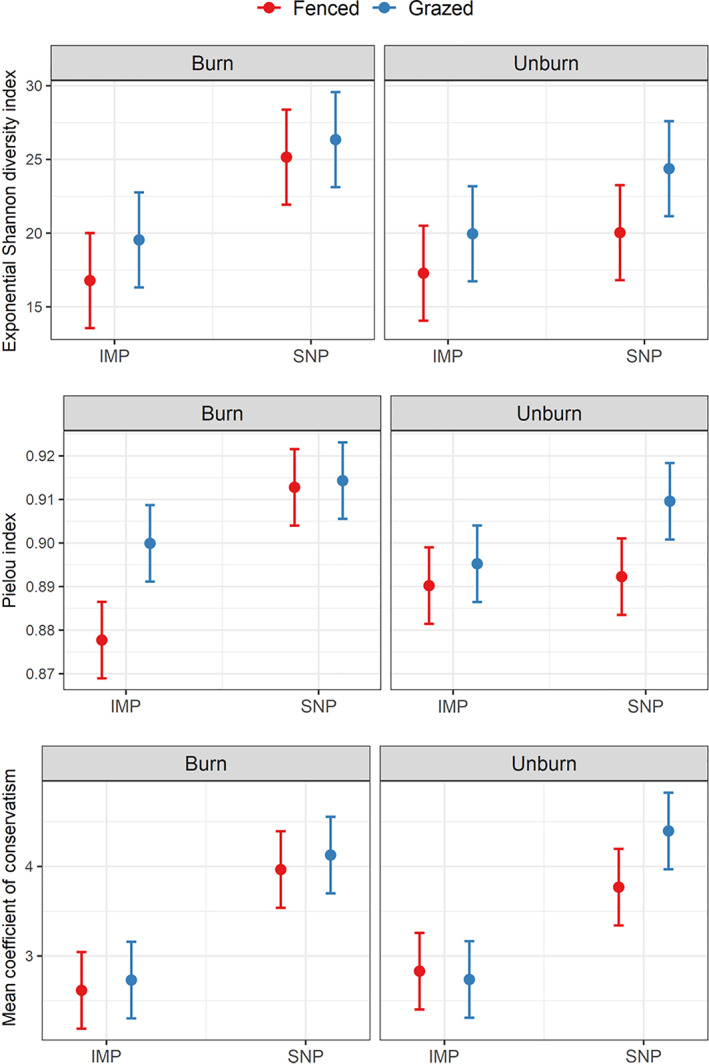
Effects of pasture type (intensively managed pasture [IMP] vs. seminatural pasture [SNP]), grazing (fenced vs. grazed), and prescribed fire (burned vs. unburned) on the exponential of Shannon diversity, Pielou evenness index, and mean coefficient of conservatism. Each panel provides the predicted mean (dot) and predicted confidence interval at 95% (error bar) associated with each combination of treatment and for each diversity metric.
We observed a significant three‐way interaction between the three treatments on species evenness (measured using Pielou's index, Figure 2; Appendix S2: Table S2). Overall, evenness was higher in wetlands in SNPs, unless the wetlands were both fenced and left unburned, in which case wetlands in both IMPs and SNPs had similar evenness (Figure 2). Prescribed fire generally increased evenness, but when combined with fencing it decreased evenness in wetlands in IMPs.
Species floristic composition in response to treatment
Forbs were more frequent in wetlands in IMPs (mean 55.74%, CI95% [53.42, 58.05]) than in wetlands in SNPs (mean 48.98%, CI95% [46.66, 51.30]) (Figure 3; Appendix S2: Table S3). Conversely, graminoids were more common in wetlands in SNPs (mean 47.38%, CI95% [45.05, 49.70]) than in wetlands in IMPs (mean 42.24%, CI95% [39.92, 44.57]). Trees and shrubs were more common in SNPs (mean 2.66%, CI95% [1.85, 3.47] vs. mean 0.98%, CI95% [0.17, 1.79]) and in fenced wetlands (mean 2.36%, CI95% [1.55, 3.17]) compared to grazed wetlands (mean 1.28%, CI95% [0.47, 2.09]).
FIGURE 3.
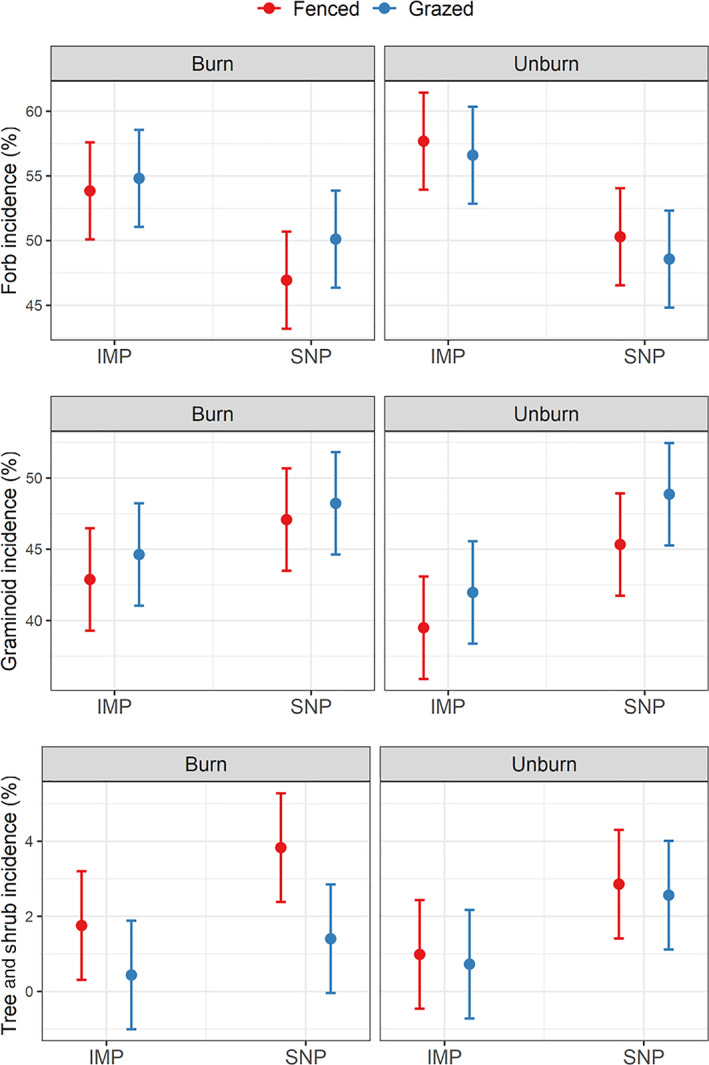
Effects of pasture type (intensively managed pasture [IMP] vs. seminatural pasture [SNP]), grazing (fenced vs. grazed), and prescribed fire (burned vs. unburned) on forb incidence (%), graminoid (%), and tree + shrub incidence (%). Each panel provides the predicted mean (dot) and predicted confidence interval at 95% (error bar) associated with each combination of treatment and for each diversity metric.
