Abstract
Introduction
The impact of life‐course traumatic brain injury (TBI) on dementia is unclear.
Methods
Within the Swedish Twin Registry (STR), 35,312 dementia‐free twins were followed for up to 18 years. TBI history was identified via medical records. Data were analyzed using generalized estimating equation (GEE) and conditional logistic regression.
Results
In multi‐adjusted GEE models, the odds ratio (OR, 95% confidence interval [CI]) of dementia was 1.27 (1.03–1.57) for TBI at any age, 1.55 (1.04–2.31) for TBI at 50 to 59 years, and 1.67 (1.12–2.49) for TBI at 60 to 69 years. Cardiometabolic diseases (CMDs) increased dementia risk associated with TBI at age 50 to 69 years. The ORs in GEE and conditional logistic regression did not differ significantly (P = .37).
Discussion
TBI, especially between ages 50 and 69 years, is associated with an increased risk of dementia, and this is exacerbated among people with CMDs. Genetic and early‐life environmental factors may not account for the TBI–dementia association.
Keywords: cardiometabolic diseases, dementia, life‐course traumatic brain injury, population‐based twin study
1. INTRODUCTION
Dementia is a burdensome disease for patients, caregivers, and society as a whole. An estimated 50 million people worldwide were living with dementia in 2018, and this number is projected to more than triple over the next 30 years. 1 The identification of modifiable risk factors has been prioritized as a strategy to prevent or delay the development of dementia.
Traumatic brain injury (TBI)—a non‐degenerative, non‐congenital insult to the brain from an external mechanical force—can cause temporary or permanent cognitive impairment. 2 , 3 , 4 Several studies have explored the association between TBI and cognitive function or dementia, but with conflicting findings. 5 , 6 , 7 , 8 Overall, TBI has been reported to confer a 1.6‐ to 3.7‐fold increased risk of dementia, 5 , 7 though some studies have found no such association. 6 , 8 Furthermore, TBI may impact cognitive function to a different degree depending on the age of onset. Previous research has suggested that slow cognitive decline may occur after a TBI at any age, 9 though this decline appears to be more severe among individuals who experience a TBI at an older versus younger age. 10 , 11 This may be related to brain plasticity. Older individuals may have less ability to compensate for TBI‐related brain damage during the initial recovery period or may experience greater brain degeneration after the initial recovery period due to reduced plasticity of the aging brain. 9 Therefore, when exploring the relationship between TBI and dementia, the timing of TBI over the life course deserves more attention. Despite this, most studies assessing the association between TBI and dementia have focused instead on people with a history of TBI in certain age groups (e.g., over 55 years or 18 to 65 years), 5 , 7 and no studies, to our knowledge, have used a life‐course approach to examine the impact of TBI in different periods of life on dementia.
RESEARCH IN CONTEXT
Systematic review: PubMed and Web of Science databases were searched, and titles and abstracts were screened. Traumatic brain injury (TBI) may impact cognitive function to a different degree depending on the age of onset. However, no studies have explored the association between TBI occurring during the lifespan and dementia. In our study, we used a life‐course approach to examine the impact of TBI on dementia at different periods of life.
Interpretation: In this population‐based study, we found that TBI, especially occurring between ages 50 and 69 years, is associated with an increased risk of dementia, and this risk is exacerbated among individuals with cardiometabolic diseases. Genetic and early‐life environmental factors may not account for the TBI–dementia association.
Future directions: Future studies need to examine the role of severity and location of TBI on dementia and explore the biological pathways linking TBI and dementia.
HIGHLIGHTS
Traumatic brain injury (TBI), especially occurring between ages 50 and 69 years, is associated with an increased risk of dementia.
Cardiometabolic diseases (CMDs) increase dementia risk associated with TBI at the age of 50 to 69 years.
Genetic and early‐life environmental factors may not account for the TBI–dementia association.
It is important to avoid brain damage, especially among people with CMDs, to prevent the development of dementia.
Accumulating evidence has shown that cardiometabolic diseases (CMDs) are associated with dementia risk, 12 and TBI is related to CMDs. 13 , 14 Thus, it is plausible that CMDs could play a moderating role in the association between TBI and dementia, but to date this issue remains unclear. Moreover, mounting evidence has suggested that both dementia and outcomes after TBI may be affected by genetic and early‐life environmental factors. 15 , 16 , 17 , 18 It is unknown whether and to what extent these factors contribute to the TBI–dementia association. A twin study design provides us with the possibility of evaluating these unmeasured factors because twins generally share a common early‐life environment and genetic background.
In this study, using data from a large cohort of nationwide Swedish twins, we aimed to (1) examine the association between life‐course TBI and dementia, exploring the role of CMDs in this association, and (2) assess whether genetic and early‐life environmental factors contribute to the TBI–dementia association using co‐twin matched analysis.
