Abstract
Objective
The aim of this study is to demonstrate the role of proton magnetic resonance spectroscopy (1H-MRS) in the detection of brain microstructural changes in patients with Crigler-Najjar syndrome type-I (CNs-I), and its correlation with demographic, neurodevelopmental and laboratory findings.
Methods
Prospective study was conducted on 25 children with CNs-I and 25 age and sex-matched children, who served as control. They underwent multivoxel 1H-MRS of basal ganglion at echo time 135–144 ms. N-acetyl aspartate/Creatine (NAA/Cr) and Choline (Ch)/Cr were calculated and correlated with demographic, clinical, and laboratory findings of patients with CNs-I.
Results
There was a significant difference in NAA/Cr and Ch/Cr between patients and controls. The cut-off value for NAA/Cr and Ch/Cr used to differentiate patients from controls were 1.8 and 1.2 with an area under the curve (AUC) of 0.91 and 0.84 respectively. There was a significant difference in MRS ratios between patients with neurodevelopmental delay (NDD) and patients without NDD. The cut-off values for NAA/Cr and Ch/Cr used to differentiate between patients with NDD and patients without NDD were 1.47 and 0.99, with AUC of 0.87 and 0.8 respectively. The NAA/Cr and Ch/Cr were well correlated with family history (p = 0.006 and p < 0.001) respectively, consanguinity (p < 0.001 and p = 0.001), neurodevelopmental delay (p = 0.001 and p = 0.004), serum bilirubin level (r = −0.77, p < 0.001), (r = −0.49, p = 0.014), phototherapy (p < 0.001 and p = 0.32), blood transfusion (p < 0.001 and p = 0.001) respectively.
Conclusion
1H-MRS can be a useful tool in the detection of neurological changes in patients with CNs-I; NAA/Cr and Ch/Cr parameters are well correlated with demographic, clinical, and laboratory findings.
Advances in knowledge
Our study is the first report on using MRS in assessing neurological manifestations in CNs. 1H-MRS can be a useful tool in the detection of neurological changes in patients with CNs-I.
Introduction
Crigler-Najjar syndrome (CNs) is a rare autosomal recessive disorder characterized by the absence or decreased activity of UDP-glucuronosyltransferase, an enzyme required for glucuronidation of unconjugated bilirubin in the liver. The disease severity depends upon the number of enzymes produced required for the glucuronidation of bilirubin. CNs has two types; type I which is the most severe form with a complete absence of UDP-glucuronosyltransferase enzyme activity, and type II is less severe with a decreased level of enzyme activity. Central nervous system affection by kernicterus is mainly detected in CNs type I. Patients are presented mainly with jaundice due to the inability to conjugate bilirubin. The increased concentration of unconjugated bilirubin is the main cause of clinical manifestations. Newborns present with hyperbilirubinemia, and other neurological manifestations progressively develop later. Unconjugated hyperbilirubinemia leads to devastating encephalopathy due to bilirubin deposition in the cranial nerves, hippocampus, subthalamic nuclei, and basal ganglia (mainly globus pallidus). 1–4
Patients also may have a severe neurological impairment which can progress to cerebral palsy. Treatment of all patients with CNs-I, and some patients with CNs-II, is by using intensive phototherapy; daily intermittent phototherapy (about 6–12 h per day) is the mainstay for treatment. This can improve early neurological manifestations of bilirubin toxicity if treatment is initiated immediately. Orthotopic liver transplantation and gene therapy are the treatments of choice used to replace the missing enzyme. 4–10
MR imaging
Conventional MRI can detect bilirubin-induced encephalopathy; it shows signal abnormalities in the basal ganglionic regions that differ according to the stage of affection whether acute or chronic. In acute encephalopathy, an abnormal high signal could be seen mainly affecting the globus pallidus and subthalamic nucleus on T 1WI, while T2-signal in these regions is often unremarkable or shows subtle hyperintensity. In chronic encephalopathy, there is an abnormally high T2 signal at the sites of involvement. Signal changes that occur during the normal myelination process, as well as signal variability during the disease, affects the sensitivity and specificity of conventional MRI, even MRI can appear completely normal in the presence of bilirubin encephalopathy. 11–20
MRS
Proton magnetic resonance spectroscopy (1H-MRS) is an advanced, non-invasive MR technique that has great potential in the diagnosis and follow-up of patients having bilirubin encephalopathy through monitoring of brain metabolism. It can detect signals from protons of small molecules in the cerebral tissue. The major peaks of 1H-MRS spectrum are N-acetyl aspartate (NAA), Creatine (Cr), and Choline (Ch). 21–27
NAA and choline peaks are better delineated in the intermediate MRS study (echo time (TE) 135–144 ms). NAA shows the largest peak of the spectra; it is located primarily in neuronal tissues, so can be used as a marker for neuronal density and integrity. It is markedly reduced or even absent in any disease leading to axonal loss. Cr signal is a marker for intracellular energy and its peak is used as an internal reference standard for characterizing other peaks of brain tissues. Ch peak in MRS spectra reflects increased cell membrane synthesis and thus increased cellularity. 28
Decreased NAA/Cr and Ch/Cr ratios in the basal ganglia of patients with bilirubin encephalopathy indicate neuronal and axonal loss or dysfunction together with abnormal gliosis. 25
To the best of our knowledge, this study is the first to use 1H-MRS in the detection and assessment of neurological manifestations in CNs. Specific novel findings on MRS might be a useful tool in the detection of neurological changes in patients with CNs-I. Early detection and proper management of CNs-I patients may decrease the risk of developing bilirubin encephalopathy and neurological complications.
In our study, we aim to demonstrate the added role of 1H-MRS as a non-invasive MR imaging technique in monitoring brain metabolism in patients with CNs-I and correlating it with the demographic, clinical, and laboratory findings.