Floristic quality (unitless and measured as the mean coefficient of conservatism) was significantly higher in wetlands embedded in SNPs (mean 4.06, CI95% [3.83, 4.30] vs. mean 2.73, CI95% [2.49, 2.97] in IMP), but it was not affected by grazing or burning treatments (Figure 2; Appendix S2: Table S2).
Temporal change in response to treatments
We calculated the rate of compositional change through time (unitless) in each of the wetlands (Figure 4; Appendix S2: Table S4). We observed that the rate of change was higher in fenced wetlands (mean 0.60, CI95% [0.49, 0.71]) than in grazed wetlands (mean 0.23, CI95% [0.12, 0.34]) and that neither prescribed fire nor management intensity influenced species compositional change through time. Additionally, we calculated the MRS (unitless) in each wetland indicating the degree of species reordering between two time points. We observed that MRS was higher in wetlands within SNPs (mean 4.40, CI95% [4.03, 4.78] vs. mean 3.21, CI95% [2.83, 3.58] in IMP) and in grazed wetlands (mean 4.07, CI95% [3.69, 4.44] vs. mean 3.54, CI95% [3.17, 3.92]).
FIGURE 4.
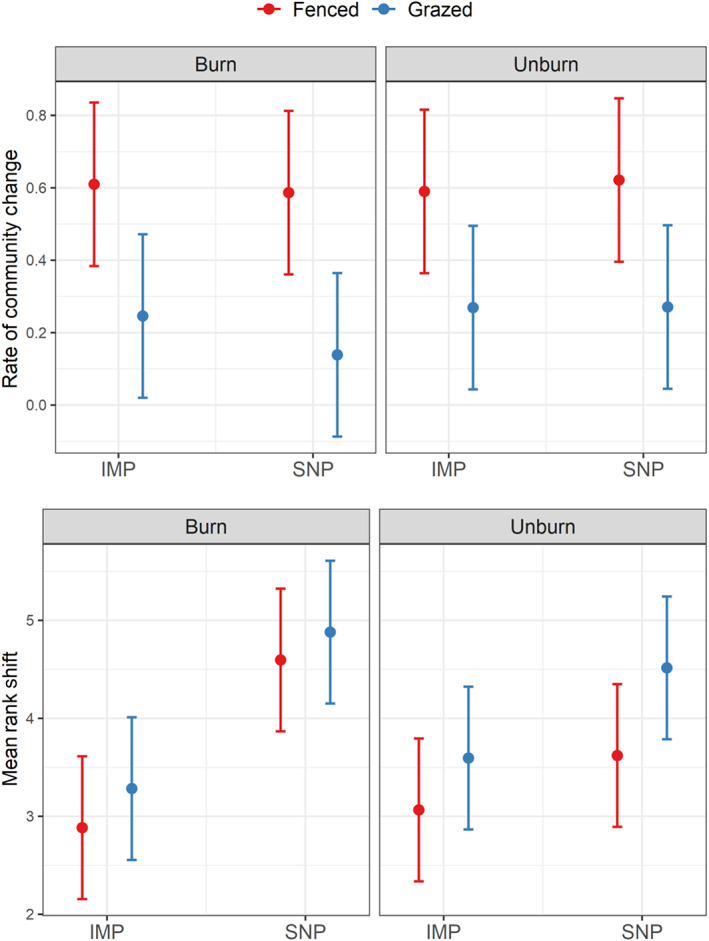
Effects of pasture type (intensively managed pasture [IMP] vs. seminatural pasture [SNP]), grazing (fenced vs. grazed), and prescribed fire (burned vs. unburned) on rate of community change (unitless), and mean rank shift (unitless). Each panel provides predicted mean (dot) and predicted confidence interval at 95% (error bar) associated with each combination of treatment and for each diversity metric.
We used rank clock plots to describe the changes in the dominance of the four most frequent species in wetlands in IMPs and SNPs (Figure 5). The relative incidence of P. hemitomon (the most frequent grass species in both wetland types) increased through time in fenced wetlands, especially in IMPs. P. hemitomon's relative incidence was significantly higher in fenced wetlands (mean frequency 61.54%, CI95% [51.86, 70.38] vs. mean 43.66%, CI95% [34.29, 53.51]). J. effusus, which occurred primarily in wetlands within IMPs (46.77%), was influenced by both grazing exclusion and prescribed fire. The frequency of J. effusus was higher in grazed wetlands (mean 69.31%, CI95% [55.65, 80.25] vs. mean 25.75%, CI95% [16.07, 38.58]) and higher in unburned wetlands (mean 56.36%, CI95% [41.78, 69.92], mean 37.75%, CI95% [25.08, 52.34]). We also observed that cattle exclusion reduced the incidence of the small prostrate forbs A. philoxeroides, C. asiatica, and D. virginiana. We explored the response of the two most abundant shrub species, Cephalanthus occidentalis L. and Baccharis halimifolia L. C. occidentalis occurred primarily in wetlands in SNPs, and its occurrence was higher in burned wetlands. B. halimifolia's abundance was higher when cattle were excluded in both SNPs and IMPs wetlands.
FIGURE 5.
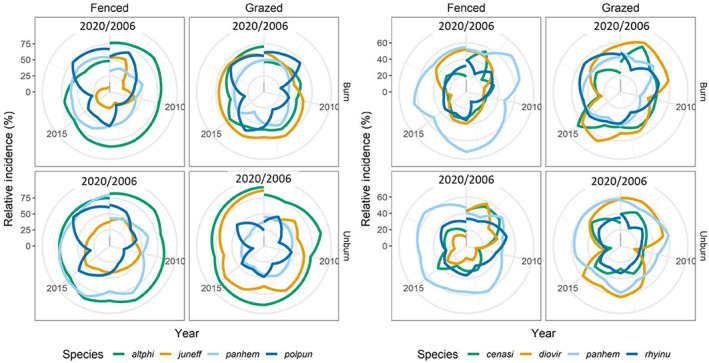
Rank clock plots of four most common wetland species found in intensively managed pasture (IMP) (left panel) and in seminatural pasture (SNP) (right panel). Plot shows change in mean relative incidence observed from 2006 to 2020 in each treatment. Species are as follows: althpi (Althernanthera philoxeroides), juneff (Juncus effusus), panhem (Panicum hemitomon), polpun (Persicaria punctata), cenasi (Centella asiatica), diovir (Diodia virginiana), and rhyinu (Rhynchospora inundata).
