Abstract
Background
Progression-free survival was significantly longer in patients who received avelumab plus axitinib versus sunitinib as first-line treatment for advanced renal cell carcinoma (aRCC) in a randomized phase III trial. We report long-term safety and efficacy of avelumab plus axitinib as first-line treatment for patients with aRCC from the JAVELIN Renal 100 phase Ib trial (NCT02493751).
Materials and Methods
In this open-label, multicenter, phase Ib study, patients with untreated aRCC received avelumab 10 mg/kg every 2 weeks plus axitinib 5 mg twice daily or with axitinib for 7 days followed by avelumab plus axitinib. Safety and efficacy were assessed in all patients receiving at least one dose of avelumab or axitinib.
Results
Overall, 55 patients were enrolled and treated. Median follow-up was 55.7 months (95% CI, 54.5-58.7). Treatment-related adverse events of any grade or grade ≥3 occurred in 54 (98.2%) and 34 (61.8%) patients, respectively. The confirmed objective response rate was 60.0% (95% CI, 45.9-73.0), including complete response in 10.9% of patients. Median duration of response was 35.9 months (95% CI, 12.7-52.9); the probability of response was 65.8% (95% CI, 46.7-79.4) at 2 years. Median progression-free survival was 8.3 months (95% CI, 5.3-32.0). Median overall survival was not reached (95% CI, 40.8-not estimable); the 5-year overall survival rate was 57.3% (95% CI, 41.2-70.5).
Conclusion
Five-year follow-up for combination treatment with avelumab plus axitinib in previously untreated patients with aRCC showed long-term clinical activity with no new safety signals, supporting use of this regimen within its approved indication in clinical practice (Clinicaltrials.gov NCT02493751).
Keywords: clinical trials as topic, drug therapy, immunotherapy, kidney neoplasms
Based on the results of the JAVELIN Renal 101 trial, avelumab plus axitinib has been approved as first-line treatment for advanced renal cell carcinoma. To provide long-term safety and efficacy data, this article reports 5-year follow-up results from the JAVELIN Renal 100 phase Ib trial.
Implications for Practice.
To the authors’ knowledge, this article presents the longest follow-up reported to date for patients with previously untreated advanced renal cell carcinoma receiving combination treatment with an immune checkpoint inhibitor and a tyrosine kinase inhibitor. Avelumab plus axitinib treatment in this population was associated with long durations of response and long-term overall survival. Safety findings were consistent with previous studies. These data support the use of avelumab plus axitinib combination within its approved indication.
Introduction
Renal cell carcinoma is the most common form of kidney cancer and constitutes approximately 2.2% of all malignant tumors in adults.1,2 The most common form of renal cell carcinoma is clear-cell renal cell carcinoma, which is characterized by mutations that increase the production of vascular endothelial growth factor (VEGF) and drive angiogenesis.2 Antiangiogenic drugs that target VEGF and its receptors (VEGFRs) have shown significant treatment benefits in patients with advanced renal cell carcinoma.2 Axitinib, a highly selective VEGFR tyrosine kinase inhibitor, is approved as monotherapy for second-line treatment of advanced renal cell carcinoma.3 Immune checkpoint inhibitors have also shown antitumor activity in renal cell carcinoma.4-6 Avelumab is a human immunoglobulin G1 monoclonal antibody that binds to PD-L1, inhibiting its interaction with PD-1.7 Combination treatment with an immune checkpoint inhibitor and a tyrosine kinase inhibitor is an established first-line treatment approach for patients with advanced renal cell carcinoma.8,9
The efficacy and safety of avelumab plus axitinib as first-line treatment for patients with advanced renal cell carcinoma was first shown in the JAVELIN Renal 100 phase Ib trial.10 After a median follow-up of 52.1 weeks, the confirmed objective response rate was 58% (95% CI, 44-71), and the median duration of response was not reached. Progression-free survival and overall survival were not reported in the previous publication.10 Treatment-related adverse events of any grade or grade ≥3 occurred in 96% and 58% of patients, respectively. Subsequently, the JAVELIN Renal 101 phase III trial showed significantly longer progression-free survival (hazard ratio, 0.69; 95% CI, 0.56-0.84; P < .001) and a higher objective response rate (51.4% vs 25.7%) with avelumab plus axitinib than with sunitinib in patients with advanced clear-cell renal cell carcinoma.11,12 Overall survival data were immature, and follow-up is ongoing. Based on the results of the JAVELIN Renal 101 trial, avelumab plus axitinib has been approved as first-line treatment for advanced renal cell carcinoma in various countries worldwide.7,13 To provide long-term safety and efficacy data for avelumab plus axitinib as first-line treatment for patients with advanced renal cell carcinoma, we report 5-year follow-up from the JAVELIN Renal 100 phase Ib trial.
