Abstract
Introduction
The relationship between pulmonary function (PF) and mild cognitive impairment (MCI), dementia, and brain pathologies remains unclear.
Methods
A total of 1312 dementia‐free participants, including a cognitively intact group (n = 985) and an MCI group (n = 327), were followed for up to 21 years to detect incident MCI and dementia. PF was assessed at baseline with a composite score and tertiled. Over follow‐up, 540 participants underwent autopsies for neuropathological assessment.
Results
Compared to the highest PF, the hazard ratios (95% confidence intervals [CIs]) of the lowest PF were 1.95 (1.43‐2.66) for MCI in the cognitively intact group and 1.55 (1.03‐2.33) for dementia in the MCI group. Low PF was further related to Alzheimer's disease pathology (odds ratio [OR] 1.32, 95% CI 1.19‐1.47) and vascular pathology (OR 3.05, 95% CI 1.49‐6.25).
Discussion
Low PF increases MCI risk and accelerates MCI progression to dementia. Both neurodegenerative and vascular mechanisms may underlie the PF‐dementia association.
Keywords: brain pathology, cohort study, dementia, mild cognitive impairment, pulmonary function
1. INTRODUCTION
Aging is accompanied by changes in lung function due to factors such as loss of lung elasticity and weakened respiratory muscles. 1 As with other organ systems, the function of the pulmonary system gradually declines with age. 2 Poor pulmonary function (PF) has been related to low quality of life and mortality among older adults in many studies. 3 , 4 As a complete evaluation of the overall respiratory condition, PF is often described using a composite measure including forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and peak expiratory flow (PEF). 5 , 6 , 7
There is no curative treatment for dementia, but proactive management of modifiable risk factors can delay the onset and slow the progression of dementia. 8 The association between PF and cognitive outcomes has been investigated in recent years. Several epidemiological studies have associated poor PF with increased risk of cognitive decline 9 , 10 , 11 and dementia, 10 , 11 , 12 , 13 with some inconsistent findings. 9 , 11 , 13 Mild cognitive impairment (MCI) is a transitional state between normal cognition and dementia. 14 So far, only two studies have examined the association between PF and MCI, suggesting that lower PF is associated with higher MCI risk. 12 , 15 In these studies, PF was tested with single measures (FEV1 or FVC). 12 , 15 No studies to date have evaluated the impact of lower PF on the progression from MCI to dementia.
Several degenerative brain pathologies can be assessed by postmortem neuropathological evaluation, including Alzheimer's disease (AD) pathology and vascular pathology. 16 Brain pathologies can provide insight into the pathogenesis associated with various risk factors for dementia. 17 , 18 Studies have suggested that poor pulmonary health—including chronic obstructive pulmonary disease (COPD) and poor respiratory muscle strength—may lead to an aggregation of AD and vascular pathologies in the brain. 7 , 19 Although hypoxia has been suggested as an explanation for the association between poor PF and cognitive decline, the underlying mechanisms linking poor PF and cognitive decline remain unclear. 6 To date, no studies have explored the association between PF and brain pathologies.
We hypothesized that poor PF is associated with an increased risk of MCI and may accelerate MCI's progression to dementia, and that both vascular and neurodegenerative processes may underlie this association. To test this hypothesis, we aimed to (1) examine the association of PF with the risk of MCI and its progression to dementia, and (2) explore the relationship of PF to AD and vascular pathologies in the brain using data from a long‐term community‐based cohort study including brain pathological assessments from participants who died during follow‐up.
2. METHODS
2.1. Study design and participants
The Rush Memory and Aging Project (MAP) is an ongoing prospective clinical‐pathological study on common age‐related chronic conditions. 20 The MAP study participants were recruited from continuous care retirement communities, senior and subsidized housing, church groups, and social service agencies in Chicago and northeastern Illinois (USA). 20 All participants underwent a comprehensive clinical evaluation, neurological examination, and extensive cognitive tests at the time of enrollment and thereafter. 21
HIGHLIGHTS
Poor pulmonary function (PF) is associated with an increased risk of mild cognitive impairment (MCI) and accelerated progression from MCI to dementia.
Low PF is further related to both Alzheimer's disease pathology and cerebral vascular disease pathology in the brain.
Interventions designed to maintain pulmonary health may represent significant opportunities for maintaining cognitive health in late life.
RESEARCH IN CONTEXT
Systematic review: We have searched the literature on pulmonary function (PF), mild cognitive impairment (MCI) and its progression to dementia, and brain pathology by accessing PubMed and Web of Science databases. So far, few studies have examined the association between PF and MCI risk, and no studies have evaluated the impact of lower PF on the progression from MCI to dementia. Moreover, studies examining the association of PF with brain pathology were sparse.
Interpretation: In this community‐based cohort study, we found that poor PF was associated with an increased risk of MCI and accelerated progression from MCI to dementia. Both Alzheimer's disease pathology and cerebral vascular disease pathology may underlie the association.
Future directions: Further longitudinal studies are warranted to examine the effect of PF on MCI and its progression and explore the mechanisms linking PF, brain pathologies, and cognitive impairment progression.
A total of 2155 participants agreed to annual follow‐up for a maximum of 21 years (from 1997 to 2019). Of them, we excluded 843 with prevalent dementia (n = 115), COPD (n = 98), or missing data on PF (n = 591) at baseline. We further excluded those who died before the first follow‐up evaluation (n = 32), had no data available on cognitive status after baseline (n = 94), and were newly recruited in 2019 (n = 106). Therefore, 1312 dementia‐free participants remained for the current study.
