Abstract
This article will identify the state of science on the generation, production, and transport of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA). Additionally, this article will focus on the transport of these environmental contaminants through air sources. It is important to explore why air exposure is critical to bring awareness to a problem that is not always immediately apparent. From a biological standpoint, clean air is necessary to sustain healthy life. Thus, it is key to understand the environmental transport of chemicals such as PFOS and PFOA with regard to their ability to migrate (i.e., air to water and water to air) and thus create unsafe air. The fluorinated backbone of these substances is both hydrophobic and oleophobic/lipophobic, while the terminal functional group is hydrophilic (water loving). Therefore, PFOS and PFOA compounds tend to partition to interfaces, such as between air and water with the fluorinated backbone residing in air and the terminal functional group residing in water. This article will identify opportunities for research to further the understanding of their potential impacts to human health.
Introduction
Since the late 1960s, perfluorinated compounds (PFCs) were originally produced for numerous industrial applications including refrigerants, polymers, pharmaceuticals, adhesives, and fire retardants (Key et al., 1997). PFCs comprise a large group of fluorinated chemicals that are synthetic and man-made with unique properties. PFCs are now recognized as a new class of emerging, persistent contaminants. Their basic structural elements include a partially or fully fluorinated alkyl chain typically 4–14 in length (hydrophobic part) and a terminated functional group (carboxylates, sulfonates, sulfonamides, phosphonates) that constitutes the hydrophilic part of the molecule. Due to the presence of both hydrophobic and hydrophilic parts, PFCs exhibit surfactant properties, reducing surface tension more strongly than all other major classes of surfactants. The carbon-fluorine bonds are the strongest bonds in organic chemistry because of a high electronegativity and the fluorine atom’s small size (O’Hagan, 2008). PFCs are nonflammable and resistant towards acids, bases, oxidizers, and reductants (Ding & Peijnenburg, 2013). These chemical properties are utilized for numerous consumer products such as water-, oil- and stain-resistant coatings for clothing fabrics, leather, and carpets, as well as oil-resistant coatings for paper products for the food industry (Chen et al., 2012; Giesy & Kannan, 2001; Lindstrom, Strynar, & Libelo, 2011; Tsai et al., 2002).
Another application of these chemicals includes their use to extinguish fuel fires, allowing an aqueous film to spread over the flammable liquid and further act as a vapor sealant during firefighting on military bases, airports, and oil refineries (Schultz et al., 2003). The stability and application of these compounds make them practically nonbiodegradable and therefore persistent in the environment (Key et al., 1998).
Characteristics and Production of Perfluorinated Compounds
The most encountered or investigated PFCs persistent in the environment are perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA). PFOS and PFOA are both stable in air at high temperatures (>150 °C); nonflammable; not readily degraded by strong acids, alkalis, or oxidizing agents; and are not subject to photolysis (Kissa, 2001). PFOS and PFOA have been made by two major manufacturing methods: electrochemical fluorination (ECF) and telomerization (Buck et al., 2011; Lindstrom, Strynar, Delinsky, et al., 2011). ECF produces a mixture of compounds including branched, linear, and cyclic isomers of various chain lengths, while telomerization produces primarily straight chain (linear) compounds with an even number of carbons, such as PFOA. Isomer profiling methods can be used to assess the relative contribution from each of these manufacturing processes to PFOA found in environmental and biological media (Benskin et al., 2010, De Silva & Mabury, 2006; De Silva et al., 2009).
There is now major environmental concern over these compounds due to studies indicating serious health effects associated with PFOS and PFOA (Organization for Economic Co-operation and Development, 2002; U.S. Environmental Protection Agency [U.S. EPA], 2002). Consequently, these concerns have led to voluntary cessation of the production of PFOS in the U.S. as well as reductions in factory emissions of PFOA and therefore a reduction in residual chemicals from PFOA in finished products (U.S. EPA, 2002). In 2000, the production and use of PFOS (approximately 3,500 metric tons) greatly outnumbered the production of PFOA (approximately 500 metric tons).
After the 3M Company, the major manufacturer of PFOS, phased out production in 2002, the global production of this chemical dropped dramatically to 175 metric tons in 2003 (3M Company, 2003). In contrast, global production of PFOA increased to 1,200 metric tons/year in 2004 and has seemingly become the most common PFC in commerce. Currently, there are many companies worldwide that still produce and/or use a wide range of different PFCs in a variety of products (Prevedouros et al., 2006). In 2006, the U.S. Environmental Protection Agency (U.S. EPA) initiated the PFOA Stewardship Program, in which eight key companies in the industry committed to reducing facility emissions, product contents of PFOA, and related chemicals on a global basis by 95% (U.S. EPA, 2018a).
While the routes of exposure and the associated risks are largely unknown, it has been determined that residents living in industrialized countries have detectable levels of many PFCs in their blood (Kannan et al., 2004). Possible routes of exposure source could include inhalation, dermal contact, food, food packaging, house dust, and drinking water.
Perfluorinated Compounds Released in the Air Through Manufacture and Production Facilities
There are both direct and indirect sources of PFOS and PFOA emissions to the environment. Direct sources are a result from the manufacture and use of these compounds. Indirect sources in the environment occur as chemical reaction impurities or when substances degrade to form by-products. Figure 1 demonstrates a possible environmental transport pathway of PFOA and PFOS by air deposition. Comparable to other groundwater contaminants, PFOA can reach drinking water wells through the pathway of migration of a contaminated groundwater plume (Butt et al., 2010; DuPont Corporate Remediation Group & URS Diamond, 2003; Lau et al., 2007). PFOA can also reach groundwater from air emissions from nearby industrial facilities, followed by deposition from air onto soil and migration through the soil to groundwater (Davis et al., 2007). In West Virginia and Ohio, drinking water wells were contaminated by releases from a nearby industrial manufacturing facility for fluoropolymers (Steenland, Jin, et al., 2009). The hypothesis is that the contamination occurred through soil deposition of PFOA emitted into the air that leached downward and migrated to groundwater and/or contaminated surface water from the Ohio River (Shin et al., 2011).