Patients and Methods
Full details of the study design have been reported previously.10 Briefly, JAVELIN Renal 100 was a multicenter, single-arm, phase Ib trial designed to evaluate the safety and efficacy of avelumab plus axitinib (ClinicalTrials.gov identifier, NCT02493751). Eligible adults had confirmed advanced renal cell carcinoma with a clear-cell component, a resected primary tumor, at least one measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, an Eastern Cooperative Oncology Group performance status of ≤1, and no previous systemic therapy for advanced renal cell carcinoma.10 The trial was conducted in accordance with the ethics principles of the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice Guidelines. All patients provided written informed consent.
Patients enrolled in the dose-finding phase received axitinib 5 mg twice daily for 7 days, followed by combination therapy with avelumab 10 mg/kg every 2 weeks and axitinib 5 mg twice daily. Patients enrolled into the dose-expansion phase of the study received either combination therapy from the start of treatment or axitinib alone followed by combination treatment. All patients continued treatment until confirmed disease progression, unacceptable toxicity, withdrawal, or loss to follow-up.
The primary endpoint of the study was dose-limiting toxicity within the first 4 weeks (2 cycles) of treatment with avelumab in combination with axitinib, as reported previously.9 Secondary endpoints included safety assessments, confirmed objective response (per RECIST 1.1), disease control rate response (per RECIST 1.1), time to response, duration of response, progression-free survival, and overall survival.
Efficacy and safety endpoints were assessed in all patients who received at least one dose of avelumab or axitinib. Adverse events were classified according to the Medical Dictionary for Regulatory Activities and graded per National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. Antitumor activity was assessed at baseline, every 6 weeks up to 1 year after the first dose, and every 12 weeks thereafter, with responses classified per RECIST 1.1. Objective response rate and disease control rate were estimated and the corresponding exact 2-sided 95% CIs were calculated using the Clopper-Pearson method. Duration of response, progression-free survival, and overall survival were analyzed using the Kaplan-Meier method, and 95% CIs were calculated using the Brookmeyer and Crowley method. Full details of the statistical analyses have been reported previously.10 In this article, results for patients enrolled in the dose-finding and dose-expansion parts are reported together.
Results
Patients and Treatment
Between October 30, 2015, and September 30, 2016, a total of 55 patients were enrolled, including 6 in the dose-finding phase and 49 in the dose-expansion phase. In the dose-expansion phase, 10 patients were assigned to a 7-day lead-in with axitinib therapy before the first cycle of combination therapy.9 Patient characteristics are summarized in Supplementary Table S1. The International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk score was favorable, intermediate, and poor in 18.2%, 61.8%, and 18.2% of patients, respectively. At the data cutoff for this analysis (March 4, 2021), the study had ended and all patients had discontinued study treatment. The median follow-up was 55.7 months (95% CI, 54.5-58.7), and the median duration of treatment was 10.4 months with avelumab (range, 0.5-58.4 months) and 10.6 months with axitinib (range, 0.7-58.7 months). Patients received a median of 19.5 infusions of avelumab (range, 1.0-120.0).
Safety
All 55 patients had at least one adverse event during the trial, and 43 (78.2%) had a grade ≥3 adverse event. Treatment-related adverse events of any grade or grade ≥3 occurred in 54 (98.2%) and 34 (61.8%) patients, respectively (Table 1). The most common grade ≥3 treatment-related adverse events were hypertension (27.3%), lipase increased (9.1%), and palmar-plantar erythrodysesthesia syndrome (9.1%). Serious adverse events occurred in 24 patients (43.6%) and were judged to be treatment related in 13 (23.6%). Ten patients (18.2%) discontinued avelumab and 5 (9.1%) discontinued axitinib due to treatment-related adverse events; the most common reasons were increased alanine aminotransferase level (n = 3) for avelumab and proteinuria (n = 2) for axitinib. One patient died following a treatment-related adverse event (autoimmune myocarditis; reported previously).9
Table 1.
Treatment-related adverse events occurring in ≥10% of patients for any grade events (N=55).
| Any grade | Grade ≥3 | |
|---|---|---|
| Adverse event | 54 (98.2) | 34 (61.8) |
| Diarrhea | 36 (65.5) | 2 (3.6) |
| Fatigue | 28 (50.9) | 2 (3.6) |
| Dysphonia | 27 (49.1) | 0 |
| Hypertension | 26 (47.3) | 15 (27.3) |
| Palmar-plantar erythrodysesthesia syndrome | 19 (34.5) | 5 (9.1) |
| Rash | 16 (29.1) | 0 |
| Alanine aminotransferase increase | 15 (27.3) | 4 (7.3) |
| Aspartate aminotransferase increase | 15 (27.3) | 1 (1.8) |
| Hypothyroidism | 15 (27.3) | 0 |
| Amylase increased | 14 (25.5) | 4 (7.3) |
| Decreased appetite | 13 (23.6) | 1 (1.8) |
| Mucosal inflammation | 13 (23.6) | 1 (1.8) |
| Arthralgia | 12 (21.8) | 2 (3.6) |
| Lipase increased | 12 (21.8) | 5 (9.1) |
| Nausea | 12 (21.8) | 1 (1.8) |
| Infusion-related reaction | 11 (20.0) | 1 (1.8) |
| Pruritus | 11 (20.0) | 0 |
| Proteinuria | 9 (16.4) | 4 (7.3) |
| Weight decreased | 9 (16.4) | 1 (1.8) |
| Stomatitis | 8 (14.5) | 0 |
| Vomiting | 7 (12.7) | 0 |
| Chills | 6 (10.9) | 0 |
| Cough | 6 (10.9) | 0 |
| Dysgeusia | 6 (10.9) | 0 |
| Hypophosphatemia | 6 (10.9) | 2 (3.6) |
| Myalgia | 6 (10.9) | 1 (1.8) |
Values represent n (%).
