Abstract
Background
Epidemiological studies of mild cognitive impairment (MCI) and subtypes of MCI have rarely focused on rural residents in China.
Methods
This population‐based study included 5068 participants (age ≥60 years) who were living in rural communities. We defined MCI, amnestic MCI (aMCI), and non‐amnestic MCI (naMCI) following the Petersen's criteria that integrated neuropsychological assessments with in‐person clinical evaluations.
Results
The overall prevalence of MCI, aMCI, and naMCI was 26.48%, 22.30%, and 4.18%, respectively. The prevalence of MCI increased with age. The adjusted odds ratio (OR) of MCI was 0.71 (95% confidence interval [CI] 0.61 to 0.82) for primary school (vs. illiteracy), 0.30 (0.24 to 0.39) for middle school or above, 1.35 (1.09 to 1.67) for being farmers, 0.65 (0.54 to 0.78) for alcohol consumption, 1.43 (1.20 to 1.70) for stroke history, and 1.14 (0.95 to 1.36) for any apolipoprotein E (APOE) ε4 allele (vs ε3/ε3).
Conclusions
MCI affects over one‐fourth of rural older adults in China. Overall MCI was associated with demographic factors, non‐alcohol consumption, and stroke, but not with APOE genotype and cardiometabolic factors.
Keywords: mild cognitive impairment, population‐based study, prevalence, rural, subtype
1. INTRODUCTION
Mild cognitive impairment (MCI) is considered an intermediate stage between normal aging and dementia. The concept of MCI has evolved in the past 20 years to accommodate heterogeneity in etiologies and prognostic outcomes by differentiating MCI into various subtypes. 1 Amnestic MCI (aMCI), the most common subtype of MCI, involves impairment in episodic memory that is more likely to progress to typical Alzheimer's disease (AD), whereas non‐amnestic MCI (naMCI) involves impairment in cognitive domains other than memory that more often evolves to atypical AD (eg, posterior cortical atrophy and logopenic variant of primary progressive aphasia) and other types of dementia (eg, vascular dementia, Lewy body dementia, and frontotemporal dementia). 2 , 3
Over the past two decades, several population‐based studies have reported the prevalence of MCI among older adults in China, with the prevalence ranging from 5% to 28%. 4 The wide range of MCI prevalence is due partly to differences in demographic features of the study populations, as well as the neuropsychological tests and operational criteria used to define MCI. 5 Most of the previous studies have targeted urban populations with relatively high education or high socioeconomic position, whereas data from rural populations are sparse. 6 Furthermore, most epidemiological studies in China have used only brief cognitive screening tests to define MCI such as the Mini‐Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA), 7 and the standard neuropsychological test battery has been rarely used to define MCI and subtypes of MCI. Thus epidemiological data of MCI in China may not be comparable with those from studies where the guidelines‐based approaches have been used in defining MCI and its subtypes. Indeed, both brief cognitive screening instruments and comprehensive neuropsychological test battery have been recommended in assessing and defining MCI by the practice guidelines. 8 In addition, current guidelines have recommended that the diagnosis of MCI should be based ultimately on a clinical evaluation of cognitive function and functional status and not solely on a specific test score. 8 Taken together, there remains a knowledge gap with regard to epidemiology of MCI and main MCI subtypes among Chinese older adults, especially among rural‐dwelling older adults, where MCI and subtypes of MCI are defined following the internationally standard criteria.
In this community‐based study of rural‐dwelling Chinese older adults, we defined MCI and MCI subtypes by using the standard approach that integrated neuropsychological evaluations with the consensus clinical diagnosis. Our aims were to (1) determine the prevalence of MCI, aMCI, and naMCI; and (2) examine the cross‐sectional associations of MCI and its subtypes with demographic features, lifestyle, clinical factors, and apolipoprotein E (APOE) genotype.
RESEARCH IN CONTEXT
Systematic Review: We searched PubMed for literature reporting prevalence of mild cognitive impairment (MCI) and subtypes. Most studies have targeted urban populations and used only brief cognitive screening tests to define MCI. The practice guidelines‐based approaches, which require both neuropsychological assessment and clinical evaluation, are rarely used in defining MCI and subtypes.
Interpretations: In this community‐based study of rural‐dwelling older adults, we defined MCI, amnestic MCI (aMCI), and non‐amnestic MCI (naMCI) via in‐depth neuropsychological assessments and clinical evaluations. We found that MCI affected over one‐fourth of older adults and that over four‐fifths of them were of the amnestic type. MCI was associated with illiteracy, being farmers, non‐alcohol consumption, and stroke, but not with apolipoprotein E (APOE) genotype.
Future directions: Future follow‐up data will help determine the incidence and natural history of MCI and subtypes, and clarify demographic, genetic, and modifiable factors for progression from normal cognition through MCI to dementia in rural Chinese older adults.
HIGHLIGHTS
Mild cognitive impairment (MCI) was defined via neuropsychological and clinical assessments.
MCI affected over one‐fourth of Chinese rural older adults, and over four‐fifths were amnestic type.
MCI was correlated with demographic factors and stroke, but not with apolipoprotein E (APOE) genotype.
2. METHODS
2.1. Study participants
This community‐based cross‐sectional study included baseline participants in the Multimodal Interventions to Delay Dementia and Disability in Rural China (MIND‐China) project, 9 , 10 which is part of the World‐Wide Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (WW‐FINGERS) Network. 11 Figure 1 shows the flowchart of the study participants. In brief, MIND‐China targets all registered residents (n = 7698) who were ≥60 years of age by the end of 2017 and were living in Yanlou Town, Yanggu County, western Shandong Province, China. The Yanlou Town was a typical rural area, with a population of ≈52 800 residents who were living in 52 villages that were spread over 67 km2. Most adult residents in the town engaged in agricultural work. In March to September 2018, the baseline examination for MIND‐China was incorporated in the annual health check‐up at the Yanlou Town hospital provided by local government for all residents who were ≥65 years of age, but we additionally invited residents who were 60 to 64 years of age for the MIND‐China project. Of all the 7698 eligible participants, 1933 were dropouts due to death prior to the examination (n = 128), refusal (n = 1200), being not reachable (n = 569), and severe mental illness (n = 36). Thus, a total number of 5765 residents (74.9% of all eligible) participated in the baseline examination. Individuals who either refused to participate or were not reachable (n = 1769) were older (mean age 71.38 vs 70.89 years, P = .010) and more likely to be men (45.56% vs 42.81%, P = .041) than those examined participants. For the present study, of the 5765 examined participants, we further excluded 307 persons who were diagnosed with prevalent dementia according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) criteria and 390 who had missing or insufficient information for defining MCI, leaving 5068 participants for the analysis (Figure 1).
