Summary
Consuming a greater proportion of total energy intake earlier in the day rather than in the evening is proposed to positively influence weight loss and health, potentially due to greater synchronization of human body circadian rhythms. This systematic review provides an update on existing evidence regarding earlier distributed eating patterns in weight loss interventions. Using a robust search strategy in five electronic databases, nine randomized controlled trials investigating the impact of energy intake distribution on weight loss were identified. Following critical appraisal, a random‐effects meta‐analyses found that, in the context of an energy‐reduced diet, distributing energy intake with a focus on earlier intake resulted in significantly greater weight loss (−1.23 kg; 95% CI 2.40, −0.06, p = 0.04). Improvements in HOMA‐IR, fasting glucose, and LDL cholesterol were also seen. The current study provides a timely update on the evidence linking distribution of total daily energy intake and health, showing that a focus on earlier intakes can result in greater short‐term weight loss compared with later intakes. Future studies are needed to elucidate the impact that earlier intakes may have on weight management and metabolic health.
Keywords: chrononutrition, energy distribution, obesity, weight loss
Abbreviations
- BMI
body mass index
- HbA1c
glycated hemoglobin
- HDL cholesterol
high‐density lipoprotein cholesterol
- HOMA‐IR
homeostatic model assessment for insulin resistance
- LDL cholesterol
low‐density lipoprotein cholesterol
- OGTT
oral glucose tolerance test
1. INTRODUCTION
Globally, rates of obesity continue to increase, with an estimated 33% of the adult population being classified as having overweight and 13% having obesity. 1 Obesity increases an individual's risk of metabolic disease, such as diabetes and cardiovascular disease, musculoskeletal disorders such as osteoarthritis, and some cancers. 1 , 2 , 3 Current dietary weight loss interventions primarily focus on maintaining decreased total energy intake to create a sustained energy deficit, 4 , 5 leading to metabolism of fat, protein, and glycogen stores to make up for the energy deficit, and resulting in a reduction in body mass. 6 , 7 However, not all individuals appear to respond to simple energy restriction for weight loss, and in the search for the mechanisms behind responders and nonresponders to traditional weight loss diets, it has been found that the distribution of energy intake across the day may also be factor in achieving weight loss success. 8 , 9
“Chrononutrition” relates to the timing of meals and distribution of total energy intake across the day. 10 Evidence is building for chrononutrition as a potential target in both weight loss and metabolic disease interventions. 8 , 10 Thus far, there are no recommendations on how energy intake should be distributed throughout the day. However, there is increasing evidence that distributing total energy intake towards the morning and early afternoon, as compared with late afternoon and evening, is favored for weight loss and metabolic improvements. 8 , 11 , 12 It has been suggested that later eating patterns, where total energy intake is distributed towards evenings, may cause the desynchronization of peripheral and central circadian rhythms. 13 , 14 Our central circadian rhythm is responsible for sleep, body temperature, and melatonin production and is controlled by the suprachiasmatic nuclei with light as the major synchronizer. 15 Peripheral circadian rhythms are, on the other hand, responsible for regulation of metabolic hormones and enzymes, such as insulin and ghrelin, and as such a desynchronization may cause disruption to metabolic pathways and hunger signals. 13 , 15 It is hypothesized that earlier distributed energy intakes result in enhanced synchronization of circadian rhythms and improved metabolic health. 10 , 16
Distribution of total energy intake favoring the late afternoon and evening, and late‐night eating, have become the norm in many countries. This is seen particularly in many western societies, where dinner is viewed as the main meal of the day, is the meal with the largest proportion of daily total energy intake, and is commonly designated a social occasion. 17 , 18 , 19 , 20 Restrictions placed on individuals by common work hours also allow for greater meal preparation and eating time in the evenings and can lead to individuals skipping breakfast in the mornings when they are more time poor. 21 , 22 Foods consumed in the evening are also typically higher in energy density compared with those consumed during the day, 23 , 24 leading to a later overall distribution of total energy intake. Later distribution of energy intake, including night eating, has also been associated with increased weight, 11 , 25 with shift workers having a higher body mass index (BMI) than their daytime working counterparts. 26 Studies have also shown the potential for higher energy intakes in the evening to have a negative impact metabolic health, with later eating patterns and distribution of intake associated with reduced insulin sensitivity. 27 , 28
The aim of this systematic review was to examine the impact of earlier versus later distribution of total daily energy intake on weight loss, and to evaluate the potential for utilizing altered energy distribution as a tool in weight loss interventions. We build on previous findings by Fong et al. (2017), 9 by limiting eligible studies to clinical trials only, and investigating how earlier distribution of total energy intake affects body weight and metabolic outcomes in adults.
2. METHODS
2.1. Literature search strategy
The following bibliographic databases were searched for articles from inception to 20/11/2021; MEDLINE, SCOPUS, Cochrane Library of Clinical Trials, CINAHL, PsycINFO, and Embase. Google Scholar was also used to search gray literature. The search terms included the MeSH terms and key words for overweight, obesity, BMI, weight loss, energy metabolism, body composition, glucose, insulin, ghrelin, hunger, and combinations of meal timing, nutrient timing, food timing, and chrononutrition. These were combined appropriately using the Boolean operators. The search was not limited by publication date. References that resulted from the search were entered into Covidence (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org) where duplicates were removed. The search strategies used in the search have been presented in the Supporting Information for this review. The reference lists of included studies were also hand‐searched for additional relevant studies; however, no studies were added from this search. This study was registered with the International Prospective Register for Systematic Reviews, PROPSERO ID: CRD42020160741. The study protocol for this review was not submitted for publication.
2.2. Study selection
2.2.1. Exclusion criteria
Studies in children (under 18 years) and animal studies were excluded. Review articles were not included; however, bibliographies of these were hand‐searched for additional relevant primary studies. Articles on shift workers were excluded as their eating and sleeping schedules are driven by external factors other than hunger and sleep cycles, and shift work has been shown to affect weight management. 29 , 30 , 31 As this review focused on how the distribution of energy intake across the day affects weight loss in the general population, studies that involved a period of fasting such as time restricted feeding, intermittent fasting, or Ramadan were excluded. Studies on drug or surgical interventions, such as bariatric surgery, were also excluded as these interventions may have an impact on metabolism and weight loss independently of eating patterns.
2.2.2. Inclusion criteria
For inclusion in this review, studies were limited to clinical trials (randomized or nonrandomized) which reported the effect of dietary intervention on body weight in adults (≥18 years) of any BMI, with at least two treatment arms, where participants were advised to alter eating patterns to meet a specified distribution of energy intake across the day. Details of the dietary changes that participants were advised to make to their distribution of total energy intake across the day was required as an independent variable, and this needed to be quantified or quantifiable by researchers. For example, this needed to be reported as a percentage of energy intake or number of total calories/kilojoules consumed per meal (±snacks) across the day. For inclusion in this review, studies were required to report body weight (preintervention and postintervention or preintervention and change in body weight). Compliance to the advised energy distribution needed to be reported by the researchers.
2.3. Data management and data extraction
The titles and abstracts of all identified citations identified in the systematic search were screened by two of the researchers independently (IY and HP). The full texts of all potentially eligible studies were then screened independently by the same two researchers. Any discrepancies were screened by a third researcher (KS) to gain consensus.
Data were extracted by one researcher (IY) from each relevant full‐text study and tabulated (Tables 1 and 2), including participant demographic characteristics, distribution of total energy intake across the day (as a percentage or in calories/kilojoules), study outcomes including anthropometry (body weight, BMI, and waist circumference), biochemistry (lipid profile, fasting blood glucose, and hormones including insulin, ghrelin, and leptin) and measures of sleep which were measured via self‐reported survey and arm‐worn trackers. Outcome data were extracted: mean and standard deviation at baseline, postintervention, and change score if reported by the authors.
