Abstract
Climate change‐related environmental stress has been recognized as a driving force in accelerating forest mortality over the last decades in Central Europe. Here, we aim to elucidate the thermal sensitivity of three native conifer species, namely Norway spruce (Picea abies), Scots pine (Pinus sylvestris) and silver fir (Abies alba), and three non‐native species, namely Austrian pine (Pinus nigra), Douglas fir (Pseudotsuga menziesii) and Atlas cedar (Cedrus atlantica).
Thermal sensitivity, defined here as a decline of the maximum quantum yield of photosystem II (Fv/Fm) with increasing temperature, was measured under varying levels of heat stress and compared with the turgor loss point (πtlp) as a drought resistance trait. We calculated three different leaf thermotolerance traits: the temperature at the onset (5%) of the Fv/Fm decline (T5), the temperature at which Fv/Fm was half the maximum value (T50) and the temperature at which only 5% Fv/Fm remained (T95).
T5 ranged from 38.5 ± 0.8 °C to 43.1 ± 0.6 °C across all species, while T50 values were at least 9 to 11 degrees above the maximum air temperatures on record for all species. Only Austrian pine had a notably higher T5 value than recorded maximum air temperatures. Species with higher T5 values were characterized by a less negative πtlp compared to species with lower T5.
The six species could be divided into ‘drought‐tolerant heat‐sensitive’ and ‘drought‐sensitive heat‐tolerant’ groups. Exposure to short‐term high temperatures thus exhibits a considerable threat to conifer species in Central European forest production systems.
Keywords: Conifers, heat stress, physiological limitations, tree mortality, water stress
Temperatures recorded during heat waves in Central Europe exceed the thermal tolerance threshold of native and non‐native conifer species.

INTRODUCTION
Forest disturbance is a natural part of forest ecosystem dynamics (Seidl et al. 2017), but increasing climate‐induced tree mortality has been observed in a wide range of forest ecosystems worldwide (Allen et al. 2010; Cobb et al. 2017). In European temperate forests, mortality of canopy trees has doubled over the last three decades (Senf et al. 2018) and has been primarily linked to climate‐induced drought (Senf et al. 2020). Tree mortality results in changes to ecological communities, shifts in ecosystem functions with a reduction in ecosystem services, together with unexpected land–climate feedbacks (Anderegg et al. 2013). A mechanistic understanding of tree mortality is crucial to lessen economic and cultural harm (Allen et al. 2010) and sustain forest production systems. The predicted increases in frequency and intensity of temperature extremes (heatwaves) are unequivocally linked to climate change and occur independently of drought events (IPCC, 2019). However, heatwaves can cause drought stress due to high potential evapotranspiration in combination with little or no rainfall. The duration and intensity of heatwaves can seriously impact the physiological performance of trees (Billon et al. 2020), some of which are important timber trees.
Over centuries, Norway spruce and Scots pine have been the most important timber species for the forestry sector in Central Europe. Large‐scale wind disturbance and associated bark beetle outbreaks have been historically major disturbances in forest production systems consisting mainly of Norway spruce (Eriksson et al. 2007). However, the increasing frequency and longer duration of heatwaves has favoured bark beetle infestation and replaced windthrow as the main cause of mortality (Hentschel et al. 2014). Although Scots pine has been considered relatively drought‐tolerant compared to e.g., Norway spruce, an accelerated mortality of Scots pine has been observed in recent years. Besides Norway spruce and Scots pine, other species in Europe are expected to be impacted by the expected rise in drought and heatwaves in future climate change scenarios (irrespective of the actual cause of tree mortality) (Hartmann et al. 2018).
Several advantages of conifers over broadleaved tree species, e.g., optimal mechanical wood properties, potentially faster growth rates, larger carbon sink per area and increased economic yield, means forest owners would, in the future, preferably plant a drought‐adapted conifer species. Consequently, the native silver fir has in recent years been planted in increasing numbers across Central Europe, aiming to achieve higher drought resistance in forest stands (Muck et al. 2008). Likewise, the substitution of former Norway spruce and Scots pine forests by non‐native drought‐tolerant conifer species or the intermixing of these with native species became common practice (Bolte et al. 2009). The three non‐native species, Douglas fir, Austrian pine or Atlas cedar, have been considered as potentially suitable alternatives in forest production systems under a future climate (De Avila & Albrecht 2018). However, criticism arises on admixing non‐native species due to their potentially invasive character and potential detrimental impact on forest ecosystems (Pötzelsberger et al. 2020). Also, it remains unknown how such species will perform under a changing climate.
The actual drivers and mechanisms causing tree mortality are still under debate and are most probably a result of complex interdependencies of mutually inclusive mechanics (Hajek et al. 2020). Most studies on tree mortality focus predominantly on drought stress as the initial trigger of tree mortality (e.g., Pretzsch et al. 2020), neglecting heat‐induced changes in morphological, physiological and biochemical processes, although these do affect the overall performance, growth and ultimately the survival of plants (Song et al. 2014). However, there is growing evidence that heat‐induced tree mortality can cause abrupt changes in forest biomass stocks (Chaste et al. 2019; Breshears et al. 2021), which suggests a limited ability of trees to locally acclimate to higher temperatures (Konôpková et al. 2018). Drought‐induced effects on forest performance could potentially be mitigated by the application of silvicultural practices, such as stand density reduction (Sohn et al. 2013), which in turn reduces the canopy cover, negatively affecting the forest understorey during warm periods (von Arx et al. 2012). Thus, short‐term management options to increase forest resistance to heat events are even more limited than forest management options to increase forest drought resistance (Burschel & Huss 2003). Therefore, a better understanding of temperature thresholds that trigger forest decline and mortality is necessary for choosing suitable species for future forest production systems.
