Abstract
Introduction
Delirium is associated with new onset dementia and accelerated cognitive decline; however, its pathophysiology remains unknown. Cerebral glucose metabolism previously seen in delirium may have been attributable to acute illness and/or dementia. We aimed to statistically map cerebral glucose metabolism attributable to delirium.
Methods
We assessed cerebral glucose metabolism using 18F‐fluorodeoxyglucose positron emission tomography (FDG‐PET) in sick, older patients with and without delirium, all without clinical dementia (N = 20). Strict exclusion criteria were adopted to minimize the effect of established confounders on FDG‐PET.
Results
Patients with delirium demonstrated hypometabolism in the bilateral thalami and right superior frontal, right posterior cingulate, right infero‐lateral anterior temporal, and left superior parietal cortices. Regional hypometabolism correlated with delirium severity and performance on neuropsychological testing.
Discussion
In patients with acute illness but without clinical dementia, delirium is accompanied by regional cerebral hypometabolism. While some hypometabolic regions may represent preclinical Alzheimer's disease (AD), thalamic hypometabolism is atypical of AD and consistent with the clinical features that are unique to delirium.
Keywords: cerebral glucose metabolism, delirium, dementia, 18F‐fluorodeoxyglucose positron emission tomography, neuroimaging
1. INTRODUCTION
Delirium affects up to 40% of hospitalized patients over the age of 65 and is associated with a 3‐fold increase in the odds of mortality, 1 yet the neural mechanisms leading to delirium remain unknown.
Delirium and dementia are distinct but interconnected causes of cognitive impairment. Dementia is a strong risk factor for delirium. 2 Delirium independently predicts new‐onset dementia, and increased exposure to delirium is associated with poor cognitive outcomes. 3 , 4 Although delirium indicates vulnerability to dementia, evidence of neuroaxonal injury during delirium supports the possibility of a causal relationship between delirium and dementia. 5 Therefore, investigating the neurobiology of delirium and identifying therapeutic targets has potential to not only improve outcomes for patients with delirium but also reduce global dementia burden. 6
Due to the epidemiological and clinical overlap between delirium and dementia, examining their shared pathophysiological mechanisms makes clinical sense. However, histopathological studies demonstrate that cognitive decline after delirium results from mechanistically unique pathways, rather than those classically associated with dementia such as amyloid plaques, neurofibrillary tangles, and vascular damage. 7 Furthermore, clinical trials of pharmaceutical agents targeting these traditional dementia pathologies have failed to alter the trajectory of dementia, 8 so alternative avenues in both delirium and dementia research must be explored.
Cerebral glucose hypometabolism is a core feature of dementia. The pattern of hypometabolism on 18F‐fluorodeoxyglucose positron emission tomography (FDG‐PET) can assist with diagnosing dementia and predicting progression from baseline cognition to mild cognitive impairment (MCI) and from MCI to Alzheimer's disease (AD). 9 The uptake of FDG, which is a glucose analog, is a surrogate measurement of glucose metabolism. 10 The rate of FDG uptake is a reliable indicator of neural and synaptic activity. 10
Cerebral glucose hypometabolism, as well as being a biomarker, plays an important role in the pathophysiology of AD. 11 , 12 Clinical trials targeting cerebral bioenergetic pathways in humans have shown some promising early results. 11
Cerebral hypometabolism is also a feature of delirium. The cerebrospinal fluid (CSF) of older patients with delirium has increased levels of lactate and altered levels of key carbohydrate enzymes, suggesting suppressed aerobic metabolism. 13 , 14 Because regional cerebral perfusion is coupled with glucose utilization, 10 neuroimaging studies also indicate altered metabolism by demonstrating reversible cerebral hypoperfusion, decreased cerebral oxygenation, and dysfunctional autoregulation during delirium. 15
Two studies used FDG‐PET to assess glucose metabolism during delirium and both found cortical hypometabolism. 16 , 17 However, dementia and acute illness were likely significant confounders in both studies. 18
In this exploratory study, we sought to determine whether any component of cerebral glucose hypometabolism is primarily attributable to delirium rather than acute illness or dementia. We used FDG‐PET to compare unwell patients with delirium but without dementia diagnoses nor symptoms to acutely unwell controls without delirium and similarly without dementia. Statistical analysis was used to assess the relationship between regional hypometabolism and clinical features and outcomes of delirium.
2. METHODS
2.1. Setting and recruitment
This single‐center exploratory study was conducted at a tertiary hospital in Sydney, Australia. Our target population was acutely ill in‐patients aged ≥ 65 years. Two groups of patients were recruited: a delirium group and a control group. The delirium group included patients with ongoing delirium formally diagnosed by an experienced geriatrician and documented by a positive Confusion Assessment Method (CAM). 19 Patients had no prior diagnosis of dementia and an Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) 20 score of ≤ 3.44, as an IQCODE score of > 3.44 has high sensitivity and specificity in identifying undiagnosed dementia. 21 The eligibility criteria for enrolment in the control group were: age ≥ 65, negative CAM, no history of delirium or dementia, Mini‐Mental State Examination (MMSE) score ≥ 26, IQCODE score ≤ 3.44, and acutely ill and hospitalized.
RESEARCH IN CONTEXT
Systematic Review: We reviewed the literature using traditional biomedical databases. Human and animal model neuroimaging and cerebrospinal fluid (CSF) studies exploring cerebral metabolism in delirium and dementia are discussed and appropriately cited in the paper.
Interpretation: Cerebral glucose hypometabolism is a feature of delirium and links the pathophysiology of delirium and dementia. Cortical and subcortical hypometabolism accompanies delirium in a pattern that overlaps with, but is atypical of, Alzheimer's disease.
Future Directions: The article highlights opportunities for future hypothesis‐driven research. This includes: (a) understanding the precise mechanism of altered cerebral glucose metabolism using CSF and neuroimaging modalities, (b) examining shared metabolic pathways in delirium and dementia, (c) determining whether hypometabolism can be therapeutically targeted to improve clinical outcomes in delirium, and (d) exploring the relationship between cerebral glucose metabolism and delirium in other populations.
