Abstract
Therapy and prognosis of several solid and hematologic malignancies, including non-small cell lung cancer (NSCLC), have been favourably impacted by the introduction of immune checkpoint inhibitors (ICIs). Their mechanism of action relies on the principle that some cancers can evade immune surveillance by expressing surface inhibitor molecules, known as “immune checkpoints”. ICIs aim to conceal tumoural checkpoints on the cell surface and reinvigorate the ability of the host immune system to recognize tumour cells, triggering an antitumoural immune response.
In this review, we will focus on the imaging patterns of different responses occurring in patients treated by ICIs. We will also discuss imaging findings of immune-related adverse events (irAEs), along with current and future perspectives of metabolic imaging. Finally, we will explore the role of radiomics in the setting of ICI-treated patients.
Introduction
Therapy and prognosis of non-small cell lung cancer (NSCLC) have been favorably impacted by the introduction of immune checkpoint inhibitors (ICIs), which trigger an antitumoural immune response against tumors that escape from immune surveillance by expressing surface inhibitors, termed “immune checkpoints”. 1–3 These checkpoints act against the activation of T-cells, hampering an efficient immune reaction against tumour cells. This down-modulatory effect of tumours on T-cells is counteracted by ICIs, resulting in an enhanced immune response. 4 ICIs target “programmed cell death protein 1” (PD-1; Nivolumab, Pembrolizumab, Cemiplimab) and “programmed cell death protein ligand 1” (PD-L1; Atezolizumab, Avelumab, Durvalumab), and they are proposed for both advanced and earlier-stages NSCLC. 2,4–8 Compared to chemotherapy (ChT), which directly inhibits cancer cell growth and may cause a swift reduction of the tumour burden, ICIs might have a delayed effect and demonstrate a slower tumour burden decrease, yet durable even after treatment interruption (Figure 1). The immunohistochemical assessment of tumour PD-L1 expression (defined as the percentage of PD-L1 positive tumour cells, from 0 to 100%) represents the only validated predictive biomarker in metastatic NSCLC, with variable response rates based on different thresholds of PD-L1 expression. 9–11
Figure 1.
Long-lasting response after immune checkpoint inhibitors. (A) Baseline CT scan of an 81-male patient shows a heterogeneous mass with right hilum involvement (white arrow). (B) CT scan performed after two years after diagnosis shows a reduction of the right hilar mass consistent with prolonged disease response.
ICIs, however, are not expected to have beneficial effects on all patients, and accurate selection of patients is mandatory because of their potential toxicities and costs. A tight collaboration between radiologists and oncologists is of paramount relevance in managing NSCLC patients. It will help recognise new response patterns to treatment, potential pitfalls, and immune-related adverse events (irAEs). 12–14
Approaches to longitudinal evaluation of ICIs-treated patients: the need for dedicated classification systems
Since the introduction of ICIs in advanced melanoma, the World Health Organization (WHO) criteria and the Response Evaluation Criteria in Solid Tumours (RECIST) have proved to be insufficient for evaluating peculiar responses to ICIs. 15 Radiologists had to reconsider how to assess responses in patients treated with ICIs, as they might display atypical imaging presentations posing critical diagnostic dilemmas. 16,17 According to WHO criteria and RECIST, a decrease in lesion size (or absence of new lesions) accounts for a positive response to cancer therapy. In contrast, a size increase (or appearance of new lesions) would result in progressive disease (PD), indicating treatment failure and thus leading to therapy discontinuation. 18 The effect of ICIs might take longer to manifest than standard ChT, and patients undergoing ICIs may display different imaging patterns at follow-up. 19 These include complete tumour disappearance (complete response), decrease in size with numerical stability of tumour lesions (stable disease), partial response and progressive disease, all of which are established patterns of response to therapy. ICI patients might also have a delayed response after an initial increase in total tumour burden (TTB), and new lesions might develop before a decrease in tumour size. However, these two patterns of response to ICIs do not necessarily account for PD, unlike ChT. 18 The advent of ICIs changed the approach to lung cancer imaging interpretation, requiring radiologists to familiarize themselves with these unusual patterns of response, further underlining the need for a multidisciplinary approach given the role of clinical status in evaluating disease stability. 20
Newer classification systems in the context of immunotherapy were adopted to overcome the limitations of the WHO criteria and RECIST. These new systems, currently applied in the context of clinical trials, 15,16,20 include Immune-Related Response Criteria (irRC), Immune-Related RECIST (irRECIST), and iRECIST. irRC system was developed from WHO criteria and requires bidimensional measurements; thus, it is associated with lower reproducibility of lesions’ size assessment and increased measuring time. irRECIST and iRECIST, derived from RECIST, allow unidimensional measurements and define the number of lesions that should be evaluated. One major implementation of iRECIST is the concept of variable referring time-point beyond baseline and nadir, resetting the bar after a lesion change during treatment. According to iRECIST, new or growing lesions needs to be confirmed after 4/8 weeks; furthermore, new target lesions are evaluated separately, while in the irRC and irRECIST, they are included in the sum of measures of the baseline target lesions. Recently, the immune-modified RECIST (imRECIST) criteria have been proposed to adapt response patterns to progression-free survival (PFS) analysis. 9,16
Differences between the classification systems can be summarized as follows:
-
Immune-Related Response Criteria (irRC)
Arisen from WHO criteria
Number of measurable lesions: not defined
Measurements: bidimensional
-
Immune-Related RECIST (irRECIST)
Adapted from the RECIST system
Number of measurable lesions: standardized
Measurements: unidimensional
New lesions: incorporated into TTB
-
immune RECIST (iRECIST)
Adapted from the RECIST system
Number of measurable lesions: standardized
Measurements: unidimensional
New lesions: incorporated into a separate cluster
Beyond the differences among these classification systems, it should be noted that imaging assessment maintains a central role in all of them, providing crucial information on dimensional changes and tumour burden.
Atypical imaging patterns in ICI-treated patients' follow-up
Pseudoprogression
The increase in tumour size or the appearance of new lesions followed by either tumour shrinkage or stability is defined as pseudoprogression. This phenomenon can be observed in less than 10% of cancer patients treated with ICI, specifically in about 5% of those with NSCLC 21,22 (Figures 2 and 3) The definition of pseudoprogression relies on a ≥ 20% increase of the sum of longest diameter compared with nadir (minimum 5 mm) or progression of non-target lesions or new lesion not confirmed in the following studies. 23,24 Therefore, according to iRECIST, growth of pre-existing lesions or appearance of new lesions at first follow-up should be reported with “interim” terminology (i.e., immune “unconfirmed” PD) until second imaging follow-up that can either confirm (i.e., immune “confirmed” PD) or rule out disease progression.
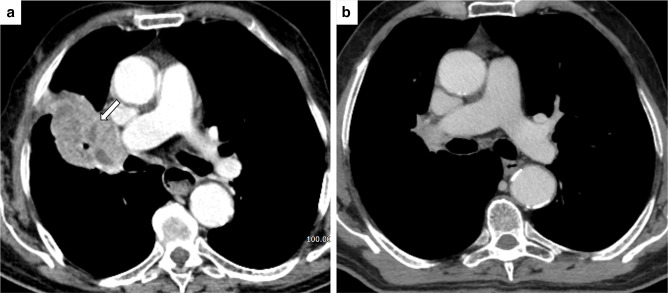
Figure 2.
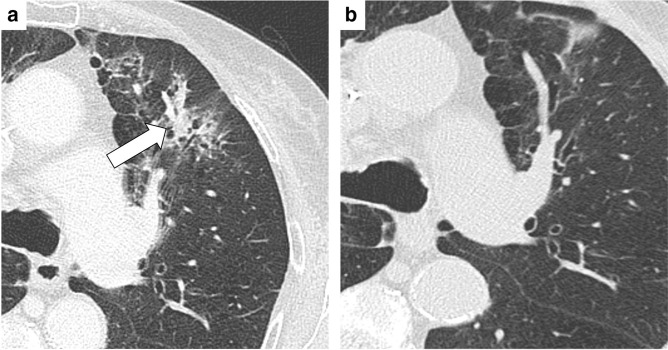
Pseudoprogressive disease. (A) PET-CT scan (lung window) of a 67 female patient shows a right lower lobe mass. (B) A Follow-up CT scan performed one month after immunotherapy initiation shows a size increase of the pulmonary mass with cavitation. ICI therapy was not interrupted: after a further one month (C), the pulmonary mass showed a dimensional reduction, consistent with pseudoprogressive disease.
