Abstract
Background
The impact of donor age on the immune reconstitution of patients with hematological malignancies who underwent hematopoietic cell transplantation (HCT) is unclear.
Method
We retrospectively compared the outcomes of 381 patients who underwent allogeneic peripheral blood stem cell transplantation (PBSCT) from 308 donors under 50 years of age and 73 donors over 50 years of age. IVIG was regularly supplemented for patients in the first 3 months post‐HCT.
Results
The counts of CD8+CD45RA+ naïve T cells were significantly lower in patients of the older donor group than in the younger donor group in the first year after PBSCT (190.6 cells/μl vs. 239.6 cells/μl, p = .018). Patients in the older donor group had significantly fewer CD19+ B cells on day +270 (123.4 cells/μl vs. 183.5 cells/μl, p = .021) and day +365 (169 cells/μl vs. 271.1 cells/μl, p = .01) after PBSCT. Serum IgA (.76 g/L vs. .97 g/L, p < .001) and IgM levels (.75 g/L vs. 1.04 g/L, p < .001) were significantly lower in patients in the older donor group from day +60 to +365 after PBSCT. The EBV reactivation rate within the first 3 months after PBSCT was significantly higher in patients in the older donor group (48.6% vs. 38.3%, p = .034). However, the incidences of CMV reactivation, II‐IV acute graft‐versus‐host disease (aGvHD), chronic GvHD (cGvHD), 3‐year relapse rate, 3‐year transplant‐related mortality (TRM) and 3‐year overall survival (OS) were not significantly different between the two groups.
Conclusion
In conclusion, donors ≥50 years old were associated with inferior immune reconstitution and higher EBV reactivation in patients after PBSCT, but no change in OS.
Keywords: allogeneic hematopoietic cell transplantation (HCT), donor age, hematological malignancies, immune reconstitution, lymphocytes
1. INTRODUCTION
Allogeneic peripheral blood stem cell transplantation (PBSCT) is a curative modality for most hematological malignancies. Hematopoietic stem cells, like all other cells, are subjected to aging mechanisms, and will gradually lose their self‐renewal and regenerative potential. 1 , 2 Stuart et al. and HK et al. showed that donors over 50 years old had inferior mobilization of CD34+ cells in the PBSCT setting. 3 , 4 Therefore, older donors may experience a higher rate of mobilization failure and lead to engraftment failure of recipients. In recent years, haploidentical transplantation has given rise to more donor sources. 5 In some cases, a matched unrelated or a haploidentical related younger donor may be preferable to a matched older donor.
Immune reconstitution plays an important role in the outcome of HCT since post‐transplantation infection leads to high morbidity and mortality. 6 , 7 However, the role of donor age on the immune reconstitution of their recipients remains largely unknown, and whether older donors will lead to inferior transplant outcomes is unclear. Therefore, we divided patients into donors < 50 years old group and donors ≥50 years old group. We retrospectively analyzed data from our center and compared grafts, engraftment, immune reconstitution, GvHD and survival of patients transplanted from the two donor age groups.
2. METHODS
2.1. Patients and donors
A total of 381 patients who underwent allogeneic PBSCT from January 2017 to December 2020 in our center were retrospectively analyzed. They received allograft from their HLA‐matched related donors (n = 109) or haploidentical related (n = 272) donors. This study had ethical approval from hospital ethical committees and was conducted in accordance with the Declaration of Helsinki. All patients included in the study signed informed consent.
2.2. Conditioning regimen
Myeloablative conditioning regimen for patients with acute myeloid leukemia (AML) and myelodysplasia syndrome (MDS) consisted of intravenous fludarabine (150 mg/m2) or cladribine (25 mg/m2), cytarabine (5 g/m2‐10 g/m2) and busulfan (12.8 mg/kg). For patients with acute lymphoblastic leukemia (ALL) and non‐Hodgkin lymphoma (NHL), conditioning regimen consisted of intravenous etoposide (20 mg/kg‐30 mg/kg), cyclophosphamide (100 mg/kg) and total body irradiation (TBI, 10 Gy).
2.3. GvHD prophylaxis
For HLA‐matched related transplantation, GvHD prophylaxis consisted of intravenous cyclosporine (2 mg/kg from day ‐5), methotrexate (15 mg/m2 on day +1, 10 mg/m2 intravenous on day +3, +6), and mycophenolate (MPA, 720 mg bid orally from day +1 to day +30). Rabbit anti‐thymocyte globulin (ATG, Thymoglobin®, Genzyme Polyclonals S.A.S, 2.5 mg/kg on day‐2 and day‐1, total dose 5 mg/kg) was added to 22 patients. For haploidentical transplantation, 71 patients received prophylaxis with ATG (2.5 mg/kg from day‐1 to day‐4, total dose 10 mg/kg), cyclosporine, methotrexate and MPA, 158 patients received low dose ATG (2.5 mg/kg on day‐2 and day‐1, total dose 5 mg/kg) and low dose posttransplant cyclophosphamide (50 mg/kg), 8 and the others received posttransplant cyclophosphamide (50 mg/kg on day +3, +4), cyclosporine (2 mg/kg from day +5) and MPA (720 mg tid from day +5 to day +35).
2.4. Engraftment and GvHD
Neutrophil engraftment was defined as the first of three consecutive days of achieving a sustained peripheral blood neutrophil count of > .5 × 109/L. Platelet engraftment is defined as independence from platelet transfusion for at least 7 days with a platelet count of more than > 20 × 109/L.9 aGvHD and cGvHD were graded according to the modified Glucksberg grading of aGvHD 10 and 2014 National Institutes of Health consensus criteria, respectively. 11
2.5. Supplement of intravenous immunoglobulin
Patients were given intravenous immunoglobulin (IVIg, Baxter, the USA) 10 g/day for 2–3 days per month in the first 3 months post‐HCT, and intermittent supplement of IVIg if their serum IgG was lower than the normal range thereafter.
