Abstract
Background
Cytomegalovirus (CMV) infection and disease are preventable complications following pediatric liver transplantation (PLT), despite the use of prophylaxis to minimize the risk of CMV disease. We evaluated the incidence and complications of CMV disease in PLT recipients in South Africa (SA), with particular reference to potential differences in outcome between state and private sector patients.
Methods
Medical records of patients younger than 16 years of age who received liver transplants between January 1, 2012, and August 31, 2018 were analyzed.
Results
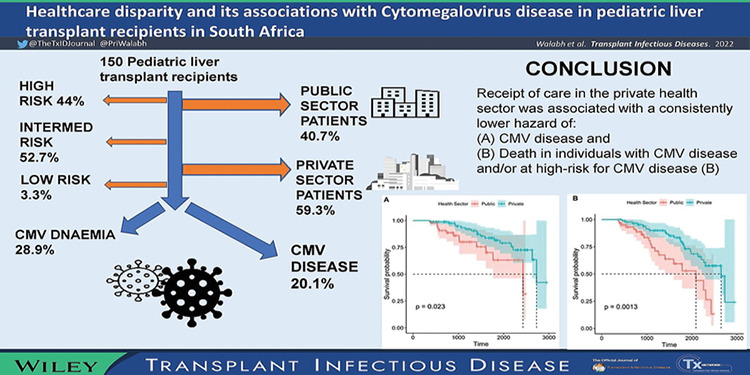
Records of all 150 PLT patients were retrieved. The median age at transplant was 29.2 months (95% confidence interval 15.6–58.4) and follow‐up was 46.3 months (interquartile range 27.6–63.1). Sixty‐six (44%) patients were high risk, 79 (52.7%) were intermediate risk, and five (3.3%) were low risk for CMV infection. Forty‐three (28.9%) patients had CMV DNAemia following transplantation, and 30 (20.1%) developed CMV disease. Receipt of care in the private sector was consistently associated with a lower hazard of CMV disease (adjusted hazard ratio [aHR] ranging from 0.36 to 0.43) and a consistently lower hazard of death among recipients at high risk for CMV disease and/or those who developed CMV disease (aHR ranging from 0.28 to 0.33).
Conclusion
Receipt of care in the private health sector was associated with a consistently lower hazard of CMV disease and death in individuals with CMV disease and/or at high risk for CMV disease. Policies aimed at creating a more equitable healthcare system in SA may mitigate the differential burden of illness associated with CMV in PLT recipients.
Keywords: end‐organ disease, equitable healthcare, ganciclovir prophylaxis, immunosuppression, mortality, pediatric liver transplantation
Graphical abstract:

Abbreviations
- aHR
adjusted hazard ratio
- CMV
cytomegalovirus
- D
donor
- HHV
human herpes virus
- HIC
high‐income countries
- HR
hazard ratio
- IQR
interquartile range
- NHI
National Health Insurance
- PLT
pediatric liver transplant
- PPP
public‒private partnership
- R
recipient
- SA
South Africa
1. INTRODUCTION
Cytomegalovirus (CMV) infection and disease is one of the most important preventable and treatable infectious complications following liver transplantation. 1 , 2 , 3 , 4 Multiple risk factors are associated with the occurrence of CMV infection in transplant patients, the most significant of which are donor (D)–recipient (R) serostatus and the extent of pharmacologic immunosuppression. 4 , 5 , 6 , 7
Based on D and R CMV serostatus, recipients are categorized as low (D‐R‐), intermediate (D+R+; D‐R+), or high risk (D+R‐) for CMV disease after transplantation, and this stratification can be useful to determine which prophylactic regimen is used for different risk categories. 4 , 8 Interpretation of D and R serostatus for children younger than 18 months is not reliable, as it may be affected by transplacentally acquired maternal CMV antibodies, and these patients are often considered to be CMV negative. 9 , 10 Children may be CMV naïve at the time of transplantation and acquire CMV infection post‐transplantation.
Strategies that are used to reduce the rate of CMV infection following pediatric liver transplantation (PLT) include universal antiviral prophylaxis, preemptive therapy or a sequential (hybrid) approach. 10 , 11 However, to our knowledge, no study has confirmed the superiority of any one of these strategies. 11
The choice of the appropriate antiviral prophylaxis regimen is further complicated in our South African context, which consists of a dichotomous population in terms of socioeconomic and financial distribution, which has implications in terms of healthcare. 12 Our current healthcare system is two‐tiered and highly unequal, with 84% of the population accessing state‐funded public sector health services and the rest accessing private healthcare through individual contributions to medical insurance schemes. 12 , 13 , 14
The purpose of this study was to evaluate the incidence and complications of CMV disease in PLT recipients in a transplant service in South Africa (SA) and to assess the similarities and differences in outcomes related to CMV disease between state‐ and private sector patients. This would assist in tailoring prophylaxis and treatment regimens in PLT recipients in our context.
2. PATIENT AND METHODS
2.1. Study design and population
This study was a retrospective record review of 150 PLT patients aged less than 16 years whose transplants were performed at Wits Donald Gordon Medical Centre (WDGMC), Johannesburg, from January 1, 2012, to August 31, 2018. Four of the patients were retransplanted, but for the purposes of this study, we excluded the second transplant. All 150 patients were followed up for a minimum of 1 year after transplant. Follow‐up was at Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) for state‐funded (public sector) patients and WDGMC for private patients with medical insurance. CMJAH is a tertiary academic hospital, and WDGMC is a private academic hospital where all liver transplantations are performed by transplant surgeons employed in the private sector. The facilities are closely situated in Johannesburg, Gauteng Province, SA. There is currently a public‒private partnership (PPP) between CMJAH and WDGMC whereby public sector transplants are funded by the state, which is responsible for funding the pre‐ and post‐transplant care of these patients.
Resources and medication availability in both public and private sector hospitals are similar, with the major discrepancy being the lack of liver transplant surgeons and interventional radiologists in the public sector hospital (CMJAH). This discrepancy is ameliorated by the PPP, and hence, liver transplantation and immediate postoperative intensive care unit management and radiological intervention are performed at WDGMC. Public sector patients are discharged to CMJAH once they no longer require immediate postoperative intervention for complications. The time period for transitioning public sector patients varies from a few days to months. During the study period, there was substantial overlap of staff (hepatologists, transplant coordinators, transplant surgeons) caring for both public and private sector patients with combined academic discussions regarding all patients. Timeous accessibility and availability of assistance from experienced medical staff for symptomatic post‐transplant patients was the same for both sectors.
