Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) are the leading causes of hepatocellular carcinoma (HCC) worldwide. Limited data exist on surgical outcomes for NAFLD/NASH-related HCC compared with other HCC etiologies. We evaluated differences in clinicopathological characteristics and outcomes of patients undergoing surgical resection for NAFLD/NASH-associated HCC compared with other HCC etiologies.
Methods
Demographic, clinicopathological features, and survival outcomes of patients with surgically resected HCC were collected. NAFLD activity score (NAS) and fibrosis score were assessed by focused pathologic review in a subset of patients.
Results
Among 492 patients screened, 260 met eligibility (NAFLD/NASH [n = 110], and other etiologies [n = 150]). Median age at diagnosis was higher in the NAFLD/NASH HCC cohort compared with the other etiologies cohort (66.7 vs. 63.4 years, respectively, P = .005), with an increased percentage of female patients (36% vs. 18%, P = .001). NAFLD/NASH-related tumors were more commonly >5 cm (66.0% vs. 45%, P = .001). There were no significant differences in rates of lymphovascular or perineural invasion, histologic grade, or serum AFP levels. The NAFLD/NASH cohort had lower rates of background liver fibrosis, lower AST and ALT levels, and higher platelet counts (P < .01 for all). Median overall survival (OS) was numerically shorter in NAFLD/NASH vs other etiology groups, however, not statistically significant.
Conclusions
Patients with NAFLD/NASH-related HCC more commonly lacked liver fibrosis and presented with larger HCCs compared with patients with HCC from other etiologies. No differences were seen in rates of other high-risk features or survival. With the caveat of sample size and retrospective analysis, this supports a similar decision-making approach regarding surgical resection for NAFLD/NASH and other etiology-related HCCs.
Keywords: steatohepatitis, NAFLD, NASH, hepatocellular carcinoma, outcome, metabolic syndrome
This article evaluates differences in clinicopathological characteristics and outcomes of patients undergoing surgical resection for non-alcoholic fatty liver disease-associated and non-alcoholic steatohepatitis-associated hepatocellular carcinoma compared with other etiologies.
Implications for Practice.
There is an increasing prevalence of NAFLD/NASH-related HCC, especially in the Western world. The complexity of disease biology and the absence of effective screening guidelines, approved biomarkers, or effective treatment for NAFLD/NASH, makes it imperative to understand the key aspects of this disease better. Our study findings contribute to an enhanced understanding of the disease biology and outcome of NAFLD/NASH-related HCC in patients who underwent surgical resection. The fact that NAFLD/NASH patients were diagnosed with larger tumors highlights the importance of developing biomarkers to predict which patients with NAFLD and NASH will develop HCC to aid in the ability to better screen appropriate patients for the development of HCC.
Introduction
Hepatocellular carcinoma (HCC) accounts for approximately 80% of primary liver cancers and is the fourth leading cause of cancer-related deaths worldwide.1 HCC most commonly occurs in the setting of chronic inflammation and cirrhosis although it can occur in the absence of cirrhosis. Risk factors for HCC include infections (chronic viral hepatitis B [HBV] and hepatitis C [HCV]), excessive alcohol intake, metabolic syndrome (MS)-associated non-alcoholic fatty liver disease (NAFLD), and non-alcoholic steatohepatitis (NASH), exposure to environmental toxins such as aflatoxin, inherited genetic diseases such as hemochromatosis or alpha1-antitrypsin deficiency, autoimmune hepatitis, and primary biliary sclerosis.2,3 Treatment options for HCC vary by the stage of the disease. For early-stage HCC, the treatments of choice include surgical resection, ablation, and orthotopic liver transplant (OLT).4,5
Metabolic syndrome (MS) is a clinical diagnosis defined by the presence of 3 of the following 5 conditions: abdominal obesity, elevated triglycerides, reduced high-density lipoprotein (HDL), hypertension, and impaired fasting glucose.6 It is the major risk factor for the development of NAFLD which is characterized by hepatic steatosis by histology or imaging in the absence of a history of significant alcohol consumption or other known liver diseases. Non-alcoholic steatohepatitis (NASH), a progressive form of NAFLD, currently can only be accurately diagnosed histologically and is characterized by ballooning hepatocellular injury often accompanied by intracytoplasmic aggregated cytokeratin intermediate microfilaments (Mallory–Denk bodies) and lobular inflammation.7 Patients with NASH have an increased risk for the development of liver fibrosis, cirrhosis, and HCC.6
Currently, NAFLD is estimated to be the most common cause of chronic liver disease in the United States, affecting approximately 24% of adults and up to 10% of children, with approximately 10%-25% of those with NAFLD progressing to NASH over time.8,9 A meta-analysis of 9 studies that included greater than 1.5 million participants reported that obesity, defined as a body mass index (BMI) greater than 30 kg/m2, was associated with a 2-fold increased risk of developing HCC.10,11 Recent studies have also shown that lean individuals, especially in Asia, can develop NAFLD11 although the risk of HCC in patients with “lean NAFLD” is not as well defined. An estimated 20%-30% of patients with NASH will develop fibrosis, with 3%-15% developing cirrhosis. The annual incidence rate of HCC in those with NASH is estimated at 5.29 per 1000 person-years.12 The global prevalence of NAFLD and NASH continues to rise, and they are emerging as an increasingly common etiology for HCC in both developed and developing countries.12,13
Previous studies have sought to identify differences in patient demographics and co-morbidities between patients with NAFLD/NASH HCC and non-NASH HCC, as well as different pathways of tumorigenesis.12,14–18 Studies evaluating survival outcomes of patients with HCC arising in NASH versus other etiologies (primarily viral and/or alcohol-related) treated with definitive local therapy (various combinations of transplant, surgery, and ablation) have reached mixed conclusions. While most studies report equivalent relapse-free and overall survival (OS), some studies have demonstrated worse OS with NASH-related HCC compared with combined cohorts of other etiologies, worse OS compared with alcohol-related liver disease (ALD)-associated HCC, and improved survival compared with viral hepatitis-associated HCC.14–18 Few studies have reported specifically on the subset of patients who have had a surgical resection for HCC. Therefore, to address potential differences in this population, we compared patient demographics, co-morbidities, disease characteristics, and clinical outcomes between patients with surgically resected HCC due to NAFLD/NASH and or other etiologies in a large multi-institutional study.
