Abstract
Background
BK polyomavirus‐associated nephropathy (BKPyVAN) carries a risk of irreversible allograft injury. While detection of BK viremia and biopsy assessment are the current diagnostic gold standard, the diagnostic value of biomarkers reflecting tissue injury (donor‐derived cell‐free DNA [dd‐cfDNA]) or immune activation (C‐X‐C motif chemokine ligand [CXCL]9 and CXCL10) remains poorly defined.
Methods
For this retrospective study, 19 cases of BKPyVAN were selected from the Vienna transplant cohort (biopsies performed between 2012 and 2019). Eight patients with T cell‐mediated rejection (TCMR), 17 with antibody‐mediated rejection (ABMR) and 10 patients without polyomavirus nephropathy or rejection served as controls. Fractions of dd‐cfDNA were quantified using next‐generation sequencing and CXCL9 and CXCL10 were detected using multiplex immunoassays.
Results
BKPyVAN was associated with a slight increase in dd‐cfDNA (median; interquartile range: .38% [.27%‐1.2%] vs. .21% [.12%‐.34%] in non‐rejecting control patients; p = .005). Levels were far lower than in ABMR (1.2% [.82%‐2.5%]; p = .004]), but not different from TCMR (.54% [.26%‐3.56%]; p = .52). Within the BKPyVAN cohort, we found no relationship between dd‐cfDNA levels and the extent of tubulo‐interstitial infiltrates, BKPyVAN class and BK viremia/viruria, respectively. In some contrast to dd‐cfDNA, concentrations of urinary CXCL9 and CXCL10 exceeded those detected in ABMR, but similar increases were also found in TCMR.
Conclusion
BKPyVAN can induce moderate increases in dd‐cfDNA and concomitant high urinary excretion of chemokines, but this pattern may be indistinguishable from that of TCMR. Our results argue against a significant value of these biomarkers to reliably distinguish BKPyVAN from rejection.
Keywords: biomarker, BK polyomavirus‐associated nephropathy, chemokines, donor‐derived cell‐free DNA, kidney transplantation
1. INTRODUCTION
BK polyomavirus‐associated nephropathy (BKPyVAN) is a serious infectious complication affecting approximately 5% of kidney transplant recipients. 1 BK virus replication in renal allografts may cause a continuous anti‐viral immune reaction, and may in some instances induce irreversible tissue damage and ultimately result in allograft failure. Detection of BK viremia and histologic evaluation of transplant biopsies including immunohistochemistry for SV40 large T antigen represent the current diagnostic gold standard, guiding tailored adjustment of baseline immunosuppression. 2 However, even in the presence of high levels of BK viremia, sampling error or inter‐observer variability may complicate the accurate diagnosis of BKPyVAN. 3 , 4 , 5 Currently, non‐invasive monitoring and disease prediction is mainly based on the quantification of BK virus copy numbers in plasma (with a cut‐off > 10 4 copies/ml for presumptive BKPyVAN) 6 , 7 and/or decoy cells in urine. 2 , 8 These parameters, however, may lack proper standardization, and BK viremia may not inevitably indicate ongoing allograft injury. 2 The use of additional non‐invasive biomarkers reflecting allograft injury and/or immune cell activation may therefore be of potential interest, and as an adjunct to current diagnostic strategies, such markers could contribute to improved prediction, diagnosis and/or monitoring of BKPyVAN.
One promising non‐invasive biomarker reflecting active allograft injury may be the relative quantification of donor‐derived cell‐free DNA (dd‐cfDNA) in peripheral blood, for example, via analysis of single nucleotide polymorphisms in the absence of recipient or donor genotyping. 9 , 10 , 11 In kidney transplant recipients, several recent studies have investigated the diagnostic and discriminative value of dd‐cfDNA as a marker of transplant rejection including antibody‐mediated rejection (ABMR), 12 , 13 , 14 , 15 , 16 , 17 T cell‐mediated rejection (TCMR) or borderline lesions, 18 , 19 respectively. In the context of TCMR or borderline rejection—which in their morphologic presentation may mirror BKPyVAN—the release of dd‐cfDNA may be less pronounced, which in some cases may limit its diagnostic value. 20 , 21 , 22 Currently, there is only scarce data on the course of dd‐cfDNA in the context of BKPyVAN. A small sub‐study performed within the DART trial 12 revealed increased levels of plasma dd‐cfDNA in BKPyVAN when compared to patients with BK viremia in absence of histologic evidence of renal involvement (in median 3.38% vs. .58%, respectively). 23 However, results were limited by a small sample size (10 patients; seven biopsies) and a retrospective study design. 23 In addition, two recent studies have discussed a diagnostic value of dd‐cfDNA and/or viral cfDNA in urine. 24 , 25
Beside quantification of dd‐cfDNA, assessment of serum and urinary chemokines, in particular, C‐X‐C motif chemokine ligand (CXCL) 9 and CXCL10, have evolved as promising markers of allograft immune activation. In donor‐specific antibody (DSA)‐positive kidney allograft recipients, elevations of urinary CXCL9 and CXCL10 were shown to associate with ABMR. 26 In addition, a recent longitudinal analysis of 56 kidney allograft recipients with positive BKPyV PCR showed increased serum and urinary CXCL10 levels during the course of BK viremia. 27
The primary objective of our present study was to investigate whether and to which extent BKPyVAN influences dd‐cfDNA fractions in plasma and, in addition, levels of serum and urinary chemokines CXCL9 and CXCL10. We included 19 patients diagnosed with BKPyVAN and, for comparison, eight patients with TCMR, 17 with ABMR and 10 without rejection, respectively. Plasma dd‐cfDNA fractions and urinary chemokines were analyzed in relation to BK viremia/viruria, decoy cells, Banff single lesion scores, and BKPyVAN severity.