TABLE 1.
Characteristics of studies included in meta‐analysis
| Study and country | Participant characteristics (mean [SD]) | Duration | Intervention | Total energy intake distribution (%) a | Compliance |
|---|---|---|---|---|---|
|
Keim et al. (1997) USA 37 |
n = 10; female only Age: 29.4 (5.4) years BMI: 28.0 (4.2) kg/m2 |
15 weeks (3‐week stabilization + 2 × 6‐week crossover interventions) |
|
Early: 35:35:15 + 15 after dinner Late: 15:15:35 + 35 after dinner |
Intake was controlled for each participant as all food items were provided. |
|
Jakubowicz et al. (2012) Israel 40 |
n = 193; 115 = female, 78 = male Age: 46.1 (7.3) years BMI: 32.3 (1.8) kg/m2 |
16 + 16‐weeks follow‐up |
|
Early: 43:36:21 Late: 21:36:43 |
Body weight and daily dietary intake checklists were kept by participants for all food consumed. These were monitored every 4 weeks by a dietitian and counseling to ensure compliance was provided. |
|
Jakubowicz et al. (2013) Israel 41 |
n = 93; female only Age: 45.8 (7.1) years BMI: 32.2 (1.2) kg/m2 |
12 weeks |
|
Early: 50:36:14 Late: 14:36:50 |
Weekly 3‐day food diaries were recorded; participants met with a dietitian biweekly to review these. Noncompliance was defined as ±10% or more deviation from recommended daily energy intake. Total noncompliant days were recorded per participant and divided by seven to determine a weekly percentage of noncompliance. Noncompliance of 43% or greater resulted in participant withdrawal. |
|
Lombardo et al. (2014) Italy 39 |
n = 36; female only Age: 41 (16.4) years BMI: 35.5 (4.8) kg/m2 |
12 weeks |
|
Early: 70 for breakfast, morning tea and lunch, 30 for afternoon tea and dinner Late: 55 for breakfast, morning tea and lunch, 45 for afternoon tea and dinner |
Participants were telephoned weekly by a dietitian to ensure compliance with the diet plans; they also completed 3‐day food diaries at baseline, weekly throughout the study, and at its completion. |
|
Rabinovitz et al. (2014) Israel 42 |
n = 59; female, male Age: 46.0 (17.2) years BMI: 32.4 (3.7) kg/m2 |
12 weeks |
|
Early: 33:25:25 (fat and protein enriched) Late: 13:33:33 (high carbohydrate) Remaining energy as snacks |
Meetings with dietitian at 1‐ to 3‐week intervals to estimate adherence to diet and calorie intake. Participants were also asked to record daily intake via food diary three times every 2 weeks to assess compliance. |
|
Madjd et al. (2016) Iran 38 |
n = 80; female only Age: 33.6 (7.0) years BMI: 32.2 (2.3) kg/m2 |
12 weeks |
|
Early: 15:50:20 (+15 for snacks) Late: 15:20:50 (+15 for snacks) |
Participants met with a dietitian every 2 weeks and presented them with diet diaries for that period. They also received phone calls every weekday during the intervention |
|
Versteeg et al. (2018) The Netherlands 35 |
n = 23; male only Age: 59.8 (8.0) years BMI: 34.2 (3.9) kg/m 2 |
5 weeks (1‐week run‐in with weight maintenance diet followed by 4‐week energy‐restricted diet) |
|
Early: 50:35:15 Late: 15:35:50 |
Weekly visits to the research unit for dietary monitoring and weight control. REE was performed weekly, and participants reported energy intake and meal timing via online food diary. |
|
Raynor et al. (2018) USA 34 |
n = 8; female only Age: 53.1 (6.4) years BMI: 36.0 (2.4) kg/m2 |
8 weeks |
|
Early: 50:30:20 Late: 20:30:50 |
Participants self‐monitored their intake and 1 day/week was randomly selected by researchers to check adherence to diet plan. Meals consumed within one hour of prescribed time and ±50 kcal of energy goals were considered compliant. |
|
Madjd et al. (2020) Iran 36 |
N = 82; female only Age: 35.0 (7.2) years BMI: 32.8 (2.0) kg/m2 |
12 weeks |
|
Early: 15:50:20 Late: 15:50:20 Remaining energy split into snacks |
Participants completed logbooks that included their evening mealtime. Food diaries were completed at 0, 6, and 12 weeks. These were checked by a dietitian fortnightly. Noncompliance was defined as deviation from recommended evening mealtime on more than 10% of occasions. |
Abbreviations: BMI, body mass index; REE, resting energy expenditure.
Total energy intake shown as percentages split between the three main meals of the day (Breakfast:Lunch:Dinner).
TABLE 2.
Summary of study outcomes and results
| Study | Risk of bias | Completion/retention | Results (mean [standard deviation or interquartile range]) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Keim et al. (1997) 37 | High | 83% | Body weight (kg) | Morning mean change | Evening mean change | ||||
| −2.91 (0.08) | −3.69 (0.15) | ||||||||
| Jakubowicz et al. (2012) 40 | Moderate | 74% | Morning pretest | Posttest 16 weeks | Posttest 32 weeks | Evening pretest | Posttest 16 weeks | Posttest 32 weeks | |
| Body weight (kg) | 91.2 (9.8) | 77.6 (9.0) | 70.6 (8.7) | 90.4 (9.2) | 75.2 (8.1) | 86.9 (9.7) b | |||
| BMI (kg/m2) | 32.2 (1.9) | 27.4 (1.8) | 24.9 (1.9) | 32.3 (1.9) | 26.9 (1.7) | 30.9 (2.0) b | |||
| Waist circumference (cm) | 110.7 (3.1) | 103.3 (4.3) | 96.4 (5.3) | 110.4 (3.2) | 102.5 (4.3) | 108.7 (3.6) b | |||
| Triglycerides (mmol/L) | 2.0 (0.2) | 1.6 (0.1) | 1.4 (0.1) | 2.0 (0.3) | 1.5 (0.1) b | 2.0 (0.2) b | |||
| Total cholesterol (mmol/L) | 5.5 (0.5) | 4.9 (0.3) | 4.6 (0.3) | 5.5 (0.5) | 4.9 (0.3) | 4.9 (0.5) b | |||
| LDL (mmol/L) | 4.1 (0.5) | 3.5 (0.3) | 3.2 (0.3) | 4.0 (0.5) | 3.4 (0.4) | 3.5 (0.5) | |||
| HDL (mmol/L) | 1.2 (0.1) | 1.3 (0.1) | 1.3 (0.1) | 1.2 (0.1) | 1.3 (0.1) | 1.2 (0.1) | |||
| Glucose (mg/dl) | 94.4 (7.0) | 86.2 (5.6) | 84.2 (4.6) | 94.6 (7.4) | 85.1 (6.7) | 95.5 (4.9) b | |||
| Insulin (μU/ml) | 21.7 (3.6) | 12.6 (3.4) | 8.9 (3.9) | 21.7 (3.6) | 13.9 (4.8) | 23.69 (3.8) b | |||
| HOMA‐IR | 5.0 (0.9) | 2.5 (0.5) | 1.6 (0.4) | 5.1 (0.9) | 2.4 (0.5) | 5.9 (0.9) b | |||