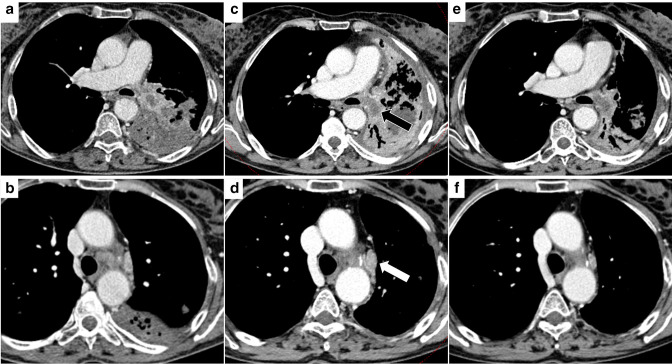
Figure 3.
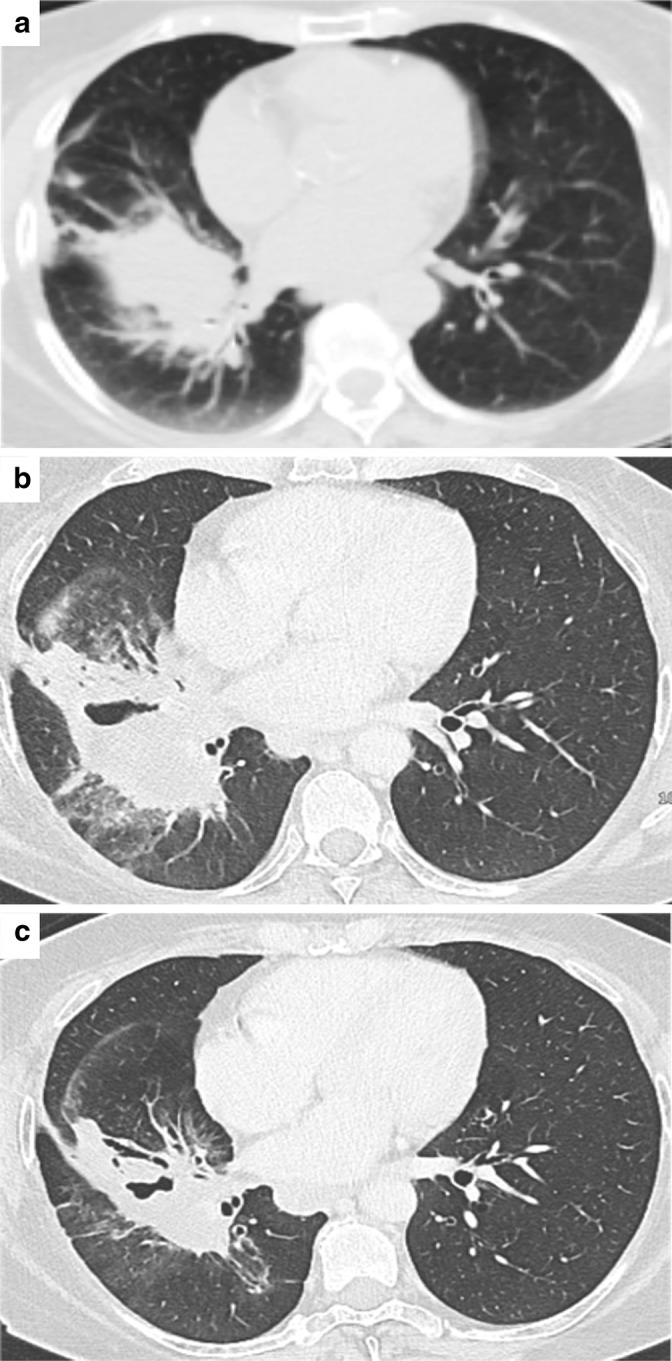
Pseudoprogressive disease. (A-B) CT image of a 59 female patient showing a mass involving the left hilum with necrotic component and post-obstructive atelectasis of the left lower lobe and a mediastinal lymph node in station 5. (C-D) A three months CT control after ICI treatment showed an increased necrotic component (black arrow), increased size of hilar tissue and of thoracic lymph nodes, including that shown in station 5 (white arrow). (E-F) The further imaging follow-up performed after one month demonstrated a dimensional reduction of the mass and improved ventilation of the left lung, and the dimensional reduction of the abovementioned mediastinal lymph node.
The biological basis of pseudoprogression resides either in transient immune cell infiltration, necrosis or oedema or in the initial growth of the tumour before complete activation of the immune response. 12,17 The appearance of new lesions is usually due to the lymphocytic infiltration of tumour sites undetectable at baseline imaging. 17,25 A predisposition toward pseudoprogression cannot be predicted from histopathological tumour features, such as PD-L1 expression. Time-to-pseudoprogression is variable, ranging from days to months since treatment initiation. A large real-world study reported that most patients with pseudoprogression first developed progressive disease within two months of treatment. 22,26
Hyperprogression
An unfavourable phenomenon described among ICI morphological responses is termed hyperprogression, which reflects a severe tumour surge occurring early after the administration of ICIs, associated with poor overall survival (OS) (Figures 4 and 5). 17–19 As per a recent meta-analysis, the pooled incidence of hyperprogression is 13.4%, varying from 5.9 to 43.1%. Such a large range of incidence is thought to reflect different methods of hyperprogression assessment, posing the need for establishing uniform criteria (Table 1). 27
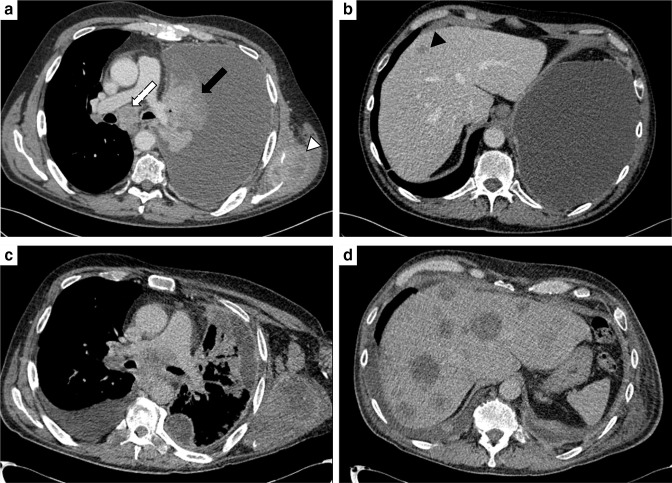
Figure 4.
Hyperprogressive disease. (A, B) CT images at two different levels of a 46-male patient admitted to the emergency department because of dyspnoea. CT scan depicted a large left pleural effusion and a heterogeneous lesion (black arrow) obstructing the upper bronchus with left lung atelectasis. Other findings included: enlarged and necrotic mediastinal (white arrow) and left axillary lymph nodes; osteolytic lesion involving the left scapula (white arrowhead); liver hypodense lesion (black arrowhead). The patient was administered immune checkpoint inhibitors-therapy, and at the first follow-up CT scan (C, D), a tumour surge was observed, with an increase in size and number of the neoplastic lesions.
Figure 5.
Hyperprogressive disease. (A) CT of a 73-male patient with a right upper lobe mass that showed a dimensional increase (B, three month-follow up) during chemotherapy. The patient was administered immune checkpoint inhibitors-therapy, with evidence of further dimensional and numerical growth (C, CT performed one month after) confirmed at a further one-month follow-up CT (D).
