ABSTRACT
The pervasiveness of irreproducible research remains a thorny problem for the progress of scientific endeavor, spawning an abundance of opinion, investigation, and proposals for improvement. Irreproducible research has negative consequences beyond the obvious impact on achieving new scientific discoveries that can advance healthcare and enable new technologies. The conduct of science is resource intensive, resulting in a large environmental impact from even the smallest research programs. There is value in making explicit connections between the conduct of more rigorous, reproducible science and commitments to environmental sustainability. Shared research resources (also commonly known as cores) often have an institutional role in supporting researchers in the responsible conduct of research through training, informal mentorship, and services and are particularly well suited to promulgating essential principles of scientific rigor, reproducibility, and transparency. Shared research resources can also play a role in advancing sustainability by virtue of their inherently efficient science model in which singular shared equipment, technology, and expertise resources can serve many different research programs. Programs that elevate shared research resources, scientific rigor, reproducibility, transparency, and environment sustainability in harmony may achieve a unique synergy. Several case studies and quality paradigms are discussed that offer tools and concepts that can be adapted whole or in part by individual shared research resources or research-intensive institutions as part of an overall program of sustainability.
INTRODUCTION
The pervasiveness of irreproducible research remains a thorny problem for the progress of scientific endeavor, spawning an abundance of opinion, investigation, and proposals for improvement. A PubMed search on the keyword “reproducibility” yields over 500 000 titles across the depth and breadth of scientific disciplines, with the vast majority published in the last 20 years. This seems to indicate a general concurrence among scientists that research irreproducibility is a problem. However, there also appears to be a certain inertia when it comes to changing individual researcher practices even in assessing the work of others. The journal Nature published a survey in 2016 of more than 1500 scientists, revealing that over 70% of them reported failure to reproduce other scientists’ experiments.1 More than 50% of those surveyed agreed there is a crisis of reproducibility, yet, paradoxically, fewer than 31% thought their own failure to reproduce an experiment meant that the published result could be wrong. The National Institutes of Health (NIH), the major source of funding for biomedical research in the United States, has sought to incentivize the application of principles of rigor, reproducibility, and transparency (RR&T) by making them essential criteria for grant awards. In a 2015 announcement,2 the NIH emphasized 4 broad areas that should be addressed in demonstrating rigor and transparency in grant applications: 1) the scientific premise of the proposed research, 2) rigorous experimental design for robust and unbiased results, 3) the consideration of relevant biological variables, and 4) the authentication of key biological and/or chemical resources. While the NIH initiative has raised some awareness of the need to address the problem of research irreproducibility, it is unclear the extent to which it has promoted improved practices and outcomes.
CONNECTION BETWEEN REPRODUCIBILITY AND ENVIRONMENTAL SUSTAINABILITY
Beyond the implications as an administrative criterion for receiving NIH or other sponsored grant funding, irreproducible research in and of itself has immediate negative consequences: research funding from sponsors (and often taxpayers) is spent on experiments that cannot succeed. This devalues the positive societal impact that might otherwise be achieved with these funds; the negative effect is extended when research programs and scientists experience delays associated with “dead ends” that often result from trying to build on irreproducible work. Society at large thus suffers by waiting longer for new scientific discoveries that can, for example, advance healthcare and enable new technologies. The potential for wasted funds, effort, and resources is further amplified by the significant environmental footprint of scientific research. Laboratories are energy intensive, typically using 3 to 10 times more energy than commercial spaces.3 A survey of the University of Virginia operations revealed that laboratories, though making up only 13% of the university’s physical footprint, are responsible for 33% of the university’s building energy use.4 Laboratory research also frequently generates large waste streams of single-use plastics. As a result, science based on irreproducible research exacerbates the negative impact of large resource use on the environment. To give a sense of the scale of these negative consequences, a 2015 study estimated that “scientists in the United States spend $28 billion each year on basic biomedical research that cannot be repeated successfully.”5 It can therefore be argued that a commitment to the conduct of more rigorous and reproducible research can not only have a wide ranging and significant positive impact on society in general but can also advance efforts to improve environmental sustainability: a well-designed experiment conducted under rigorous conditions will yield quality results with fewer repetitions, enabling more sustainable use of resources including research supplies, equipment, and utilities (energy and water) for equipment and experimental processes. New studies built on such rigorously conducted science are better set up for experimental success, amplifying quality and accelerating discovery in a virtuous cycle. Finally, the adoption of a shared resource model where possible can further amplify this positive impact by consolidating use of space, equipment, and resources in professionally staffed laboratory settings already shown to enable efficiency and more reproducible science.
