Abstract
Background
Although vonoprazan has been proven to be a highly potent drug for Helicobacter pylori eradication, there have been no randomized trials comparing the effectiveness of regimens containing vonoprazan 20 mg daily with alternative standard strategies. We aimed to assess the efficacy, tolerance, and cost‐effectiveness of quadruple therapy with vonoprazan 20 mg daily as a first‐line therapy for H. pylori eradication.
Materials and Methods
We conducted a single‐center, open‐label, noninferiority, randomized controlled study in Zhejiang, China. Treatment‐naive H. pylori‐positive participants (n = 234) were randomly assigned to three groups in a 1:1:1 ratio: vonoprazan 20 mg daily with amoxicillin 1000 mg, furazolidone 100 mg and colloidal bismuth 200 mg each given twice a day for 10 days (V10) or 14 days (V14), or esomeprazole 20 mg with amoxicillin 1000 mg, furazolidone 100 mg and colloidal bismuth 200 mg each given twice a day for 14 days (E14). The primary endpoint was the eradication rates in each group. The secondary endpoints were the incidence of adverse events (AEs) and compliance.
Results
The eradication rates in the V10, V14 and E14 groups were 96.2% (89.2–99.2%), 94.9% (87.4–98.6%), and 93.6% (85.7–97.9%) in the intention‐to‐treat analysis, and 98.6% (92.7–100.0%), 97.4% (90.8–99.7%), and 94.8% (87.2–98.6%) in the per‐protocol analysis, respectively. Quadruple therapy with vonoprazan 20 mg daily was noninferior to the esomeprazole‐based regimen (Farrington and Manning test: margin 10%, significance level 2.5%). The adverse event rates were 12.8% versus 3.8% versus 6.4% in the V10, V14, and E14 groups, respectively. All regimens were well tolerated without significant differences (p = 0.096). The cost‐effectiveness ratio was 1.32, 1.88, and 3.06 for the V10, V14, and E14 groups in the intention‐to‐treat analysis, respectively. (NCT04907747).
Conclusions
Vonoprazan (20 mg daily) was as effective as esomeprazole (20 mg twice a day) in quadruple therapies for the eradication of H. pylori, was more economical, and was well tolerated. In addition, the 10‐day regimen of vonoprazan (20 mg daily) was comparable to the 14‐day regimen.
Keywords: economic efficiency, Helicobacter pylori, quadruple therapy, vonoprazan
1. INTRODUCTION
Helicobacter pylori (H. pylori) infection is a general condition that is usually lifelong, with a prevalence reaching approximately 50% worldwide 1 ; in China, the prevalence has recently reached 44%. 2 In December 2021, the U.S. Department of Health and Human Services listed chronic H. pylori as a clear carcinogen in the 15th report on carcinogens. It has also been proven to be closely related to multiple gastrointestinal (GI) diseases such as gastric adenocarcinoma, peptic ulcer (gastric and duodenal ulcer), chronic gastritis, and mucosa‐associated lymphoid tissue (MALT) lymphoma. 3 , 4 , 5 Successful H. pylori eradication would not only benefit individuals by improving histologic gastritis, preventing gastric carcinogenesis, and reducing the risk of serious complications but also block transmission and alleviate future medical expenses. 6 , 7 , 8
However, H. pylori eradication rates have been decreasing in recent years, while the antibiotic resistance rate continues to increase. 9 In general, recommended eradication regimens should be based on the best locally available resources. 6 In China, quadruple therapy (two antibiotics + bismuth + an acid inhibitor) is currently the optimal first‐line treatment for H. pylori infection. Among all antibiotics, the resistance rates of amoxicillin, tetracycline, and furazolidone are low, so it is unnecessary to consider resistance when they are part of a treatment regimen. 10 In addition, proton pump inhibitors (PPIs) are the main evidence‐based prescription acid inhibitors in China and play a key role in H. pylori treatment: They (1) raise the intragastric pH to keep H. pylori in a reproductive state and easy to eliminate; (2) reduce the minimum inhibitory concentration (MIC) of most antibiotics; and (3) inhibit urease activity and decrease the metabolic activity of H. pylori and its colonization in the stomach. 11 However, a patient's response to PPIs is largely dependent on the CYP2C19 gene polymorphism involved in proton pump inhibitor metabolism, 12 , 13 , 14 influenced by a strict dosing time of 30 min before meals, and typically requires more than 75–100 h to achieve the maximum effect. 15 These limitations have resulted in an unmet need for PPI‐containing H. pylori eradication regimens.
