Abstract
Background
Preoperative carbohydrate (CHO) loading improves patient outcomes but is not extensively studied in individuals with diabetes mellitus (DM), resulting in limited professional recommendations. This study examined postprandial glycemic responses and gastric emptying rates following consumption of a CHO drink in adults with and without DM.
Methods
A single‐arm, nonrandomized pilot trial was conducted in adults without DM (non‐DM) (47.5 ± 2.5 years), with pre‐DM (55.8 ± 3.0 years), and with type 2 DM (56.2 ± 2.5 years). Following an overnight fast, participants consumed a 50 g CHO drink followed by 1.5 g liquid paracetamol. Venous blood samples were collected at baseline (ie, t = 0 min) and 15, 30, 45, 60, 90, 120, 150, 180, 210, and 240 min for plasma glucose and serum insulin and paracetamol concentrations to assess gastric emptying.
Results
Participants with DM were older and had a higher body mass index than non‐DM participants (31.2 ± 0.9 vs 28.2 ± 0.9). Fasting glucose and hemoglobin A1c levels differed significantly across groups (non‐DM: 95.4 ± 3.6 mg/dl and 5.2% ± 0.1%; pre‐DM: 111.6 ± 3.6 mg/dl and 5.8% ± 0.1%; DM: 167.4 ± 3.6 mg/dl and 7.2% ± 0.1%). Compared with the non‐DM group, DM had increased glucose responses at 30–180 min. Glucose returned to baseline at 150 min in the non‐DM and pre‐DM groups compared with 210 min in the DM group. Paracetamol concentrations were not significantly different between the non‐DM and DM groups.
Conclusion
Blood glucose returned to baseline within ~2.5 h in non‐DM and pre‐DM groups and ~3.5 h in participants with DM following ingestion of a CHO drink. No consistent differences in gastric emptying rates were observed between participants with and without DM.
Keywords: carbohydrate loading, diabetes mellitus, enhanced recover after surgery, enhanced recovery pathways, gastric emptying, glycemic control
INTRODUCTION
Surgery is a major source of emotional and physiological stress that induces metabolic changes. 1 , 2 Specifically, postoperative insulin resistance develops and contributes to hyperglycemia. 1 , 2 Postoperative hyperglycemia is associated with adverse clinical outcomes, such as increased risk of complications and longer hospital length of stay (LOS). 2 Because of the heightened physiological stress experienced by patients during surgery, many institutions now implement enhanced recovery pathways that aim to optimize perioperative management of surgical patients with the goal of improving clinical outcomes. 3 Of the multimodal perioperative care components, there is an emerging role for nutrition, 4 including the use of preoperative carbohydrate (CHO) loading drinks. Data show that preoperative CHO loading is associated with improved patient well‐being and reduced postoperative insulin resistance. 5 , 6 Currently, the practice of preoperative CHO loading is recommended by multiple professional surgical society guidelines for various surgical specialties. 7 , 8 , 9 , 10
Compared with traditional overnight fasting or a placebo beverage, utilization of preoperative CHO loading in surgical patients with normal glycemia improved preoperative and postoperative subjective well‐being as defined by decreased thirst, hunger, nausea, and vomiting. 11 , 12 , 13 , 14 Data also report that utilization of preoperative CHO loading reduced postoperative insulin resistance. 15 In a 2010 randomized clinical trial, patients undergoing elective colorectal surgery were randomly assigned to receive either a preoperative CHO loading or placebo beverage (ie, flavored water) 3 h before induction of anesthesia or to fast overnight before surgery. At the end of surgery, patients consuming the preoperative CHO beverage had significantly lower homeostatic model assessment for insulin resistance values compared with the other two groups. 15 To date, the practice of preoperative CHO loading for patients with type 2 diabetes mellitus (T2DM) has not been extensively studied. As a result, there is a lack of consensus by professional society guidelines on this practice in patients with DM because of limited evidence and theoretical concerns. Several Enhanced Recovery After Surgery® guidelines acknowledge that more data are needed surrounding the effect of preoperative CHO loading in patients with DM. 7 , 8 , 16 Additionally, preoperative CHO loading is not endorsed in patients with type 1 diabetes. 10 A continued lack of clear direction for surgical patients with DM is problematic, as this number is only expected to increase, with some investigators reporting that current DM prevalence may be as high as 60% among patients undergoing surgery. 2
Concerns over adoption of preoperative CHO loading drinks in patients with DM primarily stems from the risk of perioperative insulin resistance and hyperglycemia, as well as delayed gastric emptying and increased risk for aspiration after induction of anesthesia. 2 , 17 A limited number of studies examined the direct effect of preoperative CHO loading on clinical outcomes in patients with DM. In patients with DM undergoing colorectal surgery, implementation of an enhanced recovery pathway, which included preoperative CHO loading, was associated with significantly reduced rates of postoperative hyperglycemic events and reduced hospital LOS (−2.55 days) when compared with a cohort of patients with DM that underwent surgery before an enhanced recovery pathway implementation. 18 In a separate study in colorectal surgery patients with DM that used a similar design, enhanced recovery pathway implementation, including preoperative CHO loading, was not associated with increased preoperative insulin requirements, rates of glycemic variability, or postoperative complications compared with a control group with DM. 19 However, these data were from retrospective studies, and preoperative CHO loading was included as part of an enhanced recovery pathway that contained other perioperative care components. In patients with controlled DM, compared with patients without DM (non‐DM), ingestion of a preoperative CHO loading drink (12.5% maltodextrin; Nutricia preOp) did not significantly alter gastric emptying rates. 20 This study was not conducted in patients undergoing surgical procedures, and thus no perioperative clinical outcomes were assessed. Despite theoretical concerns and limited data in this patient population, a recent survey of 68 US hospitals with colorectal enhanced recovery programs implementing preoperative CHO loading reported that 80.9% of these institutions administered preoperative CHO loading beverages to patients with DM not taking insulin. 21 Although available data suggest that preoperative CHO loading in patients with DM is not likely to confer negative effects, further clinical studies examining the isolated effect of preoperative CHO loading on patient well‐being and clinical outcomes are warranted to inform professional recommendations in this population.