Table 1.
definition of hyperprogression in NSCLC reported in the literature
| Lahmar | Champiata | Katoa | Saâda-Bouzida | Ferrara | Kas | |
|---|---|---|---|---|---|---|
| Population | 89 | 131 | 155 | 34 | 406 | 406 |
| RECIST | No | TTB>20% new lesion | TTB>50% | No | TTB>20% | TTB>20% |
| Time-normalized growth index | TGR ratio>1.5 | TGR ratio>2 | TGR ratio>2b | TGK ratio>2 | ΔTGR > 50 | ΔTGR > 100 |
| Clinical integration | No | No | TTF<60 days | No | No | No |
| Incidence of HPD | 10.1% | 9.2% | 3.9% | 29.4% | 13.8% | 8.4%c |
| Median OS | N.A. | 138 days | 45 days d | 183 days | 102 days | 100 daysc |
TGK: tumour growth kinetics; TGR: tumour growth rate; TTB: total tumour burden; TTF: time to treatment failure.
populations including primary cancers beyond NSCLC
observation period: 2 months before and 2 months after ICI initiation
data were derived from Figure 2C of Kas et al, according to the optimal selection of 34 patients as reported in Results paragraph [19]
time-to-treatment failure, individually reported for each patient with hyperprogression in Supplementary material [24]
Several mechanisms of hyperprogressive disease have been suggested, including T-regulatory cell expansion, T-effector cell exhaustion, modulation of pro-tumorigenic immune subset, aberrant inflammation, and oncogenic pathway activation, and both translational and in vivo studies have attempted to unveil the underlying biology for such phenomenon. A definitive answer, however, is still to be found. Diagnostic indicators for hyperprogression include tumour growth rate (TGR), TGR on treatment minus TGR before treatment (delta TGR, ΔTGR), and tumour growth kinetics (TGK), 28 with a ΔTGR>50% at first evaluation being proposed as a possible criterion. 29,30 In this rapidly evolving clinical scenario, a univocal definition of hyperprogression is still to be provided; notably, the identification of the underlying mechanisms and potential biomarkers that future studies could grant is paramount to avoiding detrimental immunotherapy and exploiting novel therapeutic targets for future immunotherapy combinations. 31,32
Dissociated response
The detection of synchronous shrinkage and progression of different lesions within the same imaging study reflects a dissociated response (DR), potentially related to tumour heterogeneity or differences in drug distribution to tissues. 33,34
What to look for: the many imaging faces of immune-related adverse events
The stimulation of the immune system prompted by ICIs might lead to autoimmune-type reactions with local and systemic adverse events (immune-related adverse events, irAEs). 35,36 Almost all trials reported irAEs, uncommonly observed in standard ChT and often requiring medical support. irAEs affect one or multiple organs simultaneously at any time (during and/or after treatment), ranging from asymptomatic to severe or even life-threatening manifestations. 19 On imaging, lung involvement may be indistinguishable from infection, pseudoprogression or disease progression. 19,37
Immune-related (ir) pneumonitis accounts for one of the most clinically relevant irAE. It exhibits a broad spectrum of imaging patterns, often coexisting, and high-resolution computed tomography (HRCT) is the imaging modality of choice for their evaluation. 37–40 Longitudinal imaging usually allows accurate characterisation in both asymptomatic and symptomatic patients. 41
Recognized imaging patterns of lung-related irAEs include organizing pneumonia (OP), nonspecific interstitial pneumonia (NSIP), diffuse alveolar damage (DAD)/acute respiratory distress syndrome (ARDS) and findings of hypersensitivity pneumonia (HP):
OP: patchy, usually bilateral, confluent peribronchial consolidations with mostly peripheral or subpleural distribution; central ground-glass opacities (GGO), surrounded by consolidation (reversed halo sign) can also be detected (Figure 6);
NSIP: patchy GGOs, usually bilateral and peripheral, with lower lung predominance are typical of cellular NSIP, whereas reticulations and traction bronchiectasis/bronchiolectasis are indicative of fibrotic NSIP;
HP: poorly defined centrilobular nodules and/or bilateral GGO; mosaic attenuation due to air trapping;
DAD/ARDS: extensive bilateral GGO with or without crazy-paving pattern, dependent consolidations, and potential development of traction bronchiectasis (Figure 7).
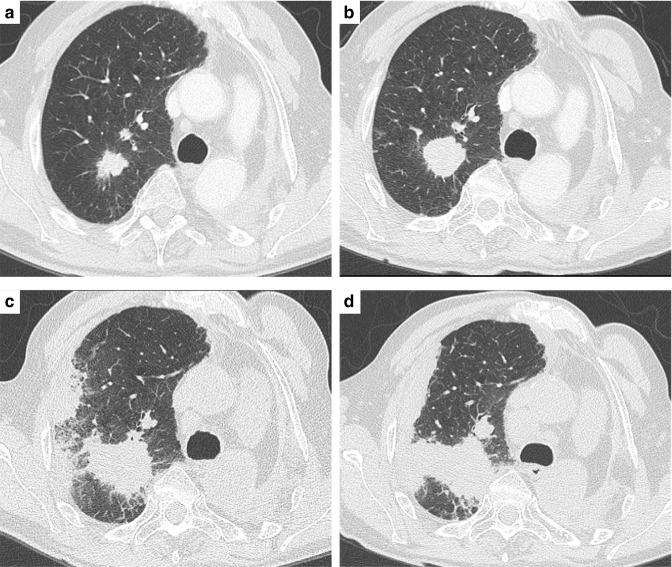
Figure 6.
Pulmonary immune-related adverse event: organizing pneumonia. (A) Magnification of High-Resolution Computed Tomography scan of an 82-female patient under immune checkpoint inhibitors treatment. CT scan showed bilateral, patchy consolidation with peribronchial distribution (white arrow). The pattern is consistent with organizing pneumonia. (B) The finding of pulmonary irAE resolved after discontinuation of ICIs and the administration of steroid therapy.
Figure 7.
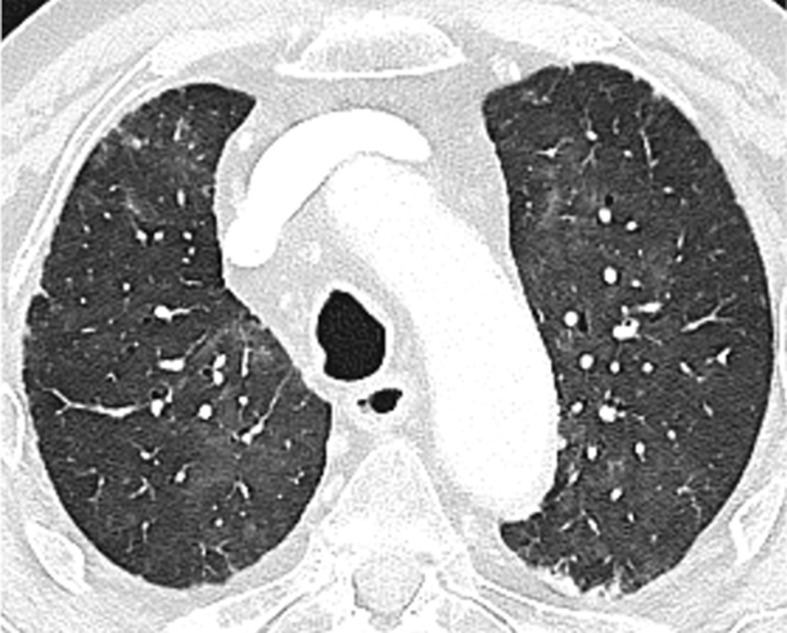
Pulmonary immune-related adverse event: diffuse alveolar damage. CT scan of an 82-male patient shows interstitial involvement with bilateral, patchy ground-glass opacities. The pattern was deemed consistent with diffuse alveolar damage in a patient undergoing immunotherapy.
Interstitial ir-pneumonitis shows different clinical severity, with DAD/ARDS pattern being the most severe, followed by OP, NSIP and HP. 42 Sarcoid-like reactions can also be detected on HRCT as new perilymphatic nodules and enlarged mediastinal/hilar lymph nodes, showing 18F - Fluorodeoxyglucose (18F - FDG) - Positron-emission-tomography (PET) uptake, findings that overlap PD (Figure 8). 43 Sarcoid-like nodules have been linked to the phenomenon of pseudoprogression, as they may be due to the infiltration of activated immune cells. 44
Figure 8.