Data collected included the patients’ demographics, indication for liver transplant, type of liver transplant, laboratory findings, CMV infection risk category, duration of follow‐up (censored at August 31, 2019) and outcome. Approval to conduct the study was obtained from the Human Research Ethics Committee of University of the Witwatersrand (Medical) (M1911041).
2.2. Study procedures
CMV infection was defined as evidence of CMV replication in tissue, blood or other body fluids regardless of symptoms, with a CMV viral load greater than 1000 IU/ml used to define significant replication. 15 Patients with CMV DNAemia but without evidence of end‐organ disease were labeled as having CMV infection. CMV disease was defined as CMV infection that was accompanied by clinical signs and symptoms and was further subdivided into CMV syndrome (fever with temperature >38.0°C, malaise, atypical lymphocytosis, leukopenia, thrombocytopenia) and end‐organ disease (gastrointestinal disease, pneumonitis, hepatitis) as evidenced by tissue invasive disease.
The National Health Laboratory Service (NHLS) for state‐funded patients and the Lancet Laboratory for private patients were used for laboratory investigations during follow‐up. These laboratories utilize the COBAS® AmpliPrep/COBAS® TaqMan® CMV Test, which is highly sensitive for the detection of CMV DNA in clinical specimens. 16 Laboratory turnaround times differed between the laboratories, with the NHLS (public sector) taking between 1 and 2 weeks for CMV viral load blood results as opposed to 48–72 h at the Lancet (private sector) laboratory.
Prior to transplantation, CMV serology was obtained from all patients older than 18 months of age, and a CMV viral load (DNA) peripheral blood test was performed for all those who were younger than 18 months of age. All patients less than 18 months were classified as high risk from June 2016. Prior to this change in departmental policy, all patients less than 18 months of age were classified according to CMV serology results as high (D+R‐), intermediate (D‐R+; D+R+), or low risk (D‐R‐). For the purposes of our analysis, we classified all patients younger than 18 months of age as high risk for CMV disease. After transplantation, CMV viral load on blood was measured monthly for the first 12 months and every alternate month thereafter for state‐funded patients. Private patients were not routinely tested for CMV disease unless symptomatic. CMV viral load assays on blood were performed in both state‐funded and privately funded patients if there was a clinical suspicion of CMV infection.
Patients were classified as high, intermediate, or low risk for CMV disease based on D and R CMV serostatus. As per our protocol, all high‐risk patients should have been prescribed 15 mg/kg valganciclovir for CMV prophylaxis daily for 3 months, after which they were either managed in the state sector with monthly viral loads or in the private sector with on‐demand testing. Intermediate‐ and low‐risk patients were given acyclovir 5 mg/kg twice daily for herpes simplex virus prophylaxis for 3 months and were monitored clinically. In patients who became symptomatic for CMV disease at any point, a viral load blood test was performed, and CMV treatment was instituted if the CMV viral load was above 1000 IU/ml. Prophylaxis for CMV was discontinued if patients experienced adverse effects or developed CMV infection or disease. Treatment of CMV comprised oral valganciclovir 15 mg/kg twice daily for CMV infection and intravenous ganciclovir 5 mg/kg twice daily for 2–3 weeks for CMV disease. Patients were treated until two consecutive CMV viral load blood test results, conducted 1 month apart, were negative. Some patients received secondary prophylaxis after completing CMV treatment, while others were monitored, and no secondary prophylaxis was instituted. Primary and secondary prophylaxis varied at the discretion of the clinician managing the patient.
The baseline immunosuppression regimen for all PLT recipients consisted of oral tacrolimus and low‐dose oral corticosteroids. A dosage of 10 mg/kg of intravenous methylprednisolone was administered after reperfusion of the graft and then given at 1 mg/kg daily for the first week after transplant, weaned to 0.5 mg/kg daily for the second week and then changed to oral prednisone, weaned to 0.25 mg/kg daily thereafter and stopped at 3 months except in patients with autoimmune hepatitis who were kept on low‐dose steroids. Tacrolimus was dosed twice a day from day 1 post‐transplantation, and titrated to maintain tacrolimus levels between 10 and 14 ng/ml for the first month, 8–10 ng/ml for the next 3 months, and 6–8 ng/ml thereafter for the first year. 17 Mycophenolate mofetil or azathioprine was added as a second agent in PLT recipients who had more than two episodes of acute cellular rejection, plasma‐rich rejection, or autoimmune hepatitis. All PLT recipients with steroid‐resistant rejection received a lymphocyte‐depleting agent such as thymoglobulin, accompanied by oral valganciclovir prophylaxis.
2.3. Statistical methods
Categorical variables were described using frequencies and percentages, and proportions were compared using Pearson's chi‐squared test or Fisher's exact test for sparse data. The Shapiro‒Wilk test was used to test for normality of continuous variables, and nonnormal continuous variables were described using the median and interquartile range (IQR). Two sets of survival analyses were conducted, examining the clinical endpoints of (1) CMV disease among all patients, and (2) death in patients who were either high risk for CMV disease and/or who developed CMV disease. For survival analysis, censoring was applied at the date of the clinical outcome of interest, death, loss to follow‐up, or at the last observation date (August 31, 2019). Univariate Cox proportional hazards analysis was conducted, followed by a series of multivariate Cox proportional hazards models. Multivariate models that were explored included: (1) parameters that on univariate testing had a p‐value of <.1; (2) parameters that on univariate testing had p‐values of <.2; (3) stepwise Cox proportional hazards modeling; and (4) least absolute shrinkage and selection operator (LASSO) regression. For all survival analysis models, the proportional hazards assumption was tested using the cox.zph function in the R survival package 18 : only covariates that did not violate the proportional hazards assumption were retained in the final models. Model selection was undertaken using the Akaike information criterion (AIC), with the most informative model designated by the lowest AIC score. All statistical analyses were conducted using R version 4.1.1. 19
3. RESULTS
3.1. Study population characteristics
One hundred and fifty pediatric patients younger than 16 years of age underwent 154 PLT at WDGMC in the study period. The first transplants of all 150 patients were included in this analysis. The median age at the time of transplantation was 29.2 months (IQR 15.7–57.70), and 44 (29.3%) were younger than 18 months of age (Table 1). The median weight‐for‐age Z‐score of the patients at the time of transplant was ‐0.8 (IQR ‐1.85 to 0.06). Seventy‐seven of the transplants (51.3%) were living‐related liver donations (Table 1). The indication for liver transplant was biliary obstruction, fulminant liver disease or inborn errors of metabolism in most cases (n = 136) (Table 1), with biliary atresia accounting for 80 out of 150 (53.3%) patients and fulminant liver failure in 22 out of 150 (14.7%) patients. The median follow‐up time after transplant was 46.3 months (IQR 27.6–63.1).
TABLE 1.