2.6. Statistical analysis
The statistics were analyzed by SPSS version 26.0 (SPSS, Chicago, IL, USA). Continuous variables were analyzed by independent t‐test and Mann–Whitney U‐test. Categorical factors were compared by the Chi‐square test. Immune reconstitution between patients of the HLA‐matched group and the haploidentical group was analyzed by Mann–Whitney U‐test. Immune reconstitution of lymphocyte subset counts and immunoglobulin (Ig) of each time point (day +30, +60, +120, +180, +270, and +365 posttransplant) between the two donor age groups were analyzed by multiple linear regression to adjust covariates of patient age and gender, donor gender, use of ATG, disease status, aGvHD, HLA disparity and virus reactivation. To clarify the impact of donor age on immune reconstitution, donor age was used as both a categorical variable (< 50 and ≥50 year) and a continuous variable in multiple linear regression. A generalized linear mixed model (GLMM) was applied to analyze the overall impact of donor age on immune reconstitution, and data of lymphocyte subset counts and immunoglobulin levels on day +60, +90, +120, +180, +270, and +365 after HCT were collected and analyzed. All the covariates used in multiple linear regression were adjusted in GLMM as well. Data of lymphocytes subsets were available for 69 recipients on day +30, 178 recipients on day +60, 171 recipients on day +90, 214 recipients on day +120, 231 recipients on day +180, 178 recipients on day +270 and 168 recipients on day +365 after HCT. Data of serum immunoglobulin levels were available for 96 recipients on day +60, 102 recipients on day +90, 105 recipients on day +120, 126 recipients on day +180, 91 recipients on day +270 and 95 recipients on day +365 after HCT.
Cumulative incidences of EBV and CMV reactivation were analyzed by Cox regression. Relapse rate, overall survival (OS) and transplant‐related mortality (TRM) were analyzed using the Kaplan‐Meier method, compared with the log‐rank test and adjusted by multivariate Cox regression. Covariates of HLA disparity, patient age, aGvHD, use of ATG, disease status, and hematopoietic cell transplantation comorbidity index (HCT‐CI) were adjusted in Cox regression. All risk factors with p‐values < .1 in univariate analysis were included in multivariate analysis. All p‐values were two‐sided and were defined as statistically significant if < .05.
3. RESULTS
3.1. Patient characteristics
A total of 381 patients who underwent myeloablative HLA‐matched related or haploidentical PBSCT were included, with a median follow‐up of 17 (1–54) months. The diagnosis was AML in 186, ALL in 83, MDS in 75 and NHL in 37. The median age of patients and donors was 39 (range 7–67) and 36 (range 8–69) years old respectively. Among them, 73 donors (19.2%) were older than 50 years old. Baseline characteristics of all patients and comparison of donor <50 and ≥50 year group were shown in Table 1.
TABLE 1.
Patients’ baseline characteristics
|
All patients (n = 381) |
Donor<50 year (n = 308) |
Donor ≥50 year (n = 73) |
p‐Value | |
|---|---|---|---|---|
| Patient age, year | 39 (7‐67) | 39 (7‐67) | 38 (18‐64) | .423 |
| Patient gender | .737 | |||
| Male | 231 | 188(61.0%) | 43(58.9%) | |
| Female | 150 | 120(39.0%) | 30(41.1%) | |
| Donor age, year | 36 (8‐69) | 31 (8‐49) | 55 (50‐69) | .000 |
| Donor gender | .680 | |||
| Male | 232 | 186(60.4%) | 46(63.0%) | |
| Female | 149 | 122(39.6%) | 27(37.0%) | |
| HLA | .057 | |||
| Matched | 109 | 81(26.3%) | 28(38.4%) | |
| Haploidentical | 272 | 227(73.7%) | 45(61.6%) | |
| GvHD prophylaxis | .433 | |||
| CSA+MTX+MPA | 96 | 73(23.7%) | 23(31.5%) | |
| ATG+CSA+MTX+MPA | 93 | 76(24.7%) | 17(23.3%) | |
| ATG+CSA+CTX+MPA | 158 | 129(41.9%) | 29(39.7%) | |
| CTX+CSA+MPA | 34 | 30(9.7%) | 4(5.5%) | |
| Diagnosis | .794 | |||
| AML | 186 | 154(50%) | 32(43.8%) | |
| ALL | 83 | 65(21.1%) | 18(24.7%) | |
| MDS | 75 | 59(19.2%) | 16(21.9%) | |
| NHL | 37 | 30(9.7%) | 7(9.6%) | |
| Disease status | .620 | |||
| CR | 270 | 220 (71.4%) | 50 (68.5%) | |
| NR | 111 | 88 (28.6%) | 23 (31.5%) | |
| ECOG | .437 | |||
| 0 | 276 | 226(73.4%) | 50(68.5%) | |
| 1 | 89 | 68(22.1%) | 21(28.8%) | |
| 2 | 10 | 8(2.6%) | 2(2.7%) | |
| 3 | 6 | 6(1.9%) | 0 (.0%) | |
| HCT‐CI | .840 | |||
| 0 | 301 | 241(78.2%) | 60(82.2%) | |
| 1 | 63 | 53(17.2%) | 10(13.7%) | |
| 2 | 8 | 7(2.3%) | 1(1.4%) | |
| 3‐4 | 9 | 7(2.3%) | 2 (2.7%) | |
| Donor‐recipient gender | .918 | |||
| Female donors to male recipients | 87 | 70 (22.7%) | 17 (23.3%) | |
| Others | 294 | 238 (77.3%) | 56 (76.7%) | |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ATG, rabbit‐anti‐human thymocyte globulin; CR, complete remission; CSA, cyclosporine; CTX, cyclophosphamide; ECOG, Eastern cooperative oncology group; GvHD, graft‐versus‐host disease; HCT‐CI, hematopoietic cell transplantation comorbidity index, MDS, myelodysplasia syndrome; MPA, mycophenolate; MTX, methotrexate; NHL, non‐Hodgkin lymphoma; NR, non‐complete remission.