SRRs AS A MECHANISM FOR ADVANCING EFFICIENCY/SUSTAINABILITY AND RR&T
SRRs can play a role in advancing sustainability by virtue of their inherently efficient science model in which singular shared equipment, technology, and expertise resources can serve many different research programs. This shared model avoids proliferation of duplicative equipment, enabling resource and cost efficiencies for laboratory space, general and scientific infrastructure, materials and supplies, energy usage, and, in some cases, the avoidance of waste generation. As noted above, because of expertise provided by SRR facility directors and managers, SRRs are key partners for the training and education of the next generation of scientists, as they most commonly interact directly with graduate students, postdoctoral trainees, and early career investigators. This not only makes SRRs effective agents for transmitting and applying principles of RR&T, but it also provides an opportunity for SRRs to extend their influence and capabilities toward a more efficient and sustainable conduct of research for the long term.
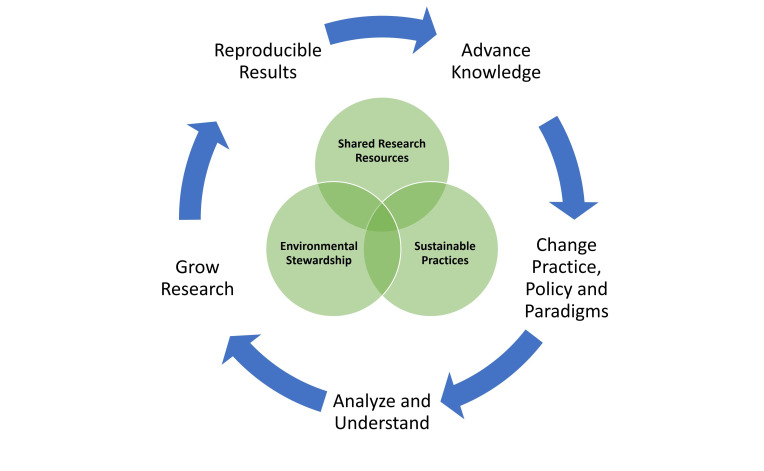
The challenge for SRRs to leverage this opportunity is considerable, however, as highlighted in an ABRF survey of SRR directors, managers, and staff.7 While many respondents were aware of the impact of irreproducible research and were actively incorporating best practices for RR&T into their SRR operations, the large majority of them reported a lack of institutional engagement in these efforts. A recent report from the Federation of American Societies for Experimental Biology (FASEB) suggests that the more intuitive institutional focus on sustainability as a financial concern could be extended to enable a more holistic view that encompasses social and environmental factors as well.8 Therefore, one possible strategy is to explicitly integrate newly emerging (and higher profile) institutional programs for broad environmental sustainability with certain essential characteristics of SRRs, which, as an existing pipeline for good science, can promote and align shared principles of scientific RR&T with those of environmental sustainability (Figure 1). These essential characteristics of SRRs include the following:
Figure 1. Integrating SRRs, environmental stewardship, and sustainable practices to advance scientific knowledge and societal benefit.
Efficient use of space, utilities, and equipment. SRRs are a mechanism for the consolidation of resources, such as equipment of various kinds, from high-end equipment to more basic equipment, that is useful to scientists in many different disciplines. Within a single laboratory space, an SRR facility can provide access to an instrument or instruments (eg, a mass spectrometer, electron microscope, UV-Vis spectrometer, floor centrifuges, etc.) that can be used by dozens or hundreds of researchers. Investigators save money, as an SRR facility is able to purchase reagents and supplies in large volumes that a single research laboratory could never achieve, and can share the cost of expert technical staff and instrument maintenance across many users. In addition, an institution benefits by serving intensive infrastructure needs that often accompany such instrument operation (cooling, electricity) to a single laboratory space instead of many.
