Summary
To update existing literature and fill the gap in meta‐analyses, this meta‐analysis quantitatively evaluated the worldwide economic burden (in 2022 US $) of childhood overweight and obesity in comparison with healthy weight. The literature search in eight databases produced 7756 records. After literature screening, 48 articles met the eligibility criteria. The increased annual total medical costs were $237.55 per capita attributable to childhood overweight and obesity. Overweight and obesity caused a per capita increase of $56.52, $14.27, $46.38, and $1975.06 for costs in nonhospital healthcare, outpatient visits, medication, and hospitalization, respectively. Length of hospital stays increased by 0.28 days. Annual direct and indirect costs were projected to be $13.62 billion and $49.02 billion by 2050. Childhood obesity ascribed to much higher increased healthcare costs than overweight. During childhood, the direct medical expenditures were higher for males than for females, but, once reaching adulthood, the expenditures were higher for females. Overall, the lifetime costs attributable to childhood overweight and obesity were higher in males than in females, and childhood overweight and obesity resulted in much higher indirect costs than direct healthcare costs. Given the increased economic burden, additional efforts and resources should be allocated to support sustainable and scalable childhood obesity programs.
Keywords: childhood obesity, costs, economics, systematic review
1. INTRODUCTION
Childhood obesity is one of the world's most threatening and alarming health problems. Global childhood obesity has skyrocketed with an increase of more than eightfold over 40 years. 1 In 2020, an estimated 39 million children under the age of 5 years and 150 million children aged 5–19 years were overweight or obese. 2 These numbers are estimated to reach 40 and 254 million in 2030. 2 The current global coronavirus disease 2019 (COVID‐19) pandemic has exacerbated this childhood obesity epidemic. A study with 432,302 United States children found that the pandemic doubled the increase rate of body mass index (BMI), with preschoolers and school‐age children experiencing the largest increase. 3 Being overweight in childhood and adolescence was found to be a strong predictor of adult obesity, which imposes serious short‐ and long‐term physical and psychological threats including type 2 diabetes, cardiovascular diseases, increased mortality, premature death, disability, 2 and decreased mental health. 4 Moreover, obesity can adversely affect children and adolescents' school performance and educational attainment because of its negative effects on cognitive functioning. 5
Childhood obesity imposes personal, societal, and economic challenges for children and their parents, communities, and countries. The direct economic consequences of childhood obesity can include medical costs (e.g., prescription drug, emergency room, and outpatient and inpatient costs), whereas the indirect economic consequences can involve labor market costs such as job absenteeism and lower productivity of caregivers because of caring for sick children. 6 Further, childhood overweight and obesity may persist through adulthood resulting in higher lifetime costs including obesity‐related comorbidities and treatments. 7 Studies conducted in the United States found that the incremental lifetime direct medical costs for a 10‐year‐old child with obesity versus the one with a healthy weight ranged from $16,310 to $19,350. 8 Additionally, the added lifetime medical costs related to obesity among US fifth graders were estimated to be $17 billion higher than those who maintained a healthy weight during childhood but gained weight during adulthood, or $25 billion higher than those who maintained a healthy weight during both childhood and adulthood. 9 By the same token, a study conducted in Germany found that individuals with overweight or obesity during childhood increased lifetime costs by 3.7 times in men and five times in women compared with children with a healthy weight. 10 The estimated excess lifetime costs due to obesity were €10,666 ($8458) for males and €15,963 ($12,659) for females. 10
Relative to overweight and obesity in adulthood, there is a scarcity of research in general and systematic reviews in particular related to the economic burden associated with childhood overweight and obesity. To the best of our knowledge, only a few systematic reviews on the economic burden of childhood overweight and obesity were published during 2012–2018. 6 , 8 , 11 For example, one systematic review of 10 studies published up to July 2010 found that six studies estimated inpatient costs and four estimated outpatient and primary care costs. 6 However, this review was not able to quantitatively synthesize the different healthcare costs because of different healthcare models. 6 Another systematic review with six US‐based studies published before May 2013 estimated increased lifetime medical costs of $19,000 for a child with obesity compared with a child with a healthy weight. 8 A more recent systematic review of 13 studies published between January 2000 and February 2016 reported average total lifetime costs of €149,206 ($112,203) for a boy and €148,196 ($111,443) for a girl with obesity compared with a child with a healthy weight. 11 The two later systematic reviews only focused on the lifetime costs of childhood obesity, and no meta‐analysis was conducted yet. Moreover, all three systemic reviews included a small number of studies (n = 6–13) published before February 2016. Given the increasing rates of childhood overweight and obesity and the annual growth in healthcare spending, an updated review is merited.
Therefore, to update the existing literature and expand the research on healthcare cost categories (e.g., overall healthcare costs, inpatient costs, outpatient costs, medication costs, and total length of hospital stays), this systematic review and meta‐analysis was conducted to quantitatively estimate the total medical costs (including all medical direct costs of inpatient care, outpatient care, and prescriptions), nonhospital healthcare costs (including costs of outpatient care and prescriptions), outpatient visit costs, prescribed medication costs, hospitalization costs, length of hospital stays, and total population or lifetime costs (i.e., annual direct medical and indirect costs and lifetime direct medical and indirect costs) due to childhood overweight and obesity.
2. METHODS
A systematic review and meta‐analysis was conducted. We followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) 12 and the Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) checklist 13 for this report.
2.1. Data sources and search strategy
Our health science librarian conducted a literature search for journal articles, conference abstracts, theses and dissertations, and research reports in the following eight databases: CINAHL, Cochrane, EconLit, Embase, Food Sciences and Technology Abstracts, PsycINFO, PubMed, and Scopus in February 2022. The search focused on three main areas: costs, children or pediatrics, and obesity. Search terms for the costs included healthcare costs, direct costs, indirect costs, economic burden, cost savings, and associated keywords and subject headings. For child, the keywords applied were child, pediatric, adolescents, youth, infants, and associated keywords and subject headings. Lastly, obesity included obesity, overweight, BMI, pediatric obesity, body mass index, and associated keywords and subject headings. The search was modified for each database to include controlled vocabulary but remained largely similar across databases. The search was not date limited. Controlled vocabulary (Medical Subject Headings [MeSH], CINAHL Subject Headings, and EMTREE) as well as keywords were used. The bibliographies of relevant review articles and included eligible articles were also reviewed for potential records.
2.2. Eligibility criteria and study screening
The following inclusion criteria were used for selecting eligible primary articles for this review: (1) mainly included children aged 0–18 years with overweight or obesity and the mean age was under 18 years, (2) the comparison group was children with a healthy weight, (3) calculated increased costs per capita with overweight or obesity compared with subjects with a healthy weight, and (4) written in English. Studies focusing on intervention costs were excluded. Moreover, conference abstracts, theses, dissertations, summary reports, editorials, and expert opinions were not selected. Following the PRISMA flow diagram, two‐step screening was conducted by two independent reviewers: (1) two trained independent reviewers screened each record's title and abstract, and the results were compared and evaluated by the first author, and (2) the first author and a second reviewer carefully screened the full text of each selected article from Step 1 and discussed any discrepancies until reaching an agreement.
