Abstract
Background
Limited data from small series have suggested that brain metastases from biliary tract cancers (BrM-BTC) affect ≤2% of patients with BTC. We sought to review our experience with patients with BrM-BTC and to identify associations of tumor-related molecular alterations with outcomes.
Materials and Methods
A retrospective review of patients with BTC seen at a tertiary referral center from 2005 to 2021 was performed; patients with BrM-BTC were identified, and clinical and molecular data were collected.
Results
Twenty-one of 823 patients with BTC (2.6%) developed BrM. For patients with BrM-BTC, median follow-up time was 27.9 months after primary BTC diagnosis and 3.1 months after BrM diagnosis. Median time from primary diagnosis to diagnosis of BrM was 14.4 [range, 1.1-66.0] months. Median overall survival (OS) from primary diagnosis was 31.5 [2.9-99.8] months and median OS from BrM diagnosis was 4.2 [0.2-33.8] months. Patients who underwent BrM-directed therapy trended toward longer OS following BrM diagnosis than patients receiving supportive care only (median 6.5 vs 0.8 months, P = .060). The BrM-BTC cohort was enriched for BRAF (30%), PIK3CA (25%), and GNAS (20%) mutations. patients with BrM-BTC with BRAF mutations trended toward longer OS following BrM diagnosis (median 13.1 vs 4.2 months, P = .131).
Conclusion
This is the largest series of patients with BrM-BTC to date and provides molecular characterization of this rare subgroup of patients with BTC. Patients with BrM-BTC may be more likely to have BRAF mutations. With advances in targeted therapy for patients with BTC with actionable mutations, continued examination of shifting patterns of failure, with emphasis on BrM, is warranted.
Keywords: cholangiocarcinoma, bile ducts, intrahepatic, bile ducts, extrahepatic, gallbladder, mutation, genomics
This article reports an institutional experience with brain metastases from biliary tract cancer and provides clinical and genomic characterizations of these patients.
Implications for Practice.
Brain metastases from biliary tract cancers are rare. In this retrospective series of 21 patients with biliary tract cancer brain metastases, tumor-related molecular alterations, and their associations with disease-related outcomes are examined. Patients with brain metastases in this series more commonly had mutations in BRAF, PIK3CA, and GNAS. Further research is warranted to better characterize the utility of screening and optimal management for molecular subgroups.
Introduction
Biliary tract cancers (BTC) are a group of invasive malignancies subdivided into 3 main types: gallbladder carcinoma (GBC), and intrahepatic (iCC) and extrahepatic cholangiocarcinoma (eCC), the latter including both hilar (Klatskin tumor) and distal cholangiocarcinomas.1,2 Prognosis for this disease is poor, with average 5-year survival rates of ~10%.3 Common metastatic sites of BTC include the liver, lymph nodes, and lungs, yet rarely do these malignancies metastasize to the brain.4,5 As such, literature on the subject of brain metastases from BTC (BrM-BTC) is limited, with previous studies reporting incidences of 0.15%-1.4%.4-8
Molecular profiling of BTC has increasingly suggested a genomically-rich landscape (particularly for iCC), with opportunities for targeted therapy. Recent clinical trials have led to the approvals of pemigatinib, ivosidenib, and dabrafenib/trametinib for targetable FGFR2, IDH1, and BRAF metastatic BTC, respectively.9-14 In addition, other targetable mutations such as EGFR and HER2 continue to be studied in clinical trials for patients with advanced BTC.4
While targeted agents may facilitate longer-term survival, it is unknown whether they will impact on patterns of disease progression seen in BTCs. No data to date have characterized the molecular profile of BrM-BTC patients, or the association of molecular status with disease trajectory for patients with BrM-BTC. We report our institutional experience of patients with BTC with BrM, providing a clinical and genomic characterization of patients with BrM-BTC.