Patients and Methods
In this retrospective study, pathology databases were used to identify patients who underwent surgery for HCC between February 2004 and April 2015 at the Massachusetts General Hospital (MGH) or Brigham and Women’s Hospital, and study follow-up was completed in 2019. The terms “HCC”, “hepatectomy”, “hepatoma,” and “hepatic resection” were used as search terms. The NAFLD/NASH cohort included patients with a NAFLD activity score (NAS) of ≥16 as scored by an attending pathologist (J.M.) or met 3 of 5 criteria for MS defined by the National Cholesterol Education Program’s Adult Treatment Panel III report (Table 1).19 Patients with cryptogenic cirrhosis were included in the NAFLD/NASH cohort, and patients with a history of significant alcohol consumption or other chronic liver diseases were excluded. The cohort of patients with other chronic liver diseases (the “other etiologies cohort”) included those with a known history of chronic HCV, chronic HBV, alcohol abuse, hemochromatosis, or other etiology. Patients were confirmed as having chronic HBV or HCV by serology (HBV surface antigen or DNA positivity; HCV antibody or RNA positivity), and/or they were receiving or had received antiviral therapy for either. Patient medical records were used to determine alcohol consumption. For the patients who received multiple curative intent surgeries for HCC, only the first operation was included in the study. Data were collected retrospectively from the electronic medical record system on a protocol approved by the Partners Institutional Review Board.
Table 1.
Cohort’s characteristics.
| Cohort #1—NAFLD/NASH + cryptogenic cohort (metabolic risk factor related HCC) is defined as: |
| No history of chronic HCV, HBV, alcohol abuse, hemochromatosis, autoimmune hepatitis, or primary sclerosing cholangitis |
| And |
| • Three of the following five characteristics for the adult treatment plan (ATPIII) definition of MS |
| ◦ BMI >28.8 |
| ◦ On an anti-hypertensive medication or BP ≥130/≥85 mmHg on two occasions |
| ◦ On a diabetes medication or HbA1c >6.5% |
| ◦ On a triglyceride-lowering agent or triglycerides ≥150 mg/dL |
| ◦ On a lipid-lowering agent or an HDL for men <40 mg/dL, for women <50 mg/dL |
| Or |
| • NASH activity score (NAS) of ≥1 with at least one point for steatosis |
| ◦ Steatosis (0–3) |
| ◦ Lobular inflammation (0–3) |
| ◦ Hepatocellular ballooning (0–2) |
| ◦ Brunt fibrosis (0–4) |
| Cryptogenic: Cryptogenic cause cirrhosis (CC) is defined as the end stage of a chronic liver disease in which its underlying etiology remains unknown after extensive clinical, serological, and pathological evaluation |
| Cohort #2—Non-NASH or other etiologies cohort is defined as |
| • Known history of chronic HCV, chronic HBV, alcohol abuse (in notes), hemochromatosis, or other etiologies besides NASH |
Abbreviations: ATPIII, adult treatment panel; BMI, body mass index; HBV, chronic viral hepatitis B; HCV, chronic viral hepatitis C; HDL, high-density lipoprotein; MS, metabolic syndrome; NASH, non-alcoholic steatohepatitis; NAS, NASH activity score.
Rationale for Inclusion of HCC Patients Arising in the Background of Cryptogenic Cirrhosis with the NAFLD/NASH Cohort
Although there are multiple potential etiologies for cryptogenic HCC, the majority of these are thought to be due to NAFLD that was not previously recognized.20 This is due to several factors. Not all of the features of MS need to be present for NAFLD and NASH to develop. The absence of central obesity is not a reliable factor for ruling out NASH as up to 25% of patients with NASH do not have central obesity, especially in some Asian countries.21 In addition, there are histologic features that suggest many cases of cryptogenic cirrhosis likely had a NASH etiology.22 Thus, especially before the past decade when NASH became recognized as a common etiology for HCC, a high percentage of these cases would likely have been interpreted as cryptogenic. Tumor size was based on explant pathology, not pre-surgical imaging, and was defined as the largest tumor diameter. For multiple tumors, the sum of the largest tumor diameter of each lesion was used.
Data Collection and Statistical Plan
The data analyzed included demographic factors, clinical risk factors, laboratory values, tumor pathologic features, and survival outcomes. Scoring fibrosis of the background liver was based upon the Brunt and Ishak scoring system,6,23 depending on the etiology of liver disease. Brunt scoring was scaled 0-4 and Ishak scoring was scaled 0-6. For fibrosis, scores of 0-1 were labeled as “absent” and ≥2 as “present”. In cases where fibrosis was not reported in the pathology report, an attending pathologist (J.M.) staged the cases with available tissue based on trichrome stains prepared at the time of initial evaluation.
Continuous variables were compared using the Student t-test. Categorical variables were compared using the chi-square test. Median recurrence-free survival (RFS) and OS after liver resection were calculated using the Kaplan-Meier method and compared using the log-rank test. Categorical and time-to-event outcomes were compared using the chi-square test and Cox proportion hazard regression. Assumptions of baseline hazard proportionality were assessed for each of the available covariates and predictors. In the first stage, a univariate Cox regression model was used to identify the eligible predictors of mortality which showed a marginal association of 10% with the primary outcome (alive vs. deceased). In the second stage, a backward stepwise multiple Cox proportional hazard model was used to identify the independent predictors of mortality with an entry P = .1 and exit P = .05. All analyses were performed using STATA 15.1 (StataCorp, College Station, TX) and 2-sided nominal P < .05 were considered for statistical significance.