3.2. Donor grafts
We compared the percentages and counts of grafts between donors<50 and ≥50 year. The percentages (.49% vs. .67%, p < .001), absolute cell counts (1410 cells/μl vs. 1900 cells/μl, p < .001), and dose of CD34+ cells (9.00 × 106/kg vs. 11.25 × 106/kg, p < .001) in the grafts of donors ≥50 year were significantly lower than those of donors < 50 year. No difference was found in absolute lymphocyte counts of grafts from older donors and younger donors (83.64 × 109 cells/L vs. 91.32 × 109 cells/L, p = .188). An age‐related increase of CD3+CD4+ T cell percentage was observed (Table 2, p < .001). Both percentage (22.92% vs. 26.92%, p = .002) and counts (18.00 × 109 cells/L vs. 23.48 × 109 cells/L, p = .002) of CD3+CD8+ T cell were significantly lower in the older donor group. In addition, differences between younger and older donor grafts were evident as decreases in CD4+CD45RA+ naïve and CD8+CD45RA+ naïve T cells, and increases in CD4+CD45RO+ and CD8+CD45RO+ memory T cells with age (Table 2). Besides, a significant increase of CD4+CD29+ T cell percentage and reductions in both percentage and counts of CD8+CD28+ T cells with donor age were identified (Table 2).
TABLE 2.
Comparison of cell subsets of grafts from two donor groups
| Cell types | Donor < 50 year | Donor ≥50 year | p‐Value | r |
|---|---|---|---|---|
| CD34+ cell counts (cells/μl) | 1900 (200‐9640) | 1410 (140‐8050) | <.001 | −.108* |
| Percentage of CD34+ cell (%) | .67 (.06‐2.23) | .49 (.05‐1.85) | <.001 | −.126* |
| CD34+ cell dose (106/kg) | 11.25 (3.11‐46.55) | 9.00 (1.23‐33.22) | <.001 | −.117* |
| Total lymphocyte (109/L) | 91.32 (33.35‐228.24) | 83.64 (25.59‐217.97) | .188 | −.070 |
| CD3+CD4+ T (%) | 35.52 (15.90‐59.80) | 38 (13.10‐57.62) | .095 | .252** |
| CD3+CD4+ T (109/L) | 31.54 (6.45‐90.70) | 30.63 (5.83‐95.47) | .707 | .085 |
| CD3+CD8+ T (%) | 26.92 (9.98‐43.68) | 22.92 (11.30‐46.50) | .002 | −.306** |
| CD3+CD8+ T (109/L) | 23.48 (7.12‐90.61) | 18 (2.89‐80.87) | .002 | −.207** |
| CD56+ NK (%) | 13.31 (1.60‐60.10) | 14.32 (3.31‐34.50) | .456 | .033 |
| CD56+ NK (109/L) | 10.34 (1.57‐86.16) | 8.97 (1.43‐40.87) | .411 | −.054 |
| CD19+ B (%) | 14.66 (2.10‐36.60) | 17.10 (5.82‐30) | .048 | .139 |
| CD19+ B (109/L) | 12 (1.01‐49.76) | 12.47 (3.74‐37.88) | .853 | .064 |
| CD4+CD45RA+ naïve T/CD4+ T (%) | 35.7 (1.70‐76.20) | 29.37 (7.70‐61.93) | .026 | −.308** |
| CD4+CD45RA+ naïve T (109/L) | 10.36 (.43‐53.88) | 6.97 (.80‐34.71) | .066 | −.154* |
| CD4+CD45RO+ memory T/CD4+ T (%) | 60.40 (16.80‐96.50) | 66 (35.04‐92.10) | .035 | .290** |
| CD4+CD45RO+ memory T (109/L) | 17.81 (4.33‐49.59) | 19.33 (4.49‐74.94) | .693 | .216** |
| CD8+CD45RA+ naïve T/CD8+ T (%) | 54.81 (.10‐86.70) | 44.37 (7.88‐82) | <.001 | −.332** |
| CD8+CD45RA+ naïve T (109/L) | 12.28 (.03‐39.21) | 6.73 (.92‐30.81) | <.001 | −.383** |
| CD8+CD45RO+ memory T/CD8+ T (%) | 38.56 (8.30‐85.80) | 49.29 (11.70‐87.20) | <.001 | .355** |
| CD8+CD45RO+ memory T (109/L) | 8.40 (1.69‐45.67) | 7.86 (1.13‐43.75) | .703 | .089 |
| CD4+CD29+ T (%) | 20.31 (6.03‐45.80) | 24.63 (8.10‐46.80) | <.001 | .378** |
| CD4+CD29+ T (109/L) | 17.12 (4.30‐53.41) | 18.93 (4.89‐83.70) | .489 | .210** |
| CD8+CD28+ T (%) | 18.96 (1‐33.70) | 15.13 (5.90‐30.10) | <.001 | −.318** |
| CD8+CD28+ T (109/L) | 17.12 (.49‐63.68) | 12.51 (2.12‐40.86) | .006 | .247** |
Note: The medians (range) of cell subsets in grafts from the two donor groups, and the correlation coefficient between donor cell subsets and donor age were shown. Asterisk (*) indicated correlation coefficient with statistical significance.
3.3. Engraftment
All but five patients engrafted successfully. Among the patients who failed to engraft, four were in the younger donor group and one was in the older donor group. For patients engrafted successfully, there were no significant differences in median days to the engraftment of neutrophil (12 days vs. 12 days, p = .667) and platelet (13 days vs. 13 days, p = .426) between the two donor age groups (Table 3).
TABLE 3.