Methods
Patients
This is a prospective multicenter study; three centers were involved. The study was carried out in the period from April 2018 to August 2020. Ethics committee approval was obtained, and informed consent was signed from the legal guardians of children and controls before they participated in the study. The study included 25 children with CNs-I, and 25 children served as controls. The control group was coming for an MRI examination due to other causes unrelated to CNs (e.g. headache, blurring of vision). Patients’ inclusion criteria included clinically and laboratory-confirmed patients with CNs-I; clinical and laboratory diagnosis was based on the presence of severe unconjugated hyperbilirubinemia developing within the first three days of life and progress in an unremitting fashion with normal liver function, absence of hemolysis, and absence of manifestation of any chronic liver disease. Genetic confirmation was not available. Personal data (age, gender, family history, and consanguinity), medical history including neonatal intensive care (NICU) admission, phototherapy utilization, blood transfusion, and neurodevelopmental delay were noted. Exclusion criteria included the presence of cognitive and neurological deficits or psychiatric illness or other major medical disorders in the control group. Patients with other causes of indirect hyperbilirubinemia or with direct hyperbilirubinemia and patients who refused to participate in the study were also excluded. Routine MRI examination with proton MRS was done for all patients and controls.
Clinical assessment
Full general, physical, and neurological examinations were done by skilled pediatric neurologists. The assessment was done for extrapyramidal symptoms and minor signs such as neuromuscular incoordination, abnormities of muscle tone, and reflexes and motor power according to Touwen’s infant neurological examination 29 and Touwen’s test modified by Hadders-Algra et al. 30
Laboratory assessment
Serial serum bilirubin levels were done to all patients which indicated the disease severity.
Conventional MR
Routine MRI was performed using an MR scanner 1.5-Tesla machine (Ingenia, Philips, Amsterdam, the Netherlands), with a standard circularly polarized head coil. Chloral hydrate sedation was used in young unco-operative children (n = 9). The conventional imaging protocol consisted of axial T 1-, T 2 weighted imaging, and fluid-attenuated inversion recovery (FLAIR) images. The imaging parameters were repetition time (TR)/TE = 500/14 ms for T 1WI and 5000/86 ms for T 2WI, field of view (FOV) = 240×240 mm, slice thickness = 5 mm, and interslice gap = 0.4 mm. Diffusion-weighted images (DWIs) were obtained using a multislice, spin echo, echoplanar image sequence. A set of multiple axial scans were obtained. The diffusion gradients were applied in three orthogonal directions (x, y, and z). Diffusion images were acquired with a diffuse b factor of 0, 600 and 1000 mm2/s, and apparent diffusion coefficient maps were reconstructed.
MRS protocol
Multivoxel MRS technique data were collected using PRESS with chemical–shift-selective water suppression. An automated shimming procedure for the water was performed aiming at a homogenous magnetic field. Automatic adjustment of the shimming and water suppression was done. Baseline correction and curve fitting were performed afterward. Spectral parameters were as follow: using intermediate TE = 144 and TR = 1500 ms: band with = 1000 Hz: average = 4; slice thickness = 10 mm; matrix size = 16×6; and FOV = 160×160 mm. The spectroscopic region of interest is the basal ganglionic region (mainly the Globus pallidus). The spatial resolution of the MRS images obtained was 1×1×1 cmᶟ. The peaks fitted included 3.22 ppm for Ch, 3.03 ppm for Cr, and 2.00 ppm for NAA. By using standardized post-processing protocols, the raw data are processed automatically, allowing for operator-independent quantifications. To minimize the amount of arbitrary operator input, no use was made of the possibility of retrospective voxel shifting. The integrals of Ch and Cr were calculated. The ratios of integrals of various metabolites calculated concerning Cr included NAA/Cr and Ch/Cr.
Image analysis
Conventional MR images (T 1WI, T 2WI, FLAIR, and DWI) were examined in all cases. 1H-MRS images were evaluated in consensus by two expert neuroradiologists (TS, AE), with 20 and 12 years’ experience, respectively. They were blinded to the clinical and laboratory findings. Assessment of the resonance of NAA peak, Cr and Ch peaks was done as well as the metabolite ratios of NAA/Cr and Ch/Cr were calculated.
Statistical analysis
Data were statistically described in terms of mean ± standard deviation (± SD), median and range, or frequencies and percentages when appropriate. For comparing numerical variables, the Student’s t-test was used for independent samples when normally distributed and Mann–Whitney U test when not normally distributed. For comparing categorical data, χ2 test was done. The exact test was used instead when the expected frequency; <5. Correlation between variables was done using the Pearson moment correlation equation for linear relation in normally distributed variables and the Spearman rank correlation equation for non-normal. Probability (p) values of <0.05 were considered statistically significant. All statistical calculations were done using the computer program SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL) v. 15 for Microsoft Windows.
Categorial variables included sex, family history, consanguinity, NDD, therapy type and NICU admission.
Quantitative variables included age, serum bilirubin, NAA/Cr, and Ch/Cr.
ROC curve analysis was done for patients with NDD and patients without NDD using NAA/Cr and Ch/Cr. ROC curve analysis was used to assess the diagnostic performance of NAA/Cr and Ch/Cr in differentiating symptomatic from asymptomatic cases, sensitivity and specificity were detected from the curve by selecting the best cut-off point, and positive-predictive value, negative-predictive value, and accuracy were calculated through cross-tabulation after transformation of the continuous variable into nominal variable according to selected cut-off point, the area under the curve (AUC) is considered good if >0.7.
Results
Demographic and clinical characteristics
Table 1 shows the demographic, clinical, and laboratory data of patients and controls. Our study included 25 patients with CNs-I, 13 males and 12 females, with ages ranging from 0.3 to 13 years, with a mean ± SD of 4.5 ± 3.7. The control group included 25 children who had MRI and MRS studies performed for other non-related causes. They were 16 males and 9 females. Their age ranged from 0.8 to 10 years with a mean ± SD of 5.6 ± 2.8. Significant statistical results were found regarding the patient’s family history and consanguinity; positive family history of the same disease condition was detected in 14 cases (56%) among the patient group, p = 0.001. Positive family history was correlated with NAA/Cr of 1.41 ± 0.27 and with Ch/Cr of 0.84 ± 0.28, p = 0.006 and p < 0.001. Consanguinity was present in 19 cases (76%) among the patients, p = 0.012. Third-degree consanguinity was detected in 11 cases, and fourth-degree consanguinity was detected in 8 cases. Consanguinity was well correlated with NAA /Cr of 1.36 and Ch/Cr of 0.95, p < 0.001 and p = 0.001 respectively.