Rigorous conduct of experiments. SRRs specialize in providing expert training, advice, and highly reproducible scientific services, resulting in reliable data that can be confidently interpreted—and therefore less likely to be challenged and more likely to be reproducible by others. Increased reproducibility and access to expertise can enable greater experimental success earlier in a project, thus saving researchers time and reducing the amount of material needed to run experiments, such as single-use plastics. In addition, because of the volume of SRR user projects, high-density experimental formats are possible, which can reduce the amount of instrument runs. SRRs thus inherently conserve the use of resources and energy while researchers benefit from receiving high-quality results.
Expert data management and analytical practices. SRRs are often the source of large datasets used in academic research and, as such, play an integral role in producing reproducible results. In addition to producing reliable primary research data, SRRs often provide expert data analysis and visualization services as well as infrastructure for managing research data that ensure integrity and security.
LEVERAGING QUALITY STANDARDS TO ADVANCE RR&T AND ENVIRONMENTAL SUSTAINABILITY
The push toward scientific RR&T in academic SRRs can benefit from examining well-established industrial quality, process improvement, and financial models, including the development of Total Quality Management (TQM) and nternational Organization for Standardization (ISO) 900014 that led to a global supply chain and subsequent advances in the complexity of product across fields as varied as automobile, medical device, and electronics manufacturing. When evaluating the role of centralized facilities from an environmental sustainability standpoint, a comparison can be further made to industries that choose to pursue certification in the ISO 14000 family.14 ISO 14000 mirrors ISO 9000 standards for process improvement and is based on TQM methodology but with a focus on environmental impact. The most common certification pursued is ISO 14001, which describes requirements for environmental management systems. The key aspects of this standard include developing an institutional-level environmental policy, generating employee buy in, setting sustainability targets, using life cycle analysis in decision-making, and having a continuous evaluation and improvement plan. While recent studies are mixed on the advantage offered through pursuing ISO 9000 certification,15 these studies focused on mature industries. However, it appears that early adopters of these techniques had a clear competitive advantage.16, 17 From this viewpoint, SRRs and the institutions that most effectively implement rigorous quality assurance programs such as TQM or ISO 9000 may well enjoy a competitive advantage over groups and institutions that are late to implement. A similar argument can be made when comparing the academic SRR model to the industrial adoption of LEED-certified construction and ISO 14000 and product life cycle management.
Numerous evaluations of the financial impacts of ISO 14000 certification have been performed, ranging from first principles analysis to an empirical analysis of financial performance.18, 19, 20 When considering the postimplementation financial performance of ISO 14000–certified corporations versus the S&P 500, the ISO 14000–certified corporations do realize cost savings in initial 3- to 5-year periods19 and were able to achieve more than twice the return over the broader S&P 500 portfolio from 1996 to 2015 in a rule-based, buy and hold, model portfolio.20 While this study was limited to showing that ISO 14000 certification was correlative with improved financial performance, the finding is in contrast to studies of companies seeking only ISO 9000 certification with less clear results.21
CASE STUDY: UNIVERSITY OF NORTH CAROLINA SCHOOL OF MEDICINE HIGH-THROUGHPUT SEQUENCING FACILITY
The standardization of next-generation sequencing workflows through the adoption of a quality management system (QMS) benefits both basic science and clinical applications.22, 23, 24 A QMS is a formal system characterized by the documentation of all processes and procedures guiding an organization’s central activities. Through the establishment of expectations for standard processes and performance, research organizations (including SRRs) engender confidence from customers, optimize workflows, reduce costs, and drive continuous operational improvements.25 The High-Throughput Sequencing Facility (HTSF) at the University of North Carolina (UNC) School of Medicine instituted a formal QMS based on principles of the ISO 9001 system 2015 requirements. Process improvement followed the principles of Six Sigma26 and included discovery, process mapping, training, drafting SOPs, metrics tracking, root cause analysis, customer meetings to gather feedback, and mock audits.27
The implementation of this QMS and the adoption of a continuous improvement mindset in the UNC HTSF has provided numerous benefits to the SRR, the customers, and the staff related to sustainability. The SRR has experienced a reduction in laboratory errors, obviating the need for rework, which leads to wasted time and revenue and customer dissatisfaction. Improved inventory management processes have resulted in a reduction in lost reagents because of expiry and a timelier processing of reagent orders based on customer demand and forecasting. The consistency and reliability of SRR services has improved, reducing material and temporal waste streams. The health and safety for SRR staff has improved, reducing accidents and associated downtime. Importantly, the HTSF has completed multiple successful quality audits of the SRR’s QMS, ensuring ongoing procurement of DNA/RNA sequencing contracts from multiple federal laboratories and agencies. The sustainability of these key scientific relationships further supports RR&T across time, eliminating the risks associated with using multiple SRRs/vendors to complete different projects. SRRs are integral to the conduct and success of biomedical research across the basic and clinical spectrum. Although SRRs may be unique related to focus, instrumentation, expertise, and customer base, all SRRs will benefit from a QMS to drive sustainability, rigor, and responsible conduct of reproducible research.