2. METHODS
2.1. Study population
Participants were derived from the nationwide Swedish Twin Registry (STR), which was initiated in the 1960s. 19 From 1998 to 2002, all living twins from the STR who were born in 1958 or earlier were invited to participate in the Screening Across the Lifespan Twin (SALT) study, a full‐scale screening via a computer‐based telephone interview. Of the 44,919 twin individuals in the SALT study, we excluded 152 with prevalent dementia, 36 who developed early‐onset dementia prior to age 60, 565 who died before age 60, and 8854 who were <50 years old at baseline and therefore had a low possibility of developing the outcome during the follow‐up. Therefore, a total of 35,312 individuals were included in the current study and followed‐up until 2016 (Figure 1).
FIGURE 1.
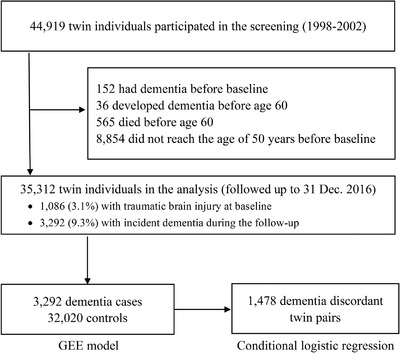
Flowchart of the study population. GEE, generalized estimating equation
2.2. Data collection
Information on demographics (age, education, and marital status), lifestyle (smoking status, alcohol consumption, and physical activity), zygosity, and anthropometrics (height and weight) was collected through the baseline SALT survey. Information on sex was collected from the STR based on self‐report. Education level was dichotomized as <8 or ≥8 years based on the maximum number of years of formal schooling. 20 Zygosity was divided into monozygotic, dizygotic, or undetermined zygosity. Marital status was categorized as married/cohabitating versus single (including divorced and widowed). Smoking status was dichotomized as never versus former/current smoking. Alcohol consumption was categorized as no/mild drinking versus heavy drinking. Physical activity was ascertained using a question from the SALT interview about annual exercise patterns and dichotomized as active (including the responses “more than average,” “much more than average,” and “maximum”) and inactive (including the responses “almost never,” “much less than average,” “less than average,” and “average”). 21 Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2) and grouped into <20.0 (underweight), 20.0 to 24.9 (normal weight), 25.0 to 29.9 (overweight), and ≥30 (obese). 22
Information on medical history was obtained via linkage with the Sweden National Patient Registry (NPR), which covers all inpatient diagnoses since the 1960s and outpatient care since 2001. 23 Each medical record in the NPR included up to eight discharge diagnoses according to International Classification of Disease (ICD) codes. The seventh revision (ICD‐7) was used until 1968, the eighth revision (ICD‐8) was used from 1969 to 1986, the ninth revision (ICD‐9) was used from 1987 to 1996, and the tenth revision (ICD‐10) has been used from 1997 to the end of available follow‐up in 2016. Survival status was identified through the Swedish Cause of Death Register. 24
Baseline type 2 diabetes (T2D), heart disease, stroke, and hypertension (ICD‐7 codes 444–447; ICD‐8 codes 400–404; ICD‐9 codes 401–405; and ICD‐10 codes I10–I15) were ascertained based on medical history. Information on baseline dyslipidemia was obtained based on self‐report in the SALT survey. Participants were asked if they have had high cholesterol or had taken treatment for lipid disorders. The presence of dyslipidemia was defined as having a medical history of high cholesterol or taking medication for lipid disorders.
Informed consent was obtained from all participants. The study was approved by the Regional Ethics Board at Karolinska Institutet, Stockholm, Sweden and the Institutional Review Board of the University of Southern California, USA.
2.3. Ascertainment of TBI
At baseline, information on participants’ previous TBI diagnoses and their corresponding recorded dates was obtained from the NPR. The ICD codes for TBI included the following: ICD‐7 codes N800–N801, N803, and N850–N856; ICD‐8 codes N800–N801, N803–N804, and N850–N854; ICD‐9 codes 800–801, 803–804, and 850–854; and ICD‐10 codes S01.0–S01.9, S02.0, S02.1, S02.3, S02.7–S02.9, S04.0, S06.0–S06.9, S07.0, S07.1, S07.8, S07.9, S09.7–S09.9, T90.1, T90.2, T90.4, T90.5, T90.8, and T90.9.
Life‐course TBI was divided into five groups according to the age at which TBI first occurred: ≤39, 40 to 49, 50 to 59, 60 to 69, and ≥70 years. Participants’ total number of TBI occurrences was summed and further divided into three categories: TBI‐free, one TBI, and two or more TBIs. Building on the current literature, 5 , 7 in exploratory analyses we further divided TBI into different severity groups (“mild” vs. “moderate‐to‐severe”) and excluded participants with undetermined TBI severity.
2.4. Assessment of dementia
Information on all‐cause dementia diagnoses—including Alzheimer's disease (AD; ICD‐8 code 290; ICD‐9 codes 290.0–290.1 and 331.0; ICD‐10 codes F00.0 and G30), vascular dementia (VaD; ICD‐9 code 290.4; ICD‐10 code F01), and other or unspecified types of dementia (ICD‐9 codes 290.8–290.9 and 331.2; ICD‐10 codes F02, F03, F05, G31.0, and G31.8) was determined based on information in the NPR and the Cause of Death Register. All dementia diagnoses were determined based on neurological examinations at neurology clinics. The age at onset of dementia was estimated based on the earliest recorded date of the dementia diagnosis.