2. METHODS
2.1. Study design and patients
In this retrospective single‐center study (Medical University of Vienna) 19 of 35 consecutive cases of biopsy‐proven polyoma virus nephropathy associated with BK viremia, which were recorded in the Vienna biopsy database over an 8‐year period, from January 2012 (initiation of systematic biobanking at our unit) to December 2019, were included. The 19 included study subjects (age > 18 years) had previously consented to participate in the Vienna Kidney Transplant Cohort Study for prospective biobanking, and at the time of index biopsy sufficient biological material was available for retrospective dd‐cfDNA analysis and chemokine measurements. The remaining 16 recipients had to be excluded from the analysis because no biologic material was available for retrospective biomarker detection. As shown in Supplemental Table S1, a comparison of baseline characteristics revealed a significantly lower proportion of deceased donor transplant recipients, a lower urinary protein/creatinine ratio and a slightly lower tubulitis (t) score among the 16 non‐included BKPyVAN patients.
For comparative analyses of biomarker patterns, we searched our biopsy database (January 2012 – December 2019) for cases of TCMR (Banff ≥I; DSA‐ and C4d‐negative; no BK or JC viremia) and HLA class I and/or II DSA‐positive ABMR (active, chronic active or chronic active phenotypes). Based on the availability of biobank material for retrospective biomarker testing, eight patients with TCMR and 17 patients with ABMR were selected for the study (biopsies performed within the first week after transplantation were excluded to rule out any influence of reperfusion injury on measured dd‐cfDNA fractions; the ABMR cohort has been described in a previous publication 13 ). In addition, 10 randomly selected DSA‐ and BK‐negative patients (index biopsies between January 2012 and December 2020) with no evidence of rejection or polyomavirus nephropathy served as negative controls.
The study was approved by the institutional ethics committee (registration numbers: 267/2011 and 1887/2020) and conducted in compliance with the Good Clinical Practice Guidelines, the principles of the Declaration of Helsinki 2008, and the Declaration of Istanbul 2018.
2.2. Measurement of dd‐cfDNA
Fractions of dd‐cfDNA were analyzed in bio‐banked plasma samples that had been collected in BD Vacutainer® ethylenediaminetetraacetic acid (EDTA) collection tubes (Becton Dickinson, Franklin Lakes, NJ, USA), as described previously. 13 Briefly, plasma was separated within <2 h in order to preclude white blood cell lysis‐induced recipient cfDNA. 28 Patient plasma was transferred to barcoded polypropylene tubes and stored at a mean temperature of equal or below ‐70°C, following a predefined protocol, as described previously (Biobank of the Medical University of Vienna 29 ). We extracted cfDNA from ≥.5 ml plasma using Qiagen's QIAamp Circulating Nucleic Acid Kit (Qiagen, Venlo, Netherlands) and employed a double AMPure clean‐up step to remove contaminating cell‐derived DNA. Donor‐derived‐cfDNA was measured by AlloSeq cfDNA assay (CareDx, Fremantle, WA, Australia), which is a targeted next‐generation sequencing assay employing bi‐allelic single nucleotide polymorphisms to quantify dd‐cfDNA without separate recipient or donor genotyping. Sequencing run results generated as FASTQ files were automatically analyzed using AlloSeq cfDNA Software (CareDx).
2.3. HLA antibody detection
For detection of HLA antibodies, we used LABscreen Single Antigen assays (One Lambda, Thermo Fisher Scientific, Canoga Park, CA, USA), as described previously. 29 HLA antibody testing was performed retrospectively on bio‐banked sera that were obtained at the time of biopsy. Serum samples were treated with EDTA (10 mM) to avoid complement interference. 30 The presence of DSA (mean fluorescence intensity [MFI] threshold > 1000 for DSA positivity) was determined according to serological and/or low‐ or high‐resolution donor/recipient HLA typing (HLA‐A, ‐B, ‐Cw, ‐DR, ‐DQ, and/or DP).