Vonoprazan, a potent first‐in‐class potassium‐competitive acid blocker (P‐CAB), was first approved in Japan in 2015. It competitively blocks the activity of potassium to hydrogen‐potassium ATPase and shows a more substantial rapid and durable acid‐inhibiting effect than PPIs. 16 , 17 Moreover, vonoprazan outperforms PPIs in many aspects, including but not limited to activation without a high acid concentration, valid inhibition of proton pumps, fast‐action and independence of CYP2C19 polymorphisms. 18 , 19 , 20 Targeted pH values (commonly above 5, possibly higher than 6) 21 are always reliably achieved by vonoprazan, which makes this novel acid inhibitor a potential adjuvant for H. pylori eradication therapy. Several previous studies reported higher eradication rates associated with vonoprazan (20 mg twice a day)‐based first‐line therapy than with PPI‐based therapies. 22 , 23 Furthermore, previous studies showed that vonoprazan 20 mg daily achieved high pH ≥4 holding time ratios (HTRs), suggesting efficacy similar to that of 20 mg esomeprazole twice a day, irrespective of CYP2C19 genotype. 19 However, there have been few studies on the effectiveness of vonoprazan 20 mg daily as a crucial component of H. pylori therapies.
We suspected that vonoprazan 20 mg daily may be effective as a component of H. pylori eradication therapy. Therefore, this study aimed to assess the effectiveness and safety of quadruple therapy with vonoprazan 20 mg daily as a first‐line treatment compared to quadruple therapy with esomeprazole 20 mg twice a day, the well‐acknowledged standard‐dose high potency PPI‐containing quadruple therapy for H. pylori eradication.
2. MATERIALS AND METHODS
2.1. Design and ethical issues
This was an open‐label, single‐center, noninferiority and randomized controlled clinical trial performed at the Second Affiliated Hospital, School of Medicine, Zhejiang University between June 2021 and October 2021. This trial was conducted according to the Declaration of Helsinki and approved by the Ethics Committee of the Second Affiliated Hospital, School of Medicine, Zhejiang University. This study was registered at ClinicalTrials.gov with the identifier NCT04907747.
2.2. Study participants
H. pylori‐positive participants aged 18–65 with no history of treatment were eligible for inclusion in this trial. Participants with alarm symptoms, a family history of gastric cancer or age over 35 years old were requested to undergo an endoscopy. The diagnosis of H. pylori infection was confirmed by one or more of the following methodologies before treatment: gastric biopsy using histochemical staining, tissue culture, the 14C‐urea breath test (UBT), and/or the 13C‐UBT. Participants were excluded if they (1) used antibiotics or bismuth within four weeks before inclusion or acid inhibitor use, including H2 receptor antagonist (H2RA), PPI or P‐CAB use, within two weeks prior to inclusion; (2) had an active peptic ulcer with complications such as hemorrhage, perforation, or obstruction; (3) had a history of esophagectomy or gastrectomy; (4) had an allergy to any study drug; (5) had severe comorbidities or physical or mental diseases; (6) were pregnant or breastfeeding; or (7) had a history of alcohol abuse or drug addiction. All subjects in this study signed written informed consent forms before enrollment.
2.3. Randomization and intervention
Participants were randomly assigned to receive V10, V14, or E14 therapy in a 1:1:1 ratio using a computer‐generated algorithm for the trial treatment. V10 therapy consisted of vonoprazan 20 mg daily, amoxicillin 1000 mg, furazolidone 100 mg, and colloidal bismuth 200 mg twice a day for 10 days. V14 therapy consisted of vonoprazan 20 mg daily and amoxicillin 1000 mg, furazolidone and colloidal bismuth 200 mg twice a day for 14 days. E14 therapy consisted of esomeprazole 20 mg, amoxicillin 1000 mg, furazolidone 100 mg, and colloidal bismuth 200 mg twice a day for 14 days. Vonoprazan was administered at a regular time without regard to meals. Colloidal bismuth and esomeprazole were administered 30 min before breakfast and dinner, and amoxicillin and furazolidone were administered immediately after breakfast and dinner. Participants and researchers were not blinded to the assigned treatment groups. Urea breath test personnel were unaware of the treatment given.
The study drugs were as follows: vonoprazan was from Tianjin Takeda Pharmaceutical Co., Ltd, and esomeprazole was from AstraZeneca Pharmaceuticals Ltd. Amoxicillin was obtained from CSPC Zhongnuo Pharmaceutical (Shijiazhuang) Co., Ltd. Furazolidone was obtained from Tianjin Lisheng Pharmaceutical Co., Ltd. Colloidal bismuth was obtained from Shanxi Ante Biopharmaceutical Co., Ltd. All study drugs were obtained from the hospital pharmacy.