At baseline, 985 participants were enrolled in the cognitively intact group. Over the follow‐up, 441 participants developed MCI and 45 participants had incident dementia without MCI observation. A total of 709 participants were enrolled in the MCI group, including participants with MCI at baseline (n = 327) and participants from the cognitively intact group who developed incident MCI (n = 382; excluded 59 participants who died before the next follow‐up). Over the follow‐up period, 642 participants died, and 540 (83.96%) of them underwent brain autopsy (Figure 1).
FIGURE 1.
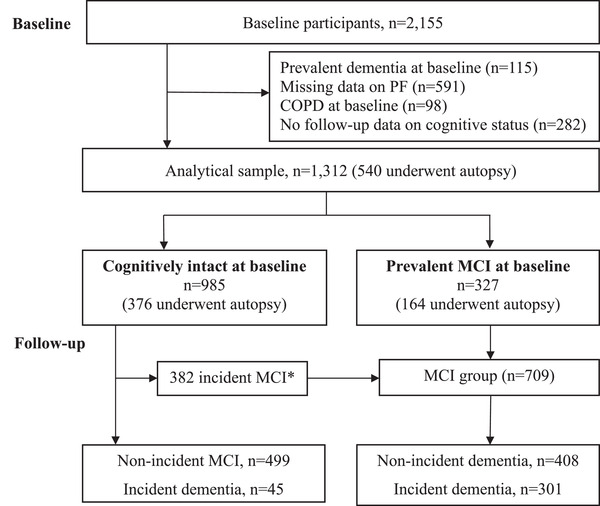
Flow chart for the study population. MCI, mild cognitive impairment. *During the follow‐up, 441 people developed incident MCI; 59 of them died, leaving 382 participants with MCI who were included in the MCI group
This study was approved by a Rush University Medical Center Institutional Review Board and conducted in accordance with the ethical standards laid out in the 1964 Declaration of Helsinki and its later amendments. All participants provided written informed consent, Uniform Anatomic Gift Act documentation, and repository consent to allow their data to be shared.
2.2. Assessment of pulmonary function
At study entry, PF was tested using a hand‐held spirometer (MicroPlus Spirometer MS03, MicroMedical LTC,. Kent, UK). Three separate indicators of PF were measured twice and averaged for each participant: FVC (the maximum amount of air that can be exhaled after a maximum inhalation), FEV1 (the volume of air exhaled with force for 1 second), and PEF (the maximum speed of expiration). Next, the raw scores of the three averaged component measures were converted into z‐scores using the means and SDs calculated from the entire study sample. Finally, a comprehensive PF score at study entry (baseline) was created by averaging the z‐scores of FVC, FEV1, and PEF, with a higher score indicating a better level of PF. 22 Participants with an FEV1/FVC ratio ≤0.7 were considered to have possible COPD. 23
2.3. Assessment of dementia, Alzheimer's dementia, and mild cognitive impairment
Dementia, Alzheimer's dementia, and MCI were assessed using a uniform, structured process including computer scoring of cognitive tests, clinical judgment by a neuropsychologist, and diagnostic classification by a clinician. 24 , 25 Dementia and Alzheimer's dementia were diagnosed in accordance with the criteria from the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS/ADRDA), taking into consideration normative cognitive function based on age, sex, and education. 25 , 26 Participants were diagnosed with MCI if they showed evidence of cognitive impairment (ie, one impaired cognitive domain based on normative cognitive data considering age, sex, and education) in the neuropsychologist's examination, but did not meet the criteria for dementia in the clinician's examination. 24 , 27 Participants who were free from both dementia and MCI at study entry were included in the cognitively intact group.
2.4. Assessment of brain pathologies
Brain removal, tissue sectioning and preservation, and uniform gross and microscopic examinations with quantification of postmortem pathological indices were conducted following a standard protocol. 16 AD pathology burden (ie, global AD pathology, amyloid beta [Aβ] load, and neurofibrillary tangles) was quantified and classified as low or high based on the median value. 28 Lewy bodies, 16 typical hippocampal sclerosis, 29 chronic gross‐ and micro‐infarcts, 30 and cerebral vascular disease pathology (including atherosclerosis, arteriolosclerosis, and cerebral amyloid angiopathy) were assessed as present or absent. 7
2.5. Assessment of other covariates
Information on participants’ demographic characteristics, socioeconomic status, and lifestyle factors was collected at baseline. These are described in detail in the Supplementary Methods. Additional details about the data collection can be found and requests for MAP data can be made online at the Rush Alzheimer's Disease Center Resource Sharing Hub (https://www.radc.rush.edu/).
2.6. Statistical analysis
Baseline characteristics of the study population were analyzed using chi‐square tests for categorical variables and one‐way analysis of variance (ANOVA) or Wilcoxon rank‐sum tests for continuous variables. The z‐score of PF was operationalized as both a continuous and a categorical variable (tertiled as lowest, middle, and highest [as reference]). The lowest tertile of PF was considered poor PF.
