Abstract
Introduction
This study was designed to compare mirror therapy and visual feedback with the control group in adhesive capsulitis patients.
Methods
Thirty-six patients, divided into three groups as mirror therapy, visual feedback and control for 15 sessions of treatment. Evaluations were made before treatment, at 6th and 10th weeks. Bilateral glenohumeral exercise was performed at the end of each session with the affected extremity behind the mirror in the mirror group, with both upper extremities in front of the mirror in the visual feedback group, and without the mirror in the control group.
Results
There were statistically significant differences between the mirror therapy and visual feedback in terms of pain severity change, and the visual feedback was superior to the change in pain severity compared to the control. Visual feedback showed significant improvement in mean change from baseline to week 10 in shoulder pain and disability index scores compared to control (p = 0.012). There was no significant difference between the groups in terms of modified constant score, proprioception and shoulder range of motion.
Conclusion
It was determined that the exercises performed by seeing the affected extremity in the mirror were more effective than mirror therapy and control group.
Keywords: frozen shoulder, visual feedback, proprioception, pain insensitivity, shoulder pain
Introduction
The shoulder junction is a very dynamic structure that has a very wide range arc of motion, can move in the sagittal, vertical and transverse planes. It provides the connection between the trunk and the upper extremity. Soft tissue pathologies are common issues at the shoulder junction due to its dynamic structure. One of the most common of these pathologies is adhesive capsulitis (AC) also known as frozen shoulder. It is a disease picture characterized by pain and limitation of movement in the shoulder joint, which is defined as inflammation in the capsule and synovium first, followed by adhesion formations, especially in the axillary fold and in the places where the capsule attaches to the anatomical neck of the humerus.1, 2 It is thought that the incidence of AC is around 2–5%, and it is more likely to be seen in women between the ages of 40–65, and those who have previously been diagnosed with AC in the contralateral shoulder joint. 3 Although the etiology of AC is not known exactly, it has been suggested that fibrosis and inflammation are triggered by the abnormal release of growth factors such as transforming growth factor-beta (TGF-β), platelet-derived growth factor (PDGF), and tumor necrosis factor-alpha (TNF-α) as a result of the inflammatory contracture present in the shoulder joint. 4 In individuals with adhesive capsulitis, high serum cytokine levels have been observed in the recent studies to cause or result in a long-term, sustained and intense inflammatory/fibrotic response affecting the synovial and capsuloligamentous structures. 3 Adhesive capsulitis is more likely to develop in patients with diabetes mellitus (DM), thyroid disorders, patients with Dupuytren's contracture, patients with autoimmune disease, patients treated for breast cancer, cerebrovascular events, and patients who have had myocardial infarction.5–9
Mirror therapy was developed by Ramachandran and Rogers in 1996 for the treatment of phantom pain in the amputated extremity. 10 In recent years, studies investigating the effectiveness of mirror therapy on chronic pain and musculoskeletal disorders have increased considerably.11–13 In the study published by Louw et al. in 2017, they found a significant improvement in pain intensity, pain catastrophe, fear of movement, and flexion range of motion after 1 session of mirror therapy in 69 people with shoulder pain and limited range of motion. 13 In a study published by Başkaya et al. in 2014, they showed that patients with AC who received mirror therapy had better results on pain, function, range of motion and quality of life than patients who did not receive mirror therapy. 14
Visual feedback can be defined as the sensory feedback given to the external environment in response to the visual feedback reaching the individual from the external environment. 15 Visual feedback in motor rehabilitation positively accelerates the process of rehabilitation by increasing motor learning and providing motivation. 16 It has been shown that individuals change their posture every time they look at visual feedback. 17 Performing exercises with visual feedback increases motivation, provides somatosensory stimulation and ensures correct movement. 18
Based on these data in the literature, it is aimed to examine the effectiveness of mirror therapy applied in addition to the conventional physiotherapy program on AC patients by comparing it with the control group and visual feedback group.
Method
Design
We established our study as a randomized controlled study. Before conducting the investigation, ethical clearance was obtained from the ethics committee of the Marmara University, Faculty of Medicine (09.2018.148, 2018-02/02), and it was performed in terms of the guidelines of the Declaration of Helsinki. All participants gave written consent before participation in any study-related activity.
Participants were randomly allocated to the mirror therapy, visual feedback or control group using a concealed method. Participants were allocated to three groups with numbers generated using a random number table sealed in opaque envelopes. Randomization was made after the recruiter had determined eligibility for the study and participants gave written consent for the participation. Randomization, evaluations and treatment were performed by the same physiotherapist.
Participants
Participants who were diagnosed with “Adhesive Capsulitis” by the specialist physician who applied to Kartal Dr Lutfi Kirdar Training and Research Hospital (Istanbul/Turkey) for our research were included in the study. For the diagnosis of adhesive capsulitis, progressive worsening of pain and stiffness, limitation of glenohumeral passive range of motion (especially external rotation), absence of glenohumeral arthritis in X-RAY, no history of dislocation, exclusion of subacromial impingement syndrome were used.2, 3 Thirty-six individuals divided into three groups as mirror therapy, visual feedback and control group, were included in the study for 15 sessions of treatment. Participants who were diagnosed with unilateral AC according to the above mentioned diagnostic criterias had normal radiographic images, and whose symptoms persisted for 2–12 months were included in the study. Those who were pregnant, had shoulder or upper extremity surgery, had nerve injury, had received any treatment (injection, physiotherapy, surgery vs) except nonsteroid anti inflamatuar drugs (NSAID) due to recent AC condition, had an unheeded diabetes mellitus and hypertension, had a history of cancer and symptoms persisted over a year or less than two months were excluded from the study.
