Abstract
Introduction
There are few randomized clinical trials in vascular cognitive impairment (VCI). This trial tested the hypothesis that the PDE5 inhibitor tadalafil, a widely used vasodilator, increases cerebral blood flow (CBF) in older people with symptomatic small vessel disease, the main cause of VCI.
Methods
In a double‐blind, placebo‐controlled, cross‐over trial, participants received tadalafil (20 mg) and placebo on two visits ≥7 days apart (randomized to order of treatment). The primary endpoint, change in subcortical CBF, was measured by arterial spin labelling.
Results
Tadalafil increased CBF non‐significantly in all subcortical areas (N = 55, age: 66.8 (8.6) years) with greatest treatment effect within white matter hyperintensities (+9.8%, P = .0960). There were incidental treatment effects on systolic and diastolic blood pressure (–7.8, –4.9 mmHg; P < .001). No serious adverse events were observed.
Discussion
This trial did not identify a significant treatment effect of single‐administration tadalafil on subcortical CBF. To detect treatment effects may require different dosing regimens.
Keywords: cerebral blood flow, clinical trials, PDE5, small vessel disease, tadalafil, vascular cognitive impairment, vascular cognitive impairment and dementia
1. BACKGROUND
Small vessel disease (SVD) is a common cause of lacunar stroke and vascular contributions to cognitive impairment and dementia. 1 , 2 SVD is common in older people, seen on brain magnetic resonance imaging (MRI) as diffuse white matter hyperintensities (WMH), focal ischemic lesions, and micro‐hemorrhages. 2 SVD is associated with reduced cerebral blood flow (CBF) particularly in subcortical areas, including deep grey nuclei, subcortical white matter, and within WMH. 3 , 4 , 5 , 6 , 7 There is currently no disease‐modifying therapy for SVD. 2 , 8
CBF is regulated by multiple factors, including nitric oxide (NO). Tonic endothelial NO activates guanylyl cyclase in overlying vascular myocytes, to drive cyclic guanosine monophosphate (cGMP) formation, leading to myocyte relaxation and vasodilation. Cytoplasmic cGMP is degraded by phosphodiesterase enzymes, in particular PDE5. Potent, selective PDE5 inhibitors (PDE5i) such as sildenafil (Viagra) and tadalafil (Cialis) are in routine use as vasodilators in erectile dysfunction and pulmonary arterial hypertension. PDE5i augment blood flow in peripheral tissues and are well tolerated across dosing regimens. 9 , 10 This study addressed the hypothesis that PDE5i increase CBF in older people, particularly in the subcortical regions affected by SVD. 11
PDE5 is present in human brain neurons 12 and in vascular myocytes within subcortical white matter. 13 Among PDE5i, tadalafil has a relatively long plasma half‐life (16 hours in healthy adults) 14 , 15 with evidence of brain penetration in rodents and primates. 16 , 17 Tadalafil is well tolerated and has been widely prescribed worldwide. 9 , 10 , 14 , 15 This article presents primary outcomes from a clinical trial with cross‐over design 8 , 18 to determine whether a single administration of tadalafil increases subcortical CBF.
2. METHODS
For Expanded Methods please see supporting information.
This trial, Perfusion by Arterial Spin Labelling Following Single Dose Tadalafil in Small Vessel Disease (PASTIS) was preregistered at http://www.clinicaltrials.gov (Unique identifier: NCT02450253) and https://eudract.ema.europa.eu (Unique identifier: 2015‐001235‐20). The data supporting this report are available from the corresponding author upon reasonable request.
2.1. Trial design, randomization, and endpoints
The trial received ethical approval from the UK National Research Ethics Service (REC reference: 15/LO/0714). Within the UK, the National Research Ethics Service, part of the National Health Service (NHS) Health Research Authority (https://www.hra.nhs.uk/) enacts the principles of the Declaration of Helsinki (and subsequent amendments; World Medical Association) for medical research involving human subjects. Written informed consent was obtained from all participants or their next of kin. Participants were enrolled by members of the trial team and randomized to order of treatment (tadalafil 20 mg, placebo; oral administration). The randomization list was generated in advance by Sharp Clinical Services, Crickhowell, Powys, UK. Each participant received, on two separate occasions—Visit#1 and Visit#2—a placebo dose and a tadalafil 20 mg dose, which were identical in size, shape, weight, and color. Two study visits were performed at least 7 days apart, with blood pressure measurement, MRI scanning, and a battery of cognitive tests up to 3 hours before and 3 to 5 hours after dosing (see Figure 1A). Participants, care providers, and those assessing outcomes were all blind to treatment allocation.
FIGURE 1.
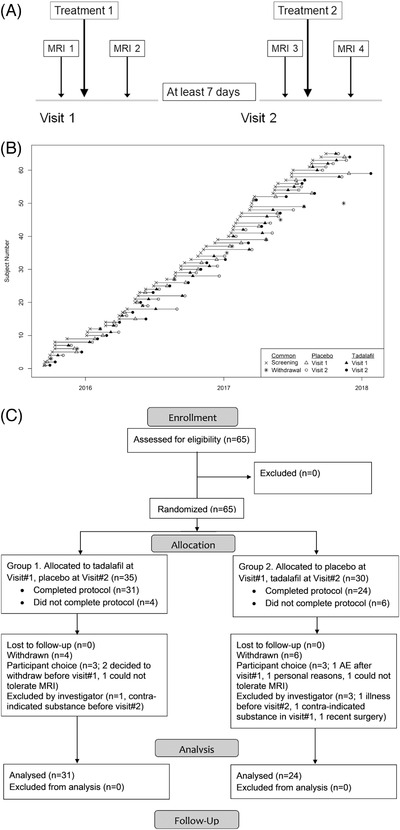
PASTIS trial design and recruitment. A, Trial design. For Group 1, Treatment 1 was tadalafil and Treatment 2 placebo, for Group 2 vice versa. B, Recruitment to PASTIS. C, CONSORT diagram. CONSORT, Consolidated Standards of Reporting Trials; MRI, magnetic resonance imaging; PASTIS, Perfusion by Arterial Spin Labelling Following Single Dose Tadalafil in Small Vessel Disease
RESEARCH IN CONTEXT
Systematic review: Brain vascular disease is a major contributor to dementia, with few treatment options. Phosphodiesterase inhibitors (PDE5i) are widely used vasodilators in peripheral vascular disease. Hence, we tested whether tadalafil, a brain‐penetrant PDE5i, changes deep brain blood flow.