2.3. Data extraction
We developed a data extraction form based on previously published reviews. 6 , 11 The form included author, publication year, country, data used for analyses and time frame, child demographic characteristics (e.g., sample size, age, sex, and race), child groups, cost included items, and results on costs (e.g., costs, currency, and year). One trained research assistant extracted relevant data from each selected eligible article following this form, and the first author conducted a thorough review to verify each entry.
We estimated the economic burden of childhood overweight and obesity in US 2022 dollars. Using the purchasing power parities (PPP), 14 we converted different currencies into US dollars for the relevant years. Then we inflated the costs to US dollars ($) in February 2022 using the Consumer Price Index Inflation calculator developed by the US Bureau of Labor Statistics. 15
2.4. Quality appraisal
We adapted the Risk of Bias in Non‐randomized Studies–of Interventions (ROBINS‐I) tool to assess each eligible study's risk of bias. 16 Our adapted evaluation tool included five domains: (1) bias due to confounding, (2) bias in the selection of participants into the study, (3) bias due to missing data, (4) bias in measurements of outcomes, and (5) bias in the selection of the reported results. Following these five domains, two independent evaluators (SC and TK) rated the risk of bias as either low, moderate, serious, or critical for each domain. Results from the two independent evaluators were compared, and inconsistencies were discussed with the first author until reaching a consensus. Studies with low or moderate risk of bias on all five domains were rated to have an overall low risk of bias, and those with any serious or critical risk of bias on any five domains were considered to have an overall high risk of bias. 16 We retained all eligible studies in this review regardless of their risk of biases, but sensitivity analyses were performed to examine the influence of risk of biases (low vs. high) on the economic costs of childhood obesity.
2.5. Data synthesis and analyses
All data analyses were conducted using the Comprehensive Meta‐Analysis Version 3 program (www.meta-analysis.com). Difference in means was calculated as the effect size using random‐effects models to compare the healthcare costs or length of hospital stays between children with a healthy weight and those with overweight or obesity, and the number of comparisons or effect sizes was the sample size in meta‐analysis. A positive effect size indicated that children with overweight or obesity had a higher healthcare cost or a longer length of hospital stays than those with a healthy weight. When mean and standard deviation (SD) were not reported, median (m) and interquartile range (IQR, q1, q3) were used to calculate mean and SD by 17 Influential outliers were identified with standardized residual >2.58 and I 2 being decreased by >10% after removing a potential outlier. 18 Heterogeneity among the included studies was assessed by the Q test and I 2 statistics. Q is the weighted sum of squared differences between individual study effects and pooled effects, and it follows a chi‐square distribution. I 2 statistics of 25%, 50%, and 75% indicated low, moderate, and high levels of heterogeneity. Publication bias was evaluated using the Begg and Mazumdar rank correlation test, Egger's regression asymmetry test, and funnel plot. When both tests' results were significant and funnel plot was asymmetric, there was evidence of publication bias. If publication bias was present, Duval and Tweedie's trim and fill method was used to adjust the effect size. Additionally, sensitivity analyses were performed to examine whether the results were robust according to studies' risk of biases, country, and age.
3. RESULTS
3.1. Study selection
Figure 1 illustrates the PRISMA flow diagram. The literature search produced 7756 records (CINAHL, 921; Cochrane, 9; EconLit, 21; Embase, 1676; Food Science and Technology Abstracts, 31; PsycINFO, 367; PubMed, 2505; Scopus, 2226), and hand searching through article bibliographies resulted in additional 239 records. After removing duplicates, we screened the titles and abstracts of 4679 records. A total of 141 articles were obtained from the first screening. Further screening the full texts of the 141 articles resulted in 48 eligible articles (see Table S1). Ninety‐three articles were excluded because of being reviews/reports/commentaries/editorials (n = 39), abstracts only (n = 23), not assessed healthcare costs (n = 18), not on childhood obesity (n = 9), not in English (n = 3), or a dissertation/thesis (n = 1). Three studies were excluded from the meta‐analysis because of lack of effect size data reported in published articles and unsuccessful contact with corresponding authors. 19 , 20 , 21
FIGURE 1.
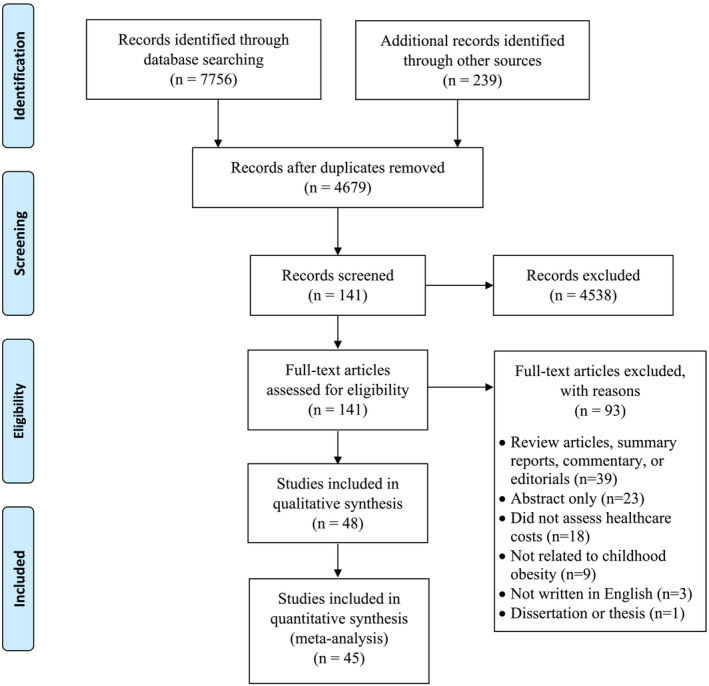
PRISMA flow diagram
3.2. Quality appraisal
Of the 48 studies maintained in this review, 16 (33.3%) were evaluated to have a high risk of bias, and the remaining with a low risk of bias (see Table 1). For the 16 studies rated having a high risk of bias, 10 were due to applying self‐ or parent‐reported height and weight for assessing children's weight status, three had missing data >50%, and three did not consider confounding factors during analysis. Regarding the missing data, 10 studies did not report any missing data information, and eight described a missing data proportion ranging between 20% and 50%. One study even did not report the data analysis approach used in the published article. 22
TABLE 1.