The goal of the current pilot study was to examine postprandial glycemic responses and gastric emptying rates following consumption of a CHO loading drink in adult participants (not surgical patients) with DM and non‐DM (specifically T2DM). It was hypothesized that gastric emptying rates would not differ between those with DM and those non‐DM and that glucose levels would return to baseline earlier in participants who are non‐DM compared with those with DM. To test this, adults with DM and those who are non‐DM completed a single‐arm, nonrandomized study in which they orally ingested 10 fl oz of a preoperative CHO loading drink containing 50 g CHO. Following ingestion, blood samples were collected to assess postprandial glycemic responses and gastric emptying rates at regular intervals during the 240‐min postprandial period.
MATERIALS AND METHODS
Study participants
The present study was conducted according to the guidelines established in the Declaration of Helsinki, and all procedures involving human patients were approved by the institutional review board (IRB) at each respective study site (Copernicus Group IRB). This study was registered at clinicaltrials. gov (NCT04313920). Written informed consent was obtained from all participants at screening before study enrollment. Participants were not surgical patients and were required to meet the following inclusion criteria: age 18–75 years, body mass index (BMI) >18.5 and ≤40.0 (calculated as weight in kilograms divided by height in meters squared), not pregnant or lactating, regular chronic medication usage (no change for ≥2 months; if any), no usage of dietary supplements during the past 4 weeks, no aversion to ingredients found in the study product, and self‐reported to be free of infection/infectious disease (ie, hepatitis and HIV), cancer, cardiovascular, renal, and noninfectious hepatic disease, bleeding disorders, and no history of gastroparesis. Before enrollment, participant glycemic status (ie, non‐DM, pre‐DM, and DM) was determined by hemoglobin A1c (HbA1c) according to the following criteria by the American Diabetes Association: non‐DM (<5.7%), pre‐DM (≥5.7% to <6.5%), and DM (≥6.5% to <8.0%). Diabetes status was also confirmed by use of oral hypoglycemic medication for at least 2 months before screening. Individuals with type 1 diabetes or those taking insulin or glucagon‐like peptide‐1 inhibitors for glucose control were excluded. Therefore, subsequent use of “DM” in this article refers to T2DM, unless otherwise specified. Participants were instructed to continue all permitted prescribed medications, with the exception that oral hypoglycemic medication was to be withheld on the morning of the study visit.
Study design
This was a single‐arm, nonrandomized clinical trial conducted at three US sites (Health Awareness, Inc; Biofortis; and Great Lakes Clinical Trials) examining postprandial glycemic responses and rates of gastric emptying following consumption of a CHO loading drink. Before visiting the study center, participants were instructed to consume an average of 150 g CHO per day for 3 days prior (verified from self‐reported food records) and to abstain from alcohol and exercise the day before in an effort to control for variability of the normal diet on postprandial effects. Participants visited the study center following an overnight fast (8–14 h). At the study visit, participants ingested 1 bottle (10 fl oz [296 ml] of 50 g complex CHO; 14.9% maltodextrin) of Ensure Presurgery (Abbott Nutrition), immediately followed by 1.5 g liquid paracetamol (ie, acetaminophen; Gericare, Geri‐Care Pharmaceuticals Corp). The study product was given to participants in a nonlabeled container.
Sample collection and laboratory analyses
Venous blood samples were collected from study participants into evacuated tubes containing ethylenediaminetetraacetic acid or sodium heparin before (t = 0 min) and at 15, 30, 45, 60, 90, 120, 150, 180, 210, and 240 min postingestion. Plasma was obtained following centrifugation and analyzed for glucose concentrations (0–240 min), and serum was obtained through natural separation and analyzed for insulin (0–240 min) and paracetamol concentrations (0–180 min). Paracetamol absorption, as assessed by measuring serum concentrations, was utilized as a noninvasive, indirect indicator of postprandial gastric emptying. Plasma glucose and serum insulin and paracetamol were measured using commercially available enzymatic assays according to the manufacturer's instructions (Roche Cobas Integra 400 Plus, Roche Diagnostics).