FIGURE 1.
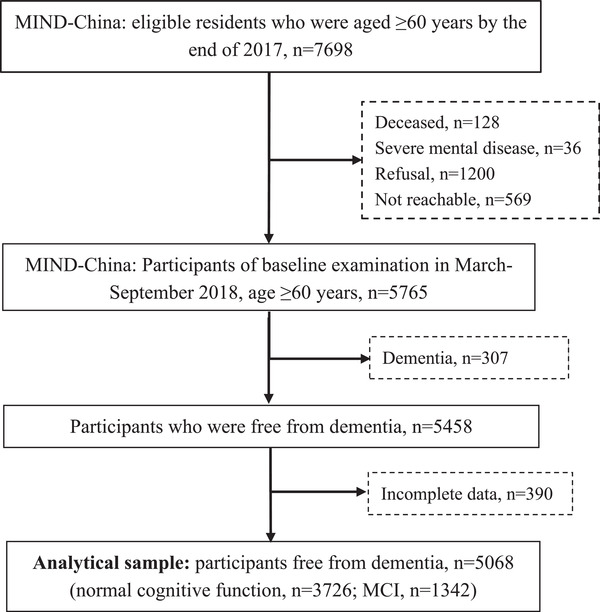
Flowchart of study participants. MIND‐China, multimodal interventions to delay dementia and disability in Rural China; MCI, mild cognitive impairment
The MIND‐China protocol was approved by the ethics committee at Shandong Provincial Hospital affiliated with the Shandong University in Jinan, Shandong. Written informed consent was obtained from all participants, or in the case of cognitively impaired persons, from a proxy (usually a guardian or a family member). MIND‐China was registered in the Chinese Clinical Trial Registry (ChiCTR, www.chictr.org.cn; Registration no.: ChiCTR1800017758).
2.2. Data collection
Trained medical staff (eg, junior neurologists or senior postgraduate students in the major of neurology from Shandong Provincial Hospital) collected data through face‐to‐face interviews, clinical examinations, neuropsychological testing, and laboratory tests (laboratory technicians at local hospital), as reported previously. 9 , 10 Sociodemographic, epidemiological, clinical, and neuropsychological data were collected following a structured questionnaire. The questionnaire included sociodemographic features (age, sex, education, and occupation), lifestyle habits (eg, smoking and alcohol drinking), and medical history. Medical history included cardiometabolic risk factors and related disorders (eg, hypertension, diabetes mellitus, hyperlipidemia, ischemic heart disease, heart failure, atrial fibrillation, and stroke). The neurological examination aimed to assess motor and sensory function, status of the cranial nerves, and reflexes. The 15‐item Geriatric Depression Scale (GDS‐15) was used to assess the presence of depressive symptoms. 12
2.3. APOE genotyping
Peripheral blood samples were taken and then DNA was extracted. APOE genotype was determined using the TaqMan single‐nucleotide polymorphism (SNP) method. APOE genotype was divided into ε2/ε2 or ε2/ε3, ε3/ε3 (reference), and any ε4 allele.
2.4. Neuropsychological assessments
Subjective cognitive decline was assessed using three questions of memory problems the participants experienced in the past year, that is, difficulty remembering, forgetting what had been planned, and worry about memory decline. We also used the Clinical Dementia Rating Scale (CDR) 13 to assess participants’ cognitive alterations. We used the Chinese version of MMSE to assess global cognitive function, 14 and the Chinese version of Activities of Daily Living Scale (ADLs) to evaluate self‐care and instrumental activities of daily living. 15 We assessed four specific cognitive domains: episodic memory (Auditory Verbal Learning Test [AVLT]‐immediate recall, long‐delayed free recall, and long‐delayed recognition), 16 language (Verbal Fluency Test‐categories of animals, fruits, and vegetables), 17 attention (Digit Span Test [DST] ‐forward 18 and Trail Making Test [TMT] A 19 ), and executive function (DST‐backward 18 and TMT B 19 ). The raw test scores were standardized into z scores using the age‐ and education‐specific means and standard deviations (SDs), derived from the study participants who were free from dementia. Because all cognitive domains were assessed using more than one test, we created the composite z score for each of the cognitive domains by averaging the z scores of the tests for that domain. Table S1 shows data of raw neuropsychological test scores by age groups and education among participants who were free from dementia.
2.5. Diagnosis of MCI, aMCI, and naMCI
All participants were divided into 10 groups according to both age (60 to 64, 65 to 69, 70 to 74, 75 to 79, and ≥80 years) and education (illiterate and non‐illiterate). Then, among dementia‐free participants, persons with a cognitive z score ≥1.0 SD below mean scores of the age‐ and education‐specific groups in any of the four cognitive domains were considered to have objective cognitive impairment. 20 , 21 However, the final judgment about objective cognitive impairment was based on both neuropsychological test scores and a consensus agreement among neurologists specialized in clinical diagnosis, treatment, and care of cognitive disorders (L.C., T.H., X.H., L.Y., Q.Z., and J.F.), while taking into account participants’ education, occupation, visual or hearing impairment, and other information.
We defined MCI and subtypes following the Petersen's criteria that were operationalized in the Mayo Clinic Study of Aging: 21 (1) cognitive concern by the subject (responses to the three questions of memory problems), informant or physician (CDR ≥0.5); (2) objective cognitive impairment evidenced in at least one of the four cognitive domains (from cognitive test battery); (3) essentially preserved function of daily activities (from the ADLs); and (4) absence of dementia (DSM‐IV criteria). MCI was then categorized into aMCI if memory domain was impaired or naMCI if there was no impairment in memory.