Infusion-related reactions occurred in 18 patients (32.7%) and were grade 1 or 2 in all except 1 patient (1.8%; grade 3). Immune-related adverse events occurred in 25 patients (45.5%) and were grade ≥3 in 5 patients (9.1%; Table 2). The most common categories of immune-related adverse events of any grade were thyroid disorders (25.5%), rash (16.4%), and hepatitis (5.5%). The most common individual immune-related adverse events of any grade were hypothyroidism (23.6%), increased alanine aminotransferase level (5.5%), and hyperthyroidism (5.5%).
Table 2.
Immune-related adverse events (N=55).
| Any grade | Grade ≥3 | |
|---|---|---|
| Adverse event | 25 (45.5) | 5 (9.1) |
| Endocrinopathies: thyroid disorders | 14 (25.5) | 0 |
| Hypothyroidism | 13 (23.6) | 0 |
| Hyperthyroidism | 3 (5.5) | 0 |
| Blood thyroid-stimulating hormone increase | 1 (1.8) | 0 |
| Rash | 9 (16.4) | 2 (3.6) |
| Dermatitis acneiform | 2 (3.6) | 0 |
| Rash | 2 (3.6) | 0 |
| Maculopapular rash | 2 (3.6) | 1 (1.8) |
| Drug eruption | 1 (1.8) | 1 (1.8) |
| Pruritis | 1 (1.8) | 0 |
| Papular rash | 1 (1.8) | 0 |
| Pustular rash | 1 (1.8) | 0 |
| Hepatitis | 3 (5.5) | 2 (3.6) |
| Alanine aminotransferase increase | 3 (5.5) | 2 (3.6) |
| Aspartate aminotransferase increase | 2 (3.6) | 1 (1.8) |
| Colitis | 1 (1.8) | 1 (1.8) |
| Diarrhea | 1 (1.8) | 1 (1.8) |
| Endocrinopathies: adrenal insufficiency | 1 (1.8) | 0 |
| Myocarditis | 1 (1.8) | 1 (1.8) |
Values represent n (%).
Efficacy
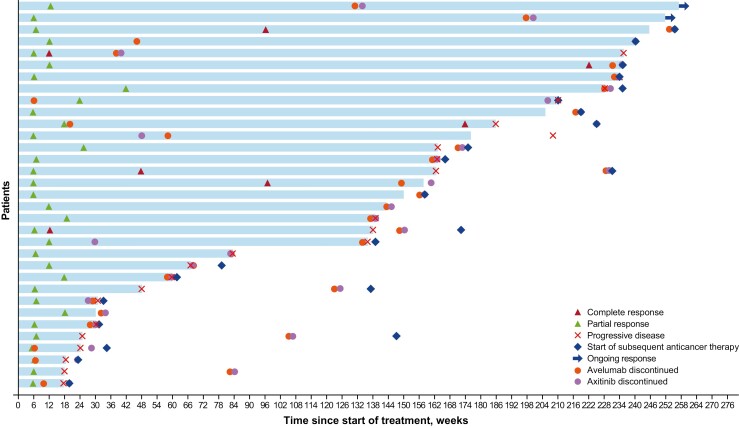
The confirmed objective response rate was 60.0% (95% CI, 45.9-73.0), including complete response in 6 patients (10.9%) and partial response in 27 (49.1%; Table 3). Three patients who had a partial response in the initial analysis converted to complete response, and 1 patient who had stable disease converted to a partial response. The median duration of response was 35.9 months (95% CI, 12.7-52.9). At 2 years, 21 patients had a long-term response; the probability of response was 65.8% (95% CI, 46.7-79.4). Responses were ongoing at data cutoff in 2 patients after 56.54 and 56.28 months, and an additional 6 patients switched treatment to commercial avelumab plus axitinib or axitinib alone while their response was ongoing (duration of response at data censoring was 54.93, 52.50, 52.34, 51.35, 45.90, and 33.18 months; Fig. 1). Four patients had a response that continued for more than 6 months after discontinuing avelumab and axitinib: in 2 patients, treatment was discontinued approximately 28 and 11 months prior to censoring, with ongoing response at last follow-up (duration of response of 56.28 and 56.54 months, respectively); in 1 patient, treatment was discontinued approximately 27 months prior to censoring for missing/inadequate assessment (duration of response, 39.20 months); and in 1 patient, treatment was discontinued approximately 45 months prior to occurrence of progressive disease (duration of response of 52.86 months).