Functional diversity response to treatments
Functional diversity (measured as functional dispersion and unitless) was surprisingly not affected by pasture management intensity, but we observed a significant interaction between grazing and prescribed fire treatments on functional diversity (Figure 6, Appendix S2: Table S5). Overall, we observed higher functional diversity in grazed wetlands (mean 1.32, CI95% [1.28, 1.37] vs. mean 1.21, CI95% [1.17, 1.25]), whereas prescribed fire increased functional diversity only in fenced wetlands (mean 1.26, CI95% [1.20, 1.31] in burned and fenced wetlands vs. mean 1.16, CI95% [1.10, 1.22] in unburned and fenced wetlands).
FIGURE 6.
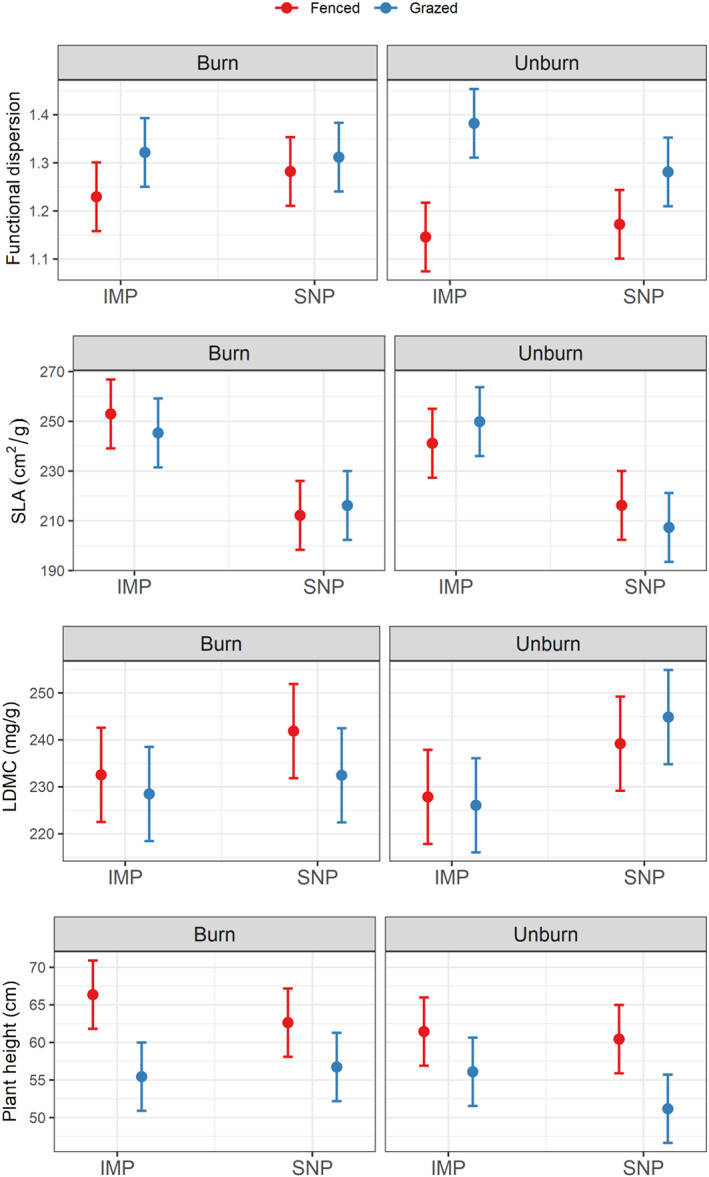
Effects of pasture type (intensively managed pasture [IMP] vs. seminatural pasture [SNP]), grazing (fenced vs. grazed), and prescribed fire (burned vs. unburned) on functional dispersion (unitless), specific leaf area (SLA, cm2/g), leaf dry matter content (LDMC, mg/g), and plant height (cm). Each panel provides the predicted mean (dot) and predicted confidence interval at 95% (error bar) associated with each combination of treatment and for each metric.
SLA (cm2/g) was lower in wetlands in SNPs (mean 212.98 cm2/g, CI95% [205.14, 220.82] vs. mean 247.33 cm2/g, CI95% [239.50, 255.17]), but it did not differ between grazing and burning treatments (Figure 6; Appendix S2: Table S5). LDMC (mg/g) followed the opposite pattern with higher LDMC in wetlands in SNPs (mean 239.6 mg/g, CI95% [233.84, 245.36] vs. mean 228.75 mg/g, CI95% [222.99, 234.51]). Plant vegetative height (cm) was lower in grazed wetlands (mean 54.86 cm, CI95% [52.03, 57.70] vs. mean 62.72 cm, CI95% [59.89, 65.56] in fenced wetlands) and slightly lower in unburned wetlands (mean 57.29 cm, CI95% [54.46, 60.13] vs. mean 60.30 cm, CI95% [57.46, 63.13] in burned wetlands).
DISCUSSION
Numerous studies have tested the effects of cattle grazing on plant diversity, plant composition, and plant traits over the long term (Bullock et al., 2001). However, only a few studies have focused on wetland plant communities and usually only assessed the effect of cattle grazing solely (Marty, 2005; Moges et al., 2017) (but see Marty, 2015a). Our study is one of the largest (40 wetlands) and longest running (14 years) experiments investigating the effect of management intensity, cattle exclusion, prescribed fire, and their interactions inx wetlands. Although some of our results are congruent with the short‐term analysis presented in Boughton et al. (2016), this study points to dissimilarities between short‐ and long‐term outcomes.
Pasture management intensity a major driver of wetland diversity and composition
Conversion of native prairies to pastures (which in our study system occurred between 1950 and 1960) and ensuing land‐use intensification had tremendous impacts on embedded seasonal wetland plant communities. Wetlands in IMPs were less diverse, dominated by a few plant species and overall had lower floristic quality than wetlands in SNPs. Wetlands in IMPs were less diverse, and they were characterized by a higher number and higher frequency of nonnative species. All these results are in line with previous studies at the site showing similar impacts of pasture type on wetland plant communities (Boughton et al., 2010, 2011, 2016; Medley et al., 2015). Notably, most of these nonnative species were not introduced intentionally by the land manager and were able to naturally disperse (via bird droppings, hurricanes, and pumping water from large conveyance canal) and establish viable populations, especially in wetlands in IMPs. In our experiment, IMP wetlands were exposed to regular fertilizer runoff from surrounding pastures (with the exception of phosphorus application, which stopped in 1986) (Swain et al., 2007) and were mowed occasionally before the onset of the experiment. Therefore, it is likely that nonnative species benefitted from both higher nutrient availability in soils and higher disturbance regime in wetlands embedded within IMPs (Saltonstall & Court Stevenson, 2007; Zedler & Kercher, 2004).