Characteristics of children undergoing liver transplantation, stratified by facility
| Overall | Public sector | Private sector | p‐Value | |
|---|---|---|---|---|
| N | 150 | 61 | 89 | |
| Male (%) | 60 (40.0) | 24 (39.3) | 36 (40.4) | 1.000 |
| Black (%) | 101 (67.3) | 49 (80.3) | 52 (58.4) | .008 |
| Median age at TP (months) [IQR] | 29.18 [15.69, 57.01] | 30.32 [17.23, 68.22] | 28.76 [13.85, 49.35] | .321 |
| Under 18 months of age at TP (%) | 44 (29.3) | 16 (26.2) | 28 (31.5) | .611 |
| Reason for TP (%) | .001 | |||
| Autoimmune | 8 (5.3) | 3 (4.9) | 5 (5.6) | |
| Fulminant liver failure | 22 (14.7) | 17 (27.9) | 5 (5.6) | |
| Idiopathic | 1 (0.7) | 0 (0.0) | 1 (1.1) | |
| Metabolic | 16 (10.7) | 4 (6.6) | 12 (13.5) | |
| Neoplastic | 3 (2.0) | 3 (4.9) | 0 (0.0) | |
| Obstructive | 98 (65.3) | 34 (55.7) | 64 (71.9) | |
| Other | 2 (1.3) | 0 (0.0) | 2 (2.2) | |
| Type of TP (%) | .277 | |||
| Reduced | 4 (2.7) | 3 (4.9) | 1 (1.1) | |
| Related living donor | 77 (51.3) | 31 (50.8) | 46 (51.7) | |
| Split | 39 (26.0) | 18 (29.5) | 21 (23.6) | |
| Whole | 30 (20.0) | 9 (14.8) | 21 (23.6) | |
| Median weight at TP [IQR] | 12.00 [9.35, 16.00] | 13.00 [9.80, 18.00] | 11.10 [9.00, 15.60] | .106 |
| Median weight‐for‐age Z‐score at TP [IQR] | ‐0.80 [‐1.85, ‐0.06] | ‐0.52 [‐1.46, ‐0.08] | ‐1.18 [‐2.17, ‐0.04] | .018 |
| ABO incompatible donor (%) | 5 (3.3) | 5 (8.2) | 0 (0.0) | .022 |
| CMV risk category (%) | .012 | |||
| Low risk (D‐/R‐) | 5 (3.3) | 0 (0.0) | 5 (5.6) | |
| Intermediate risk (D+/R+ or D‐/R+) | 79 (52.7) | 40 (65.6) | 39 (43.8) | |
| High risk (D+/R‐) | 66 (44.0) | 21 (34.4) | 45 (50.6) | |
| Prophylactic regimen used (%) | .801 | |||
| Acyclovir only | 34 (22.7) | 16 (26.2) | 18 (20.2) | .518 |
| Ganciclovir/acyclovir/valganciclovir | 1 (0.7) | 0 (0.0) | 1 (1.1) | – |
| Ganciclovir/valganciclovir | 5 (3.3) | 2 (3.3) | 3 (3.4) | – |
| Valganciclovir | 58 (38.7) | 24 (39.3) | 34 (38.2) | – |
| None | 52 (34.7) | 19 (31.1) | 33 (37.1) | .507 |
| CMV infection (%) | 43 (28.9) | 20 (32.8) | 23 (26.1) | .486 |
| CMV disease (%) | 30 (20.1) | 13 (21.3) | 17 (19.3) | .928 |
| Secondary prophylaxis (%) | 15/43 (34.9) | 6/20 (30.0) | 9/23 (39.1) | .760 |
| Secondary prophylaxis after CMV disease (%) | 13/30 (43.3) | 5/13 (38.4) | 8/17 (47.0) | .711 |
| CMI‐mediated rejection (%) | 58 (38.7) | 19 (41.3) | 39 (58.2) | .115 |
| Antibody‐mediated rejection (%) | 3 (2.0) | 2 (3.3) | 1 (1.1) | .740 |
| Chronic rejection (%) | 7 (4.7) | 4 (6.6) | 3 (3.4) | .607 |
| Any rejection (%) | 69 (46.3) | 25 (41.0) | 44 (50.0) | .358 |
| Died (%) | 45 (30.0) | 20 (32.8) | 25 (28.1) | .663 |
Note: p‐Values are derived from tests that compare public sector to private sector transplant cases, according to type of variable—chi‐squared test or Fisher's exact test, as appropriate, for categorical variables; Student's t‐test for comparison of means; Kruskal‒Wallis test for comparison of medians.
Abbreviations: CMI, cell‐mediated immunity; CMV, human cytomegalovirus; D, donor; IQR, interquartile range; R, recipient; TP, transplant.
3.2. Characteristics of the study population stratified by facility
Eighty‐nine (59.3%) PLT recipients were from the private sector. State sector patients constituted significantly more black patients than the private sector patients (p = .008). Twenty‐eight (63.6%) of the 44 children under 18 months of age were from the private sector, which was consistent with our findings that more patients from the private sector were at high risk for CMV disease (p = .012). Private sector patients were transplanted at significantly lower weight‐for‐age Z‐scores than state sector PLT recipients (‐1.18 vs. ‐0.52; p = .018), with obstructive causes being the predominant cause for PLT in private sector patients (71.9% vs. 55.7%; p = .001) (Table 1). Fulminant liver failure contributed 27.9% (17/61) of public sector indications for PLT, compared to 5.6% (5/89) in private sector patients (Table 1). The prevalence of ABO incompatible PLT in public sector patients in our cohort (5/61, 8.2%) was significantly higher than that observed in private sector patients (0/89; p = .022) (Table 1). Most PLT recipients received valganciclovir prophylaxis (38.7%) and acyclovir prophylaxis (22.7%), but 34.7% received no prophylaxis. Prophylactic regimens used in public and private sector patients were similar (Table 1). The number of PLTs performed in public sector patients increased over the study period (Figure 1).
FIGURE 1.
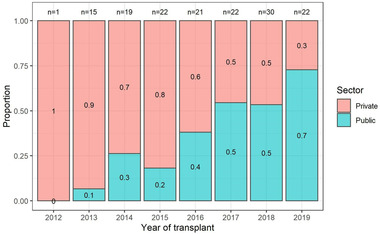
Representation of increasing public health sector pediatric liver transplantation (PLT) recipients over time.