Risk of bias assessment of included studies (n = 48)
| Study | Confounding | Participants | Missing data | Measurement | Results |
|---|---|---|---|---|---|
| Au et al., 2012 | 1 | 1 | 1 | 1 | 1 |
| Batscheider et al., 2014 | 1 | 1 | 2 | 1 | 1 |
| Bettenhausen et al., 2015 | 1 | 1 | 1 | 1 | 1 |
| Biener et al., 2020 | 1 | 1 | 1 | 3 | 1 |
| Black et al., 2018 | 1 | 1 | 1 | 1 | 1 |
| Booth et al., 2009 | 3 | 1 | 1 | 1 | 1 |
| Breitfelder et al., 2011 | 1 | 1 | 1 | 1 | 1 |
| Buescher et al., 2008 | 1 | 1 | 1 | 1 | 1 |
| Clifford et al., 2015 | 1 | 1 | 1 | 1 | 1 |
| Estabrooks et al., 2007 | 1 | 1 | 3 | 1 | 1 |
| Finkelstein et al., 2008 | 1 | 1 | 1 | 1 | 1 |
| Hampl et al., 2007 | 1 | 1 | 1 | 1 | 1 |
| Hayes et al., 2016 | 1 | 1 | 1 | 1 | 1 |
| Janicke et al., 2008 | 1 | 1 | 1 | 1 | 1 |
| Janicke et al., 2010 | 1 | 1 | 1 | 1 | 1 |
| Janssen et al., 2009 | 1 | 1 | 1 | 3 | 1 |
| Jerrell et al., 2009 | 1 | 1 | 1 | 1 | 1 |
| Johnson et al., 2006 | 1 | 1 | 2 | 3 | 1 |
| Kirk et al., 2012 | 1 | 1 | 2 | 1 | 1 |
| Kompaniyets et al., 2020 | 1 | 1 | 1 | 1 | 1 |
| Kuhle et al., 2011 | 1 | 1 | 2 | 1 | 1 |
| Kuhle et al., 2012 | 1 | 1 | 2 | 1 | 1 |
| Lightwood et al., 2009 | 1 | 1 | 1 | 1 | 1 |
| Monheit et al., 2009 | 1 | 1 | 2 | 3 | 1 |
| Nafiu et al., 2008 | 1 | 1 | 1 | 1 | 1 |
| Okubo et al., 2016 | 1 | 1 | 1 | 1 | 1 |
| Okubo et al., 2017A | 1 | 1 | 2 | 1 | 1 |
| Okubo et al., 2017B | 1 | 1 | 2 | 1 | 1 |
| Okubo et al., 2018A | 1 | 1 | 2 | 1 | 1 |
| Okubo et al., 2018B | 1 | 1 | 2 | 1 | 1 |
| Okubo et al., 2018C | 1 | 1 | 2 | 1 | 1 |
| Ramsey et al., 2020 | 1 | 1 | 3 | 1 | 1 |
| Sonntag et al., 2016 | 1 | 1 | 1 | 1 | 1 |
| Thavamani et al., 2020 | 1 | 1 | 1 | 1 | 1 |
| Trasande et al., 2009A | 1 | 1 | 2 | 3 | 1 |
| Trasande et al., 2009B | 1 | 1 | 2 | 1 | 1 |
| Trasande et al., 2010 | 1 | 1 | 2 | 1 | 1 |
| Turer et al., 2013 | 1 | 1 | 1 | 3 | 1 |
| Vellinga et al., 2008 | 3 | 1 | 1 | 1 | 1 |
| Wang et al., 2002 | 3 | 1 | 1 | 1 | 1 |
| Wang et al., 2010 | 1 | 1 | 2 | 3 | 1 |
| Ward et al., 2021 | 1 | 1 | 1 | 3 | 1 |
| Wenig et al., 2011 | 1 | 1 | 1 | 1 | 1 |
| Wenig et al., 2012 | 1 | 1 | 1 | 1 | 1 |
| Wijga et al., 2018 | 3 | 1 | 2 | 3 | 2 |
| Woolford et al., 2007 | 1 | 1 | 3 | 1 | 1 |
| Woodford et al., 2009 | 1 | 1 | 2 | 1 | 1 |
| Wright et al., 2014 | 1 | 1 | 2 | 3 | 1 |
Notes: 1 = low, 2 = moderate, 3 = serious risk of bias.
3.3. Study characteristics
Among the included 48 studies, 29 were conducted in the United States, seven in Europe (including five in Germany, one in Ireland, and one in the Netherlands), five in Australia, four in Canada, and three in Japan. Publication years ranged from 2002 to 2021: one in 2002, 17 in 2005–2009, 13 in 2010–2014, 12 in 2015–2019, and five in 2020–2021. The average sample size was 412,000, with a range from 200 to 8 million. Among the 48 studies, 16 included all age categories of children (0–18 years), nine included school‐age children and adolescents (6–17 years), three included preschoolers and school‐age children (3–8 years), and 20 included only one age category including three with young children (0–5 years), seven with school‐age children (6–11 years), and 10 with adolescents (12–18 years). About 52.9% were male (range: 41%–86.9%).
Twenty‐one studies (43.8%) examined the total medical costs including inpatient care, outpatient visits, emergency visits, and medication. Three studies (6.3%) focused on the nonhospital healthcare costs, and three other studies (6.3%) assessed only the outpatient visit costs. Eight studies (16.7%) estimated the prescribed medication costs. Fifteen (31.3%) studies focused on hospitalization‐related medical care costs and lengths of hospital stays. Four studies (8.3%) focused on annual population or lifetime direct medical or indirect costs in relation to childhood overweight and obesity.
3.4. Direct healthcare costs
3.4.1. Total medical costs
Thirty‐five comparisons, with a high level of heterogeneity (Q = 239,663.71, p < 0.001; I 2 = 99.99%), evaluated the annual total medical costs between healthy weight and overweight/obesity, and no influential outlier was identified. Overall, being overweight or obese resulted in a per capita increase of $237.55 (95%CI: 165.54, 309.56; p < 0.001; see Figure 2) total medical costs annually during childhood. Specifically, obesity increased the annual total medical costs by $307.72 per capita (k = 15; 95%CI: 241.39, 374.04; p < 0.001), whereas overweight increased the annual total medical costs by $190.51 per capita (k = 19; 95%CI: 130.14, 250.88; p < 0.001), and the differences were statistically significant (Q = 6.56, p = 0.010).
FIGURE 2.
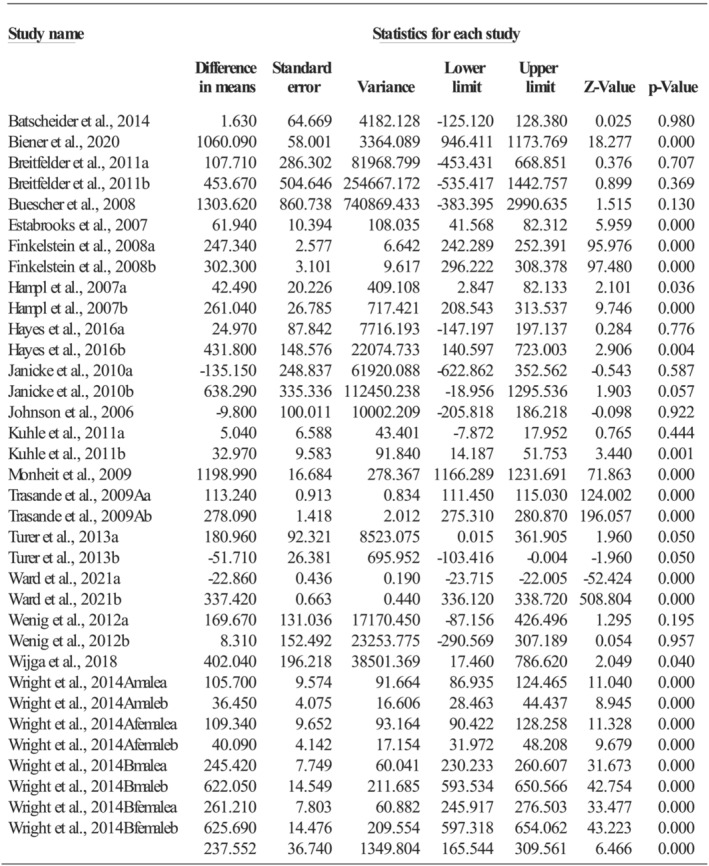
Effect size of total medical costs
3.4.2. Nonhospital healthcare costs
Seven comparisons, without any identified influential outliers, assessed the annual nonhospital healthcare costs and had a high level of heterogeneity (Q = 45.12, p < 0.001; I 2 = 86.70%). The average annual increased costs were $56.52 per capita (95%CI: 27.94, 85.09; p < 0.001) during childhood attributable to childhood overweight and obesity. Obesity increased the costs by $68.22 per capita annually (k = 3; 95%CI: 12.73, 123.70; p = 0.016), and overweight resulted in an increase of $52.28 per capita (k = 4; 95%CI: 13.55, 91.01; p = 0.008), and the increases were not significantly different (Q = 0.21, p = 0.644).