FIGURE 1.
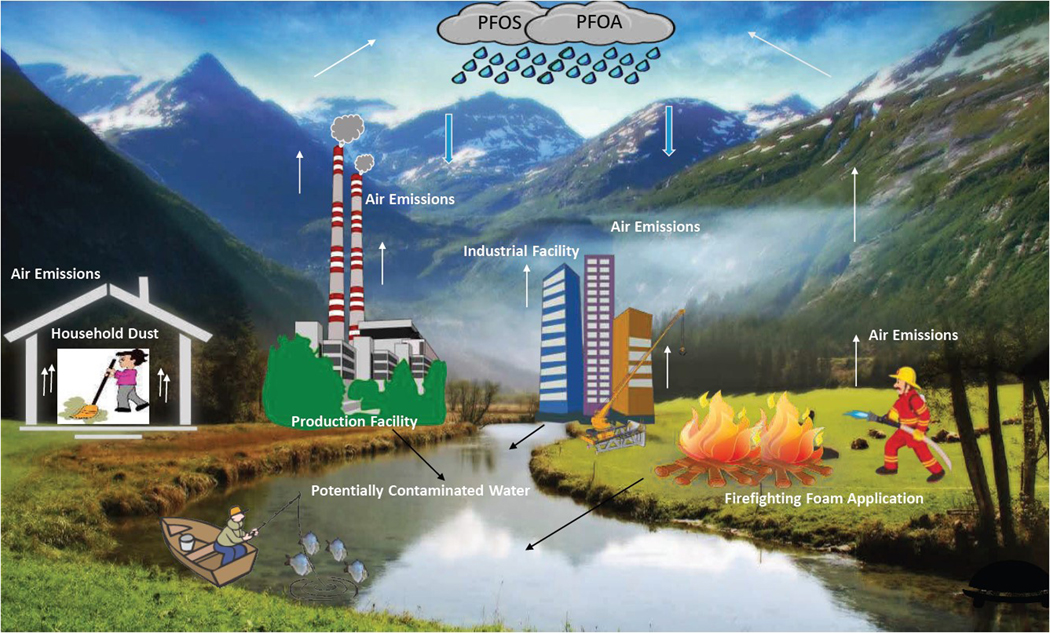
Environmental Transport Pathway Examples of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) by Air Deposition
The public water supply wells in the vicinity of the production facility had PFOA detected at levels up to >4,000 ng/L and in private wells up to >13,000 ng/L (DuPont, 2008; Hoffman et al., 2011). The impact of contamination from production facilities was also noted in New Jersey. PFOA has been detected at up to 190 ng/L in shallow, unconfined wells of a public water supply and at >40 ng/L with a maximum >400 ng/L in 59 of 104 private wells within a radius of slightly more than 2 miles from the facility (DuPont, 2009; Post et al., 2009).
PFOA can also enter groundwater or surface water used for drinking water from sources other than industrial releases. These sources include discharge from wastewater treatment plants processing domestic and/ or industrial waste street runoff, storm water runoff, release of aqueous firefighting foams, land application of sludge, land application of wastewater from industrial sources, and use of contaminated industrial waste as a soil amendment (Clarke & Smith, 2011; Kim & Kannan, 2007; Moody et al., 2003; Murakami et al., 2008; Sinclair & Kannan, 2006; Konwick et al., 2008).
Wang and coauthors (2014) estimated that 4% of the perfluoroalkyl carboxylic acids emitted due to PFOA manufacturing is released into the air, while emissions due to fluoropolymer manufacturing measured 16%. Based on information obtained from interviews with engineers at a DuPont fluoropolymer factory in the U.S., Paustenbach and coauthors (2006) concluded that PFOA is most likely emitted into the air in the form of vapors that quickly condense to fumes after they exit the stack. They also reported that DuPont characterized the particle size distribution of PFOA released from their exhaust after installing a scrubber in 1996: approximately 54% of the mass was observed on aerosols <0.1 μm and 27% on aerosols between 0.1 μm and 0.3 μm. Barton and coauthors (2006) reported that 60% of the mass of PFOA sampled along the fence line of the same fluoropolymer manufacturing facility in 2003–2004 was distributed as aerosols <0.3 μm. This size range includes aerosols that could have a residence time in the atmosphere on the order of days (Slinn & Slinn, 1980).
Kaiser and coauthors (2010) conducted a study by simulating and reconstructing a PFOA manufacturing site to better understand how neighboring communities and workers might be exposed to PFCs in the air when handling these compounds. Their study included workplace monitoring, experimental data, and modeling results to ascertain the most probable workplace exposure sources and transport mechanisms for PFOA and its ammonium salt. These two compounds were monitored due to the dramatic difference in physical properties of the anionic form and the protonated acid form. PFOA, measured as the anion PFO- in blood, is projected to have a biological half-life in humans of 2–4 years (Burris et al., 2002). Historically, levels ranging from 0–100 ppm have been found in the blood of workers with most of the results <20 ppm (Ubel et al., 1980). These levels are significantly higher than blood levels found in the U.S. general population, averaging 5 ppb based on blood bank sampling performed in 2000–2001 (Olsen et al., 2003). In their modeling study, Kaiser and coauthors (2010) used simple mass transfer to simulate volatilization from open liquids and sublimation to air from surfaces within the re-created manufacturing site applying the equation:
where E = air emissions from the liquid surface (g/s), K = mass transfer coefficient (m/s), A = liquid surface area (m2), and CL = concentration of PFOA in the liquid phase (g/m3).