Intervention
All participants in all groups were included in a 5-week treatment program with three sessions per week. Therapeutic ultrasound (1 MHz, %25 pulsed, 1,5 watt/cm²) applied to affected shoulder for 5 min, transcutaneous electrical nerve stimulation (TENS 80 Hz applied for 30 min, and cold pack applied for 20 min after exercise. Glenohumeral exercises such as Codman pendulum for 2–3 min, wand exercises (flexion, abduction, extension, internal and external rotation) 10 repetitions for each participant were administered under the supervision of a physiotherapist scapulothoracic exercises such as scapular adduction, abduction, elevation and depression 10 repetitions for each participant were administered under the supervision of a physiotherapist. Passive static stretching was applied by a physiotherapist for shoulder flexion, abduction, external and internal rotation along 20 s x 10 repetitions in every session. Those therapeutic agents and exercises were determined as conventional physiotherapy and performed on all participants in all groups. Also, participants were asked to do their exercise programs at home, three times a day. Detailed information about their diseases and treatment process was given to all participants before treatment sessions.
In the mirror therapy group, in addition to the conventional physiotherapy, the mirror was placed in the midline of the body and it was ensured that the unaffected extremity was seen on the reflected face of the mirror, and the affected extremity was left behind the mirror and not seen; in this position, first symmetrical movements for 1–2 min were made to understand the mirror effect. After that, active shoulder flexion, abduction, and rotation movements were performed bilaterally, for 15 repetitions each.
In the visual feedback group, in addition to the conventional physiotherapy, active shoulder flexion, abduction and rotation movements with 15 repetitions were performed in front of the mirror in order to see both extremities.
In the control group in addition to the conventional physiotherapy, active shoulder flexion, abduction and rotation movements were performed with 15 repetitions without feedback in order to make equal the exercise load with the other groups. All treatments were decided jointly by the specialist physician and physiotherapist due to literature. 3
Outcome measures
Evaluations were made before treatment (baseline), after treatment (week 6) and 10 weeks after baseline. Post-treatment evaluation was made one day after the 5-week treatment program ended, at the 6th week from baseline. Clinical and demographic information of each patient participating in the study was recorded. A visual analogue scale (VAS) was used for pain assessment. VAS is the patient's self-reported score used to measure pain intensity by giving a value between 0–10. Participants marked the most severe pain they had experienced in the last 24 h. 19 The functional status of the patients was evaluated using the “Shoulder Pain and Disability Index (SPADI)”20,21 and the Modified Constant Shoulder Score. 22 The SPADI score was calculated in the percentile for the total score obtained from the 50-point pain measurement and 80-point disability measurement.20, 21 The modified Constant score consists of 15 points for pain, 20 points for daily activities, 40 points for active joint range of motion measurement, and 25 points for strength parameters. 22 The shoulder proprioception assessment was performed with joint position sense test. Shoulder flexion range of motion (ROM) of the patients was used to determine the measurement target. Starting from 0 degrees, 50% of the participants maximum flexion angle was determined as the target angle. The error value of the difference between the target angle and the angle found by the participant was recorded by asking the participant to bring them back to the same position with their eyes closed. 23 Active ROM (flexion, abduction, external rotation, internal rotation) of the affected shoulder was evaluated with a universal goniometer by the physiotherapist While the participant was in the supine position, external and internal rotation ROM measured with the shoulder abducted to 45° or to 90° in the frontal plane and elbow flexed to 90°, flexion and abduction ROM measured with arm comfortably by the side.3, 24, 25 Proprioception and ROM measurements were repeated 3 times and the average was recorded as an outcome.
Statistical analysis
All Statistical analyses were performed using Statistical Package for Social Sciences (SPSS) version 23.0 (SPSS Inc., Chicago, IL, USA). A p-value of <0.05 was considered to indicate a statistically significant difference. Normality was analyzed by 3 of 5 combinations of “Shapiro-Wilk Test”, coefficient of variation, “Skewness-Kurtosis”, visual examination of Q-Q plot and histogram. In comparisons of between groups “ANOVA” variance analysis and “Bonferroni Test” for posthoc analysis was used for compliance with normal distribution. “Kruskal-Wallis Test” used for not suitable for normal distribution data and “Mann Whitney U Test” for posthoc analysis. In analyses within groups “Repeated Measures ANOVA” test and “Bonferroni Test” for posthoc analysis was used for compliance with normal distribution. Non suitable data for normal distribution were analysed with “Friedman Test” and posthoc analysis was performed with “Wilcoxon Test”.
Sample size and power calculations were made with the “GPower (ver3.1.9.2)” application. Calculations were made according to the mean of change in VAS values between groups before and after treatment in the study published by Baskaya et al. in 2018. 14 While calculating, the mean change of the experimental group was taken as 8.28 and the mean change of the control group as 6.2. The significance level was determined as 0.05 and the effect size was calculated as 0.762 (effect size d). According to these values, the total sample size was calculated as 21 people. Since we planned our study as 3 groups, a total of 43 participants were included in the study and 36 participants completed the study. When we perform a power analysis of our results and the number of patients we receive, the actual power is 0.9527 and the effect size is 0.54.
