Abstract
Background
A key tenet of clinical management of patients post liver transplantation (LT) is the prevention of thrombotic and bleeding complications. This systematic review investigated the optimal management of thromboprophylaxis after LT regarding portal vein thrombosis (PVT) or hepatic artery thrombosis (HAT) and prevention of bleeding.
Methods
Systematic review following PRISMA guidelines and recommendations using the GRADE approach derived from an international expert panel. Seven databases were used to conduct extensive literature searches focusing on the use of anticoagulation in LT and its impact on the following outcomes: PVT, HAT, and bleeding (CRD42021244288).
Results
Of the 2478 articles/abstracts screened, 16 studies were included in the final review. All articles were critically appraised by a panel of independent reviewers. There was wide variation regarding the anticoagulation protocols used. Thromboprophylaxis with therapeutic doses of heparin/Vitamin K antagonist combination did not decrease the risk of de novo or the recurrence of PVT but was associated with an increased risk of bleeding in some studies. Only the use of aspirin resulted in a small but significant decrease in the incidence of HAT post‐LT, yet it did not increase the risk of bleeding.
Conclusions
Based on existing data and expert opinion, thromboprophylaxis at therapeutic or prophylactic dose is not recommended for prevention of de novo PVT following LT in patients not at high risk. Aspirin should be considered as the standard of care following LT to prevent HAT. Thromboprophylaxis should be strongly considered in recipients at risk of HAT and PVT following LT.
Keywords: anticoagulation, antiplatelet, bleeding, hepatic artery, liver transplantation, portal vein, thromboprophylaxis, thrombosis
1. INTRODUCTION
Liver transplantation (LT) is a complex procedure. On one hand there is an attendant risk of perioperative bleeding because of major upper abdominal surgery in a context of portal hypertension, coagulopathy related to impaired liver function, thrombocytopenia related to hypersplenism and potential activation of fibrinolysis in a context of controlled infection On the other hand, there is a risk of vascular thrombosis as a consequence of decreased portal blood flow, increased intrahepatic vascular resistance, complex vascular anastomoses, technical complications, and an imbalance between pro and anticoagulant factors. Optimization of early management after LT, therefore, requires the anticipation of bleeding/thrombotic complications and early initiation of targeted interventions to prevent and/or correct these complications.
Portal vein thrombosis (PVT), either partial or complete, is not uncommon in candidates for LT with end stage cirrhosis, with a prevalence ranging from 5% to 26% according to different populations and different diagnostic criteria. 1 , 2 Prevalence of benign PVT seems to be higher in patients with NASH‐related cirrhosis as compared to patients with cirrhosis due to other causes. 3 As a consequence, since NASH is a growing indication for LT, the prevalence of PVT in transplant candidates with end stage cirrhosis is likely to increase in the near future. In addition to patients with PVT at evaluation, 5%–10% of patients develop new PVT after being waitlisted, especially in countries where waiting time is long. 4 , 5 , 6 Beyond technical difficulties during the transplant procedure, recurrence of PVT after LT is a matter of concern and requires a strategy to identify patients at risk of recurrence, justifying early anticoagulation to prevent re‐thrombosis.
In contrast to LT candidates, de novo PVT following deceased donor liver transplantation (DDLT) in adult recipients is a relatively rare complication with reported incidence ranging between 2.2% and 2.9%. 7 , 8 However, it is associated with increased risk of graft loss requiring invasive procedures including re‐transplantation and the increased risk of recipient death with reported rates as high as 67%–100%. 7 , 8 , 9 Although, de novo PVT is uncommon with conventional end‐to‐end portal anastomosis, factors such as the presence of the pre‐operative PVT, pediatric transplantation, non‐physiological portal vein reconstruction, LDLT setting and hypercoagulable states like myeloproliferative disorders contribute to higher rates of post‐operative PVT and require special consideration. 10 , 11 , 12 , 13 , 14
Adequate arterial liver graft perfusion is an absolute necessity since arterial blood flow is the only source of oxygen supply to allograft's biliary tree. 15 , 16 , 17 The incidence of hepatic artery thrombosis (HAT) ranges between 1.6% and 10% according to different series and is more prevalent in pediatric rather than adult recipients. 15 HAT is a catastrophic complication, which generally occurs during the first week after transplantation and is often associated with diffuse, irreversible ischemic cholangiopathy requiring re‐transplantation in up to 60% of cases and contributes to high recipient mortality ranging between 9% and 56%. 16 Revascularization attempts are rarely effective at preventing the ischemic damage to the bile ducts. Therefore, accurate screening by Doppler ultrasound is central in the detection of reduced arterial blood flow or thrombosis. Late HAT (>1 month post‐LT) is less common than early HAT (<1 month post‐LT), but it may also result in diffuse ischemic cholangiopathy. 18 , 19 While several risk factors for HAT have been clearly identified (pediatric transplantation, LDLT, donor age, reduced size graft, complex arterial reconstruction, aorto‐hepatic conduits, hypercoagulable state), there is no consensus on the optimal initiation time and the duration of the thromboprophylactic therapy or the nature of the thromboprophylactic agents. 14
Early postoperative bleeding is one of the most frequent and life‐threatening complications of LT, leading to increased morbidity and mortality. In early studies where post‐operative anticoagulation and antiplatelet drugs were not used routinely, the incidence of bleeding post LT has been reported to be as high as 8.4% and 16%. 20 , 21 The main reasons of perioperative and early post‐operative bleeding in LT are coagulopathy secondary to slow graft function, large operative field and extensive surgical procedure. Additional risks include previous abdominal surgery and prior history of spontaneous bacterial peritonitis that both contribute to prolonged adhesiolysis, presence of ascites (reflecting the degree of portal hypertension), high lab‐MELD score and renal failure. 22 , 23 , 24 Post‐operative variceal bleeding is very uncommon as liver transplantation reverses portal hypertension, however it remains a significant source of morbidity and mortality in patients with pre‐operative PVT requiring cavo‐portal or reno‐portal anastomosis. Although in most patients coagulation factors return to normal or near normal values within the first week after transplantation, the platelet count may remain low due to persistence of hypersplenism and other factors including adverse drug events. Thrombocytopenia may contribute to the persistent risk of bleeding especially when medical thromboprophylaxis is considered. Overall, early post‐operative course in LT is characterized by increased risk of bleeding as well as thrombotic events. Therefore, to optimize the recovery, a careful balance must be achieved to mitigate both risks of bleeding and thrombosis.
In this manuscript, we have reviewed the literature on thromboprophylaxis to prevent PVT/HAT and its associated risk of perioperative bleeding with the goal to delineate recommendations based on the existing data and the expert opinion of the panel.
2. MATERIALS AND METHODS
2.1. Information sources and protocol registration
The study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 25 A systematic literature review was performed on March 30, 2021, searching the online databases including Ovid MEDLINE, Embase, Scopus, Google Scholar, Clinical.Trials.gov, and the Cochrane Central Register of Controlled Trials. Additional review was performed using PubMed. The protocol was registered on PROSPERO (CRD42021244288).