Patients were excluded if they had a history of stroke, traumatic brain injury, or other organic brain pathology that would impact FDG‐PET scan findings. Patients suffering from major depression; patients using regular benzodiazepines, antipsychotics, and anticonvulsants; and patients with poor glycemic control were also excluded due to altered cerebral metabolism on FDG‐PET. 22 We excluded cases of delirium tremens and hepatic encephalopathy as these conditions have distinct clinical features and etiologies.
2.2. Consent and patient assessment
Written informed consent for study participation was obtained from all patients without delirium. For patients with delirium and diminished capacity, proxy consent was sought from the person responsible according to the New South Wales Guardianship Act (1987). Upon enrolment, baseline demographic data, including age, sex, and handedness, were recorded. On the day of the scan, an experienced geriatrician confirmed the presence or absence of delirium. Three trained researchers conducted all neuropsychological assessments including the CAM. We have assessed inter‐rater reliability of the CAM in our research team and found it to be high (kappa = 1.0, based on 10 patient reviews). Neuropsychological tests included the Wechsler Adult Intelligence Scale (WAIS‐IV) Digit Span test 23 of attention and working memory; Trail Making Tests A and B 24 to measure psychomotor speed, information processing speed, higher‐level divided attention, and visual scanning; Wechsler Memory Scale (WMS‐III) Mental Control 25 task of sequencing, working memory, and basic multitasking; clock‐drawing task to assess visuospatial organizational ability; and verbal fluency tasks. 26 The CAM confirmed active delirium at the time of the scan. Delirium severity was assessed using the Delirium Index, 27 an instrument based on the symptom domains of the CAM, scored from 0 to 21 (higher scores reflect greater severity). The MMSE assessed the severity of cognitive dysfunction. 28 Delirious patients were classified as hyperactive, hypoactive, or mixed according to expert geriatric assessment and the extended version of the CAM and Delirium Index. The Acute Physiology and Chronic Health Evaluation III (APACHE III) 29 was used to quantify acute illness severity, and the Charlson Comorbidity Index 30 was used to assess the burden of chronic disease. The Barthel Index 31 and Instrumental Activities of Daily Living (IADL) index 32 were used to assess functional status prior to acute illness; for patients with delirium, the person responsible or caregiver provided this information.
2.3. FDG‐PET imaging
After comprehensive assessment, all patients underwent an FDG‐PET scan. Each patient was accompanied by a family member or caregiver to provide reassurance and comfort. After a 6‐hour fast and a 10‐minute rest in a dark, quiet room, patients received an intravenous dose of 3.5 MBq/kg of 18F‐FDG, followed by a 60‐minute uptake period. All scans were performed using a Philips Ingenuity TF128 PET/CT scanner at midday. Image acquisition was conducted at the resting state, with the patient in a supine position, arms by their side. Each patient's head was stabilized using a foam brace with Velcro chin and forehead straps to minimize movement.
A helical low‐dose head computed tomography (CT) scan was acquired (64 × 0.625 mm slice thickness, 50 mA, 0.602s rotation speed, 0.828 pitch, 120 kVP), followed by a single bed‐position (18 cm axial field of view) PET acquisition for 10 minutes in a 256 × 256 image matrix. CT images were reconstructed using Philips Standard Iterative method, with an iDose factor of 3 and a standard brain filter. Reconstruction of the PET images was performed using Phillips Astonish TF, a list mode, fully 3D iterative ordered subset expectation maximization algorithm with “Blob” basis function (three iterations, three subsets, kernel width = 18.1 cm, relaxation parameter = 1). PET data were corrected for: decay, random coincidences using the delayed window method, scatter using the single scatter simulation approximation method, and point spread function effects using the Philips point spread function correction with one iteration and a regularization factor of 6. PET data were reconstructed into 90 × 2 mm slices.
2.4. Semiquantitative analysis of FDG‐PET scans
All FDG‐PET scans were analyzed semiquantitatively using Syntermed's NeuroQ version 3.0 (Philips Medicals Systems) under the supervision of two experienced nuclear medicine physicists. FDG‐PET scans were first processed and reformatted. We assessed metabolism in cortical and subcortical regions. NeuroQ has standardized regions of interest (ROI). Several ROI make up a cluster; there are 47 “clusters” across the brain. For each cluster of ROIs, NeuroQ computes the normalized regional value (NRV), a semiquantitative index that represents the average number of counts per second per pixel in each ROI, which is then normalized to the number of counts in the cerebellum. The cerebellum was chosen as the reference region for normalization as it is thought to be relatively unaffected in delirium (see Appendix B in supporting information). The NRV reflects cerebral metabolic activity in these regions.
2.5. Statistical analysis
Statistical analysis was performed using IBM SPSS version 26, and non‐parametric statistics were used due to the small sample size. Differences in baseline characteristics and assessment results between the two groups were compared using the Mann–Whitney U test for continuous data and Fisher's exact test for categorical data. Differences in the NRV of anatomical regions between groups were assessed with the Mann–Whitney U test. Depending on the variability of measurements within individual brain regions, this study was powered to detect NRV group mean differences between 0.02 and 0.2, with an alpha of 0.05 and power of 80%. Differences of this magnitude were found in a similar study assessing FDG‐PET scans in delirium. 16 No correction for multiple comparisons was made.
The relationship between hypometabolic regions and clinical features of delirium was analyzed using Spearman's rho. All statistical tests were two‐tailed, with a significance level of 0.05. For descriptive statistics, continuous data were presented as medians and interquartile range, while categorical data were presented as proportions.
3. RESULTS
3.1. Recruitment and patient characteristics
There were 20 patients recruited with a mean age of 83 years and 55% were male. Baseline characteristics are presented in Table 1. The groups were well matched on age, sex, and acute illness severity. Delirious patients showed lower IADL scores, suggesting greater baseline functional impairment.
TABLE 1.