| Jakubowicz et al. (2013) 41 | Moderate | 79% | Morning pretest | Morning posttest | Mean change | Evening pretest | Evening posttest | Mean change | |
|
Body weight (kg) BMI (kg/m2) |
86.5 (0.7) 32.3 (0.2) |
77.8 (0.7) 29.2 (0.2) |
−8.7 (1.4) |
87.1 (0.7) 32.2 (0.2) |
83.5 (0.8) b 30.9 (0.2) b |
−3.6 (1.5) b | |||
| Waist circumference (cm) | 110.1 (0.40) | 101.4 (0.43) | 111.2 (0.41) | 107.6 (0.51) | |||||
| Triglycerides (mmol/L) | 2.0 (0.03) | 1.3 (0.02) | 2.0 (0.04) | 2.3 (0.04) b | |||||
| Total cholesterol (mmol/L) | 5.6 (0.06) | 5.3 (0.06) | 5.7 (0.06) | 5.6 (0.05) b | |||||
| LDL (mmol/L) | 3.5 (0.06) | 3.4 (0.06) | 3.5 (0.06) | 3.4 (0.06) | |||||
| HDL (mmol/L) | 1.2 (0.02) | 1.3 (0.02) | 1.2 (0.02) | 1.2 (0.02) | |||||
| Glucose (mg/dl) | 94.6 (0.9) | 83.7 (0.7) | 92.9 (0.7) | 89 (0.9) b | |||||
| Insulin (mg/ml) | 20.2 (0.4) | 9.9 (0.2) | 18.6 (0.5) | 13.2 (0.2) b | |||||
| HOMA‐IR | 4.7 (0.1) | 2.0 (0) | 4.3 (0.1) | 2.9 (0.1) b | |||||
| Rabinovitz et al. (2014) 42 | Moderate | 77% |
Body weight (kg) BMI (kg/m2) |
Large breakfast pretest | Mean change | Small breakfast pretest | Mean change | ||
|
87.05 (12.2) 31.95 (3.7) |
−2.43 (0.46) −0/88 (0.7) |
89.23 (14.7) 32.8 (3.7) |
−1.86 (0.4) −0.69 (0.15) |
||||||
| Waist circumference (cm) | 106.8 (9.2) | −2.65 (0.66) | 105.8 (11.1) | −2.2 (0.47) | |||||
| Triglycerides (mmol/L) | 1.68 (0.64) | −0.03 (0.14) | 2.13 (2.23) | −0.16 (0.09) | |||||
| Total cholesterol (mmol/L) | 4.6 (0.77) | −0.04 (0.13) | 4.7 (0.97) | 0.09 (0.11) | |||||
| LDL (mmol/L) | 2.8 (0.6) | 0.05 (0.18) | 2.7 (0.85) | 0.2 (0.12) | |||||
| HDL (mmol/L) | 1.1 (0.28) | 0.03 (0.002) | 1.2 (0.26) | 0.004 (0.02) | |||||
| Glucose (mmol/L) | 7.8 (2.0) | −0.5 (0.3) | 8.1 (3.0) | −0.3 (0.2) | |||||
| Insulin (μU/ml) | 17.58 (9.07) | −4.22 (1.37) | 16.45 (7.74) | −3.3 (1.13) | |||||
| HbA1c (%) | 6.9 (1) | −0.46 (0.15) | 6.85 (1.1) | −0.15 (0.07) b | |||||
| Lombardo et al. (2014) 39 | High | 85% |
Morning pretest Morning posttest |
Mean change |
Evening pretest Evening posttest |
Mean change | |||
|
Body weight (kg) BMI (kg/m2) |
94.2 (2.1) 35.8 (5.2) |
86.3 (1.9) 32.7 (2.3) |
−8.2 (3) −3.1 (0.2) |
88.2 (0.9) 35.1 (4.5) |
81.7 (0.9) 33.3 (3.9) |
−6.5 (3.4) b −1.8 (0.4) b |
|||
| Waist circumference (cm) | 100 (3) | 93 (4) | −7 (0.6) | 100 (5) | 95 (5) | −5 (0.3) b | |||
| Energy intake (kcal) | 2008 (67) | 1992 (89) | −12 | 1993 (87) | 1967 (95) | −26 | |||
| Triglycerides (mmol/L) | 1.3 (1.1–1.6) | 1.0 (0.8–1.1) | −0.3 (0.06) | 1.4 (1.1–1.8) | 1.2 (1.0–1.5) | −0.2 (0.03) | |||
| Total cholesterol (mmol/L) | 5.1 (1.0) | 5.0 (0.8) | −0.2 (0.1) | 5.3 (1.1) | 5.1 (0.9) | −0.2 (0.1) | |||
| LDL (mmol/L) | 3.3 (0.5) | 3.2 (0.4) | −0.1 (0.1) | 3.3 (0.6) | 3.3 (0.5) | −0.03 (0.003) | |||
| HDL (mmol/L) | 1.2 (0.3) | 1.3 (0.2) | 0.1 (0.01) | 1.3 (0.4) | 1.3 (0.3) | 0.01 (0.01) b | |||
| Glucose (mmol/L) | 5.35 (0.66) | 5.21 (0.32) | −0.14 (0.06) | 5.12 (0.82) | 5.16 (0.57) | 0.04 (0.02) | |||
| Insulin (μU/ml) | 16.2 (12.4–19.1) | 9.5 (6.3–11.5) | −6.7 (1.4) | 17.3 (11.9–19.5) | 15.5 (11.3–18.7) | −1.8 (0.02) | |||
| HOMA‐IR | 4.12 (2.62) | 2.75 (1.34) | −1.37 (0.27) | 4.15 (2.51) | 3.41 (1.95) | −0.74 (0.12) b | |||
| Madjd et al. (2016) 38 | Moderate | 86% | Lunch pretest | Lunch posttest | Mean change | Dinner pretest | Dinner posttest | Mean change | |
| Body weight (kg) | 84.02 (7.25) | 78.28 (6.88) a | −5.73 (1.91) | 83.05 (6.98) | 78.75 (7.18) a | −4.31 (1.93) b | |||
| BMI (kg/m2) | 32.21 (2.24) | 30.02 (2.36) a | −2.21 (0.75) | 32.10 (2.31) | 30.45 (2.44) a | −1.67 (0.74) b | |||
| Waist circumference (cm) | 100 (7.98) | 94.38 (8.57) a | −6.05 (2.14) | 101 (8.3) | 95.75 (8.07) a | −5.33 (1.95) | |||
| Energy intake (kcal/day) | 2329 (264) | 1946 (257) | 2316 (260) | 1984 (191) | |||||
| Triglycerides (mmol/L) | 1.57 (0.27) | 1.41 (0.24) a | −0.15 (0.06) | 1.58 (0.24) | 1.43 (0.21) a | −0.13 (0.06) | |||
| Total cholesterol (mmol/L) | 4.59 (0.46) | 4.22 (0.50) a | −0.38 (0.19) | 4.56 (0.48) | 4.27 (0.43) a | −0.32 (0.16) | |||
| LDL (mmol/L) | 2.66 (0.55) | 2.29 (0.58) a | −0.37 (0.20) | 2.64 (0.49) | 2.34 (0.45) a | −0.32 (0.16) | |||
| HDL cholesterol (mmol/L) | 1.22 (0.17) | 1.29 (0.16) a | −0.06 (0.04) | 1.2 (0.13) | 1.28 (−0.17) a | 0.07 (0.04) | |||
| Insulin (mU/L) | 14.04 (2.89) | 12.01 (2.91) a | −2.03 (1.07) | 13.7 (2.34) | 12.59 (2.33) a | −1.16 (0.68) b | |||
| HbA1c (%) | 5.4 (0.61) | 5.11 (0.57) a | −0.34 (0.19) | 5.36 (0.54) | 5.08 (0.49) a | −0.30 (0.16) | |||
| HOMA‐IR | 3.16 (0.42) | 2.49 (0.68) a | −0.68 (0.31) | 3.07 (0.64) | 2.62 (0.56) a | −0.46 (0.23) b | |||
| Versteeg et al. (2018) 35 | Moderate | 92% | Morning pretest | Posttest | Mean change | Evening pretest | Posttest | Mean change | |
| Body weight (kg) | 108.3 (13) | 101.4 (13) | 7 (1.4) | 111.2 (16.6) | 104.3 (16.3) | 6.8 (2.0) | |||
| BMI (kg/m2) | 34.2 (4.2) | 32.0 (4.2) | 34.3 (3.7) | 32.1 (3.8) | |||||
| Triglycerides (mmol/L) | 1.7 (0.82) | 0.93 (0.33) | 1.8 (0.92) | 0.98 (0.4) | |||||
| Total cholesterol (mmol/L) | 5.4 (1.1) | 4.1 (0.61) | 5.4 (1.3) | 4.5 (0.79) | |||||
| LDL (mmol/L) | 3.5 (0.98) | 2.7 (0.6) | 3.4 (1.0) | 3.0 (0.71) | |||||
| HDL (mmol/L) | 1.2 (0.26) | 1.0 (0.18) | 1.2 (0.18) | 1.1 (0.15) | |||||
| Glucose (mmol/L) | 5.5 (0.83) | 4.7 (0.67) | 5.3 (0.88) | 4.9 (0.47) | |||||
| Insulin (pmol/L) | 120.5 (72.4) | 55.8 (41.9) | 110.2 (35.6) | 72.3 (24.6) | |||||
| Raynor et al. (2018) 34 | Moderate | 100% | Morning pretest | Mean change | Evening pretest | Mean change | |||
| Body weight (kg) | −7.55 (1.008) | −4.58 (1.144) b | |||||||
| BMI (kg/m2) | 35.7 (4.8) | 36.3 (2.5) | |||||||
| Madjd et al. (2020) 36 | Moderate | 91% | Early pretest | Early posttest | Mean change | Late pretest | Late posttest | Mean change | |