Results
Participant flow and demographics
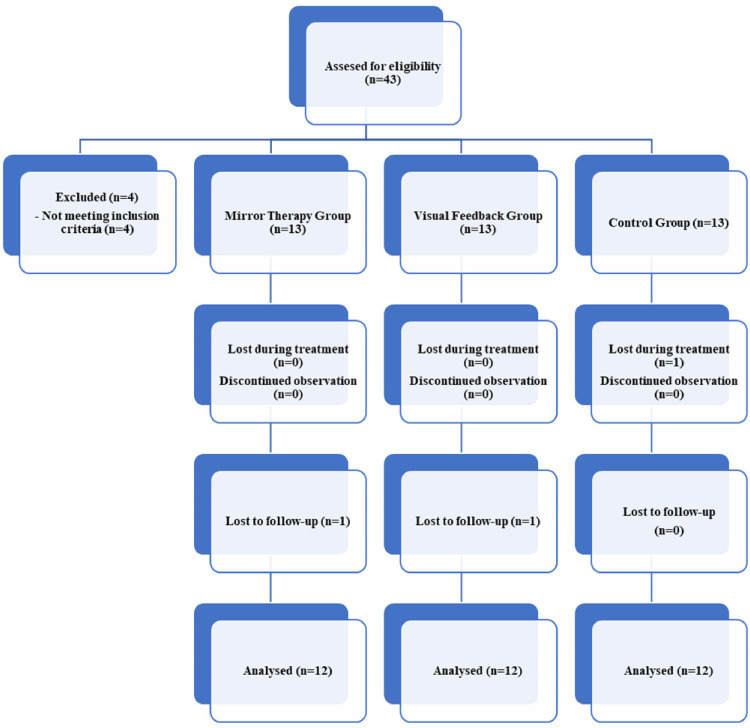
Forty-three participants were assessed for eligibility. Four participants were excluded because they did not meet inclusion criteria. Thirty-nine participants were included in the study divided into three groups. In the control group, one participant was diagnosed with “Parkinson's Disease” during treatment sessions, and therefore excluded from study. The other two lost participants didn’t show up for 10 weeks follow up evaluation. In the end, thirty-six participants (15 male, 21 female) completed the study (Figure 1). The participants had a mean age of 58.30 years (SD 1.37) and a mean body mass index of 27.9 kg/m2 (SD 0.77). There were no apparent differences at baseline between the groups for demographic factors (Table 1).
Figure 1.
Design and flow of participants through the trial.
Table 1.
Baseline characteristics of participants (MTG = mirror therapy group, VFG = visual feedback group, CG = control group, BMI = body mass index, DM = diabetes mellitus.).
| Characteristics | Randomised (n = 36) | ||
|---|---|---|---|
| MTG (n = 12) | VFG (n = 12) | CG (n = 12) | |
| Age (yr), mean (SD) | 58.91 (2.34) | 57.25 (3.01) | 58.75 (1.81) |
| Gender, n males (%) | 5 (41.7%) | 4 (33.3%) | 6 (50%) |
| Height (cm), mean (SD) | 164.67 (0.02) | 162.17 (0.01) | 168.58 (0.02) |
| Weight (kg), mean (SD) | 72.16 (4.09) | 77.25 (3.37) | 79.75 (5.27) |
| BMI (SD) | 26.53 (1.32) | 29.29 (1.10) | 27.87 (1.54) |
| DM, positive (%) | 7 (58.3%) | 9 (75.0%) | 6 (50%) |
Outcomes
Figures 2-5 show changes within groups according to evaluation sessions. Table 2 shows changes in all outcomes at Week 6 and Week 10 compared to Baseline among the three groups.
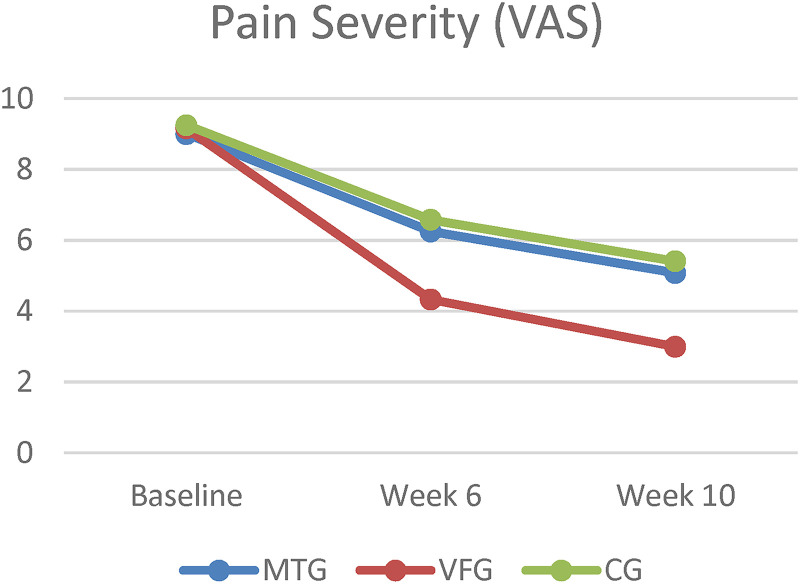
Figure 2.
Mean change in pain severity at 6 and 10 weeks from baseline, according to All groups. MTG = mirror therapy group, VFG = visual feedback group, CG = control group, VAS = visual analog scale.
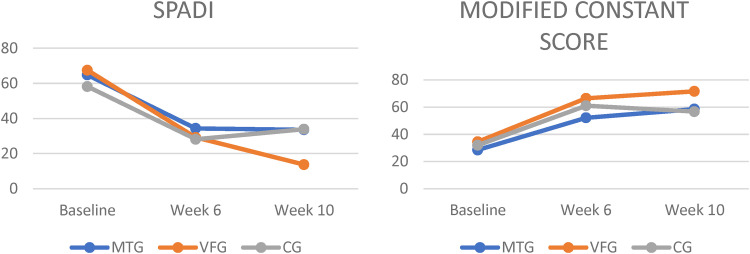
Figure 3.
Mean change in functional Status at 6 and 10 weeks from baseline, according to All groups. MTG = mirror therapy group, VFG = visual feedback group, CG = control group, VAS = visual analog scale, SPADI = shoulder pain and disability index.