2.2. Eligibility criteria
The search terms were organized according to the PICO (population, intervention, control, and outcomes) criteria. The population represents adult (18 years old and older) recipients that received a deceased donor or living donor liver transplant. Split‐liver recipients were excluded. The intervention groups included patients that received aspirin, unfractionated or low‐molecular weight heparin (UFH or LMWH), epoprostenol, vitamin K antagonist as well as monitored coagulation with viscoelastic testing during the perioperative period following liver transplantation. The control groups include patients that did not receive aspirin, UFH, epoprostenol, vitamin K antagonist or monitoring with viscoelastic testing after liver transplantation. The main outcomes were incidence of PVT, HAT, and bleeding following LT. The additional outcomes included deep venous thrombosis, pulmonary embolism rates, morbidity, mortality, intensive care, and hospital stay.
2.3. Search and study selection
The searches were performed by expert University of Zurich scientific librarians and the members of the expert panel. All selected titles were screened by two independent investigators (SB and SA) and controversies were resolved by consensus of the study group. Only studies reporting on the management of thromboprophylaxis after liver transplantation in respect to PVT, HAT, bleeding, morbidity, mortality, intensive care unit and hospital stay were included. The studies that had conference abstract with known upcoming manuscript submission were also included. Studies that reported on pediatric population alone, case reports or published in language other than English were excluded.
Both comparative and single cohort studies, retrospective or prospective, describing outcomes in patients receiving different types of anticoagulation or outcomes in patients that were monitored for bleeding with different markers and techniques were included.
2.4. QOE and recommendations grading
The recommendations were formulated using the “Grading of Recommendations Assessment, Development and Evaluation” (GRADE) approach derived from an international expert panel. 26 The GRADE guideline provided a comprehensive and structured approach to rate the quality of evidence (QOE) for systematic review and to grade strength of recommendations. Six authors (VAK, BO, CI, LM, PGN, GWS) separately rated the QOE for each outcome. The direction and strength of recommendations were assessed by the experts individually followed by the final consensus during the expert panel.
3. RESULTS
3.1. Study selection
Of 2478 articles/abstracts screened, 31 publications underwent full‐text assessment for eligibility and 16 were included in qualitative synthesis (Figure 1). Twelve studies were comparative; of those, eight were single center 17 , 27 , 28 , 29 , 30 , 31 , 32 , 33 and two were multi‐center retrospective 34 , 35 studies, one was a single center non‐randomized 36 and one was a multi‐center randomized prospective 37 study. The remaining four were single center retrospective non‐comparative studies. 16 , 38 , 39 , 40 Baseline characteristics including study type, number of subjects enrolled, and target outcomes are reported in Table 1.
FIGURE 1.
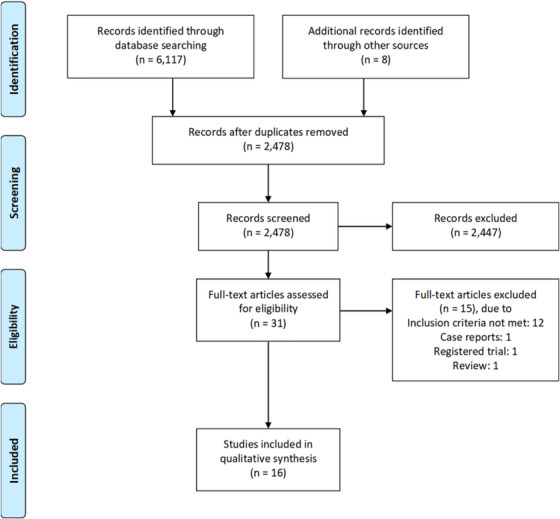
Flow diagram of study extraction and selection
TABLE 1.
Study characteristics
| Study type | No. of patients | Main outcomes assessed | |
|---|---|---|---|
| 1. Shay et al 2013 | Single center retrospective observational comparative study | Total n = 469 (Aspirin n = 165; No Aspirin n = 304) |
Early use of aspirin in liver transplantation
|
| 2. Lin et al. 28 | Single center retrospective observational comparative study | Total n = 198 (HAT n = 13, No HAT n = 185) |
Intraoperative flow measures for prediction of HAT
|
| 3. Bärthel et al. 37 | Multi‐center prospective randomized control trial, open label with and without PGI2 | Total n = 80 (PGI2 n = 40, no PGI2 n = 40) |
Seven day course of PGI2 treatment following liver transplantation
|
| 4. Wolf et al. 17 | Single center retrospective observational comparative study (Minimum f/up 3 months) | Total n = 529 (ASA Group n = 354, No ASA n = 175) |
Effect of ASA on:
|
| 5. Nguyen‐Buckley et al, 2021 | Single center retrospective observational comparative study (Intra‐op or within 1 month) | n = 2330 (no ROTEM n = 2002, ROTEM n = 328) |
Effect of Rotational Thromboelastometry and Cryoprecipitate on:
|
| 6. Roullet et al., 2014 | Single center prospective non‐randomized | n = 60 (no ROTEM n = 30, ROTEM n = 30) |
Effect of Rotational Thromboelastometry on:
|
| 7. Oberkofler C et al, 2021 (manuscript is available on request) | Multicenter retrospective observational comparative study | n = 2366 (ASA n = 1436; No ASA n = 930) |
Effect of low‐dose ASA on:
|
| 8. Widen A, 2009 | Single center retrospective observational comparative case‐matched analysis | n = 138 (n = 59 warfarin vs. N = 79 control) |
Use of anticoagulation after liver transplantation
|
| 9. Vivarelli et al. 31 | Single center retrospective observational comparative study | n = 838 (236 antiplatelet vs. 598 no‐antiplatelet prophylaxis) |
Effect of ASA on prevention of HAT
|
| 10. Alexander et al. 32 | Single center retrospective observational comparative study | n = 716 (220 heparin vs. 496 no‐heparin) |
Effect of chemoprophylaxis on
|
| 11. Bos et al. 35 | Two‐center retrospective observational comparative study | Total n = 235 with PVT (anticoagulation n = 113; no anticoagulation n = 122) |
Utility of therapeutic anticoagulation post liver transplantation in recipients with pre‐transplant Yerdel‐grade I/II PVT
|
| 12. Rizzari M, 2020 | Single center retrospective observational study, non‐comparative | Total n = 126 with PVT (Yerdel grade I n = 73; Yerdel grade II/III n = 53) |
Impact of PVT grade and intra‐op flow on:
|
| 13. Sanchez‐Ocaña et al. 33 | Single center retrospective observational comparative study |
Total n = 215 PVT n = 37 |
6 month course of oral anti‐coagulation (warfarin)
|
| 14. Kaneko et al. 39 | Single center retrospective observational study, non‐comparative | Total n = 125 |
Use of heparin in LDLT (dalteparin (25 IU/kg/d) on POD 1; then heparin with ACT goal 130–160 starting POD#3)
|
| 15. Gad et al. 40 | Single center retrospective observational study, non‐comparative | Total n = 213 |
Vascular outcomes post‐LDLT in adult and pediatric population
|
| 16. Stange et al. 16 | Single center retrospective observational study, non‐comparative | Total n = 1192 |
Outcomes on thromboprophylaxis on adult DDLT
|
3.2. QOE and recommendations grading
The results of the individual studies are reported in Table 2. The summary of findings for the main outcomes, thromboprophylaxis to prevent PVT, HAT and bleeding following LT, and the final QOE grading according to the GRADE approach are summarized in Tables 2 and 3. The QOE was rated low to very low for the reported outcomes. The main reasons for downgrading were imprecision due to large variation in study groups and interventions as well as limitations due to the retrospective observational nature of most studies. For the thromboprophylaxis for prevention of HAT, QOE was upgraded due to similarity and magnitude of the effect. The evidence to recommendation framework according to the GRADE approach are listed in Table 4.