2.5. Assessment of CMDs
Based on previous literature, CMDs were defined as T2D, heart disease, and stroke. 12 , 25 NPR data was used to ascertain baseline T2D (ICD‐7 code 260; ICD‐8 and ‐9 code 250; and ICD‐10 codes E10–E14), heart disease (ICD‐7 codes 420 and 434; ICD‐8 codes 410–414 and 427; ICD‐9 codes 410–414 and 428; ICD‐10 codes I20–I25 and I50), and stroke (ICD‐7 codes 330–334; ICD‐8 codes 430–438; ICD‐9 codes 430–437; ICD‐10 codes I60–I68 and G47). Age of CMD onset was defined based on the earliest date of T2D, heart disease, or stroke diagnosis.
2.6. Statistical analysis
The differences in baseline characteristics between the TBI‐free group and TBI group were analyzed using the χ2 tests for categorical variables and t‐tests for continuous variables.
Odds ratios (ORs) and 95% confidence intervals (CIs) for the association between TBI and dementia were estimated from generalized estimating equation (GEE) models and conditional logistic regression models. Among all twin individuals (n = 35,312, including 3292 incident dementia cases and 32,020 controls), GEE models were applied to examine the association between TBI and dementia in the unmatched case‐control analysis. 24 Next, conditional logistic regression models were used to assess the TBI–dementia association in co‐twin matched dementia‐discordant twin pairs. In these twin pairs, one twin developed dementia and the other did not, and unmeasured factors (such as genetic and early‐life environmental factors) could be controlled for, allowing us to explore the contribution of genetic and early‐life environmental factors to the TBI–dementia association. The basic model was adjusted for age, sex, and education. The multivariable model was further adjusted for marital status, smoking status, alcohol consumption, physical activity, BMI, T2D, heart disease, stroke, hypertension, and dyslipidemia.
Logistic regression was used to test the difference in ORs from the GEE model and the conditional logistic regression model by examining the difference in the proportion of TBI between unmatched and matched control participants. 21 , 24 , 26 A statistically significant OR from the logistic regression indicates that genetic and early‐life environmental factors may play a role in the TBI–dementia association; otherwise, the influence of these shared factors on the observed relationship is likely small or null.
In sensitivity analyses, we excluded individuals who developed dementia within 5 years of TBI to minimize the impact of potential preclinical dementia that could bias the association between TBI and dementia. TBI has been associated with an elevated risk of death, 27 so we further adjusted for death during the follow‐up.
The synergetic effect of TBI (yes vs. no) and CMDs (yes vs. no) on the risk of dementia was assessed by creating a dummy variable with four groups: (1) TBI‐ and CMD‐free (reference group), (2) presence of TBI but no CMDs, (3) presence of CMDs but no TBI, and (4) coexistence of TBI and CMDs. The additive interaction between TBI and CMDs on dementia risk was tested by estimating the relative excess risk due to interaction (RERI), the attributable proportion (AP), and the synergy index (S). Additionally, we examined the multiplicative interaction between TBI and CMDs on dementia risk by incorporating the two variables and an interaction term in the same model.
Missing values for education (n = 1429), marital status (n = 890), smoking status (n = 1378), alcohol consumption (n = 1472), physical activity (n = 6643), and BMI (n = 2141) were imputed by Rubin's rule for pooling estimates to obtain valid statistical inferences. Statistical analyses were performed by SPSS 25.0 (IBM Corp.) and SAS 9.4 (SAS Institute). Two‐sided P‐values <.05 were considered statistically significant.
3. RESULTS
3.1. Characteristics of the study population
Among 35,312 participants in the study (mean age = 62.8 ± 9.7 years, 54.0% female), 1086 (3.1%) had a history of TBI at baseline. Of them, 285 (26.3%) had TBI at or before 39 years of age, 253 (23.3%) between ages 40 and 49 years, 259 (23.8%) between ages 50 and 59 years, 161 (14.8%) between ages 60 and 69 years, and 128 (11.8%) at the age of 70 years or older.
Compared to TBI‐free individuals, those who experienced TBI at any age were more likely to be male; single; heavy drinkers; and have T2D, heart disease, stroke, or hypertension (Table 1). There were no significant differences between TBI‐free individuals and those with a history of TBI in terms of age, education, smoking status, physical activity, BMI, age of CMD onset, and dyslipidemia.
TABLE 1.