Subjective appetitive ratings and product liking
Before beverage ingestion and immediately following, as well as at the end of the postprandial period, participants completed a simple 100 mm visual analog scale for thirst, hunger, fullness, and desire to eat. Participants also completed a product assessment questionnaire to determine their liking and taste preference.
Statistical analyses
A primary outcome of interest in the current study was postprandial plasma glucose concentrations, including peak plasma glucose relative to baseline concentrations. A sample size of five per group (non‐DM, pre‐DM, and DM) had 80% power to detect a mean difference of 5.8 mmol/L in peak plasma glucose concentrations, with an SD of 2.5 mmol/L using a two‐group t‐test with a 0.05 two‐sided significance level. To detect mean differences of 8.8 min (with SD = 11 min) in gastric emptying half‐time between groups, a sample size of 90 participants was needed. To account for participant attrition (~10%), 26 evaluable participants per group were needed, and all evaluable participants were included in the final data analysis. There were no adjustments to P values for multiple testing because of the pilot nature of the study and small sample sizes in each study group. Data (least squared means [LSM] ± SE) for plasma glucose and serum insulin are reported as change from baseline values for each postprandial time point relative to concentrations before beverage consumption (ie, t = 0 min). Glucose values were converted from millimoles per liter to milligrams per deciliter (1 mmol/L = 18 mg/dl) for clinical reporting purposes. To determine the time point at which glucose and insulin returned to baseline values, the baseline and postprandial values were compared using paired t tests. The earliest postprandial time point at which glucose and insulin concentrations were not significantly different from the baseline value was considered as the time point when concentrations returned to baseline. Area under the curve (AUC) was calculated using the trapezoidal rule. Categorical variables were analyzed using tests of association (Cochran‐Mantel‐Haenszel tests controlling for site and Fisher exact tests). For comparison between the glycemia groups at baseline, continuous variables were analyzed using analysis of covariance (ANCOVA) with factors for glycemia group and study site. Repeated‐measures ANCOVA with factors for study site, age, glycemia group, gender, BMI, and glycemia group by gender interaction was used for comparisons between the glycemia groups on postprandial timepoints. If the distribution of the variable deviated significantly from normality, nonparametric analysis (Wilcoxon rank sum test) was used to verify the result. For serum paracetamol concentrations, undetectable levels were replaced by half the lower limit of quantification, and the Weibull model was used to fit the response for each participant. All data were analyzed using SAS version 9.4. P < .05 was considered statistically significant.
RESULTS
Participant characteristics
A total number of 335 participants were screened to achieve a target enrollment of 90 adults. Of the 90 adults enrolled in the study, 80 evaluable participants (39 males and 41 females) were included in the final analysis (Table 1). Ten participants violated criteria for evaluability, including one or more missing insulin or glucose values, fasting for >14 h, blood draws outside the sampling windows, not consuming all of the CHO drink, or early dropout from the study. All participants completed the intervention without any major adverse events. Participants were primarily White (73%) and aged ~50 years (54.1 ± 1.5 years) and had fasting plasma glucose and HbA1c values consistent with their respective glycemic group (Table 1). Participants with DM were significantly older and had a higher BMI than those who were non‐DM (P < .05). Fasting glucose and HbA1c levels differed significantly across groups (P < .05) (Table 1). Additionally, there was a significantly greater proportion of males in the DM group compared with the non‐DM and pre‐DM groups (P < .05) (Table 1).
Table 1.
Participant demographics and baseline characteristics
| non‐DM (n = 27) | pre‐DM (n = 28) | DM (n = 25) | |
|---|---|---|---|
| Age (years) | 47.5 ± 2.5a | 55.8 ± 3.0a,b | 56.2 ± 2.5b |
| Gender, n (%) | |||
| Male | 11 (40.7)a | 9 (32.1)a | 19 (76.0)b |
| Female | 16 (59.3)a | 19 (67.9)a | 6 (24.0)b |
| Race, n (%)† | |||
| Asian | 1 (3.7) | 0 (0.0) | 1 (4.0) |
| Black/African American | 7 (25.9) | 3 (10.7) | 6 (24.0) |
| White | 16 (59.3) | 24 (85.7) | 18 (72.0) |
| Other | 3 (11.1) | 1 (3.6) | 0 (0.0) |
| HbA1c | 5.2 ± 0.1a | 5.8 ± 0.1b | 7.2 ± 0.1c |
| Fasting glucose (mg/dl) | 95 ± 3.6a | 112 ± 3.6b | 167 ± 3.6c |
| BMI | 28.2 ± 0.9a | 29.6 ± 1.1a, b | 31.2 ± 0.9b |
Note: Data are reported as the LSM ± SE, and groups not sharing a common superscript are significantly different (P < .05). Age, HbA1c, fasting glucose, and BMI were analyzed using analysis of variance, with effects for glycemia group and site. Gender was analyzed using the Cochran‐Mantel‐Haenszel test, and race was analyzed using Fisher exact test. BMI is calculated as weight in kilograms divided by height in meters squared.