Photosystem II (PSII) is a pigment–protein complex located in the thylakoid membranes of the chloroplasts and is considered the most heat‐sensitive component of photosynthesis (Ashraf & Harris 2013), with temperatures above a critical value leading to irreversible damage to the photochemistry (Tiwari et al, 2021; Slot et al. 2021). One approach to quantify thermotolerance is to analyse the effect of extreme temperatures on the photochemistry of the leaves. A quantitative measure of the photochemical efficiency of PSII is the ratio between variable and maximum chlorophyll fluorescence (Fv/Fm), which is a good indicator of drought and heat stress (Yu & Guy 2004). The temperature at which the quantum efficiency declines by 50% (T50) has been used as a proxy for the critical temperature at which irreversible damage to the photochemistry occurs (Krause et al. 2010; Tiwari et al. 2021). Data on T50 values are predominantly available from subtropical and tropical regions (Sastry et al. 2017; Leon‐Garcia & Lasso 2019; Perez & Feeley 2020), where trees have adapted to elevated temperatures. However, tree species from mid‐latitudes are expected to have a very narrow thermal tolerance range and thus may be more susceptible to heatwaves (O'Sullivan et al. 2017).
In this study, we used the aforementioned temperature‐induced changes in Fv/Fm to assess thermal tolerance for three conifer species native to Central Europe and three that are potentially or increasingly being planted for future forest production systems. We aimed to address the following questions: (i) will current summer temperatures exceed the thermal thresholds of conifer species, and (ii) how will their thermal tolerance be related to hydraulic leaf properties, namely turgor loss point? The overall goal was to provide a first assessment on thermo‐tolerance of the economically most important conifer species and their suitability in forest production systems under a changing climate—in particular under the threat of the increasing frequency of heatwaves in Central Europe.
MATERIAL AND METHODS
Material from native and non‐native conifers was collected in the surroundings of Sichersdorf, in the rural district of Fürth in Middle Franconia, Germany (49°24'04.4" N 10°56'01.3" E). The district is characterized by very patchy forest distribution, with approximately 30% of the area stocked with forest. Scots pine (Pinus sylvestris L.) has been the main species cultivated in the area, followed by Norway spruce (Picea abies Karst.) and European oak (Quercus robur L.). A few autochthonous silver fir (Abies alba L.) are scattered sparsely throughout those forests. The region has been affected by high tree mortality in recent years. The mortality increased after two very dry summers combined with heatwaves in 2015 and 2016, and accelerated during the following dry and very hot conditions in 2019 and 2020. The non‐native conifer species selected for this study, i.e., Atlas cedar (Cedrus atlantica ‘Glauca group’ (Endl.) G. Manetti ex Carrière) and Douglas fir (Pseudotsuga menziesii (Mirbel) Franco), are commonly grown in urban areas in the district and the botanical material was collected in the adjacent township. Austrian pine (Pinus nigra sensu lato J.F. Arnold) was collected from a forest stand on a former military training ground planted by the US military in the 1970s (49°25'16.6" N, 10°59'47.9" E). For each species, one sun‐exposed branch was sampled from five individuals in early spring 2021. In the forests, we targeted dominant canopy trees, and a minimum diameter at breast height (DBH) of 25 cm. Per individual, needles from 1‐year‐old shoots were collected. Needles were dark‐acclimated for 30 min and tested for initial maximum photosynthetic efficiency (Fv/Fm) to ensure leaf health (Fv/Fm between 0.83 and 0.75) with a chlorophyll fluorometer (MINI‐PAM; Walz, Effeltrich, Germany). Thermal dependence of Fv/Fm was assessed following the protocol of Krause et al. (2010). Therefore, needles were placed in Microcloth, separated by cloth layers to prevent anoxic conditions, and transferred into watertight Whirlpack bags. The bags were bathed under water in a precision cooker at varying temperatures for 15 min. The exact temperature was monitored with a digital thermometer (TFA; Dostmann, Wertheim, Germany). The mean temperature during the vegetation period in the region is 15° C (DWD 2021), hence we used 15° C as starting temperature. Temperature treatments covered the range between 15 °C and 59 °C. We started with 5‐degree steps between treatments and lower steps of 2 degrees within the critical temperature range, starting at 30 °C. Per individual, about eight needles were selected and each needle was randomly assigned to a temperature treatment. Overall, 40 needles were used to establish one curve. Heat‐treated needles were incubated under controlled conditions (15 °C, ~20 μmol m−2 s−1 light) in Petri dishes containing a thin film of water (Tiwari et al. 2021). The next day, the recovery of Fv/Fm was measured after a 30‐min dark adaptation period. We used the data on turgor loss point collected by Kunert & Tomsakova (2020) to compare the thermal tolerance traits with hydraulic properties. Thermal and hydraulic traits were sampled on the same tree individuals. We used air temperature data available on the data platform of the Deutscher Wetterdienst (station number 3668; DWD, 2021) to compare the increase in frequency of hot days.