Table 3.
Response assessments.
| Response | N = 55 |
|---|---|
| Confirmed best overall response, n (%) | |
| Complete response | 6 (10.9) |
| Partial response | 27 (49.1) |
| Stable disease | 10 (18.2) |
| Progressive disease | 10 (18.2) |
| Not evaluable | 2 (3.6) |
| Objective response rate (95% CI), % | 60.0 (45.9-73.0) |
| Disease control rate (95% CI), % | 78.2 (65.0-88.2) |
Figure 1.
Durations of confirmed objective responses. Includes responses in patients on study; responses after switching to commercial avelumab plus axitinib were censored.
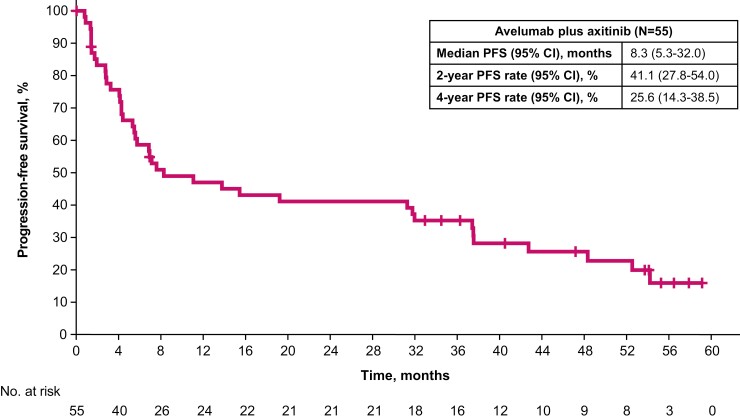
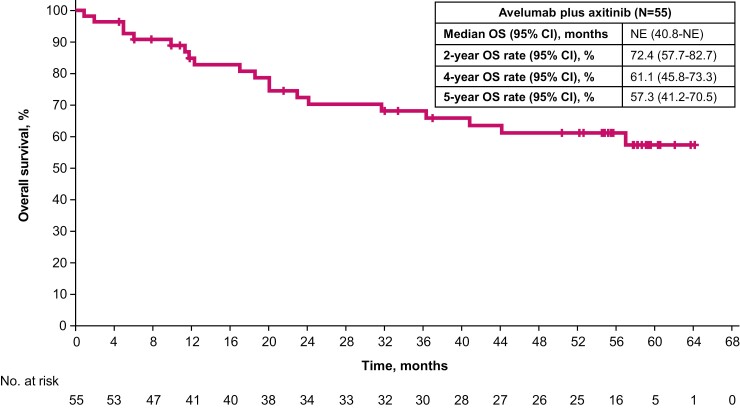
Median progression-free survival was 8.3 months (95% CI, 5.3-32.0) (Fig. 2). The progression-free survival rates at 2 and 4 years were 41.1% (95% CI, 27.8-54.0) and 25.6% (95% CI, 14.3-38.5), respectively. Median overall survival was not reached (95% CI, 40.8-not estimable) (Fig. 3). The overall survival rates at 2, 4, and 5 years were 72.4% (95% CI, 57.7-82.7), 61.1% (95% CI, 45.8-73.3), and 57.3% (95% CI, 41.2-70.5), respectively.
Figure 2.
Progression-free survival.
Figure 3.
Overall survival.
Post-Study Therapy
After discontinuing study treatment, 39 patients (70.9%) received subsequent anticancer drug treatment, including 20 (36.4%) who received ≥2 different regimens (Table 4). The most commonly received drugs (in any line) were cabozantinib (n = 17 [30.9%]), nivolumab (n = 13 [23.6%]), and axitinib (n = 10 [18.2%]).
Table 4.
Subsequent anticancer therapy.
| N = 55 | |
|---|---|
| Received subsequent anticancer drug therapy | |
| Yes | 39 (70.9) |
| No | 0 |
| Not reported | 16 (29.1) |
| Number of subsequent anticancer drug regimens received | |
| 1 | 19 (34.5) |
| 2 | 14 (25.5) |
| 3 | 4 (7.3) |
| ≥4 | 2 (3.6) |
| Not reported | 16 (29.1) |
| Subsequent anticancer drugs received by ≥5% of patientsa | |
| Cabozantinib | 17 (30.9) |
| Nivolumab | 13 (23.6) |
| Axitinib | 10 (18.2) |
| Everolimus | 7 (12.7) |
| Pazopanib | 6 (10.9) |
| Avelumab | 5 (9.1) |
| Ipilimumab | 4 (7.3) |
| Sunitinib | 4 (7.3) |
| Lenvatinib | 3 (5.5) |
| Received subsequent anticancer radiotherapy | |
| Yes | 8 (14.5) |
| No | 25 (45.5) |
| Not reported | 22 (40.0) |
| Received subsequent anticancer surgery | |
| Yes | 5 (9.1) |
| No | 21 (38.2) |
| Not reported | 29 (52.7) |
Values represent n (%).
aPatients may have received ≥1 type of agent; individual agents listed may have been received alone or in combination in any line.