3.3. CMV DNAemia and infection and rejection
Fifty‐three (53/150, 35.3%) patients had CMV DNAemia with a median maximum CMV viral load of 2252 IU/ml (IQR 545–11,350) post‐transplant. Twenty‐two (41.5%) of the 53 patients with CMV viremia post‐transplant received primary CMV prophylaxis, which was found to be statistically significant in protecting the high‐risk category of patients against developing CMV DNAemia as opposed to intermediate‐risk patients (adjusted odds ratio 2.20; 95% confidence interval [CI] 1.00–4.86; p = .049). Seventeen (11.3%) patients had CMV infection, of which seven (41%) had received primary prophylaxis. Four patients with asymptomatic CMV infection progressed to develop CMV disease, and 50% of these patients had received CMV prophylaxis. Among patients with CMV DNAemia, 17 out of 30 (56.7%) patients who developed CMV disease did not receive secondary CMV prophylaxis, while 13 out of 30 (43.3%) patients received secondary prophylaxis (p = .07) (Table 1).
3.4. Covariates associated with CMV disease
Thirty patients (30/149; with available data: 20.1%) developed CMV disease. None of these patients received intravenous ganciclovir as initial prophylaxis. One patient (3%) developed CMV disease while on primary prophylaxis. Ten (33.3%) patients with CMV disease received primary valganciclovir prophylaxis for a median duration of 79 days (IQR 57–121 days), while a median duration of prophylaxis of 100 days (IQR 76–167; p = .364) was observed for patients who did not develop CMV disease. Although CMV prophylaxis did not prevent CMV disease, patients at high risk of CMV disease who did not receive CMV prophylaxis developed CMV disease earlier than those patients who received CMV prophylaxis (Figure 2A). Overall survival was similar regardless of whether CMV prophylaxis was administered in PLT recipients who were at high risk for CMV disease. (Figure 2B). Seventeen (26%) patients in the high‐risk group developed CMV disease as opposed to 13 out of 80 (16%) patients in the intermediate‐risk group, with 47% and 15% of patients having received prophylaxis both in the high‐ and intermediate‐risk groups.
FIGURE 2.
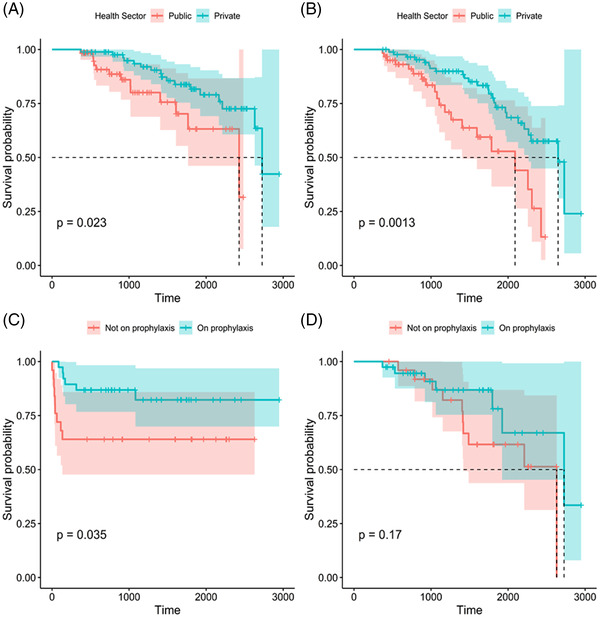
Kaplan‒Meier survival plots for private versus public sector patients: (A) cytomegalovirus (CMV) disease; (B) death in patients at high risk for CMV disease and/or in those with CMV disease; (C) timing of CMV disease with/without CMV prophylaxis; (D) overall survival with/without CMV prophylaxis.
In univariate analysis, age under 18 months (hazard ratio [HR] 2.26), biliary atresia (HR 2.54), receipt of acyclovir prophylaxis (HR 3.08), and post‐transplant lymphoproliferative disorder (HR 5.43) were associated with CMV disease (Table 2). Care in the private sector was associated with a 57% reduced hazard (95% CI 9%–80%) of CMV disease in univariate analysis (Table 2). In multivariate analyses, care in the private sector was consistently associated with an independently lower adjusted hazard of CMV disease compared to care in the public sector (adjusted HR [aHR] ranging from 0.36 to 0.43, depending on the model).
TABLE 2.
Cox proportional hazards models for covariates associated with cytomegalovirus (CMV) disease and covariates associated with death in children at high risk for CMV disease and/or those with CMV disease and death
| Covariates associated with CMV disease | Covariates associated with death in children that were at high risk for CMV disease and/or who developed CMV disease | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CMV disease (n = 30, %) | No CMV disease (n = 119, %) | Univariate (HR, 95% CI) | Model A (aHR, 95% CI) | Model B (aHR, 95% CI) | Died (n = 29, %) | Survived (n = 50, %) | Univariate (HR, 95% CI) | Model C (aHR, 95% CI) | Model D (aHR, 95% CI) | Model E (aHR, 95% CI) | |
| Cyclosporin A | 0 (0.0) | 2 (1.7) | No estimate | – | – | 1 (3.4) | 0 (0.0) | 7.80 (0.97, 62.5) | 13.2 (1.25, 140.0) | – | – |
| PTLD | 11 (36.7) | 5 (4.2) | 5.43 (2.54, 11.6) | 4.55 (1.90, 10.9) | 6.06 (2.61, 14.1) | 3 (10.3) | 12 (24.0) | 0.53 (0.16, 1.77) | – | – | – |
| Acyclovir prophylaxis | 11 (36.7) | 24 (20.2) | 3.08 (1.41, 6.70) | 3.37 (1.33, 8.56) | 4.86 (2.06, 11.5) | 2 (6.9) | 11 (22.0) | 0.58 (0.13, 2.45) | – | – | – |
| Biliary atresia | 22 (73.3) | 58 (48.7) | 2.54 (1.13, 5.73) | 1.87 (0.72, 4.88) | – | 19 (65.5) | 36 (72.0) | 1.18 (0.53, 2.65) | – | – | – |
| Under 18 months | 13 (43.3) | 31 (26.1) | 2.26 (1.08, 4.72) | 1.85 (0.75, 4.53) | 2.24 (0.96, 5.24) | 20 (69.0) | 24 (48.0) | 2.37 (1.04, 5.41) | 1.99 (0.62, 6.39) | – | – |
| Care in private | 17 (56.7) | 71 (59.7) | 0.43 (0.20, 0.91) | 0.36 (0.16, 0.80) | 0.37 (0.17, 0.83) | 18 (62.1) | 32 (64.0) | 0.43 (0.20, 0.94) | 0.33 (0.12, 0.93) | 0.29 (0.11, 0.81) | 0.28 (0.10, 0.80) |
| Azathioprine | 3 (10.0) | 13 (10.9) | 1.82 (0.55, 6.09) | – | 3.51 (0.98, 12.6) | 2 (6.9) | 4 (8.0) | 3.67 (0.80, 16.8) | 3.21 (0.64, 16.0) | – | 4.87 (0.90, 26.4) |
| High risk for CMV disease | 17 (56.7) | 48 (40.3) | 1.73 (0.84, 3.57) | – | – | 28 (96.6) | 38 (76.0) | 3.98 (0.54, 29.3) | – | 4.69 (0.59, 37.3) | – |
| Black | 18 (60.0) | 83 (69.7) | 0.94 (0.45, 1.98) | – | – | 20 (69.0) | 28 (56.0) | 1.90 (0.86, 4.20) | – | – | 2.86 (0.58, 14.1) |
| CMV prophylaxis | 8 (26.7) | 54 (45.4) | 0.46 (0.20, 1.09) | 0.45 (0.18, 1.15) | – | 13 (44.8) | 28 (56.0) | 0.66 (0.31, 1.41) | – | – | 1.15 (0.40, 3.28) |
| Valganciclovir prophylaxis | 10 (33.3) | 54 (45.4) | 0.57 (0.26, 1.25) | – | – | 11 (37.9) | 30 (60.0) | 0.49 (0.23, 1.07) | 0.55 (0.20, 1.49) | – | – |
| EBV infection | 26 (86.7) | 95/114 (83.3) | 1.11 (0.38, 3.18) | – | – | 18/23 (78.3) | 45 (90.0) | 0.73 (0.27, 1.97) | – | 0.36 (0.11, 1.16) | 0.35 (0.10, 1.29) |