After correcting for a typographical error, we used the same log‐logistic curve for the Fv/Fm response as described by Tiwari et al. (2021):
| (1) |
where T represents the temperature, c is the Fv/Fm of the lower plateau, d is the higher plateau, T50 is the temperature at which Fv/Fm reached 50% of the total decrease, b is the slope of the curve at T = T50. Because our initial results showed clearly asymmetric curves for some species, we also used an extension of Equation (1) proposed by Ricketts & Head (1999) allowing for a difference in curvature before and after T = T50:
| (2) |
where b1 and b2 now represent a possibly different curvature before and after T50. Both response curves were fitted using the ‘modelFit’ function of the ‘drc’ package using the LL.4 (Equation (1)) and baro5 (Equation (2)) methods (Ritz et al. 2016) in R. Akaike’s information criterion was used to decide between Equation (1) and Equation (2). Half of the species’ thermal Fv/Fm decline could be described by the four‐parameter logistic curve (Equation (1); Scots pine, Austrian pine and Douglas fir), whereas the best fitting model for the other half was a five‐parameter asymmetric logistic (Equation (2)) function (silver fir, Norway spruce and Atlas cedar) (see Figs 1 and 2). Five different measures were extracted from the fitted curves to compare different species. The first three measures (T5, T50, T95) correspond to temperatures at which the change in Fv/Fm is 5, 50 or 95% of the maximum change (c–d). We fitted a simpler model to species pairs to compare the deviated from the species‐specific dose–response curves with an approximate F‐test (Ritz et al. 2016). In cases where the null hypothesis was rejected, the ‘compParm’ function was used to identify species differences in T5, T50 and T95. Analogous to Tiwari et al. (2021), we also defined two decline widths for the temperature difference between different Fv/Fm levels: before the turning point (T50): T50–T5, and after the turning point: T95–T50. These decline widths are inversely related to the slope parameters b, b1 and b2 , with smaller values indicating more rapid changes in Fv/Fm. All presented means are ± SE. Data analysis was performed using the R program, version 4.0.4 (R Core Team, 2021).
Fig. 1.
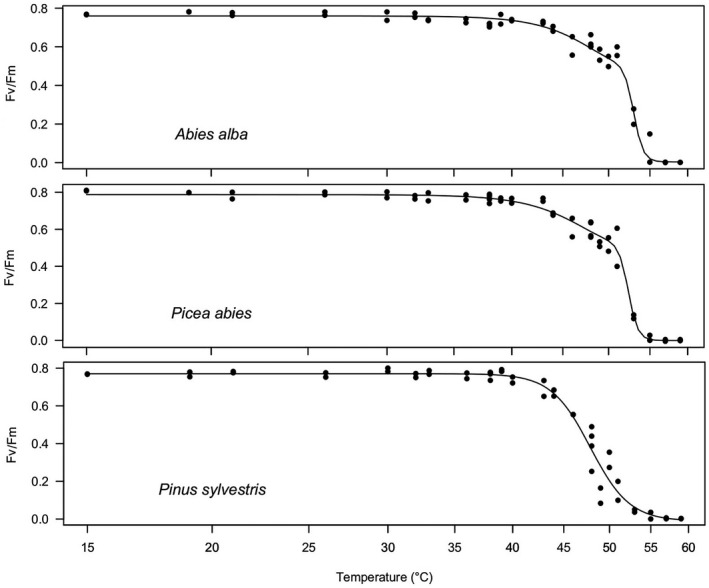
Temperature response of PSII efficiency (Fv/Fm) to 15‐min duration heat treatment of needles of three native conifer species. Top panel: Silver fir (Abies alba); middle panel: Norway spruce (Picea abies); bottom panel: Scots pine (Pinus sylvestris). Five individuals per species were measured to establish the thermal vulnerability curve.
Fig. 2.
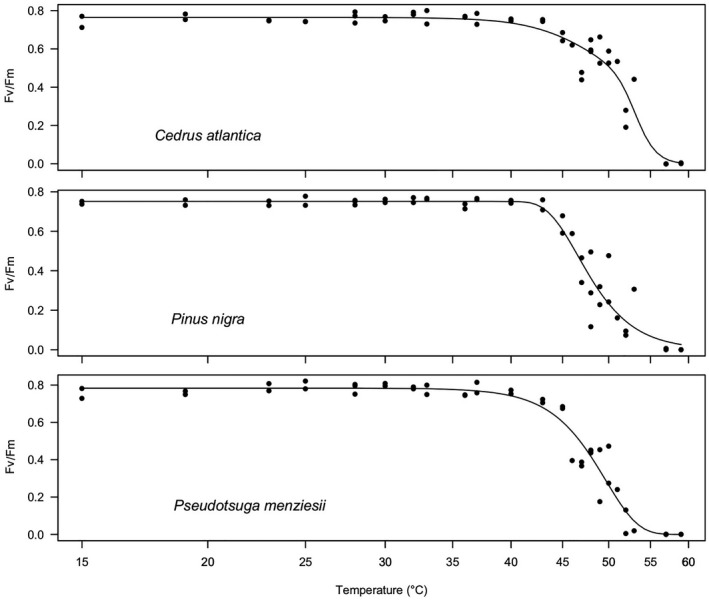
Temperature response of PSII efficiency (Fv/Fm) to 15‐min duration heat treatment of needles of the three exotic conifer species. Top panel: Atlas cedar (Cedrus atlantica); middle panel: Austrian pine (Pinus nigra); bottom panel Douglas fir (Pseudotsuga menziesii). Five individuals per species were measured to establish the thermal vulnerability curve.