Livestock impacts on species richness
Although pasture management intensity was the strongest driver of species composition in wetlands, cattle exclusion also significantly affected species diversity and composition. This contrasted with short‐term results obtained by Boughton et al. (2016), who found no main effect of cattle exclusion on species richness and Shannon diversity. This emphasizes the importance of long‐term experimental studies (Franklin, 1989), as lag effects may occur (Lira et al., 2019; Magnuson, 1990). Numerous studies have documented higher diversity in grazed compared to ungrazed ecosystems, most often in uplands (Hillebrand et al., 2007; Olff & Ritchie, 1998). However, livestock grazing remains a controversial issue in wetlands, especially in wetland restoration projects. Our results suggest that cattle removal decreased plant diversity in seasonal subtropical wetlands and that this effect persisted over more than 10 years of cattle removal, similar to results obtained in California vernal pools (Marty, 2005, 2015b). In our study, this is explained by cattle exclusion increasing the abundance of highly clonal grasses (e.g., P. hemitomon, Hemarthria altissima [Poir.] Stapf & C.E. Hubbard, and Hymenachne amplexicaulis [Rudge] Nees), which outcompeted other species to form dense monospecific stands. This increase in grass abundance following cattle removal is due to cattle preferentially grazing grasses over forbs (Marty, 2005, 2015a). Additionally, livestock grazing is generally thought to facilitate opportunities for invasive nonnative species to establish (Lyseng et al., 2018), especially in locations where ungulate grazing is a new disturbance (Cingolani, Noy‐Meir, & Diaz, 2005; Milchunas et al., 1988). In our study, cattle exclusion did not decrease the diversity and frequency of nonnative species. In fact, cattle exclusion increased the abundance of highly clonal and palatable nonnative grass species (H. altissima, H. amplexicaulis). This is in agreement with previous studies suggesting that cattle grazing could be used to manage specific nonnative invasive species (Firn et al., 2013; Silliman et al., 2014).
Temporal dynamics in species composition
In contrast to short‐term studies, our long‐term analysis of wetland plant communities allowed us to assess wetland plant community temporal dynamics. To do this, we used the MRS to measure the relative change in species rank abundances over time among species that occurred across the entire time series (Collins et al., 2008). The MRS is not directional and highlights turnover in dominant species between years. We also used the rate of community change, which indicates whether species reordering over time resulted in directional compositional change. We observed that the MRS was lower in wetlands in IMPs than in SNPs, but the rate of change was similar between pasture management intensities. Together, these results showed that wetlands in SNPs are characterized by greater turnover in the dominant species between years, but these changes are not directional. In SNP grazed wetlands, several species were codominant (e.g., P. hemitomon, P. cordata, and Cladium jamaicense Crantz), and ranking of these species varied between years, resulting in higher MRS. This could be because neutral processes are more important during the assembly of wetlands within SNPs. Previous work in these wetlands showed that regional factors related to propagule dispersal were more important in SNP wetlands than in IMP wetlands (Boughton et al., 2010; Medley et al., 2015). In contrast, grazed wetlands in IMPs were dominated by J. effusus and to a lesser extent by Persicaria spp., and these species remained dominant throughout the years. This may suggest that higher‐intensity grazing in IMPs combined with nutrient runoff creates strong and highly selective filters.
Following fencing, we observed that the rate of compositional change was higher in fenced wetlands, indicating that over time fenced wetlands diverged from grazed wetlands in terms of species composition. Although these can be attributed to changes in species identity, most of this pattern is the result of changes in abundance. For example, J. effusus, P. punctata, and small prostrate forbs, which characterized grazed wetlands in IMPs, decreased following cattle exclusion. The decrease in J. effusus following cattle exclusion was not reported in the short‐term study and showed that, despite J. effusus's capacity to form a persistent and large seed bank, it was competitively excluded by fast‐growing palatable grasses. We also observed that trees and shrubs slightly increased following cattle exclusion (e.g., B. halimifolia). This effect was likely underestimated since shrubs and trees increased primarily at the extreme edge of seasonal wetlands, where sampling points were infrequent. This result was not observed in the short‐term studies of these wetlands and could have important consequences for wetland functions, especially water‐use efficiency (Budny & Benscoter, 2016; Doody & Benyon, 2011). Another discrepancy between short‐term and long‐term responses was observed with Eupatorium capillifolium (Lam.) Small ex Porter & Britton, a weedy native species. E. capilifolium increased considerably in frequency following cattle exclusion, but this increase was only temporary (in 2007–2008) as the species was observed inconsistently throughout the remaining years. This is likely explained by the fact that this is a biennial species capable of dispersing to recently disturbed areas that were later fenced.
Functional diversity and functional composition
In this study, we found that functional diversity was not affected by land‐use intensification. This result is independent of species richness since functional dispersion is loosely related to species richness (Laliberté & Legendre, 2010). This is surprising since pasture management intensity had a strong effect on other diversity metrics measured in this study. This suggests that, despite the loss of species in wetlands in IMP, we might not observe a loss of functions in these wetlands. A recent study on these wetlands found that soil C stocks were not affected by pasture management intensity (Ho et al., 2018). Despite a lack of effect of pasture management intensity on functional diversity, we observed higher mean SLA and lower LDMC in wetlands in IMPs. This suggests that the management of wetlands in IMPs selected for species adopting a more acquisitive strategy (Wright et al., 2004), likely due to higher soil nutrient content (Bohlen & Gathumbi, 2007).
Overall, we observed higher functional diversity in grazed wetlands. This was likely because grazing selected for two different life history strategies in these wetlands. The first strategy was to tolerate/resist grazing by being able to resprout—exemplified by P. hemitomon. The second strategy was to avoid grazing by being unpalatable to cattle—exemplified by J. effusus—or by being of small prostrate stature—exemplified by Ludwigia repens J.R.Forst. This was corroborated by the lower mean plant height observed in grazed wetlands, suggesting that grazing selected for shorter species. Although both strategies were present in wetlands within IMPs and SNPs, the strategy of “avoidance by being unpalatable” was particularly dominant in IMPs. In these wetlands, higher grazing intensity resulted in wetlands with low forage value (Sonnier et al., 2020), a pattern also observed in Patagonian steppe grasslands (Cingolani, Posse, & Collantes, 2005).