2.4. Chemokine measurement
Serum and urine samples were collected at the time of kidney allograft biopsy. Serum and urine CXCL9 and CXCL10 levels were assessed using Human ProcartaPlex Simplex Immunoassays (Thermo Fisher Scientific, Waltham, MA, USA). Urine chemokines were protected immediately by adding protease inhibitors, as described previously. 26 After centrifugation (1890 × g, 10 min, 22°C) supernatants of serum and urine were aliquoted and stored at −80°C until analysis. For serum CXCL9 and CXCL10 measurements, undiluted sera were diluted in assay buffer after addition of EDTA (10 mM). For urinary CXCL9 and CXCL10 measurements, undiluted urine samples were diluted in assay buffer according to the manufacturer's protocols. Urinary chemokine levels were normalized to urinary creatinine and are presented as pg (biomarker)/mg (creatinine). Measurements were performed in duplicates and measured on a Luminex 200 instrument (Luminex Corp., Austin, TX, USA). For one patient in the ABMR group no urine sample was available.
2.5. Quantitative polyomavirus pcr
All kidney transplant recipients underwent virological routine testing consisting of PCR analyses for BKPyV DNA in urine and serum samples collected on the same day of a routine post‐transplant visit (before the biopsy) or on the day of the biopsy using a PCR protocol described previously. 27 For two patients in the BKPyVAN group, no urine testing for BKPyV was available. For another two patients in the BKPyVAN cohort, plasma and urine BKPyV levels were available only after the biopsy (12 and 15 days after the biopsy, respectively). BKPyV plasma levels were available for all control patients and 14/17 ABMR patients.
2.6. Biopsies
Histomorphology and immunohistochemistry were evaluated on formalin‐fixed paraffin‐embedded tissue sections. Single lesions, BKPyVAN, TCMR and ABMR were scored and classified according to the 2019 update of the Banff scheme. 31 BKPyVAN histological scoring included the Banff interstitial fibrosis score (ci) and the semiquantitative histologic assessment of intrarenal polyomavirus replication/load levels (pvl). The pvl score included the percentage of tubules with immunohistochemical staining of epithelial cell nuclei for SV40 large T antigen and/or detection of typical intranuclear viral inclusion bodies (pvl 1: 1% positive tubules/ducts; pvl 2: 1%–10% positive tubules/ducts; pvl 3: > 10% positive tubules/ducts). The ci and the pvl scores were used to determine the severity of BKPyVAN (class I‐III), as recently described. 1 All biopsies initially classified as BKPyVAN were re‐scored by an experienced nephropathologist blinded to clinical results (N.K.).
2.7. Statistics
Categorical variables are presented as absolute and relative frequencies; for group comparisons the Fisher's exact test was applied. Continuous data are presented as median and interquartile range (IQR); for group comparisons non‐parametric testing (Mann‐Whitney U‐test) was applied. We calculated bivariate correlations using Spearman coefficient. A two‐sided p < .05 was considered statistically significant. All analyses were performed using IBM SPSS Statistics Version 24 (IBM, Armonk, NY, USA) and illustrated with Graph Pad Prism version 9.0 (Graph Pad, San Diego, CA, USA).
3. RESULTS
3.1. Patients and biopsy results
For this retrospective study, we selected a cohort of 19 patients with biopsy‐proven BKPyVAN. Three additional cohorts were included for comparative analysis: 8 patients with TCMR (Banff grade I: n = 2; Banff grade II: n = 6), 17 patients with DSA+ ABMR (active: n = 3; chronic active: n = 13; chronic inactive: n = 1) and 10 DSA‐ control cases with no evidence of rejection or polyomavirus nephropathy (control group). As shown in Table 1, the baseline characteristics of patients with BKPyVAN did not differ significantly from those recorded for patients with TCMR, with the exception of a later index biopsy time point (.4 vs. .2 years). In contrast, BKPyVAN recipients had their biopsy earlier than ABMR+ patients, a median of .4 versus 3.8 years post‐transplant. In addition, they were older, and fewer patients had pre‐formed DSA or were re‐transplant recipients. Tacrolimus‐based immunosuppression was more common and protein excretion was lower. Compared to DSA‐ control subjects, patients with BKPyVAN had lower donor age and higher estimated glomerular filtration rate (eGFR) (Table 1).
TABLE 1.