Laplace regression models were used to estimate and compare the median time (years, with 95% confidence intervals [CIs]) to the development of MCI or dementia according to PF level. Cox proportional hazards regression models were used to estimate the hazard ratios (HRs) and 95% CIs for the incidence of MCI/dementia according to PF level. Follow‐up time (years) was calculated as the time from study entry to MCI/dementia diagnosis, death, or the final examination. Multivariate logistic models were used to estimate the odds ratios (ORs) and 95% CIs for the association between PF and brain pathologies. Models were adjusted for age, sex, education, income, smoking, alcohol consumption, physical activity, body mass index (BMI), heart disease, hypertension, stroke, diabetes, depression, and apolipoprotein E (APOE) ε4. The proportional hazards assumption was confirmed using tests based on Schoenfeld residuals. The multicollinearity of the variables included in the analysis was tested by the variance inflation factor (VIF) for each dependent variable, and no significant collinearity was identified (overall VIF = 1.21). Stratified analyses were performed to explore the role of age, sex, and behavioral factors (including smoking, physical activity, and alcohol assumption) in the association between PF and MCI and its progression to dementia. Statistical interaction between PF and other variables was examined by creating an indicator variable with the cross‐product of PF and the other variable of interest.
In sensitivity analysis, we repeated the analyses after (1) using a competing risks model (the Fine‐Gray model) with death as the competing event, (2) performing multiple imputations for subjects missing data on PF or any of the covariates, and (3) excluding participants who developed MCI during follow‐up. P‐values < .05 were considered statistically significant. All statistical analyses were performed using Stata SE 16.0 (Stata Corp, College Station, Texas, USA).
3. RESULTS
3.1. Baseline characteristics
Among 1312 dementia‐free participants (75.91% female; mean age 79.32 ± 7.75 years), 985 had normal cognition (NC) and 709 had MCI, including participants who developed MCI over follow‐up. Table 1 presents the baseline characteristics of the study population by PF group. Characteristics by incident MCI/dementia among NC/MCI participants are presented in Table S1. Compared to the participants who were included in the study population, those who were excluded were more likely to be older, male, have a higher education and income, be less physically active, have a history of stroke, and carry at least one APOE ɛ4 allele (Table S2).
TABLE 1.
Characteristics of the study population by pulmonary function (PF) group in the cognitively intact group and mild cognitive impairment (MCI) group
| Cognitively intact group (N = 985) | MCI group (N = 327) | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Lowest PF (n = 304) | Middle PF (n = 346) | Highest PF (n = 335) | P | Lowest PF (n = 127) | Middle PF (n = 100) | Highest PF (n = 100) | P |
| Follow‐up time, years | 3.93 (2.00, 6.07) | 5.03 (2.78, 8.06) | 5.99 (2.65, 8.71) | – | 3.01 (1.92, 5.92) | 4.05 (2.11, 6.72) | 3.90 (1.97, 7.48) | – |
| Age, years | 82.15 ± 6.26 | 78.16 ± 6.93 | 75.10 ± 7.83 | <0.001 | 83.35 ± 7.60 | 82.29 ± 7.47 | 80.81 ± 7.23 | 0.011 |
| Female | 288 (94.74) | 316 (91.33) | 165 (49.25) | <0.001 | 115 (90.55) | 78 (78.00) | 35 (35.00) | <0.001 |
| Education, years | 14.14 ± 3.21 | 14.48 ± 2.99 | 15.60 ± 3.40 | <0.001 | 14.20 ± 3.43 | 14.54 ± 2.69 | 15.01 ± 2.98 | 0.155 |
| Income | 6.30 ± 2.65 | 6.82 ± 2.48 | 7.64 ± 2.39 | <0.001 | 5.92 ± 2.69 | 6.72 ± 2.42 | 7.44 ± 2.47 | 0.252 |
| BMI, kg/m2 | 27.93 ± 5.60 | 27.60 ± 5.18 | 27.44 ± 5.31 | 0.587 | 27.11 ± 4.82 | 27.06 ± 5.61 | 26.62 ± 5.38 | 0.517 |
| Smoking | 0.287 | 0.326 | ||||||
| Never | 190 (62.50) | 212 (61.27) | 183 (54.63) | 65 (52.00) | 60 (60.00) | 58 (58.00) | ||
| Ever smoker | 106 (34.87) | 123 (35.55) | 140 (41.79) | 57 (45.60) | 37 (37.00) | 42 (42.00) | ||
| Current smoker | 8 (2.63) | 11 (3.18) | 12 (3.58) | 3 (2.40) | 3 (3.00) | 0 (0.00) | ||
| Alcohol consumption, g/day | 0.00 (0.00, 4.32) | 0.00 (0.00, 4.32) | 2.16 (0.00, 10.80) | <0.001 | 0.00 (0.00, 2.40) | 0.00 (0.00, 4.13) | 1.08 (0.00, 10.58) | 0.011 |
| Physical activity, h/week | 2.08 (0.63, 4.00) | 2.39 (0.75, 4.42) | 3.25 (1.70, 5.50) | <0.001 | 1.67 (0.50‐4.00) | 1.67 (0.50, 4.25) | 2.71 (0.75, 5.04) | 0.028 |
| Stroke | 26 (9.22) | 23 (7.28) | 18 (6.02) | 0.337 | 18 (15.38) | 5 (5.88) | 6 (6.98) | 0.045 |
| Heart disease | 38 (12.50) | 32 (9.25) | 36 (10.75) | 0.410 | 21 (16.54) | 20 (20.00) | 9 (9.00) | 0.086 |
| Diabetes | 43 (14.14) | 47 (13.58) | 55 (16.42) | 0.339 | 17 (13.39) | 11 (11.00) | 14 (14.00) | 0.296 |
| Hypertension | 249 (81.91) | 256 (73.99) | 221 (65.97) | <0.001 | 97 (76.38) | 71 (71.00) | 73 (73.00) | 0.646 |
| Depression | 83 (27.30) | 61 (17.63) | 60 (18.07) | 0.003 | 39 (31.20) | 35 (35.00) | 15 (15.00) | 0.003 |
| APOE ɛ4 carriers | 59 (19.47) | 61 (17.99) | 81 (24.55) | 0.092 | 28 (22.95) | 23 (23.23) | 35 (35.35) | 0.073 |
| Death during follow‐up | 183 (60.20) | 136 (39.31) | 112 (33.43) | <0.001 | 92 (72.44) | 61 (61.00) | 58 (58.00) | 0.053 |
| Autopsy | 154 (50.33) | 120 (34.68) | 102 (30.45) | <0.001 | 65 (51.18) | 49 (49.00) | 50 (50.00) | 0.948 |
Values are mean ± SD, n (%), or median (interquartile range).