Prescribed fires’ impact on diversity
Prescribed fire had little effect on plant diversity (native richness, total species richness, or mean coefficient of conservatism). This agrees with the results of the short‐term study by Boughton et al. (2016), but it contrasts with several studies in wetlands and grasslands (Boughton et al., 2013; Marty, 2015a). For example, Marty (2015a) found a temporary increase in native plant diversity in recently burned vernal pools in California, and Boughton et al. (2013) found that in a shallow marsh system, unburned plant communities had lower total richness, forb richness, and graminoid richness compared to burned plots.
Like the short‐term study, our study showed that prescribed fire affected species' evenness through its interaction with grazing treatment and pasture management intensity. Prescribed fire had no effect on the evenness of grazed wetlands in either pasture management intensity. However, in fenced wetlands within SNPs, prescribed fire increased evenness. This suggests that, in the absence of grazing, prescribed fire might be an alternative management tool to maintain evenness in wetlands in SNPs. However, the opposite pattern was observed in fenced wetlands in IMPs, because in these wetlands fire combined with fencing promoted highly clonal grass species. This effect was not documented in the short‐term study and could be a simple lag effect or the consequence of multiple prescribed fire events. It appears that the impact of fire on plant communities is context dependent and may depend on the characteristics of dominant species and species adaptations to fire.
Implications for wetland management and restoration
Wetlands are a feature of many agricultural landscapes around the world. We showed that past and present land use, more than cattle grazing, and prescribed fire had a strong legacy effect on wetland vegetation. Conversion to improved pastures led to a decrease of native plant diversity and increase in nonnative species diversity in embedded wetlands, highlighting the importance of not converting SNPs to IMPs. Despite 14 years of cattle exclusion combined with prescribed fires, wetlands in IMPs did not recover diversity levels and floristic quality observed in wetlands in SNPs. This suggests that additional steps, such as planting or seeding native plant species, might be required. However, it is unclear whether these species would establish or be outcompeted by species able to use nutrients more efficiently. Most studies that showed successful establishment of wetland species focused on dominant species that are good competitors (e.g., P. hemitomon) (De Steven & Sharitz, 2007), but mixed results were obtained with less dominant species (Buckallew, 2007). Therefore, more studies are needed to determine whether active planting of absent species would be successful.
Some groups advocate for removing cattle from wetland restoration easements altogether. Removing the ability to graze conservation easements may result in unintended consequences by reducing landowners' participation in conservation easements. Our results showed that excluding livestock completely is not necessarily needed, in agreement with results obtained in nearby wetland restoration easements (Sonnier et al., 2018). In wetlands within SNPs, grazing levels promoted species diversity by allowing short forbs to coexist with larger clonal grasses. Grazing did not increase nonnative plant species or decrease floristic quality, and removal of grazing from wetlands resulted in an increase in trees and shrubs. Historically, our studied wetlands had little to no shrub cover, so tree and shrub encroachment could be perceived as a negative effect, especially when some shrub species are known invasives (e.g., Ludwigia peruviana [L.] H. Hara). In our system, removing grazing led to the increase of some nonnative species. For example, West Indian marsh grass (H. amplexicaulis)—a category of invasive in Florida—increased in fenced wetlands to the point of forming large monoculture. Studies should investigate the costs and benefits of using cattle grazing as a way to control this particular species versus chemical application (Sellers et al., 2008).
Implementing prescribed fire is especially important in SNP areas, where grazing may be removed to maintain more even plant communities. These long‐term experimental results suggest that grazing that follows best management practices is compatible with existing desired vegetation outcomes for conservation easements in wetlands in SNPs. In this study, grazed wetlands were subjected to the grazing regimes applied to the surrounding pastures and, thus, was not experimentally controlled. Future studies should assess stocking densities and seasonality to refine grazing recommendations specific to wetlands. Network research focusing on different wetland types across multiple land uses is critical to assess context dependent effects.
AUTHOR CONTRIBUTIONS
Elizabeth H. Boughton maintained the experiment and collected data over the years. Grégory Sonnier contributed to data collection from 2016 to 2020 with help from Ruth Whittington, who also collected the plant trait data. Elizabeth H. Boughton and Grégory Sonnier developed the specific question addressed in this manuscript. Grégory Sonnier performed statistical analyses. Grégory Sonnier wrote the paper with contributions from Elizabeth H. Boughton and Ruth Whittington.
CONFLICT OF INTEREST
The authors have no conflict of interest regarding the work presented in this manuscript.
Supporting information
Appendix S1
Appendix S2
ACKNOWLEDGMENTS
This research was funded by the US Department of Agriculture (USDA) Cooperative State Research, Education, and Extension Service (2006‐35101‐17204), which provided initial funding to set up the experiment and data collection for 4 years. The USDA Department of Natural Resources Conservation Service (USDA‐NRCS 68‐4209‐16‐500), the Mosaic Company, and the Florida Cattlemen's Association later provided additional funding to support a postdoctoral position. This research was a contribution from the LTAR network. LTAR is supported by the USDA. This work would not have been possible without the help of many research interns and research assistants who contributed to data collection starting in 2006. We are grateful to BIR staff for help with prescribed burning and field infrastructure.
Sonnier, Grégory , Boughton Elizabeth H., and Whittington Ruth. 2023. “Long‐Term Response of Wetland Plant Communities to Management Intensity, Grazing Abandonment, and Prescribed Fire.” Ecological Applications 33(1): e2732. 10.1002/eap.2732
Handling Editor: Jason P. Kaye
Funding information The Florida Cattlemen Association; The Mosaic Company; U.S. Department of Agriculture, Grant/Award Number: 2006‐35101‐17204; USDA‐NRCS, Grant/Award Number: 68‐4209‐16‐500
DATA AVAILABILITY STATEMENT
Vegetation survey data, plant trait data, and metadata (Sonnier et al., 2022) are available on the Environmental Data Initiative Data Portal at https://doi.org/10.6073/pasta/f20622c01b40e1e08e72f01e29f59302.