Discussion
We report long-term follow-up of patients with advanced renal cell carcinoma who received first-line treatment with avelumab, an anti-PD-L1 immune checkpoint inhibitor, plus axitinib, a VEGFR tyrosine kinase inhibitor, in the JAVELIN Renal 100 phase Ib trial. In this final analysis, reported with a median follow-up of almost 5 years, avelumab plus axitinib demonstrated long-term clinical benefits. Continued assessment of safety did not identify any new safety signals, and the safety profile of avelumab plus axitinib in this study was consistent with that reported in the JAVELIN Renal 101 phase III trial.11
Compared with the initial analysis, 5-year results showed a slight increase in the objective response rate (from 58.2% [32 of 55] to 60.0% [33 of 55]), with a large increase in the rate of complete response (from 5.5% [3 of 55] to 10.9% [6 of 55]).10 This indicates that the best response can improve over time with avelumab plus axitinib treatment. The objective response rate in this study is similar to that reported in JAVELIN Renal 101 (52.5%).12 Responses were highly durable, as shown by the median duration of response of 35.9 months, and the probability of response was 65.8% at 2 years.
Median progression-free survival was 8.3 months (95% CI, 5.3-32.0), which was lower than that reported in JAVELIN Renal 101 (13.9 months in the most recent analysis).14 The reasons for the difference in median durations of progression-free survival observed in JAVELIN Renal 100 vs JAVELIN Renal 101 are unclear. However, differences between the studies include a smaller patient population in the current study (55 vs 442 in JAVELIN Renal 101) and/or the slightly smaller proportion with favorable IMDC status (18.2% vs 21.3% in JAVELIN Renal 101).10 Median overall survival was not reached after a median follow-up of 55.7 months, and 4- and 5-year overall survival rates were 61.1% and 57.3%, respectively. Similarly, in the JAVELIN Renal 101 trial, median overall survival has not yet been reached with avelumab plus axitinib after a median follow-up of 34.1 months14; the trial is ongoing until the final analysis. After discontinuing study treatment, a high proportion of patients (70.9%) received subsequent anticancer drug therapy with various agents, which may have contributed to the long duration of overall survival observed among study participants.
Patients treated with other immune checkpoint inhibitor plus tyrosine kinase inhibitor combinations have also shown long-term survival with extended follow-up, although cross-trial comparisons should be made with caution because of differences in trial designs and patient populations. In a phase Ib trial of first-line pembrolizumab plus axitinib in patients with advanced renal cell carcinoma, after a median follow-up of 42.7 months, the objective response rate was 73.1%, median duration of response was 22.1 months, median overall survival was not reached, and the 4-year overall survival rate was 66.8%.15 In a phase III trial of first-line pembrolizumab plus axitinib vs sunitinib in patients with advanced renal cell carcinoma, after a median follow-up of 42.8 months, median overall survival was 45.7 vs 40.1 months, and 3-year overall survival rates were 63% vs 54%, respectively.16
This trial had several limitations, including its single-arm design, which prevents direct comparison with another standard of care, and the relatively small population size, which prevents any meaningful subgroup analyses. In addition, although immune checkpoint inhibitor combination therapies have greatly improved treatment outcomes for patients with advanced renal cell carcinoma, not all patients obtain a treatment benefit. Potential strategies to improve patient outcomes that are being explored in other studies include improving drug delivery methods, using state-of-the-art sequencing methods, and increasing the characterization of molecular drivers of variant histology.17
Conclusion
Extended follow-up with the combination of avelumab plus axitinib in treatment-naïve patients with advanced renal cell carcinoma showed long-term clinical benefits with no new safety signals, supporting the use of this treatment regimen within its approved indication in clinical practice.
Supplementary Material
Acknowledgments
The authors thank the patients and their families, investigators, co-investigators, and the study teams at each of the participating centers. This trial was sponsored by Pfizer as part of an alliance between Pfizer and Merck (CrossRef Funder ID: 10.13039/100009945). Medical writing support was provided by Kakoli Parai of ClinicalThinking, Inc., and was funded by Pfizer and Merck.
Contributor Information
James Larkin, Department of Medical Oncology, Royal Marsden NHS Foundation Trust, London, UK.
Mototsugu Oya, Department of Urology, Keio University Hospital, Tokyo, Japan.
Marcella Martignoni, Clinical Development and Operations, Pfizer, Milan, Italy.