Input parameters for room air velocities and PFOA concentrations were selected to represent actual facility conditions during air monitoring of past manufacturing site conditions. Three scenarios used in the modeling included:
Volatilization of PFOA from wet sump A, which contained an aqueous solution of 340 mg/L PFOA at pH = 1.8.
Sublimation of PFOA from dry sump A, with approximately 50% of its previously wetted surface area currently covered with dry PFOA molecules.
A combination of volatilization and sublimation of PFOA from sump B, with volatilization of PFOA from an aqueous solution of 54 mg/L PFOA at pH = 6.7 and sublimation from dry walls with 10% of their previously wetted surface area covered with dry PFOA molecules.
A sump is defined as a low space that collects undesirable liquids such as water or chemicals.
During a 2-week period of air monitoring for PFOA where pH, concentration, and water level varied based upon operating activities, air samples taken near two process sumps showed quantifiable levels of PFOA (Table 1). These data suggest a major correlation among increased air concentrations, decreased sump pH, and water level.
Table 1.
Eight-Hour Time-Weighted Average Air Levels of Perfluorooctanoic Acid (PFOA) Near Process Sumps
| Day | PFOA Concentration (mg/m3) | Comment |
|---|---|---|
| 1 | 0.065 | Low pH sump |
| 1 | 0.007 | After sump pH adjusted to 7 |
| 11 | 0.061 | Low water in sump |
| 13 | 0.004 | Water level restored |
Source: Kaiser et al., 2010.
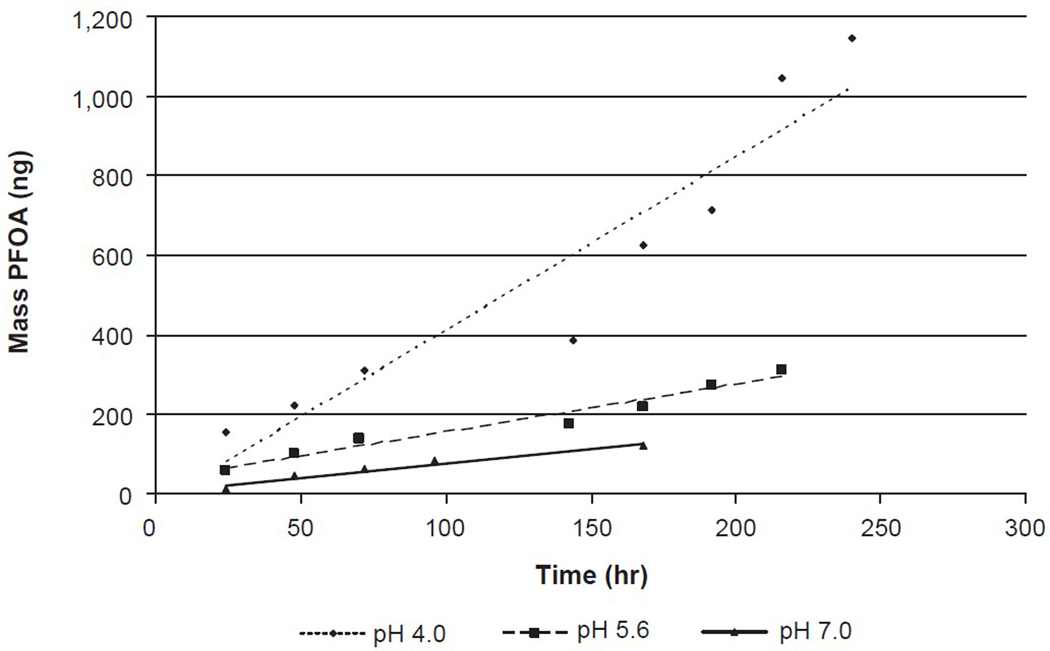
Figure 2 shows a graph of the mass of PFOA partitioned to air from aqueous solution as a function of time and pH (Kaiser et al., 2010). The graph suggests that the lower the pH, the greater the volatilization and therefore, more PFOA is partitioned into the air from the aqueous solution. This finding coincides with the monitoring data shown in Table 1. Furthermore, this research implies that in a manufacturing setting, the source of PFOA in air could be from sumps or holding reservoirs, as well as PFOA material that has condensed on walls, floors, and equipment. As PFOA contains a hydrophobic perfluoroalkyl tail, the undissociated acid is much less water soluble. In fact, the undissociated form is highly insoluble in water with a significant driving force for it to partition out of the water into the air above the water under low pH conditions. The experimental data demonstrate that a pH of >7 limited the quantity of undissociated acid leaving the surface. This understanding has direct implications in the workplace for minimizing the potential for PFOA to become airborne at high measurable concentrations. These findings suggest that keeping surfaces clean, preventing accumulation of material in unventilated areas, removing solids from waste trenches and sumps, and maintaining neutral pH in sumps can all lower workplace exposures.
FIGURE 2.