EXPANDING SRRs TO INCORPORATE MORE EQUIPMENT IN LOWER TO MID-COST CATEGORIES TO GROW RR&T AND SUSTAINABILITY
At research institutions, scientists readily think of sharing equipment that is expensive because it is often otherwise cost prohibitive, but there is an abundance of untapped opportunities to improve the sharing of research equipment in the lower to mid-cost categories, equipment that many researchers can afford to purchase for their individual laboratories. A shift in the culture of the research community to more readily include these less expensive, but important, equipment resources in staffed SRR facilities rather than individual laboratories will help expand the capability of SRR to benefit RR&T and sustainability. Just like expensive equipment types, increased sharing of more affordable but important equipment types, such as spectrophotometers, microscopes, biosafety cabinets, nanodrops, centrifuges, thermocyclers, etc., leads to avoided equipment duplication, more efficient use of space, and utility savings. Furthermore, RR&T still benefits from an SRR director or staff member with expertise overseeing equipment upkeep, providing advice on experimental design, ensuring quality of stocks, and training users on proper techniques and effective use of equipment. Additionally, as institutions elevate interdisciplinary research, widespread access by researchers to more basic equipment resources and experts in different disciplines is becoming increasingly important to support scientists with research projects bridging traditional discipline boundaries.
Because the investigative focuses of research groups will often change over time, and therefore so can the research equipment needs of the research group, it is not uncommon for equipment in lower to mid-cost ranges procured by individual principal investigators to become underutilized or unused within individual research laboratories, turning laboratory space into storage space for that equipment. This common inefficiency problem is avoided with SRR facilities, where it does not matter if the direction of an individual research group changes because that SRR equipment is serving many different research groups. Research institutions can often find underutilized equipment resources in individual research group laboratories. Through the engagement of research scientists, the BioCore Shared Instrumentation Program at University of Colorado Boulder (CU Boulder) is an example of a successful effort working to migrate underutilized resources into managed, shared equipment that many scientists can access. In the BioCore model, some equipment is moved to a shared laboratory space, while others remain in individual laboratories where access to equipment is coordinated and/or communicated by the BioCore manager.
DISCUSSION
Interest in laboratory sustainability appears to be growing generally, and the implementation of “green labs” programs are becoming increasingly prevalent at research intensive institutions. These programs are often collaborative endeavors, with scientists, students, trainees, and other campus stakeholders working together to identify and implement sustainable solutions that can support research goals while also reducing the environmental impact of research. The University of Virginia (UVA) Smart Labs Program, for example, is enabling more sustainable laboratory space by targeting laboratory ventilation inefficiencies. Once fully implemented, this Smart Labs Program envisions saving UVA an estimated $5 million per year in energy savings.30 On the “bench side,” UVA laboratory members are encouraged to make more efficient and sustainable choices through participation in the UVA Green Labs Program, which offers the UVA Green Lab Certification, the International Freezer Challenge, and other initiatives. During a recent “Shut the Sash” contest at UVA, the Office for Sustainability team reported saving $34 000 in energy costs in 1 month through the efforts of 21 laboratories closing their fume hood sashes when not in use, concluding that “maintaining those behaviors could result in energy savings of over $400 000 annually.”31
The connection between SRRs and sustainability within scientific research has also become a topic of interest over the past ~7 years for the green laboratories and laboratory sustainability movements. The CU Boulder Green Labs Program, discussed in more detail below, has been actively working to elevate awareness of SRRs as agents for improved environmental sustainability locally, regionally, nationally, and internationally through the International Institute for Sustainable Laboratories (I2SL). As a natural outcome of these efforts, new and productive relationships have also formed between I2SL and organizations such as FASEB and ABRF that have a strong SRR focus. ABRF-CCoRRe efforts to elevate the connection SRRs and best practices for RR&T have also begun to promote the more complex interplay between SRRs, RR&T, and sustainability.