Fiona Thistlethwaite, The Christie NHS Foundation Trust, Manchester, UK; Faculty of Biology, Medicine and Health, University of Manchester, Manchester, UK.
Paul Nathan, Department of Medical Oncology, Mount Vernon Cancer Centre, Northwood, UK.
Moshe C Ornstein, Department of Hematology and Medical Oncology, Cleveland Clinic, Cleveland, OH, USA.
Thomas Powles, Barts Cancer Institute, Experimental Cancer Medicine Centre, Queen Mary University of London, St Bartholomew’s Hospital, London, UK.
Kathryn E Beckermann, Department of Internal Medicine, Vanderbilt University Medical Center, Nashville, TN, USA.
Arjun V Balar, Department of Medicine, Perlmutter Cancer Center at NYU Langone Health, New York, NY, USA.
David McDermott, Department of Medical Oncology, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Sumati Gupta, Department of Medicine, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA.
George K Philips, Department of Medicine, Georgetown University Medical Center, Washington, DC, USA.
Michael S Gordon, Division of Cancer Research, HonorHealth Research Institute, Scottsdale, AZ, USA.
Hirotsugu Uemura, Department of Medicine, Kindai University Hospital, Osaka, Japan.
Yoshihiko Tomita, Department of Urology, Graduate School of Medical and Dental Sciences, Niigata University, Niigata, Japan.
Jing Wang, Biostatistics, Pfizer, Cambridge, MA, USA.
Elisabete Michelon, Safety Surveillance & Risk Management, New York, NY, USA.
Alessandra di Pietro, Global Product Development, Pfizer, Milan, Italy.
Toni K Choueiri, Lank Center for Genitourinary Oncology, Dana-Farber Cancer Institute and Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA.
Funding
This trial was sponsored by Pfizer as part of an alliance between Pfizer and Merck (CrossRef Funder ID: 10.13039/100009945).
Ethics Approval and Consent to Participate
The trial was conducted in accordance with the ethics principles of the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice Guidelines. All patients provided written informed consent.
Conflict of Interest
James Larkin has received honoraria from Bristol Myers Squibb, CRUK, Dynavax, Eisai, GSK, Incyte, iOnctura, Merck, Novartis, Pfizer, Roche, touchExperts, and touchIME; has served in a consulting role for Apple Tree, Boston Biomedical, Bristol Myers Squibb, GSK, Immunocore, Incyte, iOnctura, Iovance, Novartis, Pfizer, and YKT Global; has received speaker fees from Aptitude, AstraZeneca, Bristol Myers Squibb, Calithera, Eisai, Ervaxx, EUSA Pharma, GSK, Incyte, Ipsen, Merck, MSD, Novartis, Pierre Fabre, Pfizer, Roche, Seagen, and Ultimovacs; has received institutional research funding from Achilles Therapeutics, Aveo, Bristol Myers Squibb, Covance, Immunocore, MSD, Nektar Therapeutics, Novartis, Pfizer, Pharmacyclics, and Roche; and has received grants from Achilles Therapeutics, Aveo, Bristol Myers Squibb, Immunocore, MSD, Nektar Therapeutics, Novartis, Pfizer, Pharmacyclics, and Roche. Mototsugu Oya has received speaker fees from Bayer, Bristol Myers Squibb, MSD, Novartis, Ono, Pfizer, and Takeda. Marcella Martignoni is an employee of Pfizer and also holds stock and other ownership interests with Pfizer. Fiona Thistlethwaite has served in consulting or advisory roles for Achilles Therapeutics, AdicetBio, Bayer, Bristol Myers Squibb, Enara Bio, ESMO, Evelo Therapeutics, GSK, Ixaka, Janssen, Tknife, and Zelluna Immunotherapy; has received research funding from Novartis; has received reimbursement for travel and accommodation from GSK and Ixaka; and has received conference registration reimbursement from Janssen and Tknife. Paul Nathan has served in consulting or advisory roles for AstraZeneca, Bristol Myers Squibb, MSD, Immunocore, Pfizer, Pierre Fabre, Novartis, GSK, Ipsen, 4SC, and Merck; has served on speaker’s bureaus for Bristol Myers Squibb, Novartis, MSD, and Merck; and has received reimbursement for travel and accommodations expenses from Bristol Myers Squibb and MSD. Moshe C. Ornstein has served in consulting or advisory roles for Aveo, Bristol Myers Squibb, Eisai, Exelixis, MSD, and Pfizer; has served on speaker’s bureaus for Bristol Myers Squibb and Exelixis; has received institutional research funding from Bristol Myers Squibb, MSD, Pfizer, AstraZeneca, and Astellas; and has received reimbursement for travel and accommodation expenses from Bristol Myers Squibb, Pfizer, and Exelixis. Thomas Powles has received honoraria and research funding and served in a consulting or advisory role for Astellas Pharma, AstraZeneca, Bristol Myers Squibb, Eisai, Exelixis, Ipsen, Johnson & Johnson, Merck, MSD, Novartis, Pfizer, Roche, and Seattle Genetics; has received honoraria from and served in a consulting or advisory role for Incyte; and has received travel and accommodation expenses from AstraZeneca, Ipsen, MSD, Pfizer, and Roche. Kathryn E. Beckermann has