Comparison of engraftment, virus reactivation, GvHD and survival of patients from two donor groups
| Donor < 50 year | Donor ≥50 year | p‐Value | |
|---|---|---|---|
| Median days to neutrophil engraftment (range) | 12.0 (7‐25) | 12.0 (9‐20) | .667 |
| Median days to platelet engraftment (range) | 13.0 (8‐45) | 13.0 (9‐32) | .426 |
| EBV reactivation rate (%) (n) | 38.3 (114) | 48.6 (34) | .034 |
| CMV reactivation rate (%) (n) | 38.4 (114) | 37.1 (26) | .785 |
| Incidence of aGvHD | .742 | ||
| I° (%) (n) | 20.1 (65) | 22.2 (16) | |
| II‐IV° (%) (n) | 12.2 (37) | 15.3 (11) | |
| Incidence of 1‐year cGvHD (%) (n) | 43.7 (107) | 51.7 (31) | .265 |
| 3‐year relapse rate (%) (n) | 22.7 (57) | 22.1 (13) | .819 |
| 3‐year TRM (%) (n) | 14.5 (36) | 11.6 (8) | .989 |
| 3‐year OS (%) (n) | 68.6 (224) | 70.2 (52) | .775 |
Abbreviations: aGvHD, acute graft‐versus‐host disease; cGvHD, chronic graft‐versus‐host disease; OS, overall survival, TRM, transplant‐related mortality.
3.4. Immune reconstitution
To investigate the differences in immune reconstitution, we compared the counts of peripheral lymphocyte subsets between patients of the two donor groups by multiple linear regression. No differences in the reconstitution of total lymphocytes, CD3+CD4+ T cells, CD3+CD8+ T cells and natural killer (NK) cells were found between the two groups at all posttransplant time points (Figure 1A–D). As for T cell subsets, there were no differences in the reconstitution of CD4+CD45RA+ naïve T cells, CD4+CD45RO+ memory T cells and CD8+CD45RO+ memory T cells between the two groups (Figure 1F,G,I). However, we found a significant reduction in CD8+CD45RA+ naïve T cell counts in patients of the older donor group on day +60 (Figure 1H, 57.1 cells/μl vs. 97.2 cells/μl, p = .041) after HCT, and significant inverse correlations of CD8+CD45RA+ naïve T cell counts with donor age were found on day +60, +90, +180, +270 after HCT (Figure 2A–D, p = .002, .017, .001, .047, respectively). As for CD19+ B cells, the reconstitution kinetics began to show a difference from the 4th month post‐PBSCT (Figure 1E). Patients of the older donor group had significantly fewer CD19+ B cells on day +270 (123.4 cells/μl vs. 183.5 cells/μl, p = .021) and day +365 (169 cells/μl vs. 271.1 cells/μl, p = .01) post‐HCT. Besides, significant inverse correlations of CD19+ B cells with donor age were identified on day +270 and +365 post‐HCT (Figure 2E,F, p = .027 and .021).
FIGURE 1.
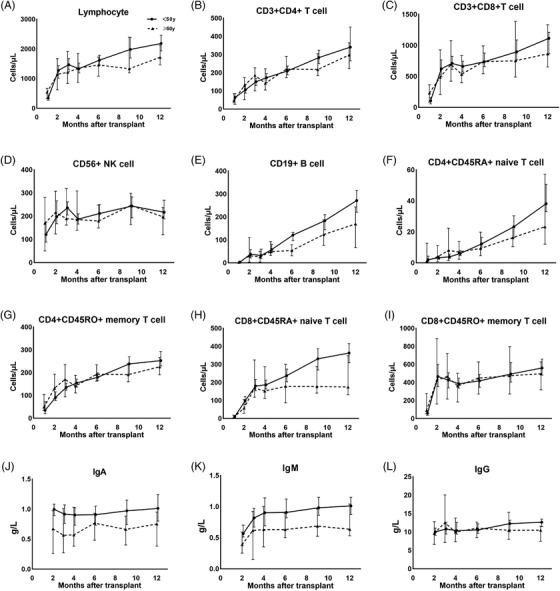
Comparison of immune reconstitution of patients between donor < 50 year group and ≥50 year group within the first year post‐PBSCT. Median lymphocyte subset counts on day+ 30, +60, +90, +120, +180, +270, and +365 post‐PBSCT, and median serum IgA, IgM and IgG level on day +60, +90, +120, +180, +270, +365 post‐PBSCT were shown
FIGURE 2.
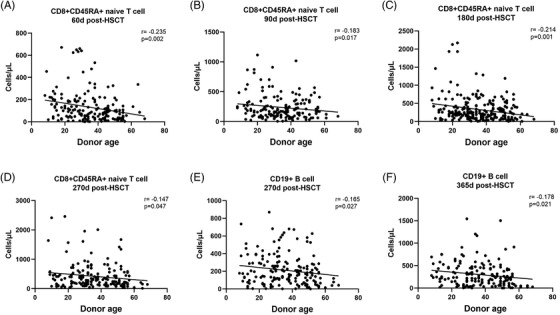
Correlations between donor age and immune reconstitution of CD8+CD45RA+ naïve T cells on (A) day +60 (B) day +90 (C) day +180 (D) day +270 and CD19+ B cells on (E) day +270 (F) day +365 after HCT. r referred to correlation coefficient
We found significant reductions in serum IgA on day +90 (Figure 1J, .57 g/L vs. .82 g/L, p = .04), +365 (Figure 1J, .75 g/L vs. .91 g/L, p = .016) and serum IgM on day +180 (Figure 1K, .63 g/L vs. .81 g/L, p = .042) in the older donor group after PBSCT. However, no significant differences in the reconstitution of serum IgG were found between the two donor age groups (Figure 1L).