Materials and Methods
After approval by the Institutional Review Board (PA14-0646), we conducted a retrospective review of BTC patients seen at a single tertiary care center between 2005 and 2021. Demographic, clinical, and molecular profiling data were extracted from patient medical records. All patients had pathologic confirmation of adenocarcinoma of bile duct origin from the primary tumor or metastasis. Diagnosis of BTC-BrM was confirmed either pathologically (ie, biopsy or resection) in 9 patients or via magnetic resonance imaging (MRI) of the brain by neuroradiologists in the remaining 12 patients. Molecular profiles from patient medical records were obtained from next-generation sequencing (NGS)-based analysis for the detection of somatic mutations, copy number variations, and gene fusions. NGS-based analysis was performed on either DNA extracted from solid tumor tissue or circulating cell free DNA (cfDNA) isolated from plasma in a Clinical Laboratory Improvement Amendments (CLIA)-certified molecular diagnostic laboratory. A comparison of mutations between the BTC-BrM cohort and an institutional cohort of patients with iCC with non-brain extrahepatic metastases (M1) was performed to identify mutations more common in patients with BTC-BrM.
Statistical analysis was performed using statistical software JMP Pro 15 (SAS Institute, Cary, NC) and Stata Version 16.0 (StataCorp, College Station, TX, USA). Fisher’s exact test was used for comparison of proportions. Overall survival (OS) was estimated using the Kaplan-Meier method and log-rank testing was used to compare survival differences between molecular subgroups of patients.
Results
Patient Characteristics
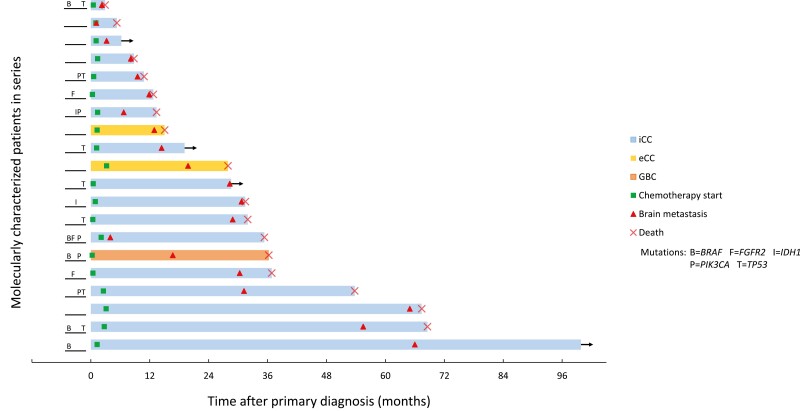
Eight-hundred twenty-three patients with BTC were identified, of whom 21 (2.6%) developed BrM. Patient demographics, clinical characteristics, and treatment details are summarized in Table 1, and the disease course of each patient with key clinical events can be visualized in Fig. 1. A majority of patients with BrM-BTC (70%) had extrahepatic metastatic disease at initial diagnosis. The most common sites of extracranial metastatic disease present at the time of BrM diagnosis were non-regional lymph nodes (62%), lung (48%), peritoneum (24%), bone (19%), and adrenal (19%). BrM in all patients occurred metachronously. The median follow-up time from primary diagnosis was 27.9 months (range, 2.9-99.8 months).
Table 1.
Patient and treatment characteristics.