Results
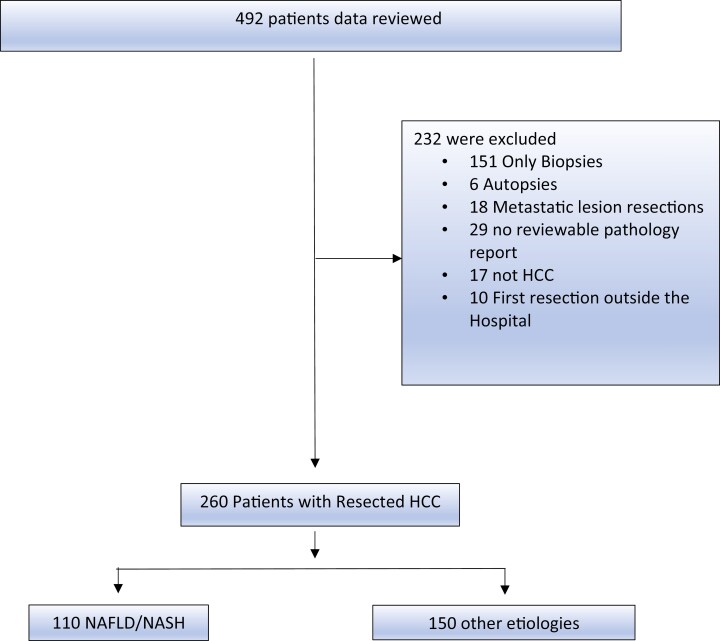
Of the 492 patients identified for this study, 260 patients met the eligibility criteria. The most common reason for exclusion included biopsy specimens only with no history of resection (n = 152) (Fig. 1). Of 260 patients with resected HCC, the presumed cause of HCC was NAFLD/NASH in 61 cases, cryptogenic in 49, and other defined etiology in 150. A subset of patients with a cryptogenic HCC were grouped into the NAFLD/NASH cohort as explained in the Patients and Methods section. The other etiologies cohort included patients with chronic HBV (27%, n = 41), chronic HCV (36%, n = 54), concurrent chronic hepatitis B and C (4%, n = 6), alcoholic-related cirrhosis (29%, n = 43), hemochromatosis (3.3%, n = 5), and alpha-1 antitrypsin deficiency (0.67%, n = 1). Baseline characteristics for the resection group are shown in Table 2.
Figure 1.
CONSORT Diagram of the study depicting screened and eligible populations, reasons for exclusion, and break down of patients into the NAFLD/NASH and other etiologies cohorts.
Table 2.
Baseline characteristics of patients with HCC in the NAFLD/NASH and other etiologies cohorts.
| Variables | NAFLD/NASH N = 110 (42%) |
Other etiologies N = 150 (58%) |
P-value |
|---|---|---|---|
| Gender, n (%) | .001 | ||
| Male | 70 (63.3) | 123 (82.0) | |
| Female | 40 (36.0) | 27 (18.0) | |
| Median age at surgery (years) | 66.7 | 63.4 | .005 |
| Race, n (%) | .001 | ||
| Caucasian | 69 (62.7) | 70 (46.6) | |
| Black | 0 (0) | 9 (6.0) | |
| Hispanic | 4(3.6) | 2 (1.3) | |
| Asian | 4 (3.6) | 34 (22.0) | |
| Other | 1 (0.9) | 0 (0) | |
| Unknown | 32 (29.0) | 35 (23.0) | |
| Risk factors, n (%) | |||
| HBV | 0 (0) | 41 (27.3) | |
| HCV | 0 (0) | 54 (36.0) | |
| HBV + HCV | 0 (0) | 6 (4) | |
| EtOH | 0 (0) | 43 (28.7) | |
| Hemochromatosis | 0 (0) | 5 (3.3) | |
| Other (alfa-1 antitrypsin deficiency) | 0 (0) | 1 (0.67) | |
| Cryptogenic | 49 (19) | 0 (0) | |
| MS | 60 (55.0) | 36 (24.0) | .001 |
| Diabetes | 51 (50.0) | 41 (25.0) | .001 |
| Hypertension | 84 (76.0) | 98 (65.3) | .037 |
| Hypercholesterolemia | 57 (51.8) | 44 (29.3) | .001 |
| Pre-surgical BMI ≥ 28.8 | 46 (43.4) | 37 (25.0) | .003 |
| Median pre-surgical labs | |||
| Total bilirubin | 0.5 | 0.6 | .14 |
| Albumin | 4.0 | 4.2 | .06 |
| Platelets | 252 | 175 | .0001 |
| INR | 1.1 | 1.1 | .58 |
| ALT | 34 | 45 | .004 |
| AST | 36 | 50 | .003 |
| AFP | 10.1 | 10.6 | .23 |
| Cr | 0.98 | 0.94 | .29 |
| MELD | 8.1 | 7.4 | .21 |
| Ascites, n (%) | 7 (6.3) | 8 (5.0) | .72 |
| Hepatic encephalopathy, n (%) | 0 (0) | 3 (2.0) | .13 |
Abbreviations: BMI, body mass index; HBV, chronic viral hepatitis B; HCC, hepatocellular carcinoma; HCV, chronic viral hepatitis C; MS, metabolic syndrome; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis.
Comparison of Patient Demographics and Co-morbidities in NAFLD/NASH and Other Etiologies Cohorts
The median age at diagnosis was higher in the NAFLD/NASH cohort compared with the other etiologies cohort (66.7 vs. 63.4 years, respectively, P = .005), as was the percentage of female patients (36% vs. 18%, P = .001). The NAFLD/NASH population had a higher percentage of White patients compared with other etiologies (63% vs. 47%; P = .001), which had a higher percentage of Asians (22% vs. 4%; P = .001), the latter due to the increased percentage of cases with chronic HBV. Compared with the cohort of patients with other etiologies HCC, the cohort of NAFLD/NASH HCC had a significantly higher percentage of patients with BMI >30.0 kg/m2 (43% vs. 25.0%, P = .003), diabetes mellitus (DM) (83.6% vs. 21.0%, P = .001), hypertension (76% vs. 65.0%, P = .037), and hypercholesterolemia (52% vs. 29%, P = .001).
Comparison of Background Liver on Pathology and of Surrogates of Liver Function on the Laboratory Analysis
Liver fibrosis scoring was available for 102 (39.0%) patients by pathology reports and performed on 260 available cases by an attending pathologist. This data subset included 78 patients in the NAFLD/NASH cohort and 24 patients in the other etiologies cohort. Liver fibrosis was found to be more commonly present in patients with other etiologies of HCC compared with NAFLD/NASH (70.8% vs. 37.1%, P = .005). On baseline laboratory analysis before resection, median ALT and AST were higher in patients with other etiologies compared with NAFLD/NASH (ALT: 45 IU/L vs. 34 IU/L, P = .004; AST: 50 IU/L vs. 36 IU/L, P = .003) (Table 2). The median platelet count for NAFLD/NASH patients was higher (252 × 109/L vs. 175 × 109/L, P < .001). Median MELD scores and the presence of ascites were not significantly different between those with NAFLD/NASH and other etiologies.