Abbreviations: BMI, body mass index; DM, diabetes mellitus; HbA1c, hemoglobin A1c; LSM, least squared means; non‐DM, without diabetes mellitus; pre‐DM, pre‐diabetes mellitus.
No significant differences between groups.
Glucose and insulin responses
Significant differences were observed between groups for postprandial changes in plasma glucose responses (P < .05) (Figure 1). Compared with the non‐DM group, the pre‐DM group had increased glucose concentrations at 60 min but lower glucose concentrations at 240 min. Additionally, the DM group had increased glucose concentrations at 30–180 min compared with the non‐DM group and at 30–210 min compared with the pre‐DM group. Glucose responses returned to baseline levels at 150 min in the non‐DM and pre‐DM groups compared with 210 min in the DM group. However, AUC did not differ among the groups (P > .05). After adjusting for covariates, peak plasma glucose values did not differ between groups. Additionally, although time to peak values occurred earliest in the non‐DM group, later in the pre‐DM group, and the latest in the DM group, these differences were not statistically significant (P > .05) (Table 2).
Figure 1.
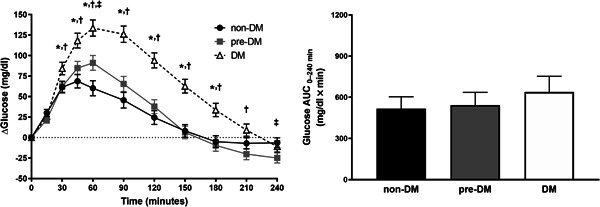
Postprandial changes in plasma glucose responses (left) following CHO drink ingestion by study participants. Data are LSM ± SE and were analyzed using repeated‐measures ANCOVA. AUC (right) for postprandial responses was calculated using the trapezoidal rule; non‐DM (n = 27), pre‐DM (n = 28), and DM (n = 25). *P < .05, non‐DM vs DM; † P < .05, pre‐DM vs DM; ‡ P < .05, non‐DM vs pre‐DM. ANCOVA, analysis of covariance; AUC, area under the curve; CHO, carbohydrate; DM, diabetes mellitus; LSM, least squared means; non‐DM, without diabetes mellitus; pre‐DM, pre‐diabetes mellitus
Table 2.
Peak glucose and insulin values and time to reach peak glucose and insulin value by participant group
| Glucose (mg/dl)† | Insulin (mIU/ml) | |||||
|---|---|---|---|---|---|---|
| non‐DM | pre‐DM | DM | non‐DM | pre‐DM | DM | |
| Peak value | 100.8 ± 10.8 | 115.2 ± 10.8 | 113.4 ± 14.4 | 134.1 ± 13.1a | 88.8 ± 15.0b | 26.6 ± 8.5c |
| Time to peak (min) | 48.3 ± 7.1 | 60.7 ± 7.5 | 74.8 ± 9.3 | 55.6 ± 7.5 | 64.2 ± 8.9 | 77.5 ± 8.2 |
Note: Data are reported as the LSM ± SE and were adjusted for baseline, age, BMI, gender, and site were analyzed using ANCOVA with effects for glycemia group, gender and site; interaction effect for glycemia group with gender and with baseline, age, and BMI as covariates. Groups not sharing a common superscript are significantly different (P < .05).
Abbreviations: DM, diabetes mellitus; LSM, least squared means; non‐DM, without diabetes mellitus; pre‐DM, pre‐diabetes mellitus.
No significant differences between groups.
Serum insulin concentrations differed significantly between groups (P < .05) (Figure 2). Participants with DM had significantly lower serum insulin concentrations at 15–60 min compared with the non‐DM group and at 15–120 min compared with those with pre‐DM. Additionally, insulin concentrations were significantly greater in participants with DM at 180 and 210 min and at 240 min compared with the non‐DM and pre‐DM groups, respectively. Compared with the non‐DM group, participants with pre‐DM had lower insulin concentrations at 15 min but greater insulin concentrations at 90–180 min (P < .05) (Figure 2). Insulin AUC was significantly lower in participants with DM compared with the non‐DM and pre‐DM groups (P < .05) (Figure 2). Insulin responses returned to baseline levels at 180, 210, and 240 min in the non‐DM, pre‐DM, and DM groups, respectively. Peak serum insulin values were significantly lower in participants with DM compared with the non‐DM and pre‐DM groups (P < .05) (Table 2). Time to peak insulin values were similar to those observed for glucose responses; however, these did not significantly differ between the groups after adjusting for covariates (Table 2).
Figure 2.