Baseline characteristics of the study population by traumatic brain injury (TBI) status (N = 35,312)
| Characteristics | TBI‐free (n = 34,226) | TBI (n = 1086) | P value |
|---|---|---|---|
| Age (years) | 62.8 ± 9.7 | 63.3 ± 10.6 | .064 |
| Female | 18606 (54.4) | 446 (41.1) | <.001 |
| Education | .252 | ||
| <8 years | 13,200 (40.1) | 383 (38.3) | |
| ≥8 years | 19,684 (59.9) | 616 (61.7) | |
| Marital status | <.001 | ||
| Married/cohabiting | 23,862 (71.5) | 646 (62.9) | |
| Single | 9533 (28.5) | 381 (37.1) | |
| Zygosity | .039 | ||
| Monozygotic | 6791 (19.8) | 199 (18.3) | |
| Dizygotic | 22,884 (66.9) | 715 (65.9) | |
| Undetermined | 4551 (13.3) | 172 (15.8) | |
| Smoking status | .073 | ||
| Never smoked | 16,526 (50.2) | 473 (47.3) | |
| Former/current smoker | 16,408 (49.8) | 527 (52.7) | |
| Alcohol consumption | <.001 | ||
| No/mild drinking | 30,697 (93.4) | 841 (84.8) | |
| Heavy drinking | 2151 (6.6) | 151 (15.2) | |
| Physical active | 13,974 (50.2) | 413 (49.2) | .573 |
| BMI | .827 | ||
| <20.0 (underweight) | 1598 (5.0) | 54 (5.5) | |
| 20.0–24.9 (normal weight) | 15,366 (47.7) | 461 (46.8) | |
| 25.0–29.9 (overweight) | 12,487 (38.8) | 389 (39.5) | |
| ≥30 (obese) | 2735 (8.5) | 81(8.2) | |
| CMDs | 5727 (16.7) | 274 (25.2) | <.001 |
| Type 2 diabetes | 2093 (6.1) | 83 (7.6) | .039 |
| Heart disease | 3399 (9.9) | 159 (14.6) | <.001 |
| Stroke | 1384 (4.0) | 103 (9.5) | <.001 |
| Age of CMD onset (years) | 60.3 ± 13.3 | 60.0 ± 14.0 | .748 |
| Hypertension | 1658 (4.8) | 73 (6.7) | .005 |
| Dyslipidemia | 4279 (12.5) | 129 (11.9) | .540 |
Abbreviations: BMI, body mass index; CMDs, cardiometabolic diseases; SD, standard deviation.
Data were presented as means ± standard deviations or number (%).
3.2. Association of TBI with dementia in unmatched case‐control analyses
During the 18‐year follow‐up period, 3292 (9.3%) participants developed dementia, including 1096 (33.3%) with AD, 692 (21.0%) with VaD, and 1504 (45.7%) with other or unspecified types of dementia. Of the 1086 participants with a history of TBI, 128 developed dementia. The mean time interval between TBI occurrence and dementia diagnosis was 20.3 ± 9.7 years. In the multi‐adjusted GEE model, compared to the TBI‐free participants, the OR (95% CI) of dementia was 1.27 (1.03–1.57) for individuals with a history of TBI at any age, 0.86 (0.43–1.72) for TBI at age ≤39 years, 1.34 (0.81–2.21) for TBI between ages 40 and 49 years, 1.55 (1.04–2.31) for TBI between ages 50 and 59 years, 1.67 (1.12–2.49) for TBI between ages 60 and 69 years, and 0.92 (0.59–1.42) for TBI at age ≥70 years. Regarding dementia subtypes, TBI at any age was not significantly associated with AD (OR 1.23, 95% CI 0.88–1.74) or VaD (OR 1.10, 95% CI 0.73–1.66). However, TBI at the age of 60 to 69 years (OR 1.84, 95% CI 1.01–3.35) was significantly associated with AD (Table 2).
TABLE 2.
Odds ratios (ORs) and 95% confidence intervals (CIs) of dementia in relation to traumatic brain injury (TBI) (N = 35,312)
| All‐cause dementia (n = 3292) | Alzheimer's disease (n = 1096) | Vascular dementia (n = 692) | |||||
|---|---|---|---|---|---|---|---|
| No. of subjects | OR (95% CI) a | OR (95% CI) b | OR (95% CI) a | OR (95% CI) b | OR (95% CI) a | OR (95% CI) b | |
| TBI‐free | 34226 | Reference | Reference | Reference | Reference | Reference | Reference |
| TBI | 1086 | 1.25 (1.01–1.54) | 1.27 (1.03–1.57) | 1.16 (0.83–1.63) | 1.23 (0.88–1.74) | 1.16 (0.77–1.74) | 1.10 (0.73–1.66) |
| Age of first TBI | |||||||
| ≤39 years | 285 | 0.84 (0.42–1.68) | 0.86 (0.43–1.72) | 1.25 (0.54–2.89) | 1.33 (0.57–3.11) | 0.42 (0.06–2.94) | 0.40 (0.06–2.75) |
| 40–49 years | 253 | 1.29 (0.78–2.12) | 1.34 (0.81‐2.21) | 1.05 (0.44–2.48) | 1.12 (0.47–2.66) | 1.70 (0.70–4.13) | 1.65 (0.67–4.04) |
| 50–59 years | 259 | 1.53 (1.03–2.28) | 1.55 (1.04–2.31) | 1.62 (0.86–3.04) | 1.71 (0.91–3.19) | 1.71 (0.80–3.65) | 1.64 (0.76–3.54) |
| 60–69 years | 161 | 1.68 (1.13–2.51) | 1.67 (1.12–2.49) | 1.81 (0.99–3.30) | 1.84 (1.01–3.35) | 1.00 (0.40–2.50) | 1.02 (0.41–2.56) |
| ≥70 years | 128 | 0.89 (0.58–1.37) | 0.92 (0.59–1.42) | 0.44 (0.18–1.08) | 0.47 (0.19–1.18) | 0.97 (0.46–2.08) | 0.88 (0.41–1.89) |
Generalized estimating equation model adjusted for age, sex, and education.