Demographics and baseline data
| Delirium (n = 10) | Controls (n = 10) | P value | |
|---|---|---|---|
| Age in years (IQR) | 84.0 (80.8–88.3) | 81.5 (78.8–88.5) | .631 |
| Female (%) | 6 (60) | 3(30) | .370 |
| Right‐handed (%) | 8(80) | 9(90) | 1 |
| History of delirium (%) | 2 (20) | 0 (0) | – |
| Duration of delirium at time of scan (days, IQR) | 4 (2.75–9.75) | – | – |
| IQCODE a /5 (IQR) | 3.2 (3.1–3.4) | 3.1 (3.0–3.2) | .052 |
| APACHE a /299 (IQR) | 34.5 (29.5–41.0) | 34.0 (23.5–40.3) | .684 |
| Charlson comorbidity score a /35 (IQR) | 5 (4–7) | 5 (3.8–6.5) | .853 |
| Accommodation type | |||
| Home n (%) | 9 (90) | 9 (90) | |
| Nursing home n (%) | 1(10) | 1 (10) | |
| Barthel index b /20 (IQR) | 19.5 (17.3–20.0) | 20.0 (20–20) | .315 |
| iADL index b /12 (IQR) | 8.5 (4.3–10.5) | 12 (11–12) | .029* |
| Blood sugar level prior to scan in mmol/L (IQR) | 5.9 (5.2–6.6) | 5.1 (4.6–6.8) | .218 |
Note: continuous data are presented in median and interquartile range, while categorical data are presented in percentages. Differences between the two groups were compared using the Mann–Whitney U test for continuous data and Fisher's exact test for categorical data.
Abbreviations: APACHE III, Acute Physiology and Chronic Health Evaluation III; iADL, Instrumental Activities of Daily Living; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly (a score of > 3.44 is suggestive of dementia); IQR, interquartile range.
Higher score indicates worse performance.
Higher score indicates better performance.
P < .05.
The delirium assessment and neuropsychological tests are outlined in Table 2. All delirious patients were CAM‐positive at the time of scanning, while all control patients were CAM‐negative. As expected, neuropsychological tests demonstrated significant differences between groups.
TABLE 2.
Delirium and neuropsychological assessment prior to FDG‐PET scan
| Delirium only N = 10 | Controls N = 10 | P value | |
|---|---|---|---|
| CAM positive n (%) | 10 (100) | 0 (0) | |
|
Predominant etiology of delirium or acute illness a Sepsis n (%) Musculoskeletal n (%) Others n (%) |
6 (60) 2 (20) 2 (20) |
9 (90) 1 (10) ‐ |
|
|
Delirium subtypes Mixed n (%) Hyperactive n (%) Hypoactive n (%) |
7 (70) 1 (10) 2 (20) |
||
| MMSEc/30 |
20.5 (18–24) |
28 (28–29) |
P < .001* |
| Delirium index b /21 |
7.5 (5.8–10.3) |
0 (0–1) |
P < .001* |
| WAIS‐IV Digit Span Forwards c /16 |
7.5 (4–8.5) |
11.0 (7.8–13) |
P = .019* |
|
WAIS‐IV Digit Span Backwards c /14 |
3.5 (3–4.3) |
6.0 (5–7) |
P = .002* |
| WMS III Mental Control c /40 |
9.5 (8.8–14) |
22.5 (21–25) |
P < .001* |
| Clock Drawing test * /5 |
1.5 (1–3) |
5.0 (3–5) |
P = .001* |
| Trail Making Test A b /seconds |
60 (45–180) |
18 (16–21) |
P < .001* |
| Trail Making Test B b /seconds |
300 (283–300) |
61 (39–83) |
P = .01* |
Note: Patients with delirium performed worse on neuropsychological assessment representing cognitive dysfunction during delirium. Continuous data are presented in median and interquartile range, while categorical data are presented in percentages.
Abbreviations: CAM, Confusion Assessment Method; FDG‐PET, 18F‐fluorodeoxyglucose positron emission tomography; MMSE, Mini‐Mental State Examination; WAIS‐IV, Wechsler Adult Intelligence Scale IV; WMS‐III, Wechsler Memory Scale‐III.
Most patients had more than one cause of delirium or acute illness.
Higher scores indicate worse performance.
Higher scores indicate better performance.
P < .05.
3.2. Comparison of FDG‐PET scans
Semiquantitative analyses of the cluster NRVs are summarized in Figures 1 and 2, and Appendix A and B in supporting information. Patients with delirium demonstrated lower NRV in the right superior frontal cortex (rSFC; NRV [interquartile range] delirium versus control: 1.07 [1.04–1.09] vs. 1.10 [1.06–1.11], P = .04), right posterior cingulate cortex (rPCC; 1.24 [1.17–1.27] vs. 1.29 [1.24–1.36], P = .035), right inferolateral anterior temporal cortex (riLAT; 0.96 [0.94–0.98] vs. 0.99 [0.98–1.01], P = .015), left superior parietal cortex (lsPL; 0.971 [0.958–0.981] vs. 0.993 [0.979–1.00], P = .015) and bilateral thalami (right 1.07 [1.04–1.09] vs. 1.11 [1.09–1.13], P = .003; left 1.04 [1.02–1.09] vs. 1.11 [1.10–1.13], P = .001). No difference was demonstrated in the left frontotemporal regions, left PCC, or right parietal regions. There were no differences in the caudate and lentiform nuclei. ,
FIGURE 1.
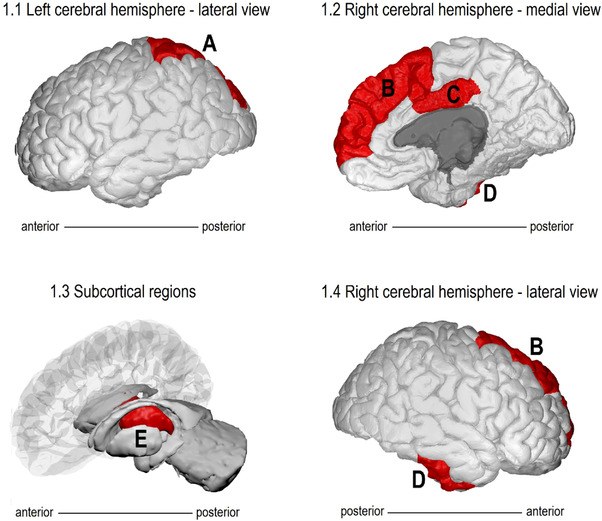
Hypometabolic regions during delirium. Diagrammatic representation of hypometabolic regions in delirious patients compared to control. A, Left superior parietal cortex, (B) right superior frontal cortex, (C) right posterior cingulate cortex, (D) right infero‐lateral anterior temporal lobe, (E) bilateral thalami. Images generated using BrainPainter 58
FIGURE 2.