Interpretation: This is the first randomized clinical trial of a PDE5i in small vessel disease. Although the data did not support a difference between single‐administration tadalafil and placebo with respect to subcortical blood flow, a trend to augmented perfusion of white matter hyperintensities (9.8%, P = .096) suggested that different treatment regimens may have clinical benefit. Combination therapy may be required to over‐ride cerebral blood flow autoregulation.
Future directions: A future trial will require a different treatment regimen in older people (age ≥ 65), with sufficient power to detect 10% blood flow augmentation in white matter hyperintensities.
HIGHLIGHTS
Single administration of the PDE5 inhibitor tadalafil:
was well tolerated in older people with symptomatic small vessel disease.
did not change cerebral blood flow (measured with arterial spin labelling), despite reduced systolic blood pressure and diastolic blood pressure.
trended enhanced perfusion within white matter hyperintensities.
The primary endpoint was change in subcortical CBF, assessed in three tissue types: deep gray matter nuclei (DGM), normal appearing white matter (NAWM), and WMH. Change in CBF for cortical gray matter was a secondary endpoint.
The trial commenced September 4, 2015 (Figure 1B). Participants were recruited from St George's Hospital and local Participant Identification Centers. All visits, data management, and trial coordination were performed at the St George's site. The trial ended when the predetermined recruitment target was met (January 25, 2018).
2.2. Study population
All data were from older adults without known diagnosis of dementia, with radiological and clinical evidence of symptomatic SVD. Inclusion criteria were as follows: (1) radiological evidence of SVD, defined as: MRI evidence of lacunar infarct(s) up to 15 mm maximum diameter and/or confluent deep WMH (grade 2 or higher on the Fazekas scale 19 ); (2) clinical evidence of SVD defined as either: lacunar stroke syndrome with symptoms lasting at least 24 hours, occurring at least 6 months prior to visit#1; or transient ischemic attack (TIA) lasting < 24 hours with limb weakness, hemi‐sensory loss, or dysarthria at least 6 months previously and with diffusion‐weighted MRI performed acutely showing lacunar infarction. If MRI was not performed within 10 days of transient ischemic attack, a lacunar infarction in an anatomically appropriate position as demonstrated on a subsequent MRI was also deemed eligible; (3) age at least 50 years; and (4) imaging of the carotid arteries in the previous 12 months, demonstrating less than 70% stenosis in both internal carotid arteries or less than 50% stenosis in both internal carotids if measured in previous 12 to 60 months. Exclusion criteria included: known diagnosis of dementia; cortical infarction (more than 15 mm maximum width); systolic blood pressure (SBP) < 90 mmHg; diastolic blood pressure (DBP) < 50 mmHg; creatinine clearance < 30 mL/min; stroke or TIA within the previous 6 months; concomitant use of PDE5i. A full list of exclusion criteria is given in the published protocol. 18
2.3. Study assessments
In the screening visit (“Visit 0″), informed consent was documented and education level and Montreal Cognitive Assessment (MoCA) scores were recorded. In study visits (Visit#1, Visit#2), participants underwent blood pressure measurements, a cognitive test battery, 18 and brain MRI. At the end of each study visit, and at least 3 hours post‐dosing, two blood samples (5 mL) were taken for full blood count and analysis of tadalafil concentration.
2.4. MRI acquisition
Whole‐brain perfusion MRI was acquired using a 3T scanner (Achieva Dual TX MRI scanner, Philips Medical Systems,) at St George's University Hospitals NHS Foundation Trust. Whole brain T1‐weighted, fluid attenuated inversion recovery (FLAIR), susceptibility‐weighted imaging (SWI), and pseudo‐continuous arterial spin labelling (pCASL) images (which included a proton‐density weighted image) were acquired. Full MRI acquisition protocol information is provided in supporting information. All MRI data were acquired from brain scans performed on a Tuesday or Thursday, pre‐dosing scans between the hours of 10:00 a.m. and 12:00 p.m. and post‐dosing scans 2:00 p.m. to 5:00 p.m. The pCASL protocol was based on the consensus recommendations of the International Society for Magnetic Resonance Medicine Perfusion study group and European ASL in Dementia consortium 20 using the Philips pCASL sequence in the scanner v5.3 software.
2.5. MRI analysis
Full MRI analysis information is included in the supporting information and Figure S1 in supporting information provides an outline of the MRI data analysis pipeline. An average pCASL map was separately computed for each pCASL data acquisition (example in Figure 2) using oxford_asl (part of the FSL‐BASIL toolset, fsl.fmrib.ox.ac.uk/fsl/fslwiki/BASIL). CBF in each voxel was calculated using the standard equation for pCASL. 20
FIGURE 2.