Generalized estimating equation model adjusted for age, sex, education, marital status, smoking status, alcohol consumption, physical activity, body mass index, type 2 diabetes, heart disease, stroke, hypertension, and dyslipidemia.
Compared to TBI‐free participants, those who experienced one TBI had an increased risk of dementia (OR 1.27, 95% CI 1.01–1.61). No statistically significant risk of dementia was observed for participants who experienced two or more TBIs in their lifetime (OR 1.27, 95% CI 0.79–2.04; Table S1 in supporting information). However, these individuals had an increased risk of mortality (OR 1.58, 95% CI 1.11–2.25).
3.3. Joint effect of TBI and CMDs on dementia risk
In joint effect analysis, we excluded participants who had TBI at ≤49 or ≥70 years of age (n = 666), as TBI during these age ranges was not significantly associated with dementia in the previous analyses. This left 34,646 participants who were TBI‐free or who had TBI between ages 50 and 69 years. The multi‐adjusted OR (95% CI) of dementia was 1.00 (0.90–1.11) for participants with CMDs but no TBI, 1.28 (0.88–1.84) for participants with TBI but no CMDs, and 2.47 (1.57–3.87) for those with both TBI and CMDs (reference: TBI‐ and CMD‐free). There was a significant additive interaction (RERI 1.19, 95% CI 0.04–2.33, P = .04; AP 0.48, 95% CI 0.19–0.77, P < .01; Figure 2 and Table S2 in supporting information) and multiplicative interaction (OR 1.93, 95% CI 1.08–3.48; P = .03) between TBI at the age of 50 to 69 years and CMDs on dementia risk.
FIGURE 2.
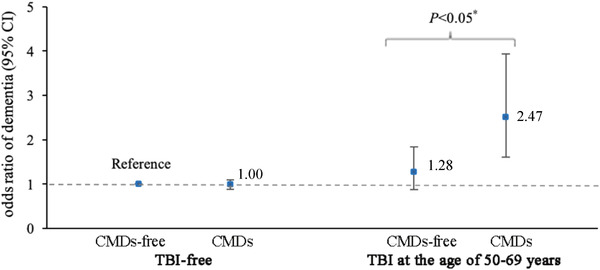
Joint effect of traumatic brain injury (TBI) at the age of 50 to 69 years and cardiometabolic diseases (CMDs) on dementia (reference: TBI‐ and CMD‐free). Note: Multi‐adjusted odds ratios (95% confidence intervals [CIs]) of dementia in relation to joint exposure of TBI at the age of 50 to 69 years and CMDs from generalized estimating equation models (adjusted for age, sex, education, marital status, smoking status, alcohol consumption, physical activity, body mass index, hypertension, and dyslipidemia). * P‐value of <.05 refers to the difference in the risk of dementia between participants with and without CMDs among participants who experienced TBI at the age of 50 to 69 years
3.4. Association between TBI and dementia in co‐twin matched case‐control analysis
In conditional logistic regression conducted among 1478 dementia‐discordant twin pairs, the TBI–dementia association remained significant (OR 1.58, 95% CI 1.02–2.44; Table 3). There was no statistically significant difference between the ORs from the GEE model and the conditional logistic model (OR 0.86, 95% CI 0.62–1.20; P = .37).
TABLE 3.
Odds ratios (ORs) and 95% confidence intervals (CIs) for the association between traumatic brain injury (TBI) and dementia in co‐twin matched case‐control analysis in dementia‐discordant twin pairs (N = 1478)
| Co‐twin with dementia | ||
|---|---|---|
| Co‐twin control | TBI‐free | TBI |
| TBI‐free | 1385 | 53 |
| TBI | 34 | 6 |
| Basic‐adjusted OR (95% CI) a | 1.58 (1.03–2.44) | |
| Multi‐adjusted OR (95% CI) b | 1.58 (1.02–2.44) | |
Conditional logistic regression model adjusted for sex and education.
Conditional logistic regression model adjusted for sex, education, marital status, smoking status, alcohol consumption, physical activity, body mass index, type 2 diabetes, heart disease, stroke, hypertension, and dyslipidemia.
3.5. Supplementary analyses
Similar results to those from the initial analyses were obtained when we performed sensitivity analyses after: (1) excluding participants diagnosed with dementia within 5 years after TBI (Table S3 in supporting information); (2) excluding participants aged <55 years at baseline (Table S4 in supporting information); (3) additionally adjusting for death during the follow‐up, as TBI was significantly related to mortality (OR 1.26, 95% CI 1.07–1.48; Table S5 in supporting information); (4) additionally including TBI cases that occurred during the follow‐up but before dementia diagnosis (n = 2441; Table S6 in supporting information); and (5) excluding participants with stroke that occurred before or within 1 month after TBI (OR 1.32, [95% CI 1.06–1.64] for TBI). Additionally, after classifying TBI according to severity, the multi‐adjusted OR (95% CI) of dementia was 1.29 (1.01–1.66) for participants with mild TBI and 1.33 (0.79–2.23) for participants with moderate‐to‐severity TBI (Table S7 in supporting information).