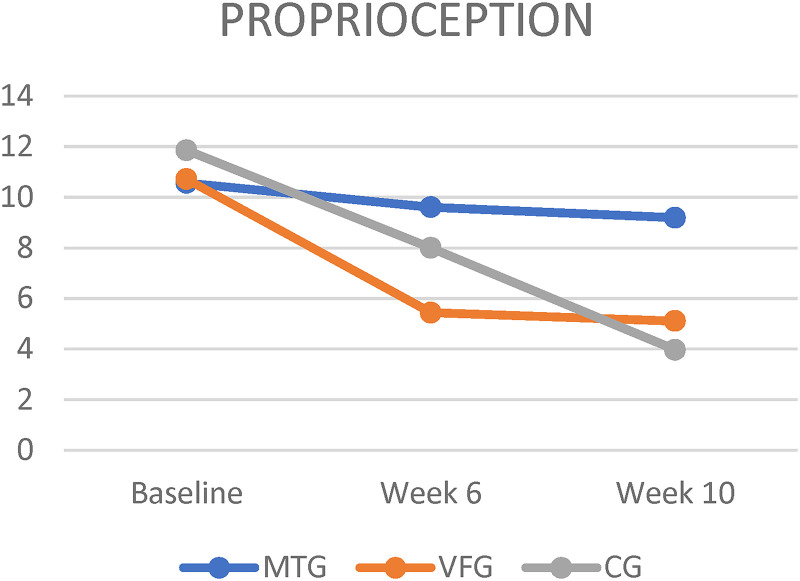
Figure 4.
Mean change in proprioception at 6 and 10 weeks from baseline, according to All groups. MTG = mirror therapy group, VFG = visual feedback group, CG = control group.
Figure 5.
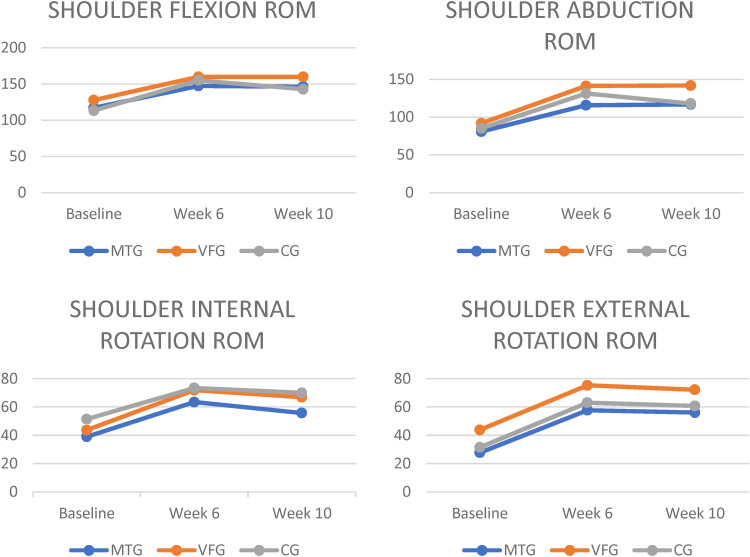
Mean change in shoulder range of motions at 6 and 10 weeks from baseline, according to All groups. MTG = mirror therapy group, VFG = visual feedback group, CG = control group, ROM = range of motion.
Table 2.
Group comparisons for mean changes from baseline. MTG = mirror therapy group, VFG = visual feedback group, CG = control group, VAS = visual analog scale, SPADI = shoulder pain and disability index, CI = confidence interval.
| Variables | Mean Change from Baseline (95% CI) | Between Group Difference (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MTG | VFG | CG | MTG vs CG | P Value | VFG vs CG | P Value | MTG vs VFG | P Value | ||
| N = 12 | N = 12 | N = 12 | ||||||||
| VAS | Week 6 | −2.75 (−3.96 to −1.53) | −4.83 (−6.38 to −3.28) | −2.66 (−4.13 to −1.19) | −0.08 (−2.38 to 2.21) | 0.616* | −2.16 (−4.46 to 0.13) | 0.029* | 2.08 (−0.21 to 4.38) | 0.028* |
| Week 10 | −3.91 (−5.72 to −2.11) | −6.16 (−7.51 to −4.81) | −3.83 (−5.56 to −2.10) | −0.08 (−2.74 to 2.57) | 0.838* | −2.33 (−4.99 to 0.32) | 0.029* | 2.25 (−0.41 to 4.91) | 0.041* | |
| SPADI | Week 6 | −30.42 (−44.67 to −16.17) | −38.33 (−51.99 to −24.67) | −30.06 (−45.31 to 14.81) | −0.36 (−23.70 to 22.97) | 1.000 | −8.26 (−31.60 to 15.06) | 1.000 | 7.90 (−15.43 to 31.24) | 1.000 |
| Week 10 | −31.19 (−50.64 to −11.74) | −53.78 (−61.91 to −45.65) | −24.29 −38.81 to −9.78) | −6.89 (−30.84 to 17.05) | 1.000 | −29.48 (−53.43 to −5.53) | 0.012 | 22.58 (−1.35 to 46.53) | 0.070 | |
| Constant | Week 6 | 23.66 (18.00 to 29.33) | 31.91 (25.03 to 38.79) | 29.08 (20.72 to 37.44) | −5.41 (−16.85 to 6.01) | 0.722 | 2.83 (−8.60 to 14.26) | 1.000 | −8.25 (−19.68 to 3.18) | 0.234 |
| Week 10 | 30.08 (21.59 to 38.57) | 37.08 (30.47 to 43.69) | 24.75 (15.12 to 34.37) | 5.33 (−8.17 to 18.84) | 0.980 | 12.33 (−1.17 to 25.84) | 0.083 | −7.00 (−20.50 to 6.50) | 0.601 | |
| Proprioception | Week 6 | −0.95 (−6.55 to 4.63) | −5.27 (−9.60 to −0.94) | −3.86 (−10.23 to 2.51) | 2.90 (−6.00 to 11.81) | 1.000 | −1.41 (−10.32 to 7.49) | 1.000 | 4.31 (−4.59 to 13.22) | 0.691 |
| Week 10 | −1.37 (−7.43 to 4.68) | −5.60 (−11.68 to 0.47) | −7.88 (−13.99 to −1.78) | 6.51 (−3.34 to 16.36) | 0.315 | 2.27 (−7.57 to 12.13) | 1.000 | 4.23 (−5.62 to 14.08) | 0.859 | |
| Flexion | Week 6 | 30.13 (21.02 to 39.25) | 32.16 (22.89 to 41.43) | 41.69 (20.92 to 62.46) | −11.55 (−34.48 to 11.37) | 0.638 | −9.52 (−32.45 to 13.39) | 0.906 | −2.02 (−24.95 to 20.89) | 1.000 |
| Week 10 | 29.11 (18.55 to 39.67) | 32.16 (23.94 to 40.38) | 29.55 (18.70 to 40.40) | −0.44 (−16.56 to 15.67) | 1.000 | 2.61 (−13.50 to 18.72) | 1.000 | −3.05 (−19.17 to 13.06) | 1.000 | |
| Abduction | Week 6 | 34.94 (21.01 to 48.87) | 49.50 (34.72 to 64.27) | 46.14 (28.90 to 63.38) | −11.19 (−36.12 to 13.72) | 0.796 | 3.35 (−21.56 to 28.28) | 1.000 | −14.55 (−39.48 to 10.37) | 0.451 |