RESULTS
The T5 was on average at 40.3 ± 0.7 °C across all six conifer species. Austrian pine had the highest T5 (43.1 ± 0.6 °C) and Norway spruce the lowest T5 (38.5 ± 0.8 °C). T5 of Norway spruce was reached by the maximum air temperature recorded in the area (Fig. 3). T50 was on average 50.0 ± 0.9 °C, and silver fir had the highest T50 (52.3 ± 0.2 °C) of all species, followed by Atlas cedar (51.8 ± 0.4 °C). The two pine species had both very low T50 values of 47.8 ± 0.3 °C. T95 averaged 55.2 ± 0.6 °C across all species and was highest in Austrian pine (57.5 ± 1.4 °C) and lowest in Norway spruce and Douglas fir (both 53.9 °C; for ±SE, see Table 1).
Fig. 3.
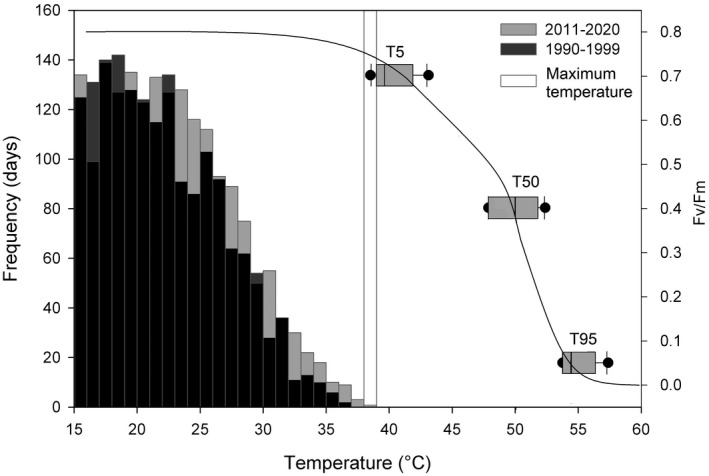
Thermal tolerance of conifer needles for six species represented by a boxplot diagram (note: boxplot y‐ordinate positioning indicative and not absolute, the logistic curve indicates combined fit for all six species). Thermal tolerance was measured as Fv/Fm temperature response to 15‐min duration heat treatment. The histogram highlights the warming trend in daily maximum air temperature relative to the two 10‐year periods between 1990–1999 and 2011–2020. Black bars are difference in frequency between the two periods. Maximum temperature represents the temperature record observed in the region as recorded in 2020.
Table 1.
Summary of the measured thermal tolerance for the six European conifer species.
| Common name | Latin name | Native/introduced | T5 | T50 | T95 |
|---|---|---|---|---|---|
| °C ± SE | °C ± SE | °C ± SE | |||
| Silver fir | Abies alba | Native | 39.5 ± 1.0a | 52.3 ± 0.2a | 54.6 ± 0.7a |
| Norway spruce | Picea abies | Native | 38.5 ± 0.8a | 51.6 ± 0.2a | 53.9 ± 0.2a |
| Scots pine | Pinus sylvestris | Native | 41.9 ± 0.7b | 47.8 ± 0.3b | 54.6 ± 1.0b |
| Douglas fir | Pseudotsuga menziesii | Non‐native | 39.8 ± 1.0a | 48.3 ± 0.3a | 53.9 ± 0.6a |
| Austrian pine | Pinus nigra | Non‐native* | 43.1 ± 0.6b | 47.8 ± 0.3b | 57.5 ± 1.4b |
| Atlas cedar | Cedrus atlantica | Non‐native | 39.0 ± 1.6a | 51.8 ± 0.4a | 56.5 ± 1.2a |
| Mean | 40.3 ± 0.7 | 50.0 ± 0.9 | 55.2 ± 0.6 |
Breaking point temperature at which PSII efficiency declines 5% (T5), temperature at which efficiency is at 50% (T50) of the maximum, and temperature at which only 5% of the maximum efficiency remains (T95). Superscript letters indicate significant differences between species (z‐test).
Austrian pine is native to Central Europe, but does not naturally occur in the study area.
The T5 and decline width (T95–T5) were not significantly related (r2 = 0.385. P = 0.188; Fig. 4a). However, T5 correlated significantly with the decline width before the turning point (T50–T5) and after the turning point (T95–T50) (see Fig. 4b and c). Species with lower T5 were characterized by a wider decline width from T50–T5. Species—namely the two pine species—characterized by a high breakpoint temperature show sudden and steeper Fv/Fm decline over a narrow decline width.
Fig. 4.
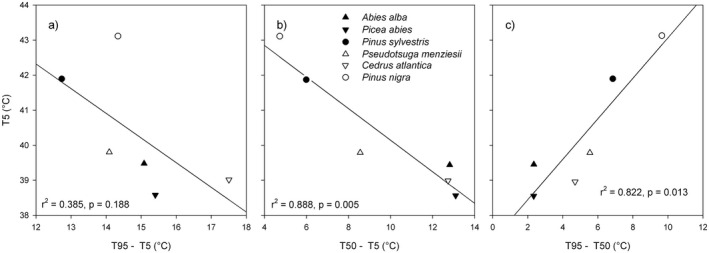
Relationships between PSII maximum quantum yield (Fv/Fm) breakpoint temperature, T5, and decline width. The temperature windows are shown when Fv/Fm declines from (a) 95% to 5% of the maximum Fv/Fm level (T95–T5), (b), the Fv/Fm decline from 5% to 50% of the maximum Fv/Fm level, and (c) Fv/Fm declines from 50% to 95% of the maximum Fv/Fm level.