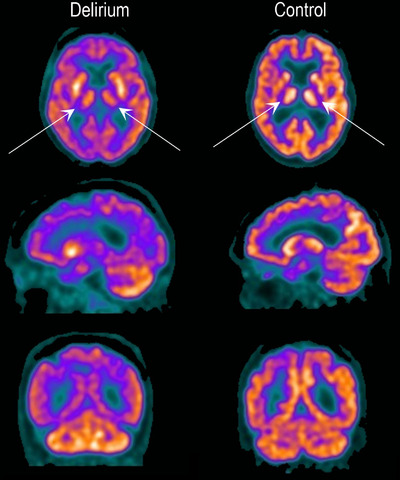
18F‐fluorodeoxyglucose positron emission tomography (FDG‐PET) images. FDG‐PET during delirium compared to control. Top: axial; middle: sagittal; bottom: coronal slices. The delirium scan is an 89‐year‐old female with delirium but no dementia with acute gastroenteritis. The control scan is a cognitively intact 80‐year‐old male with pneumonia and acute kidney injury. Darker colors indicate lower metabolism. There is relative hypometabolism in the thalamus bilaterally (arrows) and also throughout the cerebral cortex in the delirious patient compared to cognitively intact control. During delirium, relative preservation of cerebellar FDG uptake is noted. See Appendix B in supporting information for further images
3.3. Relationship between regional cerebral glucose hypometabolism and clinical features of delirium
To assess whether regional cerebral glucose hypometabolism was related to patient characteristics and clinical features of delirium, correlation analysis was performed in significantly hypometabolic regions. Correlations between clinical variables and NRVs are presented in Table 3. Delirium Index correlated with hypometabolism in the bilateral thalami (right ρ = –0.701, P = .001; left ρ = –0.705, P = .001) and rPCC (ρ = –0.469, P = .037 ρ = –0.701, P = .001), indicating that increased delirium severity was associated with more pronounced hypometabolism. Specific neuropsychological tests were chosen for correlation analysis depending on the cognitive function of individual regions. Most regions, but not all, demonstrated moderate–strong correlation; better performance correlated with greater metabolism (thalamus and Trail Making Rest B: right ρ = –0.510, P = .031; left ρ = –0.517, P = .028 and rPCC and WMS III mental control ρ = –0.566, P = .009).
TABLE 3.
Correlation of delirium severity and relevant neuropsychological tests with normalized regional value in significantly hypometabolic regions
| Region | Cognitive functions | Assessment | Correlation |
|---|---|---|---|
| Thalamus |
Enables selective attention. Regulates arousal, consciousness, motor control, impulse inhibition. |
Delirium index Right Left WMS III Mental control Right Left Trail Making Test B Right Left |
ρ = −0.701, P = .001* ρ = −0.705, P = .001* ρ = −0.375, P = .104 ρ = −0.553, P = .011* ρ = −0.510, P = .031* ρ = −0.517, P = .028* |
| rPCC |
Attention, working memory, awareness, episodic memory retrieval. Critical part of the default mode network and limbic system. |
Delirium index WMS III Mental control WAIS‐IV Digit Span Forwards |
ρ = −0.469, P = .037* ρ = −0.566, P = .009* ρ = −0.412, P = .071 |
| rSFC | Executive function. Cognitive and motor impulse control. Self‐awareness (including orientation). |
Delirium index WMS III Mental control MMSE orientation score |
ρ = −0.441, P = .051 ρ = −0.598, P = .005* ρ = −0.320, P = .168 |
| riLAT | Semantic knowledge; word, object, and facial recognition. |
Delirium index Verbal fluency |
ρ = −0.390, P = .089 ρ = −0.532, P = .016* |
| lsPL |
Sensorimotor integration. Selective attention to visual and tactile stimuli. Working memory. |
Delirium index WMS III Mental control Trail making test B |
ρ = −0.430, P = .058 ρ = 0.379, P = .099 ρ = −0.251, P = .316 |
Note: Correlation analysis was performed using Spearman's rank correlation coefficient.
Abbreviations: lsPL, left superior parietal cortex; MMSE, Mini‐Mental State Examination; rPCC, right posterior cingulate cortex; rSFC, right superior frontal cortex; riLAT, right infero‐lateral anterior temporal cortex; WMS III, Wechsler Memory Scale III; WAIS‐IV, Wechsler Adult Intelligence Scale.
P < .05.
4. DISCUSSION
This is the first study to use FDG‐PET to compare delirious and non‐delirious hospitalized patients aged ≥ 65 while controlling for acute illness and dementia. Patients with delirium demonstrated hypometabolism in both cortical and subcortical regions compared to controls. The degree of hypometabolism correlated with both delirium severity and performance on neuropsychological tests, meaning patients with more pronounced hypometabolism had increased severity of symptoms and decreased cognitive function. While some of the identified hypometabolic regions overlap with AD, thalamic hypometabolism is atypical of preclinical AD and consistent with clinical features that distinguish delirium from dementia.
This research adds important new information implicating glucose metabolic disruption in the pathophysiology of delirium. Glucose is the most crucial metabolic substrate of the brain, with baseline synaptic activity accounting for 75% of resting glucose uptake. 33 It has long been hypothesized that delirium results from inadequate glucose and oxygen supply ultimately leading to “cerebral metabolic insufficiency.” 34 Studies linking cerebral hypoperfusion and reduced cerebral oxygenation to delirium are supportive of this. 15 Furthermore, severe hypoglycemia rapidly decompensates brain function, which clinically manifests as delirium, loss of consciousness, seizures, and coma—and these are usually reversible when euglycemia is restored. 35 However, in this study patients were normoglycemic with no difference from controls. Similarly, CSF glucose levels of delirium and control patients have demonstrated no differences. 13 , 14 This suggests that blood glucose level does not drive cerebral hypometabolism in delirium, rather there is a central nervous system defect such as compromised glucose transport across the blood–brain barrier or cellular membranes, impaired neuronal glucose metabolism, or impaired insulin signalling, including brain insulin resistance.
Patients with AD demonstrate reduced glucose transporter (GLUT) 1 and GLUT 3 expression and brain insulin resistance. 11 , 12 , 36 This may confer a baseline vulnerability to acute metabolic changes, explaining why patients with pre‐existing dementia are at greatest risk of delirium. However, as the metabolic abnormalities in delirium are yet to be defined, it remains uncertain whether dementia and delirium result from shared pathophysiological pathways.