Comparison of Tumor Biology in NAFLD/NASH HCC vs HCC Due to Other Etiologies
The tumor characteristics of the patients with NAFLD/NASH HCC and non-NASH HCC were evaluated (Table 3). Patients with NAFLD/NASH HCC, when compared with those with other etiologies HCC, had a higher median tumor size (6.25 vs. 4.5 cm, P = .001) and more frequently had a tumor size ≥5 cm (66% vs. 45%, P = .001). However, no differences were seen in rates of vascular invasion, perineural invasion, or in histologic grade. Similarly, no difference was seen in rates of multifocal tumors. Median AFP values were similar in the two groups (NAFLD/NASH, 10 ng/mL; other etiologies, 10.6 ng/mL).
Table 3.
Pathologic characteristics and staging of patients with HCC in the NAFLD/NASH and other etiologies cohorts.
| Variables | NAFLD/NASH N = 110 (42%) |
Other etiologies N = 150 (58%) |
P-value |
|---|---|---|---|
| Single tumor | .45 | ||
| No | 12 (11.0) | 33 (22.0) | |
| Yes | 71 (65.0) | 79 (52.6) | |
| Unknown | 27 (24.5) | 38 (25.0) | |
| Tumor size, n (%) | .001 | ||
| <5 cm | 37 (33.0) | 81 (54.0) | |
| ≥5 cm | 73 (66.0) | 68 (45.0) | |
| Median tumor size | 6.25 | 4.5 | .001 |
| Vascular invasion | .90 | ||
| Present | 43 (39.0) | 55 (36.6) | |
| Absent | 60 (54.5) | 86 (57.3.0) | |
| Unknown | 7 (6.3) | 9 (6.0) | |
| Perineural invasion | .79 | ||
| Present | 1 (0.9) | 3 (2.0) | |
| Absent | 67 (60.1) | 93 (62.0) | |
| Unknown | 42 (38.1) | 54 (36.0) | |
| Biliary invasion | .28 | ||
| Present | 5 (4.5) | 2 (1.3) | |
| Absent | 66 (60.0) | 96 (64.0) | |
| Unknown | 39 (35.4) | 52 (34.6) | |
| Positive lymph node | .49 | ||
| Yes | 1 (0.91) | 0 (0) | |
| No | 17 (15.4) | 25 (16.6) | |
| Unknown | 92 (83.6) | 125 (83.3) | |
| Histology, n (%) | .71 | ||
| Well differentiated | 18 (16.3) | 23 (15.0) | |
| Well to moderately | 7 (6.5) | 9 (6.0) | |
| Moderately differentiated | 63 (57.0) | 83 (55.3) | |
| Moderately to poor | 10 (9.0) | 11 (7.3) | |
| Poorly differentiated | 6 (5.5) | 17 (11.3) | |
| Unknown | 6 (5.5) | 7 (5.0) | |
| Liver fibrosis at baseline | .005 | ||
| Absent | 49 (62.8) | 7 (29) | |
| Present | 29 (37.1) | 17 (70.8) | |
| Margin status (next to R1 < 1 mm) | .46 | ||
| R0 | 93 (84.5) | 123 (82.0) | |
| R1 | 15 (13.6) | 20 (13.3) | |
| R2 | 2 (1.8) | 7 (4.6) | |
| Stage | .16 | ||
| I | 40 (36.0) | 70 (46.6) | |
| II | 47 (42.7) | 55 (37.0) | |
| III | 21 (19.0) | 25 (16.7) | |
| IV | 2 (1.8) | 0 (0) |
Abbreviations: HCC, hepatocellular carcinoma; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis.
Survival Analysis for Resected Patients
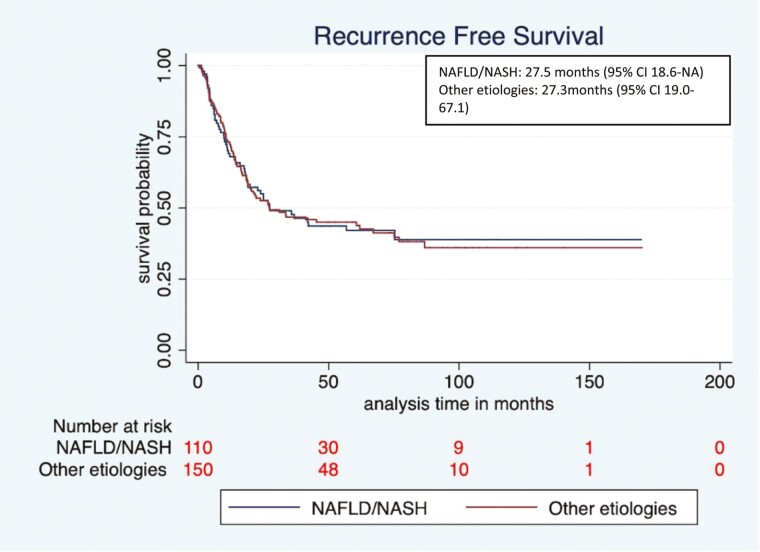
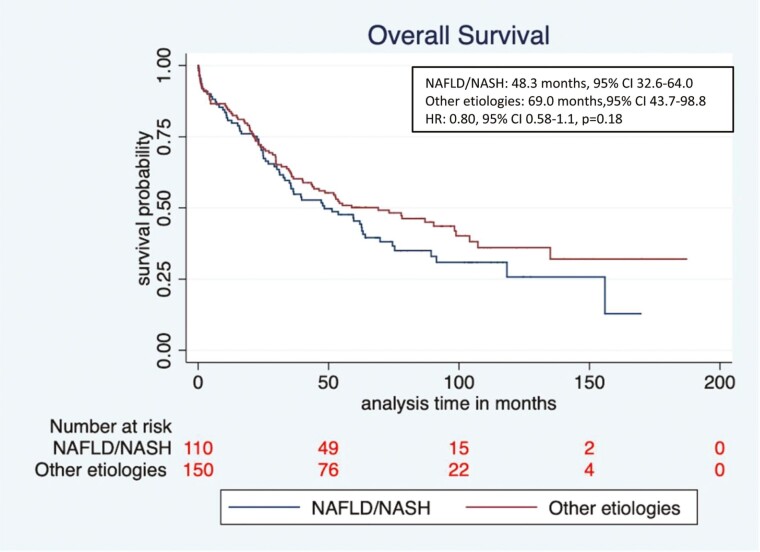
Recurrence-free survival (RFS) and OS of patients in the NAFLD/NASH and other etiologies cohorts were evaluated. Median RFS was similar among patients with NAFLD/NASH vs other etiologies (27.5 months, 95% CI 18.4–NE vs. 27.3 months, 95% CI 19.0-67.1, respectively; hazard ratio (HR) 1.0, 95% CI 0.7-1.4, P = .96). In addition, although the median OS was numerically shorter in the NAFLD/NASH vs. the other etiologies group, this was not statistically significant (48.3 months, 95% CI 33.2-64.0 vs. 69.0 months, 95% CI 43.4-98.8, respectively, HR 0.80, 95% CI 0.58-1.1, P = .18) (Figs. 2 and 3). We also evaluated the survival outcomes in patients with NAFLD/NASH versus those with a viral etiology only, and no difference was seen in RFS (27.5 months, 95% CI 18.6-NE vs. 27.2 months, 95%CI 16.5-86.8, respectively; HR 1.0, 95% CI 0.7-1.4, P = .90) but there was a trend toward lower median OS in the NAFLD/NASH group (48.3 months, 95% CI 32.6-64.0 vs. 86.9 months, 95% CI 40.1-NE respectively; HR 0.73, 95% CI 0.5-1.0, P = .09) (Supplementary Figs. S1 and S2). To be certain that the inclusion of those with cryptogenic cirrhosis combined with those with NAFLD/NASH did not modify the results, we also performed a separate analysis of those with NAFLD/NASH alone versus the combined NAFLD/NASH + cryptogenic patients. No significant difference was observed in the median OS of NAFLD/NASH + cryptogenic versus NAFLD/NASH alone (69.7 months, 95% CI 29.1-NE vs. 47.2 months, 95% CI 32.6-61.9, respectively, HR 1.39, 95% CI 0.85-2.2, P = .18). Median OS among those patients who presented with fibrosis at baseline was numerically shorter in the NAFLD/NASH cohort compared with other etiologies cohort but this was not statistically significant (33.2 months, 95% CI 12.8-59.6 vs. 52.2 months, 95% CI 11.9-NE, HR 0.58, 95% CI 0.28-1.22, P = .15).