The T5 values of the six species correlated significantly with the turgor loss point (πtlp; Fig. 5a). Species with a higher T5 were characterized by a less negative πtlp, and species with a lower T5 by a more negative πtlp. There was no significant relationship between T50 and πtlp, nor T95 and πtlp (Fig. 5b and c).
Fig. 5.
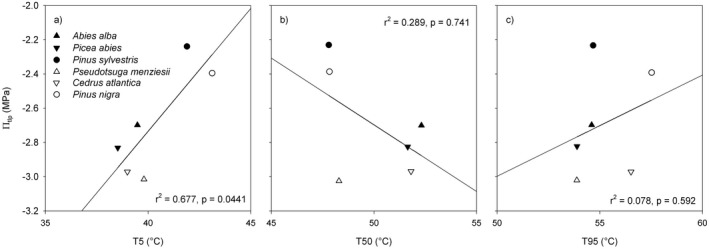
Relationship between thermal tolerance (T5, T50 and T95) and leaf turgor loss point (πtlp). πtlp data from Kunert & Tomaskova (2020), except Cedrus atlantica (Kunert, unpublished data).
DISCUSSION
We report the first temperature‐dependent Fv/Fm measurements made on foliage of mature conifers from a temperate region. The most important finding is that the breakpoint temperature T5, the temperature when Fv/Fm starts to decline, lies on average at 40.3 ± 0.7 °C. For all six species, T5 is similar or marginally above the maximum summer air temperature recorded in the study area, and T50 values that were at least 9 to 11 degrees above the maximum summer air temperatures on record. Further, T5 correlates with leaf turgor loss point, indicating two diverging mechanistic strategies of species to deal with heat and drought stress. Species are classified as either ‘drought‐tolerant heat‐sensitive’ or ‘drought‐sensitive heat‐tolerant’.
Summer temperatures reach thermal threshold of photosynthetic decline
We found clear evidence that summer temperatures measured during heatwaves exceed the thermal threshold, when the functioning of PSII of the investigated conifer species is significantly affected. The most sensitive species was Norway spruce, in which the maximum temperature measured during a heatwave in 2021 in the region (38.5 °C; see Fig. 2) will already result in initial PSII damage (T5 = 38.5 ± 0.8 °C). Ideally, the thermal tolerance thresholds should be related to leaf rather than air temperature; however, during heatwaves, limited access to soil water commonly results in stomatal closure. Stomatal closure, in turn. results in a rise of leaf temperatures to a level that even exceeds the ambient air temperature (Krause et al. 2010). In the course of a heatwave, trees in full sunlight are exposed to critical temperatures and high light intensities that can significantly change physiological and biochemical processes (Tiwari et al. 2021). The latter changes would likely affect the overall performance, growth and, ultimately, the survival of Norway spruce. Hence, thermal stress may contribute to the accelerated mortality of Norway spruce. We expect two possible consequences when the thermal threshold is surpassed, which might have a direct lethal outcome or induce long‐term physiological disorders in Norway spruce. First, thermal stress often triggers leaf senescence (Way, 2013). In the case of severe damage to the foliage, the canopy will be defoliated, leading to irreversible loss of carbon reserves as this species is unable to resprout new leaves within the same season (Galiano et al. 2011). Second, less severe damage to PSII can reduce electron transport rates that may decrease photosynthesis. A reduction in photosynthesis rate may weaken the defence mechanisms of Norway spruce and increase its susceptibility to bark beetle attacks (Huang et al. 2020) in the subsequent vegetation period.
Air temperatures above 40 °C have been observed on a regular basis at different climate stations throughout Central Europe in the last few years (e.g., Herold & Schappert, 2019). Only Scots pine and Austrian pine had a substantially higher T5 (41.9 ± 0.7 °C and 43.2 ± 0.6 °C, respectively), whereas T5 values of silver fir, Douglas fir and Atlas cedar were only marginally above the recorded temperatures. Temperature extremes, in particular more frequent and hotter heatwaves, have significantly increased on a regional and global scale over the last few decades (Perkins‐Kirkpatrick & Lewis 2020). Increasing temperature extremes have also been observed in our study area (Fig. 3). The latter trend is predicted to intensify in the near future, with forests likely to experience increasing levels of thermal stress resulting in tree mortality events, potentially amplifying positive climate–carbon cycle feedbacks.
Heat tolerant or sensitive
Tiwari et al. (2021) describe a continuous spectrum of strategies found in six tropical broadleaved tree species. Our results suggest that the investigated six temperate conifer species can be classified as either ‘heat‐sensitive’ or ‘heat‐tolerant’: Douglas fir, silver fir, Norway spruce and Atlas cedar represent the heat‐sensitive species, characterized by an early decline in Fv/Fm and wide decline width in particular between T5 and T50. Fv/Fm in these mentioned four species declined rapidly after T50 (narrow decline width from T50 to T95). Both pine species, namely Scots pine and Austrian pine, can be classified as heat‐tolerant species, with a higher T5 and a narrow decline width between T5 and T50. The decline width of the pines between T50 and T95 was wider than in the other conifers, indicating a broader tolerance to high temperatures. Differences among species in their tolerance to high temperatures supports the Tiwari et al. (2021) hypothesis of classifying species into ‘heat‐sensitive’ and ‘heat‐tolerant’; however, the total decline width between T5 and T95 was found to be a rather weak indicator of thermal sensitivity (r2 = 0.385, P = 0.188), whereas the decline widths T50–T5 (r2 = 0.888, P = 0.005) and T95–T50 (r2 = 0.822, P = 0.013) were good predictors of heat tolerance strategies in the investigated conifers.