| Body weight (kg) | 84.72 (6.37) | 77.98 (6.15) | −6.74 (1.92) | 84.40 (6.86) | 79.59 (7.08) | −4.81 (2.22) b | |||
| BMI (kg/m2) | 32.78 (2.05) | 30.18 (2.11) | −2.60 (0.71) | 32.73 (2.00) | 30.86 (2.18) | −1.87 (0.85) b | |||
| Waist circumference (cm) | 104 (7.82) | 96 (7.72) | −8 (3.25) | 103 (7.42) | 97 (7.20) | −6 (3.05) b | |||
| Total cholesterol (mmol/L) | 4.66 (0.38) | 4.14 (0.44) | −0.52 (0.26) | 4.55 (0.42) | 4.22 (0.42) | −0.33 (0.16) b | |||
| LDL (mmol/L) | 3.10 (0.49) | 2.56 (0.51) | −0.54 (0.27) | 2.45 (0.49) | 2.31 (0.47) | −0.14 (0.25) | |||
| HDL (mmol/L) | 1.24 (0.18) | 1.31 (0.16) | 0.07 (0.04) | 1.21 (0.13) | 1.27 (0.12) | 0.07 (0.04) | |||
| Glucose (mmol/L) | 5.06 (0.36) | 4.61 (0.34) | −0.46 (0.18) | 5.05 (0.39) | 4.66 (0.38) | −0.40 (0.22) | |||
| Insulin (mU/L) | 14.33 (3.07) | 11.69 (2.63) | −2.64 (1.49) | 14.23 (2.40) | 12.80 (2.28) | −1.43 (1.38) b | |||
| HbA1c (%) | 5.38 (0.60) | 5.08 (0.56) | −0.30 (0.20) | 5.39 (0.49) | 5.06 (0.48) | −0.32 (0.19) | |||
| HOMA‐IR | 3.24 (0.80) | 2.41 (0.63) | −0.83 (0.37) | 3.22 (0.69) | 2.66 (0.57) | −0.55 (0.39) b | |||
Abbreviations: BMI, body mass index; HDL, high‐density lipoprotein; HOMA‐IR, homeostatic model of assessment of insulin resistance; LDL, low‐density lipoprotein.
p < 0.05 for within group differences.
p < 0.05 for between group differences.
2.4. Risk of bias assessment
Risk of bias was assessed by two researchers (IY and HP) independently using methodology from the Joanna Briggs Institute. 32 Use of these tools allowed for classification of the risk for selection, detection, attrition, and reporting bias as low, high, or unclear. Disagreements were brought to a third researcher (KS) if required to achieve consensus.
2.5. Data analysis
Data on variables of interest were collated and reported as mean and standard deviation where available and statistically significant results noted. Where necessary, biochemical and hormone values were mathematically converted to one reporting standard. 33 Where change in weight was not reported, authors were contacted to obtain this information. 34 Two papers by Versteeg et al. reported different outcomes from the same study; however, the 2018 publication 35 was selected for inclusion as it reported metabolic outcomes relevant to this review.
Meta‐analysis was conducted using Review Manager (RevMan [computer program], Version 5.4, The Cochrane Collaboration, 2020), and random‐effects model was used. All studies reporting the outcome of interest were included in the meta‐analysis for that outcome. Body weight and other outcome measures were weighted by the inverse of the variance of their respective mean difference (MD), so that the overall weighted MDs and 95% confidence intervals of the various risk factors from all the studies could be estimated. One study 36 focused the change in energy distribution on the final meal and snack of the day only. While this study altered energy intake distribution across the day, and did not include a set period of fasting, and therefore fitted the inclusion criteria, as the focus on energy intake distribution differed markedly from the other studies, a sensitivity test was conducted with this study removed to determine the impact of this study on the meta‐analysis.
Subgroup analysis was also undertaken to explore possible causes for heterogeneity. Studies with interventions <12 and ≥12 weeks (which was the median study duration) were analyzed separately. Six of the nine studies examined females only; therefore, a subanalysis was conducted with studies reporting female participants only, and a subsequent analysis was conducted on those reporting males with or without females.
3. RESULTS
Following removal of duplicates, 13,230 records were screened by title and abstract; full‐text screening was conducted for 52 articles (further details on full‐text screening can be seen in the Supporting Information). From these, nine studies (10 papers) were identified for data extraction and quality appraisal (Figure 1).
FIGURE 1.
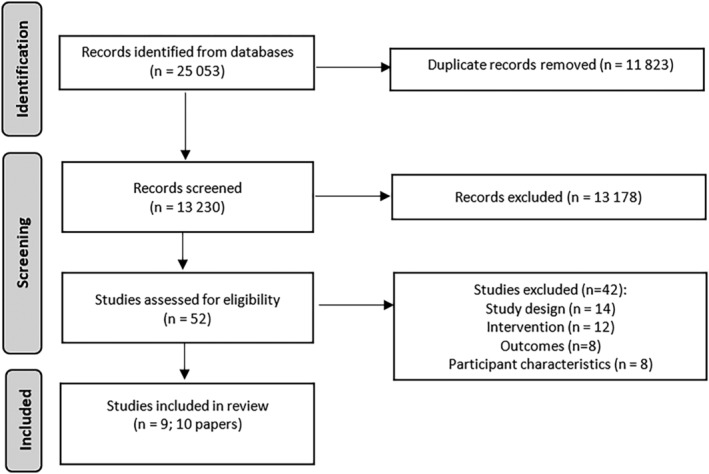
PRISMA flow diagram for study selection
3.1. Study characteristics
Nine clinical trials were included in the meta‐analysis. Studies were conducted in the United States, 34 , 37 Iran, 36 , 38 Italy, 39 the Netherlands, 35 and Israel. 40 , 41 , 42 While the exclusion criteria did not specify that studies needed to be RCTs, all included studies were found to be RCTs. Total number of participants was 485 (earlier distributed total energy intakes: n = 244, later distributed total energy intakes; n = 241), sample size ranged from n = 8 to n = 193. Six studies included females only, two males only, and one included both males and females. Mean age and BMI of participants ranged from 2953 years and 22–46 kg/m2. One study was an experimental RCT conducted under laboratory conditions, 37 and the remaining eight were pragmatic RCTs. Duration of intervention ranged from 516 weeks; one study reported a follow‐up period to 32 weeks; however, as participant compliance with dietary intervention was not measured during the 16‐week follow‐up period, only immediate postintervention data were included in the meta‐analysis. While it was not a requirement for inclusion in this systematic review, all studies employed energy‐restricted diets in both intervention arms. These diets were advised by study dietitians, with two studies providing either meals or nutritional supplements to control altered energy distribution. Compliance to dietary advice on energy distribution was measured in all studies using food diaries which were assessed for adherence by study dietitians. Completion rates ranged from 74% to 100%. Study characteristics are further summarized in Table 1.