APOE ε4, apolipoprotein ε4; BMI, body mass index; MCI, mild cognitive impairment.
The number of subjects with missing values was 33 (3.35%) for income, 13 (1.32%) for APOE ε4, 19 (1.93%) for BMI, and 88 (8.93%) for stroke in the cognitively intact group; 2 (0.61%) for alcohol consumption, 7 (2.14%) for APOE ε4 genotype, 6 (1.83%) for BMI, 29 (8.87%) for stroke, and 2 (0.61%) for smoking status in the MCI group.
3.2. Pulmonary function and the development of MCI
During the follow‐up (median: 4.98 years, interquartile range [IQR]: 2.08‐7.99 years), 441 NC participants developed MCI and 45 participants developed incident dementia without first being diagnosed with MCI. The median time of MCI onset was 4.99 (95% CI: 4.19‐5.78), 8.93 (95% CI: 7.72‐10.12), and 9.19 (95% CI: 8.12‐10.26) years in people with the lowest (PF ≤ −0.36), middle (−0.36 < PF ≤ 0.39), and highest PF (PF > 0.39), respectively (Figure 2A). Thus, among NC participants, the lowest PF accelerated MCI onset by more than 4 years compared to the highest PF (Z = 6.16, P < 0.001).
FIGURE 2.
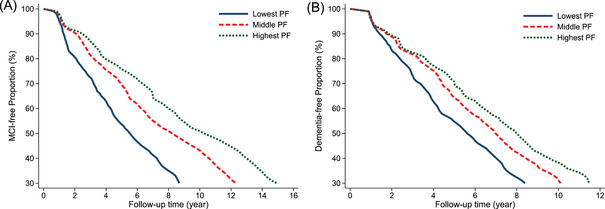
Mild cognitive impairment (MCI)–free proportion in the cognitively intact group (A) and dementia‐free proportion in the MCI group (B) by pulmonary function (PF). Note: Model adjusted for age, sex, education, income, smoking, alcohol consumption, physical activity, body mass index, heart disease, hypertension, diabetes, stroke, depression, and apolipoprotein E (APOE) ε4
In multiple‐adjusted Cox regression models, higher level of PF (as a continuous variable) was associated with lower risk of MCI (HR [95% CI]: 0.71 [0.61‐0.82]). Compared to those with the highest PF, the HR (95% CI) of MCI was 1.95 (1.43‐2.66) for participants with the lowest PF (Table 2).
TABLE 2.
Incidence rate (IR) per 1000 person‐years and hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between pulmonary function (PF) and mild cognitive impairment (MCI) in the cognitively intact group, or dementia in the MCI group
| Cognitively intact group (N = 985) | MCI group (N = 709) b | |||||||
|---|---|---|---|---|---|---|---|---|
| Pulmonary Function | No. of subjects | No. of cases | IR (95% CI) | HR (95% CI) a | No. of subjects | No. of cases | IR (95% CI) | HR (95% CI) a |
| Continuous | 985 | 486 | 96.9 (88.1‐105.7) | 0.71 (0.61‐0.82) | 709 | 301 | 103.0 (91.1‐114.8) | 0.78 (0.63‐0.96) |
| Categorical (quartiles) | ||||||||
| Highest PF | 335 | 147 | 70.9 (57.4‐84.3) | Ref. | 234 | 90 | 94.3 (70.6‐117.9) | Ref. |
| Middle PF | 346 | 145 | 82.1 (67.9‐96.3) | 1.22 (0.89‐1.65) | 240 | 100 | 98.3 (78.3‐118.3) | 1.48 (1.02‐2.15) |
| Lowest PF | 304 | 194 | 141.1 (119.3‐162.9) | 1.95 (1.43‐2.66) | 235 | 111 | 114.1 (90.9‐137.2) | 1.55 (1.03‐2.33) |
Model adjusted for age, sex, education, income, smoking, alcohol consumption, physical activity, body mass index, heart disease, hypertension, diabetes, stroke, depression, and apolipoprotein E (APOE) ε4.
During the follow‐up, 441 people developed incident MCI; of them 59 died, leaving 382 participants with MCI who were included in the MCI group.
3.3. Pulmonary function and MCI's progression to dementia
During the follow‐up (median: 6.29 years, IQR: 3.49‐9.34 years), 301 participants with MCI developed dementia. The median time from MCI to dementia development was 5.92 (95% CI: 5.13‐6.72), 6.99 (95% CI: 4.56‐9.43), and 7.62 (95% CI: 5.24‐10.01) years in people with the lowest (PF ≤ −0.60), middle (−0.60 < PF ≤ 0.19), and highest PF (PF > 0.19), respectively (Figure 2B). Among people with MCI, the lowest PF accelerated dementia onset by 1.71 years compared to the highest PF (Z = 2.11, P = .035).