Table 1.
Demographics, clinical and laboratory data of patients vs controls
| Parameter | Controls(n = 25) | Patients(n = 25) | P value |
|---|---|---|---|
| Age | 5.63 ± 2.80 6 (0.8–10) |
4.58 ± 3.71 3.0(0.3–13.0) |
Z = 0.846 p = 0.398 |
| Sex Male Female |
16 (64.0%) 9 (36.0%) |
13 (52.0%) 12 (48.0%) |
χ2 = 1.138 p = 0.286 |
| Family history No Yes |
12 (100%) 0 |
11 (44.0%) 14 (56.0%) |
χ2 = 10.81 p = 0.001* |
| Consanguinity No Yes |
8 (66.7%) 4 (33.3%) |
6 (24.0%) 19 (76.0%) |
χ2 = 6.28 p = 0.012* |
| NDD No Yes |
Not applicable | 15 (60%) 10 (40.0%) |
χ2 = 6.58 p = 0.01* |
| Serum Bilirubin | 0.8 ± 0.2 | 25.12 ± 5.11 | |
| Therapy Photo therapy Bl transfusion |
0 0 |
20 (80.0%) 8 (32.0%) |
χ2 = 20.89, p < 0.001* χ2 = 4.89, p = 0.027* |
| NICU admission | 0 | 25 (100%) 0 (0.0) |
NDD, neurodevelopmental delay; NICU, neonatal intensive care unit.
Correlation between MRS markers and other variables
Table 2 shows the correlation of NAA/Cr and Ch/Cr of patients with CNs-I with demographic, clinical, and laboratory findings.
Table 2.
Correlation of NAA/Cr and Ch/Cr of patients with demographic, clinical, laboratory findings vs controls and patients with NDD vs without NDD
| NAA/Cr | Test of significance | Choline/Cr | Test of significance | |
|---|---|---|---|---|
| age | r = 0.041 | p = 0.811 | r = −0.144 | p = 0.396 |
| Sex Male Female |
1.75 ± 0.38 1.73±0.44 |
t = 0.164 p = 0.871 |
1.54 ± 1.93 1.16±.32 |
t = 0.867 p = 0.392 |
| Family history No Yes |
1.80 ± 0.45 1.41±0.27 |
t = 2.94 p = 0.006* |
1.26 ± 0.157 0.849±0.28 | t = 5.72 P<0.001* |
| Consanguinity No Yes |
2.12 ± 0.187 1.367±0.257 | t = 9.53 P<0.001* |
1.36 ± 0.159 0.95±0.242 | t = 5.55 p = 0.001* |
| Therapy Photo therapy -ve +ve |
1.998 ± 0.238 1.415±0.38 | t = 5.25 P<0.001* |
1.228 ± 0.262 1.02±0.286 |
t = 2.24 p = 0.032* |
| Bl transfusion -ve +ve |
1.947 ± 0.307 1.265±0.222 | t = 7.48 p<0.001* |
1.241 ± 0.253 0.927±0.242 | t = 3.79 p= 0.001* |
| NDD -ve +ve |
1.79 ± 0.43 1.28±0.16 |
t = 3.64 p = 0.001* |
1.185 ± 0.274 0.889±0.225 | t = 3.04 p = 0.004* |
| Laboratory (bilirubin) | r = −0.776 | p < 0.001* | r = −0.496 | p = 0.014* |
Ch, Choline; Cr, Creatine; NAA, N-acetyl aspartate; NDD, neurodevelopmental delay.
Neurological problems with NDD were reported in 10 patients (40%), p = 0.01. These included problems in muscle tone, reflexes, co-ordination, and involuntary movements. Four patients had dystonic cerebral palsy with hearing loss, two patients had choreoathetosis, two patients had epilepsy and two patients had abnormal eye movement with impairment of upward gaze. There was a statistically significant correlation between the presence of NDD and NAA/Cr of 1.28 ± 0.16, and Ch/Cr of 0.88 ± 0.22, (p = 0.001 and 0.004) respectively. The mean bilirubin level in patients was 25.1 ± 5.1. A statistically significant positive correlation was detected between NDD and the highest serum bilirubin level to which the patient was exposed. There was also a statistically significant negative correlation between the highest serum bilirubin level and NAA/Cr and Ch/Cr, (r = −0.77, p < 0.001 and r = −0.49, p = 0.014) respectively. Among the patients' group 20 cases (80%) were on phototherapy, p < 0.001. The measured MRS ratios (NAA/Cr = 1.4 and Ch/Cr = 1.02), of those patients, were positively correlated with the history of irregular phototherapy (p < 0.001 and p = 0.03 respectively). Eight patients (32%) had a history of blood transfusion, which was correlated with NAA/Cr = 1.2 and Cho/Cr = 0.92, p < 0.001 and p = 0.001 respectively. In our study, all patients were admitted previously to NICU.
ROC curve analysis
Table 3 shows the mean and SD of NAA/Cr and Ch/Cr ratio of patients versus controls and patients with NDD vs without NDD.
Table 3.
Mean, SD and p-value of NAA/Cr, Ch/Cr in patients vs controls, and patients with NDD vs without NDD
| Patients | Controls | P-value | |
|---|---|---|---|
|
Patients vs controls
NAA/Cr Ch/Cr |
1.45 ± 0.38 0.999±0.28 |
2.07 ± 0.17 1.33±0.13 |
t = 5.46, p <0.001* t = 3.70, p = 0.001 |
|
NDD vs without NDD
NAA/Cr Ch/Cr |
NDDn = 10 | Without NDD n = 15 | |
| 1.28 ± 0.16 0.889±0.25 |
1.78 ± 0.43 1.18±0.27 |
t = 3.65, p = 0.001 t = 3.04, p = 0.004 |
Ch, Choline; Cr, Creatine; NAA, N-acetyl aspartate; NDD, neurodevelopmental delay.