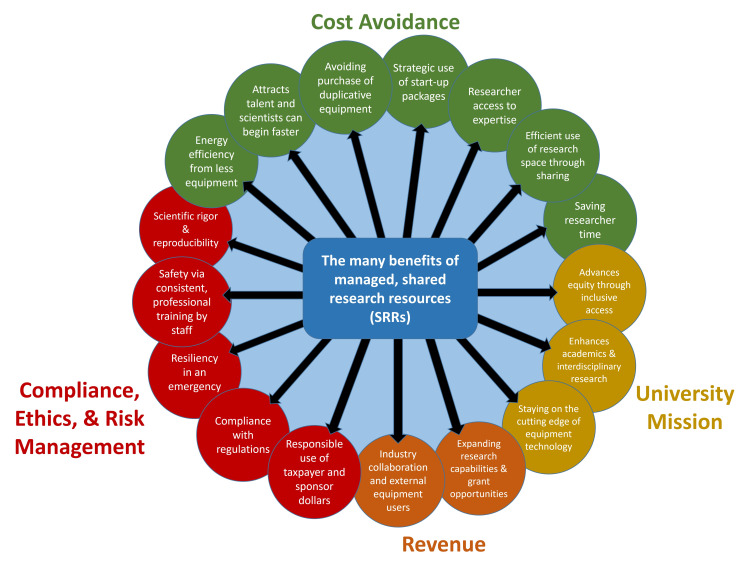
Collaborative engagement across the broader research community can result in new calls to action for improved laboratory sustainability that could also lead to better support of SRRs and staff for SRR facilities. The CU Boulder Green Labs Program, working with scientists, SRR staff, and other stakeholders, has grown a campus-wide culture of equipment sharing at CU Boulder that provides one blueprint for how other institutions could accomplish similar goals. For example, a list of the many positive benefits of managed SRRs (Figure 2) has been promoted across CU Boulder by the Green Labs Program to the scientific community and those who touch the research enterprise in others ways such as campus planners, facilities management, institutional administrators, and senior leaders. This has been accomplished through the Green Labs Program’s day-to-day interactions, partnership efforts with scientists, including core directors, and campus outreach activities organized by the program. The latter also serve to raise awareness of SRRs, providing tours of SRR facilities and posters promoting the benefits of SRRs, incorporating SRRs into new student and faculty orientations, providing presentations on the intersection between SRRs and sustainability to a wide range of stakeholders, and writing white papers on how to leverage institutional strategic visioning and goals. CU Boulder Green Labs has also led development of the CU Boulder Shared Instrumentation Network and obtained initial campus funding to start the BioCore Shared Instrumentation Program. These efforts have resulted in the growing support of SRRs at CU Boulder for new institutional priorities that align with recommendations from the FASEB “Maximizing Shared Research Resources Part III” report.8 The institutional synergy enabled by the CU Boulder Green Labs Program continues to strengthen SRRs through the establishment of new research space allocation guidelines that support SRRs, the consideration of SRRs during lab renovation and construction projects, and the creation of a new Director of Cores and Shared Instrumentation position. Nationally, such heightened awareness at academic research institutions can help these institutions respond productively to the global climate crisis by better meeting energy and greenhouse gas reduction.
Figure 2. The many benefits of managed SRRs.
Decision makers now have a road map to how the large environmental footprint of individual laboratories can be mitigated by strengthening and promoting the integration of SRRs into the research enterprise as a more efficient way of conducting science that can yield more sustainable and rigorous research.