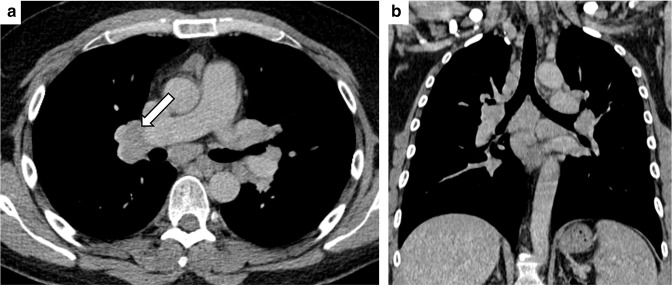
Pulmonary immune-related adverse event: sarcoid-like reaction. Axial (A) and coronal-reformatted (B) Computed Tomography images of a 46 male patients undergoing immune checkpoint inhibitors showing enlarged hilar and mediastinal lymph nodes (white arrows), homogeneous in density, consistent with the sarcoid-like reaction, which was confirmed after EBUS-TBNA.
Pre-existing pulmonary fibrosis, previous thoracic radiation therapy (RT), a combination of drugs, and various degrees of tumoural obstruction and invasion of airways are recognised risk factors for ir-pneumonitis. 45,46 Previous studies observed a positive association with active inflammation, reporting that pulmonary infections sustained by non-tuberculous mycobacteria or Pseudomonas aeruginosa can be worsened by ICI and that individuals with a pro-inflammatory state (e.g., patients with rheumatoid arthritis) are more prone to develop ir-pneumonitis. 47 Rarer thoracic irAEs include tuberculosis reactivation, recurrent allergic bronchopulmonary aspergillosis, and diaphragm myositis. 48–52
Distinguishing ir-pneumonitis from RT pneumonitis may be challenging. The differential diagnosis should consider both chronological and radiological aspects. RT-pneumonitis typically occurs 4 to 12 weeks after RT, usually involving areas included in the RT field, with GGO, nodular or focal consolidations often crossing lung fissures. 53 Future therapeutic approaches combining RT and ICI might exploit their synergistic effect. Still, the risk of an acute inflammatory response caused by a systemic agent (i.e., ICI) in a previously irradiated area should not be disregarded. In patients treated by ICIs, the pathogenesis of the so-called radiation recall pneumonitis (RRP) is thought to be driven by multiple signalling pathways prompted by ICIs, resulting in an inflammatory response of the previously irradiated field, with different HRCT patterns including OP, NSIP, HP or DAD/ARDS, 54,55 and clinical presentation can develop within a broad timeframe, as high as more than two years after the end of RT. 56,57 Of note, combined chemoradiotherapy (CRT) followed by ICI-consolidation has been approved in unresectable locally advanced NSCLC 2,5–7,12–14 and is being tested for non-metastatic settings. 58–61 Hence, owing to the widened use of ICIs, atypical responses and irAEs are likely to be more frequently observed on follow-up images. 39,59,62–65 In the PACIFIC trial, a Phase three study where Durvalumab was compared with placebo in unresectable stage III NSCLC previously treated by CRT, the risk of pneumonitis was of particular concern since large tumour size, respiratory comorbidities, sequelae of smoking and potential negative synergy with recently delivered RT may undermine lung function. Rates of severe pneumonitis were comparable across treatment arms (Durvalumab as consolidation therapy vs placebo) and safely manageable. 40,64,66
Gastro-intestinal irAE: ir-colitis typically develops 6–7 weeks after treatment; recognized CT patterns include diffuse and segmental colitis with generalized or focal bowel wall thickening, mesenteric hyperaemia, infiltration of perivisceral fat, air-fluid levels, and ascites. Although CT also allows the assessment of extraluminal complications (e.g., perforation, abscess formation) (Figure 9), its role might be of limited value in detecting subtle signs of inflammation. The clinical management of these cases can be supported by endoscopy. 67
Figure 9.
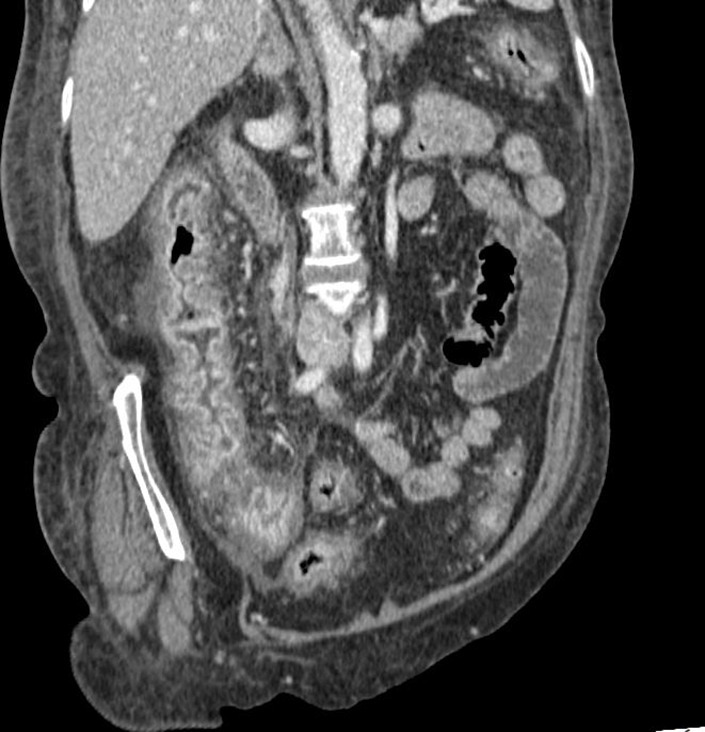
Gastro-intestinal immune-related adverse event: colitis. Coronal-reformatted image of a 67-female patient undergoing ICIs showing diffuse colonic concentric wall thickening that was deemed suspicious for irAE colitis and confirmed after endoscopic evaluation.
Liver and pancreatic irAE: ir-hepatitis and acute hepatitis display overlapping non-specific findings, including hepatomegaly and periportal lymphadenopathy. Ultrasound may show periportal/portal vein hyperechogenicity and gallbladder wall oedema; CT findings include liver hypoattenuation and periportal oedema, which is also demonstrated by increased T2 signal of the portal vein walls and periportal spaces on magnetic resonance imaging (MRI). 19,68 A liver biopsy might help the diagnostic process. 69 Imaging findings of the rare (incidence <1%) ir-pancreatitis are similar to those of acute non-ir pancreatitis and include glandular enlargement, adipose tissue stranding, and oedema. 70
Neurologic irAEs involve the central and peripheral nervous system, causing headaches, encephalopathies, and meningitis. Imaging characterization is mainly performed with MRI; findings include signal changes in the limbic system, corpus callosum or signs of posterior reversible encephalopathy. 70–72
Endocrine irAE encompasses thyroiditis and hypophysitis. 73 Enlarged and heterogeneous thyroid on ultrasound, CT, and FDG-PET avid gland account for features of thyroiditis. 15 Hypophysitis, a potentially life-threatening condition, displays an enlarged hypophysis on brain CT or MR and an avid gland 18F-FDG uptake on functional studies and has shown a substantial surge compared to the pre-ICI era, with the highest incidence rates after combination therapy. 74,75
Cardiac irAE: myocarditis and pericarditis are rare irAEs, although often severe and potentially fatal. Diagnosis and monitoring can be performed by echocardiography, cardiac MRI and, for pericarditis, CT. 76,77
The role of metabolic imaging in the evaluation of response to ICI
The functional information provided by PET imaging can be potentially applied to irRECIST and iRECIST for the evaluation of ICI patients since metabolic tumoural changes may pre-date morphological alterations due to therapy. 19,78,79 However, the role of PET-CT is still debated: an increased FDG uptake by inflammatory cells is responsible for false-positive findings, and its high cost and limited availability hamper a broad and homogeneous use. Also, PET sensitivity is relatively low in the case of respiratory artefacts, small lesions, and low cellular density. Moreover, the high brain tissue uptake observed in physiological conditions limits the ability of FDG-PET imaging to detect intracranial metastases, common in NSCLC. 75 The use of immune-PET might overcome these limitations, but its application in clinical practice is still under evaluation. 80
Various classification systems have been proposed to assess ICI response, including the PET-CT Criteria for Early Prediction of Response to Immune Checkpoint Inhibitor Therapy (PECRIT), the PET Response Evaluation Criteria for Immunotherapy (PERCIMT), and immune Positron Emission Tomography Response Criteria in Solid Tumours (iPERCIST). 81 Early assessment by PET-CT might stratify the risk of progression or predict patient survival through the identification of different response patterns according to changes in FDG uptake, as well as help assess early irAEs. 9,75 The disappearance of FDG uptake is regarded as a complete metabolic response, whereas modifications in FDG uptake relate to either partial response or progressive disease, allowing the assessment of metabolic tumoural activity. 75 FDG-uptake, however, might also be increased in case of activation of tumour immune microenvironment, potentially leading to false-positive results. Semiquantitative measurements represent a promising approach for the evaluation of response to ICIs. Higher values of SUVmax reflecting high glucose metabolism were observed in NSCLC patients with PD-L1 expression compared to those without. 82 The relation between SUVmax and PD-L1 expression has been postulated due to the activation of signalling pathways promoting tumour proliferation. 83,84 Apart from SUVmax, other metrics potentially reflecting tumoural metabolic activity have been proposed; these include metabolic tumour volume (tumoural volume with SUV above a threshold), total lesion glycolysis, and metabolic-to-morphological volume ratio. 75,85 A comprehensive discussion of these metrics, however, goes beyond the scope of this review.