TABLE 2.
Study outcomes
| Prevention of bleeding | HAT | PVT | |
|---|---|---|---|
| 1. Shay R et al 2013 |
Significant Bleeding (requiring ≥2 units RBC) 9% on Aspirin vs 19% without Aspirin, NS |
HAT
|
PVT .6% on Aspirin vs 2% without Aspirin, NS |
| 2. Lin et al. 28 | NA |
No imaging comparison, more intraoperative flow as predictor of HAT
|
NA |
| 3. Bärthel et al. 37 | Fresh Frozen Plasma requirement (units) 5.5 control vs 4 iloprost. 0 (‐4,1) p = .264. | Composite HAT/PVT AE rare and not different between groups | Composite HAT/PVT AE rare and not different between groups |
| 4. Wolf et al. 17 |
Biopsy bleeding:
|
Overall HAT
|
NA |
| 5. Nguyen‐Buckley C et al, 2021 | NA |
Overall HAT
|
NA |
| 6. Roullet S et al, 2014 |
Perioperative bleeding:
|
NA | NA |
| 7. Oberkofler C et al, 2021 (manuscript is available on request) | NA |
1‐year arterial patency:
|
NA |
| 8. Widen A, 2009 |
Number of patients who bled
|
NA | NA |
| 9. Vivarelli et al. 31 | N/A |
The effect of aspirin on early HAT was not assessable due to median presentation 5 days post‐op and ASA was started at median time 8 days.
|
N/A |
| 10. Alexander et al. 32 |
|
N/A | N/A |
| 11. Bos et al. 35 |
Bleeding events:
|
HAT: 4.3% anticoagulation vs. 4.5% no anticoagulation, NS |
Overall for Yerdel I and II PVT recurrence within 1 yr post‐LT:
|
| 12. Rizzari M, 2020 | NA | NA |
Recurrent post‐op PVT:
|
| 13. Sanchez‐Ocaña et al. 33 |
|
NA |
Intra‐op: Short (Grades I‐II) 26/37 (70%) Long (Grades III‐IV) 11/37 (30%) Cavernous Transformation 5/37 (13.5%) Post‐op PVT recurrence: 0% recurrence of PVT (all patients received low molecular‐weight heparin (1 mg/kg twice a day) in the immediate postoperative period and were discharged with OAC (warfarin) for 6 months) |
| 14. Kaneko et al. 39 |
Incidence of bleeding:
|
Early HAT
|
PVT
|
| 15. Gad et al. 40 |
Incidence of bleeding:
|
Incidence of HAT
|
Incidence of PVT
|
| 16. Stange et al.16 |
Incidence of bleeding:
|
Incidence of early HAT
|
NA |
TABLE 3.
Summary of findings leading to the QOE Assessment according to the GRADE approach
| Summary of Findings | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Effect from comparative studies | Limitations | Inconsistency | Indirectness | Imprecision | Publication Bias | Quality of Evidence (GRADE) | ||
| RCT | Observational comparative | Observational non‐comparative | |||||||
| Outcome 1: Thromboprophylaxis for prevention of PVT following liver transplantation | |||||||||
| 1 | 3 | 4 | Thromboprophylaxis does not prevent PVT | Very serious | Not serious | Very serious | Very serious | Likely | Very low ∘∘∘ |
| Outcome 2: Thromboprophylaxis and monitoring for prevention of HAT following liver transplantation | |||||||||
| 1 | 6 | 3 | Aspirin decreases rate of HAT | Serious | Not serious | Serious | Serious | Likely | Low •∘∘ |
| Outcome 3: Thromboprophylaxis and prevention of bleeding following liver transplantation | |||||||||
| 2 | 6 | 3 | Effect is not similar but favors increased bleeding rate with the use of heparin/vitamin K antagonists at therapeutic levels | Very serious | Erious | Very serious | Serious | Likely | Very low ∘∘∘ |
TABLE 4.
Evidence to recommendation framework according to the GRADE approach
| Question: Does thromboprophylaxis prevent PVT and HAT following liver transplantation while limiting bleeding complications? | |||
|---|---|---|---|
| Decision domain | Judgment | Reason for Judgment | |
| Yes | No | ||
| Balance between desirable and undesirable outcomes (estimated effects), with consideration of values and preferences (estimated typical) | ✓ |
The thromboprophylaxis did not have a desired outcome on prevention of primary PVT or recurrent of PVT following liver transplantation. Aspirin was the only thromboprophylaxis that was shown to have small but desirable effect on prevention of HAT following liver transplantation. Use of aspirin did not correlate with increased number of undesirable effects such as bleeding. The thromboprophylaxis with heparin products or heparin/warfarin combination had moderate undesirable consequences including bleeding, increased length of stay and positive correlation between thromboprophylaxis and death following transplantation. |
|
| Confidence in the magnitude of estimates of effect of the interventions on important outcomes (overall QOE for outcomes) | ✓ |
Thromboprophylaxis does not prevent PVT following liver transplantation: very low •∘∘∘ Thromboprophylaxis with aspirin may decrease the incidence of HAT: low ••∘∘ Only Aspirin thromboprophylaxis did not correlate with the increased risk of bleeding following liver transplantation: low ••∘∘ |
|
| Confidence in Values and Preference, and their Variability | ✓ |
Based on the available data and the clinical experience of all authors, there is a limited application of thromboprophylaxis in prevention of the PVT for all liver transplant recipients when weighted against the moderate risk of post‐transplant bleeding complications. Aspirin may provide a small protective effect in prevention of HAT at the same time balancing an acceptable low risk of bleeding following the liver transplantation. Intra‐operative flow monitoring may predict the risk of HAT or recurrence of PVT, which in turn may help to stratify the liver recipients who are at risk for the post‐operative thrombosis and would benefit from thromboprophylaxis following the transplantation (Lin et al, 2002, Rizzari, 2020). Intra‐operative coagulation monitoring using ROTEM did not significantly decrease the intra‐operative bleeding but may increase the number of major thrombotic complications including HAT (Nguyen‐Buckley C et al, 2020, Roullet S et al, 2014) |
|
| Resource implications | ✓ | The resources required to provide aspirin thromboprophylaxis to liver transplant recipients are rated lower than the resources that are needed for management of the devastating complications of HAT. The thromboprophylaxis for PVT does not prevent thrombotic events but increases utilization of resources for management of bleeding complications. | |
| Overall QOE: Very low for thromboprophylaxis to prevent PVT. Low for thromboprophylaxis to prevent HAT. Very low for thromboprophylaxis to prevent bleeding | |||
| Overall strength of recommendations: | Strong |