Figure 2.
Recurrence-free survival (RFS) of NAFLD/NASH versus other etiologies cohorts.
Figure 3.
Overall survival (OS) NAFLD/NASH vs other etiologies cohorts.
Univariate and Multivariate Analysis
Univariate and multivariate analysis was performed to determine the independent predictors of survival outcome for the patients who underwent resection of HCC-related to NAFLD/NASH or other etiologies. Factors including age, HBV infection, MS, DM, total bilirubin, AST, platelet count, albumin, INR, serum creatinine, tumor histology, tumor size, tumor involvement of resected margin, and vascular invasion were associated with outcome after liver resection in univariate analysis (P < .10) and hence selected for the multivariate analysis (Table 4). The etiology of liver disease was not significantly associated with outcome in the univariate analysis and therefore was not included in the multivariate analysis.
Table 4.
Stepwise multiple Cox regression model to identify the independent predictors of mortality in resected NAFLD/NASH and other etiologies HCC.
| Variable | HR (95% CI) | P-value |
|---|---|---|
| Age | ||
| <55 (reference) | (reference) | |
| ≥55 | 1.7 (1.0–2.7) | .033 |
| Diabetes | ||
| No (reference) | (reference) | |
| Yes | 1.7 (1.2–2.5) | .003 |
| Platelet | ||
| Normal (reference) | 1 (reference) | |
| Low | 2.6 (1.2–5.4) | .008 |
| Resection | ||
| R0 (reference) | (reference) | |
| R1 | 2.4 (1.5–3.9) | <.001 |
| R2 | 2.7 (1.3–5.7) | .008 |
| Vascular invasion | ||
| No (reference) | (reference) | |
| Yes | 1.5 (1.0–2.0) | .015 |
Abbreviations: HCC, hepatocellular carcinoma; HR, hazard ratio; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis.
Multivariate analysis was performed to determine the independent predictors of outcome for the patients who underwent resection. In the multiple Cox regression model, a higher risk of death was seen in patients with age ≥55 years (HR 1.7, 95% CI 1.0-2.7, P = .003), diabetes (HR 1.7, 95% CI 1.2-2.5, P = .003), and a low platelet count (HR 1.3, 95% CI 1.0-1.7, P = .033). As expected, compared with R0 resection (microscopically margin-negative resection), patients with R1 (microscopic margin are positive for tumor) and R2 resections (gross residual tumor and macroscopic margin involvement) had a higher likelihood of mortality (HR 2.4 [95% CI 1.5-3.9], P < .001 and HR 2.7 [95% CI 1.3-5.7], P = .008, respectively) as did those with vascular invasion compared with those without it (HR 1.5, 95% CI 1.0-2.0, P = .015) (Table 4 ).
Discussion
The rising incidence of NAFLD and NASH globally and the increasing proportion of HCC caused by these underscore the importance of understanding the subset of NAFLD/NASH-related HCC.24 In this study comparing clinicopathologic features and prognosis for patients with NAFLD/NASH-related HCC and HCC due to other etiologies, we found that patients with NASH/NAFLD were more commonly female, diagnosed with HCC at an older age, had a higher BMI, had preserved liver function, and a decreased incidence of cirrhosis, similar to what has been reported in previous studies.17,25 This study focused specifically on patients who underwent surgical resection for HCC and found that the NAFLD/NASH patients also had a greater median tumor size but did not have higher rates of other pathological features that portend a poor prognosis. The composite effect of liver factors, tumor factors, and patient factors evaluated in this study summed to no significant difference in RFS or OS between the 2 cohorts.
While the larger tumor size seen in patients with NAFLD/NASH did not translate to worse patient outcomes in our study, a trend toward worse survival was seen. Previous studies have shown variable results in differences between patients with NAFLD/NASH and other etiologies, with some studies showing comparable outcomes to other etiologies similar to our results, with others showing worse outcomes than those with alcoholic liver disease but comparable survival to viral hepatitis.17,26 These inconsistent findings on the prognosis of NAFLD/NASH-related HCC may be explained by differences in sample size, treatment modalities included, definitions of NAFLD/NASH, and reliability of data capture, all resulting in differences in statistical power to detect distinctions.14–18,27–30 Importantly, other studies have included patients treated with a variety of approaches whereas our study evaluated only patients who underwent surgical resection and this may also contribute to differences from some previous studies.