received institutional grant funding for a young investigator award from LCFA-IASLC-BMS and grant funding for a postdoctoral fellowship from MSD; has served in a consulting role for Aravive; and has served on external advisory boards for Astellas, Bristol Myers Squibb, Exelixis, and Seagen. Arjun V. Balar has received institutional fees for contracted research from AstraZeneca/MedImmune, Genentech, Immunomedics/Gilead, MSD, and Seagen; has served in consulting or advisory roles for AstraZeneca/MedImmune, Bristol Myers Squibb, Genentech, Immunomedics/Gilead, Incyte, Istari Oncology, Janssen, MSD, Nektar, Pfizer, and Seagen; has served on non-speaker’s bureaus for AstraZeneca/MedImmune, Genentech, and MSD; has participated in a steering/scientific advisory committee for MSD and Nektar; has received fees for contracted research from Nektar; has served as a scientific advisory board member for EpiVax Oncology and GT Biopharma; and has equity in EpiVax Oncology and GT Biopharma. David McDermott has received research funding from Bristol Myers Squibb, MSD, Genentech/Roche, Novartis, Peloton Therapeutics, Alkermes, and Prometheus Laboratories; has served in consulting or advisory roles for Bristol Myers Squibb, MSD, Genentech/Roche, Pfizer, Exelixis, Novartis, X4 Pharma, Array BioPharma, Peloton Therapeutics, Merck, Jounce Therapeutics, Alkermes, and Lilly; and has received other fees from Beth Israel Deaconess Medical Center. Sumati Gupta has received institutional fees from Pfizer; has received honoraria from SITC; has received support for attending meetings and/or travel from QED Biopharmaceutical; has participated on a data safety monitoring board or advisory committee for Huntsman Cancer Institute; has conducted correlative studies for the University of Utah; and has other financial or nonfinancial interests with AstraZeneca, Bristol Myers Squibb, Clovis, Debiopharm, Five Prime, Rexahn, Immunocore, Incyte, LSK, LSK/Elevar Therapeutics, MedImmune, MSD, Mirati, Novartis, Pfizer, QED Biopharmaceutical, and Seattle Genetics. George K. Philips has nothing to disclose. Michael S. Gordon has received research funding from AbbVie, Amgen, Array BioPharma, Calithera Biosciences, Celldex, Deciphera, Endocyte, ESSA Pharma, Gilead Sciences, GSK, Incyte, Lilly, Lilly/ImClone, MedImmune, Merck, Millennium, OncoMed, Pfizer, Plexxikon, Roche/Genentech, Seattle Genetics, Tokai Pharmaceuticals, and TRACON Pharma; and has received consulting or advisory honoraria from Castle Biosciences, Deciphera, and RedHill Biopharma. Hirotsugu Uemura has served on speaker’s bureaus for Bayer, Bristol Myers Squibb, Janssen, MSD, and Pfizer; and has received research funding from Astellas, AstraZeneca, Daiichi Sankyo, Janssen, Kissei, Ono, Sanofi, and Takeda. Yoshihiko Tomita has received honoraria from Pfizer, Astellas Pharma, Novartis, Ono Pharmaceutical, Bristol Myers Squibb, and Chugai Pharma; has served in a consulting or advisory role for Novartis, Ono Pharmaceutical, and Taiho Pharmaceutical; and has received research funding from Pfizer, Ono Pharmaceutical, Takeda, Astellas Pharma, AstraZeneca, Novartis, Chugai Pharma, MSD, and Eisai. Jing Wang is an employee of Pfizer. Elisabete Michelon is an employee of Pfizer. Alessandra di Pietro is an employee of Pfizer and holds stock with Pfizer. Toni K. Choueiri reports institutional and personal, paid and unpaid support for research, advisory boards, consultancy, and honoraria from AstraZeneca, Aravive, Aveo, Bayer, Bristol Myers-Squibb, Calithera, Circle Pharma, Eisai, EMD Serono, Exelixis, GlaxoSmithKline, IQVA, Infinity, Ipsen, Jansen, Kanaph, Lilly, Merck, Nikang, Nuscan, Novartis, Pfizer, Roche, Sanofi/Aventis, Surface Oncology, Takeda, Tempest, Up-To-Date, and CME events (Peerview, OncLive, MJH and others), outside the submitted work; reports institutional patents filed on molecular alterations and immunotherapy response/toxicity, and ctDNA; reports equity for Tempest, Pionyr, Osel, and Precede Bio; has served in committees for NCCN, GU Steering Committee, ASCO/ESMO, ACCRU, and KidneyCan; reports that medical writing and editorial assistance support may have been funded by communications companies in part; reports no speaker’s bureau; has mentored several non-US citizens on research projects with potential funding (in part) from non-US sources/foreign components; reports that the institution (Dana-Farber Cancer Institute) may have received additional independent funding of drug companies or/and royalties potentially involved in research around the subject matter; and is supported in part by the Dana-Farber/Harvard Cancer Center Kidney SPORE (2P50CA101942-16) and Program 5P30CA006516-56, the Kohlberg Chair at Harvard Medical School and the Trust Family, Michael Brigham, Pan Mass Challenge and Loker Pinard Funds for Kidney Cancer Research at DFCI.