2.4. Study outcomes
The primary endpoint of this study was eradication rates in each group. The eradication situation was confirmed by 13C‐UBT 6–8 weeks after the treatment. The cutoff value of the 13C‐UBT was 4.0‰ (delta over baseline, DOB) and that of the 14C‐UBT was 100 (disintegrations per minute, DPM). Participants were not permitted to take PPIs or P‐CABs two weeks prior to the urea breath test or any antibiotics four weeks prior to the urea breath test.
The secondary endpoints were the incidence of adverse events (AEs) and compliance. We measured AEs and compliance with a questionnaire following the completion of treatment. Good compliance was defined as taking at least 80% of the pills. According to the impact on daily life, there were three categories of side effects: mild (transient, well tolerated), moderate (some discomfort that interfered with daily life), and severe (significant discomfort that adversely affected daily life).
2.5. Sample size calculation
In a previous study, similar regimens for first‐line H. pylori treatment achieved a 95% eradication rate. 24 We assumed eradication rates of 95% in three regimens and used a noninferiority design in the trial. A noninferiority margin of 10% was suggested for noninferiority clinical trials of antimicrobial drugs and H. pylori treatments. Supposing a power of 80% and an alpha of 0.025 (one‐sided), at least 222 patients (74 patients per treatment group) would be required for the noninferiority study. Supposing the rate of loss to follow‐up was 5%, a total of 234 subjects were required for recruitment. The sample size was calculated using PASS software, version 11.0.10 (NCSS, LLC).
2.6. Cost‐effectiveness analysis
We carried out the cost‐effectiveness analysis as a pharmacoeconomic model. In the present study, we only assessed the direct medical cost consisting of the costs of the study drugs. Effectiveness is determined by H. pylori eradication rates. The cost‐effectiveness ratio (CER) was calculated by dividing the costs by eradication rates. An incremental cost‐effectiveness ratio (ICER) was calculated by dividing the incremental costs by incremental eradication rates and expressed in terms of the additional cost of obtaining a 1% increase in eradication rates.
We performed sensitivity analysis to investigate the robustness of the outcomes. Sensitivity analysis was based on the different H. pylori eradication rates while the costs were fixed. We defined the worst effectiveness as the minimal acceptable effectiveness (90%) and the best effectiveness as the highest eradication rate (98.6%) in the present study. Moderate effectiveness is the average effectiveness (94.3%) between them.
2.7. Statistical analysis
Categorical variables are presented as absolute frequencies (proportions), and continuous variables are shown as the means ± standard deviations (SDs). Categorical variables were compared by the chi‐square test or Fisher's exact test. Continuous variables were compared using analysis of variance (ANOVA). The statistical significance level (p value) was less than 0.05. SPSS software, version 26.0 (SPSS Inc.) was used for statistical analyses.
Intention‐to‐treat (ITT) analysis included all enrolled participants, and those subjects who did not complete the urea breath test 6–8 weeks after eradication therapy were considered to have treatment failure in the ITT analysis. Study participants who discontinued the regimen because of AEs or were lost to follow‐up were excluded from the per‐protocol (PP) analysis. Noninferiority was accessed by the Farrington and Manning test with a noninferiority margin of 10% at a significance level of 2.5% (one‐sided), using SAS software, version 9.04.01M6 (SAS Institute Inc.). There might be evidence of superiority if the lower limit of the 95% confidence interval of the difference between regimens was greater than zero.
3. RESULTS
3.1. Participant enrollment and baseline characteristics
A total of 234 participants were registered and randomly allocated to each regimen group. The flow chart for study patient enrollment is shown in Figure 1. An overview of demographic information and clinical characteristics is shown in Table 1. There was no significant difference between the three groups. One participant in the V10 regimen missed follow‐up visits. Three participants in the V10 regimen, two participants in the V14 regimen, and one participant in the E14 regimen discontinued the treatment because of diverse adverse events. Participants who did not complete the urease breath test after the treatment were considered to have eradication failure in the intention‐to‐treat analysis.
FIGURE 1.