Baseline characteristics
| Patient cohorts | p‐Value | ||||||
|---|---|---|---|---|---|---|---|
| BKPyVAN n = 19 | TCMR n = 8 | DSA+ABMR+ n = 17 | Control group n = 10 | BKPyVAN versus | |||
| Variables | TCMR | ABMR | Control | ||||
| Variables recorded at the time of transplantation | |||||||
| Female sex, n (%) | 4 (21.1) | 1 (12.5) | 9 (52.9) | 6 (60) | >.99 | .08 | .05 |
| Recipient age (years), median (IQR) | 62 (48‐75) | 52 (46‐65) | 56 (34‐60) | 69 (64‐74) | .23 | .03 | .31 |
| Deceased donor, n (%) | 18 (94.7) | 5 (62.5) | 14 (82.4) | 10 (100) | .07 | .33 | >.99 |
| Prior kidney transplant, n (%) | 1 (5.3) | 0 (0) | 8 (47.1) | 1 (10) | >.99 | .06 | >.99 |
| Preformed anti‐HLA DSA, n (%) | 2 (10.5) | 0 (0) | 6 (42.9) a | 0 (0) | >.99 | .047 | .53 |
| Donor age (years), median (IQR) | 57 (48‐70) | 64 (42‐71) | 56 (32‐66) | 72 (67‐77) | .94 | .23 | .01 |
| HLA mismatch (A, B, DR), median (IQR) | 4 (2‐4) | 4 (3‐5) | 3 (3‐4) | 3 (3‐5) | .70 | >.99 | .96 |
| Initial immunosuppression | |||||||
| IL‐2 receptor antibody induction, n (%) | 17 (89.5) | 8 (100) | 8 (47.1) | 10 (100) | >.99 | .01 | .53 |
| Tacrolimus‐based immunosuppression, n (%) | 19 (100) | 7 (87.5) | 13 (76.5) | 10 (100) | .30 | .04 | >.99 |
| Peri‐transplant immunoadsorption, n (%) | 2 (10.5) | 0 (0) | 6 (35.3) | 0 (0) | >.99 | .11 | .53 |
| Variables recorded at the time of index biopsy | |||||||
| Years after transplantation, median (IQR) | .4 (.3‐.5) | .2 (.1‐.4) | 3.8 (2.6‐10.7) | .4 (.2‐.7) | .004 | .001 | .7 |
| Renal parameters | |||||||
| eGFR CKD‐EPI (ml/min/1.73m2), median (IQR) | 35.6 (24.2‐44.9) | 24.9 (17.6‐63.4) | 44.6 (24.9‐55.9) | 24.3 (17.1‐32.7) | .55 | .14 | .04 |
| Protein/creatinine ratio (mg/g), median (IQR) | 330 (192‐487) | 311 (171‐582) | 1003 (305‐2985) | 421 (203‐1570) | .78 | .01 | .34 |
| Maintenance immunosuppression | |||||||
| Triple immunosuppression, n (%) | 16 (94.1) b | 8 (100) | 17 (100) | 8 (100) c | >.99 | >.99 | >.99 |
| Tacrolimus, n (%) | 18 (100) b | 7 (87.5) | 13 (76.5) | 10 (100) c | .31 | .045 | >.99 |
| MPA, n (%) | 16 (88.9) b | 7 (87.5) | 17 (100) | 8 (100) c | >.99 | .49 | >.99 |
| Steroids, n (%) | 18 (100) b | 8 (100) | 17 (100) | 8 (100) c | >.99 | >.99 | >.99 |
Abbreviations: DSA, donor‐specific antibody; eGFR, estimated glomerular filtration rate; HLA, human leukocyte antigen; IQR, interquartile range; MPA, mycophenolic acid; BKPyVAN, BK polyomavirus‐associated nephropathy; TCMR, T cell‐mediated rejection.
For 3 recipients transplanted before 2009, solid‐phase HLA antibody screening on the wait list was not available.
For one subject, no data on maintenance immunosuppression were available.
For two subjects, no data on immunosuppression were available.
Biopsy results obtained in the BKPyVAN cohort are provided in Table 2. All included patients showed positive immunohistochemical staining for SV40 large T antigen. The average BKPyVAN class was 2 (IQR: 2‐2). Banff single lesion scores were in median 2 (2‐3) for tubulitis (t) and 1 (0‐2) for inflammation (i). Median BK viremia was 2.4 × 104 copies per ml (IQR: 1.0 × 104/ml‐1,2 × 105/ml) and viruria was 2.5 × 109/ml (6.3 × 108/ml‐8.7 × 109/ml). Decoy cells were detectable in all BKPyVAN patients with available urine cytology (n = 15; median percentage of decoy cells: 71% [IQR: 24%–90%]) (Table 2).
TABLE 2.
Biopsy results and biomarkers
| Variables | BKPyVAN cohort (n = 19) |
|---|---|
| Biopsy results | |
| Single lesion scores | |
| i, median (IQR) | 1 (0‐2) |
| t, median (IQR) | 2 (2‐3) |
| ti, median (IQR) | 2 (1‐3) |
| ci, median (IQR) | 2 (1‐3) |
| ct, median (IQR) | 1 (0‐2) |
| SV 40 positivity, n (%) | 19 (100) |
| BKPyVAN class, median (IQR) | 2 (2‐2) |
| Biomarker | |
| BKPyV DNAemia (copies/ml), median (IQR) | 2.4 × 104 (1 × 104‐1.2 × 105) |
| BKPyV DNAuria (copies/ml), median (IQR) | 2.5 × 109 (6.3 × 108‐8.7 × 109) |
| % Decoy cells in urine, median (IQR) | 60 (20‐90) |
Abbreviations: BKPy, BK polyoma virus; BKPyVAN, BK polyomavirus‐associated nephropathy; IQR, interquartile range.