Mass of Perfluorooctanoic Acid (PFOA) Transported From Aqueous Solution to Air as a Function of Time and pH
There has been major concern in North Carolina where the Chemours Company (a DuPont subsidiary) Fayetteville Works Plant allowed its effluent discharge of the compound GenX upstream from the city of Wilmington into the Cape Fear River (Clabby, 2017, October 18). A map of the work plant site can be viewed at www.northcarolinahealthnews.org/2017/07/17/genx-pollutionmysteries. Chemours proclaimed GenX as an improved substitute for PFOA due to differences in its chemical structure that make it less persistent in the environment and thus reduce potential health risks to the public (Clabby, 2017, August 17). According to U.S. EPA, the North Carolina plant might have committed federal violations by failing to notify U.S. EPA before it started manufacturing and repurposing new industrial compounds (Dalesio, 2019). Federal law requires the producers of potentially toxic substances that could present an unreasonable risk of injury to health or the environment to notify U.S. EPA before the company starts making new chemicals or using an existing compound for a significantly new use (U.S. EPA, 1976). Whereas U.S. EPA classifies GenX as an “emerging contaminant,” some scientists are finding reasons to be concerned about how PFOA exposure in the local population has been associated with adverse human health outcomes, such as affecting kidneys, blood, the immune system, liver, and developing fetuses (MacNeil et al., 2009; Nakayama et al., 2007; Steenland, Tinker, et al., 2009; U.S EPA, 2018b). In November 2018, current litigations involving the Chemours plant awarded restitution to North Carolina for $12 million to cover cleanup and provide permanent replacement drinking water to homes and businesses with contaminated wells (North Carolina Department of Environmental Quality, 2018).
Perfluorinated Compounds Released in the Air of Indoor Environments
Indoor air has been hypothesized as a primary contributor for atmospheric PFC contamination. Yao and coauthors (2018) evaluated indoor air and indoor dust samples from the rooms of residential homes, hotel buildings, textile shops, and movie theaters in China. The fluorotelomer alcohols (FTOHs) were the most frequently detected PFCs found in air (250–82,300 pg/m3) and hotel dust (24.8–678 ng/g). Polyfluoroalkyl phosphoric acid diesters were found at much lower level concentrations in air (not detected-125 pg/ m3) and in dust (0.32–183 ng/g). Perfluoroalkyl carboxylic acids were also detected in the air samples at a total concentration range of 121–20,600 pg/m3 where C4-C7 PFCs contributed up to 54% of the profiles. The high contribution of perfluoroalkyl carboxylic acids suggests that shorter-chain PFCs likely are used in China as an alternative to longchain PFCs.
Yao and coauthors (2018) included the monitoring of direct and indirect human exposure to PFCs by estimating the daily intake of PFCs through air inhalation and dust ingestion. They estimated daily intake of PFCs via air inhalation and dust ingestion at 1.04–14.1 ng/kg/body weight/day and 0.108.17 ng/kg/body weight/day. This estimation confirmed that for PFCs in adults, inhalation of air suspended with fine particles was a more important exposure pathway than dust ingestion. The major pathway for PFOA exposure in toddlers, however, was dust ingestion because of crawling and their hand/foot-tomouth contact with carpets and floors.
In Finland, Winkens and coauthors (2017) investigated the contamination levels and patterns of PFCs in air samples from children’s bedrooms. Children’s bedrooms were examined as part of a larger study focusing on environmental exposures to children. Indoor air samples were taken from 57 households and analyzed for 17 perfluoroalkyl acids and 9 precursors. Two unique acrylate compounds, 6:2 FTAC (2-perfluorohexyl ethyl acrylate) and 6:2 FTMAC (2-perfluorohexyl ethyl methacrylate), were detected in 28% and 58% of the air samples, respectively. These two compounds are not typically reported in the scientific literature. Of the fluorotelomer alcohols, 8:2 FTOH was detected at the highest median concentration of 3,570 pg/m3. Due to the reduction of use or elimination of PFCs by some industry manufacturers, the C8 perfluoroalkyl acids were still the most abundant acids. From this study, the comparison with previous studies of the measured fluorotelomer alcohols, perfluoroalkyl acids, and the pathway of PFOA and PFOS by air deposition indicated a correlation that indoor air levels of PFCs display a time delay to changes in manufacturer production over several years.
Perfluorinated Compounds Released in the Air From Firefighting Foam
Throughout the U.S., many fire departments on military bases and civilian airports are still using aqueous film-forming foams for fire suppression, fire training, and flammable vapor suppression (Hu et al., 2016). The U.S. Department of Defense is currently reviewing the use, impact, and disposal practices for firefighting foam (Hatton et al., 2018; Sullivan et al., 2017). Anderson and coauthors (2016) noted that environmental releases and exposure to firefighting foam can occur from line leaks in supply tanks, fire suppression systems, firefighting activities, and equipment maintenance. PFC vapors released in the air migrate to groundwater and can severely injure those working in the area who don’t have proper safety ventilation equipment, as well as communities living in close proximity to the affected site, such as military personnel and their families (Rak & Vogel, 2010). The Norwegian Pollution Control Authority (2008) determined that ground and soil samples near four fire training facilities were contaminated by PFCs from routine use of firefighting foams that contain PFOS. Concentrations from soil samples taken within 200 m of the training facilities exceeded the proposed Norwegian guideline value for PFOS of 100 ng/g. It was also noted that migration of PFCs to soil, water, and sediments can have a significant impact on the surrounding terrestrial animals near these contaminated sites.