3.4.3. Outpatient visit costs
One study evaluated the mean outpatient visit costs per year but found no difference between healthy weight and overweight/obesity. 23 Four comparisons, having a high level of heterogeneity (Q = 190.70, p < 0.001; I 2 = 98.43%), evaluated the outpatient visit costs per capita per visit, with an average increase of $14.27 (95%CI: 3.76, 24.78; p = 0.008) during childhood among children with overweight or obesity. Moreover, obesity resulted in a significantly larger per capita increase of $20.86 per visit (k = 2; 95%CI: 12.28, 29.44; p < 0.001), compared with increased costs of $6.95 (k = 2; 95%CI: −0.02, 13.91; p = 0.050) for being overweight (Q = 6.09, p = 0.014).
3.4.4. Prescribed medication costs
Thirteen comparisons, with no influential outlier but a high level of heterogeneity (Q = 19,271.33, p < 0.001; I 2 = 99.94%), assessed the annual prescribed medication costs during childhood. Being overweight or obese increased the annual prescribed medication costs by $46.38 per capita (95%CI: −5.03, 97.78; p = 0.077; see Figure 3). The increased annual prescribed medication costs per capita were $64.69 (k = 5; 95%CI: 13.68, 115.71; p = 0.013) for being obese and $33.23 (k = 8; 95%CI: −8.98, 75.44; p = 0.123) for being overweight, but the differences were not statistically significant (Q = 0.87, p = 0.352).
FIGURE 3.
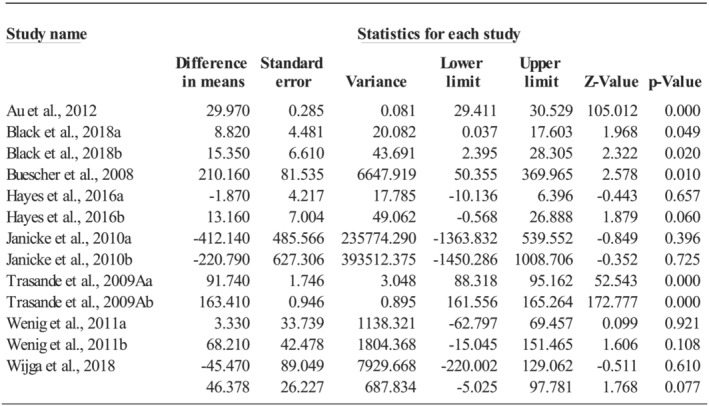
Effect size of prescribed medication costs
3.4.5. Hospitalization costs
The primary diagnoses for hospitalization varied across studies including asthma (k = 7), pneumonia (k = 6), adenotonsillectomy (k = 2), appendicitis (k = 2), affective disorder (k = 2), acute pancreatitis (k = 1), urinary tract infection (k = 1), and obesity (k = 1); resulting in a very high level of heterogeneity (Q = 56,051,897.3, p < 0.001; I 2 = 100%). The average increased per capita hospitalization costs for being overweight or obese were $1975.06 per hospitalization (95%CI: 1816.85, 2133.27; p < 0.001; see Figure 4) during childhood. The increased hospitalization costs were much higher (Q = 40.70, p < 0.001) for being obese ($2439.14, k = 19; 95%CI: 2135.93, 2742.36; p < 0.001) than for being overweight ($142.27, k = 4; 95%CI: −494.92, 779.47; p = 0.662). When obesity was the primary diagnosis, the increased hospitalization costs were $6997.29 per capita per hospitalization (95%CI: 6864.40, 7130.18; p < 0.001). Additionally, when the primary diagnosis was appendicitis, urinary tract infection, affective disorder, acute pancreatitis, asthma, pneumonia, or adenotonsillectomy, the increased hospitalization costs were $5503.95 (95%CI: 5370.67, 5637.22; p < 0.001), $2128.58 (95%CI: 1365.46, 2891.70; p < 0.001), $1936.45 (95%CI: 1807.25, 2065.66; p < 0.001), $1846.20 (95%CI: 1707.20, 1985.20; p < 0.001), $1825.38 (95%CI: 1759.10, 1891.66; p < 0.001), $1318.37 (95%CI: 1258.90, 1377.84; p < 0.001), or $902.64 (95%CI: 497.19, 1308.08; p < 0.001), respectively.
FIGURE 4.
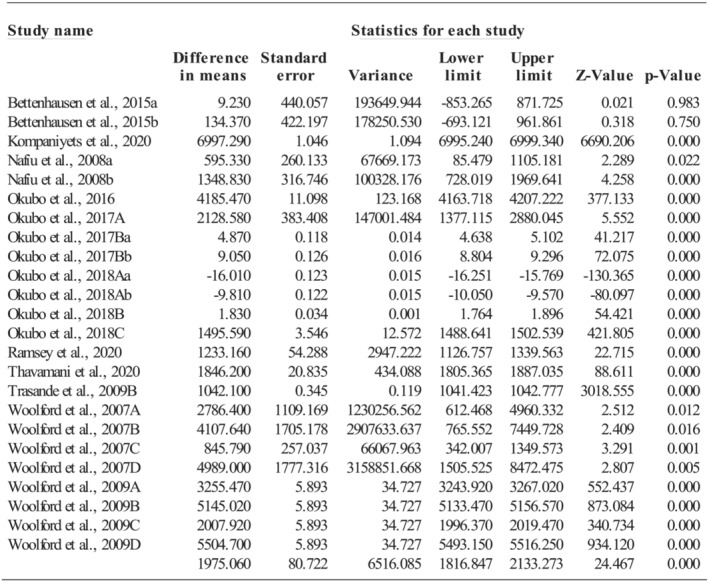
Effect size of hospitalization costs
3.4.6. Length of hospital stays
Twenty‐three comparisons, with no influential outliers but a very high level of heterogeneity (Q = 358,237,293, p < 0.001; I 2 = 100%), evaluated the length of hospital stays during childhood attributed to being overweight or obese. The average length of hospital stays increased by 0.28 days (95%CI: −0.44, 0.99; p = 0.446; see Figure 5) for being overweight or obese, with 0.36 days (k = 18; 95%CI: −0.47, 1.18; p = 0.398) for being obese and 0.002 (k = 4; 95%CI: −1.74, 1.75; p = 0.998) for being overweight, and the differences were not significant (Q = 0.13, p = 0.720).