2.6. Statistical analysis
Characteristics of the study participants were compared using Mann‐Whitney U test for continuous variables and chi‐square test for categorical variables. The crude prevalence of MCI and MCI subtypes in the total sample and by sex was calculated, along with 95% confidence intervals (CIs). Then, because the age‐ and sex‐structures of our study sample differed from those of the national population, which might confound the crude prevalence rates, we used demographic data of rural population from the Sixth China National Population Census (2010) to standardize the overall prevalence rates of MCI and subtypes (direct method). Furthermore, because many population‐based studies in the literature targeted people ≥65 years of age, to make our results comparable with these studies, we also reported the overall and standardized prevalence rates of MCI and its subtypes among people ≥65 years of age. In addition, we conducted additional analysis by using a cut‐off cognitive z score ≥1.5 SD below the age‐ and education‐specific mean scores to define objective cognitive impairment and evaluated the impact of a different cut‐off score of cognitive tests on prevalence and correlates of MCI and subtypes of MCI. We used the bar graphs to show the age‐ and sex‐specific prevalence rates (95% CIs) of MCI, aMCI, and naMCI. Finally, we used logistic regression models to examine the associations of MCI, aMCI, and naMCI with sociodemographic features, lifestyles, clinical conditions, and APOE genotype. We conducted this analysis in the total sample and by sex. SAS 9.4 software (SAS Institute Inc., 2013, Cary, NC, USA) was used for all statistical analyses.
3. RESULTS
3.1. Characteristics of the study participants
The mean age of all participants was 70.17 years (SD 5.23), 56.45% were women, 37.12% were illiterate, and 82.48% were farmers. Compared to men, women were less educated, more likely to be farmers, and had lower scores on domains of language, attention, and executive function, and a higher GDS‐15 score (P < .01). In addition, 15.87% of all participants carried the APOE ε4 allele (Table 1).
TABLE 1.
Characteristics of study participants in the total sample and by sex
| Characteristics a | Total sample (n = 5068) | Men (n = 2207) | Women (n = 2861) | P‐value* |
|---|---|---|---|---|
| Age (years), mean (SD) | 70.17 (5.23) | 70.16 (5.16) | 70.18 (5.28) | .978 |
| Age group (years), n (%) | .067 | |||
| 60‐64 | 499 (9.85) | 213 (9.65) | 286 (10.00) | |
| 65‐69 | 2051 (40.47) | 905 (41.01) | 1146 (40.06) | |
| 70‐74 | 1548 (30.54) | 654 (29.63) | 894 (31.25) | |
| 75‐79 | 675 (13.32) | 321 (14.54) | 354 (12.37) | |
| ≥80 | 295 (5.82) | 114 (5.17) | 181 (6.33) | |
| Educational level, n (%) | <.001 | |||
| Illiterate | 1881 (37.12) | 241 (10.92) | 1640 (57.32) | |
| Primary school | 2238 (44.16) | 1174 (53.19) | 1064 (37.19) | |
| Middle school and above | 949 (18.73) | 792 (35.89) | 157 (5.49) | |
| Occupation, n (%) | <.001 | |||
| Farmers | 4161 (82.48) | 1476 (66.88) | 2685 (93.85) | |
| Non‐farmers | 884 (17.52) | 731 (33.12) | 176 (6.15) | |
| Smoking, n (%) | <.001 | |||
| Never or former smoking | 3954 (78.05) | 1127 (51.11) | 2827 (98.81) | |
| Current smoking | 1112 (21.95) | 1078 (48.89) | 34 (1.19) | |
| Alcohol consumption, n (%) | <.001 | |||
| Never or former drinking | 3508 (69.78) | 851 (39.22) | 2657 (93.00) | |
| Current consumption | 1519 (30.22) | 1319 (60.78) | 200 (7.00) | |
| Clinical conditions, n (%) | ||||
| Hypertension | 3360 (66.87) | 1433 (65.40) | 1927 (68.00) | .053 |
| Diabetes mellitus | 717 (14.15) | 248 (11.24) | 469 (16.39) | <.001 |
| Hyperlipidemia | 1216 (23.99) | 362 (16.40) | 854 (29.85) | <.001 |
| Ischemic heart disease | 1056 (20.84) | 378 (17.13) | 678 (23.70) | <.001 |
| Heart failure | 136 (2.68) | 54 (2.45) | 82 (2.87) | .360 |
| Atrial fibrillation | 75 (1.48) | 36 (1.63) | 39 (1.37) | .436 |
| Stroke | 751 (14.82) | 341 (15.45) | 410 (14.33) | .266 |
| APOE ε4 allele, n (%) | 779 (15.87) | 314 (14.69) | 465 (16.78) | .047 |
| Subjective cognitive complaints, n (%) | 4415 (87.12) | 1827 (82.78) | 2588 (90.46) | <.001 |
| GDS‐15 score, mean (SD) | 1.41 (2.14) | 1.25 (1.91) | 1.53 (2.29) | <.001 |
| Cognitive domains (z score), mean (SD) | ||||
| Memory | −0.02 (0.88) | −0.02 (0.87) | −0.03 (0.89) | .686 |
| Language | −0.00 (0.80) | 0.15 (0.79) | −0.12 (0.79) | <.001 |
| Attention | −0.04 (0.86) | 0.30 (0.81) | −0.31 (0.80) | <.001 |
| Executive function | −0.10 (0.91) | 0.32 (0.81) | −0.42 (0.85) | <.001 |
Abbreviations: APOE, apolipoprotein E; GDS‐15, the 15‐item Geriatric Depression Scale; SD, standard deviation.
Information was missing in 23 for occupation, 2 for smoking, 41 for alcohol consumption, 43 for hypertension, 2 for heart failure, 10 for atrial fibrillation, and 159 for APOE genotype.
P‐value is for the test of differences between women and men.