3.2. Patterns of dd‐cfDNA fractions and chemokine profiles
As illustrated in Figure 1, patients diagnosed with BKPyVAN showed moderate increases in median dd‐cfDNA levels (.38% [IQR: .27%‐1.2%]). Comparative analyses showed that recipients with BKPyVAN had significantly lower dd‐cfDNA levels than ABMR+ subjects (1.2% [IQR: .82%–2.5%]; p = .004], but higher levels than DSA‐ subjects (.21% [.12%–.34%]; p = .005). Median levels were not significantly different to those in TCMR patients (.54% [.26%–3.56%]; p = .52) (Figure 1).
FIGURE 1.
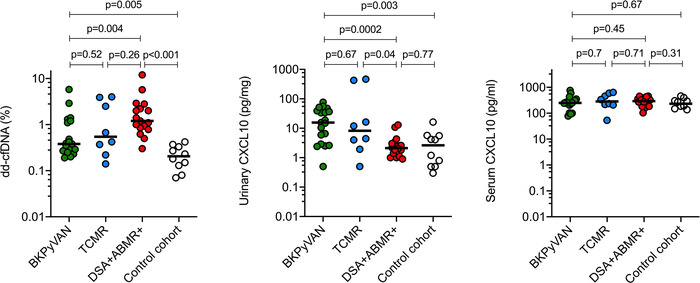
Levels of dd‐cfDNA and CXCL10 at the time of the index biopsy. Horizontal lines indicate median values. For group comparisons, the Mann‐Whitney U‐test was applied. ABMR, antibody‐mediated rejection; CXCL10, C‐X‐C motif chemokine ligand 10; dd‐cfDNA, donor‐derived cell‐free DNA; DSA, donor‐specific antibodies; BKPyVAN, BK polyomavirus‐associated nephropathy; TCMR, T cell‐mediated rejection.
As shown in Figures 1 and S1, urinary markers of immune cell activation (CXCL9; CXCL10 [pg/mg creatinine]) were significantly higher in the BKPyVAN cohort (CXCL9: 47.9 [median; IQR: 22.8‐138]; CXCL10: 15.6 [2.9‐38.9]) than in ABMR+ (CXCL9: 6.6 [3.4‐8.5]; CXCL10: 2.1 [1.2‐3.4]) and DSA‐ cohorts (CXCL9: 5.8 [1.6‐12.2]; CXCL10: 2.7 [.5‐5.6]). Urinary chemokine levels in patients with TCMR showed considerable variability (CXCL9: 9.2 [median, IQR: 2.6‐601.2]; CXCL10: 8.2 [2.5‐323.1]), without significant differences to BKPyVAN. Furthermore, while we did not observe any differences between patient groups in terms of CXCL10 concentrations in serum, CXCL9 levels, which showed substantial variation in the small TCMR group, were marginally different between BKPyVAN and TCMR (Figures 1 and S1).
3.3. Donor‐derived cfDNA and urinary chemokines in relation to BK‐specific markers and biopsy results
Among patients with BKPyVAN, we found no correlation between dd‐cfDNA levels and BK viremia (Figure 2). Moreover, there were no associations with BKPyVAN class, Banff single lesion scores, BK viruria or decoy cell excretion (Figure 2). As shown in Figures 3 and S2, there were also no associations between chemokine levels in urine and BK viremia. There was, however, a marginal inverse association with BKPyVAN severity and BK viruria, respectively. Chemokine levels were not related to Banff single lesion scores or decoy cell excretion (Figures 3 and S2).
FIGURE 2.
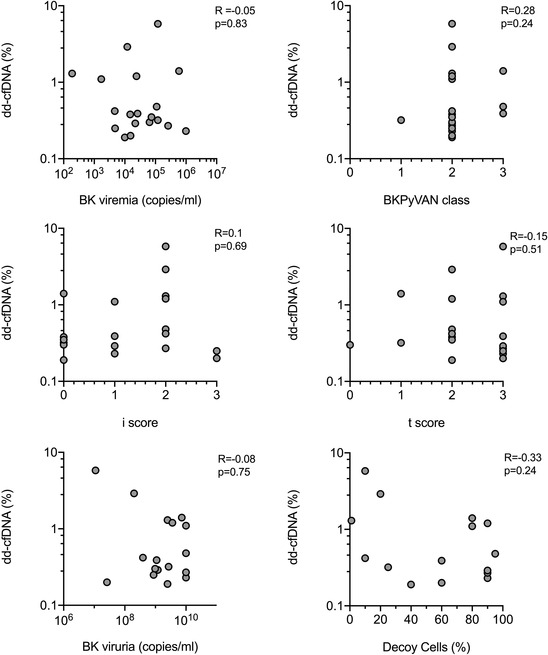
Correlation between dd‐cfDNA levels and allograft histologic analysis or BKPy‐specific biomarkers. Bivariate correlations were calculated using the Spearman coefficient. BKPyVAN, BK polyomavirus‐associated nephropathy; dd‐cfDNA, donor‐derived cell‐free DNA.
FIGURE 3.
