Abstract
Anabolic androgenic steroids (AAS) abuse is a global health‐related concern, as most of the related studies showed increasing trends and deleterious effects, mostly on sexual and fertility health. Unfortunately, there are no consensuses about the management pathways due to the lack of specific guidelines. We aimed to confirm the deleterious effects of AAS abuse, monitor the spontaneous recovery, and demonstrate the effects of treatment regimens on recovery. We enrolled 520 patients with a confirmed history of AAS intake within 1 year of presentation and evaluated their symptoms, hormones levels, and semen every 3 months until 12 months. All patients were monitored for spontaneous recovery in the first 3 months; if they showed no recovery, they were randomized to undergo either continued observation or commence medications. The most common presentation (84%) was a combination of sexual symptoms while some patients (18%) were infertile. Most patients (90%) reported low levels of luteinizing hormone, follicle‐stimulating hormone, and total testosterone. After the 3‐month observation, most patients (89%) started treatment, but some (11%) continued observation only. Treated patients showed faster improvement regarding the International Index of Erectile Function (IIEF) values, hormone levels, testicular size and semen parameters compared to non‐treated patients (p < 0.005). Among the 94 patients who presented with infertility (18%), 61 had oligospermia and 33 had azoospermia. All received treatment, but only 14 (15%) achieved successful pregnancy at 12 months while all azoospermic's patients continued to have infertility at the end of the follow‐up period. These findings demonstrated the significant negative impact of AAS abuse on sexual health and fertility, and the need for medical treatment to have faster recovery from their adverse effect.
Keywords: anabolic steroid‐induced hypogonadism, androgenic anabolic steroids, body building, infertility
1. INTRODUCTION
The use of performance‐enhancing drugs (PEDs) to enhance sports, performance and /or physical appearance has progressively increased among young and middle‐aged men. One of the most abused PEDs is anabolic androgenic steroids (AAS) (Pope, 2014). AAS abuse results in supraphysiological testosterone levels with eventual negative effects on the hypothalamic–pituitary Adrenal (HPA) axis, leading to a unique condition known as anabolic steroid‐induced hypogonadism (ASIH) and manifestations of hormonal disturbances, gynecomastia, testicular dysfunction, and infertility, all of which are well‐described but poorly understood (Boregowda et al., 2011; Coward et al., 2013; Cyrus et al., 2014; Liu et al., 2006). The lifetime prevalence of AAS abuse is estimated to be 6% in men; therefore, the resultant adverse effects constitute a public health concern since these medications can result in deleterious health effects (Sagoe & molde, 2014; Fronczak, 2012).
Even though the AAS abuse is underreported in many communities, and up to 50% of AAS users do not disclose their use to their physician, it is becoming an alarming global phenomenon and well noticed in many regional and global communities (Cohen et al., 2007; Graham et al., 2008; Maha et al., 2019; Pany & Panigraphy, 2019).
Spontaneous recovery of the negative effects caused by AAS abuse can be achieved after discontinuing their usage, but this requires several months to years; however, in many patients, the effects may be permanent (Kanayama et al., 2015; Rasmussen et al., 2016; Shankara‐Narayana et al., 2020). Presently, the peer‐reviewed literature contains limited information in describing the demographics, characteristics, and psychologic profile of AAS users. Furthermore, no comprehensive management recommendations or guidelines have been proposed for the treatment of AAS‐induced adverse effects, such as infertility and ASIH, and all available medication regimens are off‐label (Menon, 2003; Tan & Vasudevan, 2003; De Luis et al., 2001; Wenker et al., 2015; Ramasamy et al., 2015; Abram McBride & Coward, 2016; Tatem et al., 2020). Particularly in our region (the Middle East), studies on the prevalence and significant negative effects of AAS abuse, as well as management strategies are unavailable, therefore, we aimed to provide objective evidence of the deleterious effects of this condition on sexual health and fertility, and possible effects of treatment medications on early recovery from their adverse effects.
2. PATIENTS AND METHODS
This single‐center prospective randomized study was conducted on patients who presented to the urology–andrology clinic at Burjeel Hospital, Abu Dhabi, UAE, between June 2012 and June 2019. All the patients who confirmed intake of non‐prescribed AAS in the last year complained of sexual dysfunction and/or infertility.