3.2. Prevalence of MCI and MCI subtypes
Of all 5068 participants who were ≥60 years of age, MCI was defined in 1342 persons, including 1130 with aMCI and 212 with naMCI, and the crude prevalence was 26.48% for overall MCI, 22.30% for aMCI, and 4.18% for naMCI (Table 2). The crude prevalence rates among people ≥65 years of age were 27.25% for MCI, 23.05% for aMCI, and 4.20% for naMCI. Amnestic MCI accounted for 84.20% of all participants with MCI. Compared with men, women had higher crude prevalence rates of MCI (30.90% vs 20.75%, P < .001), aMCI (25.52% vs 18.12%, P < .001), and naMCI (5.38% vs 2.63%, P < .001). Using the 2010 China National Census data (rural population) as standard population, the age‐ and sex‐standardized prevalence rates of MCI, aMCI, and naMCI decreased slightly among older adults age ≥60 years, but MCI and aMCI increased slightly among those age ≥65 years (Table 2). In addition, among people who were age ≥70 years (n = 2518), the prevalence rates of overall MCI, aMCI, and naMCI were 29.19% (95% CI 27.41% to 30.97%), 25.06% (23.37% to 26.75%), and 4.13% (3.35% to 4.91%), respectively.
TABLE 2.
Crude and standardized prevalence (per 100 population) of mild cognitive impairment and subtypes in the total sample and by sex
| Crude prevalence (95% CI), per 100 population | Standardized prevalence (95% CI)a, per 100 population | ||||
|---|---|---|---|---|---|
| MCI or subtypes | No. of cases | Age ≥60 years | Age ≥65 years | Age ≥60 years | Age ≥65 years |
| Total sample (n = 5068) | |||||
| MCI | 1342 | 26.48 (25.27‐27.69) | 27.25 (25.96‐28.54) | 25.60 (24.40‐26.81) | 28.77 (27.46‐30.09) |
| aMCI | 1130 | 22.30 (21.15‐23.44) | 23.05 (21.83‐24.27) | 21.60 (20.46‐22.73) | 24.75 (23.50‐26.00) |
| naMCI | 212 | 4.18 (3.63‐4.73) | 4.20 (3.62‐4.78) | 4.01 (3.47‐4.55) | 4.03 (3.46‐4.60) |
| Women (n = 2861) | |||||
| MCI | 884 | 30.90 (29.21‐32.59) | 32.00 (30.20‐33.80) | 30.28 (28.60‐31.96) | 34.55 (32.71‐36.38) |
| aMCI | 730 | 25.52 (23.92‐27.11) | 26.49 (24.78‐28.19) | 25.17 (23.58‐26.76) | 29.01 (27.26‐30.77) |
| naMCI | 154 | 5.38 (4.56‐6.21) | 5.51 (4.63‐6.40) | 5.11 (4.31‐5.92) | 5.53 (4.65‐6.42) |
| Men (n = 2207) | |||||
| MCI | 458 | 20.75 (19.06‐22.44) | 21.11 (19.32‐22.90) | 20.78 (19.08‐22.47) | 22.56 (20.72‐24.39) |
| aMCI | 400 | 18.12 (16.52‐19.73) | 18.61 (16.90‐20.31) | 17.91 (16.31‐19.51) | 20.15 (18.39‐21.91) |
| naMCI | 58 | 2.63 (1.96‐3.30) | 2.51 (1.82‐3.19) | 2.87 (2.17‐3.56) | 2.40 (1.73‐3.07) |
Abbreviations: aMCI, amnestic mild cognitive impairment; CI, confidence interval; MCI, mild cognitive impairment; naMCI, non‐amnestic mild cognitive impairment.
The prevalence of MCI increased with age, from 19.44% in people aged 60 to 64 years to 40.00% in those aged ≥80 years, and the prevalence rate was higher in women than in men across all age groups (Figure 2). The age‐ and sex‐specific prevalence patterns of aMCI were similar to those of overall MCI, but the age‐specific trends and sex differences in prevalence of naMCI were less pronounced (Figure 2).
FIGURE 2.
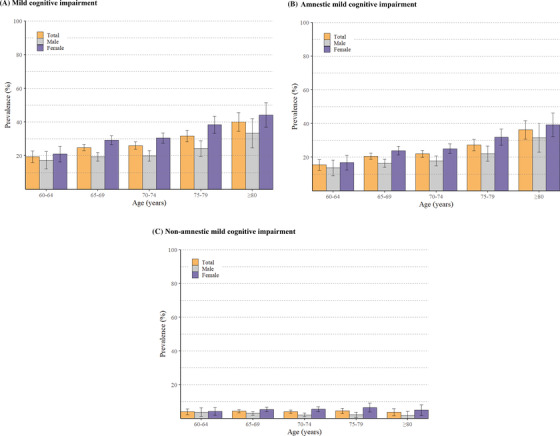
Age‐ and sex‐specific prevalence (per 100 population) and 95% confidence intervals of mild cognitive impairment (MCI) (A), amnestic MCI (B), and non‐amnestic MCI (C)
3.3. Correlates of MCI and MCI subtypes
After controlling for age, education, clinical conditions, and APOE genotype, there was no significant sex difference in the prevalence of overall MCI, aMCI, and naMCI (Figure 3). High educational attainment was significantly associated with a low likelihood of MCI, aMCI, and naMCI (P for trend < .001). Being a farmer was significantly associated with an increased likelihood of MCI, aMCI, and naMCI. Alcohol consumption was associated with a reduced likelihood of MCI and aMCI, but not with naMCI. Hypertension and stroke history were significantly associated with an increased likelihood of naMCI, but not aMCI. None of diabetes, hyperlipidemia, ischemic heart disease, heart failure, atrial fibrillation, and APOE ε4 allele was significantly associated with overall MCI, aMCI, or naMCI (Figure 3). We also examined the correlates of MCI, aMCI, and naMCI in men and women separately. Overall, there were no significant sex differences in factors associated with MCI and subtypes, except that being a farmer was significantly associated with an increased likelihood of MCI, aMCI, and naMCI in men, but not in women (Figure S1).
FIGURE 3.