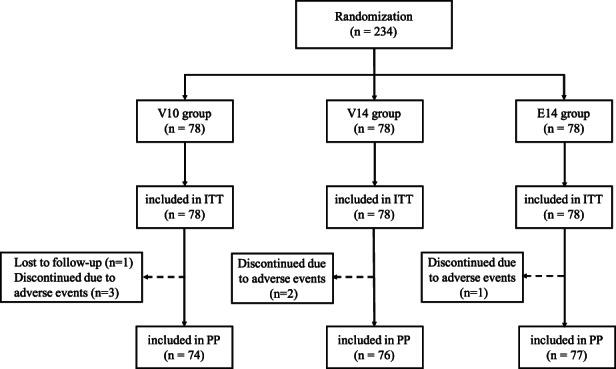
Flow diagram of this study. V10, vonoprazan‐containing 10‐day therapy; V14, vonoprazan‐containing 14‐day therapy; E14, esomeprazole‐containing 14‐day therapy. ITT, intent‐to‐treat; PP, per protocol.
TABLE 1.
Baseline characteristics of study participants
| Variables | V10 (n = 78) | V14 (n = 78) | E14 (n = 78) | p Value |
|---|---|---|---|---|
| Gender | 0.719 | |||
| Male | 32 | 37 | 34 | |
| Female | 46 | 41 | 44 | |
| Age (mean, SD), y | 37.14 (19.65) | 35.88 (11.51) | 36.54 (10.96) | 0.777 |
| Range | 23–65 | 22–65 | 19–63 | |
| BMI (mean, SD) kg/m2 | 21.49 (2.96) | 22.08 (3.43) | 22.10 (3.60) | 0.437 |
| Family history | 3 | 3 | 5 | 0.796 |
| Education level | 0.545 | |||
| Low | 14 | 20 | 18 | |
| High | 64 | 58 | 60 | |
| Marriage | 0.541 | |||
| Unmarried | 18 | 24 | 20 | |
| Married | 60 | 54 | 58 | |
| Basic diseases | ||||
| Hypertension | 2 | 2 | 2 | 1.000 |
| Diabetes | 0 | 6 | 0 | 0.004 |
Abbreviations: BMI, body mass index; E14, esomeprazole‐containing 14‐day therapy; V10, vonoprazan‐containing 10‐day therapy; V14, vonoprazan‐containing 14‐day therapy.
3.2. H. pylori eradication rates
In the ITT analysis, the overall eradication rates and 95% confidence intervals (CIs) were 96.2% (95% CI 89.2% to 99.2%) in the V10 group, 94.9% (95% CI 87.4% to 98.6%) in the V14 group, and 93.6% (95% CI 85.7% to 97.9%) in the E14 group. According to the PP analysis, the eradication rates were 98.6% (95% CI 92.7% to 100.0%) in the V10 group, 97.4% (95% CI 90.8% to 99.7%) in the V14 group, and 94.8% (95% CI 87.2% to 98.6%) in the E14 group (overall values are shown in Table 2).
TABLE 2.
Eradication rates of each therapy group
| Analysis | V10 | V14 | E14 |
|---|---|---|---|
| ITT | 96.2% (75/78) | 94.9% (74/78) | 93.6% (73/78) |
| 95% CI | 89.2% to 99.2% | 87.4% to 98.6% | 85.7% to 97.9% |
| Difference from E14 | 2.56% | 1.28% | |
| 95% CI | −6.08% to 11.2% | −7.46% to 10.02% | |
| p for non‐inferiority | 0.002 | 0.006 | |
| PP | 98.6% (73/74) | 97.4% (74/76) | 94.8% (73/77) |
| 95% CI | 92.7% to 100.0% | 90.8% to 99.7% | 87.2% to 98.6% |
| Difference from E14 | 3.84% | 2.56% | |
| 95% CI | −4.54% to 12.23% | −5.79% to 10.92% | |
| p for non‐inferiority | <0.001 | 0.002 |
Abbreviations: E14, esomeprazole‐containing 14‐day therapy; ITT, intent‐to‐treat; PP, per protocol; V10, vonoprazan‐containing 10‐day therapy; V14, vonoprazan‐containing 14‐day therapy.
By ITT analysis, the differences between the V10, V14, and E14 groups were 2.56% (95% CI: −6.08% to 11.2%) and 1.28% (95% CI: −7.46% to 10.02%), respectively. Similarly, the results were found by PP analysis: The differences with the E14 group were 3.84% (95% CI: −4.54% to 12.23%) for the V10 group and 2.56% (95% CI: −5.79% to 10.92%) for the V14 group, The lower bounds of the 95% CIs for the differences in H. pylori eradication rates in the V10 group and V14 group compared with E14 group were more remarkable than the pre‐specified noninferiority margin in both the ITT analysis (p = 0.002, p = 0.006, respectively) and the PP analysis (p < 0.001, p = 0.002, respectively).