2.1. Inclusion criteria
•History of intake of non‐prescribed AAS on any occasion over the year preceding presentation at our clinic.
•Sexual symptoms (erectile dysfunction [ED], low sexual desire, defective orgasm, and/or defective ejaculation) for at least 3 months and/or infertility (trying to conceive for >1 year before presentation at our clinic)
•Discontinuation of AAS intake after study enrolment
•Continuous follow‐up for 1 year after study enrolment
2.2. Exclusion criteria
•Sexual symptoms or infertility without a definite history of androgen use or caused by reasons other than AAS abuse.
•Patients lost to follow‐up before completing 1 year
•Relapse, which is a result of androgen use after study enrolment
2.3. Tests and examinations
•For evaluation of sexual symptoms, we used the International Index of Erectile Function (IIEF) and its sub‐domains (erection, orgasm, desire and sexual satisfaction), while for infertility, we used semen analysis
•Testicular ultrasound to follow‐up testicular size and exclude varicocele.
All patients underwent the same hormonal tests, including measurements of follicular stimulating hormone (FSH), luteinizing hormone (LH), prolactin, estradiol, testosterone, and sex hormone‐binding globulin (SHBG), at presentation and at 3, 6, 9 and 12 months of follow‐up and should be performed between 7 AM‐11 AM . Semen analysis was performed for all patients at intervals of 3 months for a period of 12 months (After 48 h of abstinence and evaluated according to WHO semen analysis criteria). Basic hormones tests reports and semen analysis before starting the courses should be available for comparison. All patients were followed up for 12 months after the initial presentation. All hormones were assessed using electrochemiluminescence immunoassays.
2.4. Patient treatment pathways
•All patients underwent a mandatory 3‐month observation before starting treatment medications.
•The patients had the option to either continue the observation or start receiving treatment medications if they could not tolerate AAS withdrawal symptoms. The patients were randomized to untreated and treated groups based on their choice.
•Patients were allowed to start treatment only if they showed at least moderate to severe symptoms with confirmed objective findings (IIEF values, hormone levels, and/or abnormal results in semen analysis).
•Treatment courses of 3 months continued until the patients showed improvement in symptoms, hormone levels and/or clinical pregnancy for infertility.
• Treatment was repeated if the patients showed any relapse of symptoms or deterioration of hormone levels during follow‐up intervals.
2.5. Medications and treatment regimens
The patients were informed that all medications were off‐label, and in the absence of specific guidelines, the medications and the duration of treatment were based on data from similar studies on the treatment of adverse effects of AAS abuse.
The treatment regimen included human chorionic gonadotrophin (HCG) 1500 IU injections /three times weekly and clomiphene citrate (CC) 25 mg tablets once daily, both of which were used for low testosterone and/or oligospermia or azoospermia. HCG is a direct luteinizing hormone (LH) analog that has been shown to stimulate testosterone production by Leydig cells and CC is a well‐known centrally acting selective oestrogen receptor modulator (SERM) that acts by inhibiting oestrogen's negative feedback on the hypothalamus, thereby increasing serum LH, FSH, and endogenous testosterone levels while preserving and even potentially improving sperms parameters (Menon, 2003; Tan & Vasudevan, 2003; Wenker et al., 2015). The duration of treatment was initially 30 days. Subsequent treatment was either continued at the same doses or titrated according to the results of follow‐up hormone tests (FSH, LH, testosterone, and estradiol) that were performed monthly during treatment and seminal fluid analysis that was performed every 3 months.
Patients who showed gynecomastia with ASIH and/or infertility at presentation were administered tamoxifen tablets (10 mg twice daily) instead of CC because it has similar gonadotrophin stimulatory effects, but unlike CC, tamoxifen is quite active in the periphery, making it effective in the treatment of early‐onset gynecomastia in men (De Luis et al., 2001; Mannu et al., 2018).
Patients who experienced gynecomastia with high estradiol levels and/or low testosterone/estradiol (T/E) ratio (N: >1/10) were treated with the aforementioned regimens with the addition of letrozole (2.5 mg) or anastrozole (1 mg) tablets every other day for 30 days. Subsequently, the dose was titrated according to the results of follow‐up hormone tests. They are both aromatase inhibitors (AIs) that effectively block the production of oestrogen without any effects on other steroidogenic pathways, thereby reducing oestrogen levels and the associated gynecomastia (Tatem et al., 2020).