Currently, research is focused on developing specific radiopharmaceutical agents, as ICIs labelled with PET isotopes (e.g., 89Zirconium - 89Zr, or 64Copper - 64Cu). For instance, 89Zr-atezolizumab imaging before treatment has shown promising results in assessing ICI response and implementing PFS and OS stratification in patients with NSCLC, bladder and breast cancer. 86–88
The potential impact of radiomics on the workflow of patients treated with ICIs
The identification of features that serve the purpose of differentiating responders from pseudoprogressors and hyper-progressors has had limited success so far. Radiomics - allowing the analysis of quantitative biomarkers (i.e., radiomic features, RFs) from the whole tumour - might help identify those patients who benefit from ICIs. 89,90 This approach could overcome the major limitation of biopsy-derived tissue, where the sampling represents only a minor proportion of the neoplastic systemic disease. 91 Radiomics could predict PD-L1 expression, and RFs describing heterogeneous lesions with non-uniform density patterns and compact borders were mostly found in ICI responders. 92 Moreover, RFs derived from intratumoural and peritumoural regions and vasculature were shown to allow the identification of patients at risk of hyperprogression. 93,94 Prognostic models combining CT-RFs and immunophenotypic features in surgically resected NSCLC predicted outcomes in various retrospective studies. 95–97 Radiomics could also quantify changes in tumour compositions after ICIs, leading to the concept of delta-radiomics, whereby the modification of RFs over time is tested for outcome prediction. 97
Nevertheless, several issues will need to be addressed before radiomics being incorporated into clinical practice, including data consistency in cancer patients who might undergo imaging in different hospitals (e.g., stability and reproducibility of features and radiomics outputs) and ethical aspects related to the “black-box” nature of radiomics itself. Furthermore, increased methodological quality and reproducibility of studies testing radiomics for predicting the ICI response in NSCLC is awaited. 98,99
Conclusion
The increasing use of ICIs in clinical practice demands a deep understanding of the associated imaging manifestations of both therapeutic effects and adverse events. Imaging is the cornerstone for the longitudinal evaluation of tumour burden in ICI-treated patients: close collaboration between radiologists and oncologists is vital for proper imaging interpretation and optimization of patient management.
Contributor Information
Gianluca Milanese, Email: gianluca.milanese@unipr.it.
Giulia Mazzaschi, Email: giulia.mazzaschi@studenti.unipr.it.
Roberta Eufrasia Ledda, Email: robertaeufrasia.ledda@unipr.it.
Maurizio Balbi, Email: balbi.m@libero.it.
Sveva Lamorte, Email: sveva.lamorte@unipr.it.
Caterina Caminiti, Email: CCaminiti@ao.pr.it.
Davide Colombi, Email: d.colombi@ausl.pc.it.
Marcello Tiseo, Email: marcello.tiseo@unipr.it.
Mario Silva, Email: mario.silva@unipr.it.
Nicola Sverzellati, Email: nicola.sverzellati@unipr.it.
REFERENCES
- 1.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018; 359: 1350–55. doi: 10.1126/science.aar4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasello G, Pavan A, Attili I, Bortolami A, Bonanno L, Menis J, et al. Real world data in the era of immune checkpoint inhibitors (icis): increasing evidence and future applications in lung cancer. Cancer Treat Rev 2020; 87: 102031: S0305-7372(20)30069-4. doi: 10.1016/j.ctrv.2020.102031 [DOI] [PubMed] [Google Scholar]
- 3.Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open 2019; 2: e192535. doi: 10.1001/jamanetworkopen.2019.2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang S, Qin C, Hu H, Liu T, He Y, Guo H, et al. Immune checkpoint inhibitors in non-small cell lung cancer: progress, challenges, and prospects. Cells 2022; 11(3): 320. doi: 10.3390/cells11030320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol 2020; 38: 1505–17. doi: 10.1200/JCO.19.03136 [DOI] [PubMed] [Google Scholar]
- 6.Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Soto Parra H, Mazières J, et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol 2020; 15: 1657–69: S1556-0864(20)30500-1. doi: 10.1016/j.jtho.2020.06.015 [DOI] [PubMed] [Google Scholar]
- 7.Gray JE, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Three-Year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC-update from Pacific. J Thorac Oncol 2020; 15: 288–93: S1556-0864(19)33529-4. doi: 10.1016/j.jtho.2019.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai R, Li L, Chen X, Chen N, Song W, Cui J. Neoadjuvant and adjuvant immunotherapy: opening new horizons for patients with early-stage non-small cell lung cancer. Front Oncol 2020; 10: 575472. doi: 10.3389/fonc.2020.575472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dromain C, Beigelman C, Pozzessere C, Duran R, Digklia A. Imaging of tumour response to immunotherapy. Eur Radiol Exp 2020; 4: 2. doi: 10.1186/s41747-019-0134-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US food and drug administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer 2019; 7: 278. doi: 10.1186/s40425-019-0768-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lantuejoul S, Sound-Tsao M, Cooper WA, Girard N, Hirsch FR, Roden AC, et al. Pd-L1 testing for lung cancer in 2019: perspective from the IASLC pathology Committee. J Thorac Oncol 2020; 15: 499–519: S1556-0864(19)33847-X. doi: 10.1016/j.jtho.2019.12.107 [DOI] [PubMed] [Google Scholar]
- 12.Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol 2015; 33: 3541–43. doi: 10.1200/JCO.2015.61.6870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 2017; 23: 1920–28. doi: 10.1158/1078-0432.CCR-16-1741 [DOI] [PubMed] [Google Scholar]
- 14.Vani V, Regge D, Cappello G, Gabelloni M, Neri E. Imaging of adverse events related to checkpoint inhibitor therapy. Diagnostics (Basel) 2020; 10(4): E216. doi: 10.3390/diagnostics10040216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter BW. Immunotherapy in lung cancer and the role of imaging. Semin Ultrasound CT MR 2018; 39: 314–21: S0887-2171(18)30019-2. doi: 10.1053/j.sult.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 16.Hodi FS, Ballinger M, Lyons B, Soria J-C, Nishino M, Tabernero J, et al. Immune-modified response evaluation criteria in solid tumors (imrecist): refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol 2018; 36: 850–58. doi: 10.1200/JCO.2017.75.1644 [DOI] [PubMed] [Google Scholar]
- 17.Zhou L, Zhang M, Li R, Xue J, Lu Y. Pseudoprogression and hyperprogression in lung cancer: a comprehensive review of literature. J Cancer Res Clin Oncol 2020; 146: 3269–79. doi: 10.1007/s00432-020-03360-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beer L, Hochmair M, Prosch H. Pitfalls in the radiological response assessment of immunotherapy. Memo 2018; 11: 138–43. doi: 10.1007/s12254-018-0389-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shroff GS, Shroff S, Ahuja J, Truong MT, Vlahos I. Imaging spectrum of adverse events of immune checkpoint inhibitors. Clin Radiol 2021; 76: 262–72: S0009-9260(20)30625-5. doi: 10.1016/j.crad.2020.11.117 [DOI] [PubMed] [Google Scholar]