| Recipient CMV positive | 13 (43.3) | 66 (55.5) | 0.71 (0.34, 1.46) | – | – | 1 (3.4) | 12 (24.0) | 0.25 (0.03, 1.85) | – | – | 0.18 (0.02, 1.49) |
Note: Statistically significant associations are shown in bold font. Models A and B reflect the two best‐performing models in the Cox proportional hazards analysis of covariates that were associated with CMV disease: Model A included all covariates which in the univariate analysis had a p‐value of <.1, and which did not violate the proportional hazards assumption in the multivariate model; Model B was a stepwise proportional Cox regression model inclusive of covariates which did not violate the proportional hazards assumption. In the model comparison, Model A had an Akaike information criterion (AIC) of 225.6 and a corrected AIC weight (AICcWt) of 40.2%, whereas Model B had an AIC of 224.8 and an AICcWt of 59.8%, reflecting that Model B encapsulated the available data most efficiently. Models C, D, and E were the three best‐performing models in the Cox proportional hazards analysis of covariates associated with death in children at high risk of CMV disease or with CMV disease: Model C included all covariates that had a p‐value of <.1 in the univariate analysis and did not violate the proportional hazards assumption in the multivariate model; Model D was a stepwise proportional Cox regression model inclusive of covariates that did not violate the proportional hazards assumption; Model E was derived through least absolute shrinkage and selection operator (LASSO) regression and included only covariates that did not violate the proportional hazards assumption. In the model comparison, Model C had an AIC of 346.8 and an AICcWt of 37.2%, Model D had an AIC of 346.5 and an AICcWt of 43.8%, and Model E had an AIC of 348.5 and an AICcWt of 16.2%.
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; EBV, Epstein‒Barr virus; HR, hazard ratio; PTLD, post‐transplant lymphoproliferative disease.
3.5. Covariates associated with death in PLT recipients at high risk for CMV disease and/or CMV disease
Of the 79 children who were either at high risk for CMV disease or developed CMV disease, 29 (36.7%) died. Case fatality was lower (16/71, 22.5%), although not significantly so at the 5% significance level, among children who were not at high risk for CMV disease and/or who never developed CMV disease (p = .087). In univariate analysis, age under 18 months (HR 2.37) was the only factor associated with death in children who were at high risk for CMV disease or who developed CMV disease (Table 2; Figure 2C). In univariate and all multivariate models, care in the private sector was consistently associated with a reduced hazard (HR 0.43; 95% CI 0.20–0.94) or adjusted hazard (aHR ranging from 0.28 to 0.33, depending on the model used) of death in PLT recipients with CMV disease or at high risk for CMV disease (Table 2; Figure 2D).
3.6. Sensitivity analysis with inclusion of HHV 6 or 8 infection
A subset of children (46/150, 30.7%) were tested for human herpes virus (HHV) 6 and 8 infection during their follow‐up. A sensitivity analysis of covariates associated with CMV disease or death in children at high risk for CMV disease elucidated HHV 6 or 8 infections as being an important independent association with CMV disease (aHR 6.48; 95% CI 1.91–22.0; p = .003) (Table S1). Even in the multivariate models that incorporated the limited number of children that were tested for HHV 6 or 8 coinfection, care in private retained statistical significance in terms of its association with CMV disease: aHR 0.32 (95% CI 0.10–0.98) and aHR 0.23 (95% CI 0.07–0.77) for the two best‐performing models, respectively (TableS1).
4. DISCUSSION
In this retrospective study, we assessed the incidence and complications of CMV disease in PLT recipients and stratified our findings between public and private sector care. To the best of our knowledge, this is the largest cohort of PLT recipients reviewed in southern Africa for the incidence of CMV infection and disease. A study performed at Red Cross Children's Hospital in SA, the only other PLT center in SA, showed that CMV infection and disease occurred in 12 of 81 (15%) and 10 of 81 (12%) pediatric patients, respectively. 20
Studies from high‐income countries (HIC), such as the United Kingdom, Korea, and Japan, reflected the overall incidence of CMV infection in PLT recipients to be 65%, 21 43%, 22 and 26.5%, 23 respectively, which was higher than what we found in our cohort. A study from Japan found the incidence of CMV disease to be 7.5%. 23 Mohan et al. 24 reported the incidence of CMV disease in India to be 17%, which, although higher than that of HIC, was lower than what we found in our patient population. The higher incidence of CMV disease in public sector patients in our cohort than in private sector patients could be attributable to many factors and mirrors the difference between CMV disease in low‐middle income countries and HICs.
The seroprevalence of CMV infection in sub‐Saharan Africa is increased in infants and young children, with a pooled seroprevalence of approximately 88.1% (range 80%–100%). 25 Our public sector patient population is at increased risk of community acquisition of primary CMV infection due to poor socioeconomic circumstances leading to overcrowded living conditions known to be a major contributory factor correlating with a higher incidence of CMV seropositivity. 25 , 26 A recent analysis of data from an SA birth cohort study by Martinez et al. 27 found that 42% of infants were CMV positive in the first year of life, with breastfeeding probably a major transmission pathway for acquisition of CMV.