Considering all decision domains, the guideline panel recommends that I) Therapeutic/prophylactic dose of thromboprophylaxis should not be routinely used in prevention of PVT, ii) Aspirin can be considered for thromboprophylaxis to prevent HAT iii) New and existing parameters for intra‐operative assessment such as coagulation monitoring or flow measures should be adopted to minimize the pro‐thrombotic events or predict the recipient groups that are at high‐risk of thrombosis following the transplantation 14 (Lin M., et al, 2002, Rizzari M, et al., 2020). iv) Prophylactic doses of UFH or LMWH should be judiciously considered for LT recipients to prevent DVT/PE early post LT. 48 |
|
| Evidence to recommendation synthesis: |
i) moderate evidence for recommending against use of the thromboprophylaxis for prevention of PVT at therapeutic or prophylactic dose ii) moderate evidence for recommending for the use of aspirin for prevention of HAT iii) moderate evidence for resource utilization for both (i & ii recommendations) |
||
3.3. Results of individual studies
3.3.1. Thromboprophylaxis and monitoring for prevention PVT following LT
Based on the literature review conducted by the authors, the evidence to support the post‐transplant thromboprophylaxis at therapeutic or prophylactic doses for prevention of de novo PVT as a standard of care for all adult LT recipients is lacking. Shay et al. retrospectively compared the rates of PVT occurrence in 469 LT recipients (the majority were DDLTs, 99%) with and without early post‐operative use of aspirin. The incidence of PVT was .6% versus 2% respectively without statistical significance. 27 Similarly, in randomized control trial, the administration of PGI2 analog during the early post‐operative period did not affect the rate of PVT following DDLT. 37
Kaneko et al. examined a combination therapy of UFH (ACT goal 130–160 s), dalteparin, prostaglandin E1, antithrombin III (AT III) concentrates and a protease inhibitor (mesilate gabexate) for anti‐thrombotic prophylaxis following LDLT in adult recipients. The rate of PVT was 1.6%, which was lower than de novo PVT rates following LDLT reported by other authors, 6.8% and 9%; however, the rate of post‐operative bleeding and the need for take‐back due to bleeding was 15% under the presented protocol. 9 , 39 Another study of early UFH administration (target: ACT 180–200 s or PTT 50–70 s) for one week followed by 3 months of dipyridamole therapy for adult and pediatric LDLT recipients found that the rate of PVT was 2.3%, which was comparable to the report of PVT rate by Kaneko et al, but with significantly lower incidence of post‐operative bleeding at 1.8% (Gad, 2016). 40
Several studies examined the use of anticoagulation protocols for LT recipients with pre‐operative diagnosis of PVT. 33 , 35 , 38 A two‐center retrospective study on the utility of UFH infusion followed by Vitamin K antagonist (target INR 2–3) for LT recipients with pre‐operative Yerdel I and II PVT showed no significant difference in recurrence of PVT between anticoagulation (Yerdel I: 5.3%; Yerdel II: 5.7%) and no anticoagulation (Yerdel I: 2.5%; Yerdel II: 5.5%) groups; however, the incidence of bleeding and length of stay were significantly higher among recipients in the anticoagulation group (see Figure 2). 35 , 41 In contrast, Sanchez‐Ocaña et al. used LMWH (1 mg/kg twice a day) followed by Vitamin K antagonist for a minimum of 6 months for all recipients with pre‐ or intra‐operative diagnosis of PVT (Yerdel I‐2: 70%; Yerdel III‐IV: 30%). The authors reported no PVT recurrence and no associated bleeding complications. 33 Rizzari et al. utilized the combination of subcutaneous (SQ) UFH 5,000 IU three times a day and 325 mg aspirin for all recipients with pre‐ or intra‐operative diagnosis of Yerdel I (58%) and grade Yerdel II‐III (42%) PVT. The recurrence rates of PVT in this study were significantly higher (Yerdel I: 9.6%; Yerdel II‐III: 22.6%) compared to the rates reported by Bos et al. and Sanchez‐Ocaña et al.; however, the authors found that the intra‐operative low portal flow measurements correlated with increased risk of PVT recurrence suggesting that other factors outside of anti‐coagulation should be considered. 38
FIGURE 2.
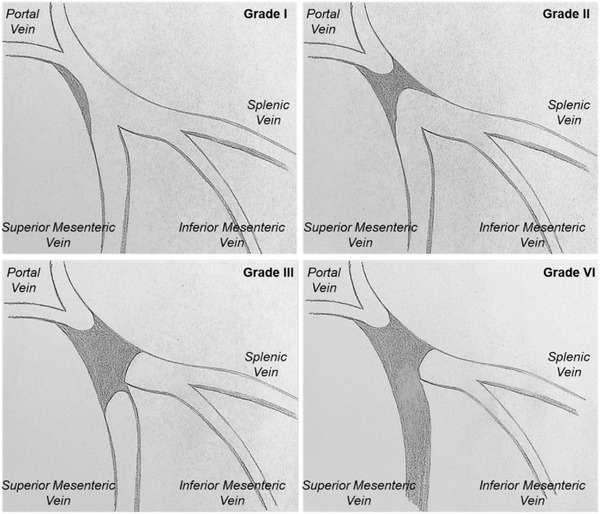
Portal vein thrombosis classification according to Yerdel et al.
Grade I – thrombus confined to <50% of the portal vein (PV) lumen, with or without minimal extension into the superior mesenteric vein (SMV)
Grade II – >50% occlusion of the PV, including total occlusion, with or without minimal extension into the SMV
Grade III – Complete thrombosis of both PV and proximal SMV
Grade IV – Complete thrombosis of the PV and proximal/distal SMV
Lastly, two other special considerations should be discussed including LT recipients with non‐physiological portal vein (PV) reconstruction (cavo‐portal hemi‐transposition, reno‐portal anastomosis, PV arterialization) and recipients with underlying hypercoagulability disorders. Hibi et al. reported post‐transplant re‐thrombosis rate of 24% among recipients who underwent non‐physiological portal vein reconstruction when compared to 4.7% re‐thrombosis rate in recipients with physiological reconstruction (p < .001). Furthermore, patient survival in the non‐physiological bypass group was significantly lower than the control group whereas the rate of hypercoagulable conditions was higher among recipients with the non‐physiological bypass. 12 Liver recipients with etiologies such as Budd‐Chiari Syndrome and underlying hypercoagulable states have a documented high‐rate of post‐transplant thrombotic complications thus prophylaxis with vitamin K antagonists post‐transplant has been a standard of care. 42
In conclusion, there is no substantial evidence to support the systematic thromboprophylaxis for prevention of de novo PVT following LT in adult DDLT recipients. Given serious consequences of PVT and the increased risk of PVT occurrence, thromboprophylaxis should be considered in the special circumstances (LDLT, pre‐transplant PVT, non‐physiological PV reconstruction, hypercoagulable states, pediatric LT); although, other factors such as intra‐operative technical challenges and intra‐operative reduced PV flows may impact the outcomes. If thromboprophylaxis is needed, UFH, LMWH, vitamin K antagonists, or direct oral anticoagulants (DOACs) should be preferred to aspirin.