It should also be noted that cerebral glucose hypometabolism during delirium may simply be a marker of a diseased brain, reflecting reduced neural and synaptic activity due to acute insults. 10
The finding of cerebral hypometabolism does not exclude a role for other putative aspects of delirium pathology but rather is complementary. Recent studies of delirium in mouse models demonstrate that acute systemic inflammation leads to abnormal energy metabolism, which drives neurocognitive outcomes. 14 Psychotropics, opioids, and anticholinergics, which are hypothesized to induce delirium via effects on neurotransmitter pathways, are also associated with cerebral hypometabolism on FDG‐PET. 37 Functional network dysconnectivity has been demonstrated during delirium 38 and functional connectivity is closely linked to glucose metabolism. 39 Patients in our study, with delirium resulting from various precipitants, demonstrate a relationship between regional cerebral glucose metabolism and severity of delirium, suggesting that disturbed metabolism is a feature of delirium irrespective of the cause, possibly a final common pathway.
The neuroanatomical localization of hypometabolism during an episode of delirium has implications for understanding delirium symptomatology.
The most striking finding in our study was bilateral thalamic hypometabolism. Growing evidence implicates thalamic abnormalities in delirium pathophysiology. Patients with thalamic white matter tract abnormalities more frequently develop delirium after cardiac surgery. 40 Reversible thalamic hypoperfusion and impaired functional connectivity between the thalamus and subcortical structures have also been observed during delirium. 38 , 41 In animal models, cognitively intact mice with thalamic synaptic loss were more susceptible to acute and fluctuating cognitive dysfunction during acute illness than mice with hippocampal atrophy alone or normal controls. 44 As underlying thalamic pathology advanced, the risk of cognitive dysfunction during acute illness progressively increased.
The thalamus is a critical node with extensive cortical and subcortical connections whose primary function is to filter cortical inputs and render outputs to enable selective attention on a specific task. 42 In addition, the thalamus receives projections from the ascending reticular activating system. Thalamic intralaminar nuclei and the mesencephalic tegmental nuclei regulate sleep–wake transitions and arousal, and therefore thalamic dysfunction may explain the altered and fluctuating levels of consciousness typical of delirium. 43
Consistent with the results of previous FDG‐PET and magnetic resonance imaging studies, 15 , 16 , 38 our results also implicate the PCC. The PCC, a highly connected and metabolic region in the posteromedial cortex, is responsible for arousal, awareness, internally directed thought, and attention 38 —cognitive processes that are typically disturbed during an episode of delirium. In our study, reduced metabolism in the right PCC correlated with increased delirium severity and impaired attention, a cardinal feature of delirium.
The PCC also plays a central role within the default mode network (DMN), being activated at rest and deactivated during externally directed attentional tasks. 38 Abnormal functional connectivity of the DMN may also account for the cognitive fluctuations and sleep—wake cycle disturbances that occur in delirium. 45 Older patients with postoperative neurocognitive disorder (pNCD, previously referred to as postoperative cognitive dysfunction), 46 a syndrome with many similarities to postoperative delirium, also demonstrate functional network dysconnectivity in the DMN. 47 , 48 Specifically, perioperative resting state connectivity changes in the PCC and SFC, both of which were hypometabolic in our study, were associated with pNCD. 48 These findings suggest delirium and pNCD share common neuroanatomical substrates.
The dorsolateral SFC is a key component of the executive network (EN), whose functions include attention, working memory, and reasoning. 49 Reduced metabolism in the rSFC may manifest as impulsivity and impaired goal‐directed behaviors, including self‐removing medical equipment and wandering, which can occur during delirium and also dementia.
Correlation between riLAT glucose metabolism and semantic fluency may account for receptive and expressive communication errors, altered object recognition, and misperception of faces and emotions observed during delirium. 50
The superior parietal cortex is not only responsible for sensorimotor integration (engagement in visual and tactile stimuli) but is also of critical importance in working memory. 51 Impairments within this region could affect visual perception and motor control accounting for hallucinations and increased falls during delirium.
Our findings of predominant right‐sided cortical hypometabolism are consistent with longitudinal studies demonstrating that delirium is associated with greater right‐sided new abnormalities in the frontal and temporal white matter. 52 Similarly, pre‐operative right‐sided white matter abnormalities correlate with the severity of postoperative delirium. 53 Sustained attention involves predominant activation of right hemispheric regions. 54 Correlations between metabolic activity and neuropsychological tests may also suggest impaired neuroplasticity in delirious patients in which the brain cannot functionally adapt or reorganize in response to stress or trauma.
5. LIMITATIONS
Our study has several limitations. First, the generalizability is limited due to strict eligibility criteria for FDG‐PET. Conditions commonly affecting hospitalized older patients, including cortical infarcts, impaired glycemic control, and use of certain centrally acting medications led to a high exclusion rate. Additionally, we were only able to scan patients on 4 days of the week because of PET availability, further hindering recruitment. As a result, our sample size was small, making the study underpowered and at risk of type II error. As this is an exploratory study, we did not correct for multiple statistical comparisons and this study is also at risk of a type I error. However, we hope that this work will generate future hypothesis‐driven research. The relationship between regional metabolic deficits and delirium should be more definitively examined in larger appropriately powered studies.
Patients in the delirium group demonstrated greater IADL deficits than controls, the 98% negative predictive value of the IQCODE for dementia (cutoff ≥3.4) suggests non‐cognitive conditions may be driving these differences. 55 Although we rigorously confirmed that patients in the delirium group had no clinical dementia through IQCODE assessment and comprehensive history and medical record review, we cannot exclude the possibility that some may have had undiagnosed MCI. Furthermore, patients in both groups may have had clinically silent dementia neuropathology, such as amyloid, tau, TAR DNA‐binding protein 43 (TDP‐43), and vascular pathology, which can be present in cognitively normal older people. 56 This is important because common FDG‐PET abnormalities in MCI and preclinical AD involve hypometabolism of the parieto‐temporal lobes and PCC. 9 , 57 Comparing FDG‐PET scans of older patients with delirium but without dementia to scans of older patients with established dementia subtypes (AD, vascular dementia, and dementia with Lewy bodies) may help ascertain whether thalamic hypometabolism is specific to delirium.