3.3. Adverse events
The most common adverse events among participants were skin rash, fever, abdominal pain, nausea, vomiting, diarrhea, dizziness, and so on, which are summarized in Table 3. Among these events, five participants complained of skin rash: One case resolved without intervention in two days, one case resolved with self‐medication with oral anti‐allergic agents, and the other three patients with skin rash discontinued treatment due to concerns about the rashes. Seven participants suffered from fever: One was lost to follow‐up, one was diagnosed with upper respiratory tract infection and cured with Tylenol, and five discontinued treatment and were cured without intervention. One participant in the V10 group reported perineal pruritus on the eighth day of therapy and was diagnosed with Candida vaginitis. These AEs were mild and fully recovered in the short term (Table S1). The overall AE rates among the V10 group, V14 group, and E14 group were 12.8%, 3.8%, and 6.4%, respectively, and there was no significant difference among the regimens (p = 0.096).
TABLE 3.
Adverse events in each regimen group
| Adverse events | V10 | V14 | E14 | p Value |
|---|---|---|---|---|
| Total, n (%) | 10 (12.8%) | 3 (3.8%) | 5 (6.4%) | 0.096 |
| Skin rash | 3 | 0 | 2 | |
| Fever | 3 | 1 | 3 | |
| Abdominal pain | 1 | 2 | 0 | |
| Nausea | 1 | 0 | 1 | |
| Vomiting | 0 | 0 | 1 | |
| Diarrhea | 1 | 0 | 0 | |
| Dizziness | 0 | 1 | 0 | |
| Candida Vaginitis | 1 | 0 | 0 |
Abbreviations: E14, esomeprazole‐containing 14‐day therapy; V10, vonoprazan‐containing 10‐day therapy; V14, vonoprazan‐containing 14‐day therapy.
3.4. Cost‐effectiveness analysis
The direct medical costs, CERs, and ICERs compared to the E14 group are presented in Table 4. The highest CER was for 14‐day esomeprazole‐based therapy. Sensitivity analysis was performed to quantify the impact of variations in eradication rates on cost‐effectiveness (Table S2).
TABLE 4.
Cost‐effectiveness ratios
| Analysis | Group | Total cost | Eradication rates | CER | ICER |
|---|---|---|---|---|---|
| ITT | V10 | 127.20 | 96.2% (75/78) | 1.32 | −61.25 |
| V14 | 178.08 | 94.9% (74/78) | 1.88 | −83.35 | |
| E14 | 286.44 | 93.6% (73/78) | 3.06 | ||
| PP | V10 | 127.20 | 98.6% (73/74) | 1.29 | −41.91 |
| V14 | 178.08 | 97.4% (74/76) | 1.83 | −41.68 | |
| E14 | 286.44 | 94.8% (73/77) | 3.02 |
Note: ICER was calculated compared V10 group and V14 group to E14 group.
Abbreviations: CER, cost‐effectiveness ratio; E14, esomeprazole‐containing 14‐day therapy; ICER, incremental cost‐effectiveness ratio; ITT, intent‐to‐treat; PP, per protocol; V10, vonoprazan‐containing 10‐day therapy; V14, vonoprazan‐containing 14‐day therapy.
4. DISCUSSION
The continuously declining eradication rates associated with conventional PPI‐containing triple therapies have become a concern globally, and many measures have been proposed to improve strategies for H. pylori eradication, including high‐dose PPI‐based regimens or a prolonged treatment duration (extended to 10 or 14 days), which have reportedly higher eradication rates. 25 Potent acid inhibitors may optimize eradication regimens partly because H. pylori regains its ability to replicate and is more susceptible to antimicrobial agents at a pH >6. 26 In addition, nocturnal acid breakthrough (NAB) is associated with unsatisfactory eradication rates. 27 , 28 A higher eradication rate has been achieved in patients without NAB than in subjects with existing NAB. 27 Vonoprazan, a novel acid inhibitor with rapid and long‐acting efficacy, has displayed remarkable treatment performance in many acid‐related diseases, such as reflux esophagitis, peptic ulcer, and H. pylori eradication. 29 , 30 It may overcome the difficulty associated with NAB by maintaining elevating gastric pH throughout the night, 31 which probably plays a noneligible role in the outstanding eradication rates reported with vonoprazan‐based regimens. A meta‐analysis of 3 RCTs (897 patients) showed that as a first‐line strategy, vonoprazan‐based triple therapy resulted in higher eradication rates than PPI‐based triple therapy (ITT pooled eradication rates:91.4% versus 74.8%; OR: 3.68, 95% CI: 1.87–7.26), while the incidence of AEs was lower (pooled cadence:32.7% versus 40.5%; OR: 0.71, 95% CI: 0.53–0.95). 23