In multiple‐adjusted Cox regression models, PF (as a continuous variable) was associated with dementia risk (HR [95% CI]: 0.78 [0.63‐0.96]). Compared to those in the highest PF group, the HR (95% CI) of MCI was 1.55 (1.03‐2.33) for participants with the lowest PF (Table 2).
The PF‐MCI/dementia association remained significant after stratification by age (< 80 vs ≥80 years), sex (male vs female), smoking status (smoking vs non‐smoking), alcohol consumption status (drinking vs non‐drinking), and physical activity level (low vs high). Furthermore, we found no significant interactions between these factors and PF on the risk of MCI/dementia (Tables S3‐7).
3.4. Association between PF and brain pathologies
Among the 540 participants who underwent autopsies, 205 developed MCI and 232 developed dementia. In multi‐adjusted logistic regression, higher PF was related to lower AD pathology burden, Aβ load, neurofibrillary tangles, and cerebral atherosclerosis (Table 3).
TABLE 3.
Odds ratios (ORs) and 95% confidence intervals (CIs) for the relationship between pulmonary function (PF) and brain pathologies in the autopsy participants (n = 540)
| Categorical PF (quartiles) a | |||
|---|---|---|---|
| Brain pathologies | Continuous PF | Middle vs Highest | Lowest vs Highest |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| AD pathologies | |||
| Global AD pathology | 0.92 (0.86‐0.98) | 1.26 (1.15‐1.39) | 1.32 (1.19‐1.47) |
| Aβ load | 0.92 (0.87‐0.99) | 1.24 (1.13‐1.37) | 1.32 (1.18‐1.46) |
| Neurofibrillary tangles | 0.93 (0.87‐0.99) | 1.13 (1.01‐1.26) | 1.16 (1.01‐1.31) |
| Chronic infarcts | |||
| Gross infarcts | 0.89 (0.67‐1.19) | 0.98 (0.60‐1.60) | 1.19 (0.71‐1.99) |
| Microscopic infarcts | 1.18 (0.87‐1.59) | 1.00 (0.61‐1.64) | 0.73 (0.43‐1.24) |
| Vascular disease pathology | |||
| Cerebral atherosclerosis | 0.66 (0.46‐0.93) | 1.93 (1.04‐3.59) | 3.05 (1.49‐6.25) |
| Cerebral amyloid angiopathy | 0.71 (0.53‐0.96) | 2.15 (1.23‐3.74) | 2.26 (1.25‐4.07) |
| Arteriolosclerosis | 0.76 (0.55‐1.05) | 1.21 (0.69‐2.14) | 1.18 (0.63‐2.20) |
| Lewy bodies | 1.12 (0.81‐1.54) | 0.83 (0.44‐1.53) | 0.78 (0.39‐1.56) |
| Hippocampal sclerosis | 1.71 (0.96‐3.05) | 0.38 (0.14‐1.04) | 0.41 (0.15‐1.16) |
Model adjusted for age, sex, education, income, smoking, alcohol consumption, physical activity, body mass index, heart disease, hypertension, diabetes, stroke, depression, and apolipoprotein E (APOE) ε4.
Aβ, amyloid beta.
Compared to participants in the highest PF group, those in the lowest PF group had a higher burden of AD pathology, Aβ load, neurofibrillary tangles, cerebral atherosclerosis, and cerebral amyloid angiopathy (Table 3). We did not find a statistically significant association of poor PF with chronic gross‐ and micro‐infarcts, Lewy bodies, or typical hippocampal sclerosis in the brain.
Within the cognitively intact group, participants with the lowest PF had a higher burden of AD pathologies, amyloid protein, neurofibrillary tangles, and cerebral atherosclerosis, compared to those with the highest PF (Table S8). In the MCI group, participants with the lowest PF had a higher burden of AD pathologies, amyloid protein, neurofibrillary tangles, and arteriolosclerosis, compared to those with the highest PF (Table S8).
3.5. Supplementary analysis
The results were not materially altered when we repeated the initial analyses after: (1) using a competing risk model with death as the competing event (Table S9), (2) performing multiple imputations for missing values of PF and some covariates (Table S10), and (3) excluding participants who developed MCI during follow‐up (Table S11).
4. DISCUSSION
In this community‐based cohort study of dementia‐free older adults, we found that poor PF is related to a nearly two‐fold increased risk of MCI among cognitively intact people and accelerates the progression from MCI to dementia by almost 2 years among people with MCI. In addition, we found that PF is related to both AD pathology (including global AD pathology, amyloid protein, and neurofibrillary tangles) and cerebral vascular disease pathology (including cerebral atherosclerosis and cerebral amyloid angiopathy), suggesting the possible involvement of both neurodegenerative and vascular mechanisms in the association between poor PF and dementing disorders.
Poor PF has been associated with cognitive decline 9 , 10 , 11 and dementia 10 , 11 , 12 , 13 in several studies. Only two longitudinal studies so far have explored the association of single PF indicators with MCI risk. 12 , 15 One suggested that FEV1/height2 is associated with increased MCI risk, 15 whereas the other showed that poor FEV1 and FVC are associated with a higher risk of MCI. 12 Furthermore, several studies have reported that COPD is related to a higher risk of MCI. 31 , 32 However, to date, no research has focused on the relationship between PF and the risk of dementia among people with MCI. Furthermore, single PF indicators like FEV1, FVC, and PEF evaluate PF from different dimensions, and these indicators are usually evaluated jointly in clinical diagnosis. 33 In the present study, we aggregated FEV1, FVC, and PEF to create a composite PF indicator, which reflects not only pure pulmonary function but also the contribution of some respiratory muscles, 22 , 34 thereby capturing lung health more comprehensively. We found that poor PF is related to a higher risk of MCI and may accelerate its progression to dementia. To our knowledge, this is the first study to explore the relationship between a comprehensive measure of PF and the risk of MCI and its progression.