3.4. Thromboprophylaxis and monitoring for prevention of HAT following LT
Despite relatively frequent use of aspirin following LT, the data on its efficacy to prevent HAT vary. Shay et al. demonstrated that 325 mg of daily aspirin during the initial three post‐operative months followed by complete discontinuation or dose reduction did not affect the incidence of overall early and late HAT; however, the incidence of early HAT with graft loss within 30 days was significantly decreased in aspirin (0%) versus no aspirin (3.6%) groups (p < .05). The retrospective multicenter study by Oberkofler et al. examined the effect of aspirin on the incidence of HAT in 2,366 LT recipients (ASA, n = 1436; no ASA, n = 930). 34 Aspirin was beneficial in lowering the risk of HAT (within 1 year post‐transplant, Hazard Ratio .23; 95% CI: .13–.4; p < .001), but the benefit did not extend to the later incidence (>1 year) of HAT. 34 In contrast, Vivarelli et al. found that the administration of aspirin decreased the overall late HAT from 2.2% to .4% (p = .037) and the recipients of DCD livers or in need of the arterial conduit during the reconstruction had significantly higher rate of HAT if aspirin prophylaxis was not used. 31
Wolf et al compared the incidence of HAT in the group of 529 adult and pediatric recipients with and without post‐operative aspirin prophylaxis (81 mg for adults and 40 mg for children). Contrary to prior studies, there was no difference in the incidence of HAT between aspirin and no aspirin groups. 17 Of note, 14 of 28 children under the age of 6 years old received post‐operative UFH infusion at dose of 10 U/kg per hour for 5–7 days. 17 The only risk factors for HAT that were identified by the authors were recipient age of younger than 2 years and low donor liver weight. 17
Other strategies such as use of PGI2 analog during early post‐operative period did not affect the incidence of HAT in DDLT recipients. 37 Similarly, Bos et al. showed no difference of HAT among two patient groups with pre‐operative diagnosis of PVT who were randomized into UFH/Vitamin K antagonist therapy (target INR 2–3) versus no anti‐coagulation groups. 35
In the setting of the LDLT, Kaneko et al. reported the incidence of HAT as 2.3% despite the aggressive post‐operative anti‐thrombotic prophylaxis protocol (UFH, dalteparin, prostaglandin E1, antithrombin III (AT III) concentrates, a protease inhibitor). 39 The authors’ HAT rate was comparable to other reports of HAT following LDLT that did not utilize anti‐coagulation prophylaxis; at the same time, the protocol by Kaneko et al. was associated with significant post‐operative bleeding complications. 39 , 43 , 44 Similarly, Gad et al. failed to demonstrate the benefit of UFH (target: ACT 180–200 s or PTT 50–70 s) and dipyridamole prophylaxis for HAT prevention in adult and pediatric recipients with a reported overall rate of 4.2%. 40
For split‐liver transplants or complex arterial reconstruction, Stange et al. used infusion of UFH at 5000 units over 24 h for 14 days. 16 The only reconstruction that had significantly higher rate of HAT was the graft interposition to recipient supra‐celiac aorta (RR 5.76; 95%, confidence interval: 2.4–13.4, p < .05). 16 These findings have been supported by Hibi et al. demonstrating an increased rate of late HAT, ischemic cholangiopathy and lower 5‐year graft survival among patients with aorto‐hepatic conduits. 45
In summary, despite a significant variability in study designs, aspirin, irrespective of dose, following LT was the only method of thromboprophylaxis that lowered the incidence of HAT in LT recipients overall and in those with higher rate of HAT such as complex arterial reconstruction with aortic conduits.
The application of intra‐operative monitoring strategies may have a value in stratifying the post‐operative risk for the development of HAT, which in turn may guide the decision regarding prophylaxis strategies. Similarly to the findings by Rizzari et al. on the positive association between the low intra‐operative flow and recurrence of PVT, Lin et al. demonstrated that liver transplant recipients with low mean and median intra‐operative hepatic arterial flow were more likely to develop HAT post‐operatively. 28 At the same time, Nguyen Buckley et al. reported that the use of intraoperative rotational thromboelastometry (ROTEM) to guide transfusion strategy increased intra‐operative use of cryoprecipitate with increased observation of post‐operative HAT from 2.4% to 5.2% (p < .01) and major thrombotic complications from 4.2% to 9.5% (p < .001) in adult liver recipients. 29 These findings put a new perspective on the use of ROTEM as the intra‐operative monitoring technique for resuscitation of liver recipients and cautioned the liberal use of cryoprecipitate. 29
3.5. Thromboprophylaxis and prevention of bleeding following LT
Research with regard to prevention of early and late thrombotic complications post LT has mainly focused on the use of aspirin, UFH, LMWH, and Vitamin K antagonists or a combination of these. 16 , 17 , 27 , 30 , 31 , 32 , 33 , 35 , 38 , 39 , 40 The incidence of bleeding associated with each of these agents in LT recipients is described below. Of note, the majority of the included studies concern the use of antiplatelet and anticoagulation agents in LT recipients who had a specific indication for thromboprophylaxis and would therefore not be expected to be generalizable to the entire LT population. Bleeding events are, where possible, classified into either major or minor in accordance with criteria recommended by the International Society on Thrombosis and Haemostasis. 46 Major bleeding is defined as fatal bleeding or requiring re‐operation, and/or symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular, pericardial, or intramuscular with compartment syndrome, and/or bleeding causing a fall in haemoglobin levels of 20 g/L or greater, or leading to a transfusion of 2 units or more of whole blood or red cells. Minor bleeding is defined as all reported bleedings not classified as major.
Three studies describing the use of aspirin in prevention of post‐operative thromboembolic complications following LT were reviewed. These were single‐center retrospective observational studies with matched control groups. Low‐dose aspirin (81 mg or 100 mg daily) was used by Wolf et al. and Vivarelli et al. whereas a standard dose aspirin (325 mg daily) was used by Shay et al. 17 , 27 , 31 In the study by Wolf et al., hemorrhage following biopsy occurred in 1.1% of patients treated with daily aspirin (81 mg) and in .6% of patients in the control group (NS). A trend toward increased incidence of gastrointestinal bleeding was reported but did not reach statistical significance (18.9% with aspirin vs. 12.8% without aspirin). 17 In more recent studies by Vivarelli et al. and Shay et al., bleeding rates were not significantly different between aspirin and control groups. Shay et al. observed significant bleeding events in 9% of patients on aspirin (325 mg) compared to 19% of patients in the control group (NS). Vivarelli et al. reported no episodes of upper or lower gastrointestinal bleeding in patients who received aspirin (100 mg) over a median follow‐up of 1704 days (range 33–7017 days). 31 Overall, these studies suggest that the administration of aspirin (81–325 mg) does not significantly increase the risk of bleeding following LT.
Seven studies describing the use of anticoagulation (UFH, LMWH, or Vitamin K antagonist) following LT were reviewed. These were retrospective observational studies conducted in several different patient cohorts. Two studies were conducted in patients with confirmed PVT prior to LT, two in LDLT and the remaining studies were conducted in a general population of LT recipients. 16 , 30 , 32 , 33 , 35 , 39 , 40 Significant differences were observed between studies with regard to the choice of anticoagulant, dosage, timing of therapy initiation, target ranges (ACT or APTT) and duration. In the majority of the studies, intravenous (IV) UFH was initiated soon after the procedure followed by either SQ UFH, LMWH, or Vitamin K antagonist for minimum duration of 3 months or longer. 16 , 30 , 32 , 33 , 35 , 39 , 40 In studies which involved patients treated with Vitamin K antagonist for confirmed PVT, there was considerable variation in bleeding rates. Bos et al. found a significant increase in bleeding complications (23% vs. 4.1%, p < .01) and increased length of stay (21 vs. 17.5 days, p < .01) among recipients who received anticoagulation. 35 Sanchez‐Ocana et al. reported no adverse events associated with anticoagulation and no cases of recurrent thrombosis. 33 Contradicting findings were also reported in studies conducted in LDLT recipients. Kaneko et al. reported bleeding requiring blood transfusion and/or re‐laparotomy in 15% of patients who received IV UFH with a target ACT of 130–160 s. 39 In contrast, Gad et al. reported an overall incidence of bleeding of 1.9% in patients receiving IV UFH up until post‐operative day 8 with a target ACT of 180 to 200 and/or APPT of 50 to 70 s. 40
Three studies conducted in a cohort of general LT population were also reviewed. 16 , 30 , 32 Widen et al. found similar rates of bleeding in patients who received anticoagulation with Vitamin K antagonist (15%) compared to those who did not (23%) (NS). The risk of bleeding after the first week post LT was 4.2 and 2.8 per 100 person years in the anticoagulated group and control group respectively. 30 Details with regard to the anticoagulation protocol and bleeding outcomes were not well described in two remaining studies. The database review by Alexander et al. reported that thromboprophylaxis was associated with an increase in the amount of blood products administered and an increase risk of death compared to the control group (OR 3.2 [1.3–8.0] p = .01). 32 Anticoagulation was, however, initiated in only 30.5% of patients (220/496) and the groups who received anticoagulation were older, had a longer hospital stay and higher MELD scores making comparison with the control group difficult. Stange et al. reported three instances (.2%) of major bleeding complications in their study but it is not clear if these received anticoagulation. 16
All studies included in the review were retrospective observational studies and usually conducted in a single‐center. Some studies lacked specific details about the choice and dose of anticoagulation used, monitoring arrangements, bleeding outcomes, follow‐up and many did not have a control group which presents a challenge when interpreting the overall findings. Despite the contrasting results, there is evidence to suggest that therapeutic dose anticoagulation (target ACT >130 or target INR 2–3) has potential to contribute to a significant bleeding risk and that a reduction in thrombotic complications is not observed with this strategy. These conclusions are also substantiated by studies in the general population where the treatment with Vitamin K antagonist is estimated to be associated with a two‐fold increase in bleeding rates. 47 In LT recipients the risk may be even greater; careful assessment and judicious use of these medicines is therefore critical.