As scientific RR&T as well as environmental sustainability receive additional scrutiny by taxpayers and funding agencies, early adopters of best practices may develop competitive advantages when seeking funding and successfully completing research. As noted above, SRRs are uniquely positioned to disseminate and amplify these practices, and thus implementation within SRRs is more efficient than attempting to implement one Principal Investigator (PI) per laboratory at a time. With SRRs as the effector mechanism, an institution can employ a holistic strategic planning process to align new initiatives to RR&T and/or environmental sustainability goals based on the opportunity and still achieve synergistic gains across both domains. While there is a cost to implement TQM or improved environmental standards, there is also a cost to falling behind the curve and missing future opportunities. ISO 14000/LEED certification, both discussed earlier, may open access to new sources of capital from environmentally responsible investment funds and an additional customer base that values environmental sustainability when making purchasing decisions.18 From a first principles standpoint, reusing equipment as well as reducing waste, the use of toxic chemicals, and energy lowers costs of production and reduces both costs of production and the risk of long-term costs for environmental remediation. While these benefits may be associated with added costs for development, increased bureaucracy, and potentially expensive operations, SRRs provide an efficient agent for distributing these costs over many users and projects, thus minimizing financial impact while maximizing quality and sustainability.
Given the data available in the industry discussed above, one can ask why academic laboratories are not more consistently adopting best practices regarding RR&T and environmental sustainability. Critically, in the industrial model, all costs roll up to a single entity, while in the academic model, financial interests are distributed among scientists, research institution administrators, and grant funding bodies (government and nongovernment) that may have various priorities and requirements. This can lead to decisions that are wasteful in terms of capital investment, operational expenses, space allocation, and natural resource use, as the equipment purchased for single projects may be used for only a short period and then stored away rather than making the equipment broadly available to other groups with overlapping technical needs. More accessible but less well-documented alternatives to the entrenched ISO and other standards do exist, which may be more appropriate for implementation in research environments, such as AGILE32 or EQUIPD.33 SRRs are uniquely positioned to enable and support a transition to these and/or more industry-inspired life cycle and quality models (as SRRs already repurpose older instruments to perform more basic experiments as new technology becomes available), make equipment broadly available to minimize redundancy and waste generation, and centralize energy-intensive infrastructure needed to support complex instrumentation and process flows. If funding agencies were to consider the total life cycle of equipment, funded projects, and real costs to maintain equipment, the US taxpayer may be able to benefit from levels of financial performance that corporations have enjoyed in adopting rigorous quality standards while reducing waste and energy use in academic institutions.
The responsibility for the development of programs and practices that enhance RR&T and environmental sustainability need not be limited to the SRRs and their home institutions. The manufacturers and vendors of instruments and reagents used by SRRs and across academic research can also play a role. Working with vendors that offer green and recyclable packaging and/or instrumentation that incorporates energy-efficient practices such as automatic shutdown after the completion of a run can contribute to improved environmental sustainability. SRRs also have opportunities to work more directly with and leverage their vendors’ expertise and resources, engaging them as partners to develop new protocols for more efficient instrument operations and training on technology platforms. A manufacturer/vendor’s field application staff and technical support staff are focused on specific instrument platforms and applications and have both a deep knowledge and a vested interest in the successful use of their instrument platforms. Often these individuals hold advanced degrees, have multiple years of laboratory experience, and have access to well-established training and technical resources. The collaboration by SRRs with these experts to use vendor-generated educational resources can provide a consistent foundation of knowledge that can also potentially enhance the knowledge of SRRs and their client/users. In particular, clients of SRRs often have minimal training and knowledge of the technologies being utilized; this is especially true for early-stage scientists. Whether an SRR client is the operator of the instrument or not, there is always a need to provide training on proper experimental design, sample preparation, equipment usage, and data visualization tools associated with a given instrument. Proper training on instrument operation and the critical factors that impact experimental success will help avoid failed experiments and irreproducible results that waste time and resources. A successful implementation of a vendor-sponsored training program does involve close consultation with the SRR to define the needs and expectations for a given training, and it is important to ensure that trainings are not commercially focused and, most importantly, structured in a manner that focuses on building user knowledge and understanding of a technical platform. However, SRRs, their users, and vendors can all benefit from such collaboration, which may spark improvements in reagent and consumable use, energy consumption, and instrument maintenance that all support the advancement of RR&T and environmental sustainability.