| Characteristics | Value |
|---|---|
| Age at primary diagnosis, years, median (range) | 56 (36-74) |
| Female sex | 14 (67%) |
| Race/ethnicity | |
| White | 12 (57%) |
| Black/African American | 3 (14%) |
| Hispanic/Latino | 4 (19%) |
| Asian | 1 (5%) |
| Other | 1 (5%) |
| M1 disease at primary diagnosis | 70% |
| Median CA 19-9 level at diagnosis (U/mL) | 154 (<1-350,400) |
| Primary diagnosis | |
| Intrahepatic cholangiocarcinoma | 18 (86%) |
| Extrahepatic cholangiocarcinoma (including hilar) | 2 (10%) |
| Gallbladder carcinoma | 1 (5%) |
| Systemic therapies | |
| Gemcitabine-cisplatin-based therapy | 20 (95%) |
| FOLFIRINOX-based therapy | 9 (43%) |
| Primary tumor-directed therapy | |
| Radiation therapy (external beam) | 12 (57%) |
| Y-90 radioembolization | 4 (19%) |
| TACE | 2 (10%) |
| Hepatectomy | 3 (14%) |
| Liver transplantation | 1 (5%) |
| Brain metastasis-directed therapy | |
| WBRT alone | 6 (29%) |
| SRS alone | 2 (10%) |
| Surgical resection alone | 1 (5%) |
| Surgical resection and SRS | 6 (29%) |
| Surgical resection, WBRT, and SRS | 2 (10%) |
| Supportive care alone | 4 (19%) |
Abbreviations: M1, extrahepatic metastasis; CA19-9, cancer antigen 19-9; FOLFIRINOX, combination chemotherapy of 5-fluoroacil, leucovorin, irinotecan and oxaliplatin; Y-90, yttrium-90; TACE, transarterial chemoembolization; WBRT, whole brain radiation therapy; SRS, stereotactic radiosurgery.
Figure 1.
Patient-level outcomes for 21 patients with BrM-BTC. The time between initial diagnosis and outcome, either last follow-up or death, is represented by the length of each bar shown. Mutation statuses for common mutations are provided.
Nearly all patients (95%) were treated with gemcitabine/cisplatin-based systemic therapy (Table 1). With regard to local liver-directed therapies, 12 patients (57%) received ablative external-beam radiation therapy (A-RT) to the primary tumor, and 4 (19%) were treated with Yttrium-90 (Y-90) radioembolization. Three patients (14%) underwent surgical resection of the primary liver tumor and one patient (5%) underwent orthotopic liver transplantation.
Brain Metastasis Outcomes
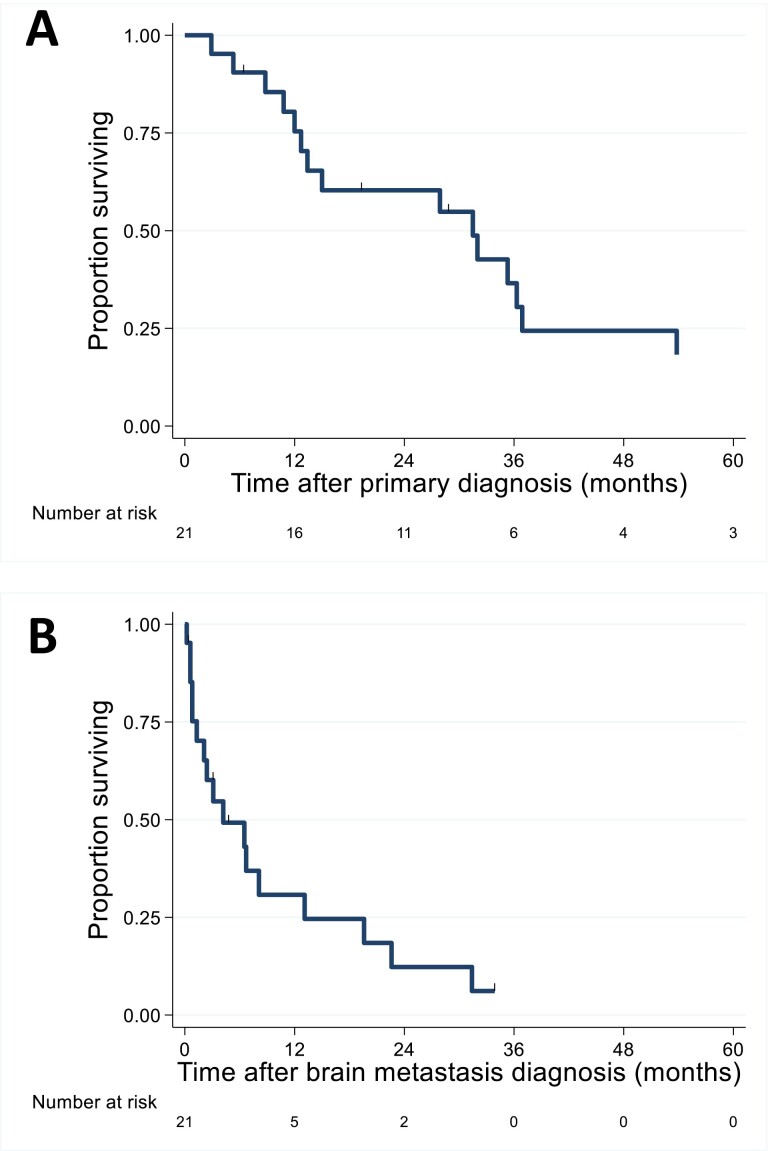