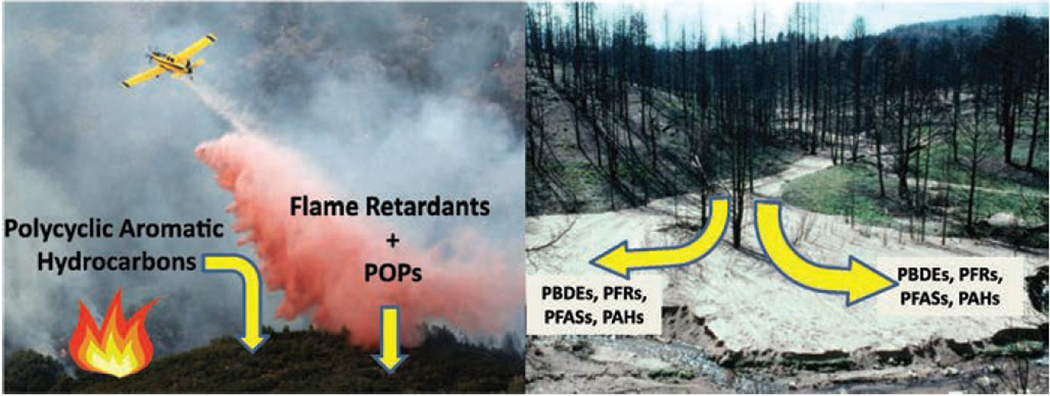
Forest fires are another potential source or pathway of PFC air contamination (Figure 3, Campo et al., 2017). As forest fires across the world have increased, aircrafts are spraying firefighting foam over more affected areas to aid in suppressing or extinguishing fires. Campo and coauthors (2017) simulated and monitored the sediment and soil from a severe fire on two Mediterranean hillslopes, one burned and one unburned, near Azuébar (SE Spain). Samples from the hillslopes were analyzed for contamination by polycyclic aromatic hydrocarbons (PAHs), indirect tri- to hepta-brominated diphenyl ethers (PBDEs), organophosphate flame retardants (PFRs), and per- and polyfluoroalkyl substances (PFASs) related to fighting forest fires.
FIGURE 3. Forest Fires as an Air Source and Exposure Pathway to Polycyclic Aromatic Hydrocarbons, Flame Retardants, Persistent Organic Pollutants, and Per- and Polyfluoroalkyl Substances.
PAHs = polycyclic aromatic hydrocarbons; PBDEs = polybrominated diphenyl ethers; PFASs = per- and polyfluoroalkyl substances; PFRs = organophosphate flame retardants; POPs = persistent organic pollutants. Source: Campo et al., 2017.
Soil samples were taken at the top of the hillslope (eroding zone), middle part (transport site), and foot of the hillslope (depositional zone). The fires were simulated, so burned soil samples were measured against control unburned samples. In the burned soil samples, low concentrations of PBDEs (7.3 ng/g), PFRs (664.4 ng/g), and PFASs (56.4 ng/g) were detected in relation to PAHs (16 PAHs = 1,255.3 ng/g). Directly after the simulated fire, concentrations of PBDEs (17.8 ng/g) and PAHs (16 PAHs = 3,154.2 ng/g) were higher in sediment than in soil. There was no definite clear pattern for the distribution of compounds over the different slope positions. Compared with samples taken from the three hillslopes, however, higher concentrations tended to be found in the middle and foot of the hillslope. It is important to note that when it rains after a fire, the erosion process can concentrate contaminants at the foot of the hillslope, possibly leading to enhanced bioaccumulation and potentially higher hazardous values (Abrahams et al., 2018).
Perfluorinated Compounds Released in the Air by Waste Incineration
An additional potential source or pathway of PFC contamination released into the air might occur by means of waste incineration. Knowledge of how PFCs behave in the incineration or combustion process is limited. Consensus in the limited scientific literature, however, is that degradation of PFOS occurs at temperatures >500 °C. In theory, fluorinated by-products are formed, which could have undesired properties and significant impacts on the environment. A study conducted by U.S. EPA and 3M stated that degradation of PFOS occurs at temperatures >600 °C and that PFOS is not released in the environment through incineration; the main degradation products, however, were the potent greenhouse gases CF4 and C2F6 (Taylor & Yamada, 2003). With fluorinated by-products resulting from waste incineration, it is clear further investigation of these compounds is needed to evaluate their chemical properties.
Conclusion and Recommendations
This article sought to identify the state of science on the generation, production, and transport of PFOS and PFOA in the environment. Specifically, this article focused on air as the primary transport route of these contaminants. It was determined that the major air contamination sources included manufacture or production facilities, indoor air contamination from household products, exposure to firefighting foam, exposure to chemicals released combating forest fires, and by-product formation of PFCs by waste incineration.
Direct sources of contamination result from the manufacture and use of these compounds. Indirect sources occur as chemical reaction impurities or when substances degrade to form by-products. With indoor air, direct exposure of PFOA through dust ingestion is the major pathway for introduction in toddlers because they crawl and have hand/foot-to-mouth contact with carpets and floors. For adults, inhalation of contaminated air with fine suspended particles is the major pathway.
The exposure pathway in the air from firefighting foam can occur from line leaks in supply tanks, fire suppression systems, fire- fighting activities, and equipment maintenance. Shortly after combating a forest fire, the exposure pathway of PFC vapors released in the air exposes communities living near or in proximity to the affected site. The information on how PFCs perform in the combustion process during incineration is still limited; however, it is clear that fluorinated by-products are formed that can have undesired properties and significant impacts on the environment.
While progress has been made to understand the environmental concerns from PFCs, there are several areas for future research. One observation is that we still know little about how people are exposed to PFCs through the air. Specific studies should:
Provide manufacturing and production facilities with further scientific knowledge to reduce air exposure of PFCs to employees and neighboring communities.
Further investigate potential sources of atmospheric PFCs released from manufacturing and production facilities and investigate the resuspension of aerosols associated with PFCs and precursor degradation.
Widen the national coverage of current monitoring to ensure the public is aware of the connections among production and use volumes of PFCs and possible exposures.