Semi‐quantitatively analyzing the scans was challenging as there is no consensus on the optimal reference region for normalization in delirium. After a comprehensive literature review and consultation with experienced nuclear medicine specialists, we chose the cerebellum as the reference region as it is relatively preserved in delirium and has low inter‐individual variability (see Figure 2 and Appendix B). Whole‐brain normalization may be less suitable as delirium is associated with global hypometabolism. 16
We acquired FDG‐PET images at a single time point. In the absence of longitudinal scans, we cannot determine whether the FDG‐PET findings are transient and due to delirium rather than a pre‐existing vulnerability. Our previous work suggests that cortical hypometabolism is at least partially reversible, 16 but the long‐term effects of acute illness on cerebral metabolism remain unclear. Should the metabolic abnormalities observed during delirium be only partially reversible, this may account for the new cognitive impairment or accelerated dementia that can occur after an episode of delirium.
6. FUTURE RESEARCH DIRECTIONS
Larger studies are required to further characterize the observed metabolic disturbance. Insulin signaling pathways, glycolytic metabolites, and glucose transporter proteins could be measured in the CSF of delirious patients. Proton magnetic spectroscopy would allow non‐invasive measurement of key metabolites. Investigating whether other potential energy sources such as ketone bodies and lactate are preferentially used during delirium may also be beneficial. Studies comparing groups with delirium, dementia, and delirium superimposed on dementia may help identify shared pathophysiological pathways. Finally, as has been explored in dementia, hypometabolism can be targeted therapeutically to determine whether enhanced cerebral metabolism can lead to improved clinical outcomes for patients with delirium.
7. CONCLUSION
In this exploratory study, delirium was associated with cortical and subcortical regional hypometabolism, and the identified regions correlate with clinical markers of delirium. Larger studies are required to confirm these findings. Future research should explore whether regional hypometabolism is dynamic, due to preclinical neuropathology, or a combination of the two. Understanding the precise mechanism of altered cerebral glucose metabolism may identify potentially targetable therapeutic pathways that could prevent progression to dementia.
CONFLICT OF INTERESTS
Anita Nitchingham is supported by an UNSW Scientia Scholarship/Australian Government Research Training Program Scholarship (paid to Anita Nitchingham) and is a committee member of the Australasian Delirium Association (ADA) and Australian and New Zealand Society for Geriatric Medicine (ANZSGM) Scientific and Research Committee (both unpaid). Anita Nitchingham has received payment for educational content from the Australian Doctors Group (paid to Anita Nitchingham). Jarett Vanz‐Brian Pereira has no disclosures to report. Eva A. Wegner is an executive board member of the Australasian Association of Nuclear Medicine Specialists (unpaid). Vincent Oxenham is the Secretary of the College of Clinical Neuropsychologists in NSW (unpaid). Jacqueline Close is an investigator on grants funded by the National Health and Medical Research Council (NHRMC); Sydney Partnership for Health, Education, Research and Enterprise (SPHERE) Leading Better Value Care Programme; and Australian and New Zealand Hip Fracture Registry (all paid to the institution). Jacqueline Close has participated in educational events for General Practice New South Wales (paid to institution) and participates on a data and safety monitoring board (unpaid). Jacqueline Close is a current board member of Agency for Clinical Innovation and Clinical Excellence Commission (paid to Jacqueline Close). Gideon A. Caplan is an investigator on grants funded by the New South Wales Health Transitional Research Grants scheme, SPHERE, NHMRC and untied funding from the Harry Triguboff Foundation (all paid to the institution). Gideon A. Caplan receives royalties from a published textbook (paid to Gideon A. Caplan). Gideon A. Caplan has received personal payment for expert testimony from Allianz Australia, NSW Crown Solicitor's Office, Crown Solicitor Insurance for NSW, AXA, Norton Rose Fulbright, Avant Insurance, and AAI Limited (not related to this manuscript). Gideon A. Caplan received travel and accommodation support for speaking at the Alzheimer's Association International Conference in Los Angeles 2019 (payments were made to Gideon A. Caplan and the institution). Gideon A. Caplan is a committee member of the ADA and ANZSGM (unpaid).
Supporting information
SUPPORTING INFORMATION
SUPPORTING INFORMATION
SUPPORTING INFORMATION
SUPPORTING INFORMATION
SUPPORTING INFORMATION
SUPPORTING INFORMATION
ACKNOWLEDGMENTS
The authors would like to acknowledge: Robert Welschinger, Lucy Haggstrom, Julia Nelson, and May Zin Aung Thein for their assistance with recruitment; Bruce McBride, Thomas Hennessy, and Megan Horsfield for providing training and support in semiquantitative analysis of PET scans; and the Department of Nuclear Medicine and PET at the Prince of Wales Hospital for performing the PET studies. This trial is financially supported by the The Julia Lowy Foundation and the Harry Triguboff Foundation. Funding was paid to the institution and not to individual co‐authors. The funding bodies have no role in trial design, trial conduct, data management, analysis, interpretation of the data, writing of the manuscript or decision to publish.
Nitchingham A, Pereira JV‐B, Wegner EA, Oxenham V, Close J, Caplan GA. Regional cerebral hypometabolism on 18F‐FDG PET/CT scan in delirium is independent of acute illness and dementia. Alzheimer's Dement. 2023;19:97–106. 10.1002/alz.12604
Anita Nitchingham and Jarett Vanz‐Brian Pereira contributed equally to the writing of the manuscript.
Anita Nitchingham and Jarett Vanz‐Brian Pereira are joint first authors.