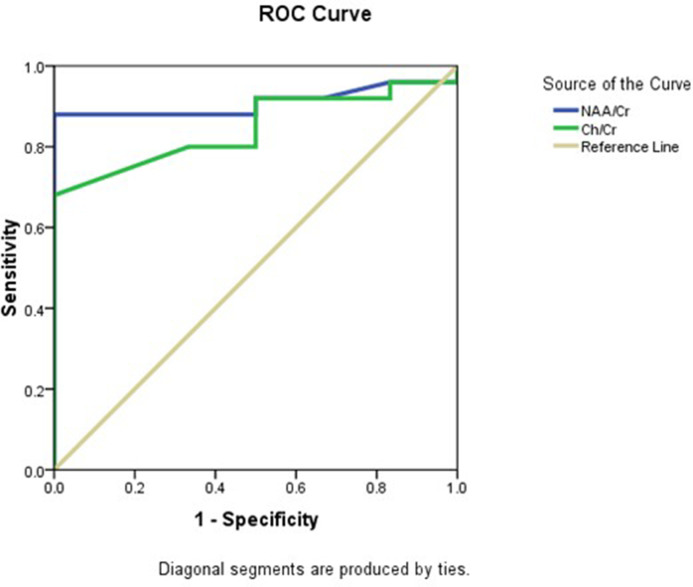
Conventional MR image analysis in all patients revealed a normal appearance of the brain with no detected significant areas of abnormal signal. At MRS examinations, despite having no significant abnormality in conventional MRI study, there was a statistically significant difference in NAA/Cr and Ch/Cr between patients and controls. NAA/Cr and Ch/Cr in patients were 1.45 ± 0.38 and 0.99 ± 0.28, while in the control group ratios were 2.07 ± 0.17 and 1.33 ± 0.13, p < 0.001, and p = 0.001 respectively. ROC curve analysis for differentiating patients from controls using NAA/Cr revealed that the AUC was 0.91, the cut-off point was 1.89 below it was considered abnormal, with 88% sensitivity, 83.3% specificity, and 86.5% accuracy. For Ch/Cr AUC was 0.84, and the cut-off point was 1.21, with 80% sensitivity, 66.7% specificity, and 75.5% accuracy (Figure 1 ).
Figure 1.
Roc curve of NAA/Cr and Ch/Cr in differentiating patients from control. Ch, Choline; Cr, Creatine; NAA, N-acetyl aspartate; ROC, receiver operating characteristic.
Also, a statistically significant difference was detected in NAA/Cr and Ch/Cr between patients with NDD and patients without NDD. NAA/Cr and Ch/Cr in patients with NDD were 1.28 ± 0.16 and 0.889 ± 0.25, while in patients without NDD were 1.78 ± 0.43 and 1.18 ± 0.27, p = 0.001, and p = 0.004 respectively.
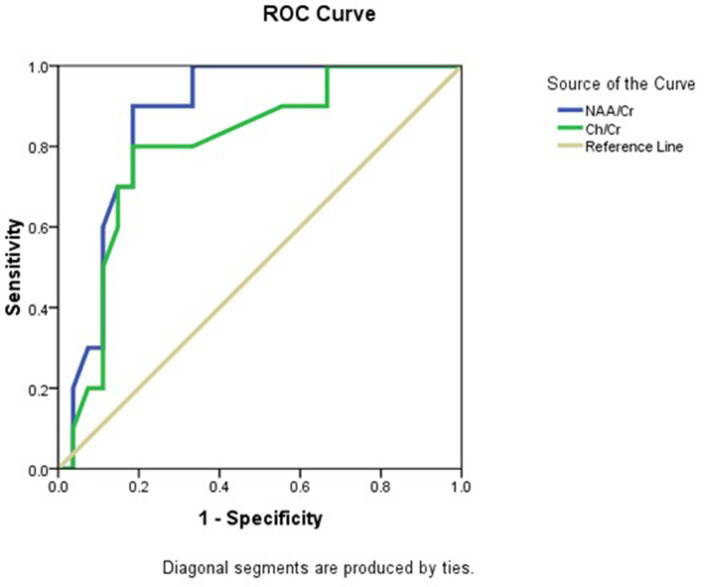
ROC curve analysis in patients with NDD and patients without NDD using NAA/Cr revealed that the AUC was 0.87, the cut-off point was 1.47 below it was considered to have NDD, with 90% sensitivity, 81.5% specificity, and 83.8% accuracy. For Ch/Cr, AUC was 0.8, and the cut-off point was 0.99, with 80% sensitivity, 81.5% specificity, and 81.1% accuracy (Figure 2 ).
Figure 2.
Roc curve of NAA/Cr and Ch/Cr in differentiating patients with NDD from patients without NDD. Ch, Choline; Cr, Creatine; NAA, N-acetyl aspartate; NDD, neurodevelopmental delay; ROC, receiver operating characteristic.
Discussion
In our study, conventional MR images examined in all patients revealed a normal appearance of the brain with no detected areas of abnormal signal, although all patients clinically had chronic encephalopathy. This might be explained by the variability and transient nature of the signal abnormalities, abnormal T1-signal may be confounding with normal T1 myelination signal. This was in agreement with previous studies that reported that abnormal high-intensity areas might not be observed after the age of one year, and are sometimes subtle and easily overlooked. 10,26
1H-MRS is considered a valuable non-invasive tool to monitor in vivo cerebral biochemistry. Three of the most important metabolites that can be studied in the brain are NAA, Ch, and Cr. The ratios of NAA/Cr and Ch/Cr are considered markers of the functional status of the neurons and axons of the brain tissue. Decreased ratios indicate neuronal or axonal loss or dysfunction. 25
The main findings in this study are that 1H-MRS ratios (NAA/Cr and Ch/Cr) can differentiate patients with CNs-I having NDD from those without NDD and normal controls, despite both having normal conventional brain MRI studies. Patients with NDD show lower NAA/Cr and Ch/Cr compared to patients without NDD. Despite being generally non-specific, with low NAA /Cr ratios indicating cell damage which could be due to ischemia or metabolic disease, the 1H-MRS ratios are well correlated with positive family history, consanguinity, neurodevelopmental delay, highest serum bilirubin level, phototherapy, and with history of blood transfusion.