Evaluate additional methods designed to reduce indoor air exposure to PFCs. These methods could range from immediate actions to enable individuals to reduce their likely burden (e.g., manipulate room ventilation, minimize products in the home treated with PFCs) to longer-term strategies (e.g., minimize chemical migration from products by modifying product formulation and design).
Better characterize the emission rates of household products treated with PFCs.
Conduct more studies to demonstrate the relationship between concentrations of PFCs in household dust and exposure to adults and children (e.g., in homes, offices, schools, and day care facilities).
Improve exposure monitoring strategies to those using firefighting foam and those combating forest fires.
Monitor a wider range of treated forest fire areas that recently have been exposed to the chemicals.
Evaluate and characterize all by-products produced during waste incineration of PFCs.
Footnotes
Disclaimer:
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 3M Company. (2003). Environmental, health and safety measures relating to perfluorooctanoic acid and its salts (PFOA) (U.S. EPA EDocket OPPTS 2003–0012-0007). http://www.fluoridealert.org/wp-content/pesticides/effect.pfoa.class.mar.13.2003.pdf
- Abrahams ER, Kaste JM, Ouimet W, & Dethier DP (2018). Asymmetric hillslope erosion following wildfire in Fourmile Canyon, Colorado. Earth Surface Processes and Landforms, 43(9), 2009–2021. [Google Scholar]
- Anderson RH, Long GC, Porter RC, & Anderson JK (2016). Occurrence of select perfluoroalkyl substances at U.S. Air Force aqueous film-forming foam release sites other than fire-training areas: Field-validation of critical fate and transport properties. Chemosphere, 150, 678–685. [DOI] [PubMed] [Google Scholar]
- Barton CA, Butler LE, Zarzecki CJ, Flaherty J, & Kaiser M. (2006). Characterizing perfluorooctanoate in ambient air near the fence line of a manufacturing facility: Comparing modeled and monitored values. Journal of the Air and Waste Management Association, 56(1), 48–55. [DOI] [PubMed] [Google Scholar]
- Benskin JP, Yeung LWY, Yamashita N, Taniyasu S, Lam PKS, & Martin JW (2010). Perfluorinated acid isomer profiling in water and quantitative assessment of manufacturing source. Environmental Science & Technology, 44(23), 9049–9054. [DOI] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, De Voogt P, … van Leeuwen SP(2011). Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integrated Environmental Assessment Management, 7(4), 513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris JM, Lundberg JK, Olsen G, Simpson C, & Mandel J. (2002). Determination of serum half-lives of several fluorochemicals (3M Company Interim Report #2, AR 226–1086). https://static.ewg.org/reports/2003/pfcs/halflife_full.pdf
- Butt CM, Berger U, Bossi R, & Tomy GT (2010). Review: Levels and trends of poly- and perfluorinated compounds in the arctic environment. Science of the Total Environment, 408(15), 2936–2965. [DOI] [PubMed] [Google Scholar]
- Campo J, Lorenzo M, Cammeraat ELH, Picó Y, & Andreu V (2017). Emerging contaminants related to the occurrence of forest fires in the Spanish Mediterranean. Science of the Total Environment, 603–604, 330–339. [DOI] [PubMed] [Google Scholar]
- Chen M-H, Ha E-H, Wen T-W, Su Y-N, Lien G-W, Chen C-Y, … Hsieh W-S (2012). Perfluorinated compounds in umbilical cord blood and adverse birth outcomes. PLOS One, 7(8), e42474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clabby C. (2017, August 17). GenX pollution-What happened? And when? North Carolina Health News. https://www.northcarolinahealthnews.org/2017/08/17/genx-pollution-whathappened-when/
- Clabby C. (2017, October 18). Local scientists uncovered Cape Fear River GenX story. North Carolina Health News. https://www.northcarolinahealthnews.org/2017/10/18/localscientists-uncovered-cape-fear-river-genx-saga/ [Google Scholar]
- Clarke BO, & Smith SR (2011). Review of ‘emerging’ organic contaminants in biosolids and assessment of international research priorities for the agricultural use of biosolids. Environment International, 37(1), 226–247. [DOI] [PubMed] [Google Scholar]
- Dalesio EP (2019). EPA hits Chemours for not notifying of new chemicals. The Times News. https://www.thetimesnews.com/news/20190215/epa-hits-chemours-for-notnotifying-of-new-chemicals [Google Scholar]
- Davis KL, Aucoin MD, Larsen BS, Kaiser MA, & Hartten AS (2007). Transport of ammonium perfluorooctanoate in environmental media near a fluoropolymer manufacturing facility. Chemosphere, 67(10), 2011–2019. [DOI] [PubMed] [Google Scholar]
- De Silva AO, & Mabury SA (2006). Isomer distribution of perfluorocarboxylates in human blood: Potential correlation to source. Environmental Science & Technology, 40(9), 2903–2909. [DOI] [PubMed] [Google Scholar]
- De Silva AO, Muir DC, & Mabury SA (2009). Distribution of perfluorocarboxylate isomers in select samples from the North American environment. Environmental Toxicology and Chemistry, 28(9), 1801–1814. [DOI] [PubMed] [Google Scholar]
- Ding G, & Peijnenburg WJGM (2013). Physicochemical properties and aquatic toxicity of poly- and perfluorinated compounds. Critical Reviews in Environmental Science and Technology, 43(6), 598–678. [Google Scholar]
- DuPont. (2008). Data assessment DuPont Washington Works (OPPT2004–0113 PFOA Site-Related Environmental Assessment Program; ). [Google Scholar]