FIGURE 5.
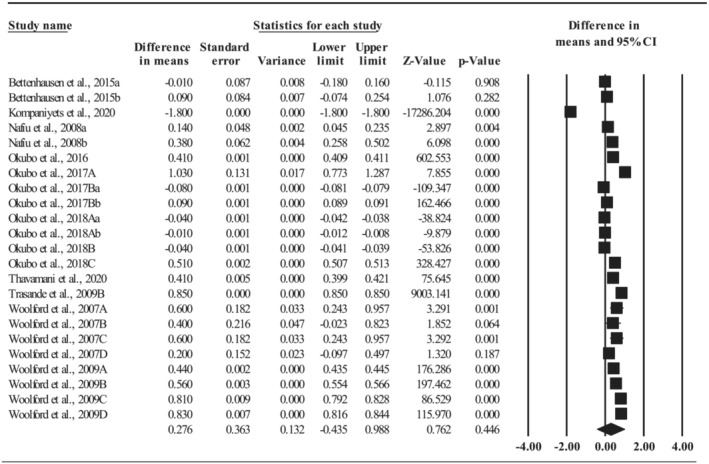
Effect size of length of hospital stays
3.5. Total population or lifetime costs
Five studies (four in the United States and one in Germany) assessed the total population or lifetime costs due to being overweight or obese. The annual hospital costs associated with childhood obesity were estimated to be $55.59 million in 1979–1981 and $203.13 million in 1997–1999. 24 The average adolescent overweight rate in 1971–2000 would result in excess annual direct medical costs of $177.02 million in 2020 and $13.62 billion in 2050 and annual indirect costs of lost productivity of $1.28 billion in 2020 and $49.02 billion in 2050. 25 The direct medical expenditures in childhood were $1.02 billion in males and $973.39 in females for being overweight in 2003–2006, whereas $1.05 billion in males and $997.46 million in females for being obese in 2003–2006. 26 The direct medical expenditures in adulthood were projected to be $401.29 million in males and $509.81 million in females because of being overweight during childhood and $1.82 billion in males and $2.33 billion in females attributed to childhood obesity. 26 The lifetime medical costs saved due to a 1% reduction in adolescent overweight and obesity in 2000 were $798.37 million ($99.41/capita). 27 One study in Germany found that the excess indirect lifetime costs due to childhood overweight and obesity were $4130.93 per male and $2399.65 per female. 28
3.6. Publication bias
Overall, no strong evidence of publication bias was identified based on the results from the Begg and Mazumdar rank correlation test and Egger's regression asymmetry test and the relative symmetry of funnel plots. For the total medical costs, the Begg and Mazumdar rank correlation test's results were significant (Tau = 0.33, z = 2.83, p = 0.005), but the Egger's regression asymmetry test's results were not significant (b = 15.44, t = 0.87, p = 0.392). For medication costs (Tau = 0.38, z = 1.83, p = 0.067; b = −10.36, t = 0.62, p = 0.548) and hospitalization length of stays (Tau = 0.25, z = 1.66, p = 0.096; b = −92.23, t = 0.39, p = 0.698), results from both tests were nonsignificant. For the hospitalization costs, results from the Begg and Mazumdar rank correlation test were not significant (Tau = 0.23, z = 1.59, p = 0.112), but the results from the Egger's regression asymmetry test were significant (b = −183.60, t = 2.15, p = 0.043).
3.7. Sensitivity analyses
The increased total medical costs (146.52 vs. 292.53, Q = 3.68, p = 0.055) and length of hospital stays (0.24 vs. 0.45, Q = 0.05, p = 0.827) due to being overweight or obese did not vary significantly between studies with a low risk of bias and those with a high risk of bias. Increased prescribed medication costs were greater among studies with a high risk of bias than those with a low risk of bias (113.38 vs. 22.34, Q = 7.75, p = 0.005). However, the increased hospitalization costs were higher in studies with a low risk of bias than those with a high risk of bias (2047.34 vs. 1309.53, Q = 7.38, p = 0.007). Studies assessing nonhospital healthcare costs and outpatient visit costs all had a low risk of bias.
As demonstrated in Table 2, increased total medical and prescribed medication costs due to being overweight or obesity were significantly higher in adolescents aged 12–18 years than among young children, but nonhospital healthcare costs were significantly higher in young children aged 0–5 years than among school‐age children. Overall, direct healthcare costs (i.e., total medical costs, prescribed medication costs, and hospitalization costs) attributable to childhood overweight and obesity were higher in the United States than in other countries.
TABLE 2.
Direct healthcare costs by country and age categories
| Category | Total medical costs ($) | Nonhospital healthcare costs ($) | Outpatient visit costs ($) | Prescribed medication costs ($) | Hospitalization costs ($) | Length of hospital stay (days) |
|---|---|---|---|---|---|---|
| Age | p < 0.001 | p < 0.001 | – | p = 0.049 | p < 0.001 | p = 0.886 |
| 0–5 | 201.12 | 74.57 | – | 13.81 | – | – |
| 6–11 | 39.90 | 31.20 | 14.27 | 12.07 | 73.72 | 0.04 |
| 12–18 | 593.00 | – | – | 91.19 | – | – |
| 0–11 | – | – | – | – | −2.01 | −0.02 |
| 6–18 | 223.18 | – | – | 124.50 | – | – |
| 0–18 | 56.83 | – | – | 32.63 | 2971.69 | 0.40 |
| Country | p = 0.243 | – | – | p = 0.001 | p < 0.001 | p = 0.678 |
| United States | 274.75 | – | – | 132.82 | 2848.25 | 0.36 |
| Europe | 132.55 | – | – | 21.44 | – | – |
| Australia | 201.31 | 56.52 | – | 13.12 | – | – |
| Canada | 19.00 | – | 14.27 | – | – | – |
| Japan | – | – | – | – | −2.01 | −0.02 |
4. DISCUSSION
This is the first systematic review and meta‐analysis in the international literature of studies that comprehensively evaluated the average increased total medical costs, nonhospital healthcare costs, outpatient visit costs, prescribed medication costs, hospitalization costs, and length of hospital stays attributable to childhood overweight and obesity. The total population or lifetime costs of childhood overweight and obesity were also synthesized. Overall, being overweight or obese during childhood significantly increased the total medical costs, nonhospital healthcare costs, outpatient visit costs, and hospitalization costs. It is clear that childhood obesity resulted in higher increased total medical costs, outpatient visit costs, and hospitalization costs than childhood overweight. Thus, reducing childhood obesity prevalence could save many preventable healthcare costs.