Non-alcoholic fatty liver disease (NAFLD)/NASH-associated HCC is often diagnosed late, possibly at least in part due to decreased screening rates for HCC in this population, and patients with NAFLD/NASH-related HCC continue to have a poor prognosis overall.12,31 In addition to surgery, a liver transplant is the other primary potentially curative approach for localized disease. NAFLD/NASH is increasingly an indication for liver transplants in the United States (,32 A retrospective study done by the United Network for Organ Sharing and Organ Transplantation (UNOS/OPTN) 2003-2014 database concluded that NASH is the most rapidly growing and second leading indication (after alcohol) of liver transplant in the United States.33 Shinginia et al reported that NASH, with or without HCC, related liver transplants have increased over time, especially among younger individuals.34
Prevention provides the best approach for decreasing the morbidity and mortality associated with NAFLD and associated HCC. Broadly educating people about the importance of a healthy diet and exercise to decrease the incidence of obesity and MS is most important. In addition, better diagnostic and prognostic biomarkers for NAFLD/NASH and HCC are needed to improve outcomes.35,36 Multiple factors influence whether and to what extent NAFLD will progress to NASH and HCC, including differences in progression or regression of causes of MS, insulin resistance, other endocrinologic factors such as growth hormone deficiency, fat storage mechanisms, lipid metabolism, presence of lobular and portal inflammation, oxidative and/or endoplasmic reticulum stress, altered immune responses, mitochondrial dysfunction, signaling networks, inflammatory cytokine production, alterations in gut microbiota, complex genetic variation, and epigenetic changes.36–41 This complexity, as well as gaps in knowledge regarding the critical factors that drive progression to HCC, has limited biomarker development. While several non-invasive circulating biomarkers or imaging approaches to monitor this NAFLD/NASH progression have been analyzed, none of these have yet been established as standards of care.42–44 Additionally, early detection of HCC remains a challenge in the NAFLD/NASH population. The population of patients with NAFLD/NASH is too large globally to cost-effectively screen everyone with current surveillance protocols, and therefore more effective, less invasive, and more accessible clinical, serum, and imaging biomarkers are needed for risk stratification.
Finally, novel therapeutic interventions are needed both to prevent the progression of NAFLD to its serious complications and to decrease the progression or recurrence of HCC. Currently, despite extensive study and drug development, no therapeutic agents have gained FDA approval for reducing the progression of NAFLD to NASH or for the treatment of NASH.45-49 Examples of ideas being explored include approaches targeting: (1) growth hormone and insulin-like growth Factor-1 (IGF-1) which may be involved in controlling the development of NAFLD50,51, (2) T-cell protein tyrosine phosphatase (TCPTP) which leads to activation of both STAT-1 and STAT-3 signaling and may be important in the development of NASH HCC in patients with MS,52 and (3) Neuregulin 4, which suppresses NASH-HCC development by restraining the tumor-prone liver microenvironment.53 Also, the recent discovery that individuals with mutations in a gene (CIDEB) that codes for a structural protein in hepatic lipid droplets are protected from developing severe liver disease suggests that targeting this protein, or possibly biology associated with it, might be an avenue for preventing the development of severe liver disease including NAFLD/NASH.49
The primary limitation of this analysis is that it was a retrospective study with limited sample size and with incomplete data in some instances. This limits the ability to determine potentially statistically significant differences between the cohorts. The manual curation of data from chart review, however, did allow for high accuracy and granularity in data collection, and comparative analyses were restricted to variables with data available for the majority of patients. In addition, assessment of steatohepatitis was not reported on all pathology reports so the absence or presence of NAFLD/NASH could not be histologically confirmed in all cases by chart review. We addressed this limitation by collaborating with an attending pathologist who manually reviewed all cases for which tissue was available. We relied on the imperfect surrogate of the presence or absence of MS for the remaining cases and acknowledge that it is possible that some of these patients did not have NAFLD or NASH. Another limitation of this retrospective review is that surgical resection was not randomized and there may have been differences inherently in the cohorts selected for resection.
Continued efforts to better understand the biology of factors that determine the progression of NAFLD to NASH and ultimately HCC are critically important for the development of more effective therapeutic interventions as well as biomarkers of progression from NAFLD to NASH and ultimately HCC allowing for early intervention and treatment as appropriate.
In parallel, as new therapeutics and biomarkers are developed, lifestyle modifications—including dietary changes, sustained weight loss, and increased physical activity—remain the cornerstone of counsel to individual patients to decrease their risk of developing HCC.
Supplementary Material
Contributor Information
Surendra Pal Chaudhary, Division of Oncology, Mass General Cancer Center and Harvard Medical School, Boston, MA, USA.
Stephanie Reyes, Duke University School of Medicine, Durham, NC, USA.
Matthew L Chase, Beth Israel Deaconess Hospital, Needham, MA, USA.
Aparna Govindan, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Lei Zhao, Department of Pathology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Jay Luther, Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Irun Bhan, Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Emily Bethea, Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Joseph W Franses, Division of Oncology, Mass General Cancer Center and Harvard Medical School, Boston, MA, USA.
Elizabeth Paige Walsh, Division of Oncology, Mass General Cancer Center and Harvard Medical School, Boston, MA, USA.
Leigh Anne Dageford, Transplantation Unit, Department of Surgery, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Shoko Kimura, Transplantation Unit, Department of Surgery, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Nahel Elias, Transplantation Unit, Department of Surgery, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Heidi Yeh, Transplantation Unit, Department of Surgery, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
James Markman, Transplantation Unit, Department of Surgery, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Adel Bozorgzadeh, Transplantation Unit, Department of Surgery, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Kenneth Tanabe, Department of Surgery, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Cristina Ferrone, Department of Surgery, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Andrew X Zhu, Jiahui Health, Jiahui International Cancer Center, Shanghai, People’s Republic of China.
Karin Andersson, Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Michael Thiim, Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Onofrio Antonio Catalano, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Avinash Kambadakone, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Parsia A Vagefi, Division of Surgical Transplantation, University of Texas Southwestern, Dallas, TX, USA.
Motaz Qadan, Department of Surgery, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Daniel Pratt, Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Nikroo Hashemi, Division of Gastroenterology, Hepatology and Endoscopy, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA.
Kathleen E Corey, Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Joseph Misdraji, Department of Pathology, Yale New Haven Hospital, Yale University, New Haven, CT, USA.
Lipika Goyal, Division of Oncology, Mass General Cancer Center and Harvard Medical School, Boston, MA, USA.
Jeffrey W Clark, Division of Oncology, Mass General Cancer Center and Harvard Medical School, Boston, MA, USA.