In the present study, statistical analysis indicated that quadruple therapy containing vonoprazan 20 mg daily for 10 or 14 days has acceptable efficacy for H. pylori eradication, compared to the well‐acknowledged high potency PPI (esomeprazole 20 mg twice a day) for 14 days. In the ITT analysis, V10 therapy showed a satisfactory eradication rate of 96.2% and V14 therapy showed a satisfactory eradication rate of 94.9%. Moreover, promising eradication rates of over 97% were observed with both therapies containing vonoprazan 20 mg daily in the PP analysis. These results indicated that quadruple therapies containing vonoprazan 20 mg daily had eradication rates comparable to those of esomeprazole‐containing quadruple regimens in both the ITT and PP analyses. Surprisingly, the V10 regimen, despite a shorter course of treatment, achieved an acceptable eradication rate compared with the E14 regimen. Similarly, previous studies in different regions have shown that 10‐day bismuth quadruple therapy (BQT) is noninferior to 14‐day BQT in terms of eradication effectiveness, 32 , 33 which means that shortening the treatment duration has multiple advantages, such as improving patient adherence, reducing antibiotic resistance and saving drug costs. Therefore, we can prudently assume that a 10‐day regimen containing vonoprazan 20 mg daily may be a potential recommended treatment as first‐line therapy in H. pylori‐positive patients.
In addition, nonadherence may partly contribute to eradication failure and severe side effects of treatment as well as the complexity of the regimen may be a major cause of poor compliance. In this study, only five participants among those who received the vonoprazan‐based therapies discontinued therapy due to not serious AEs: Three of them completed the UBT after therapy to confirm the H. pylori infection status; Of them, two patients successfully eradicated H. pylori infection, although they did not complete the entire course of treatment. Overall, the symptoms of AEs in the three groups were mild and rapidly reversible, without any serious consequences. The safety outcomes of this study were satisfactory and exhibited no significant difference among the three groups.
We further interpreted the results to indicate that H. pylori was not successfully eradicated. Twelve participants were considered to have H. pylori eradication failure according to the ITT analysis; three of them did not undergo the UBT after eradication due to loss to follow‐up or AEs. Seven of the remaining nine participants showed UBT results close to the cutoff value. Three of them retook UBT test and one underwent a monoclonal stool antigen test (SAT) and all turned out to be negative. One participant was confirmed to have H. pylori infection by biopsy, and the other two did not undergo any extra examinations. UBT is the recommended method for the confirmation of H. pylori eradication status, and monoclonal SAT is an alternative. 10 , 34 However, UBT results close to the cutoff are often less accurate, which may lead to an underestimation of eradication rates.
According to common sense, a shorter course of treatment and less frequent medication use will reduce the incidence of side effects and improve compliance. Unfortunately, this feature was not shown in this study, which may need to be confirmed by enlarging the sample size and conducting further multicenter studies.
This study demonstrated satisfactory eradication rates with furazolidone‐containing therapies. However, despite its extremely low resistance rate and cost, furazolidone is not available in some countries due to its potentially severe side effects. 35 An observational study in China showed high eradication rates with furazolidone‐based quadruple therapies, with an AE incidence of 8.2%. The total number of AEs associated with the 10‐day regimen was lower than that associated with the 14‐day regimen (6.1% vs. 17.4%). 36 Consistently, the three furazolidone‐containing quadruple regimens had similar incidence rates of AEs in our trial (12.8% vs. 3.8% vs. 6.4% for V10 vs. V14 vs. E14, p = 0.096), while the overall incidence of AEs was 7.69%.
The cost‐effectiveness of H. pylori regimens is a crucial consideration for promoting population‐based H. pylori screening and intervention programs in China. In accordance with prior data, our study showed that quadruple therapies containing vonoprazan 20 mg daily had the characteristics of lower cost and comparable efficiency in H. pylori‐naïve patients as regimens based on esomeprazole 20 mg twice a day.
This study has some limitations worth considering: (1) Lack of blinding may have introduced treatment bias and may have impacted the reporting of AEs; (2) the single‐center study design may have limited the generalizability of the results; (3) individuals over 65 years of age were excluded, which means that the findings cannot be generalized to these patients. Whether elderly patients should be treated remains controversial, and their optimal treatment options require further research, and (4) further study such as susceptibility test and 24 h pH monitor would better interpret the clinical outcomes.