The increased annual total medical costs attributable to childhood overweight and obesity were $237.55 per capita ($307.72 due to obesity and $190.51 due to overweight) in comparison with a child with a healthy weight. Among the 189 million children who were overweight or obese in 2020 worldwide, 2 the increased total medical costs are approximately $45 billion per year. With the current estimated childhood obesity prevalence of 22% in the United States, 3 the increased total medical costs are about $5 billion per year, accounting for over 1% healthcare spending in the United States. 29 This 1% estimation is within the range of 0.7% and 2.8% of a country's total healthcare expenditures on account of obesity. 30 By 2050, United States' adolescent overweight is projected to cause $13.62 billion in annual direct medical costs and $49.02 billion in annual indirect costs. 25 This study also found that the increased total medical costs attributable to overweight or obesity were highest in older adolescents aged 12–18 years. This may be due to the increased health risks of being obese on developing chronic comorbidities such as type 2 diabetes and cardiovascular diseases in adolescence. 31 , 32 These results indicate the urgent need of preventing childhood overweight and obesity early on.
Nonhospital healthcare and outpatient visit costs also increased because of childhood overweight and obesity. Unfortunately, no previous review was identified to quantitatively synthesize these outpatient care costs among children. Literature in adults found average annual physician visit costs of about $500 per capita ascribable to overweight and obesity. 33 The increased physician visit costs in adults are much higher than the increased costs (annual $40 per capita) in children. 34 The increased nonhospital healthcare costs due to childhood overweight and obesity lead to extra expenditures of approximately 11 billion per year globally and over 1 billion in the United States. 2 , 3 For the increased outpatient visit costs, childhood obesity ascribed to an increase of $20.86 per capita per visit, which is equivalent to about 1 billion annual increased costs in the United States. 34 , 35 Given the increased outpatient care costs attributable to childhood overweight and obesity, healthcare providers at the outpatient settings play the key role of focusing on childhood obesity prevention and treatment through assessing and monitoring weight status, providing healthy lifestyle promotion consultations, and referring to community‐based obesity prevention resources. 36 , 37
Childhood obesity resulted in a significant annual increase of $64.69 per capita in prescribed medication costs. This result is supported by previous literature indicating that children with obesity were more likely to use prescribed medications, especially medications for respiratory conditions, than those with a healthy weight. 38 During the current global COVID‐19 pandemic, obesity is recognized as a strong risk factor of hospitalization and death because of its suppressed effects on the immune system. 39 With the global obesity epidemic colliding with the COVID‐19 pandemic, 40 public and healthcare service actions (i.e., virtual obesity consultation, healthy food accessibility, and active lifestyle promotion) are needed to increase adequate access of effective obesity prevention or treatment resources.
Obesity significantly increased the hospitalization costs by $2439.14 per hospitalization but not the length of hospital stays. The increased hospitalization costs were much higher than the estimation of $1200 in 2000 and $1900 in 2009. 41 The average increased length of hospital stays of 0.36 days attributable to childhood obesity is much lower than the 1.5–1.8 days reported in one United States' study. 41 These mixed results may be due to the widely diverse healthcare systems with different coverages around the world: universal coverage with single‐payer system, universal coverage with multi‐payer system, multi‐payer system with no universal coverage, and no national healthcare infrastructure. 42 Surprisingly, the increased hospitalization costs were much higher when the primary diagnosis was childhood obesity ($6997.29) compared with other diseases ($902.64–5503.95) such as asthma, pneumonia, or appendicitis. This disturbing result highlights the urgent need to control the increasing childhood obesity prevalence.
In comparison with overweight, childhood obesity resulted in higher total medical, outpatient visit, and hospitalization costs but not in the nonhospital healthcare or prescribed medication costs. These results suggest that the excessive increased healthcare costs due to childhood obesity are more related to inpatient care instead of outpatient care or prescriptions. Similarly, one US study also found that the total population direct medical expenditures in both childhood and adulthood were higher in both females and males for being obese than overweight during childhood. 26 Another US study showed that with one‐unit BMI increase among an adult with obesity, the total medical expenditures would increase by $253 per capita. 43 Likewise, studies conducted in Australia and Canada also supported the higher direct healthcare costs due to childhood obesity compared with being overweight. 44 , 45 To prevent children from progressing to severe obesity and, consequently, reduce overall healthcare costs, especially in relation to inpatient care, “the big five” behaviors should be targeted early on: sweetened beverages, fast foods, family meals, media time, and habitual physical activity. 46 Moreover, compared with obesity treatments such as adolescent bariatric surgery that results in negligible effects on reducing childhood obesity prevalence, primary preventions focusing on population behavioral changes are more cost effective. 47 , 48
Interestingly, sex differences are observed in total population and lifetime costs ascribable to childhood overweight and obesity. Direct medical expenditures are higher in males than in females during childhood, but during adulthood, the costs are higher in females than in males. 26 The higher direct healthcare costs in adult women than in men may attribute to the increased functional limitation and disability and longer life expectancy in women. 49 However, the indirect lifetime costs are higher in men than in women. One study in Germany found that the estimated indirect lifetime costs due to childhood overweight and obesity were almost two times higher in males than in females. 28 Consistently, one review also concluded that the total lifetime direct and indirect costs of childhood overweight and obesity were higher in males than in females. 11 The plausible explanation for these sex differences in indirect and total lifetime costs is that women usually have lower employment and wage rates than men because of increased household responsibilities. 50 , 51 As a result, the indirect lifetime costs related to work absenteeism and low productivity due to being obesity may be lower in women than in men. Moreover, childhood overweight and obesity result in much higher indirect lifetime costs than direct healthcare costs. 25 This result is consistent with a previous review with 13 studies showing that indirect costs due to productivity losses were about seven times higher than the direct healthcare costs of childhood overweight and obesity. 11 Therefore, both direct healthcare costs and indirect costs associated with psychosocial problems, mobbing, school absences, and productivity losses should be considered when estimating the economic burden of childhood overweight and obesity.
5. LIMITATIONS
This review has a few limitations, mainly because of the high levels of heterogeneity among studies. First, the included 48 studies were conducted in different countries with different age categories of children, and our results showed cost variations by country and age. Because of the worldwide diverse healthcare systems along with different insurance coverages 42 as well as the increasing rates of obesity‐related comorbidities and decreasing quality of life from childhood to adolescence, 31 , 52 interpretation of the results needs caution. Moreover, the included studies were published from 2002 to 2021. In the past 20 years, the overall healthcare expenditures rose because of new technologies, new medications, more service provided per patient, defensive medicine, insurance system, and free rider programs. 53 , 54 Therefore, the validity of the study's results may be reduced because of the increasing healthcare expenditures and changes in costs associated with new medications and surgery in the past two decades.
6. CONCLUSIONS
Although the included 48 studies varied widely in study data, country, child characteristics such as age, risk of biases, cost included items, and currency used, this review's results consistently demonstrate the increased economic burden attributable to childhood overweight and obesity. Obesity ascribed to much higher increased healthcare costs in comparison with overweight. During childhood, the direct medical expenditures due to obesity are higher among males than among females, but the expenditures become higher in females than in males during adulthood. Overall, the total lifetime costs of childhood overweight and obesity are higher in males than in females, and childhood obesity results in much higher indirect costs than direct healthcare costs. Therefore, given the growing prevalence of childhood overweight and obesity and its increasing economic burden especially the astounding huge indirect lifetime costs, additional efforts and resources should be allocated to support sustainable and scalable obesity prevention and intervention programs. To prevent and mitigate childhood obesity‐related long‐term economic burden in healthcare and productivity losses, early prevention is the most promising tool than later treatment or care. 55 , 56
CONFLICT OF INTEREST
No conflict of interest statement.