FSH injection (75 IU thrice weekly) was administered to treat infertility that did not respond to LH and CC, in those cases, we discontinued CC and added FSH, as it directly stimulates Sertoli cells to support sperm production. The duration of treatment was initially 3 months, and treatment was subsequently continued or stopped depending on the results of semen analysis (Tatem et al., 2020).
2.6. Informed consent and ethics approval
Informed consent was obtained from all patients prior to study enrolment. The privacy and confidentiality of individual patient data were maintained throughout the study period and post‐study. Ethics committee approval was obtained before patient enrolment.
2.7. Statistical analysis
All statistical analyses were performed using Stata version 16.0. Statistical analysis was performed by comparing the patient characteristics before treatment initiation and after treatment completion. All baseline characteristics were described for the patients treated. For continuous variables, data were presented as mean ± SD. For categorical data, numbers and percentages were used in the data summaries, and the data were presented in the form of tables. Repeated‐measures analysis of variance and Chi‐squared tests were performed to compare the recovery of symptoms and hormone levels between treated and untreated patients. Statistical significance was set at p < 0.05.
3. RESULTS
Overall, 774 patients who presented with a confirmed history of AAS intake were screened. Data of 520 patients were used for data analysis since 254 patients were excluded based on the exclusion criteria (Figure 1). The mean age of the included patients was 32 ± 4 years, with the majority (52%) being aged between 20 and 30 years, and 78% of the patients were married (Table 1).
FIGURE 1.
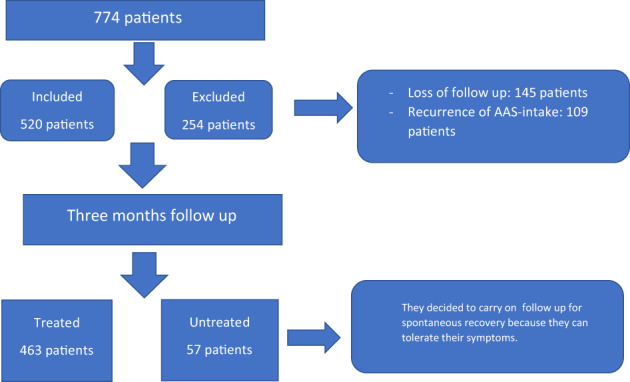
Flowchart of total patients, included and excluded patients and treated and untreated patients
TABLE 1.
Demographic characteristics of the patients
| Parameters/characteristics | N = 520 |
|---|---|
| Age (years) | 32 ± 4 |
| 20–30 | 270 (52%) |
| 30–40 | 177 (34%) |
| 40–50 | 73 (14%) |
| Marital status | |
| Married | 406 (78%) |
| Unmarried | 114 (22%) |
| Courses per year | |
| 1 | 62 (12%) |
| 2 | 182 (35%) |
| 3 | 198 (38%) |
| >3 | 78 (15%) |
| Duration of courses (weeks) | |
| 1–4 | 42 (8%) |
| 4–8 | 120 (23%) |
| 8–12 | 332 (64%) |
| >12 | 26 (5%) |
| AAS intake recommended by | |
| Coach | 395 (76%) |
| Self | 78 (15%) |
| Friends | 47 (9%) |
| Purpose of AAS use | |
| Enhanced fitness training | 328 (63%) |
| Enhanced physical appearance | 130 (25%) |
Abbreviation: AAS, anabolic androgenic steroids.
Presenting symptoms included loss of sexual desire (72%), ED (68%), gynecomastia (35%), reduced orgasmic satisfaction (23%), ejaculatory disorders (17%), and combinations of these symptoms (84%). Based on IIEF assessments, 56% (n = 291) of the patients were categorized as having severe ED (1–10; mean, 7 ± 2), 27% (n = 140) had moderate ED (11–16; mean, 14 ± 2), and 17% (n = 88) had mild ED (17–21; mean, 19 ± 1). The IIEF subdomain scores at the time of presentation were as follows: ED 14 ± 3; orgasmic function, 3 ± 2; sexual desire, 3 ± 2; intercourse satisfaction, 4 ± 2; overall satisfaction, 3 ± 2; and total IIEF, 27 ± 5. Approximately 18% (n = 74) patients had infertility.