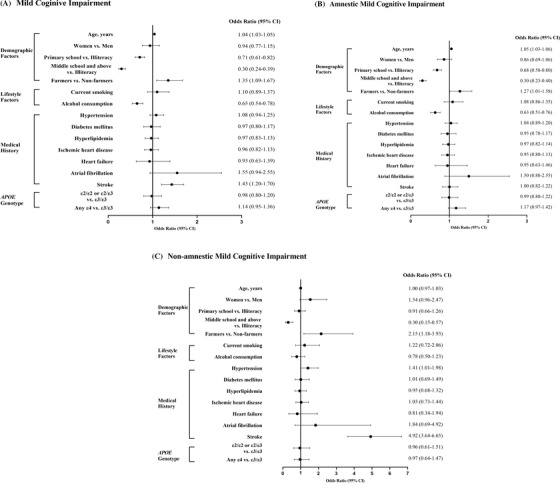
Odds ratios (95% confidence intervals) of mild cognitive impairment (MCI) (A), amnestic MCI (aMCI) (B), and non‐amnestic MCI (naMCI) associated with various factors (C). Odds ratios (95% confidence intervals) for MCI were derived from binary logistic regression models (sample size, n = 4974a); Odds ratios (95% confidence intervals) for aMCI and naMCI were derived from multinomial logistic regression models, in which participants without MCI (n = 3659) were considered as reference group in the modeling. Models were adjusted, whenever appropriate, for age, sex, education, medical history, and apolipoprotein E (APOE) genotype. aOf the 5068 participants, 94 were excluded in the analysis due to missing information, including 41 for alcohol consumption, 43 for hypertension, one for heart failure, and 9 for atrial fibrillation. In addition, information was missing in 159 persons for APOE genotype and in 23 persons for occupation, and a dummy variable for each of them was created in the analysis to represent those with missing value. CI, confidence interval; APOE, apolipoprotein E
3.4. Additional analyses
To evaluate the impact of using a different cut‐off of cognitive test scores to define objective cognitive impairment on prevalence of MCI, aMCI, and naMCI, we used the cut‐off cognitive z score ≥1.5 SD below the age‐ and education‐specific mean scores to define objective cognitive impairment. The overall prevalence and the age‐ and sex‐specific prevalence data were reported in Table S2 and Figure S2. In brief, the crude prevalence of MCI, aMCI, and naMCI among people age ≥60 years was 15.15% (95% CI 14.17% to 16.14%), 12.53% (11.62% to 13.44%), and 2.62% (2.18% to 3.06%), respectively (Table S2). The crude prevalence of MCI and subtypes was higher in women than in men. In addition, the prevalence of MCI increased with advancing age; from 12.63% in people aged 60 to 64 years to 18.31% in those aged ≥80 years and was higher in women than in men across all age groups. The age‐ and sex‐specific prevalence patterns of aMCI were similar to those of MCI, but the age‐specific trends and sex differences in the prevalence of naMCI were less pronounced (Figure S2). Finally, we examined various factors associated with MCI and subtypes in the total sample and by sex, as shown in Figure S3. Overall, the results of factors associated with MCI and subtypes were similar to those reported in Figure 3 and Figure S1 when the cut‐off cognitive z score ≥1.0 SD below the age‐ and education‐specific mean scores was used to define objective cognitive impairment (Figure S3).
4. DISCUSSION
To the best of our knowledge, this report is the largest single‐center study to examine the prevalence of MCI and main subtypes of MCI that targets older adults living in the rural communities in China. We applied the same definition and similar operational approaches as used in the Mayo Clinic Study of Aging to defining MCI, aMCI, and naMCI, in which comprehensive neuropsychological assessments were integrated with a consensus agreement of clinical diagnosis. Our data showed that MCI affected over one‐fourth of the rural‐dwelling adults age ≥60 years in our research communities, and that aMCI accounted for over four‐fifths of all people with MCI. Older age, low education, and being a farmer were associated with an increased likelihood of MCI and subtypes of MCI. Of the modifiable or manageable lifestyle and clinical factors, alcohol consumption was associated with a reduced likelihood of MCI and aMCI, whereas hypertension and stroke history were associated with naMCI but not with aMCI. Notably, APOE genotype was not associated with overall MCI and subtypes of MCI.
Previous studies have shown that the prevalence of MCI among older adults ranges from 10% to 74%, depending on the demographic features of the study population (eg, age and sex) and the criteria used to define MCI. 22 Different cut‐off values of cognitive test scores for defining objective cognitive impairment in the definition of MCI greatly affect prevalence of MCI. 23 Using a more stringent cut‐off value of cognitive test scores would result in a lower prevalence of MCI. For instance, the Leipzig Longitudinal Study of the Aged (LEILA 75+) from Germany showed that the prevalence of MCI was decreased by over 50% when the cut‐off of cognitive test scores changed from 1.0 SD (prevalence, 19%) to 1.5 SD (prevalence, 9%). 24 Similarly, in our study, the prevalence of overall MCI was decreased by over 40% when the cut‐off of cognitive test score changed from 1.0 SD (prevalence, 26.48%) to 1.5 SD (prevalence, 15.15%). In addition, the Rotterdam Study (age ≥55 years) showed that the overall prevalence of MCI was 10% when objective cognitive impairment was defined using a cognitive score ≥1.5 SD below the age‐ and education‐adjusted mean scores of the study population. 25 This is comparable to the prevalence of MCI in our study sample (age ≥60 years) (15.2%) when the same cut‐off of cognitive test score is used to define objective cognitive impairment. However, among people age ≥70 years, the overall MCI prevalence was ≈80% higher in our study sample compared with that of the Mayo Clinic Study of Aging (29.2% vs 16.0%), driven primarily by a higher prevalence of aMCI (25.1% vs 11.1%), whereas the prevalence of naMCI was comparable (4.1% vs 4.9%). The distinct demographic features of the study populations (eg, Han Chinese in a typical rural area vs Caucasian in an urban region; 81.3% had no schooling or only primary school vs 92.8% had education ≥9 years) and differences in certain methodological issues (eg, use of different neurocognitive instruments; the age‐ and education‐adjusted vs only age‐adjusted mean cognitive scores) might partly contribute to the higher prevalence of MCI and aMCI in our sample than that of the Mayo Clinic Study of Aging. 2 , 6 , 21 , 26 , 27 Finally, the LEILA75+ study found that MCI had the highest accuracy (balanced sensitivity and specificity) for predicting the development of dementia when MCI was defined using 1.0 SD, compared with use of 1.5 SD and 2.0 SD cut‐off scores. 28 This suggests that the cut‐off ≥1.0 SD below the mean cognitive test scores for defining objective cognitive impairment (or plus clinical assessments) may achieve a good balance between reliability and sensitivity to detect MCI. 28