Trade‐off between hydraulic and thermal vulnerability in leaves?
Heat sensitivity is intrinsically linked to drought avoidance; plants can either cool through transpiration, thus risking drought stress, or speedily close the stomata to avoid drought stress while risking heat damage (Konôpková et al. 2018). In our case, the first group comprising the two pine species has a less negative turgor loss point (−2.24 MPa and −2.39 MPa; Scots pine and Austrian pine, respectively) than the other four species (all between −2.70 MPa and −3.02 MPa; see Kunert & Tomsakova 2020). The second group might keep stomata open for longer durations under more water‐limiting conditions while pines trigger early stomatal closure in response to a water deficit. Turgor loss point is a ‘robust proxy of a species' degree of anisohydry’ (Meinzer et al. 2017) and, accordingly, the two pine species could be classified as being more drought‐sensitive than the other four conifer species, which are classified as drought‐tolerant. The drought‐tolerant species may follow another strategy by using transpirational cooling of the leaf to avoid or reduce heat stress instead of adopting a more heat‐tolerant photochemistry, as found in drought‐sensitive species. In our study, the conifer species with high drought tolerance tend to be more heat sensitive (i.e., have a lower breaking point temperature), but might compensate for this through maintaining transpirational cooling under drought and/or heat stress. The latter strategy could be described as ‘drought‐tolerant heat‐sensitive’ while, conversely, ‘drought‐sensitive heat‐tolerant’ species (e.g., Scots pine, Austrian pine, etc.) are more sensitive to a water deficit but can tolerate high temperatures. The latter confirms the findings of Kunert (2020) that pine mortality is triggered predominantly by a complex of interdependencies related to drought stress. Further, spruce mortality is more likely the result of heat stress. However, the results also show that tree species can follow different strategies to occupy the same ecological niche.
CONCLUSIONS
We found considerable variation in the thermal tolerance of the investigated temperate conifer species. During heatwaves, all six species were already operating very close to their thermal tolerance threshold. Species follow different strategies and either adapt their photochemistry to function under higher temperatures or adapt their osmotic potential to enable more transpirational cooling. Further research is required to understand how the interaction between heat stress and water limitation is affecting forest ecosystems. In the meantime, we suggest that the temperature of incipient PSII damage (T5) could be used to parameterize forest ecosystem models, enabling us to predict the effects of a future climate with increasing heatwave frequency and intensity.
References
- Allen C.D., Macalady A.K., Chenchouni H., Bachelet D., McDowell N., Vennetier M., Kitzberger T., Rigling A., Breshears D.D., Hogg E.H., Gonzalez P., Fensham R., Zhang Z., Castro J., Demidova N., Lim J.‐H., Allard G., Running S.W., Semerci A., Cobb N. (2010) A global overview of drought and heat‐induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management, 259, 660–684. [Google Scholar]
- Anderegg W.R.L., Kane J.M., Anderegg L.D.L. (2013) Consequences of widespread tree mortality triggered by drought and temperature stress. Nature Climate Change, 3, 30–36. [Google Scholar]
- von Arx G., Dobbertin M., Rebetez M. (2012) Spatio‐temporal effects of forest canopy on understory microclimate in a long‐term experiment in Switzerland. Agricultural and Forest Meteorology, 166–167, 144–155. [Google Scholar]
- Ashraf M., Harris P.J.C. (2013) Photosynthesis under stressful environments: an overview. Photosynthetica, 51, 163–190. [Google Scholar]
- Billon L.M., Blackman C.J., Cochard H., Badel E., Hitmi A., Cartailler J., Souchal R., Rorrez‐Ruiz J.M. (2020) The DroughtBox: a new tool for phenotyping residual branch conductance and its temperature dependence during drought. Plant, Cell and Environment, 43, 1584–1594. [DOI] [PubMed] [Google Scholar]
- Bolte A., Ammer C., Löf M., Madsen P., Nabuurs G.‐J., Schall P., Spathelf P., Rock J. (2009) Adaptive forest management in central Europe: climate change impacts, strategies and integrative concept. Scandinavian Journal of Forest Research, 24, 473–482. [Google Scholar]
- Breshears D.D., Fontaine J.B., Ruthrof K.X., Field J.P., Feng X., Burger J.R., Law D.J., Kala J., Hardy G.E.S.J. (2021) Underappreciated plant vulnerabilities to heat waves. New Phytologist, 231, 32–39. 10.1111/nph.17348 [DOI] [PubMed] [Google Scholar]
- Burschel P., Huss J. (2003) Grundriß des Waldbaus: Ein Leitfaden für Studium und Praxis. Ulmer, Stuttgart, Germany. [Google Scholar]