For modern monitoring techniques, viscoelastic testing (thromboelastography and rotational thromboelastometry) is gaining popularity, but Roullet S et al. demonstrated no benefit in preventing the perioperative bleeding (defined within 24 h post LT) when the intraoperative ROTEM was used. 36
3.6. Thromboprophylaxis and prevention deep vein thrombosis and pulmonary embolism
Outcomes regarding the incidence of post‐operative systemic thrombosis (PE and DVT) following LT but not post‐operative bleeding were reviewed by Yip et al. Of 999 recipients in this single‐center retrospective study, 2.8% developed PE or DVT within 30 days following LT. Multivariate analysis demonstrated that the presence of PICC line increased odds of PE/DVT 6.3 times (95% CI, 2.9–13.8, p < .0001). 48 Whereas, the administration of prophylactic SQ UFH (5000 IU TID) decreased transplant‐associated PE/DVT compared to LT recipients without SQ UFH prophylaxis whose odds of developing DVT/PE was five times greater (95%CI, .1–.7, p < .01). 48
Based on the reviewed literature, early aspirin use (as early as post‐operative day 1) does not seem to increase risk of post‐operative bleeding complications following LT whereas therapeutic UFH/Vitamin K antagonist levels may contribute to major bleeding events in the general adult LT population. When considering prophylactic doses of SQ UFH (5000 IU TID), it should be noted that in non‐LT surgical patients the use of thromboprophylaxis is now routine and the value and safety of this is well established. Further, Annamalai et al. reported 9% risk of DVT in LT population whereas Yip et al. demonstrated the benefit of prophylactic UFH in reducing the incidence of DVT/PE within 30 days following LT. 48 , 49 Therefore, strong consideration should be given to low dose SQ UFH prophylaxis in early post‐operative period following LT.
Despite the identified trends, there was a wide variation across the studies regarding the use of anticoagulation post LT reflecting the need for robust and rigorous randomised controlled studies in this cohort to delineate the best practices for thromboprophylaxis and simultaneously avoiding the increased risk of bleeding complications.
4. CONCLUSIONS AND PERSPECTIVES
Optimizing the prevention of thrombotic events and bleeding complications is typically a way to enhance success after LT. Indeed, the studies that have been included in this systematic review clearly show that both thrombotic and bleeding complications are relatively common early after LT with a potential for life threatening events such as primary non‐function, diffuse ischemic cholangiopathy or massive bleeding from the operative field.
The main points from the studies in the current review of the literature are the following: 1. There are no data to support systematic use of therapeutic or prophylactic dose of anticoagulation to prevent PVT in adult recipients after LT except in a subgroup of high risk patients (technical difficulties, occlusive PVT prior to LT, complex physiological anastomosis, or non‐physiological anastomosis); 2. If thromboprophylaxis is needed for prevention of PVT; UFH, LMWH or vitamin K antagonists should be preferred to aspirin; 3. Data on DOACs are scarce in liver transplantation but the consideration should be given to the reversibility of the agent prior to its use; 4. Long‐term aspirin, irrespective of dose, reduces the risk of HAT without increasing the risk of postoperative bleeding: 5. Therapeutic anticoagulation increases the risk of post‐operative bleeding events, regardless of the agent used; 6. Prophylactic dose of UFH or LMWH (barring renal dysfunction) should be judiciously considered for LT recipients to prevent DVT/PE in early post‐operative period. These findings are of utmost importance, and they help guide the management of LT recipients. However, these conclusions may be viewed as an oversimplification since the risks of bleeding and thrombosis are highly heterogeneous from patient to patient. In addition, it remains impossible to anticipate a number of factors leading to post‐operative bleeding such as allograft dysfunction, sepsis or the need for invasive procedures. Most of the current studies did not consider patients’ heterogeneity. New tools such as ROTEM are promising to guide thromboprophylaxis at an individual level. However, these tools still have limitations. For instance, recent studies have shown that intraoperative monitoring with ROTEM increases the use of cryoprecipitates, increases the rate of HAT and may lead to major thrombotic complications. 29 , 36
In conclusion, multicenter studies with focus on different populations of LT recipients are needed to refine strategies in thromboprophylaxis. Specifically, the field would benefit from prospective randomized trials on comparing different thromboprophylaxis regimens to decrease the incidence of HAT and PVT in high‐risk cases including complex arterial or venous reconstructions, conduits, non‐anatomical re‐vascularization, pre‐transplant PVT, LDLT, and pediatric transplantation. Incorporating the intra‐operative HA and PV flow measurements in the study design may provide additional guidance for the cases with the high‐thrombotic risk. The incidence of major bleeding events as defined above and other serious adverse drug events (i.e., heparin induced thrombocytopenia) should be analyzed within the same cohorts. With increasing use of DOACs, the research is needed to evaluate safety and efficacy of these drugs in LT recipients. Similarly with viscoelastic testing gaining popularity in the intra‐ and post‐operative settings, the prospective randomized studies should focus on establishing the optimal end‐goals for the resuscitation with the competing goal of minimizing thrombotic complications.
ERAS4OLT.ORG WORKING GROUP AUTHORSHIP
Stephanie Bogan, London, UK, Sithhipratha Arulrajan, London, UK, Claus Niemann, San Francisco, CA, USA, Joerg‐Matthias Pollok, London, UK, Marina Berenguer, Valencia, Spain, Pascale Tinguely, London, UK.
AUTHORSHIP
All authors qualify for authorship as per the International Committee of Medical Journal Editors (ICMJE) guidelines.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Data collection/analysis/interpretation, drafting article, critical revision of article, approval of article were performed by Varvara A. Kirchner, Bryan O'Farrell, Patrick Northup, Charles Imber, Lucas McCormack, Gi‐Wong Song, François Durand. Michael Spiro and Dimitri A. Raptis have conceived and designed the project, the systematic review strategies, prepared the PROSPERO protocols, supervised screening the records and assessing the full‐text articles for eligibility, prepared the structure of the statement manuscript template, revised and reviewed the manuscript draft.