3.2. Risk of bias analysis
Critical appraisal of included papers using the Joanna Briggs Institute Checklist for Randomised Controlled Trials 32 resulted in seven studies being assessed as having a moderate risk of bias 34 , 35 , 36 , 38 , 40 , 41 , 42 and two with high risk of bias. 37 , 39 Only five of the nine studies used true randomization, and three or the nine concealed allocation of treatment groups. No studies were blinded to participants or those delivering treatment; one study blinded outcome assessors. All studies reported baseline characteristics to be similar between groups, measured compliance, and had consistent collection of data; however, validation and reliability of measures were unclear. Risk of bias assessments is detailed in the Supporting Information.
3.3. Outcomes
3.3.1. Dietary intake
Eight of the nine included studies reported the percentage of total energy intake that was to be consumed at each main meal (mean percentages of energy intake per meal for earlier distributed intakes—breakfast: 34% ± 16%, lunch: 38% ± 7%, dinner: 20% ± 6%, and for later distributed intakes—breakfast: 19% ± 6%, lunch: 30% ± 10%, dinner; 40% ± 11%). The final study presented the advised percentage of energy intakes as pooled morning (breakfast, morning tea, and lunch) and afternoon/evening intakes (afternoon tea, dinner, and supper) with 70%:30% for the earlier distributed group and 55%:45% for the later distributed group. 39 Details on the energy distribution of each study are detailed in Table 1. One study altered the energy distribution of the evening period (dinner ± snacks) only. 36 Five studies specified an allowance of 18% ± 7% of total energy intake for snacks throughout the day. Compliance was high in all studies with participants removed for analysis as needed for not meeting compliance requirements.
All studies advised participants to consume energy‐restricted diets in conjunction with altering the distribution of total energy intake. Six studies specified macronutrient intake on prescribed diets. Two of these six studies also prescribed differing macronutrient distributions between intervention groups. 40 , 42 These differences in macronutrient intake were at the breakfast meal, with one study comparing a higher proportion of fat/protein to a high carbohydrate meal, 42 and the other study comparing a low carbohydrate meal to a high carbohydrate/protein meal. 40
3.3.2. Body weight
Five of the nine studies reported significantly greater weight loss with earlier compared with later distribution of total energy intake, with mean weight loss in interventions arms with earlier distributions of intake ranging from 2.9 to 13.6 kg, and later distributed arms ranging from 3.715.3 kg (Supporting Information). 34 , 36 , 38 , 41 , 42 One study 40 reported a 16‐week postintervention follow‐up period, during which the participants who were originally advised to consume a greater proportion of their total energy intake energy intake earlier in the day continued to lose weight postintervention, whereas those with later distribution of energy intake gained weight postintervention.
Meta‐analysis (Figure 2A) of weight loss difference between intervention arms showed that there was significantly greater weight loss in groups with earlier distributed energy intake compared with later distributed intakes (I 2 = 98%; −1.23 kg; 95% CI −2.40, −0.06, p = 0.04). The sensitivity test excluding Madjd et al. (2020) 36 (Figure 2B) showed that weight loss with earlier distributed intake was no longer significantly different between the groups (I 2 = 98%; −1.14 kg; 95% CI −2.38, 0.11, p = 0.07).
FIGURE 2.
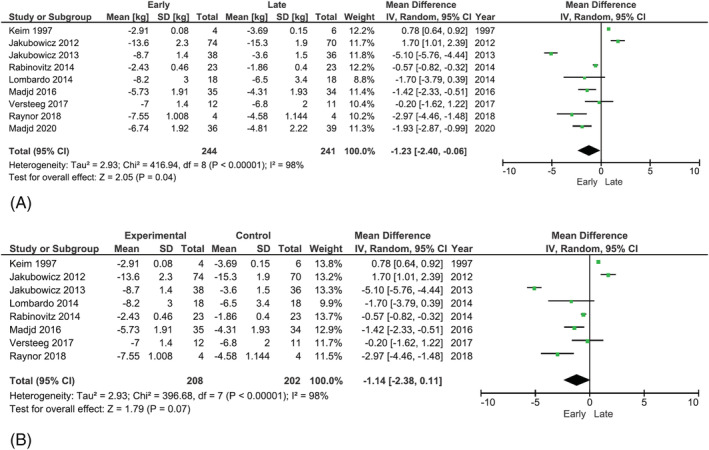
(A) Forest plot for meta‐analysis of trials reporting weight loss (kg) for earlier versus later eating patterns. (B) Forest plot for meta‐analysis of trials reporting weight loss (kg) for earlier versus later eating patterns (excluding Madjd et al. [2020] 36 )
Subgroup analysis was conducted to determine if participants gender or study duration contributed to the heterogeneity. No subanalyses results were found to be significant. Forest plots for these analyses can be seen in the Supporting Information.
3.3.3. Other outcomes
Seven studies reported changes in lipid profile with six showing changes in lipid profiles: two studies had equal improvements across both groups postintervention with no differences seen between groups, while four studies saw significantly greater improvements with earlier intakes. 36 , 39 , 40 , 41 Fasting insulin, glucose, HbA1c, and HOMA‐IR were reported in seven studies. Four of these showed no significant differences in the changes observed between groups from baseline to end of intervention, two studies reported significantly greater decreases in fasting insulin and HOMA‐IR with earlier intakes, 38 , 41 with one of these also reporting significantly greater reductions in fasting glucose. 41 One study found significantly decreased HbA1c postintervention with earlier intakes after adjusting for baseline differences. 42 Rabinovitz et al. 42 also found that earlier distributed intakes resulted in a significantly greater proportion of participants with type 2 diabetes having their medication doses reduced postintervention (31% vs. 0% for earlier vs. later distributed energy intakes, p = 0.002). Both fasting and bedtime glucose levels measured via continuous glucose monitoring were also significantly reduced in those consuming earlier distributed energy intakes (fasting: −0.83 vs. −0.27 mmol/L, p = 0.001; before sleep: −1.70 vs. −0.28 mmol/L, p = 0.009; earlier compared with later distribution of energy intake, respectively).
Meta‐analysis was performed on triglycerides, HDL, LDL, HbA1c, fasting glucose, and HOMA‐IR (preferred assessment for insulin resistance) where postintervention data were reported. Meta‐analysis was not attempted on fasting insulin as it is not a standardized measure for metabolic disease. Random‐effects models showed that there were significantly greater reductions in LDL cholesterol (MD: −0.11 mmol/L; 95% CI −0.14, −0.07, p < 0.01), fasting glucose (MD: 0.15 mmol/L, 95% CI −0.23, −0.06, p < 0.001), and HOMA‐IR (MD: −0.38; 95% CI −0.64, −0.11, p = 0.005) in groups with earlier energy intakes compared with later intakes (Figures 3, 4, 5). No significant differences between early and later intakes were seen for triglycerides, HDL, and HbA1c (figures included in the Supporting Information).
FIGURE 3.