Postprandial changes in serum insulin responses (left) following CHO drink ingestion by study participants. Data are LSM ± SE and were analyzed using repeated‐measures ANCOVA. AUC (right) for postprandial responses was calculated using the trapezoidal rule, and groups not sharing a common superscript are significantly different (P < .05; non‐DM [n = 27], pre‐DM [n = 28], and DM [n = 25]). *P < .05, non‐DM vs DM; † P < .05, pre‐DM vs DM; ‡ P < .05, non‐DM vs pre‐DM. ANCOVA, analysis of covariance; AUC, area under the curve; CHO, carbohydrate; DM, diabetes mellitus; LSM, least squared means; non‐DM, without diabetes mellitus; pre‐DM, pre‐diabetes mellitus
Paracetamol concentrations
Postprandial mean paracetamol concentrations did not significantly differ between the non‐DM group and the pre‐DM or DM groups. Statistically significant differences in serum paracetamol concentrations were observed between participants with pre‐DM and DM at 45, 60, 150, and 180 min, although the clinical relevance of these differences is unclear (P < .05) (Figure 3).
Figure 3.
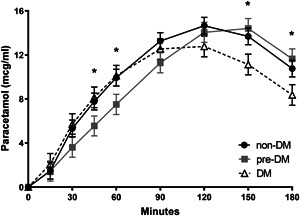
Postprandial mean serum paracetamol responses following CHO drink ingestion by study participants. Responses were fitted using the Weibull model and data are LSM ± SE and were analyzed using repeated‐measures ANCOVA (non‐DM, n = 27; pre‐DM, n = 28; DM, n = 25). *P < .05, DM vs pre‐DM. ANCOVA, analysis of covariance; CHO, carbohydrate; DM, diabetes mellitus; LSM, least squared means; non‐DM, without diabetes mellitus; pre‐DM, pre‐diabetes mellitus
Subjective appetitive responses and product liking
No significant differences in subjective appetitive responses (thirst, hunger, fullness, and desire to eat) were observed between groups at any time point (P > .05) (Figure 4). Overall, ~80% of all participants responded as “liking the product” based on the upper one‐third of responses on a 9‐point scale, as defined by “like extremely,” “like very much,” and “like moderately.” Response rates did not differ significantly between groups (P > .05) (Figure 5).
Figure 4.
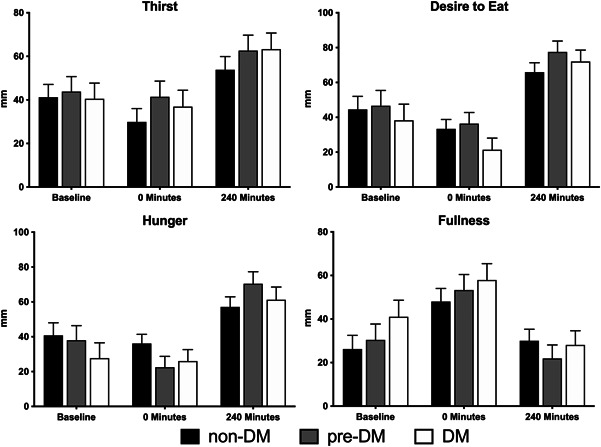
Subjective appetitive responses (thirst, desire to eat, hunger, and fullness) between participants in the non‐DM (n = 27), pre‐DM (n = 28), and DM (n = 25) groups before and following CHO drink ingestion and at the end of the 240‐min postprandial period. No significant differences were observed between the groups (P > .05). CHO, carbohydrate; DM, diabetes mellitus; non‐DM, without diabetes mellitus; pre‐DM, pre‐diabetes mellitus
Figure 5.

Product‐liking responses between participants in the non‐DM (n = 27), pre‐DM (n = 28), and DM (n = 25) groups. No significant differences were observed between the groups (P > .05). DM, diabetes mellitus; non‐DM, without diabetes mellitus; pre‐DM, pre‐diabetes mellitus
DISCUSSION
This study in adult participants without DM, with pre‐DM, or with DM demonstrates that following oral ingestion of a preoperative CHO loading drink, blood glucose responses returned to baseline levels after ~2.5 h in the non‐DM and pre‐DM groups and within ~3.5 h in participants with DM, which is consistent with the original hypothesis. Similar to other available data in participants with DM and those who are non‐DM, it was also hypothesized that gastric emptying rates following CHO drink ingestion would be similar between groups. 20 Despite observing statistically significant differences in paracetamol concentrations between participants with DM and pre‐DM at 45, 60, 150, and 180 min, these differences did not occur consistently throughout the course of the entire postprandial period. Additionally, no significant differences were observed for any postprandial timepoints in the non‐DM group compared with the DM or pre‐DM groups. Therefore, any statistically significant differences in gastric emptying rates between study participants are not likely considered to be clinically relevant, which is consistent with previous findings in the literature. 20
There is hesitancy among clinicians to adopt preoperative CHO loading regimens in patients with DM, which stems from concerns over risk of hyperglycemic events and delayed gastric emptying that outweigh potential benefit, including risk of aspiration. 2 , 17 Blood glucose responses returned to baseline levels earlier in participants who are non‐DM compared with those with DM and is consistent with a previous study. 20 Based on existing literature, 18 , 20 it was also expected that peak glucose values would be greater and occur later in participants with DM compared with non‐DM, but this was not observed. Rather, peak glucose values and time to reach peak values were not significantly different between groups after adjusting for the effects of site, gender, glycemia group with gender interaction, and for covariates age and BMI. Additionally, AUC for glucose responses over the postprandial period did not differ. Taken together, our data suggest that although participants had differing levels of glycemic control based on their health status, glycemic responses following CHO drink ingestion over the entire postprandial period were similar between groups.