The median time from primary diagnosis to BrM diagnosis was 14.4 months (range 1.1-66.0; Fig. 2A). Patients were characterized as having one solitary brain parenchymal metastasis at diagnosis (9; 43%), two to 3 metastases (6; 29%), 4 to 10 (4; 19%), or 11 to 19 (2; 10%). A majority (17/21; 81%) of patients underwent brain-directed therapy following BrM diagnosis, including surgical resection, stereotactic radiosurgery (SRS), whole brain radiation therapy (WBRT), or a combination of the 3. The choice was based on physician preference. Four patients received supportive care only for their BrM; these results are further characterized in Table 1. Patients who underwent BrM-directed therapy showed a trend toward longer survival from BrM diagnosis when compared with patients receiving supportive care only (median OS, 6.5 vs 0.8 months, P = .060). Among all 21 identified BrM-BTC patients, median OS from BrM diagnosis was 4.2 months (range, 0.2-33.8 months; Fig. 2B), and the median OS from primary diagnosis was 31.5 months (range, 2.9-99.8 months).
Figure 2.
Overall survival (A) following initial diagnosis and (B) following brain metastasis diagnosis.
Mutational Profiling Results
Tumor molecular profiling was performed for 20 out of 21 (95%) patients with BrM-BTC (Table 2; Fig. 1). TP53 mutations, detected in 8 patients (40%), were the most commonly observed alterations, followed by BRAF (30%), KRAS (25%), and PIK3CA (25%) alterations. When compared to a cohort of iCC patients with M1 disease, a significantly greater proportion of patients with BrM had BRAF (30% vs. 8%; P = .008), PIK3CA (25% vs. 8%; P = .029), or GNAS (20% vs. 4%; P = .020). A larger list of comparisons between these cohorts is shown in Table 2. Similarly, a comparison of molecular profiling results for patients with single vs multiple BrM is provided in Table 3. No statistically significant differences were found for mutations between these 2 groups.
Table 2.
List of identified mutations/alterations for patients with BrM-BTC and ICC patients with M1.
| Gene altered | Frequency of molecularly characterized patients (%) | P-value | |
|---|---|---|---|
| BrM-BTC patients (n = 20) | iCC M1 patients (n = 223) |
||
| TP53 | 8 (40%) | 69 (31%) | .454 |
| BRAF | 6 (30%) | 18 (8%) | .008* |
| KRAS | 5 (25%) | 42 (19%) | .554 |
| PIK3CA | 5 (25%) | 18 (8%) | .029* |
| CDKN2A | 4 (20%) | 45 (20%) | 1.000 |
| GNAS | 4 (20%) | 10 (4%) | .020* |
| BRCA2 | 3 (15%) | 15 (7%) | .175 |
| FGFR2 | 3 (15%) | 34 (15%) | 1.000 |
| BAP1 | 3 (15%) | 36 (16%) | 1.000 |
| IDH1 | 2 (10%) | 49 (22%) | .263 |
| MDM2 | 2 (10%) | 8 (4%) | .194 |
| ATM | 2 (10%) | 16 (7%) | .649 |
| No alterations identified | 2 (10%) | 9 (4%) | .226 |
Abbreviations: BrM-BTC, brain metastasis from biliary tract cancer; iCC, intrahepatic cholangiocarcinoma; M1, extrahepatic metastasis.