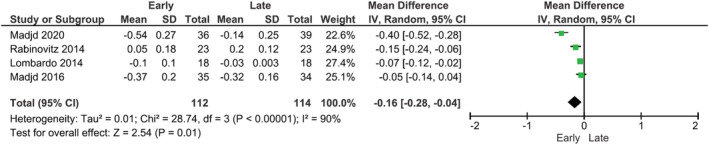
Forest plot of meta‐analysis for change in fasting serum LDL cholesterol
FIGURE 4.
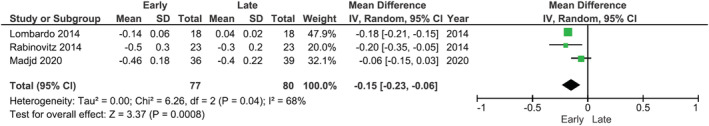
Forest plot of meta‐analysis for change in fasting glucose
FIGURE 5.
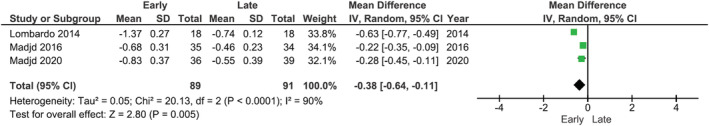
Forest plot of meta‐analysis for change in HOMA‐IR
Appetite and hunger were measured via survey in two studies 40 , 42 which found significantly improved satiety and hunger management with earlier distribution of energy intake, where urge to eat, preoccupation with food, and cravings for sweets and fats were all reduced compared with later distribution of energy intake. Ghrelin was measured as an outcome in four studies, with only one study finding that earlier intakes resulted in significantly greater suppression of postprandial ghrelin compared with later distribution of energy intake. 40 Only one study 34 measured sleep, and it was found that regularity of sleep onset and waking times were significantly greater with earlier distributed energy intakes compared with later energy intakes, and there was a significant increase in the number of hours between the last meal and sleep onset in both groups.
4. DISCUSSION
This systematic review and meta‐analysis of nine RCTs found that energy intakes with a focus on earlier distribution resulted in significantly greater weight loss when compared with similarly energy‐restricted diets with individuals consuming a larger proportion of their total energy intake later in the day and into the evening. Significantly greater improvements in metabolic outcomes including HOMA‐IR, fasting glucose, and LDL cholesterol were also seen with earlier distributed energy intakes. While the MD in weight loss was modest, and removal of one study focusing on evening intakes only resulted in loss of significance in weight loss outcomes, the results of this review still highlight the need for further investigation into the effects on weight management of distribution of total energy intake across the day and into the evening.
The greater weight loss seen with earlier intakes may be due to the superior synchronization of peripheral and central circadian rhythms in the body. 43 Feeding times have been shown to be a key environmental cue for peripheral circadian rhythms, with later meals providing conflicting stimuli to that being received by our central circadian control center from other cues such as light/darkness, hormone secretion, and changes to body temperature. 44 , 45 This results in disturbances to metabolism which may favor weight gain and hinder weight loss as well as increasing risk of metabolic diseases. 13 , 43 It has also been shown that earlier intakes result in higher thermogenesis and lower glycemic responses. 8 , 27 Taken together, these findings suggest that the human body has metabolically evolved around earlier‐weighted daily energy intake, where darkness in the evening is associated with sleep onset rather than heavy intake of food. 12 , 27 One study altered distribution of energy in the evening only with participants in that study consuming either a later or earlier dinner. While sensitivity testing showed only a small change to the MD for weight loss when excluding this study, the pooled results were no longer significant (excluding Madjd et al. [2020] 36 : 1.14 kg, p = 0.07; all studies: 1.23 kg, p = 0.04). This highlights the potential for the limiting of late‐night eating, rather than a shift in energy intake with a focus on the earlier parts of the day, as a key contributor to health improvements and greater weight loss seen in these studies. Late‐night eating, particularly intake after 8 p.m., has been shown to negatively impact metabolic outcomes and weight. 25 , 46 , 47 It has also been shown in shift workers where those working the evening and night shifts have poorer health outcomes compared with those working day shifts. 26 , 48 The impact that this study has on the results suggests that this area needs further exploration to determine whether the greater weight loss is a result of reduced late‐night eating or overall earlier distribution of intake.
The metabolic improvements seen in this review contribute to explaining some of the relationship between eating patterns and weight change and suggest that earlier distribution of energy intake may be beneficial to health, independent of weight loss. The readiness of the human body to digest and metabolize food earlier in the day may also contribute to the decreased fasting glucose and HOMA‐IR seen with earlier intakes. 8 Mechanistically, synchronization of feeding times with the natural rhythms of peripheral hormonal clocks results in improved glucose metabolism. 49 These peripheral clocks include glucagon, leptin, and insulin and their receptors and transporters. 50 , 51 , 52 Similar findings have been reported in a review by Beccuti et al., who found weight gain, hyperglycemia, and diabetes were associated with energy intake later in the day and evenings. 8 From a more clinical perspective, Rabinovitz et al., included in the present review, found that participants with type 2 diabetes were able to decrease their medication doses after weighting their meals towards the beginning of the day. 42 However, in the current review, some studies also altered macronutrient composition of meals in addition to distribution of total energy intake. 40 , 42 As macronutrient composition of meals has been shown to alter satiety, 53 these findings warrant further research into whether altering distribution of total energy intake across the day may additionally contribute to satiety benefits seen with certain dietary macronutrient compositions.
Only one study included in the meta‐analysis included a delayed follow‐up period, measuring outcomes again 16 weeks postintervention. 40 It was found that during this time, participants who were originally advised to distribute their energy intake more heavily in the morning were better able to maintain weight loss and even continue to lose weight, while the group with later distributed energy intake regained their initial weight loss. As long‐term weight maintenance continues to be an important barrier to successful weight management, 54 , 55 future studies should employ postintervention follow‐up. Additionally, poor sleep outcomes have also been associated with higher energy intake in the later part of the day and evening, as well as decreased diet quality, consumption of higher energy dense foods and disrupted metabolism. 23 In the present review, Raynor et al. 34 was the only study to include measures of sleep. With increasing evidence to show that eating in the late evening results in poor sleep quality and increased intake of energy dense foods at this time, 56 , 57 weight management interventions focusing on earlier distribution of energy intake may have clinical utility for improving sleep.
This review has both strengths and limitations: While all but one study were conducted in free‐living participants, and therefore exposed to the known difficulties of self‐reported intake in dietary interventions, all included measures of compliance to prescribed diets and protocols for exclusion, thereby strengthening the validity of their results. The studies completed in free‐living participants also provide a realistic “real‐world” representation of how these diets may be adhered to in a clinical setting. However, the studies were also relatively short in duration (5–16 weeks) with high levels of compliance, indicating that further research is needed to determine if longer duration studies with more flexibility in eating patterns are similarly as successful. In contrast, the study by Keim et (1997) 37 was conducted under laboratory conditions with strict control of intake; however, a sensitivity test removing this study did not meaningfully alter heterogeneity between studies. This review found high heterogeneity between studies, which may be due to the diversity among the methodological approaches in each study. There was no uniformity in the distributions of energy intake across the day which limited the ability to quantify the distribution of energy intake that would most effectively increase weight loss. The studies were also of varying length and sample size, and the intensity of intervention (e.g., number and frequency of education or counseling sessions) in each also differed, all of which may have contributed to the high heterogeneity seen in the meta‐analysis. While subgroup analysis was conducted, it did not identify specific contributors to the heterogeneity between study outcomes. As mentioned earlier in this discussion, one of the studies also differed in their distribution of energy across the day, choosing to change only the main evening meal. As sensitivity testing showed that exclusion of this study resulted in loss of significance between weight loss experienced by earlier and later distributed energy intakes, further research is needed to elucidate the different effects on weight loss of altering energy distribution across the whole day versus that of the evening and late night only.