1H-MRS can delineate much about brain energy metabolism in neonates with bilirubin encephalopathy. Synthesis of NAA is related to oxidative metabolism, so assessment of NAA concentrations reflects the status of mitochondrial metabolism in neonates with bilirubin encephalopathy and demonstrates energy failure and anaerobic metabolism in cases of perinatal brain injury. MRS also detects the signal of small molecules present in cerebral tissue (e.g. glutamate, and lactate). 10
NAA is a neuronal marker; decreased NAA indicates neuronal dysfunction that may be reversible. Ch is a cell membrane and myelin marker. Cr, a marker of energy metabolism, is mainly used as an internal reference for assessing the other two metabolites. 22 In all pathologies causing axonal loss, NAA is markedly reduced or even absent. 23
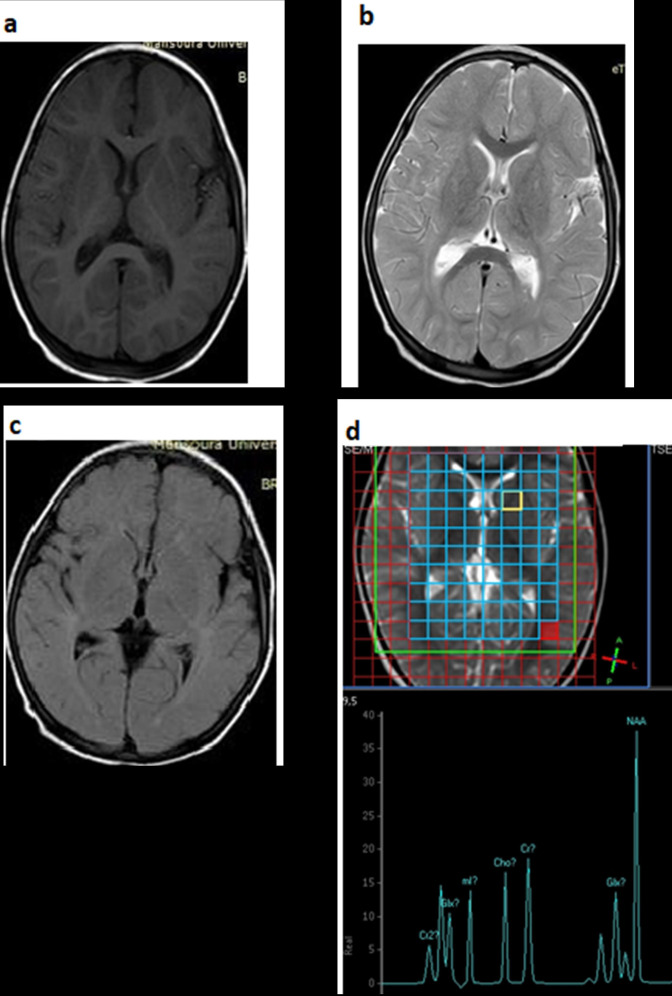
In our study, NAA/Cr ratio was significantly lower in CNs-I patients than that of the controls. A low NAA/Cr ratio denotes reduced neuronal density (Figure 3). Similarly, a study, conducted on an 18-month-old child with CNs-I, found that MRS results of the right basal ganglia showed that NAA/Cr was low in the patient with CNs-I and that NAA/Cr ratio increased in one-way after liver transplantation, which indicates that MRS may be an option of predicting brain conditions and selecting suitable patients with CNs-I for transplantation. 24 .
Figure 3.
Conventional brain MRI (a: axial T 1WI, b: axial T 2WI and c: axial FLAIR), and (d) multivoxel MRS in a patient with NDD presented with lower limbs weakness, delayed speech, and a history of attacks of loss of consciousness. The conventional images are completely normal with no apparent areas of signal abnormality, in MRS, NAA/Cr ratio is 1.49 and Ch/Cr ratio 0.79. Ch, Choline; Cr, Creatine; FLAIR, fluid-attenuated inversion-recovery; NAA, N-acetyl aspartate; NDD, neurodevelopmental delay; MRS, magnetic resonance spectroscopy; T 1WI, T 1 weighted imaging; T 2WI, T 2 weighted imaging.
The effect of metabolic changes occurring in the brain in cases of kernicterus and hepatic encephalopathy may have some similarities as they affect the same regions of the brain. In our study, Ch/Cr in CNs-I patients was significantly lower than that of the controls. A previous study reported that mild reduction in Ch/Cr ratio was detected in cases with minimal hepatic encephalopathy. In this stage, patients do not have strong clinical symptoms but have mild psychomotor and cognitive changes. 25 . Also, a previous study reported that brain proton MRS detected the development of neuropsychological changes that preceded hepatic encephalopathy. 26
In our study, NAA/Cr is well correlated with positive family history and with consanguinity and history of siblings among CNs-I parents. CNs-I is inherited in an autosomal recessive pattern which means both copies of the UGT1A1 gene in each cell have mutations. Therefore, parental consanguinity increases the risk of CNs-I. 31 . Similarly, a study done on the management of pregnancy in CNs-I found that consanguineous marriage is a risk factor for CNs. 32 In our study, the history of similar siblings affected was reported in 76% of patients. This is related to the higher rate of consanguineous marriages among CNs-I patients’ families. With each pregnancy, the risk for two carrier parents to have an affected infant is 25%, while the risk to have a carrier infant is 50%, and the chance for an infant to receive normal genes is 25%. The risk is the same for both males and females 33 ; in our study, both males and females were nearly equally affected.