| Week 10 | 35.94 (21.75 to 50.13) | 50.05 (36.26 to 63.84) | 32.88 (15.81 to 49.96) | 3.05 (−21.39 to 27.51) | 1.000 | 17.16 (−7.28 to 41.62) | 0.258 | −14.11 (−38.56 to 10.34) | 0.465 | |
| Internal rotation | Week 6 | 24.47 (9.62 to 39.32) | 28.19 (18.79 to 37.58) | 22.02 (5.64 to 38.40) | 2.44 (−20.03 to 24.92) | 1.000 | 6.16 (−16.31 to 28.64) | 1.000 | −3.72 (−26.19 to 18.75) | 1.000 |
| Week 10 | 16.74 (−1.37 to 34.86) | 23.22 (12.54 to 33.89) | 18.55 (2.76 to 34.34) | −1.80 (−26.41 to 22.79) | 1.000 | 4.66 (−19.93 to 29.27) | 1.000 | −6.47 (−31.07 to 18.13) | 1.000 | |
| External Rotation | Week 6 | 30.00 (17.01 to 42.98) | 31.47 (20.77 to 42.16) | 31.55 (16.00 to 47.11) | −1.55 (−22.99 to 19.88) | 1.000 | −0.08 (−21.52 to 21.35) | 1.000 | −1.47 (−22.90 to 19.96) | 1.000 |
| Week 10 | 28.30 (14.67 to 41.93) | 28.36 (18.89 to 37.83) | 29.27 (11.08 to 47.47) | −0.97 (−24.01 to 22.07) | 1.000 | −0.91 (−23.96 to 22.12) | 1.000 | −0.05 (−23.09 to 22.98) | 1.000 | |
At Week 6, the VFG had a statistically significant greater decrease in VAS score (−4.83 points, 95% confidence interval [CI], −6.38 to −3.28) than did the MTG (−2.75 points, 95% CI, −3.96 to −1.53) and CG (−2.66 points, 95% CI, −4.13 to −1.19), the mean between MTG and VFG difference was 2.08 points (95% CI, −0.21 to 4.38, p = 0.028) and between VFG and CG difference was −2.16 points (95% CI, −4.46 to 0.13, p = 0.029). There was no difference between MTG and CG (mean difference [MD] = −0.08, 95% CI, −2.38 to 2.21, p = 0.616).
At week 10, the VFG had a statistically significant decrease in VAS score (−6.16 points, 95% CI, −7.51 to −4.81) than did the MTG (−3.91 points, 95% CI, −5.72 to −2.11) and CG (−3.83 points, 95% CI, −5.72 to −2.11), the mean between MTG and VFG difference was 2.25 points (95% CI, −0.41 to 4.91, p = 0.041) and between VFG and CG difference was −2.33 points (95% CI, −4.99 to 0.32, p = 0.029). There was no difference between MTG and CG (MD = −0.08, 95% CI, −2.74 to 2.57, p = 0.838). At week 10, the VFG had a significantly decrease in SPADI score (−53.78 points, 95% CI, −61.91 to −45.65) than did the CG (−24.29, 95% CI, −38.81 to −9.78), the mean between VFG and CG difference was −29.48 points (95% CI, −53.43 to −5.53, p = 0.012). There was no significant difference between MTG and VFG (MD = −6.89, 95% CI, −30.84 to 17.05, p > 0.05) or MTG and CG (MD = 22.58, 95% CI, −1.35 to 46.53, p = 0.070).
All groups had significant differences within their group at week 6 and 10 for all outcomes except proprioception (Figures 2,3,4 and 5). There were no significant differences between groups for outcomes such as proprioception, ROM (flexion, abduction, external and internal rotation), and Modified Constant Score (Table 2).
No major adverse events were reported. Although three participants in MTG complained of dizziness during mirror therapy, dizziness disappeared when they stopped exercising. One participant in CG was excluded because of diagnosis ‘Parkinson's Disease’. It was clear that ‘Parkinson's Disease’ could not be linked to treatment.