- 20.Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. IRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017; 18: e143–52. doi: 10.1016/S1470-2045(17)30074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Giacomo AM, Danielli R, Guidoboni M, Calabrò L, Carlucci D, Miracco C, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother 2009; 58: 1297–1306. doi: 10.1007/s00262-008-0642-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park HJ, Kim KW, Pyo J, Suh CH, Yoon S, Hatabu H, et al. Incidence of pseudoprogression during immune checkpoint inhibitor therapy for solid tumors: a systematic review and meta-analysis. Radiology 2020; 297: 87–96. doi: 10.1148/radiol.2020200443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tazdait M, Mezquita L, Lahmar J, Ferrara R, Bidault F, Ammari S, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irrecist and irecist criteria. Eur J Cancer 2018; 88: 38–47: S0959-8049(17)31357-6. doi: 10.1016/j.ejca.2017.10.017 [DOI] [PubMed] [Google Scholar]
- 24.Sheikhbahaei S, Marcus CV, Sadaghiani MS, Rowe SP, Pomper MG, Solnes LB. Imaging of cancer immunotherapy: response assessment methods, atypical response patterns, and immune-related adverse events, from the AJR special series on imaging of inflammation. AJR Am J Roentgenol 2022; 218: 940–52. doi: 10.2214/AJR.21.26538 [DOI] [PubMed] [Google Scholar]
- 25.Ma Y, Wang Q, Dong Q, Zhan L, Zhang J. How to differentiate pseudoprogression from true progression in cancer patients treated with immunotherapy. Am J Cancer Res 2019; 9: 1546–53. [PMC free article] [PubMed] [Google Scholar]
- 26.Fujimoto D, Yoshioka H, Kataoka Y, Morimoto T, Hata T, Kim YH, et al. Pseudoprogression in previously treated patients with non-small cell lung cancer who received nivolumab monotherapy. J Thorac Oncol 2019; 14: 468–74: S1556-0864(18)33418-X. doi: 10.1016/j.jtho.2018.10.167 [DOI] [PubMed] [Google Scholar]
- 27.Park HJ, Kim KW, Won SE, Yoon S, Chae YK, Tirumani SH, et al. Definition, incidence, and challenges for assessment of hyperprogressive disease during cancer treatment with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Netw Open 2021; 4: e211136. doi: 10.1001/jamanetworkopen.2021.1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding P, Wen L, Tong F, Zhang R, Huang Y, Dong X. Mechanism underlying the immune checkpoint inhibitor-induced hyper-progressive state of cancer. Cancer Drug Resist 2022; 5: 147–64. doi: 10.20517/cdr.2021.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin M, Vanneste BGL, Yu Q, Chen Z, Peng J, Cai X. Hyperprogression under immunotherapy: a new form of immunotherapy response? -A narrative literature review. Transl Lung Cancer Res 2021; 10: 3276–91. doi: 10.21037/tlcr-21-575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol 2018; 4: 1543–52. doi: 10.1001/jamaoncol.2018.3676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Facchinetti F, Lo Russo G, Tiseo M, Garassino MC, Ferrara R. How to recognize and manage hyper-progression and pseudo- progression during immune checkpoint blockade in non-small cell lung cancer. Precis Cancer Med 2019; 2: 35. doi: 10.21037/pcm.2019.10.03 [DOI] [Google Scholar]
- 32.Lo Russo G, Facchinetti F, Tiseo M, Garassino MC, Ferrara R. Hyperprogressive disease upon immune checkpoint blockade: focus on non-small cell lung cancer. Curr Oncol Rep 2020; 22(5): 41. doi: 10.1007/s11912-020-00908-9 [DOI] [PubMed] [Google Scholar]
- 33.Humbert O, Chardin D. Dissociated response in metastatic cancer: an atypical pattern brought into the spotlight with immunotherapy. Front Oncol 2020; 10: 566297. doi: 10.3389/fonc.2020.566297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borcoman E, Kanjanapan Y, Champiat S, Kato S, Servois V, Kurzrock R, et al. Novel patterns of response under immunotherapy. Ann Oncol 2019; 30: 385–96: S0923-7534(19)31083-X. doi: 10.1093/annonc/mdz003 [DOI] [PubMed] [Google Scholar]
- 35.Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol 2016; 13: 473–86. doi: 10.1038/nrclinonc.2016.58 [DOI] [PubMed] [Google Scholar]
- 36.Nishino M, Hatabu H, Hodi FS. Imaging of cancer immunotherapy: current approaches and future directions. Radiology 2019; 290: 9–22. doi: 10.1148/radiol.2018181349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith DA, Radzinsky E, Tirumani SH, Guler E, Petraszko A, Kikano E, et al. Findings on chest CT performed in the emergency department in patients receiving immune checkpoint inhibitor therapy: single-institution 8-year experience in 136 patients. AJR Am J Roentgenol 2021; 217: 613–22. doi: 10.2214/AJR.20.24758 [DOI] [PubMed] [Google Scholar]
- 38.Sverzellati N, Lynch DA, Hansell DM, Johkoh T, King TE, Travis WD. American thoracic society-european respiratory Society classification of the idiopathic interstitial pneumonias: advances in knowledge since 2002. Radiographics 2015; 35: 1849–71. doi: 10.1148/rg.2015140334 [DOI] [PubMed] [Google Scholar]
- 39.Voong KR, Naidoo J. Radiation pneumonitis after definitive chemoradiation and durvalumab for non-small cell lung cancer. Lung Cancer 2020; 150: 249–51: S0169-5002(20)30596-1. doi: 10.1016/j.lungcan.2020.08.022 [DOI] [PubMed] [Google Scholar]
- 40.von Reibnitz D, Chaft JE, Wu AJ, Samstein R, Hellmann MD, Plodkowski AJ, et al. Safety of combining thoracic radiation therapy with concurrent versus sequential immune checkpoint inhibition. Adv Radiat Oncol 2018; 3: 391–98. doi: 10.1016/j.adro.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johkoh T, Lee KS, Nishino M, Travis WD, Ryu JH, Lee HY, et al. Chest CT diagnosis and clinical management of drug-related pneumonitis in patients receiving molecular targeting agents and immune checkpoint inhibitors: a position paper from the fleischner Society. Radiology 2021; 298: 550–66. doi: 10.1148/radiol.2021203427 [DOI] [PubMed] [Google Scholar]
- 42.Nishino M, Ramaiya NH, Awad MM, Sholl LM, Maattala JA, Taibi M, et al. Pd-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res 2016; 22: 6051–60. doi: 10.1158/1078-0432.CCR-16-1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montaudié H, Pradelli J, Passeron T, Lacour J-P, Leroy S. Pulmonary sarcoid-like granulomatosis induced by nivolumab. Br J Dermatol 2017; 176: 1060–63. doi: 10.1111/bjd.14808 [DOI] [PubMed] [Google Scholar]