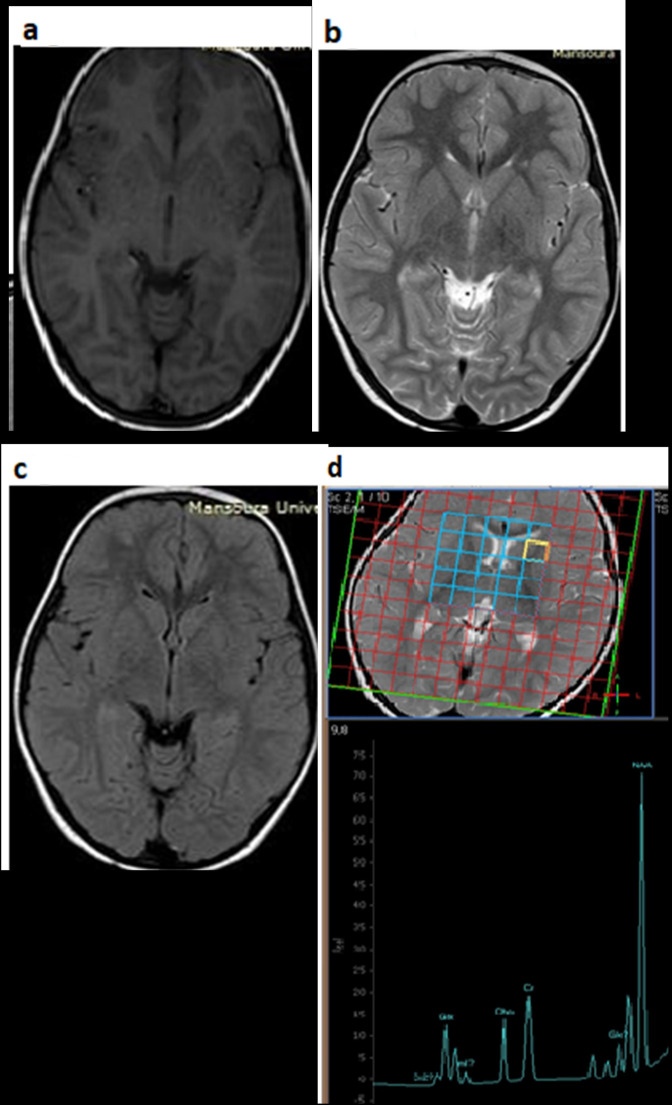
In this study, there was a significant decrease in NAA/Cr and Ch/Cr in patients with NDD which were reported in about 40% of our patients. Lodoso Torrecilla et al reported that patients with CNs are at greater risk of developing kernicterus, mostly associated with indirect bilirubin levels of around 25 mg dl−1. 34 The remaining patients (60%) in our study did not have neurological problems, which was probably related to early detection and meticulous management of our patients. However, CNs-I patients remain at an increased risk, probably throughout their life span, which necessitates treatment. Most of our patients received treatment regularly to decrease the risk of kernicterus and/or developmental problems (Figure 4 ).
Figure 4.
Conventional brain MRI in another patient, (a: axial T 1WI, b: axial T 2WI, and c: axial FLAIR), and (d) multivoxel MRS, the patient had no clinical manifestations of NDD. The conventional images are completely normal with no apparent areas of signal abnormality, in MRS, NAA/Cr ratio is 1.78 and Ch/Cr ratio is 0.91. Ch, Choline; Cr, Creatine; FLAIR, fluid-attenuated inversion-recovery; NAA, N-acetyl aspartate; NDD, neurodevelopmental delay; MRS, magnetic resonance spectroscopy; T 1WI, T 1 weighted imaging; T 2WI, T 2 weighted imaging
We found a good correlation between the presence of NDD and the decrease in NAA/Cr and Ch/Cr. Similarly, a study found that in children with developmental delay who were older than 2 years, decreased NAA/Cr ratio was detected in frontal (p < 0.001) and parieto-occipital (p < 0.017) subcortical white matter and elevated Ch/Cr ratio were detected in the frontal (p < 0.24) and parieto-occipital (p < 0.002) subcortical white matter compared with age-matched control subjects. 27
On the other hand, the same study found that in children with developmental delay who were aged 2 years or younger, no statistically significant differences were detected in the NAA/Cr or Ch/Cr ratios compared with those of the controls. 27 NAA/Cr and Ch/Cr were correlated to neurological manifestations which were more prominent in patients with lower NAA/Cr and/or Cho/Cr.
To the best of our knowledge, our study is the first report on using MRS in assessing neurological manifestations in CNs. Our results indicate that the amount of reduction of NAA is associated with the severity of clinical and neurological manifestations.
In this study, there was a statistically significant negative correlation between the highest serum bilirubin level and NAA/Cr and Ch/Cr. A previous study 27 reported a decrease in Ch/Cr in the early stage of kernicterus, as a result of neurotoxicity, as well as an increase in myoinositol mI/Cr. The study also suggested that an increase in Ch is more evident in the late stages of the disease rather than in the acute phase.
In this study, patients with lower NAA/Cr and Ch/Cr had a more aggressive disease and required different therapy. Exchange transfusion is an effective emergency management of acute bilirubin encephalopathy by removing bilirubin-saturated albumin and providing free protein, which draws bilirubin from tissues; however, it may have various complications arising from umbilical catheterization including embolism and transmission of infection. 14
In our study, all patients were admitted previously to NICU during their neonatal period for severe, persistent unconjugated hyperbilirubinemia. Newborn infants with CNs are usually admitted to neonatal care units to receive phototherapy for their severe unremitting jaundice. 13
Limitations
Regarding the limitations of this study, first: the small number of studied patients may affect the end conclusion that is reached by our study, further follow-up studies could be done on larger patient groups. Second: different ages of patients and controls may lead to bias as there is a range of ages from infant to adolescent and MRS peak values can be variable with age.
Conclusion
1H-MRS can be a useful tool in the detection of neurological changes in patients with CNs-I, NAA/Cr, and Ch/Cr parameters are well correlated with demographic, clinical, and laboratory findings.
Footnotes
Acknowledgments: I would like to thank Dr Ahmed Abdel Razek, who passed away lately, for his valuable contributions to the paper, may Allah bless his soul.
Competing interests: The authors declare that they have no competing interests.