REFERENCES
- 1. Aung Thein MZ, Pereira JV, Nitchingham A, Caplan GA. A call to action for delirium research: meta‐analysis and regression of delirium associated mortality. BMC Geriatrics. 2020;20(1):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gual N, Morandi A, Pérez LM, et al. Risk factors and outcomes of delirium in older patients admitted to postacute care with and without dementia. Dement Geriatr Cogn Disord. 2018;45(1‐2):121‐129. [DOI] [PubMed] [Google Scholar]
- 3. Vanz‐Brian Pereira J, Aung Thein MZ, Nitchingham A, Caplan GA. Delirium in older adults is associated with development of new dementia: a systematic review and meta‐analysis. Int J Geriatr Psychiatry. 2021;36(7):993‐1003. [DOI] [PubMed] [Google Scholar]
- 4. Richardson SJ, Davis DHJ, Stephan BCM, et al. Recurrent delirium over 12 months predicts dementia: results of the Delirium and Cognitive Impact in Dementia (DECIDE) study. Age Ageing. 2021;50(3):914‐920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Casey CP, Lindroth H, Mohanty R, et al. Postoperative delirium is associated with increased plasma neurofilament light. Brain. 2020;143(1):47‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khachaturian AS, Hayden KM, Devlin JW, et al. International drive to illuminate delirium: a developing public health blueprint for action. Alzheimers Dement. 2020;16(5):711‐725. [DOI] [PubMed] [Google Scholar]
- 7. Davis DHJ, Muniz‐Terrera G, Keage HAD, et al. Association of delirium with cognitive decline in late life: a neuropathologic study of 3 population‐based cohort studies. JAMA Psychiatry. 2017;74(3):244‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anderson RM, Hadjichrysanthou C, Evans S, Wong MM. Why do so many clinical trials of therapies for Alzheimer's disease fail? Lancet North Am Ed. 2017;390(10110):2327‐2329. [DOI] [PubMed] [Google Scholar]
- 9. Kato T, Inui Y, Nakamura A, Ito K. Brain fluorodeoxyglucose (FDG) PET in dementia. Ageing Res Rev. 2016;30:73‐84. [DOI] [PubMed] [Google Scholar]
- 10. Mosconi L. Glucose metabolism in normal aging and Alzheimer's disease: methodological and physiological considerations for PET studies. Clin Transl Imaging. 2013;1(4). 10.1007/s40336-013-0026-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kellar D, Craft S. Brain insulin resistance in Alzheimer's disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol. 2020;19(9):758‐766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. An Y, Varma VR, Varma S, et al. Evidence for brain glucose dysregulation in Alzheimer's disease. Alzheimers Dement. 2018;14(3):318‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Caplan GA, Kvelde T, Lai C, Yap SL, Lin C, Hill MA. Cerebrospinal fluid in long‐lasting delirium compared with Alzheimer's dementia. J Gerontol A Biol Sci Med Sci. 2010;65(10):1130‐1136. [DOI] [PubMed] [Google Scholar]
- 14. Kealy J, Murray C, Griffin EW, et al. Acute inflammation alters brain energy metabolism in mice and humans: role in suppressed spontaneous activity, impaired cognition, and delirium. JNeurosci. 2020;40(29):5681‐5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nitchingham A, Kumar V, Shenkin S, Ferguson KJ, Caplan GA. A systematic review of neuroimaging in delirium: predictors, correlates and consequences. Int J Geriatr Psychiatry. 2018;33(11):1458‐1478. [DOI] [PubMed] [Google Scholar]
- 16. Haggstrom LR, Nelson JA, Wegner EA, Caplan GA. 2‐18F‐fluoro‐2‐deoxyglucose positron emission tomography in delirium. J Cereb Blood Flow Metab. 2017;37(11):3556‐3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ma H, Liao Y, Mo Y, et al. Decreased cerebral glucose metabolism in elderly patients with postoperative delirium: a case‐control study. J Educ Perioper Med. 2017;4. [Google Scholar]
- 18. Semmler A, Hermann S, Mormann F, et al. Sepsis causes neuroinflammation and concomitant decrease of cerebral metabolism. J Neuroinflammation. 2008;5(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method: a new method for detection of delirium. Ann Intern Med. 1990;113(12):941‐948. [DOI] [PubMed] [Google Scholar]
- 20. Jorm AF. The Informant Questionnaire on cognitive decline in the elderly (IQCODE): a review. Int Psychogeriatr. 2004;16(3):275‐293. [DOI] [PubMed] [Google Scholar]
- 21. Harwood DM, Hope T, Jacoby R. Cognitive impairment in medical inpatients. I: screening for dementia‐is history better than mental state? Age Ageing. 1997;26(1):31‐35. [DOI] [PubMed] [Google Scholar]
- 22. Su L, Cai Y, Xu Y, Dutt A, Shi S, Bramon E. Cerebral metabolism in major depressive disorder: a voxel‐based meta‐analysis of positron emission tomography studies. BMC Psychiatry. 2014;14(1):321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wechsler D. Wechsler adult intelligence scale. 4th ed. San Antonio, TX: Pearson, 2008. [Google Scholar]
- 24. Tombaugh T. Trail making test A and B: normative data stratified by age and education. Arc Clin Neuropsychol. 2004;19(2):203‐214. [DOI] [PubMed] [Google Scholar]
- 25. Tulsky DS, Chiaravalloti ND, Palmer BW, Chelune GJ. Chapter 3—The Wechsler Memory Scale, Third Edition: a new perspective. In: Tulsky DS, Saklofske DH, Heaton RK, eds. Clinical Interpretation of the WAIS‐III and WMS‐III. Academic Press; 2003:93‐139. [Google Scholar]
- 26. Wechsler D. Wechsler Adult Intelligence Scale‐Third Edition and Wechsler Memory Scale—Third Edition Technical Manual. 1997b. San Antonio, TX: The Psychological Corporation. 10.1037/t49755-000. [DOI] [Google Scholar]
- 27. McCusker J, Cole MG, Dendukuri N, Belzile E. The delirium index, a measure of the severity of delirium: new findings on reliability, validity, and responsiveness. J Am Geriatr Soc. 2004;52(10):1744‐1749. [DOI] [PubMed] [Google Scholar]
- 28. Tombaugh TN, McIntyre NJ. The mini‐mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922‐935. [DOI] [PubMed] [Google Scholar]
- 29. Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system: risk prediction of hospital mortality for critically III hospitalized adults. Chest. 1991;100(6):1619‐1636. [DOI] [PubMed] [Google Scholar]
- 30. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245‐1251. [DOI] [PubMed] [Google Scholar]
- 31. Collin C, Wade D, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud. 1988;10(2):61‐63. [DOI] [PubMed] [Google Scholar]
- 32. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged: the index of adl: a standardized measure of biological and psychosocial function. JAMA. 1963;185(12):914‐919. [DOI] [PubMed] [Google Scholar]
- 33. Petit‐Taboué MC, Landeau B, Desson JF, Desgranges B, Baron JC. Effects of healthy aging on the regional cerebral metabolic rate of glucose assessed with statistical parametric mapping. Neuroimage. 1998;7(3):176‐184. [DOI] [PubMed] [Google Scholar]
- 34. Engel GL, Romano J. Delirium, a syndrome of cerebral insufficiency. J Chronic Dis. 1959;9(2):260‐277. [DOI] [PubMed] [Google Scholar]
- 35. Mosconi L. Glucose metabolism in normal aging and Alzheimer's disease: methodological and physiological considerations for PET studies. Clin Transl Imaging. 2013;1(4):217‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Winkler EA, Nishida Y, Sagare AP, et al. GLUT1 reductions exacerbate Alzheimer's disease vasculo‐neuronal dysfunction and degeneration. Nat Neurosci. 2015;18(4):521‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Waxman AD, Herholz K, Lewis DH, et al. Society of Nuclear Medicine procedure guideline for FDG PET brain imaging. 2009; Accessed October 27, 2018. http://snmmi.files.cms‐plus.com/docs/Society%20of%20Nuclear%20Medicine%20Procedure%20Guideline%20for%20FDG%20PET%20Brain%20Imaging.pdf].