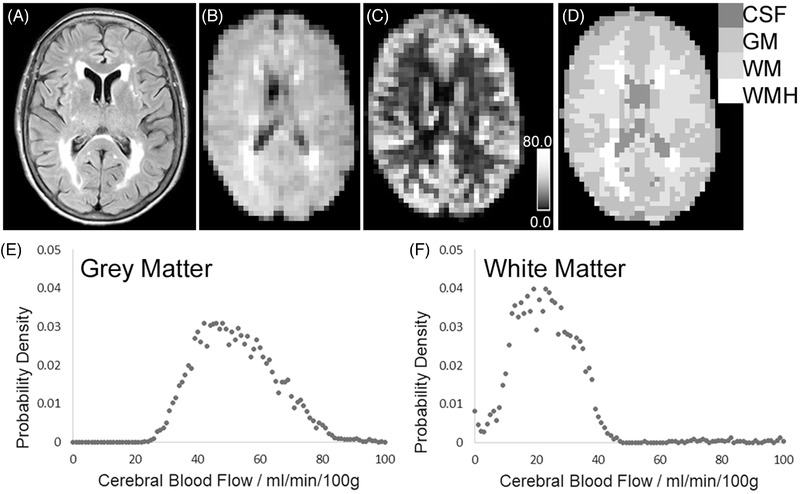
Example of anatomical and cerebral blood flow (CBF) mapping, with tissue segmentation. A, Fluid‐attenuated inversion recovery (FLAIR) image at full resolution. B, FLAIR image co‐registered to the CBF map, with voxels re‐sized to be equivalent to the pseudo‐continuous arterial spin labelling (pCASL) map. C, CBF map, derived from pCASL. Calibration bar shows 0.0–80.0 mL/min/100 g. D, Tissue segmentation map for CBF computation. Each voxel has been defined as either: cerebrospinal fluid (CSF), gray matter (GM), normal‐appearing white matter (WM), or white matter hyperintensity (WMH). E, F, Probability density functions of CBF values in voxels assigned as gray matter (E) or normal‐appearing white matter (F). For this participant, median CBF was 51.3 mL/min/100 g in gray matter and 21.8 mL/min/100 g in normal‐appearing white matter
For each scanning session, T1‐weighted images in native space were segmented into gray matter, white matter, and cerebrospinal fluid (CSF) tissue probability maps (Figure 2) using a modified form of the standard Statistical Parametric Mapping (SPM v12, https://www.fil.ion.ucl.ac.uk/spm/) described in our previous paper. 21 WMHs were delineated on FLAIR images (JIM software v7.0; Xinapse Systems Ltd). Native space T1‐weighted and FLAIR images were co‐registered to the average proton density‐weighted image, to enable alignment of the T1‐weighted tissue probability and WMH maps to the CBF data.
Each voxel in the CBF map was provisionally assigned to gray matter, NAWM, WMH, or CSF, based on the maximum tissue probability. These provisional assignments were then entered as empirical priors in a hidden Markov random field model and segmentation (FMRIB's Automated Segmentation Tool, FAST) 22 to provide an improved segmentation of gray and white matter tissue from the CBF maps. This technique reduces partial volume and tissue classification errors at the boundary between gray and white matter, caused by the relative difference between voxel sizes of the native pCASL and T1‐weighted images. For each participant at each scan session, median CBF values were calculated for total gray matter, NAWM, and WMH (example in Figure 2).
To determine CBF in DGM, the caudate, putamen, and thalamus of both hemispheres were segmented on native space T1‐weighted images using FreeSurfer (v.5.3.0, https://surfer.nmr.mgh.harvard.edu/fswiki/). Median CBF was calculated for each of these three deep gray nuclei and the average of these median values reported as CBF in DGM.
TABLE 1.
Participant demographics for the study cohort
| Participants who consented and were randomized (N = 65) | Participants who completed the protocol (N = 55) | |||||
|---|---|---|---|---|---|---|
| Variable | All | Placebo followed by tadalafil | Tadalafil followed by placebo | All | Placebo followed by tadalafil | Tadalafil followed by placebo |
| N | 65 | 32 | 33 | 55 | 25 | 30 |
| Age (y) | 66.7 | 68.0 | 65.5 | 66.8 | 67.9 | 65.9 |
| (8.7) | (8.4) | (9.0) | (8.6) | (8.4) | (8.9) | |
| Age range (y) | 52, 87 | 53, 87 | 52, 83 | 52, 87 | 53, 87 | 52, 83 |
| Female/male | 19/46 | 14/18 | 5/28 | 15/40 | 10/15 | 5/25 |
| MoCA score | 25.4 | 25.4 | 25.4 | 25.1 | 25.0 | 25.2 |
| (3.4) | (3.3) | (3.6) | (3.5) | (3.4) | (3.7) | |
| Education (y) | 12.8 (3.1) | 12.7 (2.9) | 12.8 (3.3) | 12.7 (3.2) | 12.7 (3.0) | 12.7 (3.4) |
| Time from stroke to consent (months) | 16.0 | 15.3 | 16.8 | 14.6 | 14.9 | 14.3 |
| (17.6) | (12.0) | (22.6) | (12.1) | (11.8) | (12.8) | |
| Modified Rankin score (0/1/2/3/4/5–6) | 18/26/16/3/2/0 | 7/13/10/2/0/0 | 11/13/6/1/2/0 | 16/20/14/3/2/0 | 5/10/8/2/ | 11/10/6/1/ |
| 2/0 | 0/0 | 2/0 | 2/0 | 0/0 | 2/0 | |
| NIHSS (range 0–42) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 0.5 |
| [0.0, 2.0] | [0.0, 2.0] | [0.0, 2.0] | [0.0, 2.0] | [0.0, 3.0] | [0.0, 2.0] | |
| WMH volume (mm3) | NA | NA | NA | 14,600 | 15,700 | 11,800 |
| [7,200, 31,700] | [9,200, 34,500] | [6,800, 27,600] | ||||
| Cerebral microbleeds, total count | 1 [0, 4] | 1 [0, 4] | 1 [0, 4] | 1 [0, 4] | 1.5 [0, 4] | 1 [0, 4.5] |
| SBP (mm Hg) | 145 | 147 | 144 | 145 | 147 | 144 |
| (16.6) | (17.1) | (16.4) | (16.6) | (18.7) | (14.8) | |
| DBP (mm Hg) | 81.0 | 81.0 | 81.0 | 79.9 | 79.2 | 80.5 |
| (10.7) | (9.6) | (11.9) | (10.7) | (9.7) | (11.6) | |
Notes: Data are reported as mean (SD), except for modified Rankin score (actual scores listed), NIHSS score, WMH volume, and cerebral microbleed counts, which are reported as median [interquartile range]. Scoring in MoCA ranges from 0 to 30, with a score of 26 or higher indicating normal cognitive ability. These scores have been adjusted for educational level (+1 if the participant had 12 or more years of education). SBP, DBP are the average over Visit#1 and Visit#2.
Abbreviations: DBP, diastolic blood pressure; MoCA, Montreal Cognitive Assessment; NIHSS, National Institutes of Health Stroke Scale; SBP, systolic blood pressure; SD, standard deviation; WMH, white matter hyperintensities.