ACKNOWLEDGMENTS
This manuscript was prepared for the ERAS4OLT.org Consensus Conference 2022, which is partially funded by the International Liver Transplant Society (ILTS).
Kirchner VA, B O'Farrell, Imber C, et al. What is the optimal management of thromboprophylaxis after liver transplantation regarding prevention of bleeding, hepatic artery, or portal vein thrombosis? A systematic review of the literature and expert panel recommendations. Clin Transplant. 2022;36:e14629. 10.1111/ctr.14629
Protocol registration: PROSERO protocol ID CRD42021244288
Varvara A. Kirchner and Bryan O'Farrell contributed equally to the paper and share co‐first authorship.
This work was conducted in preparation for the ILTS ‐ ERAS4OLT.org Consensus Conference on Enhanced Recovery for Liver Transplantation, January 2022, Valencia, Spain.
Contributor Information
Varvara A. Kirchner, Email: kirc0079@umn.edu.
the ERAS4OLT.org Working Group:
Stephanie Bogan, Sithhipratha Arulrajan, Claus Niemann, Joerg‐Matthias Pollok, Marina Berenguer, and Pascale Tinguely
DATA AVAILABILITY STATEMENT
All data presented in the manuscript are based on published work that is sited in the body of the manuscript.
REFERENCES
- 1. Englesbe MJ, Kubus J, Muhammad W, et al. Portal vein thrombosis and survival in patients with cirrhosis. Liver Transpl. 2010;16(1):83‐90. 10.1002/lt.21941 [DOI] [PubMed] [Google Scholar]
- 2. Francoz C, Valla D, Durand F. Portal vein thrombosis, cirrhosis, and liver transplantation. J Hepatol. 2012;57(1):203‐212. 10.1016/j.jhep.2011.12.034 [DOI] [PubMed] [Google Scholar]
- 3. Stine JG, Shah NL, Argo CK, Pelletier SJ, Caldwell SH, Northup PG. Increased risk of portal vein thrombosis in patients with cirrhosis due to nonalcoholic steatohepatitis. Liver Transpl. 2015;21(8):1016‐1021. 10.1002/lt.24134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547‐555. 10.1053/j.gastro.2014.11.039 [DOI] [PubMed] [Google Scholar]
- 5. Francoz C, Belghiti J, Vilgrain V, et al. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut. 2005;54(5):691‐697. 10.1136/gut.2004.042796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ravaioli M, Zanello M, Grazi GL, et al. Portal vein thrombosis and liver transplantation: evolution during 10 years of experience at the University of Bologna. Ann Surg. 2011;253(2):378‐384. 10.1097/SLA.0b013e318206818b [DOI] [PubMed] [Google Scholar]
- 7. You S, He XS, Hu AB, et al. [The analysis of portal vein thrombosis following orthotopic liver transplantation]. Zhonghua Wai Ke Za Zhi. 2008;46(3):176‐178. [PubMed] [Google Scholar]
- 8. Khalaf H. Vascular complications after deceased and living donor liver transplantation: a single‐center experience. Transplant Proc. 2010;42(3):865‐870. 10.1016/j.transproceed.2010.02.037 [DOI] [PubMed] [Google Scholar]
- 9. Linares I, Goldaracena N, Rosales R, et al. Splenectomy as flow modulation strategy and risk factors of de novo portal vein thrombosis in adult‐to‐adult living donor liver transplantation. Liver Transpl. 2018;24(9):1209‐1220. 10.1002/lt.25212 [DOI] [PubMed] [Google Scholar]
- 10. Lendoire J, Raffin G, Cejas N, et al. Liver transplantation in adult patients with portal vein thrombosis: risk factors, management and outcome. HPB (Oxford). 2007;9(5):352‐6. 10.1080/13651820701599033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nacoti M, Ruggeri GM, Colombo G, Bonanomi E, Lussana F. Thrombosis prophylaxis in pediatric liver transplantation: a systematic review. World J Hepatol. 2018;10(10):752‐760. 10.4254/wjh.v10.i10.752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hibi T, Nishida S, Levi DM, et al. When and why portal vein thrombosis matters in liver transplantation: a critical audit of 174 cases. Ann Surg. 2014;259(4):760‐766. 10.1097/SLA.0000000000000252 [DOI] [PubMed] [Google Scholar]
- 13. Kim JD, Choi DL, Han YS. An early single‐center experience of portal vein thrombosis in living donor liver transplantation: clinical feature, management and outcome. J Korean Surg Soc. 2011;81(1):35‐42. 10.4174/jkss.2011.81.1.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mukerji AN, Karachristos A, Maloo M, Johnson D, Jain A. Do postliver transplant patients need thromboprophylactic anticoagulation? Clin Appl Thromb Hemost. 2014;20(7):673‐677. 10.1177/1076029614538490 [DOI] [PubMed] [Google Scholar]
- 15. Bekker J, Ploem S, de Jong KP. Early hepatic artery thrombosis after liver transplantation: a systematic review of the incidence, outcome and risk factors. Am J Transplant. 2009;9(4):746‐757. 10.1111/j.1600-6143.2008.02541.x [DOI] [PubMed] [Google Scholar]
- 16. Stange BJ, Glanemann M, Nuessler NC, Settmacher U, Steinmüller T, Neuhaus P. Hepatic artery thrombosis after adult liver transplantation. Liver Transpl. 2003;9(6):612‐20. 10.1053/jlts.2003.50098 [DOI] [PubMed] [Google Scholar]
- 17. Wolf DC, Freni MA, Boccagni P, et al. Low‐dose aspirin therapy is associated with few side effects but does not prevent hepatic artery thrombosis in liver transplant recipients. Liver Transpl Surg. 1997;3(6):598‐603. 10.1002/lt.500030608 [DOI] [PubMed] [Google Scholar]
- 18. Okazaki M, Asato H, Takushima A, et al. Hepatic artery reconstruction with double‐needle microsuture in living‐donor liver transplantation. Liver Transpl. 2006;12(1):46‐50. 10.1002/lt.20550 [DOI] [PubMed] [Google Scholar]
- 19. Soliman T, Bodingbauer M, Langer F, et al. The role of complex hepatic artery reconstruction in orthotopic liver transplantation. Liver Transpl. 2003;9(9):970‐5. 10.1053/jlts.2003.50167 [DOI] [PubMed] [Google Scholar]
- 20. Liang TB, Bai XL, Li DL, Li JJ, Zheng SS. Early postoperative hemorrhage requiring urgent surgical reintervention after orthotopic liver transplantation. Transplant Proc. 2007;39(5):1549‐1553. 10.1016/j.transproceed.2007.01.080 [DOI] [PubMed] [Google Scholar]
- 21. Hendriks HG, van der Meer J, de Wolf JT, et al. Intraoperative blood transfusion requirement is the main determinant of early surgical re‐intervention after orthotopic liver transplantation. Transpl Int. 2005;17(11):673‐679. 10.1007/s00147-004-0793-5 [DOI] [PubMed] [Google Scholar]
- 22. Esmat Gamil M, Pirenne J, Van Malenstein H, et al. Risk factors for bleeding and clinical implications in patients undergoing liver transplantation. Transplant Proc. 2012;44(9):2857‐2860. 10.1016/j.transproceed.2012.09.085 [DOI] [PubMed] [Google Scholar]