In summary, it is critical to note that environmental sustainability is a value in its own right, and many academic institutions are developing specific initiatives to adopt principles of waste avoidance, energy efficiency, and carbon neutrality. While much of this necessarily begins with the low-hanging fruit of general waste streams and energy use, the resource-intensive conduct of research presents a much greater challenge. Recently, the US Department of Health and Human Services (HHS), the parent to the NIH, issued the HHS Climate Action Plan (CAP), stating that “sustainability goes hand-in-hand with the HHS mission to protect the health of all Americans.” A priority in the HHS CAP is to “Develop Climate-Resilient Grant Policies” and identifies equipment sharing as a specific action that researchers and research-intensive institutions can take to improve climate resilience. It is also of note that the recently published NIH-wide Strategic Plan calls for NIH-supported research to be “...conducted efficiently, responsibly, ethically, and with integrity.” As part of that goal, NIH restates its commitment to enhance scientific RR&T through promotion of more rigorous and transparent research practices. As argued throughout this article, these RR&T principles should be seen in natural alignment with, and connected to, those of environmental sustainability; the rigorous conduct of science can lead to more reproducible results and so is logically conserving of finite resources. Furthermore, SRRs have the inherent efficiency, scientific capabilities, and the institutional reach in many cases to advance knowledge and understanding, change practices at an enterprise scale, and steward technological and energy resources for broad research impact and ultimately societal benefit. By more comprehensively adopting learnings from industry around quality improvement and technological advancement and by thoroughly embracing their central role as disseminators of best scientific research practices, already efficient SRRs can become the unique catalyst that will make a truly rigorous, reproducible, transparent, and sustainable research enterprise possible.
References
- About NIH: what we do . National Institutes of Health. Updated August 18, 2022. Accessed 2023. https://www.nih.gov/about-nih/what-we-do/budget
- Ambec S, Paul L. Does it pay to be green? a systematic overview. Acad Manag Perspect. 2008;22(4):45-62. doi: 10.5465/amp.2008.35590353 [DOI] [Google Scholar]
- Baird TR, Kaufman D, Brown CM. Mercury free microscopy: an opportunity for core facility directors. J Biomol Tech. 2014;25(2):48-53. doi: 10.7171/jbt.14-2502-001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. 1,500 scientists lift the lid on reproducibility. Nature. 2016;533(7604):452-454. doi: 10.1038/533452a [DOI] [PubMed] [Google Scholar]
- Baker, M. Irreproducible biology research costs put at $28 billion per year. Nature. 2015. doi: 10.1038/nature.2015.17711 [DOI] [Google Scholar]
- Benner MJ, Tushman ML. Process management and technological innovation: a longitudinal study of the photography and paint industries. Adm Sci Q. 2002;47(4):676-706. doi: 10.2307/3094913 [DOI] [Google Scholar]
- Bespalov A, Bernard R, Gilis A, et al. Introduction to the EQIPD quality system. eLife. 2021;10:e63294. doi: 10.7554/eLife.63294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockalo D, Bakator M. Improving business performance with ISO 9001: a review of literature and business practice. The European Journal of Applied Economics. 2018;15(1):83-93. doi: 10.5937/EJAE15-16145 [DOI] [Google Scholar]
- Endrullat C, Glokler J, Franke P, Frohme M. Standardization and quality management in next-generation sequencing. Appl Transl Genomics. 2016;10:2-9. doi: 10.1016/j.atg.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federation of American Societies for Experimental Biology . Maximizing shared research resources part III: Addressing systemic challenges and opportunities. FASEB. Published May 2021. Accessed 2023. https://www.faseb.org/getmedia/fdce5b7d-6274-4dba-8e47-716c87ab6937/Shared-Research-Resources_Final-Report_May-2021.pdf
- Greever C, Nahreini T, Ramirez-Aguilar, K. A case study of the Biochemistry Cell Culture Facility at the University of Colorado Boulder: avoided costs and other benefits resulting from shared equipment in collaborative research space. University of Colorado Boulder. April 2018. Accessed January 1, 2022. https://www.colorado.edu/ecenter/sites/default/files/attached-files/bccf_case_study_cu_boulder_2018_1.pdf