REFERENCES
- Asner, G. P. , Elmore A. J., Olander L. P., Martin R. E., and Thomas Harris A.. 2004. “Grazing Systems, Ecosystem Responses, and Global Change.” Annual Review of Environment and Resources 29(1): 261–99. [Google Scholar]
- Bakker, E. S. , Ritchie M. E., Olff H., Milchunas D. G., and Knops J. M. H.. 2006. “Herbivore Impact on Grassland Plant Diversity Depends on Habitat Productivity and Herbivore Size.” Ecology Letters 9(7): 780–8. [DOI] [PubMed] [Google Scholar]
- Bates, D. , Mächler M., Bolker B., and Walker S.. 2015. “Fitting Linear Mixed‐Effects Models Using lme4.” Journal of Statistical Software 67(1): 1–48. [Google Scholar]
- Bohlen, P. J. , and Gathumbi S. M.. 2007. “Nitrogen Cycling in Seasonal Wetlands in Subtropical Cattle Pastures.” Soil Science Society of America Journal 71: 1058–65. [Google Scholar]
- Boughton, E. H. , Bohlen P. J., and Steele C.. 2013. “Season of Fire and Nutrient Enrichment Affect Plant Community Dynamics in Subtropical Semi‐Natural Grasslands Released from Agriculture.” Biological Conservation 158(2): 239–47. [Google Scholar]
- Boughton, E. H. , Quintana‐Ascencio P. F., Bohlen P. J., Fauth J. E., and Jenkins D. G.. 2016. “Interactive Effects of Pasture Management Intensity, Release from Grazing and Prescribed Fire on Forty Subtropical Wetland Plant Assemblages.” Journal of Applied Ecology 53(1): 159–70. [Google Scholar]
- Boughton, E. H. , Quintana‐Ascencio P. F., Bohlen P. J., Jenkins D. G., and Pickert R.. 2010. “Land‐Use and Isolation Interact to Affect Wetland Plant Assemblages.” Ecography 33(3): 461–70. [Google Scholar]
- Boughton, E. H. , Quintana‐Ascencio P. F., Nickerson D., and Bohlen P. J.. 2011. “Management Intensity Affects the Relationship between Non‐Native and Native Species in Subtropical Wetlands.” Applied Vegetation Science 14(2): 210–20. [Google Scholar]
- Bovee, K. M. , Merriam K. E., and Gosejohan M. C.. 2018. “Livestock Grazing Affects Vernal Pool Specialists More than Habitat Generalists in Montane Vernal Pools.” Applied Vegetation Science 21(1): 12–20. [Google Scholar]
- Brinson, M. M. , and Malvárez A. I.. 2002. “Temperate Freshwater Wetlands: Types, Status, and Threats.” Environmental Conservation 29(2): 115–33. [Google Scholar]
- Brooks, M. E. , Kristensen K., van Benthem K. J., Magnusson A., Berg C. W., Nielsen A., Skaug H. J., Mächler M., and Bolker B. M.. 2017. “GlmmTMB Balances Speed and Flexibility among Packages for Zero‐Inflated Generalized Linear Mixed Modeling.” The R Journal 9(2): 378–400. [Google Scholar]
- Buckallew, R. R. 2007. Comparison of Bare Root vs. Potted Plants, Species Selection, and Caging Types for Restoration of a Prairie Wetland, and Quantitative Analysis and Descriptive Survey of Plant Communities and Associations at Lewisville Lake Environmental Learning Area (LLELA), Lewisville, TX. Thesis or Dissertation, University of North Texas.
- Budny, M. L. , and Benscoter B. W.. 2016. “Shrub Encroachment Increases Transpiration Water Loss from a Subtropical Wetland.” Wetlands 36(4): 631–8. [Google Scholar]
- Bullock, J. M. , Franklin J., Stevenson M. J., Silvertown J., Coulson S. J., Gregory S. J., and Tofts R.. 2001. “A Plant Trait Analysis of Responses to Grazing in a Long‐Term Experiment.” Journal of Applied Ecology 38(2): 253–67. [Google Scholar]
- Cingolani, A. M. , Noy‐Meir I., and Diaz S.. 2005. “Grazing Effects on Rangeland Diversity: A Synthesis of Contemporary Models.” Ecological Applications 15(2): 757–73. [Google Scholar]
- Cingolani, A. M. , Posse G., and Collantes M. B.. 2005. “Plant Functional Traits, Herbivore Selectivity and Response to Sheep Grazing in Patagonian Steppe Grasslands.” Journal of Applied Ecology 42(1): 50–9. [Google Scholar]
- Cohen, M. J. , Creed I. F., Alexander L., Basu N. B., Calhoun A. J. K., Craft C., D'Amico E., et al. 2016. “Do Geographically Isolated Wetlands Influence Landscape Functions?” Proceedings of the National Academy of Sciences of the United States of America 113(8): 1978–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, S. D. , Heintzman L. J., Starr S. M., Wright C. K., Henebry G. M., and McIntyre N. E.. 2014. “Hydrological Dynamics of Temporary Wetlands in the Southern Great Plains as a Function of Surrounding Land Use.” Journal of Arid Environments 109(10): 6–14. [Google Scholar]
- Collins, S. L. , Micheli F., and Hartt L.. 2000. “A Method to Determine Rates and Patterns of Variability in Ecological Communities.” Oikos 91(2): 285–93. [Google Scholar]
- Collins, S. L. , Suding K. N., Cleland E. E., Batty M., Pennings S. C., Gross K. L., Grace J. B., Gough L., Fargione J. E., and Clark C. M.. 2008. “Rank Clocks and Plant Community Dynamics.” Ecology 89(12): 3534–41. [DOI] [PubMed] [Google Scholar]
- Costanza, R. , d'Arge R., de Groot R., Farber S., Grasso M., Hannon B., Limburg K., et al. 1997. “The Value of the World's Ecosystem Services and Natural Capital.” Nature 387(6630): 253–60. [Google Scholar]
- Costanza, R. , de Groot R., Sutton P., van der Ploeg S., Anderson S. J., Kubiszewski I., Farber S., and Kerry Turner R.. 2014. “Changes in the Global Value of Ecosystem Services.” Global Environmental Change 26(5): 152–8. [Google Scholar]
- Dahl, T. E. 2014. Status and Trends of Prairie Wetlands in the United States 1997 to 2009. Washington, D.C.: U.S. Fish & Wildlife Service. [Google Scholar]
- de Groot, R. , Brander L., van der Ploeg S., Costanza R., Bernard F., Braat L., Christie M., et al. 2012. “Global Estimates of the Value of Ecosystems and their Services in Monetary Units.” Ecosystem Services 1(1): 50–61. [Google Scholar]
- De Steven, D. , and Sharitz R. R.. 2007. “Transplanting Native Dominant Plants to Facilitate Community Development in Restored Coastal Plain Wetlands.” Wetlands 27(4): 972–8. [Google Scholar]
- Doody, T. , and Benyon R.. 2011. “Quantifying Water Savings from Willow Removal in Australian Streams.” Journal of Environmental Management 92(3): 926–35. [DOI] [PubMed] [Google Scholar]
- Firn, J. , Price J. N., and Whalley R. D. B.. 2013. “Using Strategically Applied Grazing to Manage Invasive Alien Plants in Novel Grasslands.” Ecological Processes 2(1): 26. [Google Scholar]
- Franklin, J. F. 1989. “Importance and Justification of Long‐Term Studies in Ecology.” In Long‐Term Studies in Ecology: Approaches and Alternatives, edited by Likens G. E., 3–19. New York, NY: Springer. [Google Scholar]
- Gerakis, A. , and Kalburtji K.. 1998. “Agricultural Activities Affecting the Functions and Values of Ramsar Wetland Sites of Greece.” Agriculture, Ecosystems & Environment 70(2): 119–28. [Google Scholar]
- Hallett, L. M. , Jones S. K., Andrew A., MacDonald M., Jones M. B., Flynn D. F. B., Ripplinger J., Slaughter P., Gries C., and Collins S. L.. 2016. “Codyn: An r Package of Community Dynamics Metrics.” Methods in Ecology and Evolution 7(10): 1146–51. [Google Scholar]