Table 3.
List of identified mutations/alterations for BrM-BTC patients with single vs. multiple brain metastases.
| Gene altered | Frequency of molecularly characterized patients (%) | P-value | |
|---|---|---|---|
| Single BrM (n = 9) |
Multiple BrM (n = 11) |
||
| TP53 | 4 (44%) | 4 (36%) | 1.000 |
| BRAF | 3 (33%) | 3 (27%) | 1.000 |
| KRAS | 1 (11%) | 4 (36%) | .319 |
| PIK3CA | 2 (22%) | 2 (18%) | 1.000 |
| CDKN2A | 1 (11%) | 3 (27%) | .591 |
| GNAS | 3 (33%) | 1 (9%) | .285 |
| BRCA2 | 2 (22%) | 1 (9%) | .566 |
| FGFR2 | 3 (33%) | 0 (0%) | .074 |
| BAP1 | 2 (22%) | 1 (9%) | .566 |
| IDH1 | 0 (0%) | 2 (18%) | .479 |
| MDM2 | 1 (11%) | 1 (9%) | 1.000 |
| ATM | 1 (11%) | 1 (9%) | 1.000 |
| No alterations identified | 0 (0%) | 2 (18%) | .479 |
Abbreviation: BrM-BTC= brain metastasis from biliary tract cancer.
BRAF mutation was observed in 2 patients with the longest OS from BrM diagnosis (33.8 and 31.4 months). Between patients with and without BRAF mutations, latency to BrM diagnosis following primary diagnosis (median 14.3 vs. 17 months; P = .869) was not significantly different. Similarly, OS following primary diagnosis (median 35.3 vs. 27.9 months; P = .105) was not significantly different. Patients with BrM-BTC with BRAF mutations showed a trend toward longer OS after BrM diagnosis compared to patients without BRAF mutation (median OS, 13.1 vs 4.2 months, P = .203). One patient with a BRAF mutation received BRAF-targeted therapy, a combination of MEK inhibitor binimetinib and BRAF inhibitor encorafenib; this patient exhibited the longest OS from primary diagnosis (99.8 months) and following the development of BrM (33.8 months). Two of 3 patients with FGFR2 mutations received FGFR-targeted therapy. One of 2 patients with an IDH1 mutation received IDH-directed targeted therapy.
Discussion
To date, this study represents the largest BrM-BTC series, with 21 patients, and an estimated occurrence rate of 2.6% for BrM among patients with BTC. Not characterized in prior studies, the genomic analysis presented herein suggests a differential mutational profile for BrM-BTC patients, most notably with enrichment for actionable mutations such as BRAF.
Clinical outcomes of our study are generally comparable to outcomes of prior BrM-BTC studies, although we report a slightly higher incidence of BrM-BTC (2.6% vs 0.15%-1.4%). This higher occurrence rate of BrM-BTC in our cohort may reflect the fact that patients in this cohort received care in a resource-rich environment with readily available, prompt access to diagnostic imaging. The higher incidence may also reflect some selection bias due to the volume and/or status of patients with BTC seen at our tertiary referral center. Many of these patients may have undergone prior had targeted or advanced therapies with longer survival times and therefore had more time to develop or detect BrM. The median time from primary diagnosis to BrM diagnosis in our study was 14.4 months (range 1.1-66.0), similar to 5-17 months reported in previous studies.5-8 Patients in our study also had comparable OS from BrM diagnosis (median 4.2 months). Results from the present series are summarized and compared with prior series in Table 4.