We found care in the private sector to be significantly associated with protection against CMV disease and death from CMV disease and a high risk for CMV disease patients, as evidenced through multiple Cox proportional hazards regression models. This finding is most likely attributable to patient factors related to the acquisition of CMV and the socioeconomic bracket of the public as opposed to the private sector patient population. The more rigorous surveillance processes for CMV disease in public sector PLT recipients in our cohort compared to private sector are thus warranted. SA's unique dichotomous healthcare sector and diverse patient population served as a good model where we could compare the different sectors with regard to CMV disease and its associations within one cohort. Other patient factors that would be useful to explore would be adherence factors between the public and private sector patient groups. Shemesh et al. 28 found that a higher Medication Level Variability Index (MLVI) of immunosuppression levels was predictive of increased episodes of late acute rejection leading to poor graft and patient survival. The value of MLVI calculations in our patient population to determine adherence would not have added much value, as most (95%) of our patients did not experience chronic rejection.
There are many antiviral agents that can potentially be utilized for CMV prophylaxis; however, there is a lack of clinical studies comparing the available CMV antivirals, their activities, efficacies, and adverse effects. 25 We found acyclovir prophylaxis to be strongly associated with the development of CMV disease. This was most likely secondary to a large proportion of our PLT recipients in the high‐risk category for developing CMV, receiving acyclovir instead of valganciclovir prophylaxis in error and secondary to the change in policy where the <18‐month age group was classified as high risk only after June 2016. Although most studies have described high‐dose acyclovir as a suitable preventative therapy for CMV, 29 , 30 , 31 ganciclovir and valganciclovir have still been shown to have superior outcomes in terms of CMV prevention in solid‐organ transplant recipients. 29 , 31 , 32 The lack of standardized protocols for prophylaxis and treatment of CMV DNAemia and CMV disease, as well as the erratic adherence of medical staff to protocols, may have also contributed to the poor outcomes and increased CMV disease in our collective cohort. Since this study, there is a stricter emphasis on risk stratifications of PLT patients, so antiviral prophylaxis protocols are strictly followed by all involved in ongoing management of PLT recipients in our center.
HHV 6 reactivation can precede CMV infection 33 , 34 , 35 , 36 and heightened immunosuppression is associated with reactivation of multiple herpesviruses. 37 , 38 , 39 The seroprevalence of HHV 8 in Africa is high (>50%) compared to that described in HIC settings such as Northern Europe and North America (<5%). 40 , 41 The authors thought that performing a sensitivity analysis on this group of patients would add value due to the coinfection of CMV with other herpes viruses, and the increased endemicity of HHV 8 in Africa compared with other parts of the world. Although this may have introduced additional bias due to the small numbers, further exploration into this association would be useful.
The natural history of high‐risk PLT recipients to develop CMV disease sooner was noted in our cohort; however, this did not impact overall survival. A multisite, prospective study would be better suited to assess the impact of duration and type of CMV prophylaxis on long‐term outcomes in PLT recipients. Standardization of prophylaxis and treatment protocols and guidance on routine monitoring for CMV disease in PLT recipients 42 would be anticipated to positively impact patient outcomes.
Delivery of care and differences between the resources available in the different health sectors may have impacted our study outcomes. Patient care in both sectors was performed by an experienced combined multidisciplinary transplant team. Resources lacking in the public sector, such as transplant surgeons and interventional radiologists, were ameliorated by the PPP. Although SA is an upper middle‐income country, it remains one of the most unequal societies in the world, especially regarding its fragmented healthcare system, in which more than 80% of the allocation of healthcare services benefits the highest socioeconomic sectors. 12 , 43 The South African government has recognized this disparity, and plans are underway to implement a new healthcare financing and service delivery system called National Health Insurance (NHI) to reorganize healthcare and achieve universal equal health coverage for all South Africans. The aim of NHI would be to reduce disease burden, cross‐subsidize risk, and minimize inequity through increased access to comprehensive services, increased population coverage of equal healthcare, and financial protection. 43 , 44
4.1. Limitations
Being a retrospective, single‐center study, the data used in the analyses presented here were dependent on clinical records and not always available. Furthermore, treatment and surveillance protocols were clinician dependent and unstandardized, and criteria for assignment of risk categories within the protocols changed during the study period. Delay in the initiation of CMV treatment secondary to delays in laboratory turnaround time was not interrogated in our study and may have impacted the outcomes. The delays in turn‐around time for CMV viral load results only affected public sector patients in our cohort, which potentially under‐represented CMV disease in these patients. We therefore consider our findings to be a minimal estimate of the burden of CMV disease in public sector PLT recipients in our setting. Despite the potential under‐ascertainment of CMV disease in public sector patients due to the prolonged laboratory turn‐around times, the multivariable survival models nevertheless consistently described a significantly higher adjusted hazard of CMV disease in the public sector patients compared to those treated in the private sector. Use of a dosage‐based regimen of valganciclovir rather than the recommended body surface area dosing strategy may have resulted in subtherapeutic levels, contributing to the burden of CMV disease observed in our study. 45 , 46 As the study was not randomized, a causal link between CMV disease and duration and type of CMV prophylaxis cannot be assumed; residual confounding may also account for the associations seen.
5. CONCLUSION
The incidence of CMV infection and disease in our transplant unit was 11% and 20%, respectively. The increased incidence of CMV disease in our cohort, especially in public sector patients, as opposed to HIC, is most likely secondary to a combination of financial constraints leading to overcrowded living conditions and the increased seroprevalence of CMV infection in the pediatric population served by public health sector facilities in SA. Receipt of care in the private health sector was associated with a consistently lower hazard of CMV disease and death in individuals with CMV disease and/or at high risk for CMV disease. The implementation of NHI by the SA government and addressing factors that impact the social determinants of health may lead to the resolution of healthcare‐associated disparities, thus decreasing the burden of diseases, 43 including CMV, as seen in our cohort of PLT recipients.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Conceptualizing study, data collection, writing first and subsequent drafts, and editing and drafting of final version to be published: Priya Walabh. Conceptualizing study, data analysis, writing, editing first and subsequent versions of the manuscript, and approval of final version to be published: David P. Moore. Conceptualizing study, editing first and subsequent versions of the drafts, and approval of final version to be published: Christina Hajinicolaou. Editing final draft of the paper and approval of final draft to be published: Graham Paget and Sechaba T. Palweni. Data collection, and editing and approval of final version to be published: Anja Meyer, Porai Nthabaleng M. Moshesh, and Pravina Walabh.
Supporting information
Table S1. Cox proportional hazards models for covariates associated with cytomegalovirus (CMV) disease—sensitivity analysis inclusive of human herpes virus (HHV) 6 and 8 infection status (n = 46 children with available data on HHV 6 and 8 infection status).