Author Contributions
Conception/design: M.M., A.d.P., T.K.C. Collection and/or assembly of data: J.L., M.O., M.M., F.T., P.N., M.C.O., T.P., K.E.B., A.V.B., D.M., S.G., G.K.P., M.S.G., H.U., Y.T., A.d.P., T.K.C. Data analysis and interpretation: All authors. Manuscript writing: All authors. Final approval of manuscript: All authors. Accountable for all aspects of the work: All authors.
Data Availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
- 1. Padala SA, Barsouk A, Thandra KC, et al. Epidemiology of renal cell carcinoma. World J Oncol. 2020;11(3):79-87. 10.14740/wjon1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Choueiri TK, Motzer RJ.. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med. 2017;376(4):354-366. 10.1056/NEJMra1601333. [DOI] [PubMed] [Google Scholar]
- 3. Inlyta (Axitinib). Prescribing information. New York, NY: Pfizer; 2020. [Google Scholar]
- 4. Vaishampayan U, Schöffski P, Ravaud A, et al. Avelumab monotherapy as first-line or second-line treatment in patients with metastatic renal cell carcinoma: phase 1b results from the JAVELIN Solid Tumor trial. J ImmunoTher Cancer. 2019;7(1):275. 10.1186/s40425-019-0746-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ross K, Jones RJ.. Immune checkpoint inhibitors in renal cell carcinoma. Clin Sci (Lond). 2017;131(21):2627-2642. 10.1042/CS20160894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Cancer Institute. Immune checkpoint inhibitors. Available at https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/checkpoint-inhibitors. Accessed November 24, 2021.
- 7. Bavencio (avelumab). Prescribing information. EMD Serono, Inc., Rockland, MA, USA, an affiliate of Merck KGaA; 2022. [Google Scholar]
- 8. NCCN Clinical Practice Guidelines in Oncology. Kidney Cancer. V3.2022. Accessed November 7, 2021. [DOI] [PubMed] [Google Scholar]
- 9. Escudier B, Porta C, Schmidinger M, et al. ; ESMO Guidelines Committee. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(5):706-720. 10.1093/annonc/mdz056 [DOI] [PubMed] [Google Scholar]
- 10. Choueiri TK, Larkin J, Oya M, et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): an open-label, dose-finding and dose-expansion, phase 1b trial. Lancet Oncol. 2018;19(4):451-460. 10.1016/S1470-2045(18)30107-4. [DOI] [PubMed] [Google Scholar]
- 11. Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103-1115. 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choueiri TK, Motzer RJ, Rini BI, et al. Updated efficacy results from the JAVELIN Renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol. 2020;31(8)1030-1039. 10.1016/j.annonc.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bavencio (avelumab). Summary of product characteristics. Merck Europe B.V., Amsterdam, Netherlands, an affiliate of Merck KGaA; 2022. [Google Scholar]
- 14. Haanen JBAG, Larkin J, Choueiri TK, et al. Efficacy of avelumab + axitinib (A + Ax) vs sunitinib (S) by IMDC risk group in advanced renal cell carcinoma (aRCC): extended follow-up results from JAVELIN Renal 101. Asia Pac J Clin Oncol. 2021;17(suppl 2):51(abstract 20). 10.1200/JCO.2021.39.6_suppl.302 [DOI] [Google Scholar]
- 15. Atkins MB, Plimack ER, Puzanov I, et al. Axitinib plus pembrolizumab in patients with advanced renal-cell carcinoma: long-term efficacy and safety from a phase Ib trial. Eur J Cancer. 2021;145:1-10. 10.1016/j.ejca.2020.12.009. [DOI] [PubMed] [Google Scholar]
- 16. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for advanced clear cell renal cell carcinoma (ccRCC): results from 42-month follow-up of KEYNOTE-426. J Clin Oncol. 2021;39(15 suppl): Abstract 4500. 10.1200/jco.2021.39.15_suppl.4500. [DOI] [Google Scholar]
- 17. Choueiri TK, Atkins MB, Bakouny Z, et al. Summary from the first Kidney Cancer Research Summit, September 12-13, 2019: a focus on translational research. J Natl Cancer Inst. 2021;113(3):234-243. 10.1093/jnci/djaa064 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.