Funding
L.G. receives funding from the American Cancer Society Clinical Scientist Development Grant 134013-CSDG-19-163-01-TBG, the NIH/NCI Gastrointestinal Cancer SPORE P50 CA127003. J.W.C. receives funding from the NIH/NCI Gastrointestinal Cancer SPORE P50-127003 and NIH grant R01CA247441-01A1. AK Research Grant—Philips Healthcare, Research Grant—GE Healthcare, Research Grant—PanCAN, Research Grant-Bayer, Consultant-Bayer.
Conflict of Interest
Surendra Pal Chaudhary is a full-time employee at EMD Serono, Inc. Lipika Goyal reports receiving research funding (to institution) from Adaptimmune, Bayer, Eisai, Merck, Macrogenics, Genentech, Novartis, Incyte, Eli Lilly, Loxo Oncology, Relay Therapeutics, QED, Servier, Taiho Oncology, Leap Therapeutics, Bristol Meyers Squibb, and Nucana; she also serves as an advisor/consultant to Alentis Therapeutics, AstraZeneca, Exelixis, Genentech, H3Biomedicine, Kinnate, QED Therapeutics, Servier, Sirtex Medical Ltd., Taiho Oncology, Inc., and TranstheraBio. Jeffrey William Clark reports receiving research funding (to institution) from Eisai, Exelixis, Genentech, Surface Oncology, and Trisalus Life Sciences; he also receives consulting fees from Foundation Medicine. The other authors indicated no financial relationships.
Author Contributions
Conception/design: S.P.C., L.Z., J.M., L.G., J.W.C. Provision of study material or patients: A.G., J.L., I.B., E.B., J.F., E.P.W., L.A.D., S.K., N.E., H.Y., J.M., K.T., C.F., A.X.Z., K.A., M.T., O.C., A.K., P.V., M.Q., D.P., N.H., K.E.C., J.M., L.G., J.W.C. Collection and/or assembly of data: S.P.C., S.R., L.Z., J.M., L.G., J.W.C. Data analysis and interpretation: S.P.C., S.R., M.L.C., A.G., L.Z., J.M., L.G., J.W.C. Manuscript writing: All authors. Final approval of manuscript: All authors.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2. Puoti C. New insights on hepatocellular carcinoma: epidemiology and clinical aspects. Hepatoma Res. 2018;4:57. 10.20517/2394-5079.2018.67. [DOI] [Google Scholar]
- 3. Trevisani F, Frigerio M, Santi V, Grignaschi A, Bernardi M.. Hepatocellular carcinoma in non-cirrhotic liver: a reappraisal. Dig Liver Dis. 2010;42(5):341–347. 10.1016/j.dld.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 4. Lurje I, Czigany Z, Bednarsch J, et al. Treatment strategies for hepatocellular carcinoma a multidisciplinary approach. Int J Mol Sci. 2019;20(6):1465. 10.3390/ijms20061465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jarnagin W, Chapman WC, Curley S, et al. ; American Hepato-Pancreato-Biliary Association. Surgical treatment of hepatocellular carcinoma: expert consensus statement. HPB (Oxford). 2010;12(5):302–310. 10.1111/j.1477-2574.2010.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kleiner DE, Brunt EM, Van Natta M, et al. ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 7. Noureddin M, Yates KP, Vaughn IA, et al. ; NASH CRN. Clinical and histological determinants of nonalcoholic steatohepatitis and advanced fibrosis in elderly patients. Hepatology. 2013;58(5):1644–1654. 10.1002/hep.26465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 9. Perumpail BJ, Khan MA, Yoo ER, et al. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23(47):8263–8276. 10.3748/wjg.v23.i47.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gupta A, Das A, Majumder K, et al. Obesity is independently associated with increased risk of hepatocellular cancer-related mortality: a systematic review and meta-analysis. Am J Clin Oncol. 2018;41(9):874–881. 10.1097/COC.0000000000000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Younes R, Bugianesi E.. NASH in lean individuals. Semin Liver Dis. 2019;39(1):86–95. 10.1055/s-0038-1677517. [DOI] [PubMed] [Google Scholar]
- 12. Cholankeril G, Patel R, Khurana S, Satapathy SK.. Hepatocellular carcinoma in non-alcoholic steatohepatitis: current knowledge and implications for management. World J Hepatol. 2017;9(11):533–543. 10.4254/wjh.v9.i11.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar R, Priyadarshi RN, Anand U.. Non-alcoholic fatty liver disease: growing burden, adverse outcomes and associations. J Clin Transl Hepatol. 2020;8(1):76–86. 10.14218/JCTH.2019.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pocha C, Xie C.. Hepatocellular carcinoma in alcoholic and non-alcoholic fatty liver disease-one of a kind or two different enemies? Transl Gastroenterol Hepatol. 2019;4:72. 10.21037/tgh.2019.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hester CA, Rich NE, Singal AG, Yopp AC.. Comparative analysis of nonalcoholic steatohepatitis-versus viral hepatitis- and alcohol-related liver disease-related hepatocellular carcinoma. J Natl Compr Canc Netw. 2019;17(4):322–329. 10.6004/jnccn.2018.7105. [DOI] [PubMed] [Google Scholar]
- 16. Weinmann A, Alt Y, Koch S, et al. Treatment and survival of non-alcoholic steatohepatitis associated hepatocellular carcinoma. BMC Cancer. 2015;15:210. 10.1186/s12885-015-1197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reddy SK, Steel JL, Chen HW, et al. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology. 2012;55(6):1809–1819. 10.1002/hep.25536. [DOI] [PubMed] [Google Scholar]
- 18. Yang T, Hu LY, Li ZL, et al. Liver resection for hepatocellular carcinoma in non-alcoholic fatty liver disease: a multicenter propensity matching analysis with HBV-HCC. J Gastrointest Surg. 2020;24(2):320–329. 10.1007/s11605-018-04071-2. [DOI] [PubMed] [Google Scholar]
- 19. Grundy SM, Becker D, Clark LT, et al. Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 20. Caldwell S, Marchesini G.. Cryptogenic vs. NASH-cirrhosis: the rose exists well before its name. J Hepatol. 2018;68(3):391–392. 10.1016/j.jhep.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 21. Wong RJ, Ahmed A.. Obesity and non-alcoholic fatty liver disease: disparate associations among Asian populations. World J Hepatol. 2014;6(5):263–273. 10.4254/wjh.v6.i5.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Caldwell SH, Lee VD, Kleiner DE, et al. NASH and cryptogenic cirrhosis: a histological analysis. Ann Hepatol. 2009;8(4):346–352. [PMC free article] [PubMed] [Google Scholar]