Discussion
We planned this study to compare the effectiveness of conventional physiotherapy, mirror therapy and visual feedback in patients diagnosed with AC. The visual feedback approach added to the conventional physiotherapy program was superior to the mirror therapy group and the control group in reducing the severity of pain (VAS). In addition, the visual feedback approach outperformed the control group in reducing the SPADI score at week 10.
Mirror therapy can be considered as a new practice and studies investigating its effectiveness in different rehabilitation areas are limited. There are studies showing its effectiveness in terms of pain and function, especially in amputee phantom pain, upper extremity functions in those who have had a stroke, algodystrophy and those with complex regional pain syndrome.15, 26–28 There are few studies showing the effectiveness of mirror therapy in orthopedic cases.13, 14, 29–31
In the study published by Başkaya et al., 30 patients who were diagnosed with AC (n = 15 mirror therapy, n = 15 control) were treated for 10 sessions and results showed that the mirror therapy group was superior to the control group in the development of active and passive flexion and abduction range of motion, shoulder functionality, and reduction of pain. 14 In the study published by Louw et al., they reported positive results in terms of sudden pain catastrophization, VAS, kinesiophobia and active shoulder flexion as a result of applying mirror therapy in one session in 69 patients with shoulder problems. 13 In our study, pain decreased significantly, flexion, abduction, external rotation and internal rotation active joint range of motion increased, and functionality improved in all groups after treatment compared to baseline. However, no superiority of mirror therapy was found in any outcome measure over the control or visual feedback group, and even visual feedback was found to be more effective in reducing pain.
In the prospective study published by Diercks and Stevens in 2004, 45 of 77 AC patients were followed up for 2 years and exercised within the pain limit, and no stretching or any treatment that pushed the pain limit was applied, while the other 39 patients were followed for 2 years with home programs including painful stretching and stretching exercises. As a result, they showed that the group followed with painless supervised exercises had better functional results than the group that did home-based painful exercises. 32 In the retrospective study published by Vastamaki et al., they showed that among 83 patients they followed between 2 and 27 years, those who did not receive any treatment recovered more painlessly and the duration of the disease was shorter than those who received nonoperative treatment and those who underwent manipulation under anesthesia. 33 When we compare the change between the baseline and 6-week measurements with the change between the 6-week and 10-week measurements, for all outcome measures, the change in the treatment-received period was greater in all groups than the untreated period. According to our results, performing the exercises with a supervisor may have a positive effect on the treatment process, also, it can be said that individuals who do not receive treatment will not show enough improvement compared to those who receive treatment.
Some studies in healthy individuals have suggested that appropriate feedback will affect motor recovery, and some studies have shown that it temporarily or permanently improves motor performance.18, 34–38 Visual feedback by seeing both the affected and unaffected extremities may be more effective in patients with adhesive capsulitis than feedback by hiding the affected extremity. In diseases such as AC with mechanical limitation of movement and high muscle activity, it may be more important for the patient to perform the movement correctly and activate the right muscles. Seeing the affected arm while exercising can prevent combined movements and reduce force dissipation. For example, shoulder flexion is performed with less margin of error when the patient can observe difficulties such as scapula elevation more than normal, biceps muscle overtaking, adjustment of rotation amount during shoulder flexion through visual feedback in real time. 18 In our study, this may be the mechanism that was effective in reducing the pain of the visual feedback mechanism provided by seeing the affected arm in front of the mirror.
Conventional non-invasive physiotherapy is the primary recommended treatment approach for AC (3). In drug use, NSAIDs alone are not enough, they should be used together with physiotherapy. NSAIDs are mostly used to reduce symptoms and increase compliance with physiotherapy. 39 In the review published by Eljabu et al. in 2016, they reported that standardized traditional treatment was more effective than surgery in many cases. 40 Celik, in her study published in 2016, showed an increase in ROM parameters in AC patients who applied 18 sessions of stretching exercises in 6 weeks. 41 Scapular retraction, protraction, and elevation multiplanarly adjust the optimal amount of tension of the rotator cuff muscles. 42 In a randomized controlled study in which 29 AC patients compared the effectiveness of two different exercise programs, they showed that the group to which performed scapulothoracic exercises were added had a significant difference in VAS values at the 6th week compared to the control group, and an increase in the joint range of motion values at the 12th week. 43 In the review recently published by Nakandala et al., the US is recommended for the treatment program. 44 According to the Cochrane Review published in 2014, the effects of TENS and US therapy were found to be uncertain. 45 In the guideline published by Kelley et al. in 2013, they stated that it would be beneficial to use electrotherapy applications to increase the patient's compliance with the treatment.3 In our study, as a result of conventional physiotherapy applications, significant improvements were achieved in outcome measures in all three groups compared to before treatment. In our opinion, therapeutic agents that patients cannot apply at home may both provide the persistence of participation in the sessions and may play a preparatory role before the exercise. The continuity of the patient participation in the treatment sessions may enable them to make their home programs regularly and to do the exercises more accurately by staying under observation for a longer time.
Milgrom et al. showed that approximately 30% of AC cases were diagnosed with DM in their study in 2008. 46 In our study, 7 participants in the MTG, 9 participants in the VFG, and 7 participants in the CG had DM. In other words, the prevalence of DM in our study was 63.8%. This indicates a higher rate compared to the literature. The high prevalence of DM may have affected the results of the study, since individuals with a diagnosis of DM may have a slower prognosis. But since there is no difference between the groups in terms of DM distribution, it will not affect the comparisons.