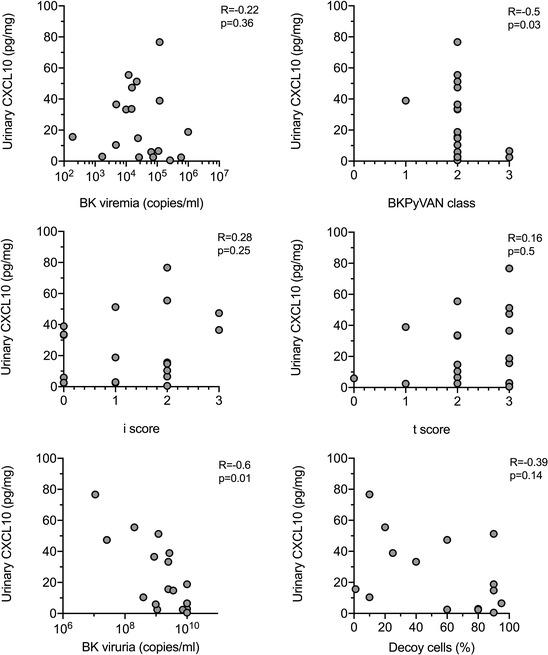
Correlation between urinary CXCL10 levels and allograft histologic analysis or BKPy‐specific biomarkers. Bivariate correlations were calculated using the Spearman coefficient. BKPyVAN, BK polyomavirus‐associated nephropathy; CXCL10, C‐X‐C motif chemokine ligand 10; dd‐cfDNA, donor‐derived cell‐free DNA.
4. DISCUSSION
A key finding of our present study was that fractions of dd‐cfDNA were only moderately elevated in patients with BKPyVAN, similar to a comparison cohort of patients with TCMR (median: .38% vs. .54%). Detected levels were in a range previously described for other TCMR cohorts. 12 , 18 At the same time, BKPyVAN was found to be associated with a substantial increase in urinary (but not serum) chemokine concentrations that far exceeded those detected in ABMR, a similar pattern to that observed in the TCMR cohort. Another result was that among patients with BKPyVAN, dd‐cfDNA fractions did not correlate with levels of BK viremia and the morphologic severity of disease.
Fractions of dd‐cfDNA in our BKPyVAN patients were lower than those reported in a previous study by Kant and coworkers. 23 In contrast to this study, we did not find any association between dd‐cfDNA and BK load in plasma or urine. Comparing the results of the two case series, however, it has to be pointed out that sample sizes are small, and a lack of biopsies in some of the patients reported in the earlier study may have complicated data interpretation. 23 Our findings may be in accordance with a recent study by Chen et al., 25 where the height of dd‐cfDNA levels did not correlate with the morphologic presentation of BKPyVAN or viral load; however, urinary instead of plasma dd‐cfDNA was analyzed.
Levels of CXCL9 and CXCL10 in urine were found to be substantially higher in patients with BKPyVAN compared to control subjects, including even those with ABMR. However, patients with TCMR showed a similar pattern. Our finding of elevated urinary chemokine levels may be in accordance with earlier studies. 27 , 32 , 33 , 34 For example, Weseslindtner et al. 27 demonstrated a significant association of CXCL10 in blood and urine with levels of BK virus replication. Different stages of progressing BKPyV infection, ranging from isolated viruria to established BKPyVAN, were thereby found to associate with stepwise increases of chemokine levels. 27 A remarkable result of our present analysis was a discrepancy between biomarker levels in blood versus urine, which led to opposite patterns among BKPyVAN versus ABMR patients. One may hypothesize that the pronounced urinary chemokine secretion in BKVPyVAN (and TCMR) could specifically mirror ongoing immune injury in the tubulo‐interstitial compartment of the allograft. Instead, ABMR may primarily affect the endothelial interface, and this could explain the more pronounced release of dd‐cfDNA into the circulation.
A strength of our study may be the high granularity of clinical and laboratory results. Moreover, for all included patients a detailed biopsy work‐up was available, whereby specimens were re‐classified by an experienced nephropathologist to strengthen the diagnostic accuracy. However, we are aware of some inherent limitations of the study. One is the retrospective study design as a potential source of bias: due to the limited availability of biosamples for retrospective biomarker testing, we were able to include only 19 out of 35 BKPyVAN cases recorded in our database. While most baseline variables were evenly distributed, it was found that the extent of tubulitis (but not interstitial inflammation) was slightly higher in the BKPyVAN cases included in our biomarker analysis. In addition, our retrospective study design did not allow for a systematic longitudinal sample analysis to assess the value of biomarker surveillance in predicting treatment responses or transition between BKPyVAN and allograft rejection. Another limitation is the small size of our studied cohorts. In this context, however, we want to point out that there is only one additional study analyzing dd‐cfDNA blood levels in relation to polyoma nephropathy. This study was even smaller (10 patients included), and only seven subjects had been subjected to renal allograft biopsies. 23 Furthermore, a limitation is the absence of bona fide control cohorts, such as patients with BK viremia but normal histology.
In conclusion, fractions of dd‐cfDNA were found to be only moderately elevated in patients with BKPyVAN, without meaningful associations with characteristics and severity of disease. ABMR showed an inverse biomarker pattern, with higher levels of dd‐cfDNA and a less pronounced increase in urinary chemokine levels, but there were no significant differences between BKPyVAN and TCMR. Our study results may argue against a diagnostic value of plasma dd‐cfDNA and urinary chemokines to reliably distinguish BKPyVAN from rejection. Future studies will have to clarify whether the combined use of these biomarkers for longitudinal monitoring of disease progression and treatment success could be useful.