- DuPont. (2009). Private drinking water well survey and sampling update. Deepwater, NJ: DuPont Chambers Works Facility. [Google Scholar]
- DuPont Corporate Remediation Group, & URS Diamond. (2003). Revised groundwater flow model for DuPont Washington Works, West Virginia: (EPA-HQ-OPPT-2003–0012-0868; ). [Google Scholar]
- Giesy JP, & Kannan K. (2001). Global distribution of perfluorooctane sulfonate in wildlife. Environmental Science & Technology, 35(7), 1339–1342. [DOI] [PubMed] [Google Scholar]
- Hatton J, Holton C, & DiGuiseppi B. (2018). Occurrence and behavior of per- and polyfluoroalkyl substances from aqueous film-forming foam in groundwater systems. Remediation, 28(2), 89–99. [Google Scholar]
- Hoffman K, Webster TF, Bartell SM, Weisskopf MG, Fletcher T, & Vieira VM. (2011). Private drinking water wells as a source of exposure to perfluorooctanoic acid (PFOA) in communities surrounding a fluoropolymer production facility. Environmental Health Perspectives, 119(1), 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, … Sunderland EM (2016). Detection of poly- and perfluoroalkyl substances (PFASs) in U.S. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environmental Science & Technology Letters, 3(10), 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser MA, Dawson BJ, Barton CA, & Botelho MA (2010). Understanding potential exposure sources of perfluorinated carboxylic acids in the workplace. The Annals of Occupational Hygiene, 54(8), 915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan K, Corsolini S, Falandysz J, Fillmann G, Kumar KS, Loganathan BG, … Aldous KM (2004). Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environmental Science & Technology, 38(17), 4489–4495. [DOI] [PubMed] [Google Scholar]
- Key BD, Howell RD, & Criddle CS (1997). Fluorinated organics in the biosphere. Environmental Science & Technology, 31(9), 2445–2454. [Google Scholar]
- Key BD, Howell RD, & Criddle CS (1998). Defluorination of organofluorine sulfur compounds by Pseudomonas sp. strain D2. Environmental Science & Technology, 32(15), 2283–2287. [Google Scholar]
- Kim S-K, & Kannan K. (2007). Perfluorinated acids in air, rain, snow, surface runoff, and lakes: Relative importance of pathways to contamination of urban lakes. Environmental Science & Technology, 41(24), 8328–8334. [DOI] [PubMed] [Google Scholar]
- Kissa E. (2001). Fluorinated surfactants and repellents. (2nd ed., Surfactant Science Series, Vol. 97), New York City, NY: Marcel Dekker, Inc. [Google Scholar]
- Konwick BJ, Tomy GT, Ismail N, Peterson JT, Fauver RJ, Higginbotham D, & Fisk AT (2008). Concentrations and patterns of perfluoroalkyl acids in Georgia, USA surface waters near and distant to a major use source. Environmental Toxicology and Chemistry, 27(10), 2011–2018. [DOI] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, & Seed J. (2007). Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicological Sciences, 99(2), 366–394. [DOI] [PubMed] [Google Scholar]
- Lindstrom AB, Strynar MJ, Delinsky AD, Nakayama SF, McMillan L, Libelo EL, … Thomas L. (2011). Application of WWTP biosolids and resulting perfluorinated compound contamination of surface and well water in Decatur, Alabama, USA. Environmental Science & Technology, 45(19), 8015–8021. [DOI] [PubMed] [Google Scholar]
- Lindstrom AB, Strynar MJ, & Libelo EL (2011). Polyfluorinated compounds: Past, present, and future. Environmental Science & Technology, 45(19), 7954–7961. [DOI] [PubMed] [Google Scholar]
- MacNeil J, Steenland NK, Shankar A, & Ducatman A. (2009). A cross-sectional analysis of type II diabetes in a community with exposure to perfluorooctanoic acid. Environmental Research, 109(8), 997–1003. [DOI] [PubMed] [Google Scholar]
- Moody CA, Hebert GN, Strauss SH, & Field JA (2003). Occurrence and persistence of perfluorooctanesulfonate and other perfluorinated surfactants in groundwater at a fire-training area at Wurtsmith Air Force Base, Michigan, USA. Journal of Environmental Monitoring, 5(2), 341–345. [DOI] [PubMed] [Google Scholar]
- Murakami M, Imamura E, Shinohara H, Kiri K, Muramatsu Y, Harada A, & Takada H. (2008). Occurrence and sources of perfluorinated surfactants in rivers in Japan. Environmental Science & Technology, 42(17), 6566–6572. [DOI] [PubMed] [Google Scholar]
- Nakayama S, Strynar MJ, Helfant L, Egeghy P, Ye X, & Lindstrom AB (2007). Perfluorinated compounds in the Cape. Fear Drainage Basin in North Carolina. Environmental Science & Technology, 41(15), 5271–5276. [DOI] [PubMed] [Google Scholar]
- North Carolina Department of Environmental Quality. (2018). State officials require Chemours to provide permanent drinking water and pay $12 million penalty [Press release]. https://deq.nc.gov/news/press-releases/2018/11/21/release-state-officials-require-chemours-provide-permanent-drinking
- Norwegian Pollution Control Authority. (2008). Screening of polyfluorinated organic compounds at four fire training facilities in Norway (TA- 2444/2008). https://www.miljodirektoratet.no/globalassets/publikasjoner/klif2/publikasjoner/2444/ta2444.pdf