The current study measured serum paracetamol concentrations to assess gastric emptying rates. This method is commonly used, as it circumvents radiation exposure, increased costs, and advanced technical expertise associated with scintigraphic methods. 22 , 23 However, despite its common use, we did observe postprandial timepoints whereby serum paracetamol concentrations were below the lower limit of quantitation (LLOQ) of the assay. This may be attributed to differences in sensitivity between commercial enzymatic assays and liquid chromatography and mass spectrometry (LC‐MS) methodologies (ie, LC‐MS/MS). 24 However, LC‐MS/MS methods typically involve more time‐intensive extraction procedures and are more costly, which was not practical in the current study given the large number of samples (~700).
In cases in which samples were below the LLOQ, half the LLOQ was used (ie, 2.5 mcg/ml) and the Weibull model was utilized to fit the postprandial response for each participant. This approach was used to smooth out erratic data patterns and mitigate measurement error. The Weibull distribution is a versatile model for describing nonzero outcomes of various skewness and peakedness (kurtosis) and is widely used in the field of medical science for survival time modeling. 25 Additionally, the Weibull model has been used in human trials similar in nature to the current study 26 and may have advantages over classical tests, such as analysis of variance, for analyzing clinical outcomes, particularly when small sample sizes are present. 27 Therefore, this approach was used in the current study given the small sample size and skewed distribution of the data, and reported paracetamol concentrations are consistent with other reports in the literature. 28 , 29
Because surgery places the body under a tremendous amount of physiological and emotional stress, 1 , 2 preoperative CHO loading beverages are often recommended to support patient well‐being. 8 , 9 , 10 Indeed, several lines of evidence support the use of preoperative CHO loading regimens with improvements such as decreased thirst and hunger. 11 , 12 , 30 However, we did not observe any differences between groups for changes in subjective appetitive ratings before and after CHO drink ingestion. This is not surprising considering all participants in this single‐arm study consumed the beverage and no comparator was used. This is in direct contrast to previous studies in which CHO drink ingestion is compared with a control group, such as overnight fasting and/or a placebo (ie, flavored water), and conducted in patients undergoing surgery. 11 , 12 , 13 , 14 , 15 Participants in the current study were required to have no history of gastroparesis to limit confounding effects on postprandial gastric emptying rates following CHO drink ingestion. Thus, data reported from the current study may not generalize to patients with a known history of gastroparesis. Gastric emptying rate primarily depends on the meal composition and macronutrient content and is less affected by the meal volume. 31 Typical ranges of gastric emptying are ~1–4 kcal/min in healthy individuals. 32 This interindividual variation of gastric emptying rates is broadened in individuals with DM, as there are reports of both delayed and occasionally rapid gastric emptying in this population. 33 In addition to adults with DM and those without, this study also examined postprandial glycemic responses in individuals with pre‐DM, which adds to previous studies that included only adults with DM and non‐DM. Understanding how a CHO loading beverage impacts glycemic responses in individuals with pre‐DM is clinically relevant, as the rate of pre‐DM is expected to increase and, if left untreated, predisposes individuals to a high probability of developing DM in the future. 34 As individuals with DM are more likely to require surgical‐related procedures and are at greater risk for complications, 35 it would be expected that adults with pre‐DM are also at increased risk. There are several additional limitations to this pilot study. Study participants were medically stable and predominantly White and were not undergoing surgery. Therefore, a multimodal enhanced recovery pathway was not implemented including full nutrition and nonnutrition components and timing. Although not addressed by the current study, future studies should seek to examine the potential benefits of preoperative CHO loading in surgical patients with pre‐DM to equip clinicians with data to provide better guidance to patients with pre‐DM surrounding the use of preoperative CHO loading regimens. 36
CONCLUSION
Blood glucose responses returned to baseline levels within ~2.5 h in participants with non‐DM and pre‐DM and within ~3.5 h in participants with DM following ingestion of CHO drink. Additionally, peak and time to reach peak glucose values were not significantly different between participants with DM and non‐DM. Furthermore, there were no consistent differences in gastric emptying rates between participants with DM and non‐DM, although paracetamol concentrations were significantly different at select timepoints. Data from the current study help to fill gaps in the literature and may help guide and support utilization and extended timing of preoperative CHO loading drinks for adults with DM as well as pre‐DM; current guidelines recommend that CHO drinks can be consumed up to 2 h before induction of anesthesia in patients who are non‐DM. 8 , 9 , 10 However, further high‐quality randomized clinical studies are needed in surgical patients to enable interpretation and translation into clinical practice. This evidence will inform future recommendations from professional societies regarding the practice and timing of preoperative CHO loading in adult surgical patients with pre‐DM and DM.