2.6. Statistical analysis
All analyses were based on the intention‐to‐treat principle (i.e., participants were analyzed according to randomized treatment group regardless of whether they received the intended treatment). Change within each treatment group was analyzed using paired sample t tests. Treatment effects were defined as {(after‐before tadalafil) – (after‐before placebo)}. Treatment effects on primary and secondary outcomes were analyzed using linear mixed effects models with fixed effects of baseline value, treatment, visit, and random effect of subject. Models were not corrected for age, blood pressure, or full blood count. Analyses were conducted using R v.3.4.1 with the lme4 and lmerTest packages (https://www.R‐project.org/). No corrections were made for multiple comparisons. P < .05 was considered significant.
3. RESULTS
Sixty‐five individuals gave consent and were randomized, 59 commenced the protocol, 55 completed the protocol, and 53 had a full set of usable CBF data (see CONSORT diagram, Figure 1C). There were no significant demographic differences between those randomized and those who completed the protocol (Table 1).
Ten participants experienced adverse events, eight while on placebo treatment and two while on tadalafil (described in Table S1 in supporting information). These included headache, nausea, sore throat, knee pain, respiratory infections, a diabetic hypoglycemic event, a panic attack in the MRI scanner. There were no serious adverse reactions. The cohort were older adults (age range: 52–87 years, Table 1), all of whom had symptomatic SVD with typical MRI manifestations. Although the protocol permitted inclusion of TIA patients, in practice all had a lacunar stroke event, verified on MRI. In all cases, Visit#1 took place at least 6 months post‐stroke. Visit#1 and Visit#2 were 20 (19) days apart (mean [standard deviation (SD)]; range 7–117 days). Four participants completed Visit#2 more than 30 days after Visit#1 (range 54–117 days). In blood samples taken 3 to 6 hours post‐drug administration, plasma tadalafil concentration was 520 (160) nmol/L (range 300–980 nmol/L, n = 53).
Following tadalafil administration, CBF increased in all three subcortical tissue types (DGM, NAWM, and WMH) as well as in total gray matter (Figure 3A‐D; Table 2). CBF also increased following placebo in DGM, NAWM, and total gray matter, but not in WMH (Table 2). Treatment effects of tadalafil on CBF were modest and not significant: 0.11 mL/min/100 g in DGM (P = .881), 0.33 mL/min/100 g in NAWM (P = .458), 0.95 mL/min/100 g in WMH (P = .0960), and 0.56 mL/min/100 g in total gray matter (P = .456). The highest treatment effect was in WMH, representing a 9.8% increase in CBF. There was no significant effect of group allocation (tadalafil at Visit#1 and placebo at Visit#2, or vice versa). No significant carry‐over effect was detectable in any of the statistical models (P > .180 for all treatment–period interactions).
FIGURE 3.
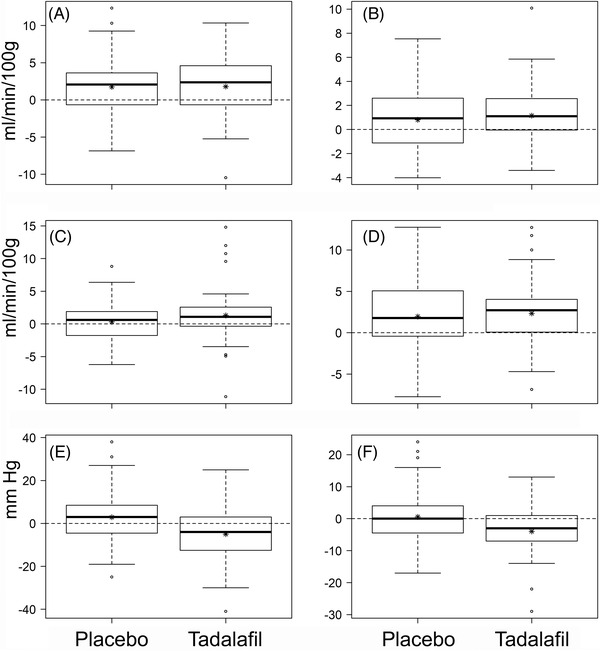
Distributions of change (after–before) after placebo or tadalafil for cerebral blood flow (CBF) and blood pressure. A‐D, Change in CBF (mL/min/100 g) in deep gray nuclei (A), normal‐appearing white matter (B), white matter hyperintensities (C), and total gray matter (D). E,F, Change in systolic blood pressure (SBP; E) and diastolic blood pressure (DBP; F) (mmHg). Box‐whisker plots show median, interquartile range (IQR), and full range. Asterisks indicate mean values. For each of the parameters presented, mean values are listed in Table 2. Individual data points shown are > 3 IQR from the median
TABLE 2.
Effect of placebo, tadalafil, and treatment effect on CBF and blood pressure
| Variable | Pre‐dose value, placebo; mean (SD) | Pre‐dose value, tadalafil; mean (SD) | Change following placebo;mean(95% CI) | Change following Tadalafil.Mean(95% CI) | Treatment effect.Mean (95% CI) |
|---|---|---|---|---|---|
| Deep gray matter CBF (mL/min/100 g) |
24.2 (6.1) |
24.5 (7.0) |
1.75 (0.74, 2.76) P = .0010 |
1.79 (0.71, 2.88) p=0.0016 |
0.11 (−1.27, 1.48) p=0.881 |
| Normal appearing white matter CBF (mL/min/100 g) |
13.5 (4.5) |
13.5 (5.2) |
0.80 (0.14, 1.47) P = .0185 |
1.15 (0.49, 1.80) p=0.0009 |
0.33 (−0.54, 1.21) p=0.458 |
| White matter hyperintensities CBF (mL/min/100 g) |
9.5 (5.6) |
9.4 (5.9) |
0.32 (–0.48, 1.12) P = .424 |
1.29 (0.21, 2.38) p=0.0203 |
0.95 (−0.15, 2.05) p=0.0960 |
| Total gray matter CBF (mL/min/100 g) |
33.0 (7.8) |
33.4 (8.7) |
2.05 (0.93, 3.17) P = .0006 |
2.54 (1.48, 3.61) p<0.0001 |
0.56 (−0.90, 2.02) p=0.456 |
| SBP (mmHg) |
145 (16.0) |
145 (15.6) |
2.9 (–0.6, 6.4) p = 0.107 |
−5.0 (−8.6, −1.4) p=0.0068 |
−7.8 (−11.8, ‐3.9) p=0.0004 |
| DBP (mmHg) |
80.6 (8.6) |
79.6 (9.4) |
0.6 (–1.6, 2.7) P = .619 |
−4.0 (−6.0, −2.0) p=0.0002 |
−4.9 (−7.3, −2.4) p=0.0003 |
Notes: Changes are presented with effect estimate, 95% confidence intervals, and associated P‐values. SBP and DBP data were derived from post hoc exploratory analyses (not prespecified endpoints). P‐values were not adjusted for age or sex. Analyses were based on 55 participants who completed the protocol. One participant had incomplete placebo CBF data and one other incomplete tadalafil CBF data, hence n = 53 for CBF data. For SBP and DBP, n = 55.