Graphical Abstract
ACKNOWLEDGMENTS
The authors thank Dr. Faith Moyo and all members of the transplant unit at Charlotte Maxeke Johannesburg Academic Hospital. David P. Moore is in part supported by a grant awarded by the Carnegie Corporation of New York.
Walabh P, Moore DP, Paget G, et al. Healthcare disparity and its associations with cytomegalovirus disease in pediatric liver transplant recipients in South Africa. Transpl Infect Dis. 2022;24:e13917. 10.1111/tid.13917
REFERENCES
- 1. Varani S, Landini MP. Cytomegalovirus‐induced immunopathology and its clinical consequences. Herpesviridae. 2011;2(1):6. doi: 10.1186/2042-4280-2-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017;64:87‐91. doi: 10.1093/cid/ciw668 [DOI] [PubMed] [Google Scholar]
- 3. Johannes Busch C, Hermann Siegler B, Werle H, et al. Risk factors for early viral infections after liver transplantation. Langenbecks Arch Surg. 2018;403(4):509‐519. doi: 10.1007/s00423-018-1672-3 [DOI] [PubMed] [Google Scholar]
- 4. Tsai KC, Danziger‐Isakov LA, Banach DB. Cytomegalovirus infection in pediatric solid organ transplant recipients: a focus on prevention. Curr Infect Dis Rep. 2016;18(2):1‐10. doi: 10.1007/s11908-015-0511-8 [DOI] [PubMed] [Google Scholar]
- 5. Green M, Michaels MG. Infections in pediatric solid organ transplant recipients. J Pediatric Infect Dis Soc. 2012;1(2):144‐151. doi: 10.1093/jpids/pir001 [DOI] [PubMed] [Google Scholar]
- 6. Lee S, Razonable RR. Current concepts on cytomegalovirus infection after liver transplantation. World J Hepatol. 2010;2(9):325‐336. doi: 10.4254/wjh.v2.i9.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marcelin JR, Beam E, Razonable RR, Jvon W. Cytomegalovirus infection in liver transplant recipients: updates on clinical management. World J Gastroenterol. 2014;20(31):10658‐10667. Jeanmonod DJ, Rebecca, Suzuki K, et al. We are I. doi: 10.3748/wjg.v20.i31.10658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Danziger‐Isakov L, Bucavalas J. Brief communication current prevention strategies against cytomegalovirus in the studies in pediatric liver transplantation (SPLIT) centers. Am J Transplant. 2014;14(8):1908‐1911. doi: 10.1111/ajt.12755 [DOI] [PubMed] [Google Scholar]
- 9. Burton CE, Dragan T, Mabilangan CA, et al. Assignment of cytomegalovirus infection status in infants awaiting solid organ transplant: viral detection methods as adjuncts to serology. Pediatr Transplant. 2018;22(5):e13229. doi: 10.1111/petr.13229 [DOI] [PubMed] [Google Scholar]
- 10. Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients—guidelines of the American society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33(9):e13512. doi: 10.1111/ctr.13512 [DOI] [PubMed] [Google Scholar]
- 11. Kotton CN, Kumar D, Caliendo AM, et al. The third international consensus guidelines on the management of cytomegalovirus in solid‐organ transplantation. Transplantation. 2018;102(6):900‐931. doi: 10.1097/TP.0000000000002191 [DOI] [PubMed] [Google Scholar]
- 12. Ngobeni V, Breitenbach MC, Aye GC. Technical efficiency of provincial public healthcare in South Africa. Cost Eff Resour Alloc. 2020;18(1):1‐19. doi: 10.1186/s12962-020-0199-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moosa MR. The state of kidney transplantation in South Africa. South African Med J. 2019;109(4):235‐240. doi: 10.7196/SAMJ.2019.v109i4.13548 [DOI] [PubMed] [Google Scholar]
- 14. Hassim A, Heywood M, Berger J. The Private Health Care Sector. Health and Democracy: A Guide to Human Rights, Health Law and Policy in Post‐Apartheid South Africa. South Africa: SiberInk; 2007:162‐199. http://section27.org.za/wp‐content/uploads/2010/04/Chapter6.pdf
- 15. Kotton CN, Kumar D, Caliendo AM, et al. The third international consensus guidelines on the management of cytomegalovirus in solid‐organ transplantation. Rev Transplant. 2018;102(6):900‐931. doi: 10.1097/TP.0000000000002191 [DOI] [PubMed] [Google Scholar]
- 16. Roh J, Kim S, Kwak E, Park J, Park Y. Performance evaluation of the Roche cobas 6800 system for quantifying cytomegalovirus DNA in plasma and urine samples. J Clin Virol. 2021;138(2020):104816. doi: 10.1016/j.jcv.2021.104816 [DOI] [PubMed] [Google Scholar]
- 17. Duncan M, DeVoll‐Zabrocki AM, Etheredge HR, et al. Blood stream infections in children in the first year after liver transplantation at Wits Donald Gordon Medical Centre, South Africa. Pediatr Transplant. 2020;24(2):1‐9. doi: 10.1111/petr.13660 [DOI] [PubMed] [Google Scholar]
- 18. Therneau T. A Package for Survival Analysis in R. R package version 3.3‐1. 2022. https://CRAN.R‐project.org/package=survival
- 19.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2021. https://www.R‐project.org/
- 20. Spearman CW, McCulloch M, Millar AJ, et al. Liver transplantation at Red Cross War Memorial Children's Hospital. S Afr Med J. 2006;96(9 pt 2):960‐963. doi: 10.7196/SAMJ.1295 [DOI] [PubMed] [Google Scholar]
- 21. Verma A, Palaniswamy K, Cremonini G, Heaton N, Dhawan A. Late cytomegalovirus infection in children: high incidence of allograft rejection and hepatitis in donor negative and seropositive liver transplant recipients. Pediatr Transplant. 2017;21(3):1‐5. doi: 10.1111/petr.12879 [DOI] [PubMed] [Google Scholar]
- 22. Kim R, Joung D, Lee S, et al. PGHN cytomegalovirus infection under a hybrid strategy in pediatric liver transplantation: a single‐center experience. Pediatr Gastroenterol Hepatol Nutr. 2017;(3):178‐185. doi: 10.5223/pghn.2017.20.3.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nafady‐Hego H, Elgendy H, Uemoto S. Contrast patterns of Cytomegalovirus and Epstein‒Barr virus infection in pediatric living‐donor liver transplant recipients. Exp Clin Transplant. 2015;13:75‐82. doi: 10.6002/ect.mesot2014.O26 [DOI] [PubMed] [Google Scholar]