- 38. Choi SH, Lee H, Chung TS, et al. Neural network functional connectivity during and after an episode of delirium. Am J Psychiatry. 2012;169(5):498‐507. [DOI] [PubMed] [Google Scholar]
- 39. Passow S, Specht K, Adamsen TC, et al. Default‐mode network functional connectivity is closely related to metabolic activity. Hum Brain Mapp. 2015;36(6):2027‐2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shioiri A, Kurumaji A, Takeuchi T, Matsuda H, Arai H, Nishikawa T. White matter abnormalities as a risk factor for postoperative delirium revealed by diffusion tensor imaging. Am J Geriatr Psychiatry. 2010;18(8):743‐753. [DOI] [PubMed] [Google Scholar]
- 41. Yokota H, Ogawa S, Kurokawa A, Yamamoto Y. Regional cerebral blood flow in delirium patients. Psychiatry Clin Neurosci. 2003;57(3):337‐339. [DOI] [PubMed] [Google Scholar]
- 42. Gaudreau J‐D, Gagnon P. Psychotogenic drugs and delirium pathogenesis: the central role of the thalamus. Med Hypotheses. 2005;64(3):471‐475. [DOI] [PubMed] [Google Scholar]
- 43. Kinomura S, Larsson J, Gulyas B, Roland PE. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science. 1996;271(5248):512‐515. [DOI] [PubMed] [Google Scholar]
- 44. Davis DHJ, Skelly DT, Murray C, et al. Worsening cognitive impairment and neurodegenerative pathology progressively increase risk for delirium. Am J Geriatr Psychiatry. 2015;23(4):403‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kyeong S, Choi SH, Eun Shin J, et al. Functional connectivity of the circadian clock and neural substrates of sleep‐wake disturbance in delirium. Psychiatry Res Neuroimaging. 2017;264:10‐12. [DOI] [PubMed] [Google Scholar]
- 46. Evered L, Silbert B, Scott DA, Eckenhoff RG. Recommendations for a new perioperative cognitive impairment nomenclature. Alzheimers Dement. 2019;15(8):1115‐1116. [DOI] [PubMed] [Google Scholar]
- 47. Browndyke JN, Berger M, Harshbarger TB, et al. Resting‐state functional connectivity and cognition after major cardiac surgery in older adults without preoperative cognitive impairment: preliminary findings. J Am Geriatr Soc. 2017;65(1):e6‐e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Browndyke JN, Berger M, Smith PJ, et al. Task‐related changes in degree centrality and local coherence of the posterior cingulate cortex after major cardiac surgery in older adults. Hum Brain Mapp. 2018;39(2):985‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Nat Acad Sci USA. 2005;102(27):9673‐9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rice GE, Caswell H, Moore P, Hoffman P, Lambon Ralph MA. The roles of left versus right anterior temporal lobes in semantic memory: a neuropsychological comparison of postsurgical temporal lobe epilepsy patients. Cereb Cortex. 2018;28(4):1487‐1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Koenigs M, Barbey AK, Postle BR, Grafman J. Superior parietal cortex is critical for the manipulation of information in working memory. J Neurosci. 2009;29(47):14980‐14986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cavallari M, Dai W, Guttmann CRG, et al. Longitudinal diffusion changes following postoperative delirium in older people without dementia. Neurology. 2017;89(10):1020‐1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tanabe S, Mohanty R, Lindroth H, et al. Cohort study into the neural correlates of postoperative delirium: the role of connectivity and slow‐wave activity. Br J Anaesth. 2020;125(1):55‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shulman GL, Pope DLW, Astafiev SV, McAvoy MP, Snyder AZ, Corbetta M. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J Neurosci. 2010;30(10):3640‐3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tang WK, Chan SSM, Chiu HFK, et al. Can IQCODE detect poststroke dementia? Int J Geriatr Psychiatry. 2003;18(8):706‐710. [DOI] [PubMed] [Google Scholar]
- 56. Wennberg AM, Whitwell JL, Tosakulwong N, et al. The influence of tau, amyloid, alpha‐synuclein, TDP‐43, and vascular pathology in clinically normal elderly individuals. Neurobiol Aging. 2019;77:26‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mosconi L, Berti V, Glodzik L, Pupi A, De Santi S, De Leon MJ. Pre‐Clinical detection of Alzheimer's disease using FDG‐PET, with or without amyloid imaging. J Alzheimers Dis. 2010;20(3):843‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Marinescu RV, Eshaghi A, Alexander DC, Golland P. BrainPainter: A software for the visualisation of brain structures, biomarkers and associated pathological processes. Multimodal Brain Image Anal Math Found Comput Anat (2019). 2019;11846:112‐120. 10.1007/978-3-030-33226-6_13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION
SUPPORTING INFORMATION
SUPPORTING INFORMATION
SUPPORTING INFORMATION
SUPPORTING INFORMATION
SUPPORTING INFORMATION