The Mayo Clinic Study of Aging suggested a 54% increased likelihood of MCI in men than women, independent of demographic features, clinical factors, and APOE genotype. 21 This is contradictory to many other population‐based studies, which show that the prevalence of MCI is either higher in women than men 20 , 29 or no differences by sex. 30 Our study showed that the crude prevalence of MCI and subtypes appeared to be higher in women than in men across all age groups. In rural China, women were more likely than men to be illiterate and to engage in agricultural work. After controlling for demographic factors and medical history, overall MCI and subtypes were not associated with sex, suggesting that the sex differences in crude prevalence of MCI were attributed to differences in socioeconomic status (eg, education and occupation) and health conditions between women and men. Indeed, our study showed that an increased educational attainment was linearly associated with a decreased likelihood of MCI, whereas being a farmer was associated with a greater likelihood of MCI. It has been suggested that higher education and occupational complexity are associated with better cognitive function, possibly through greater cognitive reserve. 31
Cardiovascular risk factors and related vascular disorders play a pivotal role in cognitive decline and dementia. 32 We found an association between stroke history and overall MCI that was driven primarily by its association with naMCI. naMCI has more vascular components than aMCI 33 and might represent prodromal stage of vascular dementia or other types of dementia (eg, Lewy body dementia and Parkinson disease dementia). 3 Stroke is a strong risk factor for vascular cognitive impairment and dementia. 34 Neuroimaging studies suggested that stroke injury could be linked with cognitive impairment (eg, impairment in memory and executive function) by causing extensive white matter lesions, triggering neurodegenerative process via disrupting amyloid clearance, and interacting with coexisting Alzheimer pathology. 34 , 35 In addition, we found that hypertension was associated specifically with naMCI. This is in line with the fact that chronic hypertension is associated with vascular brain injury, which could contribute to vascular cognitive impairment. 36 , 37 Previous studies have shown that atrial fibrillation is associated with cognitive impairment and dementia in the general elderly population, even among people without a history of stroke. 38 , 39 Our data did show that atrial fibrillation was associated with an elevated likelihood of MCI and its subtypes, and the lack of statistical significance is likely due to limited power (overall prevalence of atrial fibrillation was 1.48%). Future follow‐up data from our cohort may help clarify the potential causal relationship of cardiovascular risk factors and related disorders with MCI, subtypes of MCI, and dementia in rural elderly populations.
The relationship between alcohol consumption and cognitive impairment is complicated, depending on amount of consumption and quantitative approaches of alcohol consumption. 40 A meta‐analysis indicated that light‐to‐moderate alcohol intake was associated with a reduced risk of dementia and cognitive decline in older adults. 41 However, a categorical meta‐analysis found no association of alcohol consumption with MCI risk, whereas the dose‐response meta‐analysis suggested a linear relationship between amount of alcohol intake and risk of MCI. 42 The relationship of alcohol intake with MCI subtypes has been rarely reported. We found that regular alcohol consumption was associated with a reduced likelihood of MCI and aMCI, but not with naMCI. The temporal relationship of alcohol consumption with MCI and subtypes of MCI can be further explored when longitudinal data from MIND‐China become available.
The frequency of carrying APOE ε4 allele varies among different ethnic populations. The proportion of carrying the APOE ε4 allele in our dementia‐free participants was ≈16%, which is within the range of 5% to 18% reported among Asian populations, 43 but is much lower than that in populations from Northern Europe (≈25%), Central Africa (≈40%), Oceania (≈37%), and Australia (≈26%). 44 A meta‐analysis of 18 case‐control studies (14 studies were from China) showed that the APOE ε4 allele was associated with MCI. 45 However, population‐based studies have yielded mixed results with regard to the association of MCI with APOE genotype. For instance, data from the Mayo Clinic Study of Aging, 21 the Framingham Heart Study, 46 the Cardiovascular Health Study Cognition Study, 47 and an epidemiological study of MCI in Greece, 48 showed a higher prevalence of MCI among carriers than non‐carriers of the APOE ε4 allele. By contrast, the Multiethnic Community study (Caribbean Hispanic, Black, and non‐Hispanic White of European ancestry) 49 and the Shanghai Aging Study 23 showed no convincing evidence for the association of MCI with the APOE ε4 allele. Indeed, evidence supporting the association of APOE ε4 allele with MCI and dementia in Chinese older adults has been mostly from the case‐control studies, 45 in which the results might be affected by biases such as selection bias, information bias, or selective survival bias. Taken together, the association between APOE genotype and MCI might vary across ethnic groups. The relation of APOE genotype with MCI among Chinese population deserves further investigation.
The major strength of our study refers to the large community‐based sample of rural older adults in China and comprehensive cognitive assessments. Thus we were able to define MCI and subtypes following the definition and operational approaches that integrated objective measures of neurocognitive function with the consensus from careful clinical evaluations. 21 However, our study also has limitations. First, we used the neuropsychological test scores from our study participants who were free of dementia as normative data to define objective cognitive impairment, due to lack of normative data that fitted our study population. Second, the relationships of MCI and subtypes of MCI with various factors should be interpreted in the context of cross‐sectional design of this study, in which the observed associations are subject to selective survival bias and are potentially affected by reverse causality, and thus cannot be interpreted as any causal effect. In addition, people who refused or were not reachable were slightly older and more likely to be men than those examined participants, which might underestimate the prevalence of MCI. Finally, our study participants were recruited from a rural region in western Shandong province, and we did not recruit participants from urban populations. This should be kept in mind when generalizing our research findings to different study populations or settings.
Our study showed that MCI defined through neuropsychological assessment and clinical diagnosis affected over one‐fourth of rural‐dwelling Chinese adults ≥60 years of age. Furthermore, over four‐fifths of people with MCI were classified as having aMCI, a subtype that has the great potential to progress to typical Alzheimer's dementia. Finally, MCI was correlated with older age, low education, being a farmer, and stroke, but not with APOE genotypes. Future follow‐up data will contribute to the better understanding of the epidemiology and natural history of MCI and subtypes of MCI among rural‐dwelling older adults in China.