Table 4.
List of selected BrM-BTC case series.
| Series [Reference] (Year) |
Chindaprasirt et al7 (2012) | Frega et al 5 (2018) |
D’Andrea et al6 (2020) | Falkson et al 8 (2022) |
Present series (2022) |
|---|---|---|---|---|---|
| Number of BrM-BTC patients | 8 | 6 | 9 | 15 (6 Stanford, 9 UCSF) |
21 |
| Total number of BTC patients | 5,164 | 450 | 1,190 | Stanford: NA UCSF: 1,055 |
823 |
| Rate of BrM-BTC | 0.15% | 1.4% | 0.47% | Stanford: NA UCSF: 0.85% |
2.6% |
| Median (range) OS from primary diagnosis, months | NA | 23 (9.9-57.6) | 20.6 (0.8-83.6) | 7.1 (0.0-104.1) | 31.5 (2.9-99.8) |
| Median (range) time between primary diagnosis and BrM, months | 8 (0-96) | 13.6 (7.3-52.8) | 16.7 (0.7-66.7) | 5.2 (0.0-29.9) | 14.4 (1.1-66.0) |
| Median (range) OS from BrM diagnosis, months | 2.2 (0.2-6.5) | 3.7 (0.9-17.8) | 3.8 (0.1-16.9) | 1.9 (0.0-95.7) | 4.2 (0.2-33.8) |
Abbreviations: BrM-BTC, brain metastasis from biliary tract cancer; OS, overall survival; NA, not available.
Our molecular profile analysis reveals apparent enrichment of specific molecular alterations among patients with BrM-BTC. When compared with M1 patients with iCC without BrM, our BrM-BTC cohort was enriched for BRAF, PIK3CA, and GNAS alterations. BRAF mutations are particularly rare for patients with BTC, present in just 5%. Patients with BRAF mutations demonstrated a trend toward longer survival following BrM diagnosis when compared with patients without BRAF mutations. It is notable that one patient with a BRAF mutation who lived 33.8 months following BrM diagnosis was treated with encorafenib/binimetinib, which was found to have a 33% objective response rate in the treatment of melanoma BrM.15 BRAF inhibitors, particularly in combination with MEK inhibitors, have been shown to increase response rates,13,16-20 leading to the recent approval of dabrafenib/trameitinib for BRAF V600E metastatic solid tumors.14 While the role of systemic therapies for the management of brain metastases continues to be explored, current guidelines continue to support the use of local therapies, particularly for patients with symptoms.21 The most commonly observed genetic alteration was of TP53, occurring in 8 of 20 molecularly tested patients (40%), all of whom had iCC. However, these findings are consistent with current literature which report TP53 genetic alterations in 44.4% of iCC cases,9 suggesting no significant difference in frequency of TP53 mutations between patients with and without BrM.
This study has several limitations. As a single-institution retrospective series, selection bias is certain; in particular, there may be substantial survivorship bias, since these patients must have lived long enough to develop BrM. It is possible the reported rate of BrM among patients with BTC in this series may not be generalizable, since patients in this series likely had favorable disease control after first-line systemic therapy. Some patients with BrM-BTC may not have been identified due to loss to follow up, since patients from geographically distant areas often return to local institutions for follow-up. All patients with BrM-BTC were identified in this series by symptoms; therefore subclinical BrM-BTC cases may have been missed. Finally, only 9/21 patients had tissue diagnosis of the BrM confirmed, although none of the other patients had synchronous known malignancies that could explain radiographic intracranial findings. Nevertheless, it is possible that genomic profiles of these patients may have discordant genomic profiles between the primary site and intracranial metastatic site, which potentially limits the generalizability of our findings to genomic profiles of the brain metastases themselves.