CONFLICT OF INTERESTS
Bridget A. Cassady and Geraldine E. Baggs are employees and stockholders. Joshua D. McDonald was a contract scientist at Abbott Nutrition during the completion of this study. Menaka Yalawar is a current contract scientist at Abbott Nutrition. Kevin C. Maki is the Chief Scientist of Midwest Biomedical Research, which received a fee for service from Abbott Nutrition for conducting participant visits, and served as a consultant on the study.
AUTHOR CONTRIBUTIONS
Bridget A. Cassady and Joshua D. McDonald contributed to the conception and design of the research and drafted the manuscript; Kevin C. Maki contributed to the acquisition of the data; and Menaka Yalawar and Geraldine E. Baggs contributed to the analysis of the data. All authors contributed to the interpretation of the data, critically reviewed and revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors wish to thank the study sites, participants, and project team, including Dr Satya Jonnalagadda, Kathleen Thrush, and Sonja Acosta, for their assistance with coordinating and conducting the intervention phase of the study. This study was supported by Abbott Laboratories.
Cassady BA, McDonald JD, Yalawar M, Baggs GE, Maki KC. Pilot study on the impact of a carbohydrate loading drink on postprandial glycemic responses and gastric emptying in adults with prediabetes and type 2 diabetes mellitus. Nutr Clin Pract. 2023;38:108‐117. 10.1002/ncp.10845
REFERENCES
- 1. Gillis C, Carli F. Promoting perioperative metabolic and nutritional care. Anesthesiology. 2015;123(6):1455‐1472. [DOI] [PubMed] [Google Scholar]
- 2. Duggan EW, Carlson K, Umpierrez GE. Perioperative hyperglycemia management: an update. Anesthesiology. 2017;126(3):547‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152(3):292‐298. [DOI] [PubMed] [Google Scholar]
- 4. Gillis C, Wischmeyer PE. Pre‐operative nutrition and the elective surgical patient: why, how and what? Anaesthesia. 2019;74(Suppl 1):27‐35. [DOI] [PubMed] [Google Scholar]
- 5. Amer MA, Smith MD, Herbison GP, Plank LD, McCall JL. Network meta‐analysis of the effect of preoperative carbohydrate loading on recovery after elective surgery. Br J Surg. 2017;104(3):187‐197. [DOI] [PubMed] [Google Scholar]
- 6. Bilku DK, Dennison AR, Hall TC, Metcalfe MS, Garcea G. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl. 2014;96(1):15‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Batchelor TJP, Rasburn NJ, Abdelnour‐Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardio‐Thorac Surg. 2019;55(1):91‐115. [DOI] [PubMed] [Google Scholar]
- 8. Gustafsson UO, Scott MJ, Hubner M, et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations: 2018. World J Surg. 2019;43(3):659‐695. [DOI] [PubMed] [Google Scholar]
- 9. Weimann A, Braga M, Carli F, et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr. 2017;36(3):623‐650. [DOI] [PubMed] [Google Scholar]
- 10. Wischmeyer PE, Carli F, Evans DC, et al. American Society for Enhanced Recovery and Perioperative Quality initiative joint consensus statement on nutrition screening and therapy within a surgical enhanced recovery pathway. Anesth Analg. 2018;126(6):1883‐1895. [DOI] [PubMed] [Google Scholar]
- 11. Hausel J, Nygren J, Lagerkranser M, et al. A carbohydrate‐rich drink reduces preoperative discomfort in elective surgery patients. Anesth Analg. 2001;93(5):1344‐1350. [DOI] [PubMed] [Google Scholar]
- 12. Canbay Ö, Adar S, Karagöz AH, Çelebi N, Bilen CY. Effect of preoperative consumption of high carbohydrate drink (Pre‐Op) on postoperative metabolic stress reaction in patients undergoing radical prostatectomy. Int Urol Nephrol. 2014;46(7):1329‐1333. [DOI] [PubMed] [Google Scholar]
- 13. Hausel J, Nygren J, Thorell A, Lagerkranser M, Ljungqvist O. Randomized clinical trial of the effects of oral preoperative carbohydrates on postoperative nausea and vomiting after laparoscopic cholecystectomy. Br J Surg. 2005;92(4):415‐421. [DOI] [PubMed] [Google Scholar]
- 14. Singh BN, Dahiya D, Bagaria D, et al. Effects of preoperative carbohydrates drinks on immediate postoperative outcome after day care laparoscopic cholecystectomy. Surg Endosc. 2015;29(11):3267‐3272. [DOI] [PubMed] [Google Scholar]
- 15. Wang ZG, Wang Q, Wang WJ, Qin HL. Randomized clinical trial to compare the effects of preoperative oral carbohydrate versus placebo on insulin resistance after colorectal surgery. Br J Surg. 2010;97(3):317‐327. [DOI] [PubMed] [Google Scholar]