Abbreviations: CBF, cerebral blood flow; CI, confidence interval; DBP, diastolic blood pressure; SBP, systolic blood pressure; SD, standard deviation.
As incidental findings, there were modest but significant treatment effects on SBP (–7.8 mmHg, P < .001) and DBP (–4.9 mmHg, P < .001) in post hoc analyses (Figure 3E‐F; Table 2).
4. DISCUSSION
This article reports primary outcomes from the first double‐blind, randomized placebo‐controlled clinical trial of PDE5i treatment on CBF in older people with SVD. The main finding was that single administration of tadalafil did not significantly increase CBF relative to placebo in subcortical tissue or in total gray matter (which is dominated by cortical gray matter). As an incidental finding, tadalafil decreased SBP and DBP relative to placebo.
The dose of tadalafil (20 mg) was within the range licensed for prescribing (5–40 mg) and between the dose typically prescribed in erectile dysfunction (5–10 mg) and that used in clinical trials for pulmonary arterial hypertension (40 mg). Tadalafil‐dependent reduction in blood pressure confirmed active plasma concentrations of drug. The plasma concentrations recorded were consistent with previous pharmacokinetic studies. 14 , 15 Based on the published brain:plasma ratio of approximately 1:10 in rodents and experimental primates, 16 , 17 brain tadalafil concentration in the range 30 to 100 nmol/L is estimated for the participants in PASTIS. Hence, brain tadalafil levels in this trial were likely to be at least 6‐fold above the concentration required for half‐maximal PDE5 inhibition (pharmacological IC50 values in the range 1–5 nmol/L).
An unexpected finding was significant CBF elevation in DGM, NAWM, and total gray matter following placebo treatment. There was no effect of order of treatment (group allocation). Considering total gray matter, where the signal–noise ratio for CBF measurement is highest, the post‐placebo increase was evident in 41 (72%) participants who received placebo. This appears a high proportion for a true placebo effect. Alternatively, the observation may reflect diurnal CBF variation, possibly mediated by circadian changes in circulating hormones, vasomotor tone, or psychological arousal. Cyclical variations in hemodynamic parameters, including CBF, are reported in experimental animals 23 and in human subjects. 24 , 25 , 26 , 27 This unexpected finding emphasizes the need for a placebo group in studies of CBF, even where a cross‐over design is used. Future trials should specify the time of day for CBF measurement.
Tadalafil‐mediated treatment effects on CBF were small (range 0.4–9.8% across tissue types) and non‐significant (Table 2). As gray matter CBF data have high signal–noise and statistical power, the findings suggest that tadalafil‐mediated gray matter CBF changes are unlikely, at least in a single‐dosing regimen. Failure of target engagement is unlikely, as brain tadalafil concentration is estimated to be high and PDE5 is present in vascular myocytes of older people. 13 The greatest treatment effect was within WMH, equivalent to a 9.8% increase (P = .0960, Table 2). This finding raises two questions. First, is this a true increase (and our finding a false negative)? This trial was designed to detect treatment effects of 15% or more. 18 To confidently detect a 9.8% increase in perfusion of white matter tissue would require a substantially larger cohort. Second, what would be the clinical impact of a 9.8% increase in WMH blood flow? Such an increment, if confirmed, would at least indicate that small vessels within WMH are amenable to pharmacotherapy. Previous studies support the concept that CBF regulation within WMH differs from surrounding NAWM. 3 , 5 , 6 Given the nonlinear relation between brain perfusion and tissue damage, it is conceivable that such a modest elevation in local blood flow could be beneficial. In other tissues, chronic PDE5i treatment produced therapeutic myocardial remodeling in heart failure 28 and in pulmonary arterial hypertension. 29 Post hoc analyses revealed a trend for greater post‐tadalafil CBF change with increasing age (Figure 4). It appears reasonable to speculate that putative PDE5i‐mediated effects on CBF may be most apparent in older persons, at least 65 years of age.
FIGURE 4.