Postmortem pathological evaluation can shed light on the mechanisms of dementia development. 35 So far, only two studies have investigated the relationship between lung health and brain pathology. 7 , 19 The first also used data from MAP, demonstrating that respiratory muscle strength is related to AD pathology and macroscopic infarcts. 7 The second study indicated that COPD is related to cerebral vascular pathologies, but not to AD pathologies such as amyloid plaques and neurofibrillary tangles. 19 Furthermore, few neuroimaging studies have suggested that lower FEV1 is related to smaller gray and white matter volumes and greater white matter hyperintensity volume. 36 , 37 To our knowledge, our study is the first to demonstrate that lower PF is associated with a higher burden of AD and vascular pathologies in the brain, suggesting that both neurodegeneration and vascular lesions could underlie the association of PF with MCI and its progression to dementia.
The mechanisms behind the PF‐dementia association remain unclear. It has been proposed that the lungs and brain work in concert (ie, the lung‐brain axis), with injuries to one compromising the other, perhaps through a combination of neural, inflammatory, immunologic, and neuroendocrine signalling. 38 First, poor PF may lead to hypoperfusion and hypoxia, 38 , 39 which affect brain energy metabolism causing oxidative stress, 40 , 41 and the induction of neuroinflammation, thereby triggering neuronal apoptosis and the development of AD pathologies. 42 At the same time, sustained or intermittent hypoxia could increase the production of Aβ protein through the up‐regulation of β‐secretase mediated by hypoxia‐inducible factor 1a (HIF‐1a) in vitro, 43 , 44 or induce the formation of amyloid precursor protein in neurons, leading to the abnormal deposition of Aβ protein and hyperphosphorylation of tau. 45 Second, vascular changes in the brain could also explain this relationship. 6 , 46 PF is related to cardiovascular diseases, 47 which are associated with dementia risk. 48 In particular, cerebral atherosclerosis, arteriolosclerosis, and stroke can cause cerebral ischemia, 48 , 49 , 50 causing oxidative stress and synaptic disorders that may lead to oxidative stress–mediated damage, contributing to dementia by accelerating vascular damage and degenerative lesions. 51 Finally, PF may serve as a marker for a range of physiological, behavioral, and socioeconomic risk factors, 47 which are associated with dementia risk. 52 Thus, lung function might be a proxy of these factors in the dementing process. However, in this study, we were able to consider several possible confounding factors in our models as covariates, including education level, smoking, drinking, BMI, and other factors, and these factors did not fully explain the observed association. Further experimental studies are required to clarify the mechanisms underlying the association between PF and AD and vascular pathologies.
This study has several strengths. First, this is a community‐based cohort study with a relatively large sample size and long follow‐up, and with autopsy data available. Second, we used an aggregated indicator of PF, which provides a more comprehensive measure of respiratory function. Nonetheless, some limitations should be pointed out. First, the MAP participants were volunteers who were not randomly selected from the community. Compared to the general population in the same area, they were better educated and scored higher on cognitive tests. This might have led to an underestimation of the magnitude of the association between PF and MCI/dementia risk. However, the characteristics of participants in MAP are generally similar to those in the Rotterdam Study, 53 the Honolulu‐Asia Aging Study, 54 the Swedish National Study on Aging and Care in Kungsholmen, and the Kungsholmen project 55 in terms of demographics and chronic conditions at baseline. Still, caution is required when generalizing our results to younger populations. Second, data on brain pathology were captured only when the participants were deceased; thus, the causal inference between PF and pathologies should be interpreted with caution. Third, selection bias may have occurred due to missing data. However, we repeated the analysis after multiple imputations, and the results were not much altered compared to those from the initial analysis. Fourth, most dropouts during the follow‐up were due to poor health conditions, and this might have led to an underestimation of the observed associations. Finally, it is impossible to completely rule out potential confounders caused by unmeasured factors (including occupation, exposure to toxins, and other clinical comorbidities such as asthma and pneumonia).
In conclusion, this study provides clear evidence that poor PF—as reflected by FEV1, FVC, and PEF—is associated with an increased risk of MCI and its progression to dementia, as well as a greater burden of AD and vascular pathologies in the brain. Thus, poor PF might be a predictor of cognitive dysfunction. Our findings highlight the need for monitoring and maintenance of pulmonary function among older adults for the prevention of dementing disorders.
Weili Xu received grants from the Swedish Research Council (No. 2017‐00981 and No. 2021‐01647), the Swedish Council for Health Working Life and Welfare (2021‐01826), the National Natural Science Foundation of China (No. 81771519), Alzheimerfonden (2021‐2022), Karolinska Institutet Research Foundation (2020‐01660), the Lindhés Advokatbyrå AB (2021‐0134), Stiftelsen För Gamla Tjänarinnor (2021‐2022), and Demensfonden. David Bennett received grants from the National Institutes of Health (R01AG17917 and UH2NS100599).
The funding source had no role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data; the preparation, review, and approval of the manuscript; or the decision to submit the manuscript for publication.
CONFLICT OF INTEREST
The authors report no disclosures relevant to the manuscript.
Supporting information
Supporting information.
ACKNOWLEDGEMENT
The authors would like to express their gratitude to the participants and staff involved in data collection and management in the Rush Memory and Aging Project.