All patients underwent the initial mandatory 3‐months observation; subsequently, 463 patients (89%) decided to commence treatment because of sexual symptoms or infertility (treated group). The remaining 57 patients (11%) decided to continue without treatment until the end of the follow‐up (untreated group).
IIEF evaluations revealed no significant changes between the treated and untreated groups, before starting the course, at presentation and after 3 months of watchful waiting, although statistically significant differences in favour of the treatment group were evident at the 6‐, 9‐, and 12‐month assessments (Table 2). The untreated group showed no significant difference between the IIEF value before starting the course, at presentation and those at 3, 6, and 9 months (p > 0.05), with a significant change appearing only at the 12‐month assessment (p < 0.005). However, the IIEF values in the treated group showed significant improvements at the 6‐, 9‐ and 12‐month assessments (p < 0.005).
TABLE 2.
Mean International Index of Erectile Function values for treated and non‐treated patients at different follow‐up intervals
| Time of examination | Treated (n = 463) | Untreated (n = 57) | p value (significant ≤0.05) |
|---|---|---|---|
| Before the AAS—courses | 23 ± 1.2 | 21 ± 1.1 | 1.0000 |
| At presentation | 10 ± 1.0 | 09 ± 1.2 | 1.0000 |
| 3 months | 09 ± 1.0 | 10 ± 1.0 | 1.0000 |
| 6 months | 18 ± 2.0 | 09 ± 1.4 | 1.0000 |
| 9 months | 20 ± 2.0 | 10 ± 2.0 | <0.0001 |
| 12 months | 24 ± 2.0 | 12 ± 2.0 | <0.0001 |
Ultrasound measurements of testicular size showed small testes (<12 cc) in 177 patients (34%) at presentation (mean size, 8 ± 1.6 cc), 213 patients (41%) at 3 months (mean size, 7 ± 1.8 cc), 135 patients (26%) at 9 months (mean size, 9 ± 1.0 cc), and 109 patients (21%) at 12 months (mean size, 11 ± 1.5 cc). At the end of the year, 61% (35/57) of the patients in the untreated group still had small‐sized testes, while the corresponding proportion in the treated group was 20% (93/463; p < 0.001).
All hormones values before the AAS course were normal for both treated and untreated patients, while at presentation, showed low serum LH levels in 489 patients (94%; mean, 0.01 ± 0.2 mIU/ml), low serum FSH levels in 478 patients (92%; mean, 0.1 ± 0.2 IU/L), low total testosterone levels in 468 patients (90%; mean, 3.3 ± 1.2 nmol/L), low SHBG levels in 374 patients (72%; mean, 12 ± 3.2 nmol/L), and high estradiol levels in 286 patients (55%; mean, 65 pg/ml). Follow up hormones assessment showed significant improvement in the treatment group at 6th, 9th, and 12th month, while, for the untreated group there was no significant differences in all hormones at 3 and 6 months but significant differences were only seen in the FSH and LH levels at 9 months and in the testosterone levels at 12 months (p < 0.005) (Table 3).
TABLE 3.