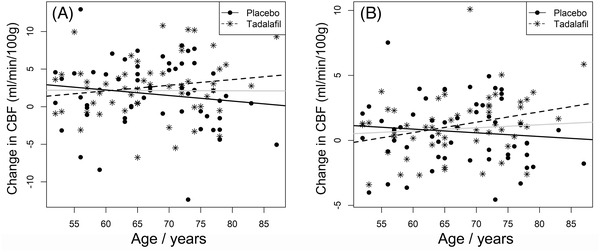
Change in cerebral blood flow (CBF) after placebo or tadalafil as a function of age. Change in CBF in total gray matter (A), and normal‐appearing white matter (B). Lines of best fit are shown for placebo (solid line), or tadalafil (dashed line) or for all data points (gray line)
Changes in CBF following PDE5i administration have been reported in older people with brain disease, 30 , 31 , 32 though the results were quite diverse. In older male subjects with a history of ischemic stroke (combining large vessel and lacunar subtypes), a mosaic of changes in regional CBF followed tadalafil treatment 31 . Sildenafil also gave a mosaic of regional CBF changes in men with erectile dysfunction. 30 In subjects with mild cognitive impairment (MCI), diagnosed clinically as “early Alzheimer's disease (AD),” there was a small (8%) elevation of global CBF following acute administration of sildenafil (50 mg). 32 All these studies lacked a placebo‐treated control group, 30 , 31 , 32 hence small PDE5i‐dependent effects cannot be distinguished from confounding factors, such as diurnal CBF changes. 27 Healthy young adults showed no change in CBF following acute PDE5i treatment. 33 , 34 In young adult male patients with Becker muscular dystrophy, 4 weeks of sildenafil treatment produced a small increase in cerebrovascular reactivity (1.6%) though CBF did not change significantly. 35
Other vasoactive agents that have been tested in older people, either with documented hypertension or with a history of SVD, include angiotensin‐converting enzyme inhibitors, 36 , 37 angiotensin receptor antagonists, 38 , 39 beta‐adrenoceptor blockers, 36 and calcium channel antagonists. 40 All reduced systemic blood pressure without significant effects on CBF. Most of these prior studies used drug treatment for at least 2 weeks in small cohorts (8–28 participants). In a larger study of 67 hypertensive older people with SVD, either intensive or standard ambulatory blood pressure–lowering treatment achieved 27 mmHg or 8 mmHg fall in SBP, respectively. 41 After 3 months of treatment, there was no significant change in global CBF from baseline in either group, and no difference in CBF between the intensive and standard treatment groups. 41 There are exceptions to this pattern. A modest, significant increase in gray matter CBF (9.5%) was reported in older hypertensive patients following intensive blood pressure lowering, relative to those on usual blood pressure treatment. 42 In AD patients, 6 months of treatment with the calcium antagonist nilvadipine suppressed blood pressure with no change in global CBF, though post hoc analyses showed a substantial (20%) increase in hippocampal CBF. 43
The present results and the bulk of published data 36 , 37 , 38 , 39 , 40 , 41 , 44 support the view that CBF autoregulation is maintained following drug treatment, even in older people with manifest SVD. The present findings support the safety of tadalafil in older people with SVD. Sustained CBF augmentation may require a combination of interventions. 45 , 46
The interplay among hypertension, SVD, and cognitive decline is an area of high interest. A recent large study (SPRINT‐MIND) compared intensive to standard blood pressure–lowering (target SBP < 120 mmHg, < 140 mmHg, respectively). 47 While there was no difference in the primary outcome of incident dementia (median treatment duration 3.3 years), intensive treatment significantly reduced risk of MCI and the combined risk of MCI or probable dementia. 47 In a subgroup with MRI data, WMH volume increase was significantly lower in the intensive treatment group (between‐group difference: 540 mm3, P < .001). 48 These findings support an earlier study (PROGRESS) in which blood pressure lowering (using an angiotensin‐converting enzyme inhibitor combined with a diuretic) reduced WMH accumulation and lowered the risk of cognitive decline. 46 Recent meta‐analyses support a beneficial effect of midlife blood pressure lowering. 49 Overall, these findings suggest that cognitive impairment due to SVD may be tractable to intensive antihypertensive strategies.
This study has strengths. First, it recruited a well‐characterized cohort with clinical and MRI evidence for symptomatic SVD. Second, CBF maps were derived from a long pCASL sequence (20 minutes), all performed on a single MRI scanner. Third, sufficient participants were recruited to attain the predetermined statistical power.
This study also has limitations. First, only a single administration of tadalafil was examined. While this was anticipated to attain the expected biological effect of near‐complete PDE5 inhibition, additional effects could emerge from a longer dosing regimen. Second, relatively young participants were included (age ≥50 years, Table 1). Of the 55 who completed the protocol, 22 (40%) were aged < 65 years. As tadalafil does not affect CBF in young, healthy adults, 33 , 34 this may have diluted a possible treatment effect. Third, only resting CBF was measured. It may be that PDE5i affects changes in cerebrovascular reactivity in response to a stimulus (such as a visual image, breath‐holding, or motor task). 35
5. CONCLUSION
This study found insufficient evidence to support a significant difference between single‐dose tadalafil (20 mg) and placebo with respect to increase in resting subcortical CBF. Modest reduction in blood pressure was observed and did not result in hypoperfusion in SVD. A trend to augmented WMH perfusion suggests that PDE5i treatment, possibly of longer duration, may yield clinical benefit.
CONFLICTS OF INTEREST
MMHP and LRB were employed as part of the PASTIS trial; JDI was Principal Investigator and AHH was Chief Investigator. CK is a PI on clinical trials with Bristol‐Myers‐Squibb and Bayer, and has received funding from NovoNordisk, Bayer, and Bristol‐Myers‐Squibb, all not relevant to the present trial. JDI has been a PI on clinical trials funded by Roche, Merck, and Lupin Pharmaceuticals and has received funds from Biogen and Roche, none relevant to the present trial. AHH has received honoraria from Eli‐Lilly and from NIA; he chairs the Vascular Cognitive Disorders PIA within ISTAART; and he leads MRC‐Dementias Platform UK Vascular Experimental Medicine group. All other authors report no relevant disclosures. The trial was subject to an ICH‐Good Clinical Practice (GCP) inspection by the UK medicines regulator, the MHRA, in September 2019, which identified a number of regulatory findings associated with the management of the trial. These are outlined in the supporting information.
Supporting information
Expanded Methods.
Supplementary Figure S1, MRI data analysis workflow.
Supplemental Table S1, list of adverse events.
ACKNOWLEDGMENTS
The authors thank our colleagues at St George's, in particular the Atkinson‐Morley Radiography team, Stroke service, South London CRN Stroke Research Network, Joint Research and Enterprise Office and Clinical Research Facility. They also acknowledge our late colleague and friend Debbie Rolfe (deceased) for her support. AHH thanks Dr. HMTC Mulrooney for helpful comments. This study was joint‐funded by UK Alzheimer's Society and Alzheimer's Drug Discovery Foundation (Grant Ref. 20140901). The funding sources had no involvement in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The sponsor was St George's University of London (contact: sponsor@sgul.ac.uk).