AUTHOR CONTRIBUTIONS
Katharina A. Mayer, Haris Omic, Konstantin Doberer, Roman Reindl‐Schwaighofer, Farsad Eskandary, Andreas Heinzel, Željko Kikić, Georg A. Böhmig, Michael Eder: participated in the research design, performance of the research, data analysis, interpretation of results, and writing of the manuscript. Lukas Weseslindtner, and Nicolas Kozakowski: participated in data analysis and writing of the manuscript. Amanda Tillgren, Thierry Viard and Susanne Haindl: participated in performance of the research. Silvia Casas: participated in writing of the manuscript.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The study was funded by an investigator‐initiated unrestricted grant from CareDx Inc., Brisbane, South San Francisco, CA, USA (to G.A. Böhmig). The authors wish to thank Dr. Anke Steiner for her technical assistance.
Mayer KA, Omic H, Weseslindtner L, et al. Levels of donor‐derived cell‐free DNA and chemokines in BK polyomavirus‐associated nephropathy. Clin Transplant. 2022;36:e14785. 10.1111/ctr.14785
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Nickeleit V, Singh HK, Randhawa P, et al. The Banff working group classification of definitive polyomavirus nephropathy: morphologic definitions and clinical correlations. J Am Soc Nephrol. 2018;29(2):680‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adam BA, Kikic Z, Wagner S, et al. Intragraft gene expression in native kidney BK virus nephropathy versus T cell–mediated rejection: prospects for molecular diagnosis and risk prediction. Am J Transplant. 2020;20(12):3486‐3501. [DOI] [PubMed] [Google Scholar]
- 3. Adam B, Randhawa P, Chan S, et al. Banff initiative for quality assurance in transplantation (BIFQUIT): reproducibility of polyomavirus immunohistochemistry in kidney allografts. Am J Transplant. 2014;14(9):2137‐2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nankivell BJ, Renthawa J, Sharma RN, Kable K, O'Connell PJ, Chapman JR. BK virus nephropathy: histological evolution by sequential pathology. Am J Transplant. 2017;17(8):2065‐2077. [DOI] [PubMed] [Google Scholar]
- 5. Drachenberg CB, Papadimitriou JC, Chaudhry MR, et al. Histological evolution of BK virus–associated nephropathy: importance of integrating clinical and pathological findings. Am J Transplant. 2017;17(8):2078‐2091. [DOI] [PubMed] [Google Scholar]
- 6. Boan P, Hewison C, Swaminathan R, et al. Optimal use of plasma and urine BK viral loads for screening and predicting BK nephropathy. BMC Infect Dis. 2016;16(1):342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Renoult E, Coutlée F, Pâquet M, et al. Evaluation of a preemptive strategy for BK polyomavirus‐associated nephropathy based on prospective monitoring of BK viremia: a kidney transplantation center experience. Transplant Proc. 2010;42(10):4083‐4087. [DOI] [PubMed] [Google Scholar]
- 8. Chakera A, Dyar O‐J, Hughes E, Bennett S, Hughes D, Roberts ISD. Detection of polyomavirus BK reactivation after renal transplantation using an intensive decoy cell surveillance program is cost‐effective. Transplantation. 2011;92(9):1018‐1023. [DOI] [PubMed] [Google Scholar]
- 9. Paul RS, Almokayad I, Collins A, Raj D, Jagadeesan M. Donor‐derived cell‐free DNA: advancing a novel assay to new heights in renal transplantation. Transplant Direct. 2021;7(3):e664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Filippone EJ, Farber JL. The monitoring of donor‐derived cell‐free DNA in kidney transplantation. Transplantation. 2021;105(3):509‐516. [DOI] [PubMed] [Google Scholar]
- 11. Kataria A, Kumar D, Gupta G. Donor‐derived cell‐free DNA in solid‐organ transplant diagnostics: indications, limitations, and future directions. Transplantation. 2021;105(6):1203‐1211. [DOI] [PubMed] [Google Scholar]
- 12. Bloom RD, Bromberg JS, Poggio ED, et al. Cell‐free DNA and active rejection in kidney allografts. J Am Soc Nephrol. 2017;28(7):2221‐2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mayer KA, Doberer K, Tillgren A, et al. Diagnostic value of donor‐derived cell‐free DNA to predict antibody‐mediated rejection in donor‐specific antibody‐positive renal allograft recipients. Transpl Int. 2021;34(9):1689‐1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jordan SC, Bunnapradist S, Bromberg JS, et al. Donor‐derived cell‐free DNA identifies antibody‐mediated rejection in donor specific antibody positive kidney transplant recipients. Transplant Direct. 2018;4(9):e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang E, Sethi S, Peng A, et al. Early clinical experience using donor‐derived cell‐free DNA to detect rejection in kidney transplant recipients. Am J Transplant. 2019;19(6):1663‐1670. [DOI] [PubMed] [Google Scholar]