The current review strengthens the evidence for the relationship between weight loss and distribution of total energy intake across the day with earlier eating patterns being favored. While the average difference in weight loss between groups was modest at 1.23 kg, earlier intakes may be a promising tool to be used in conjunction with other weight loss strategies such as energy restriction to enhance weight loss. As a result, further research is needed with longer follow‐up periods and more uniform energy distributions to elucidate the additional positive impacts that earlier distributed total energy intakes may have on weight and metabolic health.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
All authors designed the study; Isabel E. Young, Helen M. Parker, and Amudha Poobalan conducted the literature search. Isabel E. Young and Helen M. Parker completed title and abstract screening completed full‐text screening and appraisals of included studies. Isabel E. Young completed data extraction, meta‐analysis, and drafted the initial manuscript. Katharine Steinbeck, Amudha Poobalan, and Helen M. Parker contributed to the editing and completion of the final manuscript. Helen T. O'Connor passed away during the conduct of this research but is listed here as a member of the authorship team in consideration of her important contribution to this work.
Supporting information
Data S1. Supporting Information
ACKNOWLEDGMENT
The authors would like to thank Ms Kanchana Ekanayake (Health Sciences Library, The University of Sydney) for her invaluable assistance with the literature searches. Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
Young IE, Poobalan A, Steinbeck K, O'Connor HT, Parker HM. Distribution of energy intake across the day and weight loss: A systematic review and meta‐analysis. Obesity Reviews. 2023;24(3):e13537. doi: 10.1111/obr.13537
REFERENCES
- 1. World Health Organization . Obesity and Overweight Updated 16/02/2018. Accessed 26/06/2019. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 2. Dixon JB. The effect of obesity on health outcomes. Mol Cell Endocrinol. 2010;316(2):104‐108. doi: 10.1016/j.mce.2009.07.008 [DOI] [PubMed] [Google Scholar]
- 3. Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co‐morbidities related to obesity and overweight: a systematic review and meta‐analysis. BMC Public Health. 2009;9(1):88. doi: 10.1186/1471-2458-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jakicic JM, Clark K, Coleman E, et al. Appropriate intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2001;33(12):2145‐2156. doi: 10.1097/00005768-200112000-00026 [DOI] [PubMed] [Google Scholar]
- 5. Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight‐loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360(9):859‐873. doi: 10.1056/NEJMoa0804748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hall KD. What is the required energy deficit per unit weight loss? Int J Obes. 2008;32(3):573‐576. doi: 10.1038/sj.ijo.0803720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McArdle WD, Katch FI, Katch VL. Exercise Physiology: Energy, Nutrition, and Human Performance. LWW; 1991. [Google Scholar]
- 8. Beccuti G, Monagheddu C, Evangelista A, et al. Timing of food intake: sounding the alarm about metabolic impairments? A systematic review. Pharmacol Res. 2017;125(Pt B):132‐141. doi: 10.1016/j.phrs.2017.09.005 [DOI] [PubMed] [Google Scholar]
- 9. Fong M, Caterson ID, Madigan CD. Are large dinners associated with excess weight, and does eating a smaller dinner achieve greater weight loss? A systematic review and meta‐analysis. Br J Nutr. 2017;118(8):616‐628. doi: 10.1017/S0007114517002550 [DOI] [PubMed] [Google Scholar]
- 10. Henry CJ, Kaur B, Quek RYC. Chrononutrition in the management of diabetes. Nutr Diabetes. 2020;10(1):6. doi: 10.1038/s41387-020-0109-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity. 2011;19(7):1374‐1381. doi: 10.1038/oby.2011.100 [DOI] [PubMed] [Google Scholar]
- 12. Garaulet M, Gómez‐Abellán P, Alburquerque‐Béjar JJ, Lee Y‐C, Ordovás JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond). 2013;37(4):604‐611. doi: 10.1038/ijo.2012.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Froy O. Metabolism and circadian rhythms—implications for obesity. Endocr Rev. 2009;31(1):1‐24. doi: 10.1210/er.2009-0014 [DOI] [PubMed] [Google Scholar]
- 14. Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330(6009):1349‐1354. doi: 10.1126/science.1195027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Albrecht U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron. 2012;74(2):246‐260. doi: 10.1016/j.neuron.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 16. Katsi V, Papakonstantinou IP, Soulaidopoulos S, Katsiki N, Tsioufis K. Chrononutrition in cardiometabolic health. J Clin Med. 2022;11(2):296. doi: 10.3390/jcm11020296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Castro JM, Brewer EM, Elmore DK, Orozco S. Social facilitation of the spontaneous meal size of humans occurs regardless of time, place, alcohol or snacks. Appetite. 1990;15(2):89‐101. doi: 10.1016/0195-6663(90)90042-7 [DOI] [PubMed] [Google Scholar]
- 18. Garriguet D. Canadians' eating habits. Health Rep. 2007;18(2):17‐32. [PubMed] [Google Scholar]
- 19. Wang JB, Patterson RE, Ang A, Emond JA, Shetty N, Arab L. Timing of energy intake during the day is associated with the risk of obesity in adults. J Hum Nutr Diet. 2014;27(s2):255‐262. doi: 10.1111/jhn.12141 [DOI] [PubMed] [Google Scholar]
- 20. Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015;22(5):789‐798. doi: 10.1016/j.cmet.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bove CF, Sobal J, Rauschenbach BS. Food choices among newly married couples: convergence, conflict, individualism, and projects. Appetite. 2003;40(1):25‐41. doi: 10.1016/S0195-6663(02)00147-2 [DOI] [PubMed] [Google Scholar]
- 22. Cho S, Dietrich M, Brown CJ, Clark CA, Block G. The effect of breakfast type on total daily energy intake and body mass index: results from the Third National Health and Nutrition Examination Survey (NHANES III). J Am Coll Nutr. 2003;22(4):296‐302. doi: 10.1080/07315724.2003.10719307 [DOI] [PubMed] [Google Scholar]
- 23. Gallant A, Lundgren J, Drapeau V. Nutritional aspects of late eating and night eating. Curr Obes Rep. 2014;3(1):101‐107. doi: 10.1007/s13679-013-0081-8 [DOI] [PubMed] [Google Scholar]
- 24. Kreitzman L, Sassone‐Corsi P. The 24 Hour Society. Profile London; 1999. [Google Scholar]
- 25. McHill AW, Phillips AJ, Czeisler CA, et al. Later circadian timing of food intake is associated with increased body fat. Am J Clin Nutr. 2017;106(5):1213‐1219. doi: 10.3945/ajcn.117.161588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peplonska B, Bukowska A, Sobala W. Association of rotating night shift work with BMI and abdominal obesity among nurses and midwives. PloS One. 2015;10(7):e0133761. doi: 10.1371/journal.pone.0133761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bo S, Fadda M, Castiglione A, et al. Is the timing of caloric intake associated with variation in dietinduced thermogenesis and in the metabolic pattern? A randomized cross‐over study. Int J Obes (Lond). 2015;39(12):1689‐1695. doi: 10.1038/ijo.2015.138 [DOI] [PubMed] [Google Scholar]