ETHICS STATEMENT
The MIND‐CHINA protocol was approved by the ethics committee at Shandong Provincial Hospital affiliated to Shandong University in Jinan, Shandong, China. Written informed consent was obtained from all participants, or in the case of cognitively impaired persons, from a proxy (usually a guardian or a family member).
AUTHOR CONTRIBUTIONS
The study concept and design: Lin Cong, Yongxiang Wang, Yifeng Du, and Chengxuan Qiu. Obtaining data: Lin Cong, Yifei Ren, Yongxiang Wang, Xiaojuan Han, Tingting Hou, Yi Dong, Ling Yin, Qinghua Zhang, Jianli Feng, Shi Tang, and Yifeng Du. Data analysis: Yifei Ren, Yi Dong, and Lin Cong. Writing of the manuscript: Lin Cong and Chengxuan Qiu. Interpretation of the results, critical revisions of the manuscript, and approval of the version for submission: All authors.
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
We would like to thank all the participants of the MIND‐China Project as well as our staff who were involved in the organization of field survey as well as data collection and management. The MIND‐China Project was supported in part by grants from the National Key R&D Program of China (grant no.: 2017YFC1310100) and the National Natural Science Foundation of China (grants no.: 81861138008 and 8191101618). This work was support by additional grants from the Integrated Traditional Chinese and Western Medicine Program in Shandong Province, the Academic Promotion Program of Shandong First Medical University (grant no.: YXH2019ZXY008), and the Taishan Scholar Program of Shandong Province, China. Chengxuan Qiu received grants from the Swedish Research Council (VR, grants no.: 2017‐00740, 2017‐05819, and 2020‐01574) for the Sino‐Sweden Network and Research Projects, the Swedish Foundation for International Cooperation in Research and Higher Education (STINT, grant no.: CH2019‐8320) for the Joint China‐Sweden Mobility program, and Karolinska Institute, Stockholm, Sweden. The funding agency had no role in the study design, data collection and analysis, and writing of this manuscript, and in the decision to submit the work for publication.
Cong L, Ren Y, Wang Y, et al. Mild cognitive impairment among rural‐dwelling older adults in China: A community‐based study. Alzheimer's Dement. 2023;19:56–66. 10.1002/alz.12629
Yifeng Du and Chengxuan Qiu shared senior authorship.
Contributor Information
Yifeng Du, Email: du-yifeng@hotmail.com.
Chengxuan Qiu, Email: chengxuan.qiu@ki.se.
REFERENCES
- 1. Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Intern Med. 2014;275(3):214‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Knopman DS, Amieva H, Petersen RC, et al. Alzheimer disease Alzheimer disease. Nat Rev Dis Primers. 2021;7(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferman TJ, Smith GE, Kantarci K, et al. Nonamnestic mild cognitive impairment progresses to dementia with Lewy bodies. Neurology. 2013;81(23):2032‐2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nie H, Xu Y, Liu B, et al. The prevalence of mild cognitive impairment about elderly population in China: a meta‐analysis. Int J Geriatr Psychiatry. 2011;26(6):558‐563. [DOI] [PubMed] [Google Scholar]
- 5. Ward A, Arrighi HM, Michels S, Cedarbaum JM. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement. 2012;8(1):14‐21. [DOI] [PubMed] [Google Scholar]
- 6. Lu Y, Liu C, Yu D, et al. Prevalence of mild cognitive impairment in community‐dwelling Chinese populations aged over 55 years: a meta‐analysis and systematic review. BMC Geriatr. 2021;21(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xue J, Li J, Liang J, Chen S. The prevalence of mild cognitive impairment in China: a systematic review. Aging Dis. 2018;9(4):706‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cong L, Ren Y, Hou T, et al. Use of cardiovascular drugs for primary and secondary prevention of cardiovascular disease among rural‐dwelling older Chinese adults. Front Pharmacol. 2020;11:608136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han X, Jiang Z, Li Y, et al. Sex disparities in cardiovascular health metrics among rural‐dwelling older adults in China: a population‐based study. BMC Geriatr. 2021;21(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kivipelto M, Mangialasche F, Snyder HM, et al. World‐Wide FINGERS Network: a global approach to risk reduction and prevention of dementia. Alzheimers Dement. 2020;16(7):1078‐1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burke WJ, Roccaforte WH, Wengel SP. The short form of the Geriatric Depression Scale: a comparison with the 30‐item form. J Geriatr Psychiatry Neurol. 1991;4(3):173‐178. [DOI] [PubMed] [Google Scholar]
- 13. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412‐2414. [DOI] [PubMed] [Google Scholar]
- 14. Katzman R, Zhang MY, Ouang Ya Q, et al. A Chinese version of the Mini‐Mental State Examination; impact of illiteracy in a Shanghai dementia survey. J Clin Epidemiol. 1988;41(10):971‐978. [DOI] [PubMed] [Google Scholar]
- 15. Guo M, Gao L, Zhang G, et al. Prevalence of dementia and mild cognitive impairment in the elderly living in nursing and veteran care homes in Xi'an, China. J Neurol Sci. 2012;312(1‐2):39‐44. [DOI] [PubMed] [Google Scholar]
- 16. Maj M, Satz P, Janssen R, et al. WHO neuropsychiatric AIDS study, cross‐sectional phase II. Neuropsychological and neurological findings. Arch Gen Psychiatry. 1994;51(1):51‐61. [DOI] [PubMed] [Google Scholar]
- 17. Mok EH, Lam LC, Chiu HF. Category verbal fluency test performance in chinese elderly with Alzheimer's disease. Dement Geriatr Cogn Disord. 2004;18(2):120‐124. [DOI] [PubMed] [Google Scholar]
- 18. Dai XY, Ryan JJ, Paolo AM, Harrington RG. Factor analysis of the mainland Chinese version of the Wechsler Adult Intelligence Scale (WAIS‐RC) in a brain‐damaged sample. Int J Neurosci. 1990;55(2‐4):107‐111. [DOI] [PubMed] [Google Scholar]