As for HLA disparity, immune reconstitution of CD4+CD45RA naïve T cells was significantly higher in patients of the HLA‐matched group than in the haploidentical group on day +60, +90, +120, +180, +270 (All p < .001) and day +365 (p = .009) post‐HCT. In addition, patients of the HLA‐matched group had significantly fewer CD8+CD45RO+ memory T cells on day +120, +180, +270 and +365 post‐HCT (p = .002 on day +120, < .001 on day +180, +270 and +365). The differences in immune reconstitution between patients of the HLA‐matched group and the haploidentical group were shown in Table S1.
We then conducted a GLMM analysis to further clarify the overall impact of donor age on immune reconstitution. No differences in the reconstitution of total lymphocytes (p = .172), NK cells (p = 925), CD19+ B cells (p = .892), CD3+CD4+ T cells (p = .403), CD3+CD8+ T cells (p = .179), CD4+CD45RA+ naïve T cells (p = .105), CD4+CD45RO+ memory T cells (p = .671), CD8+CD45RO+ memory T cells (p = .789) and serum IgG level (p = .602) were found between the two donor age groups. However, significant reductions were found in the reconstitution of CD8+CD45RA+ naïve T cells (190.6 cells/μl vs. 239.6 cells/μl, p = .018), serum IgA (.76 g/L vs. .97 g/L, p < .001) and serum IgM (.75 g/L vs. 1.04 g/L, p < .001) in patients of the older donor group. In addition, we found that the development of I‐IV aGvHD and male gender were beneficial to the reconstitution of CD8+CD45RA+ T cells. The factors relating to the reconstitution of CD8+CD45RA+ T cells, serum IgA and IgM were shown in Table 4.
TABLE 4.
Multivariate analysis of the factors relating to CD8+CD45RA+ T cells, serum IgA and IgM reconstitution within the first year post‐HCT by GLMM
| Multivariate analysis | |||
|---|---|---|---|
| Type of reconstitution | Variables | Medians (range) | p‐Value |
| CD8+CD45RA+ T cell | Donor age | .018 | |
| <50 year | 239.6 cells/μl (.8‐6291) | ||
| ≥50 year | 190.6 cells/μl (2.7‐1672) | ||
| I‐IV aGvHD | .021 | ||
| no | 195.8 cells/μl (.8‐2415) | ||
| yes | 234.3 cells/μl (5.4‐6291) | ||
| Donor gender | .001 | ||
| male | 241.6 cells/μl (3.5‐6291) | ||
| female | 188.5 cells/μl (.8‐2009) | ||
| Serum IgA | Donor age | .000 | |
| <50 year | .97 g/L (.03‐4.60) | ||
| ≥50 year | .76 g/L (.05‐3.60) | ||
| HLA | .000 | ||
| Matched | 1.00 g/L (.05‐4.60) | ||
| Haploidentical | .73 g/L (.03‐3.20) | ||
| Recipient gender | .049 | ||
| male | .82 g/L (.03‐4.60) | ||
| female | .91 g/L (.04‐3.49) | ||
| Serum IgM | Donor age | .000 | |
| <50 year | 1.04 g/L (.03‐18.5) | ||
| ≥50 year | .75 g/L (.01‐2.73) | ||
| EBV reactivation within 100 days post HCT | .034 | ||
| no | .96 g/L (.01‐18.5) | ||
| yes | .82 g/L (.04‐5.19) | ||
| Recipient gender | .001 | ||
| male | .78 g/L (.01‐5.49) | ||
| female | 1.00 g/L (.04‐18.5) | ||
| Recipient age | .014 | ||
3.5. EBV and CMV reactivation
A total of 148 (40.2%, 95%CI: 35.2%–45.3%) and 152 (38.21%, 95%CI: 33.2%–43.1%) patients developed EBV and CMV reactivation within 90 days after HCT respectively. The EBV reactivation rate was significantly higher in patients of donor ≥50 year group than donor < 50 year group (Figure 3A, 48.6%, 95%CI: 36.6%–60.6%, vs. 38.3%, 95%CI: 32.7%–43.8%, p = .034, hazard ratio = 1.52, 95%CI: 1.03–2.23). In addition, we compared naïve CD8+ T cell counts in patients before EBV reactivation, and found the CD8+CD45RA+ naïve T cell counts were significantly lower in EBV reactivated patients than in patients without EBV reactivation (40.0 cells/μl vs. 121.1 cells/μl, p < .001). However, no difference in CMV reactivation rate was observed between the two groups (Figure 3B, 38.4%, 95%CI: 32.8%–43.9% in the younger donor group, 37.1%, 95%CI:25.5%–48.7% in the older donor group, p = .785).
FIGURE 3.
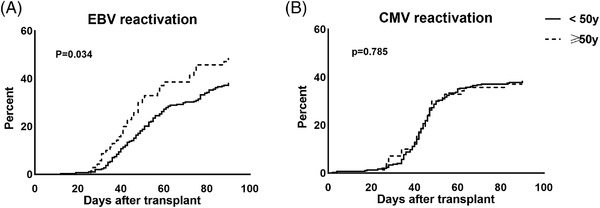
Cumulative incidence of EBV and CMV reactivation of patients between donor<50 year group and ≥50 year group within 90 days after HCT (A) EBV reactivation (B) CMV reactivation
3.6. GvHD
The cumulative incidence of grade I and grade II‐IV aGvHD in all patients was 21.5% (95%CI: 17.4%–25.7%, n = 81) and 12.8% (95%CI: 9.4%–16.2%, n = 48) respectively. No difference was identified in the two donor age groups (p = .742). As for the incidence of 1‐year cGvHD, no difference was found between the two groups (51.7%, 95%CI: 38.6%–64.7%, n = 31 vs. 43.7%, 95%CI: 37.4%–49.9%, n = 107, p = .265).