Supporting information
Table S1 Summary Table (N = 48)
ACKNOWLEDGMENTS
We would like to thank Michigan State University undergraduate students Nandini Koneru and Madison Penetrante for their assistance in literature screening and data extraction. Also, we want to acknowledge our College of Nursing master‐prepared health science librarian Jessica Sender for her comprehensive literature search for this systematic review.
Ling J, Chen S, Zahry NR, Kao T‐SA. Economic burden of childhood overweight and obesity: A systematic review and meta‐analysis. Obesity Reviews. 2023;24(2):e13535. doi: 10.1111/obr.13535
REFERENCES
- 1. NCD Risk Factor Collaboration . Worldwide trends in body‐mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population‐based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627‐2642. doi: 10.1016/S0140-6736(17)32129-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Draft recommendations for the prevention and management of obesity over the life course, including potential targets. 2021. https://cdn.who.int/media/docs/default-source/obesity/who-discussion-paper-on-obesity---final190821.pdf?sfvrsn=4cd6710a_1&download=true
- 3. Lange SJ, Kompaniyets L, Freedman DS, et al. Longitudinal trends in body mass index before and during the COVID‐19 pandemic among persons aged 2‐19 years—United States, 2018‐2020. MMWR Morb Mortal Wkly Rep. 2021;70(37):1278‐1283. doi: 10.15585/mmwr.mm7037a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carsley S, Tu K, Parkin PC, Pullenayegum E, Birken CS. Overweight and obesity in preschool aged children and risk of mental health service utilization. Int J Obes (Lond). 2019;43(7):1325‐1333. doi: 10.1038/s41366-018-0280-1 [DOI] [PubMed] [Google Scholar]
- 5. Mamrot P, Hanć T. The association of the executive functions with overweight and obesity indicators in children and adolescents: a literature review. Neurosci Biobehav Rev. 2019;107:59‐68. doi: 10.1016/j.neubiorev.2019.08.021 [DOI] [PubMed] [Google Scholar]
- 6. Pelone F, Specchia ML, Veneziano MA, et al. Economic impact of childhood obesity on health systems: a systematic review. Obes Rev. 2012;13(5):431‐440. doi: 10.1111/j.1467-789X.2011.00968.x [DOI] [PubMed] [Google Scholar]
- 7. Llewellyn A, Simmonds M, Owen CG, Woolacott N. Childhood obesity as a predictor of morbidity in adulthood: a systematic review and meta‐analysis. Obes Rev. 2016;17(1):56‐67. doi: 10.1111/obr.12316 [DOI] [PubMed] [Google Scholar]
- 8. Finkelstein EA, Graham WC, Malhotra R. Lifetime direct medical costs of childhood obesity. Pediatrics. 2014;133(5):854‐862. doi: 10.1542/peds.2014-0063 [DOI] [PubMed] [Google Scholar]
- 9. Levitt DE, Jackson AW, Morrow JR. An analysis of the medical costs of obesity for fifth graders in California and Texas. Int J Exerc Sci. 2016;9(1):26‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sonntag D, Ali S, Lehnert T, Konnopka A, Riedel‐Heller S, König HH. Estimating the lifetime cost of childhood obesity in Germany: results of a Markov model. Pediatr Obes. 2015;10(6):416‐422. doi: 10.1111/ijpo.278 [DOI] [PubMed] [Google Scholar]
- 11. Hamilton D, Dee A, Perry IJ. The lifetime costs of overweight and obesity in childhood and adolescence: a systematic review. Obes Rev. 2018;19(4):452‐463. doi: 10.1111/obr.12649 [DOI] [PubMed] [Google Scholar]
- 12. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(jul21 1):b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of observational studies in epidemiology (MOOSE) group. Jama. 2000;283(15):2008‐2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 14. Organisation for Economic Co‐operation and Development . Purchasing power parities (PPP). 2020. Accessed June 01, 2021. https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm
- 15. U.S. Bureau of Labor Statistics . CPI Inflation Calculator. 2021. Accessed June 01, 2021. https://www.bls.gov/data/inflation_calculator.htm
- 16. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin L, Chu H, Hodges JS. Alternative measures of between‐study heterogeneity in meta‐analysis: reducing the impact of outlying studies. Biometrics. 2017;73(1):156‐166. doi: 10.1111/biom.12543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vellinga A, O'Donovan D, De La Harpe D. Length of stay and associated costs of obesity related hospital admissions in Ireland. BMC Health Serv Res. 2008;8(1):88. doi: 10.1186/1472-6963-8-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Janicke DM, Harman JS, Kelleher KJ, Zhang J. The association of psychiatric diagnoses, health service use, and expenditures in children with obesity‐related health conditions. J Pediatr Psychol. 2009;34(1):79‐88. doi: 10.1093/jpepsy/jsn051 [DOI] [PubMed] [Google Scholar]
- 21. Booth ML, Dobbins T, Aitken R, et al. Costs of managing conditions associated with obesity among Australian teenagers. J Paediatr Child Health. 2009;45(7–8):448‐456. doi: 10.1111/j.1440-1754.2009.01503.x [DOI] [PubMed] [Google Scholar]
- 22. Wijga AH, Mohnen SM, Vonk JM, Uiters E. Healthcare utilisation and expenditure of overweight and non‐overweight children. J Epidemiol Community Health. 2018;72(10):940‐943. doi: 10.1136/jech-2017-210222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Janssen I, Lam M, Katzmarzyk PT. Influence of overweight and obesity on physician costs in adolescents and adults in Ontario, Canada. Obes Rev. 2009;10(1):51‐57. doi: 10.1111/j.1467-789X.2008.00514.x [DOI] [PubMed] [Google Scholar]
- 24. Wang G, Dietz WH. Economic burden of obesity in youths aged 6 to 17 years: 1979‐1999. Pediatrics. 2002;109(5):E81. doi: 10.1542/peds.109.5.e81 [DOI] [PubMed] [Google Scholar]
- 25. Lightwood J, Bibbins‐Domingo K, Coxson P, Wang YC, Williams L, Goldman L. Forecasting the future economic burden of current adolescent overweight: an estimate of the coronary heart disease policy model. Am J Public Health. 2009;99(12):2230‐2237. doi: 10.2105/AJPH.2008.152595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trasande L. How much should we invest in preventing childhood obesity? Health Aff. 2010;29(3):372‐378. doi: 10.1377/hlthaff.2009.0691 [DOI] [PubMed] [Google Scholar]
- 27. Wang LY, Denniston M, Lee S, Galuska D, Lowry R. Long‐term health and economic impact of preventing and reducing overweight and obesity in adolescence. J Adolesc Health. 2010;46(5):467‐473. doi: 10.1016/j.jadohealth.2009.11.204 [DOI] [PubMed] [Google Scholar]