Wang J, Dove A, Song R, et al. Poor pulmonary function is associated with mild cognitive impairment, its progression to dementia, and brain pathologies: A community‐based cohort study. Alzheimer's Dement. 2022;18:2551–2559. 10.1002/alz.12625
David A. Bennett and Weili Xu contributed equally as last authors.
REFERENCES
- 1. Thomas ET, Guppy M, Straus SE, Bell KJL, Glasziou P. Rate of normal lung function decline in ageing adults: a systematic review of prospective cohort studies. BMJ Open. 2019; 9(6): e028150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan ED, Welsh CH. Geriatric respiratory medicine. Chest. 1998; 114(6): 1704–1733. [DOI] [PubMed] [Google Scholar]
- 3. Baughman P, Marott JL, Lange P, et al. Combined effect of lung function level and decline increases morbidity and mortality risks. Eur J Epidemiol. 2012; 27(12): 933–943. [DOI] [PubMed] [Google Scholar]
- 4. Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax. 2003; 58(5): 388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brusasco V, Warner DO, Beck KC, Rodarte JR, Rehder K. Partitioning of pulmonary resistance in dogs: effect of tidal volume and frequency. J Appl Physiol (1985). 1989; 66(3): 1190–1196. [DOI] [PubMed] [Google Scholar]
- 6. Russ TC, Kivimäki M, Batty GD. Respiratory disease and lower pulmonary function as risk factors for dementia: a systematic review with meta‐analysis. Chest. 2020; 157(6): 1538–1558. [DOI] [PubMed] [Google Scholar]
- 7. Buchman AS, Yu L, Wilson RS, et al. Post‐mortem brain pathology is related to declining respiratory function in community‐dwelling older adults. Front Aging Neurosci. 2015; 7: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. WHO . Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines. World Health Organization; 2019. [PubMed] [Google Scholar]
- 9. Richards M, Strachan D, Hardy R, Kuh D, Wadsworth M. Lung function and cognitive ability in a longitudinal birth cohort study. Psychosom Med. 2005; 67(4): 602–608. [DOI] [PubMed] [Google Scholar]
- 10. Gilsanz P, Mayeda ER, Flatt J, Glymour MM, Quesenberry CP Jr, Whitmer RA. Early midlife pulmonary function and dementia risk. Alzheimer Dis Assoc Disord. 2018; 32(4): 270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pathan SS, Gottesman RF, Mosley TH, Knopman DS, Sharrett AR, Alonso A. Association of lung function with cognitive decline and dementia: the Atherosclerosis Risk in Communities (ARIC) Study. Eur J Neurol. 2011; 18(6): 888–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lutsey PL, Chen N, Mirabelli MC, et al. Impaired lung function, lung disease, and risk of incident dementia. Am J Respir Crit Care Med. 2019; 199(11): 1385–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sibbett RA, Russ TC, Allerhand M, Deary IJ, Starr JM. Physical fitness and dementia risk in the very old: a study of the Lothian Birth Cohort 1921. BMC Psychiatry. 2018; 18(1): 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011; 364(23): 2227–2234. [DOI] [PubMed] [Google Scholar]
- 15. Vidal JS, Aspelund T, Jonsdottir MK, et al. Pulmonary function impairment may be an early risk factor for late‐life cognitive impairment. J Am Geriatr Soc. 2013; 61(1): 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilson RS, Boyle PA, Yu L, Barnes LL, Schneider JA, Bennett DA. Life‐span cognitive activity, neuropathologic burden, and cognitive aging. Neurology. 2013; 81(4): 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buchman AS, Yu L, Wilson RS, Boyle PA, Schneider JA, Bennett DA. Brain pathology contributes to simultaneous change in physical frailty and cognition in old age. J Gerontol A Biol Sci Med Sci. 2014; 69(12): 1536–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jack CR Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011; 7(3): 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cleutjens FA, Spruit MA, Beckervordersandforth J, et al. Presence of brain pathology in deceased subjects with and without chronic obstructive pulmonary disease. Chron Respir Dis. 2015; 12(4): 284–290. [DOI] [PubMed] [Google Scholar]
- 20. Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis. 2018; 64(s1): S161–s189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the rush Memory and Aging Project. Curr Alzheimer Res. 2012; 9(6): 646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buchman AS, Boyle PA, Wilson RS, Gu L, Bienias JL, Bennett DA. Pulmonary function, muscle strength and mortality in old age. Mech Ageing Dev. 2008; 129(11): 625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buchman AS, Boyle PA, Wilson RS, Leurgans S, Shah RC, Bennett DA. Respiratory muscle strength predicts decline in mobility in older persons. Neuroepidemiology. 2008; 31(3): 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002; 59(2): 198–205. [DOI] [PubMed] [Google Scholar]
- 25. Wilson RS, Boyle PA, Yu L, et al. Temporal course and pathologic basis of unawareness of memory loss in dementia. Neurology. 2015; 85(11): 984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bennett DA, Schneider JA, Aggarwal NT, et al. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community‐based cohort studies compared to standard practice in a clinic‐based cohort study. Neuroepidemiology. 2006; 27(3): 169–176. [DOI] [PubMed] [Google Scholar]
- 27. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984; 34(7): 939–944. [DOI] [PubMed] [Google Scholar]