3.7. Relapse
A total of 70 patients had disease relapse. The 3‐year relapse rate was 22.7% (95%CI: 17.5%–27.9%) in the younger donor group, and 22.1% (95%CI: 11.1%–32.9%) in the older donor group, with no statistical difference observed (Figure 4A, p = .819). In addition, no difference in the 3‐year relapse rate was observed between patients of the HLA‐matched group and the haploidentical group (26.8% vs. 20.8%, p = .257).
FIGURE 4.
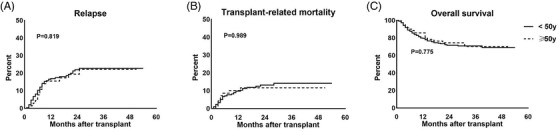
Outcomes of patients between the two donor age groups. (A) relapse rate (B) transplant‐related mortality (C) overall survival
3.8. TRM and OS
At the end of the follow‐up, 98 patients died, and 44 of them died of TRM. The 3‐year TRM of donor < 50 year group and ≥50 year group was 14.5% (95%CI: 10.1%–18.9%) and 11.6% (95%CI: 3.8%–19.3%), respectively. There was no significant difference between the two groups (Figure 4B, p = .989). Besides, no difference in 3‐year TRM was identified between patients of the HLA‐matched group and the haploidentical group (11.6% vs. 14.6%, p = .444).
In addition, there was no significant difference in the estimated 3‐year OS between donor < 50 and ≥50 year group (68.5%, 95%CI: 63.4%–73.6% vs. 70.3%, 95%CI: 59.6%–80.9% (Figure 4C, p = .775). Similarly, there was no difference in the estimated 3‐year OS between patients of the HLA‐matched group and the haploidentical group (65.6% and 71.0%, p = .732).
4. DISCUSSION
Our study compared donor grafts and immune reconstitution of 381 recipients between donors < 50 and ≥50 year, and found the grafts from older donors contained higher percentages of CD3+CD4+ T lymphocytes and CD4/CD8+CD45RO memory T lymphocytes, but lower percentages of CD3+CD8+ T lymphocytes and CD4+/CD8+CD45RA+ naïve T lymphocytes. Patients from the older donor group had significantly slower reconstitution of CD8+CD45RA+ T cells, B cells, serum IgA and IgM levels, and a higher incidence of EBV reactivation after transplant. However, there was no impact of donor age on acute and chronic GvHD, relapse and survival of patients.
The present study found an age‐related increase of CD3+CD4+ T cell percentage, but no impact of donor age on CD3+CD4+ T cell counts in donor grafts, which were different from the previous study on peripheral blood. Jiao Y et al. reported a trend of decreased peripheral blood CD3+CD4+ T cell counts and percentage with age, 12 and Melzer et al. identified the same results of CD3+CD4+ T cell counts in peripheral blood. 13 In addition, our study showed age‐related increases of CD4+CD45RO+ memory T cell and CD4+CD29+ memory effector T cell percentages, which were consistent with previous studies on peripheral blood, revealing their age‐dependent effect, 14 , 15 , 16 and this might explain the age‐related increase of CD3+CD4+T cell percentage in our study.
In our study, grafts from the older donor group contained significantly fewer CD34+ cells. However, there were no differences in the engraftment of neutrophil and platelet between the two donor age groups. The reason might be the median dose of CD34+ cells of both groups was higher than 8 × 106/kg, which was sufficient for the engraftment of donor grafts.
Studies about the impact of donor age on the immune reconstitution after HCT are lacking. Our study showed an inferior overall immune reconstitution in patients of the older donor group, which was similar to the study by Marta et al. Their study showed a faster immune reconstitution of CD3+ T cells and B cells in pediatric patients using younger donors in a T cell‐depleted haploidentical setting. 17 The reconstitution of CD4+CD45RA+ naïve T cells depended on the patient's thymic output, therefore, the CD4+CD45RO+ memory/effector T cells originating from mature T cells in the donor grafts predominated in the CD4+ T cells after HCT. 18 , 19 Indeed, our study showed slow recovery of naïve CD4+ T cells, and a major expression of CD45RO in the reconstituting CD4+ T cells. We did not find differences in the reconstitution of naïve CD4+ T cells between the two donor groups, the reason might be the reconstitution of naïve CD4+ T cells was thymus‐dependent, which was related to recipient age. However, we found significantly lower counts of naïve CD8+ T cells in older donor group patients. The generation of CD8+T cells after HCT was largely thymic‐independent, therefore, the reconstitution of naïve CD8+ T cells might be influenced by more factors including donor age. 18 We found decreased CD8+CD45RA+ T cell counts in grafts from older donors, and this might be associated with the reductions in naïve CD8+ T cell reconstitution in patients of the older donor group. As for humoral immunity, we found significant reductions in CD19+ B cell reconstitution in patients of the older donor group in the later stage, but not early stage after HCT, and this was in line with the fact that B cell counts are nearly zero within the first few months and the reconstitution begins from 4 to 6 months after HCT. 7 , 18 , 20 Furthermore, we found significant reductions in serum IgA level and serum IgM level in the older donor group patients, and this was different from the finding of Storek et al. They failed to show any impact of donor age on immunoglobulin reconstitution. 21 However, there was no difference in IgG reconstitution between the two donor groups in our study. Regular supplement of intravenous IgG post‐HCT might be the reason.
Our study showed a higher EBV reactivation in the older donor group. The first 90 days after allogeneic HCT are characterized by cellular immunodeficiencies, which renders patients susceptible to EBV and CMV reactivation. 18 The link between immune recovery and EBV reactivation after HCT is ambiguous. Z Bian et al. found a delayed recovery of CD4/CD8 double‐negative T cells correlated with EBV reactivation after haplo‐HCT. 22 We found CD8+CD45RA+ T cell counts were lower in EBV reactivated patients, indicating a possible role of CD8+CD45RA+ T cells recovery in preventing EBV reactivation.