- 16. Zhang H, Zheng C, Li X, et al. Diagnostic performance of donor‐derived plasma cell‐free DNA fraction for antibody‐mediated rejection in post renal transplant recipients: a prospective observational study. Front Immunol. 2020;11(342). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whitlam JB, Ling L, Skene A, et al. Diagnostic application of kidney allograft‐derived absolute cell‐free DNA levels during transplant dysfunction. Am J Transplant. 2019;19(4):1037‐1049. [DOI] [PubMed] [Google Scholar]
- 18. Oellerich M, Shipkova M, Asendorf T, et al. Absolute quantification of donor‐derived cell‐free DNA as a marker of rejection and graft injury in kidney transplantation: results from a prospective observational study. Am J Transplant. 2019;19(11):3087‐3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stites E, Kumar D, Olaitan O, et al. High levels of dd‐cfDNA identify patients with TCMR 1A and borderline allograft rejection at elevated risk of graft injury. Am J Transplant. 2020;20(9):2491‐2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gielis EM, Ledeganck KJ, Dendooven A, et al. The use of plasma donor‐derived, cell‐free DNA to monitor acute rejection after kidney transplantation. Nephrol Dial Transplant. 2019;35(4):714‐721. [DOI] [PubMed] [Google Scholar]
- 21. Wijtvliet VPWM, Plaeke P, Abrams S, et al. Donor‐derived cell‐free DNA as a biomarker for rejection after kidney transplantation: a systematic review and meta‐analysis. Transpl Int. 2020;33(12):1626‐1642. [DOI] [PubMed] [Google Scholar]
- 22. Xiao H, Gao F, Pang Q, et al. Diagnostic accuracy of donor‐derived cell‐free DNA in renal‐allograft rejection: a meta‐analysis. Transplantation. 2021;105(6):1303‐1310. [DOI] [PubMed] [Google Scholar]
- 23. Kant S, Bromberg J, Haas M, Brennan D. Donor‐derived cell‐free DNA and the prediction of BK virus‐associated nephropathy. Transplant Direct. 2020;6(11):e622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burnham P, Dadhania D, Heyang M, et al. Urinary cell‐free DNA is a versatile analyte for monitoring infections of the urinary tract. Nat Commun. 2018;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen X‐T, Chen W‐F, Li J, et al. Urine donor–derived cell‐free DNA helps discriminate BK polyomavirus‐associated nephropathy in kidney transplant recipients with bk polyomavirus infection. Front Immunol. 2020;11(1763). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mühlbacher J, Doberer K, Kozakowski N, et al. Non‐invasive chemokine detection: improved prediction of antibody‐mediated rejection in donor‐specific antibody‐positive renal allograft recipients. Front Med 2020;7(114). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weseslindtner L, Hedman L, Wang Y, et al. Longitudinal assessment of the CXCL10 blood and urine concentration in kidney transplant recipients with BK polyomavirus replication‐a retrospective study. Transpl Int. 2020;33(5):555‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Danesi R, Lo YMD, Oellerich M, et al. What do we need to obtain high quality circulating tumor DNA (ctDNA) for routine diagnostic test in oncology? – Considerations on pre‐analytical aspects by the IFCC workgroup cfDNA. Clin Chim Acta. 2021;520:168‐171. [DOI] [PubMed] [Google Scholar]
- 29. Eskandary F, Bond G, Kozakowski N, et al. Diagnostic contribution of donor‐specific antibody characteristics to uncover late silent antibody‐mediated rejection—results of a cross‐sectional screening study. Transplantation. 2017;101(3):631‐641. [DOI] [PubMed] [Google Scholar]
- 30. Schwaiger E, Wahrmann M, Bond G, Eskandary F, Böhmig GA. Complement component C3 activation: the leading cause of the prozone phenomenon affecting HLA antibody detection on single‐antigen beads. Transplantation. 2014;97(12):1279‐1285. [DOI] [PubMed] [Google Scholar]
- 31. Loupy A, Haas M, Roufosse C, et al. The Banff 2019 kidney meeting report (I): updates on and clarification of criteria for T cell– and antibody‐mediated rejection. Am J Transplant. 2020;20(9):2318‐2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ho J, Schaub S, Wiebe C, et al. Urinary CXCL10 chemokine is associated with alloimmune and virus compartment‐specific renal allograft inflammation. Transplantation. 2018;102(3):521‐529. [DOI] [PubMed] [Google Scholar]
- 33. Jackson JA, Kim EJ, Begley B, et al. Urinary chemokines CXCL9 and CXCL10 are noninvasive markers of renal allograft rejection and BK viral infection. Am J Transplant. 2011;11(10):2228‐2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mühlbacher J, Doberer K, Kozakowski N, et al. Non‐invasive chemokine detection: improved prediction of antibody‐mediated rejection in donor‐specific antibody‐positive renal allograft recipients. Front Med (Lausanne). 2020;7:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