- 24. Mohan N, Karkra S, Rastogi A, et al. Outcome of 200 pediatric living donor liver transplantations in India. Indian Pediatr. 2017;54(11):913‐918. doi: 10.1007/s13312-017-1181-4 [DOI] [PubMed] [Google Scholar]
- 25. Bates M, Brantsaeter AB. Human cytomegalovirus (CMV) in Africa : a neglected but important pathogen. J Virus Erad. 2016;2(CMV):136‐142. doi: 10.1185/03007995.2015.1019608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20(4):202‐213. doi: 10.1002/rmv.655 [DOI] [PubMed] [Google Scholar]
- 27. Martinez L, Nicol MP, Wedderburn CJ, et al. Cytomegalovirus acquisition in infancy and the risk of tuberculosis disease in childhood: a longitudinal birth cohort study in Cape Town, South Africa. Lancet Glob Heal. 2021;9(12):e1740‐e1749. doi: 10.1016/S2214-109X(21)00407-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shemesh E, Bucuvalas JC, Anand R, et al. The Medication Level Variability Index (MLVI) predicts poor liver transplant outcomes: a prospective multi‐site study. Am J Transplant. 2017;17(10):2668‐2678. doi: 10.1111/ajt.14276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jorgenson MR, Descourouez JL, Leverson GE, et al. High‐dose acyclovir for cytomegalovirus prophylaxis in seropositive abdominal transplant recipients. Ann Pharmacother. 2018;52(1):5‐10. doi: 10.1177/1060028017728296 [DOI] [PubMed] [Google Scholar]
- 30. Barkholt L, Lewensohn‐Fuchs I, Ericzon BG, Tydén G, Andersson J. High‐dose acyclovir prophylaxis reduces cytomegalovirus disease in liver transplant patients. Transpl Infect Dis. 1999;1(2):89‐97. doi: 10.1034/j.1399-3062.1999.010202.x [DOI] [PubMed] [Google Scholar]
- 31. Winston DJ, Wirin D, Shaked A, Busuttil RW. Randomised comparison of ganciclovir and high‐dose acyclovir for long‐term cytomegalovirus prophylaxis in liver‐transplant recipients. Lancet. 1995;346(8967):69‐74. doi: 10.1016/S0140-6736(95)92110-9 [DOI] [PubMed] [Google Scholar]
- 32. Zhang Y, Zhou T, Huang M, Gu G, Xia Q. Prevention of cytomegalovirus infection after solid organ transplantation: a Bayesian network analysis. Ann Clin Microbiol Antimicrob. 2020;19(1):1‐14. doi: 10.1186/s12941-020-00372-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mysore KR, Phan TL, Himes RW, et al. Human herpesvirus 6 infection in pediatric liver transplantation: single‐center study of incidence, outcomes, and management. J Pediatric Infect Dis Soc. 2021;10(5):599‐606. doi: 10.1093/jpids/piaa166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Humar A, Malkan G, Moussa G, Greig P, Levy G, Mazzulli T. Human herpesvirus‐6 is associated with cytomegalovirus reactivation in liver transplant recipients. J Infect Dis. 2000;181(4):1450‐1453. doi: 10.1086/315391 [DOI] [PubMed] [Google Scholar]
- 35. Singh N, Winston DJ, Razonable RR, et al. Association of HHV‐6 with outcomes in CMV‐seronegative liver transplant recipients with CMV‐seropositive donors receiving preemptive antiviral therapy. Transplantation. 2020;105(11):2427‐2434. doi: 10.1097/tp.0000000000003604 [DOI] [PubMed] [Google Scholar]
- 36. Nasimfar A, Sadeghi E, Alborzi A, et al. The activation of cytomegalovirus and human herpes virus 6 after liver transplantation. Hepat Mon. 2018;18(2):e11987. doi: 10.5812/hepatmon.11987 [DOI] [Google Scholar]
- 37. Razonable RR, Brown RA, Humar A, Covington E, Alecock E, Paya CV. Herpesvirus infections in solid organ transplant patients at high risk of primary cytomegalovirus disease. Society. 2005;55905:1331‐1339. doi: 10.1086/466529 [DOI] [PubMed] [Google Scholar]
- 38. Razonable RR. Human herpesviruses 6, 7 and 8 in solid organ transplant recipients. Am J Transplant. 2013;13(suppl 3):67‐78. doi: 10.1111/ajt.12008 [DOI] [PubMed] [Google Scholar]
- 39. Pellett Madan R, Hand J. Human herpesvirus 6, 7, and 8 in solid organ transplantation: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13518. doi: 10.1111/ctr.13518 [DOI] [PubMed] [Google Scholar]
- 40. Ariza‐Heredia EJ, Razonable RR. Human herpes virus 8 in solid organ transplantation. Transplantation. 2011;92(8):837‐844. doi: 10.1097/TP.0b013e31823104ec [DOI] [PubMed] [Google Scholar]
- 41. Jenkins FJ, Hoffman LJ, Liegey‐Dougall A. Reactivation of and primary infection with human herpesvirus 8 among solid‐organ transplant recipients. J Infect Dis. 2002;185(9):1238‐1243. doi: 10.1086/340237 [DOI] [PubMed] [Google Scholar]
- 42. Ganapathi L, Blumenthal J, Alawdah L, et al. Impact of standardized protocols for cytomegalovirus disease prevention in pediatric solid organ transplant recipients. Pediatr Transplant. 2019;23(7):e13568. doi: 10.1111/petr.13568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Murphy SD, Moosa S. The views of public service managers on the implementation of National Health Insurance in primary care: a case of Johannesburg Health District, Gauteng Province, Republic of South Africa. BMC Health Serv Res. 2021;21(1):1‐9. doi: 10.1186/s12913-021-06990-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Young M. Private vs Public Healthcare in South Africa. Honor Thesis. Western Michigan University; 2016.
- 45. Pescovitz MD, Ettenger RB, Strife CF, et al. Pharmacokinetics of oral valganciclovir solution and intravenous ganciclovir in pediatric renal and liver transplant recipients. Transpl Infect Dis. 2010;12(3):195‐203. doi: 10.1111/j.1399-3062.2009.00478.x [DOI] [PubMed] [Google Scholar]
- 46. Shaikh S, Jasiak‐Panek N, Park JM. A national survey of valganciclovir dosing strategies in pediatric organ transplant recipients. Clin Transplant. 2018;32(9):e13369. doi: 10.1111/ctr.13369 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cox proportional hazards models for covariates associated with cytomegalovirus (CMV) disease—sensitivity analysis inclusive of human herpes virus (HHV) 6 and 8 infection status (n = 46 children with available data on HHV 6 and 8 infection status).
Graphical Abstract