- O’Hagan D. (2008). Understanding organofluorine chemistry. An introduction to the C-F bond. Chemical Society Reviews, 37(2), 308–319. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Burlew MM, & Mandel JH (2003). Epidemiologic assessment of worker serum perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations and medical surveillance examinations. Journal of Occupational and Environmental Medicine, 45(3), 260–270. [DOI] [PubMed] [Google Scholar]
- Organization for Economic Co-operation and Development. (2002). Hazard assessment of perfluorooctane sulfonate (PFOS) and its salts (ENV/JM/RD (2002) 17/FINAL). Paris, France: Author. https://www.oecd.org/env/ehs/risk-assessment/2382880.pdf [Google Scholar]
- Paustenbach DJ, Panko JM, Scott PK, & Unice KM (2006). A methodology for estimating human exposure to perfluorooctanoic acid (PFOA): A retrospective exposure assessment of a community (1951–2003). Journal of Toxicology and Environmental Health, Part A, 70(1), 28–57. [DOI] [PubMed] [Google Scholar]
- Post GB, Louis JB, Cooper KR, Boros-Russo BJ, & Lippincott RL (2009). Occurrence and potential significance of perfluorooctanoic acid (PFOA) detected in New Jersey public drinking water systems. Environmental Science & Technology, 43(12), 4547–4554. [DOI] [PubMed] [Google Scholar]
- Prevedouros K, Cousins IT, Buck RC, & Korzeniowski SH (2006). Sources, fate and transport of perfluorocarboxylates. Environmental Science & Technology, 40(1), 32–44. [DOI] [PubMed] [Google Scholar]
- Rak A, & Vogel C. (2010). Increasing regulation of perfluorinated compounds and the potential impacts at Air Force installations. San Antonio, TX: Federal Facilities Forum. [Google Scholar]
- Schultz MM, Barofsky DF, & Field JA (2003). Fluorinated alkyl surfactants. Environmental Engineering Science, 20(5), 487–501. [Google Scholar]
- Shin HM, Vieira VM., Ryan PB, Steenland K, & Bartell SM (2011). Retrospective exposure estimation and predicted versus observed serum perfluorooctanoic acid concentrations for participants in the C8 Health Project. Environmental Health Perspectives, 119(12), 1760–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair E, & Kannan K. (2006). Mass loading and fate of perfluoroalkyl surfactants in wastewater treatment plants. Environmental Science & Technology, 40(5), 1408–1414. [DOI] [PubMed] [Google Scholar]
- Slinn SA, & Slinn WGN (1980). Predictions for particle deposition on natural waters. Atmospheric Environment, 14(9), 1013–1016. https://www.sciencedirect.com/science/article/abs/pii/0004698180900323?via%3Dihub [Google Scholar]
- Steenland K, Jin C, MacNeil J, Lally C, Ducatman A, Vieira V, & Fletcher T. (2009). Predictors of PFOA levels in a community surrounding a chemical plant. Environmental Health Perspectives, 117(7), 1083–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Tinker S, Frisbee S, Ducatman A, & Vaccarino V. (2009). Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant. American Journal of Epidemiology, 170(10), 1268–1278. [DOI] [PubMed] [Google Scholar]
- Sullivan M, Leeson A, & Sedlak D. (2017). Research and development needs for management of DoD’s PFAS contaminated sites [Webinar]. https://www.serdp-estcp.org/Tools-and-Training/WebinarSeries/09-07-2017
- Taylor P, & Yamada T. (2003). Final report: Laboratory-scale thermal degradation of perfluoro-octanyl sulfonate and related precursors. Dayton, OH: University of Dayton Research Institute and 3M. https://clu-in.org/download/contaminantfocus/pfas/UDRTR-03-00044.pdf [Google Scholar]
- Tsai W-T, Chen H-P, & Hsien W-Y (2002). A review of uses, environmental hazards and recovery/recycle technologies of perfluorocarbons (PFCs) emissions from the semiconductor manufacturing processes. Journal of Loss Prevention in the Process Industries, 15(2), 65–75. [Google Scholar]
- Ubel FA., Sorenson SD, & Roach DE (1980). Health status of plant workers exposed to fluorochemicals-A preliminary report. American Industrial Hygiene Association Journal, 41(8), 584–589. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. (1976). An overview of the Toxic Substances Control Act (Public Law 94–469). Washington, DC: Author. https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=91021VCC.TXT [Google Scholar]
- U.S. Environmental Protection Agency. (2002). Revised draft: Hazard assessment of perfluorooctanoic acid (PFOA) and its salts. Washington, DC: Author. [Google Scholar]
- U.S. Environmental Protection Agency. (2018a). Fact sheet: 2010/2015 PFOA stewardship program. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/fact-sheet-20102015-pfoa-stewardship-program
- U.S. Environmental Protection Agency. (2018b). Basic information on PFAS. https://www.epa.gov/pfas/basic-information-pfas
- Wang Z, Cousins IT, Scheringer M, Buck RC, & Hungerbuhler K. (2014). Global emission inventories for C4-C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, Part I: Production and emissions from quantifiable sources. Environment International, 70, 62–75. [DOI] [PubMed] [Google Scholar]
- Winkens K, Koponen J, Schuster J, Shoeib M, Vestergren R, Berger U, … Cousins IT (2017). Perfluoroalkyl acids and their precursors in indoor air sampled in children’s bedrooms. Environmental Pollution, 222, 423–432. [DOI] [PubMed] [Google Scholar]
- Yao Y, Zhao Y, Sun H, Chang S, Zhu L, Alder AC, & Kannan K. (2018). Per- and polyfluoroalkyl substances (PFAS) in indoor air and dust from homes and various microenvironments in China: Implications for human exposure. Environmental Science & Technology, 52(5), 3156–3166. [DOI] [PubMed] [Google Scholar]