- 16. Nelson G, Bakkum‐Gamez J, Kalogera E, et al. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery After Surgery (ERAS) Society recommendations‐2019 update. Int J Gynecol Cancer. 2019;29(4):651‐668. [DOI] [PubMed] [Google Scholar]
- 17. Bharucha AE, Kudva YC, Prichard DO. Diabetic gastroparesis. Endocr Rev. 2019;40(5):1318‐1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cua S, Humeidan M, Beal EW, et al. The effect of an enhanced recovery protocol on colorectal surgery patients with diabetes. J Surg Res. 2021;257:153‐160. [DOI] [PubMed] [Google Scholar]
- 19. Talutis SD, Lee SY, Cheng D, Rosenkranz P, Alexanian SM, McAneny D. The impact of preoperative carbohydrate loading on patients with type II diabetes in an enhanced recovery after surgery protocol. Am J Surg. 2020;220(4):999‐1003. [DOI] [PubMed] [Google Scholar]
- 20. Gustafsson UO, Nygren J, Thorell A, et al. Pre‐operative carbohydrate loading may be used in type 2 diabetes patients. Acta Anaesthesiol Scand. 2008;52(7):946‐951. [DOI] [PubMed] [Google Scholar]
- 21. Näslund E, Bogefors J, Grybäck P, Jacobsson H, Hellström PM. Gastric emptying: comparison of scintigraphic, polyethylene glycol dilution, and paracetamol tracer assessment techniques. Scand J Gastroenterol. 2000;35(4):375‐379. [DOI] [PubMed] [Google Scholar]
- 22. Willems M, Quartero AO, Numans ME. How useful is paracetamol absorption as a marker of gastric emptying? A systematic literature study. Dig Dis Sci. 2001;46(10):2256‐2262. [DOI] [PubMed] [Google Scholar]
- 23. Tan Q, Zhu R, Li H, Wang F, Yan M, Dai L. Simultaneous quantitative determination of paracetamol and its glucuronide conjugate in human plasma and urine by liquid chromatography coupled to electrospray tandem mass spectrometry: application to a clinical pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;893‐894:162‐167. [DOI] [PubMed] [Google Scholar]
- 24. Lai C‐D, Murthy DN, Xie M. Weibull distributions and their applications. In: Pham H, ed., Springer handbook of engineering statistics. Springer; 2006:63‐78. 10.1007/978-1-84628-288-1_3 [DOI] [Google Scholar]
- 25. Schuring E, Quadt F, Kovacs EMR, Meullenet J‐F, Wiseman S, Mela DJ. A quantitative method for estimating and comparing the duration of human satiety responses: statistical modeling and application to liquid meal replacers. Appetite. 2012;59(2):601‐609. [DOI] [PubMed] [Google Scholar]
- 26. McCrum WR, Sharp JT, Bluhm GB. Use of the Weibull distribution for analysis of a clinical therapeutic study in rheumatoid arthritis. Henry Ford Hosp Med J. 1976;24(3):173‐182. [Google Scholar]
- 27. Blom WAM, Lluch A, Vinoy S, et al. Effects of gastric emptying on the postprandial ghrelin response. Am J Physiol Endocrinol Metab. 2006;290(2):E389‐E395. [DOI] [PubMed] [Google Scholar]
- 28. Cassady BA, Considine RV, Mattes RD. Beverage consumption, appetite, and energy intake: what did you expect? Am J Clin Nutr. 2012;95(3):587‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rizvanović N, Nesek Adam V, Čaušević S, Dervišević S, Delibegović S. A randomised controlled study of preoperative oral carbohydrate loading versus fasting in patients undergoing colorectal surgery. Int J Colorectal Dis. 2019;34(9):1551‐1561. [DOI] [PubMed] [Google Scholar]
- 30. Hostalek U. Global epidemiology of prediabetes ‐ present and future perspectives. Clin Diabetes Endocrinol. 2019;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Phillips LK, Deane AM, Jones KL, Rayner CK, Horowitz M. Gastric emptying and glycaemia in health and diabetes mellitus. Nat Rev Endocrinol. 2015;Feb 11(2):112‐128. [DOI] [PubMed] [Google Scholar]
- 32. Brener W, Hendrix TR, McHugh PR. Regulation of the gastric emptying of glucose. Gastroenterology. 1983;85(1):76‐82. [PubMed] [Google Scholar]
- 33. Schwartz JG, Green GM, Guan D, McMahan CA, Phillips WT. Rapid gastric emptying of a solid pancake meal in type II diabetic patients. Diabetes Care. 1996;19(5):468‐471. [DOI] [PubMed] [Google Scholar]
- 34. Albalawi Z, Laffin M, Gramlich L, Senior P, McAlister FA. Enhanced Recovery After Surgery (ERAS®) in individuals with diabetes: a systematic review. World J Surg. 2017;41(8):1927‐1934. [DOI] [PubMed] [Google Scholar]
- 35. Wang J, Chen K, Li X, et al. Postoperative adverse events in patients with diabetes undergoing orthopedic and general surgery. Medicine (Baltimore). 2019;98(14):e15089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Robinson KN, Cassady BA, Hegazi RA, Wischmeyer PE. Preoperative carbohydrate loading in surgical patients with type 2 diabetes: Are concerns supported by data? Clin Nutr ESPEN. 2021;Oct 45:1‐8. [DOI] [PubMed] [Google Scholar]