Ethics approval and consent to participate: Ethics approval and consent to participate were approved and obtained by Mansoura faculty of medicine institutional Research Board (MFM-IRB).
Author’s contribution: Saher Taman: Choosing title of the study, doing MRI examinations, reviewing and preparing images for processing, performing images processing and registering DTI readings, statistical data preparation together with manuscript writing and preparation for submission. Mortada El-Shabrawi: Performing clinical examinations, collecting laboratory results, participating in full history taking, collecting clinical and demographic data together with statistical data preparation. Mohamed Ezz El Regal: Performing clinical examinations, collecting laboratory results, participating in full history taking, collecting clinical and demographic data together with statistical data preparation. Ahmed Megahed: Performing clinical examinations, collecting laboratory results, participating in full history taking, collecting clinical and demographic data together with statistical data preparation. Sherine Elzeny and Noha El Tantawi: Performing clinical examinations, collecting laboratory results, participating in full history taking, collecting clinical and demographic data together with statistical data preparation. Ahmed Abdel Razek: Choosing the title of the study, putting study framework, doing MRI examinations, participating in statistical data preparation together with manuscript reviewing and submission. Ebrahim Abd El Halem: Collecting clinical and demographic data together with statistical data preparation, manuscript writing & reviewing. Eman Alnaghy: Sharing in doing MRI examinations, MR image processing & participating in statistical data preparation together with manuscript writing, reviewing and submission.
Consent for publication: Obtained.
Availability of data and material: available
Contributor Information
Eman Alnaghy, Email: emnaghi@hotmail.com.
Saher Taman, Email: sahertmn@gmail.com.
Ebrahim Abdelhalim, Email: ebrahimhalim40@gmail.com.
Ahmed Abdel Razek, Email: arazek@mans.edu.eg.
Mortada El-Shabrawi, Email: melshabrawi@medicine.cu.edu.eg.
Mohamed Ezz El Regal, Email: ezzregal@yahoo.com.
Ahmed Megahed, Email: megahed732000@gmail.com.
Sherine Elzeny, Email: dr.shery_elzeny@yahoo.com.
Noha El Tantawi, Email: Nohatharwat123@mans.edu.eg.
REFERENCES
- 1.Dhawan A, Lawlor MW, Mazariegos GV, McKiernan P, Squires JE, Strauss KA, et al. Disease burden of crigler-najjar syndrome: systematic review and future perspectives. J Gastroenterol Hepatol 2020; 35: 530–43. doi: 10.1111/jgh.14853 [DOI] [PubMed] [Google Scholar]
- 2.Bhandari J, Thada PK, Yadav D. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing . In: Crigler Najjar Syndrome. ; 2020. [PubMed] [Google Scholar]
- 3.Strauss KA, Ahlfors CE, Soltys K, Mazareigos GV, Young M, Bowser LE, et al. Crigler-najjar syndrome type 1: pathophysiology, natural history, and therapeutic frontier. Hepatology 2020; 71: 1923–39. doi: 10.1002/hep.30959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebrahimi A, Rahim F. Crigler-Najjar syndrome: current perspectives and the application of clinical genetics. Endocr Metab Immune Disord Drug Targets 2018; 18: 201–11. doi: 10.2174/1871530318666171213153130 [DOI] [PubMed] [Google Scholar]
- 5.Kumar P, Sasmal G, Gupta S, Saxena R, Kohli S. Crigler najjar syndrome type 2 (CNS type 2): an unwonted cause of jaundice in adults. J Clin Diagn Res 2017; 11: D05–6. doi: 10.7860/JCDR/2017/28195.10221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Toorn R, Brink P, Smith J, Ackermann C, Solomons R. Bilirubin-Induced neurological dysfunction: a clinico-radiological-neurophysiological correlation in 30 consecutive children. J Child Neurol 2016; 31: 1579–83. doi: 10.1177/0883073816666473 [DOI] [PubMed] [Google Scholar]
- 7.Watchko JF. Bilirubin-induced neurotoxicity in the preterm neonate. Clin Perinatol 2016; 43: 297–311. doi: 10.1016/j.clp.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 8.Rose J, Vassar R. Movement disorders due to bilirubin toxicity. Semin Fetal Neonatal Med 2015; 20: 20–25: S1744-165X(14)00087-0. doi: 10.1016/j.siny.2014.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karimzadeh P, Fallahi M, Kazemian M, Taslimi Taleghani N, Nouripour S, Radfar M. Bilirubin induced encephalopathy. Iran J Child Neurol 2020; 14: 7–19. [PMC free article] [PubMed] [Google Scholar]
- 10.Wisnowski JL, Panigrahy A, Painter MJ, Watchko JF. Magnetic resonance imaging of bilirubin encephalopathy: current limitations and future promise. Semin Perinatol 2014; 38: 422–28. doi: 10.1053/j.semperi.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribeiro BN de F, Lima G de A, Ventura N, Gasparetto EL, Marchiori E. Chronic kernicterus: magnetic resonance imaging findings. Radiol Bras 2016; 49: 407–8. doi: 10.1590/0100-3984.2015.0190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Toorn R, Brink P, Smith J, Ackermann C, Solomons R. Bilirubin-Induced neurological dysfunction: a clinico-radiological-neurophysiological correlation in 30 consecutive children. J Child Neurol 2016; 31: 1579–83. doi: 10.1177/0883073816666473 [DOI] [PubMed] [Google Scholar]