In conclusion, quadruple therapies with vonoprazan 20 mg daily were noninferior to esomeprazole 20 mg twice a day for H. pylori eradication in Chinese patients. Vonoprazan‐containing therapies were well tolerated with a favorable safety profile and cost‐effectiveness. Lower doses, more flexible dosing schedules, and shorter durations are highly convenient for patients. Vonoprazan therefore would be a promising alternative to esomeprazole as a first‐line regimen for H. pylori eradication at the recommended dose of 20 mg daily. Further studies are required to explore the availability of vonoprazan‐based therapies for H. pylori infection.
AUTHOR CONTRIBUTIONS
Lifen Lu and Huihui Yan performed the research; Yuehua Han, Guochun Lou, and Yan Li collected the data; Lifen Lu and Yujing Wang designed the research study, analyzed the data, and wrote the paper; Qin Du, Huihui Yan, and Jun Ye contributed to the design of the study and revised the paper. All authors approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Supporting information
Table S1.
Appendix S1.
ACKNOWLEDGMENTS
The authors would like to thank all the investigators and participants who contributed to this study.
Lu L, Wang Y, Ye J, et al. Quadruple therapy with vonoprazan 20 mg daily as a first‐line treatment for Helicobacter pylori infection: A single‐center, open‐label, noninferiority, randomized controlled trial. Helicobacter. 2023;28:e12940. doi: 10.1111/hel.12940
Lifen Lu and Yujing Wang should be considered joint first author.
Qin Du and Huihui Yan should be considered joint senior author.
Contributor Information
Huihui Yan, Email: yandoublehui@zju.edu.cn.
Qin Du, Email: duqin@zju.edu.cn.
REFERENCES
- 1. Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta‐analysis. Gastroenterology. 2017;153:420‐429. [DOI] [PubMed] [Google Scholar]
- 2. Ren S, Cai P, Liu Y, et al. Prevalence of Helicobacter pylori infection in China: a systematic review and meta‐analysis. J Gastroenterol Hepatol. 2022;37:464‐470. [DOI] [PubMed] [Google Scholar]
- 3. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet (London, England). 2020;396:635‐648. [DOI] [PubMed] [Google Scholar]
- 4. Doorakkers E, Lagergren J, Engstrand L, Brusselaers N. Helicobacter pylori eradication treatment and the risk of gastric adenocarcinoma in a Western population. Gut. 2018;67:2092‐2096. [DOI] [PubMed] [Google Scholar]
- 5. Choi IJ, Kook MC, Kim YI, et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med. 2018;378:1085‐1095. [DOI] [PubMed] [Google Scholar]
- 6. Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353‐1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. [DOI] [PubMed] [Google Scholar]
- 8. Sheu BS, Wu MS, Chiu CT, et al. Consensus on the clinical management, screening‐to‐treat, and surveillance of Helicobacter pylori infection to improve gastric cancer control on a nationwide scale. Helicobacter. 2017;22:e12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta‐analysis in World Health Organization regions. Gastroenterology. 2018;155:1372‐1382.e1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu WZ, Xie Y, Lu H, et al. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter. 2018;23:e12475. [DOI] [PubMed] [Google Scholar]
- 11. Scott DR, Sachs G, Marcus EA. The role of acid inhibition in Helicobacter pylori eradication. F1000Res. 2016;5. doi: 10.12688/f1000research.8598.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tang HL, Li Y, Hu YF, Xie HG, Zhai SD. Effects of CYP2C19 loss‐of‐function variants on the eradication of H. pylori infection in patients treated with proton pump inhibitor‐based triple therapy regimens: a meta‐analysis of randomized clinical trials. PloS One. 2013;8:e62162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao F, Wang J, Yang Y, et al. Effect of CYP2C19 genetic polymorphisms on the efficacy of proton pump inhibitor‐based triple therapy for Helicobacter pylori eradication: a meta‐analysis. Helicobacter. 2008;13:532‐541. [DOI] [PubMed] [Google Scholar]
- 14. McNicholl AG, Linares PM, Nyssen OP, Calvet X, Gisbert JP. Meta‐analysis: esomeprazole or rabeprazole vs. first‐generation pump inhibitors in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 2012;36:414‐425. [DOI] [PubMed] [Google Scholar]
- 15. Sugimoto M, Yamaoka Y. Role of Vonoprazan in Helicobacter pylori eradication therapy in Japan. Front Pharmacol. 2018;9:1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Graham DY, Dore MP. Update on the use of Vonoprazan: a competitive acid blocker. Gastroenterology. 2018;154:462‐466. [DOI] [PubMed] [Google Scholar]