- Hillebrand, H. , Gruner D. S., Borer E. T., Bracken M. E. S., Cleland E. E., Elser J. J., Stanley Harpole W., et al. 2007. “Consumer Versus Resource Control of Producer Diversity Depends on Ecosystem Type and Producer Community Structure.” Proceedings of the National Academy of Sciences 104(26): 10904–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, J. , Boughton E. H., Jenkins D. G., Sonnier G., Bohlen P. J., and Chambers L. G.. 2018. “Ranching Practices Interactively Affect Soil Nutrients in Subtropical Wetlands.” Agriculture, Ecosystems & Environment 254(2): 130–7. [Google Scholar]
- Jansen, L. S. , Pierre S., and Boughton E. H.. 2019. “Interactions of Fire, Grazing and Pasture Management: Short‐Term and Long‐Term Responses of Water Quality to Management Regimes in Subtropical Isolated Wetlands.” Agriculture, Ecosystems & Environment 280: 102–13. [Google Scholar]
- Jones, S. K. , Ripplinger J., and Collins S. L.. 2017. “Species Reordering, Not Changes in Richness, Drives Long‐Term Dynamics in Grassland Communities.” Ecology Letters 20(12): 1556–65. [DOI] [PubMed] [Google Scholar]
- Jost, L. 2010. “Independence of Alpha and Beta Diversities.” Ecology 91(7): 1969–U104. [DOI] [PubMed] [Google Scholar]
- Junk, W. J. , Shuqing An C. M., Finlayson B. G., Květ J., Mitchell S. A., Mitsch W. J., and Robarts R. D.. 2013. “Current State of Knowledge Regarding the World's Wetlands and their Future under Global Climate Change: A Synthesis.” Aquatic Sciences 75(1): 151–67. [Google Scholar]
- Kattge, J. , Bönisch G., Díaz S., Lavorel S., Prentice I. C., Leadley P., Tautenhahn S., et al. 2020. “TRY Plant Trait Database – Enhanced Coverage and Open Access.” Global Change Biology 26(1): 119–88. [DOI] [PubMed] [Google Scholar]
- Kelly, S. L. , Song H., and Jenkins D. G.. 2015. “Land Management Practices Interactively Affect Wetland Beetle Ecological and Phylogenetic Community Structure.” Ecological Applications 25(4): 891–900. [DOI] [PubMed] [Google Scholar]
- Kleinman, P. J. A. , Spiegal S., Rigby J. R., Goslee S. C., Baker J. M., Bestelmeyer B. T., Boughton R. K., et al. 2018. “Advancing the Sustainability of US Agriculture through Long‐Term Research.” Journal of Environmental Quality 47(6): 1412–25. [DOI] [PubMed] [Google Scholar]
- Koerner, S. E. , Smith M. D., Burkepile D. E., Hanan N. P., Avolio M. L., Collins S. L., Knapp A. K., et al. 2018. “Change in Dominance Determines Herbivore Effects on Plant Biodiversity.” Nature Ecology & Evolution 2(12): 1925–32. [DOI] [PubMed] [Google Scholar]
- Laliberté, E. , and Legendre P.. 2010. “A Distance‐Based Framework for Measuring Functional Diversity from Multiple Traits.” Ecology 91(1): 299–305. [DOI] [PubMed] [Google Scholar]
- Laliberté, E. , Legendre P., and Shipley B.. 2014. “FD: Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology.” R Package (version 1.0‐12). https://CRAN.R-project.org/package=FD [DOI] [PubMed]
- Lira, P. K. , de Souza Leite M., and Metzger J. P.. 2019. “Temporal Lag in Ecological Responses to Landscape Change: Where Are we Now?” Current Landscape Ecology Reports 4(3): 70–82. [Google Scholar]
- Lyseng, M. P. , Bork E. W., Hewins D. B., Alexander M. J., Carlyle C. N., Chang S. X., and Willms W. D.. 2018. “Long‐Term Grazing Impacts on Vegetation Diversity, Composition, and Exotic Species Presence across an Aridity Gradient in Northern Temperate Grasslands.” Plant Ecology 219(6): 649–63. [Google Scholar]
- Lüdecke, D. 2021. “SjPlot: Data Visualization for Statistics in Social Science.” R package version 2.8.9. https://CRAN.R-project.org/package=sjPlot
- Magnuson, J. J. 1990. “Long‐Term Ecological Research and the Invisible Present.” Bioscience 40(7): 495–501. [Google Scholar]
- Main, M. B. , and Barry M. J.. 2002. “Influence of Season of Fire on Flowering of Wet Prairie Grasses in South Florida, USA.” Wetlands 22(2): 430–4. [Google Scholar]
- Marty, J. T. 2005. “Effects of Cattle Grazing on Diversity in Ephemeral Wetlands.” Conservation Biology 19(5): 1626–32. [Google Scholar]
- Marty, J. T. 2015a. “Fire Effects on Plant Biodiversity across Multiple Sites in California Vernal Pool Grasslands.” Ecological Restoration 33(3): 266–73. [Google Scholar]
- Marty, J. T. 2015b. “Loss of Biodiversity and Hydrologic Function in Seasonal Wetlands Persists over 10 Years of Livestock Grazing Removal.” Restoration Ecology 23(5): 548–54. [Google Scholar]
- Medley, K. A. , Boughton E. H., Jenkins D. G., Fauth J. E., Bohlen P. J., and Quintana‐Ascencio P. F.. 2015. “Intense Ranchland Management Tips the Balance of Regional and Local Factors Affecting Wetland Community Structure.” Agriculture, Ecosystems & Environment 212(12): 207–44. [Google Scholar]
- Menges, E. S. , and Kohfeldt N.. 1995. “Life History Strategies of Florida Scrub Plants in Relation to Fire.” Bulletin of the Torrey Botanical Club 122(4): 282–97. [Google Scholar]
- Milchunas, D. G. , Sala O. E., and Lauenroth W. K.. 1988. “A Generalized Model of the Effects of Grazing by Large Herbivores on Grassland Community Structure.” American Naturalist 132(1): 87–106. [Google Scholar]
- Moges, A. , Beyene A., Ambelu A., Mereta S. T., Triest L., and Kelbessa E.. 2017. “Plant Species Composition and Diversity in Wetlands under Forest, Agriculture and Urban Land Uses.” Aquatic Botany 138(2): 9–15. [Google Scholar]
- Mortellaro, S. , Barry M., Gann G., Zahina J., Channon S., Hilsenbeck C., Scofield D., Wilder G., and Wilhelm G.. 2012. “Coefficients of Conservatism Values and the Floristic Quality Index for the Vascular Plants of South Florida.” Southeastern Naturalist 11: 1–62. [Google Scholar]
- Olff, H. , and Ritchie M. E.. 1998. “Effects of Herbivores on Grassland Plant Diversity.” Trends in Ecology & Evolution 13(7): 261–5. [DOI] [PubMed] [Google Scholar]
- Pakeman, R. J. , and Quested H. M.. 2007. “Sampling Plant Functional Traits: What Proportion of the Species Need to be Measured?” Applied Vegetation Science 10(1): 91–6. [Google Scholar]
- Peco, B. , Carmona C. P., de Pablos I., and Azcarate F. M.. 2012. “Effects of Grazing Abandonment on Functional and Taxonomic Diversity of Mediterranean Grasslands.” Agriculture Ecosystems & Environment 152(5): 27–32. [Google Scholar]
- Pérez‐Harguindeguy, N. , Diaz S., Garnier E., Lavorel S., Poorter H., Jaureguiberry P., Bret‐Harte M. S., et al. 2013. “New Handbook for Standardised Measurement of Plant Functional Traits Worldwide.” Australian Journal of Botany 61(3): 167–234. [Google Scholar]
- Pielou, E. C. 1974. Population and Community Ecology. New York, NY: Gordon and Breach Science. [Google Scholar]
- R Core Team . 2020. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing.