It should be noted that there are a few limitations in the current study. Our follow-up period may have been too short to see a difference between MTG, VFG and CG. Additionally, the fact that the physiotherapist evaluating and administering the treatment is not blind increases the possibility of bias. In future studies, it should be noted that people who evaluate and administer the treatment should be blinded and a longer follow-up period should be determined.
Conclusion
In conclusion, adding visual feedback approach to adhesive capsulitis treatment may be beneficial in terms of pain and functionality. Visual feedback provided by seeing the affected extremity in front of a mirror in AC rehabilitation can provide more improvement in outcome measures compared to mirror therapy.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ömer Hekim https://orcid.org/0000-0002-7977-6797
References
- 1.Neviaser AS, Neviaser RJ. Adhesive capsulitis of the shoulder. J Am Acad Orthop Surg 2011; 19: 536–542. [DOI] [PubMed] [Google Scholar]
- 2.Hanchard NC, Goodchild L, Thompson J, et al. Evidence-based clinical guidelines for the diagnosis, assessment and physiotherapy management of contracted (frozen) shoulder: quick reference summary. Physiotherapy 2012; 98: 117–120. [DOI] [PubMed] [Google Scholar]
- 3.Kelley MJ, Shaffer MA, Kuhn JE, et al. Shoulder pain and mobility deficits: adhesive capsulitis. J Orthop Sports Phys Ther 2013; 43: A1–A31. [DOI] [PubMed] [Google Scholar]
- 4.Tamai K, Akutsu M, Yano Y. Primary frozen shoulder: brief review of pathology and imaging abnormalities. J Orthop Sci 2014; 19: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neviaser AS, Hannafin JA. Adhesive capsulitis: a review of current treatment. Am J Sports Med 2010; 38: 2346–2356. [DOI] [PubMed] [Google Scholar]
- 6.Whelton C, Peach CA. Review of diabetic frozen shoulder. Eur J Orthop Surg Traumatol 2018; 28: 363–371. [DOI] [PubMed] [Google Scholar]
- 7.Cohen C, Tortato S, Silva OBS, et al. Association between frozen shoulder and thyroid diseases: strengthening the evidences. Rev Bras Ortop (Sao Paulo) 2020; 55: 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryu JD, Kirpalani PA, Kim JM, et al. Expression of vascular endothelial growth factor and angiogenesis in the diabetic frozen shoulder. J Shoulder Elbow Surg 2006; 15: 679–685. [DOI] [PubMed] [Google Scholar]
- 9.Cohen C., Ejnisman B. Epidemiology of frozen shoulder. In:Itoi E., et al. (eds) Shoulder stiffness. Berlin, Heidelberg: Springer, 2015, pp. 21–28. [Google Scholar]
- 10.Ramachandran VS, Rogers-Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proc Biol Sci 1996; 263: 377–386. [DOI] [PubMed] [Google Scholar]
- 11.Moseley GL, Flor H. Targeting cortical representations in the treatment of chronic pain: a review. Neurorehabil Neural Repair 2012; 26: 646–652. [DOI] [PubMed] [Google Scholar]
- 12.Wittkopf PG, Johnson MI. Mirror therapy: a potential intervention for pain management. Rev Assoc Med Bras (1992) 2017; 63: 1000–1005. [DOI] [PubMed] [Google Scholar]
- 13.Louw A, Puentedura EJ, Reese D, et al. Immediate effects of mirror therapy in patients with shoulder pain and decreased range of motion. Arch Phys Med Rehabil 2017; 98: 1941–1947. [DOI] [PubMed] [Google Scholar]
- 14.Başkaya MÇ, Erçalık C, Karataş Kır Ö, et al. The efficacy of mirror therapy in patients with adhesive capsulitis: a randomized, prospective, controlled study. J Back Musculoskelet Rehabil 2018; 31: 1177–1182. [DOI] [PubMed] [Google Scholar]
- 15.Sato K, Fukumori S, Matsusaki T, et al. Nonimmersive virtual reality mirror visual feedback therapy and its application for the treatment of complex regional pain syndrome: an open-label pilot study. Pain Med 2010; 11: 622–629. [DOI] [PubMed] [Google Scholar]
- 16.Ma M, et al. Adaptive virtual reality games for rehabilitation of motor disorders. In:Stephanidis C.(eds)Universal access in human-computer interaction. Ambient interaction. UAHCI 2007. Lecture notes in computer science, vol 4555. Berlin, Heidelberg: Springer, 2007, pp. 681–690. [Google Scholar]
- 17.Kaya L, Öksüz Ç. Investigation of the effects of visual feedback on posture. Ergoterapi ve Rehabilitasyon Dergisi 2017; 5: 1–10. [Google Scholar]
- 18.Barandas M, Gamboab H, Fonsecaa JM. A real time biofeedback system using visual user interface for physical rehabilitation. Procedia Manuf 2015; 3: 823–828. [Google Scholar]
- 19.Boonstra AM, Schiphorst Preuper HR, Reneman MF, et al. Reliability and validity of the visual analogue scale for disability in patients with chronic musculoskeletal pain. Int J Rehabil Res 2008; 31: 165–169. [DOI] [PubMed] [Google Scholar]
- 20.Breckenridge JD, McAuley JH. Shoulder pain and disability Index (SPADI). J Physiother 2011; 57: 197. [DOI] [PubMed] [Google Scholar]
- 21.Bumin G, Tuzun EH, Tonga E. The shoulder pain and disability index (SPADI): cross-cultural adaptation, reliability and validity of the turkish version. J Back Musculoskeletal Rehab 2008; 21: 57–62. [Google Scholar]
- 22.Çelik D. Turkish version of the modified constant-murley score and standardized test protocol: reliability and validity. Acta Orthop Traumatol Turc 2016; 50: 69–75. [DOI] [PubMed] [Google Scholar]