- 28. Dashti HS, Gómez‐Abellán P, Qian J, et al. Late eating is associated with cardiometabolic risk traits, obesogenic behaviors, and impaired weight loss. Am J Clin Nutr. 2020;113(1):154‐161. doi: 10.1093/ajcn/nqaa264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao I, Bogossian F, Song S, Turner C. The association between shift work and unhealthy weight: a cross‐sectional analysis from the nurses and midwives' e‐cohort study. J Occup Environ Med. 2011;53(2):153‐158. doi: 10.1097/JOM.0b013e318205e1e8 [DOI] [PubMed] [Google Scholar]
- 30. Antunes L, Levandovski R, Dantas G, Caumo W, Hidalgo M. Obesity and shift work: chronobiological aspects. Nutr Res Rev. 2010;23(1):155‐168. doi: 10.1017/S0954422410000016 [DOI] [PubMed] [Google Scholar]
- 31. Proper KI, van de Langenberg D, Rodenburg W, et al. The relationship between shift work and metabolic risk factors: a systematic review of longitudinal studies. Am J Prev Med. 2016;50(5):e147‐e157. doi: 10.1016/j.amepre.2015.11.013 [DOI] [PubMed] [Google Scholar]
- 32. Joanna Briggs Institute . Checklist for randomized controlled trials. https://jbi.global/research/criticalappraisal-tools.html
- 33. Rugge B, Balshem H, Sehgal R, Relevo R, Gorman P, Helfand M. Appendix A: lipid conversion factors. In: Screening and Treatment of Subclinical Hypothyroidism or Hyperthyroidism. Agency for Healthcare Research and Quality; 2011. [PubMed] [Google Scholar]
- 34. Raynor HA, Li F, Cardoso C. Daily pattern of energy distribution and weight loss. Physiol Behav. 2018;192:167‐172. doi: 10.1016/j.physbeh.2018.02.036 [DOI] [PubMed] [Google Scholar]
- 35. Versteeg R, Ackermans M, Nederveen A, Fliers E, Serlie M, La Fleur S. Meal timing effects on insulin sensitivity and intrahepatic triglycerides during weight loss. Int J Obes (Lond). 2018;42(2):156‐162. doi: 10.1038/ijo.2017.199 [DOI] [PubMed] [Google Scholar]
- 36. Madjd A, Taylor MA, Delavari A, Malekzadeh R, Macdonald IA, Farshchi HR. Effects of consuming later evening meal versus earlier evening meal on weight loss during a weight loss diet: a randomized clinical trial. Br J Nutr. 2020;126(4):1‐25. doi: 10.1017/S0007114520004456 [DOI] [PubMed] [Google Scholar]
- 37. Keim NL, Van Loan MD, Horn WF, Barbieri TF, Mayclin PL. Weight loss is greater with consumption of large morning meals and fat‐free mass is preserved with large evening meals in women on a controlled weight reduction regimen. J Nutr. 1997;127(1):75‐82. doi: 10.1093/jn/127.1.75 [DOI] [PubMed] [Google Scholar]
- 38. Madjd A, Taylor MA, Delavari A, Malekzadeh R, Macdonald IA, Farshchi HR. Beneficial effect of high energy intake at lunch rather than dinner on weight loss in healthy obese women in a weightloss program: a randomized clinical trial. Am J Clin Nutr. 2016;104(4):982‐989. doi: 10.3945/ajcn.116.134163 [DOI] [PubMed] [Google Scholar]
- 39. Lombardo M, Bellia A, Padua E, et al. Morning meal more efficient for fat loss in a 3‐month lifestyle intervention. J Am Coll Nutr. 2014;33(3):198‐205. doi: 10.1080/07315724.2013.863169 [DOI] [PubMed] [Google Scholar]
- 40. Jakubowicz D, Froy O, Wainstein J, Boaz M. Meal timing and composition influence ghrelin levels, appetite scores and weight loss maintenance in overweight and obese adults. Steroids. 2012;77(4):323‐331. doi: 10.1016/j.steroids.2011.12.006 [DOI] [PubMed] [Google Scholar]
- 41. Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity. 2013;21(12):25042512. [DOI] [PubMed] [Google Scholar]
- 42. Rabinovitz HR, Boaz M, Ganz T, et al. Big breakfast rich in protein and fat improves glycemic control in type 2 diabetics. Obesity. 2014;22(5):E46‐E54. doi: 10.1002/oby.20654 [DOI] [PubMed] [Google Scholar]
- 43. Engin A. Circadian rhythms in diet‐induced obesity. In: Obesity and Lipotoxicity. Springer; 2017:19‐52. doi: 10.1007/978-3-319-48382-5_2 [DOI] [PubMed] [Google Scholar]
- 44. Stephan FK. The “other” circadian system: food as a Zeitgeber. J Biol Rhythms. 2002;17(4):284‐292. doi: 10.1177/074873002129002591 [DOI] [PubMed] [Google Scholar]
- 45. Flanagan A, Bechtold DA, Pot GK, Johnston JD. Chrono‐nutrition: from molecular and neuronal mechanisms to human epidemiology and timed feeding patterns. J Neurochem. 2021;157(1):53‐72. doi: 10.1111/jnc.15246 [DOI] [PubMed] [Google Scholar]
- 46. Xiao Q, Garaulet M, Scheer FA. Meal timing and obesity: interactions with macronutrient intake and chronotype. Int J Obes (Lond). 2019;43(9):1701‐1711. doi: 10.1038/s41366-018-0284-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lopez‐Minguez J, Saxena R, Bandín C, Scheer FA, Garaulet M. Late dinner impairs glucose tolerance in MTNR1B risk allele carriers: a randomized, cross‐over study. Clin Nutr. 2018;37(4):1133‐1140. doi: 10.1016/j.clnu.2017.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lim YC, Hoe VC, Darus A, Bhoo‐Pathy N. Association between night‐shift work, sleep quality and metabolic syndrome. Occup Environ Med. 2018;75(10):716‐723. doi: 10.1136/oemed-2018-105104 [DOI] [PubMed] [Google Scholar]
- 49. Hatanaka F, Matsubara C, Myung J, et al. Genome‐wide profiling of the core clock protein BMAL1 targets reveals a strict relationship with metabolism. Mol Cell Biol. 2010;30(24):5636‐5648. doi: 10.1128/MCB.00781-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kalsbeek A, Fliers E, Romijn J, et al. The suprachiasmatic nucleus generates the diurnal changes in plasma leptin levels. Endocrinology. 2001;142(6):2677‐2685. doi: 10.1210/endo.142.6.8197 [DOI] [PubMed] [Google Scholar]
- 51. Ruiter M, La Fleur SE, van Heijningen C, van der Vliet J, Kalsbeek A, Buijs RM. The daily rhythm in plasma glucagon concentrations in the rat is modulated by the biological clock and by feeding behavior. Diabetes. 2003;52(7):1709‐1715. doi: 10.2337/diabetes.52.7.1709 [DOI] [PubMed] [Google Scholar]
- 52. Cailotto C, Lei J, van der Vliet J, et al. Effects of nocturnal light on (clock) gene expression in peripheral organs: a role for the autonomic innervation of the liver. PloS One. 2009;4(5):e5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Westerterp‐Plantenga MS, Lemmens SG, Westerterp KR. Dietary protein–its role in satiety, energetics, weight loss and health. Br J Nutr. 2012;108(S2):S105‐S112. [DOI] [PubMed] [Google Scholar]
- 54. Jeffery RW, Epstein LH, Wilson GT, Drewnowski A, Stunkard AJ, Wing RR. Long‐term maintenance of weight loss: current status. Health Psychol. 2000;19(1S):5‐16. [DOI] [PubMed] [Google Scholar]
- 55. Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long‐term weight control. Obes Res. 2004;12(S12):151S‐162S. doi: 10.1038/oby.2004.282 [DOI] [PubMed] [Google Scholar]
- 56. Lopes TVC, Borba ME, Lopes RVC, et al. Eating late negatively affects sleep pattern and apnea severity in individuals with sleep apnea. J Clin Sleep Med. 2019;15(3):383‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Katagiri R, Asakura K, Kobayashi S, Suga H, Sasaki S. Low intake of vegetables, high intake of confectionary, and unhealthy eating habits are associated with poor sleep quality among middleaged female Japanese workers. J Occup Health. 2014;56(5):359‐368. doi: 10.1539/joh.14-0051-OA [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information