Average values of hormones levels between treated and untreated groups at different time intervals
| Time of examination | Treated (n = 463) | Untreated (n = 57) | p value (significant ≤0.05) |
|---|---|---|---|
| FSH (N: 1.5–12.4 mIU/ml) | |||
| Before the AAS—courses | 6.8 ± 1.1 | 6.7 ± 1.3 | 0.8734 |
| At presentation | 0.3 ± 0.1 | 0.3 ± 0.2 | 1.0000 |
| 3 months | 0.3 ± 0.2 | 0.3 ± 0.1 | 1.0000 |
| 6 months | 6.4 ± 1.6 | 0.4 ± 0.1 | <0.0001 |
| 9 months | 5.8 ± 1.8 | 0.8 ± 0.4 | <0.0001 |
| 12 months | 7.4 ± 2.2 | 1.2 ± 0.4 | <0.0001 |
| LH (N: 1.7–8.6 mIU/ml) | |||
| Before the AAS—courses | 7.2 ± 1.2 | 6.9 ± 1.1 | 0.0730 |
| At presentation | 0.3 ± 0.2 | 0.4 ± 0.2 | 0.7218 |
| 3 months | 0.4 ± 0.2 | 0.3 ± 0.2 | 0.1322 |
| 6 months | 5.2 ± 1,2 | 0.6 ± 0.1 | <0.0001 |
| 9 months | 4.8 ± 2.2 | 1.0 ± 0.6 | <0.0001 |
| 12 months | 6.4 ± 2.0 | 1.4 ± 2.0 | <0.0001 |
| Testosterone (N: 12–32 nmol/L) | |||
| Before the AAS – courses | 16.1 ± 1.2 | 15.8 ± 1.2 | 0.0755 |
| At presentation | 7.0 ± 1.2 | 7.3 ± 1.0 | 0.2278 |
| 3 months | 6.2 ± 2.2 | 7.1 ± 1.2 | 1.0000 |
| 6 months | 12.1 ± 1.2 | 8.2 ± 1.4 | <0.0001 |
| 9 months | 14.1 ± 2.2 | 8.6 ± 1.2 | <0.0001 |
| 12 months | 14.4 ± 1.2 | 9.0 ± 1.0 | <0.0001 |
| Estradiol (N: 20–55 pg/ml) | |||
| Before the AAS – courses | 19.2 ± 0.3 | 20.1 ± 0.2 | 0.4340 |
| At presentation | 85 ± 1.1 | 84 ± 1.2 | 0.1354 |
| 3 months | 75 ± 1.0 | 74 ± 1.0 | 0.1782 |
| 6 months | 26 ± 1.0 | 55 ± 2.0 | <0.0001 |
| 9 months | 30 ± 3.0 | 61 ± 1.0 | <0.0001 |
| 12 months | 24 ± 2.0 | 55 ± 2.0 | <0.0001 |
Abbreviation: AAS, anabolic androgenic steroid; FSH, follicle‐stimulating hormone; LH, luteinizing hormone.
Semen analyses before AAS courses were normal (normal sperms concentration >15 M/ml and total motility>40%) in all enrolled patients, in both treated and untreated groups (31 ± 2.2 M and 32 ± 2.1 M, p < 0.223) and (74 ± 3.2% and 76 ± 2.1%, p < 0.342), respectively. At presentation, semen analysis revealed abnormal semen characteristics in 411 patients (79%), of which 337 patients (82%) showed a combination of oligospermia, azoospermia, and asthenozoospermia. Oligospermia (<15 M/ml) was noted in 279 patients (68%; mean, 6 ± 1.8 M/ml), azoospermia in 33 (8%) patients and asthenospermia in 251 patients (61%). The rate of oligospermia at presentation was 71% in the treated group and 69% in the untreated group (p = 0.6795), however, at the 12‐month assessment, the rate was significantly less in the treated group (33% vs. 55%; p = 0.001). All azoospermia patients (n = 33) chose to start treatment; 11 and 16 patients showed sperm in their ejaculate at the 9‐ and 12‐month assessments, respectively, but all still showed severe oligospermia (<10 M/ml). Regarding asthenozoospermia, the rate at presentation was 65% in the treated group and 61% in the untreated group (p = 0.6795), however, at the 12‐month assessment, the rate was significantly less in the treated group (37% vs. 61%; p = 0.001). Among the 94 patients who presented with infertility (18%), 61 had oligospermia and 33 had azoospermia. All received treatment, but only 14 (15%) achieved successful pregnancy at 12 months. All patients who presented with azoospermia continued to have infertility at the end of the follow‐up period.
Among the 463 treated patients, 150 (32%) maintained the improvements in symptoms and hormone levels until 12 months after receiving treatment with one course (1–3 months), while 210 (45%) showed persistence or relapse of symptoms and required continuation or resumption of medical treatment for 4–6 months; the remaining 103 (23%) required >6 months of treatment and still showed persistent abnormal symptoms or hormones at 1 year of follow‐up. Patients who required only one treatment course had an AAS abuse duration of <3 months (mean, 6 ± 1.1 weeks), while those who required more treatment courses had a longer history of AAS abuse (mean, 12 ± 2.1 weeks; p < 0.001). Moreover, the average timing of presentation among those who required one course of treatment was 6 ± 1.5 weeks after the last dose of AAS versus 10 ± 2 weeks for those who required more than one course of treatment (p < 0.001). Gynecomastia was observed in 182 patients (35%), mostly with other sexual symptoms; it was more common in those with longer AAS abuse histories (>12 weeks) (mean, 14 ± 1.5 weeks) than in those with shorter histories (mean, 7 ± 2 weeks) (p < 0.001).