- 23. Thompson MA, Redden DT, Glueckert L, et al. Risk Factors Associated with Reoperation for Bleeding following Liver Transplantation. HPB Surg. 2014;2014:816246. 10.1155/2014/816246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moghadamyeghaneh Z, Alameddine M, Jue JS, et al. A nationwide analysis of re‐exploration after liver transplant. HPB (Oxford). 2018;20(3):216‐221. 10.1016/j.hpb.2017.08.024 [DOI] [PubMed] [Google Scholar]
- 25. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006‐1012. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 26. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383‐94. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 27. Shay R, Taber D, Pilch N, et al. Early aspirin therapy may reduce hepatic artery thrombosis in liver transplantation. Transplant Proc. 2013;45(1):330‐334. 10.1016/j.transproceed.2012.05.075 [DOI] [PubMed] [Google Scholar]
- 28. Lin M, Crawford M, Fisher J, Hitos K, Verran D. Hepatic artery thrombosis and intraoperative hepatic artery flow rates in adult orthotopic liver transplantation. ANZ J Surg. 2002;72(11):798‐800. 10.1046/j.1445-2197.2002.02552.x [DOI] [PubMed] [Google Scholar]
- 29. Nguyen‐Buckley C, Gao W, Agopian V, Wray C, Steadman RH, Xia VW. Major thromboembolic complications in liver transplantation: the role of rotational thromboelastometry and cryoprecipitate transfusion. Transplantation. 2021;105(8):1771‐1777. 10.1097/TP.0000000000003427 [DOI] [PubMed] [Google Scholar]
- 30. Widen A, Rolando N, Manousou P, et al. Anticoagulation after liver transplantation: a retrospective audit and case‐control study. Blood Coagul Fibrinolysis. 2009;20(8):615‐8. 10.1097/MBC.0b013e32832c87c8 [DOI] [PubMed] [Google Scholar]
- 31. Vivarelli M, La Barba G, Cucchetti A, et al. Can antiplatelet prophylaxis reduce the incidence of hepatic artery thrombosis after liver transplantation? Liver Transpl. 2007;13(5):651‐654. 10.1002/lt.21028 [DOI] [PubMed] [Google Scholar]
- 32. Alexander BR, Antigua AD, Rosenberg AF, Caruso LJ, Voils SA, LeClaire AC. Chemoprophylaxis use and risk of venous thromboembolism and death in adult patients following orthotopic liver transplantation. J Pharm Pract. 2016;29(3):218‐223. 10.1177/0897190014566304 [DOI] [PubMed] [Google Scholar]
- 33. Sanchez‐Ocaña R, Tejedor‐Tejada J, Cimavilla‐Roman M, et al. Utility of oral anticoagulants as prophylaxis of recurrent portal thrombosis after liver transplantation. Transplant Proc. 2019;51(1):83‐86. 10.1016/j.transproceed.2018.07.014 [DOI] [PubMed] [Google Scholar]
- 34. Oberkofler, CE , Raptis, et al. Low‐dose aspirin confers protection against acute cellular allograft rejection after primary liver transplantation – a novel finding with practise implications. Abstract, Eur Surg Assoc Cologne. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bos I, Blondeau M, Wouters D, et al. Therapeutic anticoagulation after liver transplantation is not useful among patients with pre‐transplant Yerdel‐grade I/II portal vein thrombosis: a two‐center retrospective study. J Thromb Haemost. 2021;19(11):2760‐2771. 10.1111/jth.15472 [DOI] [PubMed] [Google Scholar]
- 36. Roullet S, Freyburger G, Cruc M, et al. Management of bleeding and transfusion during liver transplantation before and after the introduction of a rotational thromboelastometry‐based algorithm. Liver Transpl. 2015;21(2):169‐179. 10.1002/lt.24030 [DOI] [PubMed] [Google Scholar]
- 37. Bärthel E, Rauchfuss F, Hoyer H, et al. Impact of stable PGI₂ analog iloprost on early graft viability after liver transplantation: a pilot study. Clin Transplant. 2012;26(1):E38‐E47. 10.1111/j.1399-0012.2011.01516.x [DOI] [PubMed] [Google Scholar]
- 38. Rizzari MD, Safwan M, Sobolic M, et al. The impact of portal vein thrombosis on liver transplant outcomes: does grade or flow rate matter? Transplantation. 2021;105(2):363‐371. 10.1097/TP.0000000000003235 [DOI] [PubMed] [Google Scholar]
- 39. Kaneko J, Sugawara Y, Tamura S, et al. Coagulation and fibrinolytic profiles and appropriate use of heparin after living‐donor liver transplantation. Clin Transplant. 2005;19(6):804‐809. 10.1111/j.1399-0012.2005.00425.x [DOI] [PubMed] [Google Scholar]
- 40. Gad EH, Abdelsamee MA, Kamel Y. Hepatic arterial and portal venous complications after adult and pediatric living donor liver transplantation, risk factors, management and outcome (A retrospective cohort study). Ann Med Surg (Lond). 2016;8:28‐39. 10.1016/j.amsu.2016.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yerdel MA, Gunson B, Mirza D, et al. Portal vein thrombosis in adults undergoing liver transplantation: risk factors, screening, management, and outcome. Transplantation. 2000;69(9):1873‐1881. 10.1097/00007890-200005150-00023 [DOI] [PubMed] [Google Scholar]
- 42. Ibach M, Eurich D, Dobrindt E, et al. Orthotopic liver transplantation for Budd‐Chiari syndrome: observations from a 30‐year liver transplant program. Medicina (Kaunas). 2021;57(8). 1–12. 10.3390/medicina57080821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Choi HJ, Kim DG, Kim Y, et al. Clinical course of hepatic artery thrombosis after living donor liver transplantation using the right lobe. Liver Transpl. 2018;24(11):1554‐1560. 10.1002/lt.25065 [DOI] [PubMed] [Google Scholar]
- 44. Park GC, Moon DB, Kang SH, et al. Overcoming hepatic artery thrombosis after living donor liver transplantations: an experience from Asan Medical Center. Ann Transplant. 2019;24:588‐593. doi: 10.12659/AOT.919650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hibi T, Nishida S, Levi DM, et al. Long‐term deleterious effects of aortohepatic conduits in primary liver transplantation: proceed with caution. Liver Transpl. 2013;19(8):916‐925. 10.1002/lt.23689 [DOI] [PubMed] [Google Scholar]
- 46. Schulman S, Angerås U, Bergqvist D, et al. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. 2010;8(1):202‐204. 10.1111/j.1538-7836.2009.03678.x [DOI] [PubMed] [Google Scholar]
- 47. Shoeb M, Fang MC. Assessing bleeding risk in patients taking anticoagulants. J Thromb Thrombolysis. 2013;35(3):312‐319. 10.1007/s11239-013-0899-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yip J, Bruno DA, Burmeister C, et al. deep vein thrombosis and pulmonary embolism in liver transplant patients: risks and prevention. Transplant Direct. 2016;2(4):e68. 10.1097/TXD.0000000000000578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Annamalai A, Kim I, Sundaram V, Klein A. Incidence and risk factors of deep vein thrombosis after liver transplantation. Transplant Proc. 2014;46(10):3564‐3569. 10.1016/j.transproceed.2014.09.113 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data presented in the manuscript are based on published work that is sited in the body of the manuscript.