4. DISCUSSION
In this nationwide population‐based study of Swedish twins, we found that (1) TBI, especially occurring between the ages of 50 and 69 years, was associated with almost 30% increased risk of dementia; (2) the risk of dementia associated with TBI at age 50 to 69 years became stronger among people with CMDs; and (3) genetic and early‐life environmental factors are unlikely to account for the TBI–dementia association.
Several studies have indicated that TBI is associated with an increased risk of dementia or AD. 5 , 28 , 29 Among these, one further identified that TBI patients aged ≥55 and ≥65 years were particularly vulnerable to dementia. 5 However, that study assessed the association between TBI and dementia among participants aged ≥55 years. In the current investigation, we found that TBI occurring between ages 50 and 69 years was associated with increased dementia risk, although TBI at ≤49 or ≥70 years was not associated with dementia. This may be due to greater brain plasticity in individuals who experienced TBI ≤49 years of age, 5 and greater post‐TBI mortality among individuals who experienced TBI ≥70 years of age. 6 , 27 , 30 In addition, we found that TBI occurring between ages 60 and 69 years was related to AD but not VaD, suggesting that neurodegeneration may play a role in the TBI–dementia association. To our knowledge, our study is among the first to use a life‐course approach to investigate the association between TBI and dementia.
Though we hypothesized that dementia risk may be even higher among people who experienced multiple TBIs in their lifetime, there was no significant association between a history of two or more TBIs and dementia. Given that only 220 study participants had two or more TBIs, this could be due to limited statistical power. Another possibility is that the increased mortality among these participants could have led to selective survival.
Previous studies have associated CMDs with an increased risk of dementia. 31 In the present study, we explored the effect modification of CMDs on the TBI–dementia association. We found a significant synergetic effect between CMDs and TBI occurring between ages 50 and 69 years, which suggests that individuals with CMDs who experience TBI at this stage of the life course represent an especially high‐risk group for dementia. Our findings highlight the need to monitor TBI patients with CMDs for early detection of dementia.
Emerging evidence has suggested that genetic and early‐environmental factors are related to the development of TBI and neurodegenerative disease. 15 , 16 , 32 However, to our knowledge, no studies have specifically assessed the role of these unmeasured factors in the TBI–dementia association. In our co‐twin matched case‐control analysis, we found that the TBI–dementia association was not much altered compared to the results from unmatched control analysis, suggesting that genetic and early‐life environmental factors are unlikely to contribute to the association between TBI and dementia.
Several biological mechanisms have been proposed to explain the TBI–dementia association. A head injury can lead to the activation of microglia and the release of oxygen radicals, which might disrupt the blood‐brain barrier, expose the brain to neurotoxins or inflammatory agents, induce chronic neuronal degeneration, and eventually increase individuals’ susceptibility to neurodegenerative diseases such as AD. 33 , 34 , 35 , 36 Additionally, human and animal studies have shown that TBI can induce the accumulation of abnormal proteins associated with neurodegeneration in neuronal cell bodies and injured axons. 36 , 37 , 38 These abnormal proteins—including tau, amyloid beta, and alpha‐synuclein—may trigger progressive neurodegenerative cascades, ultimately leading to progression to dementia. 5 , 39 Another mechanism for the link between TBI and dementia could be reduced cognitive reserve in people with TBI. Some preclinical brain pathologies that were previously insufficient to cause cognitive symptoms could begin to do so after a TBI that impairs the brain's compensatory ability. 40 , 41
This study has several strengths. First, the access to information on lifelong TBI history in the STR allowed for the exploration of the association between TBI over the life course and dementia. Second, the twin study design provided us an opportunity to further explore the role of unmeasured confounders such as genetic and early‐life environmental factors in the observed TBI–dementia association. However, some limitations need to be acknowledged. First, clinical diagnoses were obtained from the NPR, which included records from people who sought treatment in inpatient or outpatient clinics. Therefore, some participants with mild forms of medical conditions, including mild forms of TBI, might have been missed. However, these misclassifications are more likely to be non‐differential, leading to an underestimation of the reported associations. Therefore, caution is needed when comparing our findings to those from other population‐based cohort studies with regular follow‐up examinations. Second, we did not have information on the location of TBI in the brain, which may moderate the impact of TBI on dementia. Third, despite controlling for a range of confounders in our analyses, residual confounding from unmeasured factors—including apolipoprotein E ε4 genotype, the use of intensive care unit care and neurosurgical interventions post‐TBI, and subsequent epilepsy after TBI—could not be ruled out. Finally, dementia and its subtypes were diagnosed clinically without neuroimaging and pathological confirmation. Without this pathological confirmation, the clinical diagnosis of AD could instead represent other types of neurodegenerative disease, such as chronic traumatic encephalopathy.
In conclusion, our study provides further evidence that TBI, especially occurring between ages 50 and 69 years, is associated with a 30% increased risk of dementia, and this risk is exacerbated among individuals with CMDs. Moreover, genetic and early‐life environmental factors are unlikely to account for the TBI–dementia association. Our findings highlight the importance of avoiding brain damage, especially among people with CMDs, to prevent the development of dementia.