4. DISCUSSION
Our study confirmed the increasing global trend of AAS abuse in the young population; the largest user population in our study was 20–40 years old, consistent with results from other global and regional studies, such as those conducted in Saudi Arabia (Maha et al., 2019). Surprisingly, despite cultural differences worldwide, the aims of AAS users are almost identical. Most of our patients' decisions to use AAS were driven by their coaches' advice and friends' recommendations. Their primary aim was to improve training fitness and physical appearance as in other studies (Cohen et al., 2007; Graham et al., 2008; Pany & Panigraphy, 2019). They commonly used a combination of oral and injectable AAS, like Nandrolone decanoate, Testosterone enanthate, Metandienone, and Testosterone isocaproate. The most common source of AAS for our patients was their coaches; only some patients received their medicines through online purchases, which may be the main source of AAS in other societies (Pirola et al., 2010). These medicines were usually not registered and were illegally imported from the international market without any consideration for appropriate storage and dispensed without prescription by non‐licensed personnel and coaches.
Most patients (83%) complained of moderate to severe sexual symptoms, while a substantial proportion (18%) also complained of infertility; this explains why such a large majority of patients preferred to start early treatment. Additionally, while a small proportion of patients showed spontaneous recovery after 3 months of follow‐up, most still complained of severe symptoms and had low hormone levels. This contradicts the findings of the study, which reported a high recovery rate of 79% after 3 months (Lykhonosov et al., 2020). We confirmed the AAS impacts is based on many factors, the basic physiological status of patients, the duration and the doses of the courses, and weather early or late at the time of seeking treatment, all these factors are predictors of treatment responses like what was suggested by other studies (Kohn et al., 2017).
Other remarkable findings in our study included the high rate of abnormal semen findings (79%), the significant delay in spontaneous recovery in the untreated group, and the considerable percentage of patients presenting with infertility. Although all patients presenting with infertility were treated, the outcome was disappointing since only 15% of the patients reported a successful pregnancy before the end of the 12 months follow‐up period, these findings are highlighting the long‐term negative influence of AAS abuse on fertility and the need for longer‐than‐expected time for recovery (Boregowda et al., 2011; de Oliveira Vilar Neto et al., 2021; Windfeld‐Mathiasen et al., 2021).
In our study, the treatment regimens used for ASIH, infertility, and gynecomastia were well tolerated by all patients and were according to the recommendations of many similar studies (Mannu et al., 2018; Tatem et al., 2020; Corona et al., 2022). The regimes and the doses of the medications should be followed closely as there are variant patient's responses and should be adjusted individually; the best results were obtained when they followed up monthly for hormones and every 3 months for semen analysis. With this treatment, the recovery of AAS abuse adverse effects was faster and there was good complaince and patient's satisfaction. However, in the absence of specific guidelines for treating AAS‐induced adverse effects, uncertainty regarding the use of specific medicines and doses for individuals, and used medications are off‐label, all factors urge the need for more studies to prove their specific use and doses.
5. CONCLUSIONS
Our study characterizes the harmful effects of AAS abuse on sexual and reproductive health, especially in patients who use AAS repeatedly and over prolonged periods. These patients show a significantly high rate of sexual symptoms (ED and loss of desire), and untreated patients show a very low recovery rate over 12 months. There is also a high incidence of abnormal semen with a low recovery rate, especially in patients with azoospermia. Infertility may persist even after 1 year of treatment. The use of medications was well tolerated, and patients who received them showed significantly faster recovery of AAS withdrawal symptoms and hormone levels than untreated patients did. However, considering the absence of relevant guidelines and the off‐label use of medications, insights for individual‐specific usage of these agents are not available. Therefore, highlighting the urgent need for specific treatment guidelines.
6. LIMITATIONS
lack of testicular biopsies and DNA fragmentation monitoring, the need for a longer follow‐up period, and being a single‐center study were limitations of this study.
CONFLICT OF INTEREST
The author have norelevant conflicts of interest to disclose.