Pauls MMH, Binnie LR, Benjamin P, et al. The PASTIS trial: Testing tadalafil for possible use in vascular cognitive impairment. Alzheimer's Dement. 2022;18:2393–2402. 10.1002/alz.12559
Clinical Trial Registration. http://www.clinicaltrials.gov. Unique identifier: NCT02450253. https://eudract.ema.europa.eu. Unique identifier: 2015‐001235‐20.
REFERENCES
- 1. Esiri MM, Wilcock GK, Morris JH. Neuropathological assessment of the lesions of significance in vascular dementia. J Neurol Neurosurg Psychiatry. 1997;63:749‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cannistraro RJ, Badi M, Eidelman BH, Dickson DW, Middlebrooks EH, Meschia JF. CNS small vessel disease: a clinical review. Neurology. 2019;92:1146‐1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O'Sullivan M, Lythgoe DJ, Pereira AC, et al. Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology. 2002;59:321‐326. [DOI] [PubMed] [Google Scholar]
- 4. Yao H, Sadoshima S, Ibayashi S, Kuwabara Y, Ichiya Y, Fujishima M. Leukoaraiosis and dementia in hypertensive patients. Stroke. 1992;23:1673‐1677. [DOI] [PubMed] [Google Scholar]
- 5. Bernbaum M, Menon BK, Fick G, et al. Reduced blood flow in normal white matter predicts development of leukoaraiosis. J Cereb Blood Flow Metab. 2015;35:1610‐1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Dalen JW, Moll van Charante EP, Caan MWA, et al. Effect of long‐term vascular care on progression of cerebrovascular lesions: magnetic Resonance Imaging Substudy of the PreDIVA Trial (Prevention of Dementia by Intensive Vascular Care). Stroke. 2017;48:1842‐1848. [DOI] [PubMed] [Google Scholar]
- 7. van der Veen PH, Muller M, Vincken KL, et al. Longitudinal relationship between cerebral small‐vessel disease and cerebral blood flow: the second manifestations of arterial disease‐magnetic resonance study. Stroke. 2015;46:1233‐1238. [DOI] [PubMed] [Google Scholar]
- 8. Smith EE, Markus HS. New treatment approaches to modify the course of cerebral small vessel diseases. Stroke. 2020;STROKEAHA119024150, 51:38–46. [DOI] [PubMed] [Google Scholar]
- 9. Kloner RA, Jackson G, Hutter AM, et al. Cardiovascular safety update of Tadalafil: retrospective analysis of data from placebo‐controlled and open‐label clinical trials of Tadalafil with as needed, three times‐per‐week or once‐a‐day dosing. Am J Cardiol. 2006;97:1778–1784. [DOI] [PubMed] [Google Scholar]
- 10. Kloner RA, Goldstein I, Kirby MG, Parker JD, Sadovsky R. Cardiovascular safety of phosphodiesterase Type 5 inhibitors after nearly 2 decades on the market. Sex Med Rev. 2018;6:583‐594. [DOI] [PubMed] [Google Scholar]
- 11. Pauls MM, Moynihan B, Barrick TR, et al. The effect of phosphodiesterase‐5 inhibitors on cerebral blood flow in humans: a systematic review. J Cereb Blood Flow Metab. 2018;38:189‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Teich AF, Sakurai M, Patel M, et al. PDE5 exists in human neurons and is a viable therapeutic target for neurologic disease. J Alzheimers Dis. 2016;52:295‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vasita E, Yasmeen S, Andoh J, et al. The cGMP‐degrading enzyme phosphodiesterase‐5 (PDE5) in cerebral small arteries of older people. J Neuropathol Exp Neurol. 2019;78:191‐194. [DOI] [PubMed] [Google Scholar]
- 14. Forgue ST, Patterson BE, Bedding AW, et al. Tadalafil pharmacokinetics in healthy subjects. Br J Clin Pharmacol. 2006;61:280‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forgue ST, Phillips DL, Bedding AW, et al. Effects of gender, age, diabetes mellitus and renal and hepatic impairment on tadalafil pharmacokinetics. Br J Clin Pharmacol. 2007;63:24‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia‐Osta A, Cuadrado‐Tejedor M, Garcia‐Barroso C, Oyarzabal J, Franco R. Phosphodiesterases as therapeutic targets for Alzheimer's disease. ACS Chem Neurosci. 2012;3:832‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garcia‐Barroso C, Ricobaraza A, Pascual‐Lucas M, et al. Tadalafil crosses the blood‐brain barrier and reverses cognitive dysfunction in a mouse model of AD. Neuropharmacology. 2013;64:114‐123. [DOI] [PubMed] [Google Scholar]
- 18. Pauls MMH, Clarke N, Trippier S, et al. Perfusion by Arterial Spin labelling following Single dose Tadalafil In Small vessel disease (PASTIS): study protocol for a randomised controlled trial. Trials. 2017;18(1), 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wahlund LO, Barkhof F, Fazekas F, et al. European Task Force on Age‐Related White Matter. A new rating scale for age‐related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318‐1322. [DOI] [PubMed] [Google Scholar]
- 20. Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin‐labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73:102‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spilling CA, Jones PW, Dodd JW, Barrick TR. White matter lesions characterise brain involvement in moderate to severe chronic obstructive pulmonary disease, but cerebral atrophy does not. BMC Pulm Med. 2017;17:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation‐maximization algorithm. IEEE Trans Med Imaging. 2001;20:45‐57. [DOI] [PubMed] [Google Scholar]
- 23. Wauschkuhn CA, Witte K, Gorbey S, Lemmer B, Schilling L. Circadian periodicity of cerebral blood flow revealed by laser‐Doppler flowmetry in awake rats: relation to blood pressure and activity. Am J Physiol Heart Circ Physiol. 2005;289:H1662‐1668. [DOI] [PubMed] [Google Scholar]
- 24. Diamant M, Harms MP, Immink RV, Van Lieshout JJ, Van Montfrans GA. Twenty‐four‐hour non‐invasive monitoring of systemic haemodynamics and cerebral blood flow velocity in healthy humans. Acta Physiol Scand. 2002;175:1‐9. [DOI] [PubMed] [Google Scholar]