- 19. Arnett JA, Labovitz SS. Effect of physical layout in performance of the Trail Making Test. Psychol Assess. 1995;7(2):220‐221. [Google Scholar]
- 20. Di Carlo A, Lamassa M, Baldereschi M, et al. CIND and MCI in the Italian elderly: frequency, vascular risk factors, progression to dementia. Neurology. 2007;68(22):1909‐1916. [DOI] [PubMed] [Google Scholar]
- 21. Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010;75(10):889‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jak AJ, Bondi MW, Delano‐Wood L, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry. 2009;17(5):368‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ding D, Zhao Q, Guo Q, et al. Prevalence of mild cognitive impairment in an urban community in China: a cross‐sectional analysis of the Shanghai Aging Study. Alzheimers Dement. 2015;11(3):300‐309. e302. [DOI] [PubMed] [Google Scholar]
- 24. Busse A, Hensel A, Gühne U, Angermeyer MC, Riedel‐Heller SG. Mild cognitive impairment: long‐term course of four clinical subtypes. Neurology. 2006;67(12):2176‐2185. [DOI] [PubMed] [Google Scholar]
- 25. de Bruijn RF, Akoudad S, Cremers LG, et al. Determinants, MRI correlates, and prognosis of mild cognitive impairment: the Rotterdam Study. J Alzheimers Dis. 2014;42(3):S239‐249. [DOI] [PubMed] [Google Scholar]
- 26. Jia L, Du Y, Chu L, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross‐sectional study. Lancet Public Health. 2020;5(12):e661‐e671. [DOI] [PubMed] [Google Scholar]
- 27. Jia J, Zhou A, Wei C, et al. The prevalence of mild cognitive impairment and its etiological subtypes in elderly Chinese. Alzheimers Dement. 2014;10(4):439‐447. [DOI] [PubMed] [Google Scholar]
- 28. Busse A, Bischkopf J, Riedel‐Heller SG, Angermeyer MC. Subclassifications for mild cognitive impairment: prevalence and predictive validity. Psychol Med. 2003;33(6):1029‐1038. [DOI] [PubMed] [Google Scholar]
- 29. Subramaniapillai S, Almey A, Natasha Rajah M, Einstein G. Sex and gender differences in cognitive and brain reserve: implications for Alzheimer's disease in women. Front Neuroendocrinol. 2021;60:100879. [DOI] [PubMed] [Google Scholar]
- 30. Kivipelto M, Helkala EL, Hänninen T, et al. Midlife vascular risk factors and late‐life mild cognitive impairment: a population‐based study. Neurology. 2001;56(12):1683‐1689. [DOI] [PubMed] [Google Scholar]
- 31. Qiu C, Fratiglioni L. Aging without dementia is achievable: current evidence from epidemiological research. J Alzheimer's Dis. 2018;62(3):933‐942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qiu C, Fratiglioni L. A major role for cardiovascular burden in age‐related cognitive decline. Nat Rev Cardiol. 2015;12(5):267‐277. [DOI] [PubMed] [Google Scholar]
- 33. Logue MW, Panizzon MS, Elman JA, et al. Use of an Alzheimer's disease polygenic risk score to identify mild cognitive impairment in adults in their 50s. Mol Psychiatry. 2019;24(3):421‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuźma E, Lourida I, Moore SF, Levine DA, Ukoumunne OC, Llewellyn DJ. Stroke and dementia risk: a systematic review and meta‐analysis. Alzheimers Dement. 2018;14(11):1416‐1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kalaria RN, Akinyemi R, Ihara M. Stroke injury, cognitive impairment and vascular dementia. Biochim Biophys Acta. 2016;1862(5):915‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chui HC. Vascular cognitive impairment: today and tomorrow. Alzheimers Dement. 2006;2(3):185‐194. [DOI] [PubMed] [Google Scholar]
- 38. Kalantarian S, Stern TA, Mansour M, Ruskin JN. Cognitive impairment associated with atrial fibrillation: a meta‐analysis. Ann Intern Med. 2013;158(5 Pt 1):338‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ding M, Fratiglioni L, Johnell K, et al. Atrial fibrillation, antithrombotic treatment, and cognitive aging: a population‐based study. Neurology. 2018;91(19):e1732‐e1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Malarcher AM, Giles WH, Croft JB, et al. Alcohol intake, type of beverage, and the risk of cerebral infarction in young women. Stroke. 2001;32(1):77‐83. [DOI] [PubMed] [Google Scholar]
- 41. Anstey KJ, Mack HA, Cherbuin N. Alcohol consumption as a risk factor for dementia and cognitive decline: meta‐analysis of prospective studies. Am J Geriatr Psychiatry. 2009;17(7):542‐555. [DOI] [PubMed] [Google Scholar]
- 42. Lao Y, Hou L, Li J, Hui X, Yan P, Yang K. Association between alcohol intake, mild cognitive impairment and progression to dementia: a dose‐response meta‐analysis. Aging Clin Exp Res. 2021;33(5):1175‐1185. [DOI] [PubMed] [Google Scholar]
- 43. Liang S, Pan M, Geng HH, et al. Apolipoprotein E polymorphism in normal Han Chinese population: frequency and effect on lipid parameters. Mol Biol Rep. 2009;36(6):1251‐1256. [DOI] [PubMed] [Google Scholar]
- 44. Belloy ME, Napolioni V, Greicius MD. A quarter century of APOE and Alzheimer's disease: progress to date and the path forward. Neuron. 2019;101(5):820‐838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jiang Y, He T, Deng W, Sun P. Association between apolipoprotein E gene polymorphism and mild cognitive impairment: a meta‐analysis. Clin Interv Aging. 2017;12:1941‐1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jefferson AL, Beiser AS, Seshadri S, Wolf PA, Au R. APOE and mild cognitive impairment: the Framingham Heart Study. Age Ageing. 2015;44(2):307‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lopez OL, Jagust WJ, Dulberg C, et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 2. Arch Neurol. 2003;60(10):1394‐1399. [DOI] [PubMed] [Google Scholar]
- 48. Vlachos GS, Kosmidis MH, Yannakoulia M, et al. Prevalence of mild cognitive impairment in the elderly population in Greece: results from the HELIAD Study. Alzheimer Dis Assoc Disord. 2020;34(2):156‐162. [DOI] [PubMed] [Google Scholar]
- 49. Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63(4):494‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