Al Hashimi, M. (2022). The deleterious effects of anabolic androgenic steroid abuse on sexual and reproductive health and comparison of recovery between treated and untreated patients: Single‐center prospective randomized study. Andrologia, 54(11), e14576. 10.1111/and.14576
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Abram McBride, J. , & Coward, R. M. (2016). Recovery of spermatogenesis following testosterone replacement therapy or anabolic‐androgenic steroid use. Asian Journal of Andrology, 18, 373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boregowda, K. , Joels, L. , Stephens, J. W. , & Price, D. E. (2011). Persistent primary hypogonadism associated with anabolic steroid abuse. Fertility and Sterility Journal, 96, e7–e8. [DOI] [PubMed] [Google Scholar]
- Cohen, J. , Collins, R. , Darkes, J. , & Gwartney, D. (2007). A league of their own: Demographics, motivations and patterns of use of 1,955 male adult non‐medical anabolic steroid users in the United States. Journal of the International Society of Sports Nutrition, 4, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona, G. , Rastrelli, G. , Marchiani, S. , Filippi, S. , Morelli, A. , Sarchielli, E. , Sforza, A. , Vignozzi, L. , & Maggi, M. (2022). Consequences of anabolic‐androgenic steroid abuse in males; sexual and reproductive perspective. The World Journal of Men's Health, 40(2), 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coward, R. M. , Rajanahally, S. , Kovac, J. R. , Smith, R. P. , Pastuszak, A. W. , & Lipshultz, L. I. (2013). Anabolic steroid–induced hypogonadism in young men. Journal of Urology, 190, 2200–2205. [DOI] [PubMed] [Google Scholar]
- Cyrus, D. , Rahnema, B. S. , Larry, I. , & Lipshultz, M. D. (2014). Anabolic steroid‐induced hypogonadism: Diagnosis and treatment. Fertility and Sterility Journal, 101(5), 1271–1279. [DOI] [PubMed] [Google Scholar]
- De Luis, D. A. , Aller, R. , Cuellar, L. A. , Terroba, C. , & Romero, E. (2001). Anabolic steroids and gynecomastia. Review of the literature. Anales de Medicina Interna, 18, 489–491. [PubMed] [Google Scholar]
- de Oliveira Vilar Neto, J. , da Silva, C. A. , da Silva, C. A. B. , Pinto, D. V. , de Sá Roriz Caminha, J. , Matos, R. S. , Filho, J. C. C. N. , Alves, F. R. , Magalhães, S. C. , & Daher, E. D. F. (2021). Anabolic androgenic steroid‐induced hypogonadism, a reversible condition in male individuals? A systematic review. Andrologia, 53(7), e14062. [DOI] [PubMed] [Google Scholar]
- Fronczak, C. M. (2012). The insults of illicit drug use on male infertility. Journal of Andrology, 33, 515–528. [DOI] [PubMed] [Google Scholar]
- Graham, M. R. , Davies, B. , Grace, F. M. , Kicman, A. , & Baker, J. S. (2008). Anabolic steroid use: Patterns of use and detection of doping. Sports Medicine Journal, 38, 505–525. [DOI] [PubMed] [Google Scholar]
- Kanayama, G. , Hudson, J. I. , DeLuca, J. , Isaacs, S. , Baggish, A. , Weiner, R. , Bhasin, S. , & Pope, H. G., Jr. (2015). Prolonged hypogonadism in males following withdrawal from anabolic‐androgenic steroids: An Underrecognized problem. Addiction, 110(5), 823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn, T. P. , Louis, M. R. , Pickett, S. M. , Lindgren, M. C. , Kohn, J. R. , Pastuszak, A. W. , & Lipshultz, L. I. (2017). Age and duration of testosterone therapy predict time to return of sperm count after human chorionic gonadotropin therapy. Fertility and Sterility Journal, 107, 351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, P. Y. , Swerdloff, R. S. , Christenson, P. D. , Handelsman, D. J. , & Wang, C. (2006). Rate, extent, and modifiers of spermatogenic recovery after hormonal male contraception: An integrated analysis. Lancet, 367, 1412–1420. [DOI] [PubMed] [Google Scholar]
- Lykhonosov, M. P. , Babenko, A. Y. , Makarin, V. A. , & Fedotov, Y. N. (2020). Peculiarity of recovery of the hypothalamic‐pituitary‐gonadal (hpg) axis, in men after using androgenic anabolic steroids. Probl Endokrinol, 66(1), 104–112. [DOI] [PubMed] [Google Scholar]