CONFLICTS OF INTEREST
The authors report no conflicts of interest.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
We would like to thank the Swedish Twin Registry for access to data and are grateful to all the twins who took part in the study, as well as the members of the survey teams. The Swedish Twin Registry is managed by Karolinska Institutet and receives funding through the Swedish Research Council under grant No. 2017‐00641. W.L.X. received grants from the Swedish Research Council (No. 2017‐00981 and No. 2021‐01647), the Swedish Council for Health Working Life and Welfare (2021‐01826), the National Natural Science Foundation of China (No. 81771519), Alzheimerfonden (2021‐2022), Karolinska Institutet Research Foundation (2020‐01660), Lindhés Advokatbyrå AB (2021‐0134), Stiftelsen För Gamla Tjänarinnor (2021‐2022), and Demensfonden. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of manuscript; and decision to submit the manuscript for publication.
Zhang L, Yang W, Li X, et al. Association of life‐course traumatic brain injury with dementia risk: A nationwide twin study. Alzheimer's Dement. 2023;19:217–225. 10.1002/alz.12671
Kuan‐Yu Pan and Weili Xu contributed equally as last authors.
REFERENCES
- 1. Calsolaro V, Antognoli R, Okoye C, Monzani F. The use of antipsychotic drugs for treating behavioral symptoms in Alzheimer's disease. Front Pharmacol. 2019;10:1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paterno R, Folweiler KA, Cohen AS. Pathophysiology and treatment of memory dysfunction after traumatic brain injury. Curr Neurol Neurosci Rep. 2017;17(7):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lawton T, Huang MX. Dynamic cognitive remediation for a Traumatic Brain Injury (TBI) significantly improves attention, working memory, processing speed, and reading fluency. Restor Neurol Neurosci. 2019;37(1):71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Luca R, Maggio MG, Maresca G, et al. Improving cognitive function after traumatic brain injury: a clinical trial on the potential use of the semi‐immersive virtual reality. Behav Neurol. 2019;2019:9268179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K. Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol. 2014;71(12):1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dams‐O'Connor K, Gibbons LE, Bowen JD, McCurry SM, Larson EB, Crane PK. Risk for late‐life re‐injury, dementia and death among individuals with traumatic brain injury: a population‐based study. J Neurol Neurosurg Psychiatry. 2013;84(2):177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Raj R, Kaprio J, Korja M, Mikkonen ED, Jousilahti P, Siironen J. Risk of hospitalization with neurodegenerative disease after moderate‐to‐severe traumatic brain injury in the working‐age population: a retrospective cohort study using the Finnish national health registries. PLoS Med. 2017;14(7):e1002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mehta KM, Ott A, Kalmijn S, et al. Head trauma and risk of dementia and Alzheimer's disease: the Rotterdam Study. Neurology. 1999;53(9):1959–1962. [DOI] [PubMed] [Google Scholar]
- 9. Senathi‐Raja D, Ponsford J, Schönberger M. Impact of age on long‐term cognitive function after traumatic brain injury. Neuropsychology. 2010;24(3):336–344. [DOI] [PubMed] [Google Scholar]
- 10. Vasquez BP, Tomaszczyk JC, Sharma B, Colella B, Green REA. Longitudinal recovery of executive control functions after moderate‐severe traumatic brain injury: examining trajectories of variability and ex‐gaussian parameters. Neurorehabil Neural Repair. 2018;32(3):191–199. [DOI] [PubMed] [Google Scholar]
- 11. Moretti L, Cristofori I, Weaver SM, Chau A, Portelli JN, Grafman J. Cognitive decline in older adults with a history of traumatic brain injury. Lancet Neurol. 2012;11(12):1103–1112. [DOI] [PubMed] [Google Scholar]
- 12. Wang Z, Marseglia A, Shang Y, Dintica C, Patrone C, Xu W. Leisure activity and social integration mitigate the risk of dementia related to cardiometabolic diseases: a population‐based longitudinal study. Alzheimer's Dement. 2020;16(2):316–325. [DOI] [PubMed] [Google Scholar]
- 13. Driver S, Juengst S, Reynolds M, et al. Healthy lifestyle after traumatic brain injury: a brief narrative. Brain Inj. 2019;33(10):1299–1307. [DOI] [PubMed] [Google Scholar]
- 14. Burke JF, Stulc JL, Skolarus LE, Sears ED, Zahuranec DB, Morgenstern LB. Traumatic brain injury may be an independent risk factor for stroke. Neurology. 2013;81(1):33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sundström A, Nilsson LG, Cruts M, Adolfsson R, Van Broeckhoven C, Nyberg L. Increased risk of dementia following mild head injury for carriers but not for non‐carriers of the APOE epsilon4 allele. Int Psychogeriatr. 2007;19(1):159–165. [DOI] [PubMed] [Google Scholar]
- 16. Lourida I, Hannon E, Littlejohns TJ, et al. Association of lifestyle and genetic risk with incidence of dementia. JAMA. 2019;322(5):430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fazel S, Lichtenstein P, Grann M, Långström N. Risk of violent crime in individuals with epilepsy and traumatic brain injury: a 35‐year Swedish population study. PLoS Med. 2011;8(12):e1001150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Graham DP, Cardon AL. An update on substance use and treatment following traumatic brain injury. Ann NY Acad Sci. 2008;1141:148–162. [DOI] [PubMed] [Google Scholar]