- 13.Bortolussi G, Zentilin L, Baj G, Giraudi P, Bellarosa C, Giacca M, et al. Rescue of bilirubin-induced neonatal lethality in a mouse model of Crigler-Najjar syndrome type I by AAV9-mediated gene transfer. FASEB J 2012; 26: 1052–63. doi: 10.1096/fj.11-195461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapoor D, Singh P, Seth A. Current perspective on exchange transfusion. Indian Pediatr 2017; 54: 961–62. doi: 10.1007/s13312-017-1191-2 [DOI] [PubMed] [Google Scholar]
- 15.Wisnowski JL, Panigrahy A, Painter MJ, Watchko JF. Magnetic resonance imaging of bilirubin encephalopathy: current limitations and future promise. Semin Perinatol 2014; 38: 422–28. doi: 10.1053/j.semperi.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gkoltsiou K, Tzoufi M, Counsell S, Rutherford M, Cowan F. Serial brain MRI and ultrasound findings: relation to gestational age, bilirubin level, neonatal neurologic status and neurodevelopmental outcome in infants at risk of kernicterus. Early Hum Dev 2008; 84: 829–38. doi: 10.1016/j.earlhumdev.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 17.Katar S, Akay HO, Taskesen M, Devecioglu C. Clinical and cranial magnetic resonance imaging (MRI) findings of 21 patients with serious hyperbilirubinemia. J Child Neurol 2008; 23: 415–17. doi: 10.1177/0883073807309780 [DOI] [PubMed] [Google Scholar]
- 18.Razek AAKA, Taman SE, El Regal ME, Megahed A, Elzeny S, El Tantawi N. Diffusion tensor imaging of microstructural changes in the gray and white matter in patients with Crigler-Najjar syndrome type I. J Comput Assist Tomogr 2020; 44: 393–98. doi: 10.1097/RCT.0000000000001008 [DOI] [PubMed] [Google Scholar]
- 19.Yan R, Han D, Ren J, Zhai Z, Zhou F, Cheng J. Diagnostic value of conventional MRI combined with DTI for neonatal hyperbilirubinemia. Pediatr Neonatol 2018; 59: 161–67. doi: 10.1016/j.pedneo.2017.07.009 [DOI] [PubMed] [Google Scholar]
- 20.Watchko JF, Painter MJ, Panigrahy A. Quantitative ADC in bilirubin encephalopathy. Jpn J Radiol 2013; 31: 299–300. doi: 10.1007/s11604-013-0181-0 [DOI] [PubMed] [Google Scholar]
- 21.Razek AAKA, Abdalla A, Gaber NA, Fathy A, Megahed A, Barakat T, et al. Proton MR spectroscopy of the brain in children with neuronopathic Gaucher’s disease. Eur Radiol 2013; 23: 3005–11. doi: 10.1007/s00330-013-2924-9 [DOI] [PubMed] [Google Scholar]
- 22.Razek AAKA, Abdalla A, Ezzat A, Megahed A, Barakat T. Minimal hepatic encephalopathy in children with liver cirrhosis: diffusion-weighted MR imaging and proton MR spectroscopy of the brain. Neuroradiology 2014; 56: 885–91. doi: 10.1007/s00234-014-1409-0 [DOI] [PubMed] [Google Scholar]
- 23.Razek AAKA, Nada N. Correlation of choline/creatine and apparent diffusion coefficient values with the prognostic parameters of head and neck squamous cell carcinoma. NMR Biomed 2016; 29: 483–89. doi: 10.1002/nbm.3472 [DOI] [PubMed] [Google Scholar]
- 24.El-Mewafy ZMH, Razek AAKA, El-Eshmawy MM, El-Eneen NRA, El-Biaomy AAB. Magnetic resonance spectroscopy of the frontal region in patients with metabolic syndrome: correlation with anthropometric measurement. Pol J Radiol 2018; 83: e215–19. doi: 10.5114/pjr.2018.76024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarı S, Yavuz A, Batur A, Bora A, Caksen H. Brain magnetic resonance imaging and magnetic resonance spectroscopy findings of children with kernicterus. Pol J Radiol 2015; 80: 72–80. doi: 10.12659/PJR.892643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamei A, Sasaki M, Akasaka M, Soga N, Kudo K, Chida S. Proton magnetic resonance spectroscopic images in preterm infants with bilirubin encephalopathy. J Pediatr 2012; 160: 342–44. doi: 10.1016/j.jpeds.2011.09.036 [DOI] [PubMed] [Google Scholar]
- 27.Oakden WK, Moore AM, Blaser S, Noseworthy MD. 1H MR spectroscopic characteristics of kernicterus: a possible metabolic signature. AJNR Am J Neuroradiol 2005; 26: 1571–74. [PMC free article] [PubMed] [Google Scholar]
- 28.Verma A, Kumar I, Verma N, Aggarwal P, Ojha R. Magnetic resonance spectroscopy-revisiting the biochemical and molecular milieu of brain tumors. BBA Clin 2016; 5: 170–78. doi: 10.1016/j.bbacli.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Touwen BCL. Clinics inDevelopmental Medicine. In: Examination of the Child with Minor Neurological Dysfunctions. ; 1979. [Google Scholar]
- 30.Hadders-Algra M, Heineman KR, Bos AF, Middelburg KJ. The assessment of minor neurological dysfunction in infancy using the touwen infant neurological examination: strengths and limitations. Dev Med Child Neurol 2010; 52: 87–92. doi: 10.1111/j.1469-8749.2009.03305.x [DOI] [PubMed] [Google Scholar]
- 31.Hamamy H. Consanguineous marriages: preconception consultation in primary health care settings. J Community Genet 2012; 3: 185–92. doi: 10.1007/s12687-011-0072-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaubal AN, Patel R, Choksi D, Shah K, Ingle M, Sawant P. Management of pregnancy in crigler najjar syndrome type 2. World J Hepatol 2016; 8: 530–32. doi: 10.4254/wjh.v8.i11.530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maruo Y, Nakahara S, Yanagi T, Nomura A, Mimura Y, Matsui K, et al. Genotype of UGT1A1 and phenotype correlation between Crigler-Najjar syndrome type II and Gilbert syndrome. J Gastroenterol Hepatol 2016; 31: 403–8. doi: 10.1111/jgh.13071 [DOI] [PubMed] [Google Scholar]
- 34.Lodoso Torrecilla B, Palomo Atance E, Camarena Grande C, Díaz Fernández MC, Hierro Llanillo L, De la Vega Bueno A, et al. Crigler-Najjar syndrome: diagnosis and treatment. An Pediatr (Barc) 2006; 65: 73–78. doi: 10.1157/13090900 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Availability of data and material: available