- Gregory CW. Building a quality management system in a core facility: a genomics core case study. J Biomol Tech. 2020;31(2):57-65. doi: 10.7171/jbt.20-3102-004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler I, Guillén MF, Macpherson JM. Global competition, institutions, and the diffusion of organizational practices: the international spread of the ISO 9000 quality standards. Adm Sci Q. 2002;47(2):207-232. doi: 10.2307/3094804 [DOI] [Google Scholar]
- International Organization for Standardization . ISO. https://www.iso.org/home.html
- Kauffmann H-M, Kamp H, Fuchs R, et al. Framework for the quality assurance of ‘omics technologies considering GLP requirements. Regul Toxicol Pharmacol. 2017;91(Suppl 1):S27-S35. doi: 10.1016/j.yrtph.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudtson KL, Carnahan RH, Hegstad-Davies RL, et al. Survey on scientific shared resource rigor and reproducibility. J Biomol Tech. 2019;30(3):36-44. doi: 10.7171/jbt.19-3003-001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanati A, Marzano M, Manzari C, et al. Management at the service of research: ReOmicS, a quality management system for omics sciences. Palgrave Commun. 2019;5:75. doi: 10.1057/s41599-019-0283-0 [DOI] [Google Scholar]
- Mische SM, Fisher NC, Meyn SM, et al. A review of the scientific rigor, reproducibility, and transparency studies conducted by the ABRF research groups. J Biomol Tech. 2020;31(1):11-26. doi: 10.7171/jbt.20-3101-003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health . Enhancing reproducibility through rigor and transparency. NIH Grants. Released June 9, 2015. Accessed January 1, 2022. https://grants.nih.gov/grants/guide/notice-files/not-od-15-103.html
- National Institutes of Health . Guidance: rigor and reproducibility in grant applications. NIH Grants & Funding. Updated January 26, 2023. Accessed January 1, 2022. https://grants.nih.gov/policy/reproducibility/guidance.htm
- National Institutes of Health . Rigor and reproducibility. NIH. Accessed January 1, 2022. https://www.nih.gov/research-training/rigor-reproducibility
- Nga JKH. The influence of ISO 14000 on firm performance. Social Responsibility Journal. 2009;5(3):408-422. doi: 10.1108/17471110910977311 [DOI] [Google Scholar]
- Rigby DK, Sutherland J, Noble A. Agile at scale. Harvard Business Review. May–June 2018:88-96. https://hbr.org/2018/05/agile-at-scale
- Sebastianelli R, Tamimi N, Iacocca K. Improving the quality of environmental management: impact on shareholder value. The International Journal of Quality & Reliability Management. 2015;32(1):53-80. doi: 10.1108/IJQRM-03-2013-0056 [DOI] [Google Scholar]
- Sfreddo LS, Vieira GBB, Vidor G, Santos CHS. ISO 9001 based quality management systems and organisational performance: a systematic literature review. Total Quality Management & Business Excellence. 2021;32(3-4):389-409. doi: 10.1080/14783363.2018.1549939 [DOI] [Google Scholar]
- Smart Labs . Introduction to the Smart Labs toolkit. Smart Labs. Accessed January 1, 2022. https://smartlabs.i2sl.org/introduction.html
- Snee RD, Hoerl RW. Six Sigma Beyond the Factory Floor. Prentice Hall; 2004. [Google Scholar]
- Taatjes DJ, Ghule PN, Bouffard NA, et al. The shared core resource as a partner in innovative scientific research: illustration from an academic microscopy imaging center. J Biomol Tech. 2022;33(1):3fc1f5fe.2507f36c. doi: 10.7171/3fc1f5fe.2507f36c [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Green Building Council . LEED rating system. USGBC. Accessed January 1, 2022. https://www.usgbc.org/leed
- University of Virginia . Green labs. UVA Sustainability. Accessed January 1, 2022. https://sustainability.virginia.edu/programs/green-labs
- University of Virginia . Smart labs. UVA Sustainability. Accessed January 1, 2022. https://sustainability.virginia.edu/smart-labs
- University of Virginia . Two UVA labs, one sustainability goal. UVA Sustainability. December 9, 2021. Accessed January 1, 2022. https://sustainability.virginia.edu/two-uva-labs-one-sustainability-goal
- Wayhan VB, Balderson EL. TQM and financial performance: what has empirical research discovered? Total Quality Management & Business Excellence. 2007;18(4):403-412. doi: 10.1080/14783360701231716 [DOI] [Google Scholar]