- 23. Everhart JE, Wright EC, Goodman ZD, et al. ; HALT-C Trial Group. Prognostic value of Ishak fibrosis stage: findings from the hepatitis C antiviral long-term treatment against cirrhosis trial. Hepatology. 2010;51(2):585–594. 10.1002/hep.23315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grandison GA, Angulo P.. Can NASH be diagnosed, graded, and staged noninvasively? Clin Liver Dis. 2012;16(3):567–585. 10.1016/j.cld.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14(1):124–31.e1. 10.1016/j.cgh.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62(6):1723–1730. 10.1002/hep.28123. [DOI] [PubMed] [Google Scholar]
- 27. Schotten C, Bechmann LP, Manka P, et al. NAFLD-associated comorbidities in advanced stage HCC do not alter the safety and efficacy of yttrium-90 radioembolization. Liver Cancer. 2019;8(6):491–504. 10.1159/000501484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hashimoto E, Tokushige K.. Hepatocellular carcinoma in non-alcoholic steatohepatitis: growing evidence of an epidemic? Hepatol Res. 2012;42(1):1–14. 10.1111/j.1872-034X.2011.00872.x. [DOI] [PubMed] [Google Scholar]
- 29. Dhamija E, Paul SB, Kedia S.. Non-alcoholic fatty liver disease associated with hepatocellular carcinoma: an increasing concern. Indian J Med Res. 2019;149(1):9–17. 10.4103/ijmr.IJMR_1456_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mikolasevic I, Filipec-Kanizaj T, Mijic M, et al. Nonalcoholic fatty liver disease and liver transplantation—where do we stand? World J Gastroenterol. 2018;24(14):1491–1506. 10.3748/wjg.v24.i14.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rimini M, Kudo M, Tada T, et al. Nonalcoholic steatohepatitis in hepatocarcinoma: new insights about its prognostic role in patients treated with lenvatinib. ESMO Open. 2021;6(6):100330. 10.1016/j.esmoop.2021.100330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wong RJ, Cheung R, Ahmed A.. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59(6):2188–2195. 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 33. Kwong AJ, Ebel NH, Kim WR, et al. OPTN/SRTR 2020 annual data report: liver. Am J Transpl. 2022;22(Suppl 2):204–309. 10.1111/ajt.16978. [DOI] [PubMed] [Google Scholar]
- 34. Shingina A, DeWitt PE, Dodge JL, et al. Future trends in demand for liver transplant: birth cohort effects among patients with NASH and HCC. Transplantation. 2019;103(1):140–148. 10.1097/TP.0000000000002497. [DOI] [PubMed] [Google Scholar]
- 35. Fujiwara N, Kobayashi M, Fobar AJ, et al. A blood-based prognostic liver secretome signature and long-term hepatocellular carcinoma risk in advanced liver fibrosis. Med (New York). 2021;2(7):836–850.e10. 10.1016/j.medj.2021.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fujiwara N, Kubota N, Crouchet E, et al. Molecular signatures of long-term hepatocellular carcinoma risk in nonalcoholic fatty liver disease. Sci Transl Med. 2022;14(650):eabo4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Anstee QM, Targher G, Day CP.. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10(6):330–344. 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 38. Utzschneider KM, Kahn SE.. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91(12):4753–4761. 10.1210/jc.2006-0587. [DOI] [PubMed] [Google Scholar]
- 39. Kutlu O, Kaleli HN, Ozer E.. Molecular pathogenesis of nonalcoholic steatohepatitis-(NASH-) related hepatocellular carcinoma. Canadian J Gastroenterol Hepatol. 2018;2018:8543763. 10.1155/2018/8543763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tilg H, Moschen AR.. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52(5):1836–1846. 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 41. Stål P. Liver fibrosis in non-alcoholic fatty liver disease—diagnostic challenge with prognostic significance. World J Gastroenterol. 2015;21(39):11077–11087. 10.3748/wjg.v21.i39.11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nascimbeni F, Pais R, Bellentani S, et al. From NAFLD in clinical practice to answers from guidelines. J Hepatol. 2013;59(4):859–871. 10.1016/j.jhep.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 43. Sanyal AJ, Friedman SL, McCullough AJ, Dimick-Santos L; American Association for the Study of Liver Diseases. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and drug administration joint workshop. Hepatology. 2015;61(4):1392–1405. 10.1002/hep.27678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. He L, Deng L, Zhang Q, et al. Diagnostic value of CK-18, FGF-21, and related Biomarker panel in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Biomed Res Int. 2017;2017:9729107. 10.1155/2017/9729107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oseini AM, Sanyal AJ.. Therapies in non-alcoholic steatohepatitis (NASH). Liver Int. 2017;37(Suppl 1):97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholics. N Engl J Med. 2006;355(22):2297–2307. 10.1056/nejmoa060326. [DOI] [PubMed] [Google Scholar]
- 47. Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, Vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675–1685. 10.1056/nejmoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen M-M, Cai J-J, Yu Y, She Z-G, Li H.. Current and emerging approaches for nonalcoholic steatohepatitis treatment. Gene Expr. 2019;19(3):175–185. 10.3727/105221619X15536120524171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Verweij N, Haas ME, Nielsen JB, et al. Germline mutations in CIDEB and protection against liver disease. N Engl J Med. 2022;387(4):332–344. 10.1056/nejmoa2117872. [DOI] [PubMed] [Google Scholar]
- 50. Dichtel L eaOPaEamJ-Ahm. Dichtel L, et al. OR27. Presented at: ENDO annual meeting; June 11–14; Atlanta (hybrid meeting).
- 51. Dichtel LE, Cordoba-Chacon J, Kineman RD.. Growth hormone and insulin-like growth factor 1 regulation of nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2022;107(7):1812–1824. 10.1210/clinem/dgac088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dhanasekaran R, Felsher DW.. A tale of two complications of obesity: NASH and hepatocellular carcinoma. Hepatology (Baltimore, MD). 2019;70(3):1056–1058. 10.1002/hep.30649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang P, Chen Z, Kuang H, et al. Neuregulin 4 suppresses NASH-HCC development by restraining tumor-prone liver microenvironment. Cell Metab. 2022;34(9):1359–1376.e7. 10.1016/j.cmet.2022.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.