- 23.Salles JI, Velasques B, Cossich V, et al. Strength training and shoulder proprioception. J Athl Train 2015; 50: 277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soucie JM, Wang C, Forsyth A, et al. (Hemophilia treatment center network) Range of motion measurements: reference values and a database for comparison studies. Haemophilia 2011; 17: 500–507. [DOI] [PubMed] [Google Scholar]
- 25.Carley P, Burkhart KL, Sheridan C. Virtual reality vs goniometry: intraclass correlation coefficient to determine inter-rater reliability for measuring shoulder range of motion. J Allied Health 2021; 50: 161–165. [PubMed] [Google Scholar]
- 26.Breivik H, Allen SM, Stubhaug A. Mirror-therapy: an important tool in the management of Complex regional pain syndrome (CRPS). Scand J Pain 2013; 4: 190–197. [DOI] [PubMed] [Google Scholar]
- 27.Deconinck FJ, Smorenburg AR, Benham A, et al. Reflections on mirror therapy: a systematic review of the effect of mirror visual feedback on the brain. Neurorehabil Neural Repair 2015; 29: 349–361. [DOI] [PubMed] [Google Scholar]
- 28.Yun GJ, Chun MH, Park JY, et al. The synergic effects of mirror therapy and neuromuscular electrical stimulation for hand function in stroke patients. Ann Rehabil Med 2011; 35: 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayon-Calatayud M, Benavente-Valdepeñas AM. Del prado vazquez-muñoz M. Mirror therapy for distal radial fractures: a pilot randomized controlled study. J Rehabil Med 2016; 48: 829–832. [DOI] [PubMed] [Google Scholar]
- 30.Abolfazli M, Lajevardi L, Mirzaei L, et al. The effect of early intervention of mirror visual feedback on pain, disability and motor function following hand reconstructive surgery: a randomized clinical trial. Clin Rehabil 2019; 33: 494–503. [DOI] [PubMed] [Google Scholar]
- 31.Rostami HR, Arefi A, Tabatabaei S. Effect of mirror therapy on hand function in patients with hand orthopaedic injuries: a randomized controlled trial. Disabil Rehabil 2013; 35: 1647–1651. [DOI] [PubMed] [Google Scholar]
- 32.Diercks RL, Stevens M. Gentle thawing of the frozen shoulder: a prospective study of supervised neglect versus intensive physical therapy in seventy-seven patients with frozen shoulder syndrome followed up for two years. J Shoulder Elbow Surg 2004; 13: 499–502. [DOI] [PubMed] [Google Scholar]
- 33.Vastamäki H, Kettunen J, Vastamäki M. The natural history of idiopathic frozen shoulder: a 2- to 27-year follow-up study. Clin Orthop Relat Res 2012; 470: 1133–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zipp GP, Gentile AM. Practice schedule and the learning of motor skills in children and adults: teaching implications. J Coll Teach Learn 2010; 7. [Google Scholar]
- 35.Khan MA, Franks IM. The effect of practice on component submovements is dependent on the availability of visual feedback. J Mot Behav 2000; 32: 227–240. [DOI] [PubMed] [Google Scholar]
- 36.Newell KM, Carlton MJ. Augmented information and the acquisition of isometric tasks. J Mot Behav 1987; 19: 4–12. [DOI] [PubMed] [Google Scholar]
- 37.Young DE, Schmidt RA. Augmented kinematic feedback for motor learning. J Mot Behav 1992; 24: 261–273. [DOI] [PubMed] [Google Scholar]
- 38.Woldag H, Hummelsheim H. Evidence-based physiotherapeutic concepts for improving arm and hand function in stroke patients: a review. J Neurol 2002; 249: 518–528. [DOI] [PubMed] [Google Scholar]
- 39.Fields BKK, Skalski MR, Patel DB, et al. Adhesive capsulitis: review of imaging findings, pathophysiology, clinical presentation, and treatment options. Skeletal Radiol 2019; 48: 1171–1184. [DOI] [PubMed] [Google Scholar]
- 40.Eljabu W, Klinger HM, Von Knoch M. Prognostic factors and therapeutic options for treatment of frozen shoulder: a systematic review. Arch Orthop Trauma Surg 2016; 136: 1–7. [DOI] [PubMed] [Google Scholar]
- 41.Celik D, Kaya Mutlu E. Does adding mobilization to stretching improve outcomes for people with frozen shoulder? A randomized controlled clinical trial. Clin Rehabil 2016; 30: 786–794. [DOI] [PubMed] [Google Scholar]
- 42.Akbas E, Güneri S, Erdem EU, et al. The effects of additional proprioceptive neuromuscular facilitation over conventional therapy in patients with adhesive capsulitis. Turk J Physiother Rehabil 2015; 26: 78–85. [Google Scholar]
- 43.Celik D. Comparison of the outcomes of two different exercise programs on frozen shoulder. Acta Orthop Traumatol Turc 2010; 44: 285–292. [DOI] [PubMed] [Google Scholar]
- 44.Nakandala P, Nanayakkara I, Wadugodapitiya S, et al. The efficacy of physiotherapy interventions in the treatment of adhesive capsulitis: a systematic review. J Back Musculoskelet Rehabil 2021; 34: 195–205. [DOI] [PubMed] [Google Scholar]
- 45.Page MJ, Green S, Kramer S, et al. Electrotherapy modalities for adhesive capsulitis (frozen shoulder). Cohrane Database Syst Rev 2014; 10: 1465–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Milgrom C, Novack V, Weil Y, et al. Risk factors for idiopathic frozen shoulder. Isr Med Assoc J 2008; 10: 361–364. [PubMed] [Google Scholar]