- Chaste E., Girardin M.P., Kaplan J.O., Bergeron Y., Hély C. (2019) Increases in heat‐induced tree mortality could drive reductions of biomass resources in Canada’s managed boreal forest. Landscape Ecology, 34, 403–426. [Google Scholar]
- Cobb R.C., Ruthrof K.X., Breshears D.D., Lloret F., Aakala T., Adams H.D., Anderegg W.R.L., Ewers B.E., Galiano L., Grünzweig J.M., Hartmann H., Huang C.‐Y., Klein T., Kunert N., Kitzberger T., Landhäusser S.M., Levick S., Preisler Y., Suarez M.L., Trotsiuk V., Zeppel M.J.B. (2017) Ecosystem dynamics and management after forest die‐off: a global synthesis with conceptual state‐and‐transition models. Ecosphere, 8, e02034. [Google Scholar]
- De Avila A., Albrecht A. (2018) Alternative Baumarten im Klimawandel: Artensteckbriefe – eine Stoffsammlung. Forstliche Versuchs‐ und Forschungsanstalt Baden‐Württemberg (FVA) . Freiburg, Germany. [Google Scholar]
- DWD (2021) Deutscher Wetterdienst, Climate Data Center. Available from https://opendata.dwd.de/climate_environment/CDC/ (accessed 3 April 2021). [Google Scholar]
- Eriksson M., Neuvonen S., Roininen H. (2007) Retention of wind‐felled trees and the risk of consequential tree mortality by the European spruce bark beetle Ips typographus in Finland. Scandinavian Journal of Forest Research, 22, 516–523. [Google Scholar]
- Fang‐Yuan Y.U., Guy R.D. (2004) Variable chlorophyll fluorescence in response to water plus heat stress treatments in three coniferous tree seedlings. Journal of Forestry Research, 15, 24–28. [Google Scholar]
- Galiano L., Martínez‐Vilalta J., Lloret F. (2011) Carbon reserves and canopy defoliation determine the recovery of Scots pine 4 years after a drought episode. New Phytologist, 190, 750–759. [DOI] [PubMed] [Google Scholar]
- Hajek P., Link R.M., Nock C., Bauhus J., Gebauer T., Gessler A., Kovach K., Messier C., Paquette A., Saurer M., Scherer‐Lorenzen M., Rose L., Schuldt B. (2020). Mutually inclusive mechanisms of drought‐induced tree mortality. bioRxiv, 423038. 10.1101/2020.12.17.423038 [DOI] [PubMed] [Google Scholar]
- Hartmann H., Moura C.F., Anderegg W.R.L., Ruehr N.K., Salmon Y., Allen C.D., Arndt S.K., Breshears D.D., Davi H., Galbraith D., Ruthrof K.X., Wunder J., Adams H.D., Bloemen J., Cailleret M., Cobb R., Gessler A., Grams T.E.E., Jansen S., Kautz M., Lloret F., O'Brien M. (2018) Research frontiers for improving our understanding of drought‐induced tree and forest mortality. New Phytologist, 218, 15–28. [DOI] [PubMed] [Google Scholar]
- Hentschel R., Rosner S., Kayler Z.E., Andreassen K., Børja I., Solberg S., Tveito O.T., Priesack E., Gessler A. (2014) Norway spruce physiological and anatomical predisposition to dieback. Forest Ecology and Management, 322, 27–36. [Google Scholar]
- Herold C. & Schappert S. (2019) Wetterextreme 2019—Teil 2, Deutscher Wetter Dienst. Available from https://www.dwd.de/DE/wetter/thema_des_tages/2019/12/21.html (accessed 5 May 2021)
- Huang J., Kautz M., Trowbridge A.M., Hammerbacher A., Raffa K.F., Adams H.D., Goodsman D.W., Xu C., Meddens A.J.H., Kandasamy D., Gershenzon J., Seidl R., Hartmann H. (2020) Tree defence and bark beetles in a drying world: carbon partitioning, functioning and modelling. New Phytologist, 225, 26–36. [DOI] [PubMed] [Google Scholar]
- IPCC (2019) Summary for policymakers. In: Shukla P. R., Skea J., Calvo Buendia E., Masson‐Delmotte V., Pörtner H.‐O., Roberts D. C., Zhai P., Slade R., Connors S., van Diemen R. , Ferrat M., Haughey E., Luz S., Neogi S., Pathak M., Petzold J., Pereira J., Vyas P., Huntley E., Kissick K., Belkacemi M., Malley J. (Eds), Climate Change and Land: an IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems. Available from https://www.ipcc.ch [Google Scholar]
- Konôpková A., Kurjak D., Kmeť J., Klumpp R., Longauer R., Ditmarová Ľ., Gömöry D. (2018) Differences in photochemistry and response to heat stress between silver fir (Abies alba Mill.) provenances. Trees, 32, 73–86. [Google Scholar]
- Krause G.H., Winter K., Krause B., Jahns P., García M., Aranda J., Virgo A. (2010) High‐temperature tolerance of a tropical tree, Ficus insipida: methodological reassessment and climate change considerations. Functional Plant Biology, 37, 890–900. [Google Scholar]
- Kunert N. (2020) Preliminary indications for diverging heat and drought sensitivities in Norway spruce and Scots pine in Central Europe. iForest, 13, 89‐91. [Google Scholar]
- Kunert N., Tomaskova I. (2020) Leaf turgor loss point at full hydration for 41 native and introduced tree and shrub species from Central Europe. Journal of Plant Ecology, 13, 754–756. [Google Scholar]
- Leon‐Garcia I., Lasso E. (2019) High heat tolerance in plants from the Andean highlands: Implications for paramos in a warmer world. PLoS One, 14, e0224218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer F.C., Smith D.D., Woodruff D.R., Marias D.E., McCulloh K.A., Howard A.R., Magedman A.L. (2017) Stomatal kinetics and photosynthetic gas exchange along a continuum of isohydric to anisohydric regulation of plant water status. Plant, Cell & Environment, 40, 1618–1628. [DOI] [PubMed] [Google Scholar]