- 44.Beer L, Hochmair M, Kifjak D, Haug AR, Prayer F, Mayerhoefer ME, et al. Particular findings on lung CT in patients undergoing immunotherapy for bronchogenic carcinoma. Wien Klin Wochenschr 2020; 132: 467–74. doi: 10.1007/s00508-020-01667-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cui P, Liu Z, Wang G, Ma J, Qian Y, Zhang F, et al. Risk factors for pneumonitis in patients treated with anti-programmed death-1 therapy: a case-control study. Cancer Med 2018; 7: 4115–20. doi: 10.1002/cam4.1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isono T, Kagiyama N, Takano K, Hosoda C, Nishida T, Kawate E, et al. Outcome and risk factor of immune-related adverse events and pneumonitis in patients with advanced or postoperative recurrent non-small cell lung cancer treated with immune checkpoint inhibitors. Thorac Cancer 2021; 12: 153–64. doi: 10.1111/1759-7714.13736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moda M, Saito H, Kato T, Usui R, Kondo T, Nakahara Y, et al. Tumor invasion in the central airway is a risk factor for early-onset checkpoint inhibitor pneumonitis in patients with non-small cell lung cancer. Thorac Cancer 2020; 11: 3576–84. doi: 10.1111/1759-7714.13703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delaunay M, Prévot G, Collot S, Guilleminault L, Didier A, Mazières J. Management of pulmonary toxicity associated with immune checkpoint inhibitors. Eur Respir Rev 2019; 28: 154: 190012. doi: 10.1183/16000617.0012-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haddox CL, Shenoy N, Shah KK, Kao JC, Jain S, Halfdanarson TR, et al. Pembrolizumab induced bulbar myopathy and respiratory failure with necrotizing myositis of the diaphragm. Ann Oncol 2017; 28: 673–75. doi: 10.1093/annonc/mdw655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Picchi H, Mateus C, Chouaid C, Besse B, Marabelle A, Michot JM, et al. Infectious complications associated with the use of immune checkpoint inhibitors in oncology: reactivation of tuberculosis after anti PD-1 treatment. Clin Microbiol Infect. 2018;24(3):216-8. [DOI] [PubMed] [Google Scholar]
- 51.Pradere P, Michot JM, Champiat S, Danlos FX, Marabelle A, Lambotte O, et al. Allergic broncho-pulmonary aspergillosis following treatment with an anti-programmed cell death protein 1 monoclonal antibody therapy. Eur J Cancer. 2017;75:308-9. [DOI] [PubMed] [Google Scholar]
- 52.Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017;28(3):583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi YW, Munden RF, Erasmus JJ, Joo Park K, Chung WK, Jeon SC, et al. Effects of radiation therapy on the lung: radiologic appearances and differential diagnosis. RadioGraphics 2004; 24: 985–97. doi: 10.1148/rg.244035160 [DOI] [PubMed] [Google Scholar]
- 54.Shibaki R, Akamatsu H, Fujimoto M, Koh Y, Yamamoto N.. Nivolumab induced radiation recall pneumonitis after two years of radiotherapy. Ann Oncol. 2017;28(6):1404-5. [DOI] [PubMed] [Google Scholar]
- 55.Nobashi TW, Nishimoto Y, Kawata Y, Yutani H, Nakamura M, Tsuji Y, et al. Clinical and radiological features of immune checkpoint inhibitor-related pneumonitis in lung cancer and non-lung cancers. Br J Radiol 2020; 93: 1115: 20200409. doi: 10.1259/bjr.20200409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Azria D, Magné N, Zouhair A, Castadot P, Culine S, Ychou M, et al. Radiation recall: a well recognized but neglected phenomenon. Cancer Treat Rev 2005; 31: 555–70. doi: 10.1016/j.ctrv.2005.07.008 [DOI] [PubMed] [Google Scholar]
- 57.Teng F, Li M, Yu J. Radiation recall pneumonitis induced by PD-1/PD-L1 blockades: mechanisms and therapeutic implications. BMC Med 2020; 18(1): 275. doi: 10.1186/s12916-020-01718-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pirker R, Filipits M. Adjuvant therapy in patients with completely resected non-small-cell lung cancer: current status and perspectives. Clin Lung Cancer 2019; 20: 1–6: S1525-7304(18)30261-4. doi: 10.1016/j.cllc.2018.09.016 [DOI] [PubMed] [Google Scholar]
- 59.Naidoo J, Nishino M, Patel SP, Shankar B, Rekhtman N, Illei P, et al. Immune-Related pneumonitis after chemoradiotherapy and subsequent immune checkpoint blockade in unresectable stage III non-small-cell lung cancer. Clin Lung Cancer 2020; 21: e435–44: S1525-7304(20)30076-0. doi: 10.1016/j.cllc.2020.02.025 [DOI] [PubMed] [Google Scholar]
- 60.Chaft JE, Dahlberg SE, Khullar OV, Edelman MJ, Simone CB, Heymach J, et al. EA5142 adjuvant nivolumab in resected lung cancers (anvil). JCO 2018; 36: TPS8581. doi: 10.1200/JCO.2018.36.15_suppl.TPS8581 [DOI] [Google Scholar]
- 61.Paz-Ares L, Hasan B, Dafni U, Menis J, De Maio E, Oselin K, et al. A randomized, phase 3 trial with anti-PD-1 monoclonal antibody pembrolizumab (MK-3475) versus placebo for patients with early stage NSCLC after resection and completion of standard adjuvant therapy (EORTC/ETOP 1416-PEARLS). Annals of Oncology 2017; 28: ii23. doi: 10.1093/annonc/mdx085.013 [DOI] [Google Scholar]
- 62.Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, et al. Treatment-Related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol 2019; 5: 1008–19. doi: 10.1001/jamaoncol.2019.0393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jung HA, Noh JM, Sun J-M, Lee S-H, Ahn JS, Ahn M-J, et al. Real world data of durvalumab consolidation after chemoradiotherapy in stage III non-small-cell lung cancer. Lung Cancer 2020; 146: 23–29: S0169-5002(20)30469-4. doi: 10.1016/j.lungcan.2020.05.035 [DOI] [PubMed] [Google Scholar]
- 64.Vansteenkiste J, Naidoo J, Faivre-Finn C, Özgüroğlu M, Villegas A, Daniel D, et al. MA05.02 Pacific subgroup analysis: pneumonitis in stage III, unresectable NSCLC patients treated with durvalumab vs. placebo after crt. Journal of Thoracic Oncology 2018; 13: S370–71. doi: 10.1016/j.jtho.2018.08.350 [DOI] [Google Scholar]
- 65.Shaverdian N, Thor M, Shepherd AF, Offin MD, Jackson A, Wu AJ, et al. Radiation pneumonitis in lung cancer patients treated with chemoradiation plus durvalumab. Cancer Med 2020; 9: 4622–31. doi: 10.1002/cam4.3113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tonk EHJ, van Lindert ASR, Verhoeff JJC, Suijkerbuijk KPM. Acute-Onset pneumonitis while administering the first dose of durvalumab. Case Rep Oncol 2019; 12: 621–24. doi: 10.1159/000502202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Durbin SM, Mooradian MJ, Fintelmann FJ, Zubiri L, Chute DF, Kambadakone A, et al. Diagnostic utility of CT for suspected immune checkpoint inhibitor enterocolitis. J Immunother Cancer 2020; 8(2): e001329. doi: 10.1136/jitc-2020-001329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim KW, Ramaiya NH, Krajewski KM, Jagannathan JP, Tirumani SH, Srivastava A, et al. Ipilimumab associated hepatitis: imaging and clinicopathologic findings. Invest New Drugs 2013; 31: 1071–77. doi: 10.1007/s10637-013-9939-6 [DOI] [PubMed] [Google Scholar]
- 69.Calandri M, Solitro F, Angelino V, Moretti F, Veltri A. The role of radiology in the evaluation of the immunotherapy efficacy. J Thorac Dis 2018; 10: S1438–46. doi: 10.21037/jtd.2018.05.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang YZ, Szabados B, Leung C, Sahdev A. Adverse effects and radiological manifestations of new immunotherapy agents. BJR 2019; 92: 1093. doi: 10.1259/bjr.20180164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mazzaschi G, Bordi P, Fioretzaki R, Gnetti L, Milanese G, Tommasi C, et al. Nivolumab-induced Guillain-Barré syndrome coupled with remarkable disease response in a case of heavily pretreated lung adenocarcinoma. Clin Lung Cancer 2020; 21: e65–73: S1525-7304(19)30318-3. doi: 10.1016/j.cllc.2019.11.001 [DOI] [PubMed] [Google Scholar]