In the present study, there were no significant differences in the incidences of aGvHD and cGVHD between the two donor age groups. A retrospective registry study by the National Marrow Donor Program based on 6978 patients revealed both increased incidences of aGvHD and cGvHD with advanced donor age. 23 On the contrary, Rezvani et al. found that both myeloablative and nonmyeloablative recipients with older sibling donors had significantly a lower incidence of grade II to IV aGvHD than recipients with grafts from younger unrelated donors, but no difference in cGvHD. 24
There were no significant differences in relapse rate, TRM and OS between the two donor age groups in our study. Kollman et al. revealed an inferior outcome with advanced donor age in an unrelated donor bone marrow transplantation setting. 23 Bastida et al. showed donors over 50 years old were associated with poorer outcomes in patients diagnosed with MDS and AML. 25 Servais et al. also found a similar correlation between donor age and clinical outcome post‐HCT. 26 On the contrary, M Robin et al. found no difference between donor age and clinical outcome in patients with MDS and AML. 27 A study from Mottló et al. showed donors ≥ 55 years old mobilized fewer CD34+ cells in the PBSCT setting. However, the transplant outcome was not influenced by donor age. 28 Rezvani et al. identified similar results, the donor age beyond 60 did not impair the clinical outcome when compared with younger donors. 24 Our study only found inferior immune reconstitution and a higher EBV reactivation rate in the older donor group. However, a higher incidence of EBV reactivation did not bring inferior transplant outcomes in our study, which was consistent with previous studies by Peric Z et al. and Ru Y et al., they showed that a higher EBV reactivation rate was not associated with inferior transplant outcome. 29 , 30 Limitations of this study are mainly attributed to the relatively small number of patients and the retrospective nature of our single‐center study. Therefore, more prospective studies are needed to further clarify the outcomes of patients between younger and older donors, and the outcomes between HLA‐matched and haploidentical transplantation.
In conclusion, our study showed grafts from donors ≥50 years old contained lower CD3+CD8+ T cells and CD8+CD45RA+ naïve T cells than younger donors. Recipients from donors ≥50 years old had relatively inferior immune reconstitution of CD8+ CD45RA+ naïve T cells, CD19+ B cells, serum IgA, serum IgM and a higher incidence of EBV reactivation, but similar outcomes in engraftment, GvHD, disease relapse, TRM and OS.
CONFLICTS OF INTEREST
The authors declared no competing interests.
Supporting information
Supporting Information.
ACKNOWLEDGEMENTS
This work was supported by the Clinical Research Plan of Shanghai Shen Kang Hospital Development Center under Grants SHDC2020CR3028B, SHDC2020CR1012B, 16CR1009A, 16CR1010A, and SHDC12018X09; Clinical Research Innovation Plan of Shanghai General Hospital under Grant CTCCR‐2018B02, CTCCR‐2018BP03, CTCCR‐2019B03 and CTCCR‐2019D02; Shanghai Municipal Health and Family Planning Commission under Grant 201840043; and National Clinical Research Center for Hematologic Disease under Grant 2020ZKPC02.
Jiang P, Cai Y, Zhou X, et al. Immune reconstitution and survival of patients after allogeneic hematopoietic stem cell transplantation from older donors. Clin Transplant. 2023;37:e14844. 10.1111/ctr.14844
Contributor Information
Peiyao Jiang, Email: 904975409@qq.com.
Yu Cai, Email: butterflymmyu@hotmail.com.
Xiao Zhou, Email: m18796281338@163.com.
Jun Yang, Email: yangjuan74@hotmail.com.
Yin Tong, Email: tongyin_cn2@hotmail.com.
Chongmei Huang, Email: huangchongmei616@163.com.
Huiying Qiu, Email: qiuhy5@126.com.
Kun Zhou, Email: zhkzhw@163.com.
Xiaowei Xu, Email: xuxiaowei1616@126.com.
Ying Zhang, Email: zhangyingm03@163.com.
Jiahua Niu, Email: 215069249@qq.com.
Chang Shen, Email: sc452@163.com.
Xinxin Xia, Email: xiasmmu2003@163.com.
Yu Wei, Email: fxbeckham7@163.com.
Xianmin Song, Email: shongxm@sjtu.edu.cn.