- 19. Lichtenstein P, De Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. J Intern Med. 2002;252(3):184–205. [DOI] [PubMed] [Google Scholar]
- 20. Yang W, Li X, Pan KY, et al. Association of life‐course depression with the risk of dementia in late life: a nationwide twin study. Alzheimer's Dement. 2021;17(8):1383–1390. [DOI] [PubMed] [Google Scholar]
- 21. Li X, Yang R, Yang W, et al. Association of low birth weight with cardiometabolic diseases in Swedish twins: a population‐based cohort study. BMJ open. 2021;11(6):e048030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang R, Pedersen NL, Bao C, et al. Type 2 diabetes in midlife and risk of cerebrovascular disease in late life: a prospective nested case‐control study in a nationwide Swedish twin cohort. Diabetologia. 2019;62(8):1403–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rahman I, Humphreys K, Bennet AM, Ingelsson E, Pedersen NL, Magnusson PK. Clinical depression, antidepressant use and risk of future cardiovascular disease. Eur J Epidemiol. 2013;28(7):589–595. [DOI] [PubMed] [Google Scholar]
- 24. Xu W, Qiu C, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Mid‐ and late‐life diabetes in relation to the risk of dementia: a population‐based twin study. Diabetes. 2009;58(1):71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keenan T, Zhao W, Rasheed A, et al. Causal assessment of serum urate levels in cardiometabolic diseases through a mendelian randomization study. J Am Coll Cardiol. 2016;67(4):407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang W, Li X, Pan KY, et al. Association of life‐course depression with the risk of dementia in late life: a Nationwide Twin Study. Alzheimer's Dement. 2021;17(8):1383–1390. [DOI] [PubMed] [Google Scholar]
- 27. Lystad RP, Cameron CM, Mitchell RJ. Excess mortality among adults hospitalized with traumatic brain injury in australia: a Population‐Based Matched Cohort Study. J Head Trauma Rehabil. 2019;34(3):E1–E9. [DOI] [PubMed] [Google Scholar]
- 28. Hicks AJ, James AC, Spitz G, Ponsford JL. Traumatic brain injury as a risk factor for dementia and Alzheimer disease: critical review of study methodologies. J Neurotrauma. 2019;36(23):3191–219. [DOI] [PubMed] [Google Scholar]
- 29. Xu XJ, Yang MS, Zhang B, Niu F, Dong JQ, Liu BY. Glucose metabolism: a link between traumatic brain injury and Alzheimer's disease. Chin J Traumatol. 2021;24(1):5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cheng PL, Lin HY, Lee YK, Hsu CY, Lee CC, Su YC. Higher mortality rates among the elderly with mild traumatic brain injury: a nationwide cohort study. Scand J Trauma Resusc Emerg Med. 2014;22:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goins RT, Winchester B, Jiang L, et al. Cardiometabolic conditions and all‐cause dementia among American Indian and Alaska native peoples. J Gerontol A, Biol Sci Med Sci. 2021;77(2):323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hayes JP, Logue MW, Sadeh N, et al. Mild traumatic brain injury is associated with reduced cortical thickness in those at risk for Alzheimer's disease. Brain. 2017;140(3):813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Helmy A, De Simoni MG, Guilfoyle MR, Carpenter KL, Hutchinson PJ. Cytokines and innate inflammation in the pathogenesis of human traumatic brain injury. Prog Neurobiol. 2011;95(3):352–372. [DOI] [PubMed] [Google Scholar]
- 34. Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics. 2010;7(4):366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fang F, Chen H, Feldman AL, Kamel F, Ye W, Wirdefeldt K. Head injury and Parkinson's disease: a population‐based study. Mov Disord. 2012;27(13):1632–1635. [DOI] [PubMed] [Google Scholar]
- 36. McKee AC, Daneshvar DH. The neuropathology of traumatic brain injury. Handb Clin Neurol. 2015;127:45–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Uryu K, Chen XH, Martinez D, et al. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp Neurol. 2007;208(2):185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tran HT, LaFerla FM, Holtzman DM, Brody DL. Controlled cortical impact traumatic brain injury in 3xTg‐AD mice causes acute intra‐axonal amyloid‐β accumulation and independently accelerates the development of tau abnormalities. J Neurosci. 2011;31(26):9513–9525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gardner RC, Yaffe K. Traumatic brain injury may increase risk of young onset dementia. Ann Neurol. 2014;75(3):339–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alosco ML, Tripodis Y, Baucom ZH, et al. Late contributions of repetitive head impacts and TBI to depression symptoms and cognition. Neurology. 2020;95(7):e793–e804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alosco ML, Stern RA. The long‐term consequences of repetitive head impacts: chronic traumatic encephalopathy. Handb Clin Neurol. 2019;167:337–355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