- 72.Cuzzubbo S, Javeri F, Tissier M, Roumi A, Barlog C, Doridam J, et al. Neurological adverse events associated with immune checkpoint inhibitors: review of the literature. Eur J Cancer 2017; 73: 1–8: S0959-8049(16)33068-4. doi: 10.1016/j.ejca.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 73.Garon-Czmil J, Petitpain N, Rouby F, Sassier M, Babai S, Yéléhé-Okouma M, et al. Immune check point inhibitors-induced hypophysitis: a retrospective analysis of the French pharmacovigilance database. Sci Rep 2019; 9(1): 19419. doi: 10.1038/s41598-019-56026-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol 2018; 4: 173–82. doi: 10.1001/jamaoncol.2017.3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Costa LB, Queiroz MA, Barbosa FG, Nunes RF, Zaniboni EC, Ruiz MM, et al. Reassessing patterns of response to immunotherapy with PET: from morphology to metabolism. Radiographics 2021; 41: 120–43. doi: 10.1148/rg.2021200093 [DOI] [PubMed] [Google Scholar]
- 76.Palaskas N, Lopez-Mattei J, Durand JB, Iliescu C, Deswal A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc 2020; 9(2): e013757. doi: 10.1161/JAHA.119.013757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Altan M, Toki MI, Gettinger SN, Carvajal-Hausdorf DE, Zugazagoitia J, Sinard JH, et al. Immune checkpoint inhibitor-associated pericarditis. J Thorac Oncol 2019; 14: 1102–8: S1556-0864(19)30196-0. doi: 10.1016/j.jtho.2019.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Amrane K, Le Goupil D, Quere G, Delcroix O, Gouva S, Schick U, et al. Prediction of response to immune checkpoint inhibitor therapy using 18F-FDG PET/CT in patients with melanoma. Medicine (Baltimore) 2019; 98: 29: e16417. doi: 10.1097/MD.0000000000016417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seith F, Forschner A, Schmidt H, Pfannenberg C, Gückel B, Nikolaou K, et al. 18F-Fdg-Pet detects complete response to PD1-therapy in melanoma patients two weeks after therapy start. Eur J Nucl Med Mol Imaging 2018; 45: 95–101. doi: 10.1007/s00259-017-3813-2 [DOI] [PubMed] [Google Scholar]
- 80.Galldiks N, Abdulla DSY, Scheffler M, Wolpert F, Werner J-M, Hüllner M, et al. Treatment monitoring of immunotherapy and targeted therapy using 18F-FET PET in patients with melanoma and lung cancer brain metastases: initial experiences. J Nucl Med 2021; 62: 464–70. doi: 10.2967/jnumed.120.248278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cho SY, Lipson EJ, Im HJ, Rowe SP, Gonzalez EM, Blackford A, et al. Prediction of Response to Immune Checkpoint Inhibitor Therapy Using Early-Time-Point (18)F-FDG PET/CT Imaging in Patients with Advanced Melanoma. J Nucl Med. 2017;58(9):1421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu X, Huang Y, Zhao Q, Wang L, Song X, Li Y, et al. PD-L1 expression correlation with metabolic parameters of FDG PET/CT and clinicopathological characteristics in non-small cell lung cancer. EJNMMI Res 2020; 10(1): 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takada K, Toyokawa G, Tagawa T, Kohashi K, Akamine T, Takamori S, et al. Association Between PD-L1 Expression and Metabolic Activity on (18)F-FDG PET/CT in Patients with Small-sized Lung Cancer. Anticancer Res. 2017;37(12):7073-82. [DOI] [PubMed] [Google Scholar]
- 84.Kaira K, Kuji I, Kagamu H. Value of (18)F-FDG-PET to predict PD-L1 expression and outcomes of PD-1 inhibition therapy in human cancers. Cancer Imaging 2021; 21(1): 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tao X, Li N, Wu N, He J, Ying J, Gao S, et al. The efficiency of (18)F-FDG PET-CT for predicting the major pathologic response to the neoadjuvant PD-1 blockade in resectable non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2020;47(5):1209-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bensch F, Veen EL, Lub-de Hooge MN, Jorritsma-Smit A, Boellaard R, Kok IC, et al. 89)zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med 2018; 24: 1852–58. [DOI] [PubMed] [Google Scholar]
- 87.Flavell RR, Evans MJ, Villanueva-Meyer JE, Yom SS.. Understanding Response to Immunotherapy Using Standard of Care and Experimental Imaging Approaches. Int J Radiat Oncol Biol Phys. 2020;108(1):242-57. [DOI] [PubMed] [Google Scholar]
- 88.Yoon JK, Park BN, Ryu EK, An YS, Lee SJ. Current perspectives on 89zr-PET imaging. IJMS 2020; 21: 4309. doi: 10.3390/ijms21124309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Silva M, Milanese G, Seletti V, Ariani A, Sverzellati N. Pulmonary quantitative CT imaging in focal and diffuse disease: current research and clinical applications. BJR 2018; 91: 20170644. doi: 10.1259/bjr.20170644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 2017; 14: 749–62. doi: 10.1038/nrclinonc.2017.141 [DOI] [PubMed] [Google Scholar]
- 91.Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology 2016; 278: 563–77. doi: 10.1148/radiol.2015151169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trebeschi S, Drago SG, Birkbak NJ, Kurilova I, Cǎlin AM, Delli Pizzi A, et al. Predicting response to cancer immunotherapy using noninvasive radiomic biomarkers. Ann Oncol 2019; 30: 998–1004: S0923-7534(19)31202-5. doi: 10.1093/annonc/mdz108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vaidya P, Bera K, Patil PD, Gupta A, Jain P, Alilou M, et al. Novel, non-invasive imaging approach to identify patients with advanced non-small cell lung cancer at risk of hyperprogressive disease with immune checkpoint blockade. J Immunother Cancer 2020; 8(2): e001343. doi: 10.1136/jitc-2020-001343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tunali I, Gray JE, Qi J, Abdalah M, Jeong DK, Guvenis A, et al. Novel clinical and radiomic predictors of rapid disease progression phenotypes among lung cancer patients treated with immunotherapy: an early report. Lung Cancer 2019; 129: 75–79: S0169-5002(19)30296-X. doi: 10.1016/j.lungcan.2019.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun R, Limkin EJ, Vakalopoulou M, Dercle L, Champiat S, Han SR, et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol. 2018;19(9):1180-91. [DOI] [PubMed] [Google Scholar]
- 96.Mazzaschi G, Milanese G, Pagano P, Madeddu D, Gnetti L, Trentini F, et al. Integrated CT imaging and tissue immune features disclose a radio-immune signature with high prognostic impact on surgically resected NSCLC. Lung Cancer. 2020;144:30-9. [DOI] [PubMed] [Google Scholar]
- 97.Khorrami M, Prasanna P, Gupta A, Patil P, Velu PD, Thawani R, et al. Changes in CT Radiomic Features Associated with Lymphocyte Distribution Predict Overall Survival and Response to Immunotherapy in Non-Small Cell Lung Cancer. Cancer Immunol Res. 2020;8(1):108-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fournier L, Costaridou L, Bidaut L, Michoux N, Lecouvet FE, de Geus-Oei L-F, et al. Correction to: incorporating radiomics into clinical trials: expert consensus endorsed by the European Society of radiology on considerations for data-driven compared to biologically driven quantitative biomarkers. Eur Radiol 2021; 31: 6408–9. doi: 10.1007/s00330-021-07721-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Castello A, Castellani M, Florimonte L, Urso L, Mansi L, Lopci E.. The Role of Radiomics in the Era of Immune Checkpoint Inhibitors: A New Protagonist in the Jungle of Response Criteria. J Clin Med. 2022;11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]