Liping Wan, Email: lipingwan@sjtu.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. de Haan G, Lazare SS. Aging of hematopoietic stem cells. Blood. 2018;131(5):479‐487. [DOI] [PubMed] [Google Scholar]
- 2. Geiger H, Denkinger M, Schirmbeck R. Hematopoietic stem cell aging. Curr Opin Immunol. 2014;29:86‐92. [DOI] [PubMed] [Google Scholar]
- 3. Ings SJ, Balsa C, Leverett D, Mackinnon S, Linch DC, Watts MJ. Peripheral blood stem cell yield in 400 normal donors mobilised with granulocyte colony‐stimulating factor (G‐CSF): impact of age, sex, donor weight and type of G‐CSF used. Br J Haematol. 2006;134(5):517‐525. [DOI] [PubMed] [Google Scholar]
- 4. Al‐Ali HK, Bourgeois M, Krahl R, et al. The impact of the age of HLA‐identical siblings on mobilization and collection of PBSCs for allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2011;46(10):1296‐1302. [DOI] [PubMed] [Google Scholar]
- 5. Khan MA, Bashir Q, Chaudhry Q‐u‐N, Ahmed P, Satti TM, Mahmood SK. Review of haploidentical hematopoietic cell transplantation. J Glob Oncol. 2018;4:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Auletta JJ, Lazarus HM. Immune restoration following hematopoietic stem cell transplantation: an evolving target. Bone Marrow Transplant. 2005;35(9):835‐857. [DOI] [PubMed] [Google Scholar]
- 7. Peggs KS, Mackinnon S. Immune reconstitution following haematopoietic stem cell transplantation. Br J Haematol. 2004;124(4):407‐420. [DOI] [PubMed] [Google Scholar]
- 8. Yang J, Jiang J, Cai Y, et al. Low‐dose anti‐thymocyte globulin plus low‐dose posttransplant cyclophosphamide as graft‐versus‐host disease prophylaxis in haploidentical peripheral blood stem cell transplantation combined with unrelated cord blood for patients with hematologic malignancies: a prospective, phase II study. Bone Marrow Transplant. 2019;54(7):1049‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doescher A, Casper J, Kraemer D, Kapels H‐H, Petershofen EK, Mueller TH. Platelet engraftment after allogenic stem cell transplantation is monitored by digital polymerase chain reaction without interference by platelet support. Exp Hematol. 2018;68:21‐29. [DOI] [PubMed] [Google Scholar]
- 10. Przepiorka D, Weisdorf D, Martin P, et al. Consensus conference on acute GVHD grading (1994 Consensus Conference on Acute GVHD Grading, Keystone, January 1994). Bone Marrow Transplant. 1995;15(6):825‐828. [PubMed] [Google Scholar]
- 11. Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft‐versus‐Host Disease: i. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2015;21(3):389‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiao Y, Qiu Z, Xie J, Li D, Li T. Reference ranges and age‐related changes of peripheral blood lymphocyte subsets in Chinese healthy adults. Sci China C Life Sci. 2009;52(7):643‐650. [DOI] [PubMed] [Google Scholar]
- 13. Melzer S, Zachariae S, Bocsi J, Engel C, Loeffler M, Tarnok A. Reference intervals for leukocyte subsets in adults: results from a population‐based study using 10‐color flow cytometry. Cytom B ‐ Clin Cytom. 2015;88(4):270‐281. [DOI] [PubMed] [Google Scholar]
- 14. Bektas A, Schurman SH, Sen R, Ferrucci L. Human T cell immunosenescence and inflammation in aging. J Leukoc Biol. 2017;102(4):977‐988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Todo‐Bom A, Mota‐Pinto A, Alves V, Santos‐Rosa M. Aging and asthma‐Changes in CD45RA, CD29 and CD95 T cells subsets. Allergol Immunopathol (Madr). 2012;40(1):14‐19. [DOI] [PubMed] [Google Scholar]
- 16. Utsuyama M, Hirokawa K, Kurashima C, et al. Differential age‐change in the numbers of CD4+CD45RA+ and CD4+CD29+ T cell subsets in human peripheral blood. Mech Ageing Dev. 1992;63(1):57‐68. [DOI] [PubMed] [Google Scholar]
- 17. Gonzalez‐Vicent M, Molina B, Deltoro N, et al. Donor age matters in T‐cell depleted haploidentical hematopoietic stem cell transplantation in pediatric patients: faster immune reconstitution using younger donors. Leuk Res. 2017;57:60‐64. [DOI] [PubMed] [Google Scholar]
- 18. Bosch M, Khan FM, Storek J. Immune reconstitution after hematopoietic cell transplantation. Curr Opin Hematol. 2012;19(4):324‐335. [DOI] [PubMed] [Google Scholar]
- 19. Ogonek J, Juric MK, Ghimire S, et al. Immune reconstitutional after allogeneic hematopoietic stem cell transplantation. Front Immunol. 2016;7:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Storek J. B‐cell immunity after allogeneic hematopoietic cell transplantation. Cytotherapy. 2002;4(5):423‐424. [DOI] [PubMed] [Google Scholar]
- 21. Storek J, Viganego F, Dawson MA, et al. Factors affecting antibody levels after allogeneic hematopoietic cell transplantation. Blood. 2003;101(8):3319‐3324. [DOI] [PubMed] [Google Scholar]
- 22. Bian Z, Liu J, Xu LP, et al. Association of Epstein‐Barr virus reactivation with the recovery of CD4/CD8 double‐negative T lymphocytes after haploidentical hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52(2):264‐269. [DOI] [PubMed] [Google Scholar]
- 23. Kollman C, Howe CWS, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98(7):2043‐2051. [DOI] [PubMed] [Google Scholar]
- 24. Rezvani AR, Storer BE, Guthrie KA, et al. Impact of donor age on outcome after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21(1):105‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bastida JM, Cabrero M, Lopez‐Godino O, et al. Influence of donor age in allogeneic stem cell transplant outcome in acute myeloid leukemia and myelodisplastic syndrome. Leuk Res. 2015;39(8):828‐834. [DOI] [PubMed] [Google Scholar]
- 26. Servais S, Porcher R, Xhaard A, et al. Pre‐transplant prognostic factors of long‐term survival after allogeneic peripheral blood stem cell transplantation with matched related/unrelated donors. Haematologica. 2014;99(3):519‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Robin M, Porcher R, Ades L, et al. Matched unrelated or matched sibling donors result in comparable outcomes after non‐myeloablative HCT in patients with AML or MDS. Bone Marrow Transplant. 2013;48(10):1296‐1301. [DOI] [PubMed] [Google Scholar]
- 28. Motllo C, Sancho J‐M, Grifols J‐R, et al. Mobilization and engraftment of peripheral blood stem cells in healthy related donors >55 years old. Cytotherapy. 2014;16(3):406‐411. [DOI] [PubMed] [Google Scholar]
- 29. Peric Z, Cahu X, Chevallier P, et al. Features of Epstein‐Barr Virus (EBV) reactivation after reduced intensity conditioning allogeneic hematopoietic stem cell transplantation. Leukemia. 2011;25(6):932‐938. [DOI] [PubMed] [Google Scholar]
- 30. Ru Y, Zhang X, Song T, et al. Epstein‐Barr virus reactivation after allogeneic hematopoietic stem cell transplantation: multifactorial impact on transplant outcomes. Bone Marrow Transplant. 2020;55(9):1754‐1762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