Conclusion
In this largest series of patients with BrM-BTC to date, genomic analysis revealed risk factors and potentially actionable mutations, including BRAF. Future studies may provide further guidance on optimal management of patients with BrM-BTC, and notably differential patterns of spread and failure by molecular profile. This may inform clinical practice with regard to surveillance imaging, staging workup, and optimal treatment selection and sequencing.
Contributor Information
Grace N Dodoo, Department of Gastrointestinal Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA; Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Brian De, Department of Gastrointestinal Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA; Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Sunyoung S Lee, Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Joseph Abi Jaoude, Department of Gastrointestinal Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA; Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Jean-Nicolas Vauthey, Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Ching-Wei D Tzeng, Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Hop S Tran Cao, Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Kalman A Katlowitz, Department of Neurosurgery, Baylor College of Medicine, Houston, TX, USA; Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Jacob J Mandel, Department of Neurology, Baylor College of Medicine, Houston, TX, USA.
Thomas H Beckham, Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Bruce D Minsky, Department of Gastrointestinal Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Grace L Smith, Department of Gastrointestinal Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Emma B Holliday, Department of Gastrointestinal Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Albert C Koong, Department of Gastrointestinal Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Prajnan Das, Department of Gastrointestinal Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Cullen M Taniguchi, Department of Gastrointestinal Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Milind Javle, Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Eugene J Koay, Department of Gastrointestinal Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Ethan B Ludmir, Department of Gastrointestinal Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA; Department of Biostatistics, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Funding
This work was supported in part by Cancer Center Support (Core) grant P30 CA016672 from the National Cancer Institute, National Institutes of Health, to The University of Texas MD Anderson Cancer Center. B.D. was supported by the RSNA Research & Education Foundation through grant number RR2111. E.K. was supported by DOD (W81XWH-21-1-0709) and NIH (U54CA210181, U54CA143837, U01CA196403, U01CA200468, U01CA200468, U01CA214263, P50CA221707, R01CA221971, R01CA218004, P30CA016672). E.B.L. was supported by the Andrew Sabin Family Fellowship and the Fund for Innovation in Cancer Informatics.
Conflict of Interest
Brian De reported consulting/advisory relationship with Sermo, Inc. Ching-Wei D. Tzeng reported consulting/advisory relationship with PanTher and Ethicon. Emma B. Holliday reported research funding from Merck Serono. Albert C. Koong is a stockholder of Aravive, Inc., a cancer biotech company. Prajnan Das reported a consulting/advisory relationship with Cullen M. Taniguchi reported a consulting/advisory role with Accuray. Milind Javle is an advisory board member/consultant for Helsinn, QED, Taiho, Incyte, AstraZeneca, Meclun, Transthera, EMD Serono, Merck, BMS, Novartis, Servier, Agios, Eli Lilly, and Boehringer Ingelheim. Eugene J. Koay reported research funding from the National Institutes of Health, Stand Up 2 Cancer, MD Anderson Cancer Center, Philips Healthcare, Elekta, GE Healthcare; honoraria from RenovoRx and Taylor and Francis; a consulting/advisory role with AstraZeneca and Augmenix. The other authors indicated no financial relationships.
Author Contributions
Conception/design: All authors. Provision of study material or patients: M.J., E.J.K., E.B.L. Collection and/or assembly of data: G.N.D., B.D. Data analysis and interpretation: G.N.D., B.D., E.J.K., E.B.L. Manuscript writing: G.N.D., B.D., E.J.K., E.B.L. Final approval of manuscript: All authors.
Data Availability
The data that support the findings of this study are available from the corresponding authors, E.J.K. and E.B.L., upon reasonable request within 1 year of publication.