- 28. Sonntag D, Ali S, De Bock F. Lifetime indirect cost of childhood overweight and obesity: a decision analytic model. Obesity. 2016;24(1):200‐206. doi: 10.1002/oby.21323 [DOI] [PubMed] [Google Scholar]
- 29. U.S. Centers for Medicare & Medicaid Services . NHE fact sheet 2021. Accessed June 14, 2022. https://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/NationalHealthExpendData/NHE‐Fact‐Sheet
- 30. Withrow D, Alter DA. The economic burden of obesity worldwide: a systematic review of the direct costs of obesity. Obes Rev. 2011;12(2):131‐141. doi: 10.1111/j.1467-789X.2009.00712.x [DOI] [PubMed] [Google Scholar]
- 31. Killedar A, Lung T, Petrou S, Teixeira‐Pinto A, Tan EJ, Hayes A. Weight status and health‐related quality of life during childhood and adolescence: effects of age and socioeconomic position. Int J Obes (Lond). 2020;44(3):637‐645. doi: 10.1038/s41366-020-0529-3 [DOI] [PubMed] [Google Scholar]
- 32. Nagata JM, Ganson KT, Liu J, Gooding HC, Garber AK, Bibbins‐Domingo K. Adolescent body mass index and health outcomes at 24‐year follow‐up: a prospective cohort study. J am Coll Cardiol. 2021;77(25):3229‐3231. doi: 10.1016/j.jacc.2021.04.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tran BX, Nair AV, Kuhle S, Ohinmaa A, Veugelers PJ. Cost analyses of obesity in Canada: scope, quality, and implications. Cost Eff Resour Alloc. 2013;11(1):3. doi: 10.1186/1478-7547-11-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ashman JJ, Rui P, Okeyode T. Characteristics of office‐based physician visits, 2016. 2019. Accessed June 15, 2022. https://www.cdc.gov/nchs/products/databriefs/db331.htm [PubMed]
- 35. Ray KN, Shi Z, Ganguli I, Rao A, Orav EJ, Mehrotra A. Trends in pediatric primary care visits among commercially insured US children, 2008‐2016. JAMA Pediatr. 2020;174(4):350‐357. doi: 10.1001/jamapediatrics.2019.5509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vine M, Hargreaves MB, Briefel RR, Orfield C. Expanding the role of primary care in the prevention and treatment of childhood obesity: a review of clinic‐ and community‐based recommendations and interventions. J Obes. 2013;2013:172035. doi: 10.1155/2013/172035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pearce C, Rychetnik L, Wutzke S, Wilson A. Obesity prevention and the role of hospital and community‐based health services: a scoping review. BMC Health Serv Res. 2019;19(1):453. doi: 10.1186/s12913-019-4262-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Solmi F, Morris S. Association between childhood obesity and use of regular medications in the UK: longitudinal cohort study of children aged 5–11 years. BMJ Open. 2015;5(6):e007373. doi: 10.1136/bmjopen-2014-007373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kwok S, Adam S, Ho JH, et al. Obesity: a critical risk factor in the COVID‐19 pandemic. Clinical Obes. 2020;10(6):e12403. doi: 10.1111/cob.12403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sanchis‐Gomar F, Lavie CJ, Mehra MR, Henry BM, Lippi G. Obesity and outcomes in COVID‐19: when an epidemic and pandemic collide. Mayo Clin Proc. 2020;95(7):1445‐1453. doi: 10.1016/j.mayocp.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wier LM, Encinosa W. Obesity in children: Hospitalizations from 2000 to 2009. 2012. Accessed June 14, 2022. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb138.pdf [PubMed]
- 42.Council on Foreign Relations. How health care works around the world. 2021. https://world101.cfr.org/global-era-issues/global-health/how-health-care-works-around-world
- 43. Ward ZJ, Bleich SN, Long MW, Gortmaker SL. Association of body mass index with health care expenditures in the United States by age and sex. PloS One. 2021;16(3):e0247307. doi: 10.1371/journal.pone.0247307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hayes A, Chevalier A, D'Souza M, Baur L, Wen LM, Simpson J. Early childhood obesity: association with healthcare expenditure in Australia. Obesity. 2016;24(8):1752‐1758. doi: 10.1002/oby.21544 [DOI] [PubMed] [Google Scholar]
- 45. Kirk SF, Kuhle S, Ohinmaa A, Colman I, Veugelers PJ. Health care utilization from prevalent medical conditions in normal‐weight, overweight, and obese children. J Pediatr. 2012;160(2):216‐221.e211. doi: 10.1016/j.jpeds.2011.08.015 [DOI] [PubMed] [Google Scholar]
- 46. Rao G. Childhood obesity: highlights of AMA expert committee recommendations. Am Fam Physician. 2008;78(1):56‐63. [PubMed] [Google Scholar]
- 47. Gortmaker SL, Wang YC, Long MW, et al. Three interventions that reduce childhood obesity are projected to save more than they cost to implement. Health Aff. 2015;34(11):1932‐1939. doi: 10.1377/hlthaff.2015.0631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Erdol S, Mazzucco W, Boccia S. Cost effectiveness analysis of childhood obesity primary prevention programmes: a systematic review. Epidemiol Biostat Public Health. 2014;11(3):e9416‐e9416‐10. [Google Scholar]
- 49. Alemayehu B, Warner KE. The lifetime distribution of health care costs. Health Serv Res. 2004;39(3):627‐642. doi: 10.1111/j.1475-6773.2004.00248.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Matteazzi E, Scherer S. Gender wage gap and the involvement of partners in household work. Work, Employment Soc. 2020;35(3):490‐508. doi: 10.1177/0950017020937936 [DOI] [Google Scholar]
- 51. Carli LL. Women, gender equality and COVID‐19. Gender Manag. 2020;35(7/8):647‐655. doi: 10.1108/GM-07-2020-0236 [DOI] [Google Scholar]
- 52. Kansra AR, Lakkunarajah S, Jay MS. Childhood and adolescent obesity: a review. Front Pediatr. 2021;8:581461. doi: 10.3389/fped.2020.581461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Muñoz E, Muñoz W III, Wise L. National and surgical health care expenditures, 2005–2025. Ann Surg. 2010;251(2):195‐200. doi: 10.1097/SLA.0b013e3181cbcc9a [DOI] [PubMed] [Google Scholar]
- 54. Dieleman JL, Cao J, Chapin A, et al. US health care spending by payer and health condition, 1996‐2016. Jama. 2020;323(9):863‐884. doi: 10.1001/jama.2020.0734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Romanelli R, Cecchi N, Carbone MG, et al. Pediatric obesity: prevention is better than care. Ital J Pediatr. 2020;46(1):103. doi: 10.1186/s13052-020-00868-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pandita A, Sharma D, Pandita D, Pawar S, Tariq M, Kaul A. Childhood obesity: prevention is better than cure. Diabetes, Metabolic Syndrome and Obesity. 2016;9:83‐89. doi: 10.2147/DMSO.S90783 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Summary Table (N = 48)