- Muck P., Borchert H., Elling W., Hahn J., Immler T., Konnert M., Walentowski H., Walter A. (2008) Die Weisstanne‐ein Baum mit Zukunft. LWF Aktuell, 67, 56–58. [Google Scholar]
- O'sullivan O.S., Heskel M.A., Reich P.B., Tjoelker M.G., Weerasinghe L.K., Penillard A., Zhu L., Egerton J.J.G., Bloomfield K.J., Creek D., Bahar N.H.A., Griffin K.L., Hurry V., Meir P., Turnbull M.H., Atkin O.K. (2017) Thermal limits of leaf metabolism across biomes. Global Change Biology, 23, 209–223. [DOI] [PubMed] [Google Scholar]
- Perez T.M., Feeley K.J. (2020) Photosynthetic heat tolerances and extreme leaf temperatures. Functional Ecology, 34, 2236–2245. [Google Scholar]
- Perkins‐Kirkpatrick S.E., Lewis S.C. (2020) Increasing trends in regional heatwaves. Nature Communication, 11, 3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pötzelsberger E., Spiecker H., Neophytou C., Mohren F., Gazda A., Hasenauer H. (2020) Growing non‐native trees in European forests brings benefits and opportunities but also has its risks and limits. Current Forestry Reports, 6, 339–353. [Google Scholar]
- Pretzsch H., Grams T., Häberle K.H., Pritsch K., Bauerle T., Rötzer T. (2020) Growth and mortality of Norway spruce and European beech in monospecific and mixed‐species stands under natural episodic and experimentally extended drought. Results of the KROOF throughfall exclusion experiment. Trees, 34, 957–970. [Google Scholar]
- R Core Team . (2021) R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available from https://www.R‐project.org/ (accessed 15 April 2021). [Google Scholar]
- Ricketts J.H., Head G.A. (1999) A five‐parameter logistic equation for investigating asymmetry of curvature in baroreflex studies. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 277, R441–R454. [DOI] [PubMed] [Google Scholar]
- Ritz C., Baty F., Streibig J.C., Gerhard D. (2016) Dose‐response analysis using R. PLoS One, 10, e0146021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry A., Guha A., Barua D. (2017) Leaf thermotolerance in dry tropical forest tree species: relationships with leaf traits and effects of drought. AoB PLANTS, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl R., Thom D., Kautz M., Martin‐Benito D., Peltoniemi M., Vacchiano G., Wild J., Ascoli D., Petr M., Honkaniemi J., Lexer M.J., Trotsiuk V., Mairota P., Svoboda M., Fabrika M., Nagel T.A., Reyer C.P.O. (2017) Forest disturbances under climate change. Nature Climate Change, 7, 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senf C., Buras A., Zang C.S., Rammig A., Seidl R. (2020) Excess forest mortality is consistently linked to drought across Europe. Nature Communications, 11, 6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senf C., Pflugmacher D., Zhiqiang Y., Sebald J., Knorn J., Neumann M., Hostert P., Seidl R. (2018) Canopy mortality has doubled in Europe’s temperate forests over the last three decades. Nature Communications, 9, 4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot M., Cala D., Aranda J., Virgo A., Michaletz S.T., Winter K. (2021) Leaf heat tolerance of 147 tropical forest species varies with elevation and leaf functional traits, but not with phylogeny. Plant, Cell and Environment. (in press). [DOI] [PubMed] [Google Scholar]
- Sohn J.A., Gebhardt T., Ammer C., Bauhus J., Häberle K.‐H., Matyssek R., Grams T.E.E. (2013) Mitigation of drought by thinning: Short‐term and long‐term effects on growth and physiological performance of Norway spruce (Picea abies). Forest Ecology and Management, 308, 188–197. [Google Scholar]
- Song Y., Chen Q., Ci D., Shao X., Zhang D. (2014) Effects of high temperature on photosynthesis and related gene expression in poplar. BMC Plant Biology, 14, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari R., Gloor E., da Cruz W.J.A., Schwantes Marimon B., Marimon‐Junior B.H., Reis S.M., de Souza I.A., Krause H.G., Slot M., Winter K., Ashley D., Béu R.G., Borges C.S., Da Cunha M., Fauset S., Ferreira L.D.S., Gonçalves M.D.A., Lopes T.T., Marques E.Q., Mendonça N.G., Mendonça N.G., Noleto P.T., de Oliveira C.H.L., Oliveira M.A., Pireda S., dos Santos Prestes N.C.C., Santos D.M., Santos E.B., da Silva E.L.S., de Souza I.A., de Souza L.J., Vitória A.P., Foyer C.H., Galbraith D. (2021) Photosynthetic quantum efficiency in south‐eastern Amazonian trees may be already affected by climate change. Plant, Cell and Environment, 44, 2428–2439. [DOI] [PubMed] [Google Scholar]
- Way D.A. (2013) Will rising CO2 and temperatures exacerbate the vulnerability of trees to drought? Tree Physiology, 33, 775–778. [DOI] [PubMed] [Google Scholar]