- 17. Sue S, Maeda S. Is a potassium‐competitive acid blocker truly superior to proton pump inhibitors in terms of Helicobacter pylori eradication? Gut Liver. 2021;15:799‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scott DR, Munson KB, Marcus EA, Lambrecht NW, Sachs G. The binding selectivity of vonoprazan (TAK‐438) to the gastric H+, K+ ‐ATPase. Aliment Pharmacol Ther. 2015;42:1315‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kagami T, Sahara S, Ichikawa H, et al. Potent acid inhibition by vonoprazan in comparison with esomeprazole, with reference to CYP2C19 genotype. Aliment Pharmacol Ther. 2016;43:1048‐1059. [DOI] [PubMed] [Google Scholar]
- 20. Nabeta H, Shinozaki S, Abe Y, et al. A potassium‐competitive acid blocker‐based regimen as second‐line therapy improves Helicobacter pylori eradication. Digestion. 2020;101:332‐338. [DOI] [PubMed] [Google Scholar]
- 21. Inatomi N, Matsukawa J, Sakurai Y, Otake K. Potassium‐competitive acid blockers: advanced therapeutic option for acid‐related diseases. Pharmacol Ther. 2016;168:12‐22. [DOI] [PubMed] [Google Scholar]
- 22. Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium‐competitive acid blocker, as a component of first‐line and second‐line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double‐blind study. Gut. 2016;65:1439‐1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lyu QJ, Pu QH, Zhong XF, Zhang J. Efficacy and safety of Vonoprazan‐based versus proton pump inhibitor‐based triple therapy for Helicobacter pylori eradication: a meta‐analysis of randomized clinical trials. Biomed Res Int. 2019;2019:9781212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang YW, Hu WL, Cai Y, et al. Outcomes of furazolidone‐ and amoxicillin‐based quadruple therapy for Helicobacter pylori infection and predictors of failed eradication. World J Gastroenterol. 2018;24:4596‐4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646‐664. [DOI] [PubMed] [Google Scholar]
- 26. Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008;5:321‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim JI, Park S‐H, Kim JK, et al. The effects of nocturnal acid breakthrough on Helicobacter pylori eradication. Helicobacter. 2002;7:331‐336. [DOI] [PubMed] [Google Scholar]
- 28. Saiki Y, Yoshida T, Tanioka Y, et al. Helicobacter pylori infection prevents nocturnal gastric acid breakthrough. Hepatogastroenterology. 2006;53:150‐154. [PubMed] [Google Scholar]
- 29. Li M, Oshima T, Horikawa T, et al. Systematic review with meta‐analysis: Vonoprazan, a potent acid blocker, is superior to proton‐pump inhibitors for eradication of clarithromycin‐resistant strains of Helicobacter pylori . Helicobacter. 2018;23:e12495. [DOI] [PubMed] [Google Scholar]
- 30. Sue S, Shibata W, Sasaki T, et al. Randomized trial of vonoprazan‐based versus proton‐pump inhibitor‐based third‐line triple therapy with sitafloxacin for Helicobacter pylori . J Gastroenterol Hepatol. 2019;34:686‐692. [DOI] [PubMed] [Google Scholar]
- 31. Suzuki T, Kagami T, Uotani T, et al. Comparison of effect of an increased dosage of vonoprazan versus vonoprazan plus lafutidine on gastric acid inhibition and serum gastrin. Eur J Clin Pharmacol. 2018;74:45‐52. [DOI] [PubMed] [Google Scholar]
- 32. Dore MP, Farina V, Cuccu M, Mameli L, Massarelli G, Graham DY. Twice‐a‐day bismuth‐containing quadruple therapy for Helicobacter pylori eradication: a randomized trial of 10 and 14 days. Helicobacter. 2011;16:295‐300. [DOI] [PubMed] [Google Scholar]
- 33. Jheng GH, Wu I‐C, Shih H‐Y, et al. Comparison of second‐line quadruple therapies with or without bismuth for Helicobacter pylori infection. Biomed Res Int. 2015;2015:163960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht V/Florence consensus report. Gut. 2017;66:6‐30. [DOI] [PubMed] [Google Scholar]
- 35. Zhuge L, Wang Y, Wu S, Zhao RL, Li Z, Xie Y. Furazolidone treatment for Helicobacter pylori infection: a systematic review and meta‐analysis. Helicobacter. 2018;23:e12468. [DOI] [PubMed] [Google Scholar]
- 36. Song C, Qian X, Zhu Y, et al. Effectiveness and safety of furazolidone‐containing quadruple regimens in patients with Helicobacter pylori infection in real‐world practice. Helicobacter. 2019;24:e12591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Appendix S1.