References
- 1. Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX.. Biliary tract cancer. Lancet. 2021;397(10272):428-444. [DOI] [PubMed] [Google Scholar]
- 2. Ghidini M, Pizzo C, Botticelli A, et al. Biliary tract cancer: current challenges and future prospects. Cancer Manag Res 2019;11:379-388 10.2147/CMAR.S157156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bile Duct Cancer (Cholangiocarcinoma) - Statistics. CancerNet 6/16/2022. Available from: https://www.cancer.net/cancer-types/bile-duct-cancer-cholangiocarcinoma/statistics
- 4. Chakrabarti S, Kamgar M, Mahipal A.. Targeted therapies in advanced biliary tract cancer: an evolving paradigm. Cancers (Basel). 2020;12(8):1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frega G, Garajová I, Palloni A, et al. Brain metastases from biliary tract cancer: a monocentric retrospective analysis of 450 patients. Oncology. 2018;94(1):7-11. [DOI] [PubMed] [Google Scholar]
- 6. D’Andrea MR, Gill CM, Umphlett M, et al. Brain metastases from biliary tract cancers: a case series and review of the literature in the genomic era. Oncologist. 2020;25(5):447-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chindaprasirt J, Sookprasert A, Sawanyawisuth K, Limpawattana P, Tiamkao S.. Brain metastases from cholangiocarcinoma: a first case series in Thailand. Asian Pac J Cancer Prev. 2012;13(5):1995-1997. 10.7314/apjcp.2012.13.5.1995 [DOI] [PubMed] [Google Scholar]
- 8. Falkson SR, Zhang K, Bhambhvani HP, et al. Biliary cancer brain metastases: a multi-institution case series with case reports. J Gastrointest Oncol. 2022;13(2):822-832. 10.21037/jgo-21-818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Valle JW, Lamarca A, Goyal L, Barriuso J, Zhu AX.. New horizons for precision medicine in biliary tract cancers. Cancer Discov. 2017;7(9):943-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21(5):671-684. 10.1016/s1470-2045(20)30109-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoy SM. Pemigatinib: first approval. Drugs. 2020;80(9):923-929. 10.1007/s40265-020-01330-y [DOI] [PubMed] [Google Scholar]
- 12. Casak SJ, Pradhan S, Fashoyin-Aje LA, et al. FDA approval summary: ivosidenib for the treatment of patients with advanced unresectable or metastatic, chemotherapy refractory cholangiocarcinoma with an IDH1 mutation. Clin Cancer Res. 2022;28(13):2733-2737. 10.1158/1078-0432.ccr-21-4462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Subbiah V, Lassen U, Élez E, et al. Dabrafenib plus trametinib in patients with <em>BRAF</em>V600E-mutated biliary tract cancer (ROAR): a phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020;21(9):1234-1243. 10.1016/s1470-2045(20)30321-1 [DOI] [PubMed] [Google Scholar]
- 14. FDA grants accelerated approval to dabrafenib in combination with trametinib for unresectable or metastatic solid tumors with BRAF V600E mutation. 2022. 06/23/2022 7/2/22. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dabrafenib-combination-trametinib-unresectable-or-metastatic-solid
- 15. Holbrook K, Lutzky J, Davies MA, et al. Intracranial antitumor activity with encorafenib plus binimetinib in patients with melanoma brain metastases: a case series. Cancer. 2020;126(3):523-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386(9992):444-451. [DOI] [PubMed] [Google Scholar]
- 17. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30-39. 10.1056/nejmoa1412690 [DOI] [PubMed] [Google Scholar]
- 18. Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17(9):1248-1260. 10.1016/s1470-2045(16)30122-x [DOI] [PubMed] [Google Scholar]
- 19. Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367(2):107-114. 10.1056/nejmoa1203421 [DOI] [PubMed] [Google Scholar]
- 20. Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19(5):603-615. 10.1016/s1470-2045(18)30142-6. [DOI] [PubMed] [Google Scholar]
- 21. Vogelbaum MA, Brown PD, Messersmith H, et al. Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol. 2022;40(5):492-516. 10.1200/jco.21.02314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors, E.J.K. and E.B.L., upon reasonable request within 1 year of publication.