- 25. Siennicki‐Lantz A, Reinprecht F, Axelsson J, Elmstahl S. Cerebral perfusion in the elderly with nocturnal blood pressure fall. Eur J Neurol. 2007;14:715‐720. [DOI] [PubMed] [Google Scholar]
- 26. White WB, Jalil F, Wakefield DB, et al. Relationships among clinic, home, and ambulatory blood pressures with small vessel disease of the brain and functional status in older people with hypertension. Am Heart J. 2018;205:21‐30. [DOI] [PubMed] [Google Scholar]
- 27. Schwartz GL, Bailey KR, Mosley T, et al. Association of ambulatory blood pressure with ischemic brain injury. Hypertension. 2007;49:1228‐1234. [DOI] [PubMed] [Google Scholar]
- 28. Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1‐year, prospective, randomized, placebo‐controlled study. Circ Heart Fail. 2011;4:8‐17. [DOI] [PubMed] [Google Scholar]
- 29. Murray F, Maclean MR, Insel PA. Role of phosphodiesterases in adult‐onset pulmonary arterial hypertension. Handb Exp Pharmacol. 2011:279‐305. [DOI] [PubMed] [Google Scholar]
- 30. Lorberboym M, Mena I, Wainstein J, Boaz M, Lampl Y. The effect of sildenafil citrate (Viagra) on cerebral blood flow in patients with cerebrovascular risk factors. Acta Neurol Scand. 2010;121:370‐376. [DOI] [PubMed] [Google Scholar]
- 31. Lorberboym M, Makhline E, Lampl Y. Regional cerebral blood flow following single‐dose and continuous‐dose tadalafil after stroke. Acta Neurol Scand. 2014;130:380‐386. [DOI] [PubMed] [Google Scholar]
- 32. Sheng M, Lu H, Liu P, et al. Sildenafil improves vascular and metabolic function in patients with Alzheimer's disease. J Alzheimers Dis. 2017;60:1351‐1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jahshan S, Dayan L, Jacob G. Nitric oxide‐sensitive guanylyl cyclase signaling affects CO2‐dependent but not pressure‐dependent regulation of cerebral blood flow. Am J Physiol Regul Integr Comp Physiol. 2017;312:R948‐R955. [DOI] [PubMed] [Google Scholar]
- 34. Kruuse C, Hansen AE, Larsson HB, Lauritzen M, Rostrup E. Cerebral haemodynamic response or excitability is not affected by sildenafil. J Cereb Blood Flow Metab. 2009;29:830‐839. [DOI] [PubMed] [Google Scholar]
- 35. Lindberg U, Witting N, Jorgensen SL, et al. Effects of sildenafil on cerebrovascular reactivity in patients with Becker Muscular Dystrophy. Neurotherapeutics. 2017;14:182‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jennings JR, Muldoon MF, Price J, Christie IC, Meltzer CC. Cerebrovascular support for cognitive processing in hypertensive patients is altered by blood pressure treatment. Hypertension. 2008;52:65‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Walters MR, Bolster A, Dyker AG, Lees KR. Effect of perindopril on cerebral and renal perfusion in stroke patients with carotid disease. Stroke. 2001;32:473‐478. [DOI] [PubMed] [Google Scholar]
- 38. Nazir FS, Overell JR, Bolster A, Hilditch TE, Reid JL, Lees KR. The effect of losartan on global and focal cerebral perfusion and on renal function in hypertensives in mild early ischaemic stroke. J Hypertens. 2004;22:989‐995. [DOI] [PubMed] [Google Scholar]
- 39. Kimura Y, Kitagawa K, Oku N, et al. Blood pressure lowering with valsartan is associated with maintenance of cerebral blood flow and cerebral perfusion reserve in hypertensive patients with cerebral small vessel disease. J Stroke Cerebrovasc Dis. 2010;19:85‐91. [DOI] [PubMed] [Google Scholar]
- 40. Kimura Y, Kitagawa K, Oku N, et al. Hemodynamic influences of azelnidipine, a novel calcium channel blocker, on cerebral circulation in hypertensive patients with ischemic white matter lesions. Hypertens Res. 2008;31:2147‐2154. [DOI] [PubMed] [Google Scholar]
- 41. Croall ID, Tozer DJ, Moynihan B, et al. Effect of standard vs intensive blood pressure control on cerebral blood flow in small vessel disease: the PRESERVE Randomized Clinical Trial. JAMA Neurol. 2018;75:720‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tryambake D, He J, Firbank MJ, O'Brien JT, Blamire AM, Ford GA. Intensive blood pressure lowering increases cerebral blood flow in older subjects with hypertension. Hypertension. 2013;61:1309‐1315. [DOI] [PubMed] [Google Scholar]
- 43. de Jong DLK, de Heus RAA, Rijpma A, et al. Effects of nilvadipine on cerebral blood flow in patients with Alzheimer disease. Hypertension. 2019;74:413‐420. [DOI] [PubMed] [Google Scholar]
- 44. Nazir FS, Overell JR, Bolster A, Hilditch TE, Lees KR. Effect of perindopril on cerebral and renal perfusion on normotensives in mild early ischaemic stroke: a randomized controlled trial. Cerebrovasc Dis. 2005;19:77‐83. [DOI] [PubMed] [Google Scholar]
- 45. Blair GW, Appleton JP, Flaherty K, et al. Tolerability, safety and intermediary pharmacological effects of cilostazol and isosorbide mononitrate, alone and combined, in patients with lacunar ischaemic stroke: the LACunar Intervention‐1 (LACI‐1) trial, a randomised clinical trial. EClinicalMedicine. 2019;11:34‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dufouil C, Chalmers J, Coskun O, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation. 2005;112:1644‐1650. [DOI] [PubMed] [Google Scholar]
- 47. Williamson JD, Pajewski NM, Auchus AP, et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321:553‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nasrallah IM, Pajewski NM, Auchus AP, et al. Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA. 2019;322:524‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Peters R, Anderson CS. Advancing dementia prevention through effective blood pressure control. Lancet Neurol. 2020;19:25‐27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded Methods.
Supplementary Figure S1, MRI data analysis workflow.
Supplemental Table S1, list of adverse events.