- 28. Buchman AS, Schneider JA, Leurgans S, Bennett DA. Physical frailty in older persons is associated with Alzheimer disease pathology. Neurology. 2008; 71(7): 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Crystal HA, Schneider JA, Bennett DA, Leurgans S, Levine SR. Associations of cerebrovascular and Alzheimer's disease pathology with brain atrophy. Curr Alzheimer Res. 2014; 11(4): 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke. 2011; 42(3): 722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kakkera K, Padala KP, Kodali M, Padala PR. Association of chronic obstructive pulmonary disease with mild cognitive impairment and dementia. Curr Opin Pulm Med. 2018; 24(2): 173–178. [DOI] [PubMed] [Google Scholar]
- 32. Yohannes AM, Chen W, Moga AM, Leroi I, Connolly MJ. Cognitive impairment in chronic obstructive pulmonary disease and chronic heart failure: a systematic review and meta‐analysis of observational studies. J Am Med Dir Assoc. 2017; 18(5): 451.e451–451.e411. [DOI] [PubMed] [Google Scholar]
- 33. Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019; 200(8): e70–e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005; 25(4): 163–175. [DOI] [PubMed] [Google Scholar]
- 35. Selvackadunco S, Langford K, Shah Z, et al. Comparison of clinical and neuropathological diagnoses of neurodegenerative diseases in two centres from the Brains for Dementia Research (BDR) cohort. J Neural Transm (Vienna). 2019; 126(3): 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sachdev PS, Anstey KJ, Parslow RA, et al. Pulmonary function, cognitive impairment and brain atrophy in a middle‐aged community sample. Dement Geriatr Cogn Disord. 2006; 21(5‐6): 300–308. [DOI] [PubMed] [Google Scholar]
- 37. Yin M, Wang H, Hu X, Li X, Fei G, Yu Y. Patterns of brain structural alteration in COPD with different levels of pulmonary function impairment and its association with cognitive deficits. BMC Pulm Med. 2019; 19(1): 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stevens RD, Puybasset L. The brain‐lung‐brain axis. Intensive Care Med. 2011; 37(7): 1054–1056. [DOI] [PubMed] [Google Scholar]
- 39. Yoon S, Kim JM, Kang HJ, et al. Associations of pulmonary function with dementia and depression in an older Korean population. Psychiatry Investig. 2015; 12(4): 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Russ TC, Starr JM, Stamatakis E, Kivimäki M, Batty GD. Pulmonary function as a risk factor for dementia death: an individual participant meta‐analysis of six UK general population cohort studies. J Epidemiol Community Health. 2015; 69(6): 550–556. [DOI] [PubMed] [Google Scholar]
- 41. Terraneo L, Samaja M. Comparative response of brain to chronic hypoxia and hyperoxia. Int J Mol Sci. 2017; 18(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Butterfield DA, Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci. 2019; 20(3): 148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Daulatzai MA. Death by a thousand cuts in Alzheimer's disease: hypoxia–the prodrome. Neurotox Res. 2013; 24(2): 216–243. [DOI] [PubMed] [Google Scholar]
- 44. Blass JP. Brain metabolism and brain disease: is metabolic deficiency the proximate cause of Alzheimer dementia? J Neurosci Res. 2001; 66(5): 851–856. [DOI] [PubMed] [Google Scholar]
- 45. Fang B, Zhao Q, Ling W, Zhang Y, Ou M. Hypoxia induces HT‐22 neuronal cell death via Orai1/CDK5 pathway‐mediated Tau hyperphosphorylation. Am J Transl Res. 2019; 11(12): 7591–7603. [PMC free article] [PubMed] [Google Scholar]
- 46. Raz L, Knoefel J, Bhaskar K. The neuropathology and cerebrovascular mechanisms of dementia. J Cereb Blood Flow Metab. 2016; 36(1): 172–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Batty GD, Gunnell D, Langenberg C, Smith GD, Marmot MG, Shipley MJ. Adult height and lung function as markers of life course exposures: associations with risk factors and cause‐specific mortality. Eur J Epidemiol. 2006; 21(11): 795–801. [DOI] [PubMed] [Google Scholar]
- 48. Stephan BC, Brayne C. Vascular factors and prevention of dementia. Int Rev Psychiatry. 2008; 20(4): 344–356. [DOI] [PubMed] [Google Scholar]
- 49. Tönnies E, Trushina E. Oxidative stress, synaptic dysfunction, and Alzheimer's disease. J Alzheimers Dis. 2017; 57(4): 1105–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kalaria RN. The pathology and pathophysiology of vascular dementia. Neuropharmacology. 2018; 134(Pt B): 226–239. [DOI] [PubMed] [Google Scholar]
- 51. Love S. Oxidative stress in brain ischemia. Brain Pathol. 1999; 9(1): 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang XJ, Xu W, Li JQ, Cao XP, Tan L, Yu JT. Early‐life risk factors for dementia and cognitive impairment in later life: a systematic review and meta‐analysis. J Alzheimers Dis. 2019; 67(1): 221–229. [DOI] [PubMed] [Google Scholar]
- 53. Hofman A, Breteler MM, van Duijn CM, et al. The Rotterdam Study: objectives and design update. Eur J Epidemiol. 2007; 22(11): 819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. White L, Petrovitch H, Ross GW, et al. Prevalence of dementia in older Japanese‐American men in Hawaii: the Honolulu‐Asia Aging Study. Jama. 1996; 276(12): 955–960. [PubMed] [Google Scholar]
- 55. Xu WL, Pedersen NL, Keller L, et al. HHEX_23 AA genotype exacerbates effect of diabetes on dementia and Alzheimer disease: a population‐based longitudinal study. PLoS Med. 2015; 12(7): e1001853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