- RStudio Team . 2020. RStudio: Integrated Development for R. Boston, MA: RStudio, PBC.
- Saltonstall, K. , and Court Stevenson J.. 2007. “The Effect of Nutrients on Seedling Growth of Native and Introduced Phragmites Australis.” Aquatic Botany 86(4): 331–6. [Google Scholar]
- Sellers, B. A. , Diaz R., Overholt W. A., Langeland K. A., and Gray C. J.. 2008. “Control of West Indian Marsh Grass with Glyphosate and Imazapyr.” Journal of Aquatic Plant Management 46(2): 189–92. [Google Scholar]
- Silliman, B. R. , Mozdzer T., Angelini C., Brundage J. E., Esselink P., Bakker J. P., Gedan K. B., van de Koppel J., and Baldwin A. H.. 2014. “Livestock as a Potential Biological Control Agent for an Invasive Wetland Plant.” PeerJ 2: e567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocum, M. G. , Platt W. J., and Cooley H. C.. 2003. “Effects of Differences in Prescribed Fire Regimes on Patchiness and Intensity of Fires in Subtropical Savannas of Everglades National Park, Florida.” Restoration Ecology 11(1): 91–102. [Google Scholar]
- Sonnier, G. , Bohlen P. J., Swain H. M., Orzell S. L., Bridges E. L., and Boughton E. H.. 2018. “Assessing the Success of Hydrological Restoration in Two Conservation Easements within Central Florida Ranchland.” PLoS One 13(7): e0199333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnier, G. , Quintana‐Ascencio P. F., Bohlen P. J., Fauth J. E., Jenkins D. G., and Boughton E. H.. 2020. “Pasture Management, Grazing, and Fire Interact to Determine Wetland Provisioning in a Subtropical Agroecosystem.” Ecosphere 11(8): e03209. [Google Scholar]
- Sonnier, G. , Whittington R., and Boughton E. H.. 2022. “Long‐Term Response of Wetland Plant Communities to Management Intensity, Grazing Abandonment, and Prescribed Fire (ver 1).” Environmental Data Initiative. [DOI] [PMC free article] [PubMed]
- Spiegal, S. , Bestelmeyer B. T., Archer D. W., Augustine D. J., Boughton E. H., Boughton R. K., Cavigelli M. A., et al. 2018. “Evaluating Strategies for Sustainable Intensification of US Agriculture through the Long‐Term Agroecosystem Research Network.” Environmental Research Letters 13(3): 034031. [Google Scholar]
- Spyreas, G. 2019. “Floristic Quality Assessment: A Critique, a Defense, and a Primer.” Ecosphere 10(8): e02825. [Google Scholar]
- Steinman, A. D. , Conklin J., Bohlen P. J., and Uzarski D. G.. 2003. “Influence of Cattle Grazing and Pasture Land Use on Macroinvertebrate Communities in Freshwater Wetlands.” Wetlands 23(4): 877–89. [Google Scholar]
- Swain, H. M. , Bohlen P. J., Campbell K. L., Lollis L. O., and Steinman A. D.. 2007. “Integrated Ecological and Economic Analysis of Ranch Management Systems: An Example from South Central Florida.” Rangeland Ecology & Management 60(1): 1–11. [Google Scholar]
- Swain, H. M. , Boughton E. H., Bohlen P. J., and Lollis L. O.. 2013. “Trade‐Offs among Ecosystem Services and Disservices on a Florida Ranch.” Rangelands 35(5): 75–87. [Google Scholar]
- Wickham, H. , M. Averick, J. Bryan, W. Chang, L. McGowan, R. François, G. Grolemund, et al. 2019. “Welcome to the Tidyverse.” Journal of Open Source Software 4(43): 1686. [Google Scholar]
- Wright, I. J. , Reich P. B., Westoby M., Ackerly D. D., Baruch Z., Bongers F., Cavender‐Bares J., et al. 2004. “The Worldwide Leaf Economics Spectrum.” Nature 428(6985): 821–7. [DOI] [PubMed] [Google Scholar]
- Zedler, J. B. , and Kercher S.. 2004. “Causes and Consequences of Invasive Plants in Wetlands: Opportunities, Opportunists, and Outcomes.” Critical Reviews in Plant Sciences 23(5): 431–52. [Google Scholar]
- Zedler, J. B. , and S. Kercher. 2005. “Wetland Resources: Status, Trends, Ecosystem Services, and Restorability.” Annual Review of Environment and Resources 30(1): 39–74. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Data Availability Statement
Vegetation survey data, plant trait data, and metadata (Sonnier et al., 2022) are available on the Environmental Data Initiative Data Portal at https://doi.org/10.6073/pasta/f20622c01b40e1e08e72f01e29f59302.