- Maha, H. , Ahmed, M. , Al‐Saud, N. S. , & Omar, A. M. (2019). Knowledge of and attitudes toward the use of anabolic‐androgenic steroids among the population of Jeddah, Saudi Arabia. Journal of Microscopy and Ultrastructure, 7, 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannu, G. S. , Sudul, M. , Bettencourt‐Silva, J. H. , Tsoti, S. M. , Cunnick, G. , & Ahmed, S. F. (2018). Role of tamoxifen in idiopathic gynecomastia: A 10‐year prospective cohort study. The Breast Journal., 24(6), 1043–1104. [DOI] [PubMed] [Google Scholar]
- Menon, D. K. (2003). Successful treatment of anabolic steroid‐induced azoospermia with human chorionic gonadotropin and human menopausal gonadotropin. Fertility and Sterility, 79(Suppl 3), 1659–1661. [DOI] [PubMed] [Google Scholar]
- Pany, S. , & Panigraphy, S. (2019). Anabolic androgenic steroid abuse and their health impacts: A cross‐sectional study among body builders in a City of eastern India. International Journal of Preventive Medicine, 10, 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirola, I. , Cappelli, C. , Delbarba, A. , Scalvini, T. , Agosti, B. , Assanelli, D. , Bonetti, A. , & Castellano, M. (2010). Anabolic steroids purchased on the internet as a cause of prolonged hypogonadotropic hypogonadism. Fertility and Sterility, 94(2331), e1–e3. [DOI] [PubMed] [Google Scholar]
- Pope, H. G. (2014). Adverse health consequences of performance‐enhancing drugs: An Endocrine Society scientific statement. Endocrine Reviews, 35(3), 341–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy, R. , Armstrong, J. M. , & Lipshultz, L. I. (2015). Preserving fertility in the hypogonadal patient: An update. Asian Journal of Andrology, 17(2), 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen, J. J. , Selmer, C. , Østergren, P. B. , Pedersen, K. B. , Schou, M. , Gustafsson, F. , Faber, J. , Juul, A. , & Kistorp, C. (2016). Former abusers of anabolic androgenic steroids exhibit decreased testosterone levels and Hypogonadal symptoms years after cessation: A case‐control study. PLoS One, 11(8), e0161208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagoe, D. , & Molde, H. (2014). The global epidemiology of anabolic‐androgenic steroid use: A meta‐analysis and meta‐regression analysis. Annals of Epidemiology, 24(5), 383–398. [DOI] [PubMed] [Google Scholar]
- Shankara‐Narayana, N. , Yu, C. , Savkovic, S. , Desai, R. , Fennell, C. , Turner, L. , Jayadev, V. , Conway, A. J. , Kockx, M. , Ridley, L. , & Kritharides, L. (2020). Rate and extent of recovery from reproductive and cardiac dysfunction due to androgen abuse in men. The Journal of Clinical Endocrinology & Metabolism, 105(6), 1827–1839. [DOI] [PubMed] [Google Scholar]
- Tan, R. S. , & Vasudevan, D. (2003). Use of clomiphene citrate to reverse premature andropause secondary to steroid abuse. Fertility and Sterility, 79, 203–205. [DOI] [PubMed] [Google Scholar]
- Tatem, A. J. , Beilan, J. , Kovac, J. R. , & Lipshultz, L. I. (2020). Management of anabolic steroid‐induced infertility: Novel strategies for fertility maintenance and recovery. World Journal of Men's Health, 38(2), 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenker, E. P. , Dupree, J. M. , Langille, G. M. , Kovac, J. , Ramasamy, R. , Lamb, D. , Mills, J. N. , & Lipshultz, L. I. (2015). The use of HCG‐based combination therapy for recovery of spermatogenesis after testosterone use. sThe Journal of Sexual Medicine, 12, 1334–1337. [DOI] [PubMed] [Google Scholar]
- Windfeld‐Mathiasen, J. , Dalhoff, K. P. , Andersen, J. T. , Klemp, M. , Horwitz, A. , & Horwitz, H. (2021). Male fertility before and after androgen abuse. The Journal of Clinical Endocrinology & Metabolism, 106(2), 442–449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


